Introduction
The Gerreidae, a family of primarily marine fishes comprising eight genera and 53 species, has been reported to host infections by species of 13 trematode families. Among these 13 are the Monorchiidae, a speciose group of trematodes that primarily infect invertivore fishes. Currently, five monorchiids are known from gerreids: Alloproctotrema gerres Machida, 1973, Gerricola queenslandensis Wee, Cutmore & Cribb, 2021, Hurleytrema shorti (Nahhas & Powell, 1965) Overstreet, 1969, Postmonorchis orthopristis Hopkins, 1941, and Pseudohurleytrema eucinostomi (Manter, 1942) Yamaguti, 1954 (see Hopkins Reference Hopkins1941; Machida Reference Machida1973; Manter Reference Manter1942; Nahhas and Powell Reference Nahhas and Powell1965; Wee et al. Reference Wee, Cutmore and Cribb2021b). Of these, G. queenslandensis is the only species to be reported from Australian waters and is also the only monorchiid from gerreids to be represented by genetic data. Here, we describe two new monorchiids from Australian gerreids, from Gerres oblongus Cuvier and Gerres oyena (Forsskål) off Lizard Island on the northern Great Barrier Reef, and from Gerres subfasciatus Cuvier from Moreton Bay in southeastern Queensland. Further, we extend the known range for G. queenslandensis to the northern Great Barrier Reef and provide sequence data for this novel host/locality combination.
Materials and methods
Host and parasite collection
Gerreid fishes were collected via seine netting from off Lizard Island on the northern Great Barrier Reef, and from western Moreton Bay, south-eastern Queensland. Fishes were euthanized via cranial pithing or an overdose of anaesthetic (AQUI-S). The gastrointestinal tract was removed and examined for parasites using the gut-wash method described by Cutmore et al. (Reference Cutmore, Bray, Huston, Martin, Miller, Wee, RQ-Y and Cribb2025). Trematodes were washed in vertebrate saline, fixed by pipetting into near-boiling saline, and preserved in 80% ethanol for combined morphological and molecular characterisation. Hologenophores (sensu Pleijel et al. Reference Pleijel, Jondelius, Norlinder, Nygren, Oxelman, Schander, Sundberg and Thollesson2008) were prepared for several specimens.
Morphological analysis
Specimens were washed in fresh water, stained in Mayer’s haematoxylin, destained in dilute HCl (1.0%), neutralized in dilute ammonium hydroxide (1.0%), dehydrated in a graded ethanol series, cleared in methyl salicylate, and mounted in Canada balsam. Measurements were made using an Olympus SC50 digital camera mounted on an Olympus BX-53 compound microscope with cellSens Standard imaging software. Measurements are in micrometres (μm) and presented as a range followed by the mean in parentheses. Drawings were made using the same Olympus BX-53 compound microscope fitted with a drawing tube. Drawings were then digitized using Adobe Illustrator 2025 software. Type- and voucher specimens are lodged in the Queensland Museum (QM), Brisbane, Australia.
Genetic sequencing
Genetic sequence data were generated for two barcoding regions, the partial cytochrome c oxidase 1 mitochondrial DNA region (cox1) and the complete second internal transcribed spacer unit ribosomal DNA noncoding region (ITS2), and one phylogenetically informative region, the partial large ribosomal subunit rDNA region (28S). Total genomic DNA was extracted using the Promega Wizard® SV Genomic DNA Purification System extraction kit following manufacturer’s instructions. Amplification of the cox1 region was performed following the protocols of Wee et al. (Reference Wee, Cribb, Bray and Cutmore2017a) using the primers Dig_cox1Fa (5′-ATG ATW TTY YTD ATG CC-3′; Wee et al. Reference Wee, Cribb, Bray and Cutmore2017a) and Dig_cox1R (5′-TCN GGR TGH CCR AAR AAY CAA AA-3′; Wee et al. Reference Wee, Cribb, Bray and Cutmore2017a). Amplification of the ITS2 and 28S regions was performed following the protocols of Wee et al. (Reference Wee, Cutmore, RQ-Y and Cribb2017b) using the primers 3S (5′-GGT ACC GGT GGA TCA CGT GGC TAG TG-3′; Morgan and Blair Reference Morgan and Blair1995) and ITS2.2 (5′-CCT GGT TAG TTT CTT TTC CTC CGC-3′; Cribb et al. Reference Cribb, Anderson, Adlard and Bray1998) for the ITS2 amplification, and LSU5 (5′-TAG GTC GAC CCG CTG AAY TTA AGC A-3′; Littlewood Reference Littlewood1994) and 1500R (5′-GCT ATC CTG AGG GAA ACT TCG -3′; Snyder and Tkach Reference Snyder and Tkach2001) for the 28S amplification. All amplifications were conducted on a Takara TP-650 PCR Thermocycler. Sanger sequencing was conducted using the amplification primers for the cox1 and ITS2 regions, and an internal set of primers of the 28S region: 300F (5′-CAA GTA CCG TGA GGG AAA GTT G-3′; Littlewood et al. Reference Littlewood, Curini-Galletti and Herniou2000) and ECD2 (5′-CCT TGG TCC GTG TTT CAA GAC GGG-3′; Littlewood et al. Reference Littlewood, Rohde and Clough1997). Contiguous sequences were assembled and edited with Geneious® software.
Alignments of the cox1 and ITS2 datasets were conducted independently in MEGA version X with the MUSCLE algorithm and UPGMA clustering for iterations 1 and 2 (Kumar et al. Reference Kumar, Stecher, Li, Knyaz and Tamura2018). To determine the correct reading frame, the cox1 alignment was translated with the echinoderm/flatworm mitochondrial code and inspected for internal stop codons in MESQUITE version 2.1.10 (Maddison and Maddison Reference Maddison and Maddison2019). Once the correct reading frame was identified, the alignment was trimmed so that the reading frame began on position 1. All codons in the alignment were then tested for non-stationarity and substitution saturation with a χ2 test run on PAUP* (Swofford Reference Swofford2002) and Xia’s test as implemented in DAMBE7 (Xia Reference Xia2018; Xia and Lemey Reference Xia, Lemey, Lemey, Salemi and Vandamme2009; Xia et al. Reference Xia, Xie, Salemi, Chen and Wang2003), respectively. No significant non-stationarity and substitution saturation was detected. Neighbour-joining (NJ) analyses were conducted in MEGA version 11 (Tamura et al. Reference Tamura, Stecher and Kumar2021) for both cox1 and ITS2 datasets using the following parameters: ‘test of phylogeny = bootstrap’, ‘no. of bootstrap replicates = 10,000’, ‘model/method = no. of differences’, ‘substitutions to include = d: Transitions + Transversions’, and ‘rates among sites = uniform rates’.
