Introduction
The anoplocephalid cestode genera Progamotaenia Nybelin, 1917 and Wallabicestus Schmidt, 1975 are the dominant intestinal and biliary cestodes of kangaroos and wallabies (Marsupialia: Macropodidae), with 36 species of Progamotaenia and two species of Wallabicestus currently recognised (Spratt and Beveridge Reference Spratt and Beveridge2016). Although many species are host-specific, several others were known to occur in multiple host species. Progamotaenia festiva (Rudolphi, 1819), P. macropodis Beveridge, Reference Beveridge1976, P. zschokkei (Janicki, 1906), and W. ewersi (Schmidt, 1975) (formerly Progamotaenia ewersi) were recognised, based on morphological studies revealing considerable variation, as potential species complexes by Beveridge (Reference Beveridge1976, Reference Beveridge1980). Subsequent molecular studies of three of these species provided genetic evidence to support the hypothesis (Beveridge et al. Reference Beveridge, Shamsi, Hu, Chilton and Gasser2007; Hu et al. Reference Hu, Gasser, Chilton and Beveridge2005), leading to taxonomic revisions of P. festiva, P. zschokkei, and W. ewersi. Combining both molecular and morphological data resulted in the descriptions of a number of new species (Beveridge Reference Beveridge2007, Reference Beveridge2009; Beveridge and Shamsi Reference Beveridge and Shamsi2009), although some genotypes within P. festiva and W. ewersi could not be separated morphologically (Beveridge Reference Beveridge2009; Beveridge and Shamsi Reference Beveridge and Shamsi2009).
While P. macropodis had been identified as a potential species complex based on molecular data (Hu et al. Reference Hu, Gasser, Chilton and Beveridge2005), no attempt has yet to be made to identify and name individual species within this complex. The species was originally described by Beveridge (Reference Beveridge1976) based primarily on specimens collected from eastern grey kangaroos (Macropus giganteus), nominated as the type host, as well as additional material from the related western grey kangaroo (M. fuliginosus) and several species of wallabies, the red-necked wallaby (Notamacropus rufogriseus), the tammar wallaby (Notamacropus eugenii), and the swamp wallaby (Wallabia bicolor). Beveridge (Reference Beveridge1976) noted considerable morphological variation within the species, particularly in the distribution of the testes, either in a single band or two groups anterior to the female genitalia and uteri but considered this to be attributable to within-species variation. Beveridge (Reference Beveridge1976) described the new species based on the type material from M. giganteus but did not provide additional morphological data on the specimens from the remaining hosts apart from illustrations of proglottids from N. eugenii and W. bicolor, and as a consequence, the full extent of the morphological variation within the species has not been described. Subsequently, Beveridge et al. (Reference Beveridge, Chilton, Johnson, Smales, Speare and Spratt1998) added the wallaroo, Osphranter robustus robustus, and the whip-tailed wallaby, Notamacropus parryi, as new records from a survey of hosts in central and northern Queensland, without any additional morphological information, but both were included in the molecular study of Hu et al. (Reference Hu, Gasser, Chilton and Beveridge2005). Their analysis included material from some of the initially identified host species, M. fuliginosus, N. eugenii, and W. bicolor, and added specimens from O. robustus robustus, O. robustus woodwardi, and N. parryi (Hu et al. Reference Hu, Gasser, Chilton and Beveridge2005). Critically, it did not include material from the type host, M. giganteus. Since the publication of the original description of the species and the molecular study of Hu et al. (Reference Hu, Gasser, Chilton and Beveridge2005), additional collections of P. macropodis have been made from various parts of the continent, and frozen material from the type host, M. giganteus, has become available for molecular analysis. The present paper includes new molecular and original morphological data allowing for the description of one new species while also indicating where additional information is needed for a more detailed resolution of this species complex.