Newly generated partial 28S sequence data were aligned with sequences of other comparable monorchiid taxa available on GenBank (Table 1) using MUSCLE version 3.7 (Edgar Reference Edgar2004) with ClustalW sequence weighting and UPGMA clustering for iterations 1 and 2. The resulting alignment was trimmed manually, and indels larger than three bases, and affecting >5% of sequences were removed; the removed bases amounted to less than 2% of the initial trimmed alignments, resulting in a final alignment of 1,266 base positions. Maximum likelihood (ML) and Bayesian inference (BI) analyses were conducted using implementations of RAxML version 8.2.6 (Stamatakis Reference Stamatakis2014) and MrBayes version 3.2.7a (Ronquist et al. Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012), respectively, in the CIPRES portal (Miller et al. Reference Miller, Pfeiler and Schwartz2010). Both analyses were run with the closest estimation of the TVM+I+Γ model of evolution, based on implementations of the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) in jModelTest version 2.1.10 (Darriba et al. Reference Darriba, Taboada, Doallo and Posada2012). The ML analysis was run with 1,000 bootstrap pseudoreplicates. The BI analysis was run over 10,000,000 generations (ngen = 10,000,000) with two runs each containing four simultaneous Markov chain Monte Carlo (MCMC) chains (nchains = 4), and every 1,000th tree saved. The following parameters were used in the analysis: ‘nst = 6’, ‘rates = invgamma’, ‘ngammacat = 4’, and the priors parameters for the combined dataset were set to ‘ratepr = variable’. Samples of substitution model parameters were ‘sump burnin = 3,000’ and ‘sumt burnin = 3,000’. Sequence data for the Lissorchiidae, sister family to the Monorchiidae, were included in this dataset, and the deropristid Skrjabinopsolus nudidorsalis Sokolov, Voropaeva & Atopkin, Reference Sokolov, Voropaeva and Atopkin2020 was designated as the outgroup, based on phylogenetic findings of Sokolov et al. (Reference Sokolov, Voropaeva and Atopkin2020).
Table 1. Collection data for 28S sequences from GenBank analysed in this study
Species recognition criteria
Species were delineated using the species recognition criteria first proposed by Bray et al. (Reference Bray, Cutmore and Cribb2023) and further expanded upon by Cribb et al. (Reference Cribb, Barton, Blair, Bott, Bray, Cutmore, De Silva Manage, Faltýnková, Gonchar, Hechinger, Herrmann, Huston, Kremnev, Kuchta, Johnson, Louvard, Miller, Ogawa, Pérez-Ponce de León, Powell, Smit, Tkach, Truter, Waki, Vermaak, Wee, Yong and Achatzin-press).
Results
Overview
A total of 43 gerreid fishes were examined, 21 individuals of G. oblongus and 21 of G. oyena from off Lizard Island and one individual of G. subfasciatus from Moreton Bay; of these, three G. oblongus, four G. oyena and the sole G. subfasciatus were infected with adult trematodes consistent with the concept of the Monorchiidae. The new specimens conform to three morphologically distinct species; one morphotype was identified as Gerricola queenslandensis, but the remaining two did not conform convincingly to any known monorchiid genus.
A total of 20 cox1 sequences were generated from the new material, producing three clades that differ from each other at 74–110 base positions (15.6–23.2%); sequences within clades differed at 0–11 (0–2.3%) base positions which we interpret as intraspecific variation. One of the cox1 genotypes matched published data for G. queenslandensis; the other two genotypes did not match any previously published monorchiid sequence data. Eight ITS2 sequences were generated for specimens relating to the three cox1 clades; these sequences related to three genotypes which differed from each other at 52–86 base positions (10.4–17.2%). One ITS2 genotype was identical to published data for G. queenslandensis; the other two genotypes did not match any previously published monorchiid sequence data. Four 28S sequences were generated for the two unmatched genotypes. Analysis of these sequences with published data for G. queenslandensis identified three lineages which differed at 69–150 base positions (5.2–11.3%). Phylograms generated from the Bayesian inference and maximum likelihood analyses of the 28S rDNA dataset demonstrate that the three gerreid-infecting species form a well-supported clade, with the two unidentified species forming a clade sister to G. queenslandensis; the three species are separated by notably long branch lengths. Based on morphological and molecular distinctions (see Discussion), the three clades are interpreted as representing species of three genera, Gerricola Wee, Cutmore & Cribb, 2021 and two new genera proposed here to accommodate these new taxa.
Taxonomy
Family Monorchiidae Odhner, 1911
Subfamily Monorchiinae Odhner, 1911
Obscuromonorchis n. g.
Diagnosis
Body elongate pyriform. Tegument thin, spined. Eye-spot pigment present in anterior half of body. Oral sucker terminal, round, with opening subterminal. Ventral sucker round, in anterior half of body. Prepharynx short. Pharynx markedly smaller than oral sucker, subspherical. Oesophagus simple, moderately long, slightly sinuous. Intestine bifurcates immediately anterior to ventral sucker. Intestinal caeca blind, long, extend to level of posterior half of testis, do not enter post-testicular zone. Testis single, entire, in posterior half of hindbody. Cirrus-sac subcylindrical, in middle third of body, extends from level of ovary to level of anterior portion of ventral sucker. Seminal vesicle unipartite, ellipsoidal. Pars prostatica short to moderately long, simple. Cirrus prominent, subcylindrical, armed with robust spines. Genital atrium aspinous. Common genital pore median, immediately anterior to ventral sucker. Ovary entire, occasionally with indistinct lobes, dextro-submedial, antero-dextral to and contiguous with testis. Uterine seminal receptacle present. Vitellarium composed of two lateral masses of densely clustered follicles in anterior third of hindbody. Uterus extensive in hindbody, extends beyond gonads posteriorly, without discernible metraterm, enters terminal organ at posterior end. Terminal organ unipartite, with occasional significant tapering in anterior half that results in bipartite appearance, armed with robust spines. Eggs numerous, unfilamented. Excretory pore terminal. In intestine of gerreid fishes.
Type and only species: Obscuromonorchis ranae n. g., n. sp.
ZooBank registration: The LSID for Obscuromonorchis n. g. is urn:lsid:zoobank.org:act:E84BBC91-5F79-4B71-AC50-7FCD5127D09E.
Etymology: The name is derived from the Latin obscurus, meaning indistinct, referring to the often difficult to discern internal and external morphology of the terminal organ, and monorchis, as the genus belongs in the Monorchiidae.
Obscuromonorchis ranae n. sp.
Type host: Gerres oblongus Cuvier, Slender silverbiddy (Gerreidae)
Type locality: off Lizard Island, northern Great Barrier Reef, Queensland, Australia (14°40′S, 145°27′E)
Site of infection: Intestine
Prevalence: 3/21 (14.3%)
Deposition of specimens: Holotype and 18 paratypes, including hologenophores (QM G241951–G241969)
Molecular sequence data: cox1 mtDNA, four sequences, three submitted to GenBank (GB PV768663–PV768665); ITS2 rDNA, two sequences, one submitted to GenBank (GB PV771108); 28S rDNA, one sequence (GB PV771111).
ZooBank registration: The LSID for Obscuromonorchis ranae n. g., n. sp. is urn:lsid:zoobank.org:act:742B6F99-34AA-495E-96F0-41055CFE3F14.
Etymology: This species is named in honour of the first author’s cousin, Randee ‘Ran’ Wee, in recognition of her unwavering long-term support and encouragement.
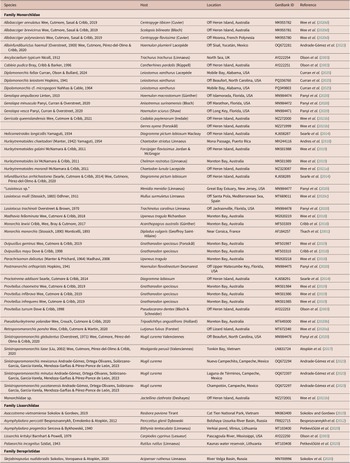
Description
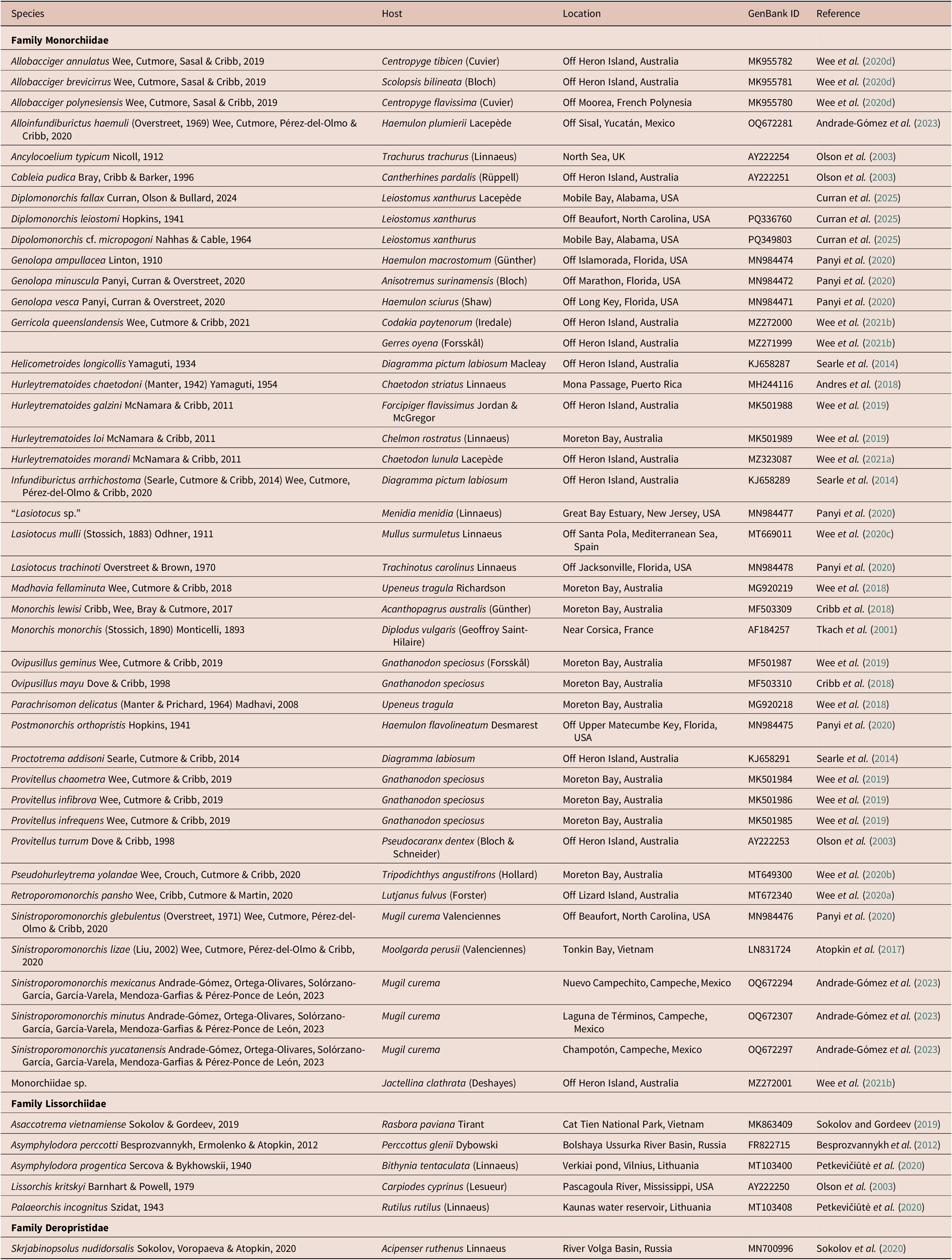
[Based on 19 gravid, unflattened specimens; Figure 1.] Body elongate pyriform, widest at mid-hindbody, strongly tapering at anterior end and moderately tapering at posterior end, 430–571 (511) × 123–170 (143), 3.3–4.1 (3.7) times longer than wide. Forebody 140–189 (165) long, or 27.3–37.2 (32.2%) of body length; hindbody 230–343 (294) long, or 51.6–62.8 (57.4%) of body length. Tegument thin, uniformly covered with small, fine, regular spines. Eye-spot pigment granules few, mostly large, restricted to level of or anterior to ventral sucker.
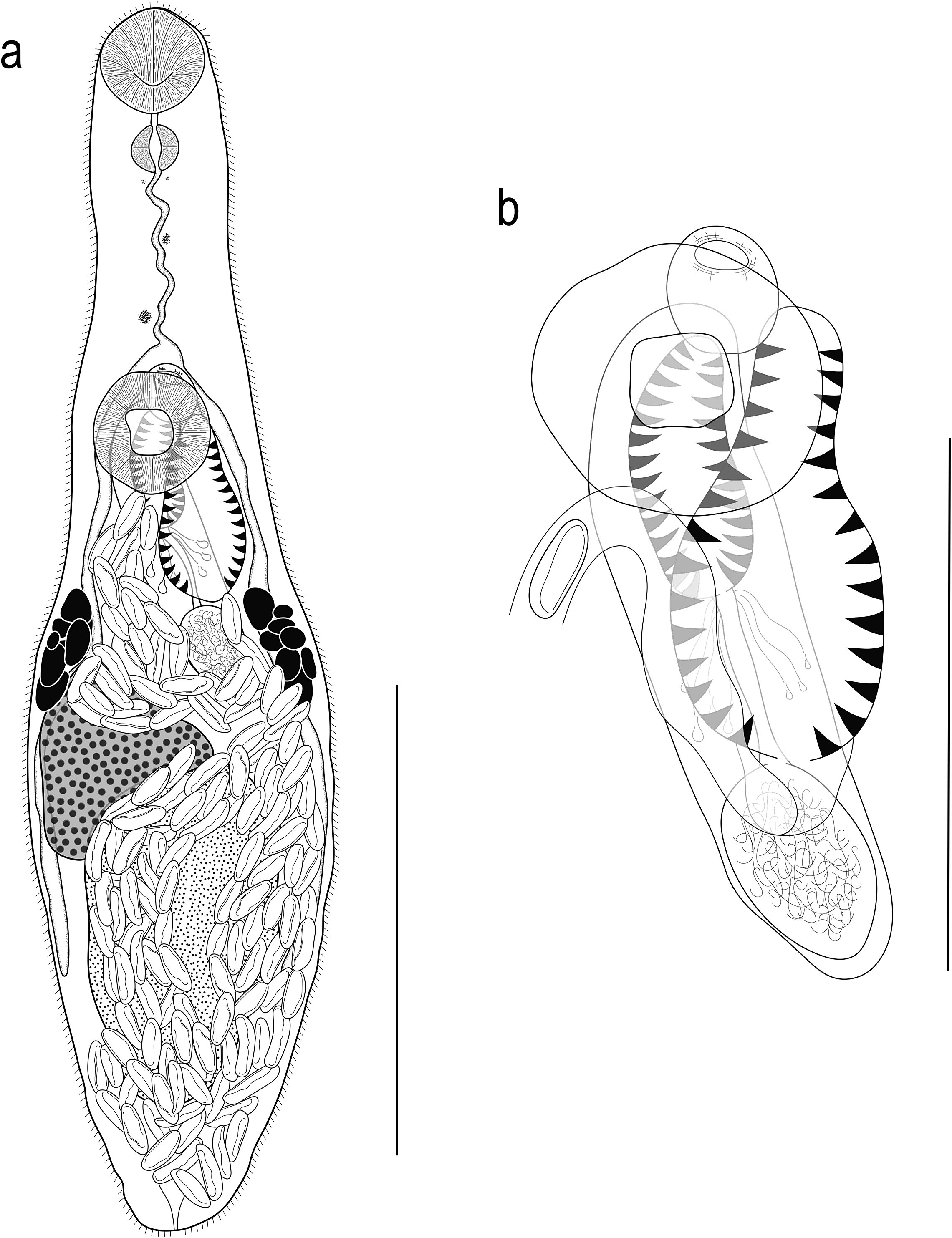
Figure 1. Obscuromonorchis ranae n. g., n. sp. from Lizard Island, Queensland, Australia. (a) Adult worm, ventral view; (b) Terminal genitalia. Scale-bars: a, 200 μm; b, 100 μm.
Oral sucker terminal, round, with opening distinctly subterminal, 34–51 (42) × 36–54 (45), 0.92–1.13 (1.05) times wider than long. Ventral sucker round, 46–62 (54) × 44–65 (56), 0.92–1.14 (1.01) times wider than long, 1.05–1.46 (1.27) times longer and 1.13–1.44 (1.25) times wider than oral sucker. Prepharynx short, 2–16 (9). Pharynx muscular, roughly spherical, 20–25 (22) × 18–23 (20); pharynx length 45.1–70.6 (53.4)% of oral sucker length; pharynx width 37.0–55.6 (45.6)% of oral sucker width. Oesophagus simple, moderately long, slightly sinuous, 65–98 (87), occupies 15.1–20.1 (16.7)% of body length. Intestinal bifurcation in posterior forebody, immediately anterior to or at level of ventral sucker; pre-bifurcal zone 25.1–33.3 (29.4)% of body length. Intestinal caeca blind, long, terminate level with posterior half of testis, well anterior to posterior extremity, 13.1–24.4 (19.5)% of body length from posterior margin of body.
Testis large, entire, ellipsoidal, occupying majority of posterior half of hindbody, partially ventrally overlaps caeca, ovary and cirrus-sac, 113–215 (167) × 73–121 (97); separated by 12.1–25.2 (18.6)% of body length from ventral sucker; pre-testicular zone 55.0–72.4 (61.2)% of body length; post-testicular zone 4.3–15.7 (7.2)% of body length. Cirrus-sac in middle third of body, subcylindrical, mostly median, typically mostly intercaecal, occasionally partially overlaps dextral caecum, 111–169 (138) × 28–41 (34), occupies 22.8–31.3 (27.1)% of body length. Seminal vesicle ellipsoidal, unipartite, 27–42 (35) × 21–31 (26), occupies 20.2–30.9 (25.3)% of cirrus-sac length. Pars prostatica simple, short to moderately long, 16–43 (29) in length, with few prostatic cells observed; prostatic cells only observed to unite with pars prostatica towards anterior end. Cirrus (eversible ejaculatory duct) moderately long, subcylindrical, armed with robust spines, 53–86 (67) × 14–25 (19), occupies 39.6–58.6 (48.2)% of cirrus-sac length. Genital atrium unspined, simple. Common genital pore small, median, immediately anterior to ventral sucker.
Ovary smooth, mostly entire, with indistinct lobes in some specimens, in anterior half of hindbody, typically distinctly posterior to ventral sucker, antero-dextral to and partially ventrally overlaps testis, 6.0–17.3 (12.5)% of body length from ventral sucker, 59–99 (78) × 50–100 (70); pre-ovarian zone 43.2–63.2 (55.4)% of body length; post-ovarian zone 26.0–41.4 (31.6)% of body length. Mehlis’ gland not observed. Uterine seminal receptacle present. Vitellarium composed of two lateral masses of densely clustered follicles, in anterior third of hindbody, extends anteriorly to posterior half of cirrus-sac and posteriorly to anterior half of testis, partially overlaps both caeca; vitelline mass length 53–79 (63), occupying 10.5–14.7 (12.4)% of body length. Uterus thin-walled, extensive, restricted to hindbody, ventral to ovary, testis, caeca and part of cirrus-sac, with coils mostly indiscernible; posterior-most coil distinctly or just anterior to posterior body margin; ascending coil entering terminal organ at posterior end. Metraterm not observed. Terminal organ sinistro-dorsal to cirrus-sac, partially overlaps cirrus-sac, unipartite, significant tapering in anterior half in some specimens can give appearance of being bipartite, 60–88 (75) × 26–49 (37), occupying 12.6–19.1 (15.0)% of body length, armed with robust spines; spines usually slightly wider than those in cirrus, but sometimes fewer and less compact, decreasing slightly in size towards anterior end of terminal organ; anterior-most spines hard to discern in some specimens. Eggs numerous, lightly tanned, operculate, unfilamented, 22–28 (25) × 7–11 (9).
Excretory vesicle small, saccular, partially overlaps testis or posterior-most coil of uterus, difficult to determine margins in most specimens. Excretory pore terminal.
Remarks
The terminal organ in this species could be misinterpreted as a bipartite rather than unipartite due to the prominent tapering in the anterior half of the organ. This impression is sometimes exacerbated by the unusual condition that there appear to be significantly fewer spines in the terminal organ in some specimens, such that they are easily missed in initial observations. However, on close inspection, despite their irregular coverage, spines can be observed throughout the terminal organ, indicating that the organ is not bipartite.
Argenticola n. g.
Diagnosis
Body small, fusiform. Tegument thin, spined. Oral sucker terminal, slightly cup-shaped, with opening subterminal. Ventral sucker round, in anterior half of body. Prepharynx short. Pharynx markedly smaller than oral sucker, subspherical. Oesophagus simple, short, slightly sinuous. Intestine bifurcates immediately anterior to ventral sucker. Intestinal caeca blind, long, extend to level of posterior half of testis, do not enter post-testicular zone. Testis single, entire, in middle and posterior third of hindbody. Cirrus-sac subcylindrical, in middle third of body, extends from anterior half of testis to level with anterior portion of ventral sucker. Seminal vesicle unipartite, subspherical to ellipsoidal. Pars prostatica mostly short, simple. Cirrus prominent, subcylindrical, armed with robust spines. Genital atrium spined; spines only in posterior half. Common genital pore median, immediately anterior to ventral sucker. Ovary entire, slightly lobed, dextro-submedial, antero-dextral to and contiguous with testis. Uterine seminal receptacle present. Vitellarium composed of two lateral masses of densely clustered follicles in middle third of body. Uterus extensive in hindbody, extends beyond gonads posteriorly, without discernible metraterm, enters terminal organ at middle third. Terminal organ unipartite, armed with robust spines. Eggs numerous, unfilamented. Excretory pore terminal.
Type and only species: Argenticola shuyinae n. g., n. sp.
ZooBank registration: The LSID for Argenticola n. g. is urn:lsid:zoobank.org:act:7562DFA6-572F-47F1-ADAF-2215E7606F44.
Etymology: The generic name Argenticola is composed from the Latin argentum, for silver, referring to the common name of the hosts, silverbiddies, and the Latin ‘-cola’ (dweller or inhabitant).
Argenticola shuyinae n. sp.
Type host: Gerres oyena (Forsskål), Blacktip silverbiddy (Gerreidae)
Other hosts: Gerres oblongus Cuvier, Slender silverbiddy (Gerreidae); Gerres subfasciatus Cuvier, Common silverbiddy
Type locality: off Lizard Island, northern Great Barrier Reef, Queensland, Australia (14°40′S, 145°27′E)
Other locality: western Moreton Bay, Queensland, Australia (27°20′S, 153°06′E).
Site of infection: Intestine
Prevalence: Lizard Island: G. oyena, 3/21 (14.3%); G. oblongus, 2/21 (9.5%). Moreton Bay: G. subfasciatus, 1/1 (100%)
Deposition of specimens: Holotype and 19 paratypes, including hologenophores (QM G241970–G241989), and 11 vouchers (QM G241990–G242000)
Molecular sequence data: cox1 mtDNA, 11 sequences, six submitted to GenBank (GB PV768668–PV768673); ITS2 rDNA, four sequences, three submitted to GenBank (GB PV771105–PV771107); 28S rDNA, three sequences, two submitted to GenBank (GB PV771109–PV771110)
ZooBank registration: The LSID for Argenticola shuyinae n. g., n. sp. is urn:lsid:zoobank.org:act:A7B96ED6-D1B4-4561-9259-5239AEFD5D51.
Etymology: This species is named in honour of the first author’s best friend, Shuyin Luo, in recognition of her constant and continued support and encouragement.
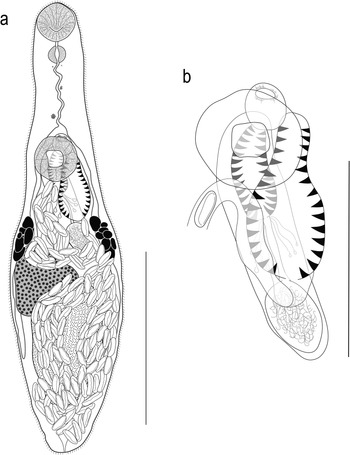
Description
[Based on 20 gravid, unflattened specimens; Figure 2]. Body small, fusiform, widest at mid-hindbody, slightly tapering to bluntly rounded anterior and posterior ends, 275–426 (325) × 97–164 (129), 2.0–3.2 (2.6) times longer than wide. Forebody 93–131 (112) long, or 30.1–40.7 (34.9)% of body length; hindbody 132–241 (168) long, or 44.5–58.6 (51.4)% of body length. Tegument thin, uniformly covered with small, fine, regular spines. Eye-spot pigment granules small, restricted to level of or anterior to ventral sucker.
Figure 2. Argenticola shuyinae n. g., n. sp. from Lizard Island, Queensland, Australia. (a) Adult worm, ventral view; (b) Terminal genitalia. Scale-bars: a, 200 μm; b, 100 μm.
Oral sucker terminal, slightly cup-shaped, with opening distinctly subterminal, 36–52 (43) × 41–58 (49), 1.02–1.39 (1.13) times wider than long. Ventral sucker round, 37–56 (46) × 41–61 (50), 0.97–1.21 (1.08) times wider than long; 0.94–1.34 (1.07) times longer and 0.79–1.21 (1.03) times wider than oral sucker. Prepharynx short, 2–12 (7). Pharynx muscular, spherical or subspherical, 19–29 (25) × 20–29 (24); pharynx length 48.9–61.9 (56.6)% of oral sucker length; pharynx width 43.9–56.9 (48.8)% of oral sucker width. Oesophagus simple, short, slightly sinuous in some specimens, 16–41 (29), occupies 5.4–12.1 (8.9)% of body length. Intestinal bifurcation in posterior forebody, just or distinctly anterior to ventral sucker; pre-bifurcal zone 23.9–34.9 (29.6)% of body length. Intestinal caeca blind, long, terminate level to posterior half of testis, 11.8–21.8 (17.5)% of body length from posterior end of body.
Testis, large, entire, roughly subspherical to longitudinally ellipsoidal, in middle and posterior third of hindbody, median, partially overlaps left caecum and ovary, 64–131 (87) × 43–109 (71); separated by 9.2–19.9 (14.9)% of body length from ventral sucker; pre-testicular zone 56.4–68.8 (63.7)% of body length; post-testicular zone 5.7–15.3 (10.3)% of body length. Cirrus-sac in middle third of body, subcylindrical, mostly median, typically intercaecal, partially overlaps right caecum and ovary in some specimens, with posterior end slightly sinistro-submedian, 71–108 (85) × 26–53 (39), occupies 24.1–30.6 (26.3)% of body length. Seminal vesicle subspherical to ellipsoidal, unipartite, 19–57 (34) × 21–52 (31), occupies 25.7–52.8 (38.9)% of cirrus-sac length. Pars prostatica simple, mostly short, 8–23 (18) in length, with few prostatic cells observed; prostatic cells difficult to observe in some specimens. Cirrus (eversible ejaculatory duct) subcylindrical, armed with robust spines, tapers slightly anteriorly, 29–48 (36) × 11–23 (16), occupies 35.1–54.9 (41.9)% of cirrus-sac length. Genital atrium armed with some robust spines on posterior margin; spines slightly longer than those in cirrus and terminal organ. Common genital pore median, immediately anterior to ventral sucker.
Ovary roughly triangular to ellipsoidal, with some lobation, partially ventrally overlaps testis, cirrus-sac and right caecum in most specimens, 1.7–12.4 (5.9)% of body length from ventral sucker, 46–87 (65) × 33–61 (65); pre-ovarian zone 46.2–60.8 (53.8)% of body length; post-ovarian zone 24.0–33.8 (28.8)% of body length. Mehlis’ gland not observed. Uterine seminal receptacle present. Vitellarium composed of two lateral masses of densely clustered follicles, at level of ovary and posterior end of cirrus-sac, never extends anteriorly to anterior half of cirrus-sac, never extends posteriorly beyond ovary; vitelline mass length 31–55 (45), occupying 10.8–19.3 (13.9)% of body length. Uterus thin-walled, extensive, restricted to middle and posterior third of body, occupying region between posterior half of ventral sucker and post-testicular zone, ventral to ovary, testis, caeca and part of cirrus-sac, some coils indiscernible, with ascending coil entering terminal organ at middle third. Metraterm not observed. Terminal organ unipartite, sinistro-ventral to and about half length of cirrus-sac, 23–61 (45) × 22–40 (28), occupying 8.4–17.4 (13.8)% of body length, armed with dense, robust spines; spines of similar size to spines in cirrus. Eggs numerous, small, lightly tanned, operculate, unfilamented, 10–16 (13) × 5–7 (6).
Excretory vesicle small, saccular, tapering at posterior end, reaches to posterior half of testis. Excretory pore terminal.
Remarks
The spination in the genital atrium in this species is unusual and caused difficulties in interpretation. We conclude that the genital atrium possesses spines in the posterior half but lacks them in the anterior half; such a conformation has not been reported for any previously described monorchiid, as spines typically either line the entire atrium or are completely absent. The difficulty in the interpretation of the form of the genital atrium spination stems from the tiny size of the worms and the overlap of the genital atrium with the anterior portion of the cirrus and terminal organ. All three organs possess robust spines, and while those in the genital atrium appear to be slightly longer than the other two, the distinction is not always clear. As such, in many specimens, the spination in the genital atrium appears only as a continuation of the cirrus and/or terminal organ. A similar organisation was observed by Panyi et al. (Reference Panyi, Curran and Overstreet2020) for Genolopa minuscula Panyi, Curran & Overstreet, 1970, who recognised that the genital atrium spines closely resembled those of the cirrus and suggested that spines observed in the genital atrium may be from a partially everted cirrus.
Specimens of A. shuyinae demonstrated minor intraspecific variation in the cox1 region, at 0–11 base positions (0–2.3%), with the largest difference between samples from Lizard Island and Moreton Bay. These differences are well within the range in intraspecific variation found for other marine trematodes in Queensland waters (see Cribb et al. Reference Cribb, Martin, Diaz, Bray and Cutmore2021; Cutmore et al. Reference Cutmore, Corner and Cribb2023; Cutmore and Cribb, Reference Cutmore and Cribb2022; Duong et al. Reference Duong, Cutmore, Cribb, Pitt, NQ-X and Bray2022). Samples from Moreton Bay and Lizard Island have identical ITS2 sequence data and 28S sequences from the two regions differed at just a single base position.
Gerricola queenslandensis Wee, Cutmore & Cribb, 2021
Type-host: Gerres oyena (Forsskål), Blacktip silverbiddy (Gerreidae).
Type-locality: Heron Island, southern Great Barrier Reef, Queensland, Australia (23°27′S, 151°55′E).
New collections
Known hosts: Gerres oyena; Gerres subfasciatus Cuvier, Common silverbiddy (Gerreidae).
New locality: Lizard Island, northern Great Barrier Reef, Queensland, Australia (14°40′S, 145°27′E)
Known locality: Western Moreton Bay, Queensland, Australia (27°20′S, 153°06′E)
Site of infection: Intestine
Prevalence: Lizard Island: G. oyena, 3/21 (14.3%). Moreton Bay: G. subfasciatus, 1/1 (100%)
Deposition of specimens: Three vouchers, including hologenophores (QM G242001–G242003).
Molecular sequence data: cox1 mtDNA, five sequences, three submitted to GenBank (GB PV768666–PV768667, PV768674); ITS2 rDNA, one sequence (GB PV856218)
Remarks
ITS2 and cox1 data for the new specimens of G. queenslandensis match those published by Wee et al. (Reference Wee, Cutmore and Cribb2021b) in the original description of the species. ITS2 sequences from Moreton Bay, Heron Island and Lizard Island are identical, and intraspecific variation in the cox1 region for this species ranges between 0 and 4 base positions (0–0.8%) The new collections from Lizard Island here extend the range of G. queenslandensis to the northern Great Barrier Reef; the previous range extended from Moreton Bay in southeast Queensland to Heron Island, on the southern Great Barrier Reef.
Phylogenetic results
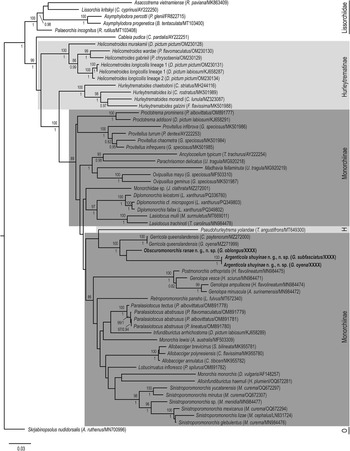
Bayesian inference (BI) and Maximum Likelihood (ML) analyses of the 28S rDNA dataset (Figure 3) produced phylograms with similar topologies. In both analyses, the two new species resolve as sister to Gerricola queenslandensis in a well-supported clade, sister to Pseudohurleytrema yolandae Wee, Crouch, Cutmore & Cribb, 2020; support for the relationship with P. yolandae is weak in both analyses. The clade of gerreid-infecting species + P. yolandae is sister to a major monorchiine clade comprising species of Allobacciger Hafeezullah & Siddiqi, 1970, Alloinfundiburictus Wee, Cutmore, Pérez-del-Olmo & Cribb, 2020, Genolopa Linton, 1910, Infundiburictus Wee, Cutmore, Pérez-del-Olmo & Cribb, 2020, Lobucirruatus Wee, Cutmore & Cribb, Reference Cutmore and Cribb2022, Monorchis Monticelli, 1893, Paralasiotocus Wee, Cutmore, Pérez-del-Olmo & Cribb, 2020, Postmonorchis Hopkins, Reference Hopkins1941, Retroporomonorchis Wee, Cribb, Cutmore & Martin, 2020, and Sinistroporomonorchis Wee, Cutmore, Pérez-del-Olmo & Cribb, 2020. The only difference between the BI and ML analyses is the placement of the taxon identified only as Monorchiidae sp. [an infection of cercaria and sporocysts from the bivalve Jactellina clathrata (Deshayes, 1835)], but notably, nodal support for its placement is poor in both analyses. In the ML analysis, Monorchiidae sp. resolves in a clade as sister to species of Diplomonorchis Hopkins, Reference Hopkins1941 and Lasiotocus Looss, 1907, whereas in the BI analysis, it resolves as sister to all represented monorchiid taxa with the exception of Cableia pudica Bray, Cribb & Barker, 1996, and all species of Helicometroides Yamaguti, 1934, Hurleytrematoides Yamaguti, 1954, Proctotrema Odhner, 1911, and Provitellus Dove & Cribb, 1998.
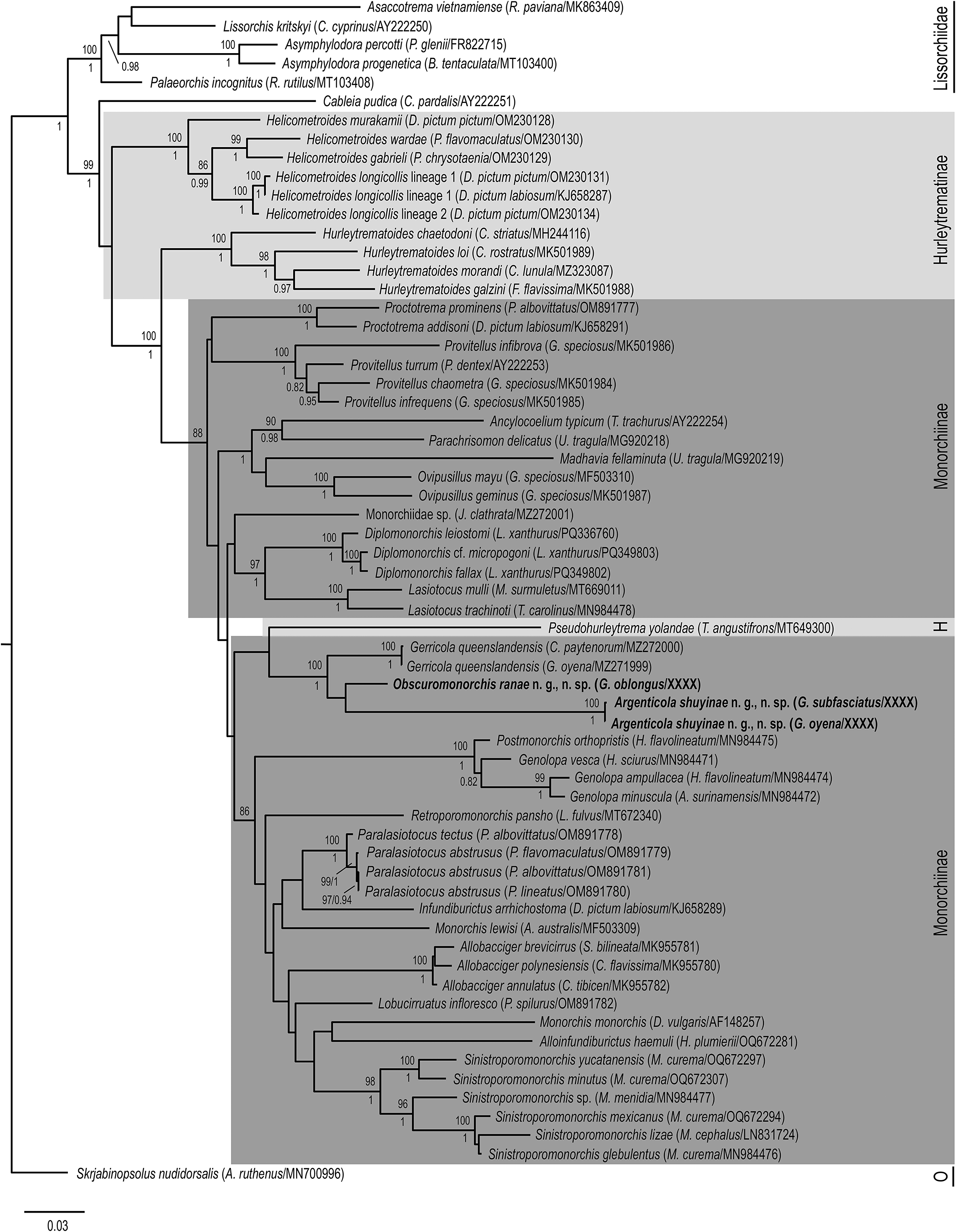
Figure 3. Relationships of monorchiid taxa based on the Maximum Likelihood analysis of the 28S rDNA dataset. Sequences for newly characterised species in this study are indicated in bold. Subfamilies are marked with a grey box, with the Hurleytrematinae in light grey, and the Monorchiinae in dark grey. Bootstrap values are shown above the nodes, and where relationships were replicated in the Bayesian inference analysis, posterior probabilities are shown below or besides, following a backslash. Nodal support below 80/0.8 not shown. Scale-bar indicates expected number of substitutions per site. Abbreviations: H, Hurleytrematinae; O, Outgroup taxon.
Discussion
Recognition of new genera
The topology of our phylogenetic analyses in which all three gerreid-infecting species resolve in a well-supported clade, makes interpretation of the generic status of the new forms subjective. Two taxonomic conclusions seem plausible: that the three species found infecting gerreids are all species of Gerricola or that all require distinct genera. Ultimately, we propose the latter course as the best interpretation, with the decision informed by the overall level of genetic dissimilarity and morphological distinction.
Comparison of the 28S genetic distance between the taxa considered here (5.6–11.5%) with that of other sequenced monorchiids is informative in guiding our interpretation of the generic status of the new species. The 12 monorchiid genera currently represented by multiple species in our 28S analyses present inter-specific variation between p-distances of 1.5% for two species of Allobacciger to 12.1% between Monorchis lewisi Cribb, Wee, Bray & Cutmore, Reference Cribb, Wee, Bray and Cutmore2018 and M. monorchis (Stossich, 1890) Looss, 1902; the high figure for the latter pair is explained by the fact that the two species do not form a clade and undoubtedly require separate genera. The highest convincing level of inter-specifc variation is 9.7% between Hurleytrematoides chaetodoni (Manter, Reference Manter1942) Yamaguti, 1954 and H. galzini. These two species are morphologically convincing as belonging to the same genus, but notably, H. chaetodoni is from Tortugas (off Florida), whereas H. galzini McNamara & Cribb, 2011 is from the central tropical Indo-Pacific. Inter-generic variation of monorchiids represented in our 28S dataset ranges from just 2.3% (Postmonorchis orthopristis relative to Genolopa vesca Panyi, Curran & Overstreet, Reference Panyi, Curran and Overstreet2020) to 20.8% (Argenticola shuyinae relative to Helicometroides murakamii Wee, Cribb, Shirakashi & Cutmore, 2022). The overall mean distinction is 13.9%. There is thus some overlap between levels of genetic distinction recognised as inter-specific and inter-generic. At the lowest end of generic distinction (P. orthopristis relative to G. vesca), it is noteworthy that the two genera form a strongly supported clade. Certainly, on the basis of morphology, the two genera are readily distinguished. However, if they were ever synonymised, the resulting genus would form a neat clade with inter-specific variation consistent with that of other monorchiid genera. The next most similar combination of genera relates to two of the three taxa considered here, Gerricola and Obscuromonorchis, which differ at only 5.6%. However, the species of these genera do not form a clade, being paraphyletic to Argenticola shuyinae, which differs from the two other gerreid-infecting species by p-distances of 11.5–13.9%; as such, inclusion of all three species in a single genus would lead to a taxon with internal branch lengths far larger than observed for any other monorchiid genus. On balance, we think that the resolution of the three considered species here as a well-supported monophyletic clade, coupled with the relatively large p-distances between A. shuyinae and both G. queenslandensis and O. ranae, and the knowledge that G. queenslandensis and O. ranae do not form a clade despite being most closely related, provide a convincing argument for the three species to be recognised as members of distinct genera.
Although the three species considered here are generally morphologically similar, they differ in important features usually considered genus-specific. The complex forms of monorchiid terminal genitalia have been consistently and reliably used to distinguish genera, with species of each genus typically exhibiting identical traits; for example, all species of Proctotrema Odhner, 1911 possess a unipartite terminal organ, and all species of Allobacciger possess a clearly bipartite terminal organ. In the case of the species studied here, although all three possess a typical spined cirrus, G. queenslandensis possesses a bipartite terminal organ, whereas both O. ranae and A. shuyinae possess a unipartite terminal organ. Further, while both G. queenslandensis and O. ranae possess an unspined genital atrium, A. shuyinae possesses a partially spined genital atrium. The nature of these differences suggests that the three species require distinct genera. However, it should be noted that a recent study by Curran et al. (Reference Curran, Olson and Bullard2025) demonstrated, with genetic evidence, that terminal genitalia can be variable within a monorchiid genus. Curran et al. (Reference Curran, Olson and Bullard2025) demonstrated that three species of Diplomonorchis, D. fallax Curran, Olson & Bullard, 2024, D. leiostomi Hopkins, Reference Hopkins1941, and D. cf. micropogoni Nahhas & Cable, 1964, form a well-supported phylogenetic clade consistent with a single genus, despite D. fallax having a clearly unipartite terminal organ and the other two species having a bipartite terminal organ. Although important, this finding is exceptional. No other combination of monorchiid congeners tested with genetic data has exhibited such significant variation in their terminal genitalia.
Ultimately, the path to satisfactorily resolving the systematics of this system will require the collection and genetic characterisation of further species within this clade. If the three genera are valid, further species should resolve as closely related to each of the three species found here, sharing the morphological distinctions we have identified. However, if other species resolve on equally long branch lengths with no consistency in terms of morphological distinctions, then the system may be better interpreted as a single genus. At present, we think the proposal of two new genera is the best representation of this system, and we provide taxonomic delimitation for them below.
Obscuromonorchis n. g
In the possession of a single testis, an ovary in the hindbody, a uterus that occupies the pre- and post-testicular region, an unspined genital atrium, and a unipartite terminal organ, O. ranae is recognised as a member of the subfamily Monorchiinae. In the possession of an unspined genital atrium and a unipartite terminal organ, the species broadly fits the concept of Pseudoametrodaptes Triveni Lakshmi & Madhavi, 2008 and Proctotrema. Obscuromonorchis ranae species can be easily differentiated from Pseudoametrodaptes in the lack of spines around the oral sucker, but differentiation from Proctotrema is complicated. As discussed by Wee et al. (Reference Wee, Cribb and Cutmore2022), the concept of Proctotrema is unhelpfully broad, accommodating significant morphological variation, and the genus likely comprises a range of unrelated species. This complexity is further exacerbated by the seemingly low host-specificity, with multiple ecologically different and unrelated families reported as hosts for some Proctotrema species. Thus, for generic level morphological comparisons, we compare our species to Proctotrema sensu stricto. The most prominent morphological character to separate the new species from species of Proctotrema is the form of the oral sucker, which is unspecialised in the new species and distinctly funnel-shaped in Proctotrema. Obscuromonorchis ranae can be further distinguished from species of Proctotrema in the location of caecal bifurcation (immediately anterior to the ventral sucker vs well anterior to the ventral sucker, in the mid-forebody) and the location of caecal termination (at the level of the testis, never extending into the post-testicular region vs well into the post-testicular region).
In our phylogenetic analyses, the new species is only distantly related to the two represented species of Proctotrema, Proctotrema addisoni Searle, Cutmore & Cribb, Reference Searle, Cutmore and Cribb2014 and Proctotrema prominens Wee, Cribb & Cutmore, Reference Wee, Cribb and Cutmore2022. Notably, the type-species, Proctotrema bacilliovatum Odhner, 1911 is not represented by genetic sequence data, and establishment of its placement in phylogenetic analyses is crucial to determine the true position of the genus. We recognise that it might appear inconsistent to compare the new species morphologically to Proctotrema sensu stricto while electing to compare our phylogenetic results to other species of Proctotrema. However, of the eight known species of Proctotrema, P. addisoni and P. prominens are the most morphologically similar to P. bacilliovatum, and until genetic data for the type-species can be obtained, we think P. addisoni and P. prominens serve as suitable proxies for the genus. Thus, based on combined current morphological and phylogenetic analyses, we think it more useful to recognise the new species as a member of a distinct genus rather than including it as a member of Proctotrema.
Further, given that Obscuromonorchis ranae forms a strongly supported clade with Gerricola queenslandensis and Argenticola shuyinae and have shared ecology in infecting gerreids, we here reiterate the morphological differences between the three genera. Obscuromonorchis can be distinguished from Gerricola in the possession of a spined unipartite terminal organ (vs. a bipartite terminal organ with a spined anterior section and an unspined posterior section), although the distinction can sometimes only be clear upon close inspection. Obscuromonorchis can be easily distinguished from Argenticola in the possession of a completely unspined genital atrium (vs. a partially spined genital atrium).
Argenticola n. g
In the possession of a single testis, an ovary in the hindbody, a uterus that occupies the pre- and post-testicular region, a unipartite, spined terminal organ without a muscular bulb, and a spined genital atrium, A. shuyinae is recognised as a member of the Monorchiinae. In the possession of a unipartite, spined terminal organ and a spined genital atrium, the species broadly fits the concept of Paraproctotrema Yamaguti, 1934 and Monorchicestrahelmins Yamaguti, 1971. Argenticola shuyinae can be easily differentiated from Paraproctotrema by the lack of a metraterm (vs. a metraterm with a distinct muscular bulb). The species more closely conforms to the generic concept of Monorchicestrahelmins. However, there are clear morphological distinctions that, when considered in combination, warrant the proposal of a distinct genus to accommodate this species. All three species of Monorchicestrahelmins possess a small testis relative to the hindbody, in that the maximum space occupied by the testis is approximately a third of the region. In addition, the three species possess a significant post-testicular region, with the caeca for all extending well into the area. In comparison, A. shuyinae possesses a large testis that occupies approximately half of the hindbody, and a relatively small post-testicular region, with the caeca terminating at the level of the posterior half of the testis, well anterior to the post-testicular region. Lastly, the generic diagnosis of Monorchicestrahelmins states the genital atrium is spined (presumably entirely spined), although we note that only the type-species, Monorchicestrahelmins lethrini (Yamaguti, 1953) Yamaguti, 1971, is explicitly described as such. Descriptions and illustrations of the two other species, Monorchicestrahelmins branchiostegi Shen, 1987, and Monorchicestrahelmins bupharynx (Barvo-Hollis, 1956) Yamaguti, 1971, do not mention or display any spination in the genital atrium. Perhaps these two species require transfer to a more appropriate genus, although that is beyond the scope of this study. Regardless, A. shuyinae possesses a genital atrium that appears to be only partially occupied by spines (at the posterior region, immediately anterior to both terminal organ and cirrus).
Notably, Monorchicestrahelmins is not represented by any genetic sequence data, which limits comparison with Argenticola. The importance of genetic sequence data in monorchiid systematics has been highlighted in recent years, particularly in the restructuring of Lasiotocus, which previously encompassed species from a wide breadth of ecologically unrelated hosts and exhibited significant morphological variation (see Wee et al. Reference Wee, Cutmore, Pérez-del-Olmo and Cribb2020c), and in investigating the validity of Genolopa, another problematic genus due to confusion and misinterpretation of some of its diagnostic morphological characters (see Panyi et al. Reference Panyi, Curran and Overstreet2020). In the current study, while genetic sequence data for Monorchicestrahelmins would undoubtedly be desirable, we think the differences between the generic concepts of Monorchicestrahelmins and Argenticola are sufficiently distinct and robust that we can be confident in the proposal of the new genus.
Similar to our approach in the morphological delineation of Obscuromonorchis, we here reemphasize the morphological differences between Argenticola, Gerricola, and Obscuromonorchis. Argenticola is most easily differentiated from Gerricola by the combination of possessing both a unipartite, spined terminal organ (vs. a bipartite terminal organ with a spined anterior section and an unspined posterior section), and a partially spined genital atrium (vs. an unspined genital atrium). The partially spined genital atrium is also the key morphological feature that separates Argenticola from Obscuromonorchis, with the latter possessing an unspined genital atrium.
Genus-level host-specificity of monorchiids
Of the 26 monorchiid genera represented in our phylogenetic analyses, 21 are known to comprise at least two species, although only 11 are represented by multiple congeners (Monorchis is not considered here, as it is polyphyletic in our analyses). Of the 11 genera, eight are known from only a single host family. The other three infect at least two, typically distantly related host families; species of Allobacciger infect the Nemipteridae and Pomacanthidae, species of Lasiotocus infect the Mullidae and Carangidae, and species of Sinistroporomonorchis infect the Mugilidae and Atherinopsidae. It should be noted that while Hurleytrematoides is reported overwhelmingly from chaetodontids, prior studies have clearly demonstrated that Hurleytrematoides justinei McNamara & Cribb, 2009 infects a tetraodontid.
When considering clades comprising at least two genera, only three infect hosts of the same family: Paralasiotocus + Infundiburictus infecting haemulids, Postmonorchis + Genolopa infecting haemulids, and Gerricola + Argenticola + Obscuromonorchis infecting gerreids. Of the three, nodal support for Paralasiotocus + Infundiburictus is weak, whereas that for Postmonorchis + Genolopa and Gerricola + Argenticola + Obscuromonorchis is robust. The two latter clades are exceptional as comprising multiple genera demonstrated convincingly with genetic sequencing (along with clear morphological distinctions) that infect a single host family.
It seems clear that patterns of host-specificity in monorchiids vary significantly. Some lineages (be it a single genus or multiple genera) evidently infect only a single host family, others principally infect a single host family but with some radiation into another family, and others clearly infect multiple host families. The implication of these distinctions is that, while it might be possible to predict the nature of host-specificity of some monorchiid groups, further testing is certainly needed for others, and genetic sequencing will be key for us to better understand the radiation of monorchiids into their hosts.
Acknowledgements
We sincerely thank Dr. Storm Martin, Dr. Tim Littlewood, Dr. Rod Bray, Dr. Delane Kritsky, Helen Armstrong, Lenin De Silva, and Tori Wang for their assistance with the collections of fish at Lizard Island, and we thank the staff of Lizard Island Research Station for their support of our work.
Financial support
This project was supported by Australian Government’s Australian Biological Resources Study National Taxonomy Research Grants Program (4-H04JDSM awarded to SCC and THC, and NTRGII000024 awarded to NW and THC).
Competing interests
The authors declare that they have no conflict of interest.
Ethical standard
All applicable institutional, national and international guidelines for the care and use of animals were followed.