Materials and methods
All material allocated to this species in the collections of the South Australian Museum, Adelaide (SAM) was examined. Within the museum, all the material is held within the Australian Helminthological Collection. Only the abbreviation SAM is used herein for this collection. Many collections consist of portions of wet material, parts of which have been mounted on slides; they are differentiated in the list of specimens examined, following the practice in the SAM database, as S for slide material and W for unmounted, wet specimens. Additional wet specimens were stained in Celestine blue, dehydrated in ethanol, cleared in methyl salicylate, and mounted in Canada balsam. Morphological specimens were re-examined using an Olympus BH2 compound microscope, and measurements were made using a micrometer eyepiece or a drawing tube. Drawings were made with a drawing tube. Measurements are presented in millimetres with the range followed by the mean and the number of specimens measured in parentheses. Apart from the standard measurements of proglottids and internal organs, the ‘asymmetry index’ of Haukisalmi and Henttonen (Reference Haukisalmi and Henttonen2007) was included, being the distance between the centre of the ovary and the lateral margin as a proportion of proglottid width, and an additional measurement, the inter-ovarian distance as a ratio of proglottid width, was measured. The latter measurement was included, as it was noticed that this distance appeared to vary considerably between specimens. The number of testes was estimated by drawing testis fields on a sheet of paper and counting testes in the drawings. While taking measurements, only a single sucker was measured on each scolex to avoid pseudo-replication; for mature and gravid proglottids, only two of each were measured per specimen, at the extremities of the mature and gravid regions, respectively. When only very few specimens were available, additional measurements were made from the same strobilae. Wherever possible, comparisons were made between gravid, fully relaxed specimens, but where this has not been possible, it has been indicated. The use of the term ‘mature’ in this study implies the presence of sperm in the seminal receptacle and the patency of the genital atrium; ‘gravid’ implies the presence of shelled eggs in proglottids. An attempt has been made to compare the rate of development in cestodes from different host species by using the following criteria: the approximate proglottid number in which the presence of all genitalia in the proglottid become visible, including the testis primordia, which develop later than the primordia of the female genitalia; the occurrence of sperm in the seminal receptacle; the dissolution of the ovary; and the appearance of shelled eggs in the uterus. Although involving a degree of subjectivity relating to counting the initial proglottids in the neck region, repeated counts suggested a difference of about 10 proglottids out of about 250 in this area of the strobila. As many of the mounted specimens were fragmented, the number of specimens examined is based on the number of scoleces present, with the remaining material being listed as ‘fragments’.
Additional museum abbreviations are as follows: BMNH, British Museum, Natural History, London, as most publications to date have used this acronym (now known as The Natural History Museum with the acronym NHMUK) and MV, Museums Victoria (formerly cited in publications as the National Museum of Victoria, NMV). Host nomenclature follows Jackson and Groves (Reference Jackson and Groves2015). Localities are listed in increasing latitude along the east coast, followed by central states and territories and then Western Australia.
Samples from the frozen cestode collections held at the School of Veterinary Science at the University of Melbourne and the South Australian Museum were characterised genetically by targeting a partial sequence of the mitochondrial region cytochrome c oxidase I as described previously (Hu et al. Reference Hu, Gasser, Chilton and Beveridge2005). Genomic DNA was extracted using a DNeasy Blood and Tissue Kit (Qiagen, USA) following the protocol provided by the manufacturer. PCR was carried out in a 25 μl volume containing 10 mM Tris-HCl (pH 8.4), 50 mM KCl (Promega, USA), 3.5 mM of MgCl2, 200 μM of each deoxynucleotide triphosphate, 50 pmol of each primer (JB3 (5’-TTTTTTGGGCATCCTGAGGTTTAT-3’) and JB4.5 (5’ – TAAAGAAAGAACATAATGAAAATG – 3’), and 1 U of GoTaq polymerase (Promega) under the following cycling conditions: 94°C for 5 min (initial denaturation); 35 cycles of 94°C for 30 s (denaturation); 52°C for 30 s (annealing) and 72°C for 30 s (extension), followed by a final extension at 72°C for 5 min. For each PCR, negative (no-DNA) and positive (Bertiella paraberrata) controls were included. No amplification was detected in any of the negative control reactions during this study. Amplicons (5 μl) were examined on 1.5% agarose gels stained with Gel Red Nucleic Acid Stain (Biotium, Inc. Hayward, CA, USA). Gels were examined using trans-illumination and were photographed using a GelDoc system (BioRad, Hercules, CA, USA). PCR amplicons were purified using FavorPrepTM GEL/PCR Purification Kit (Favorgen Biotech Corp., Taiwan) prior to automated DNA Sanger sequencing using the primers JB3 and JB4.5 in separate reactions. The quality of each sequence obtained was appraised using the program Geneious Prime 2024.0.7 (Biomatters Ltd., Auckland, New Zealand). The DNA sequences determined herein have been submitted to the GenBank database (see Table 1).
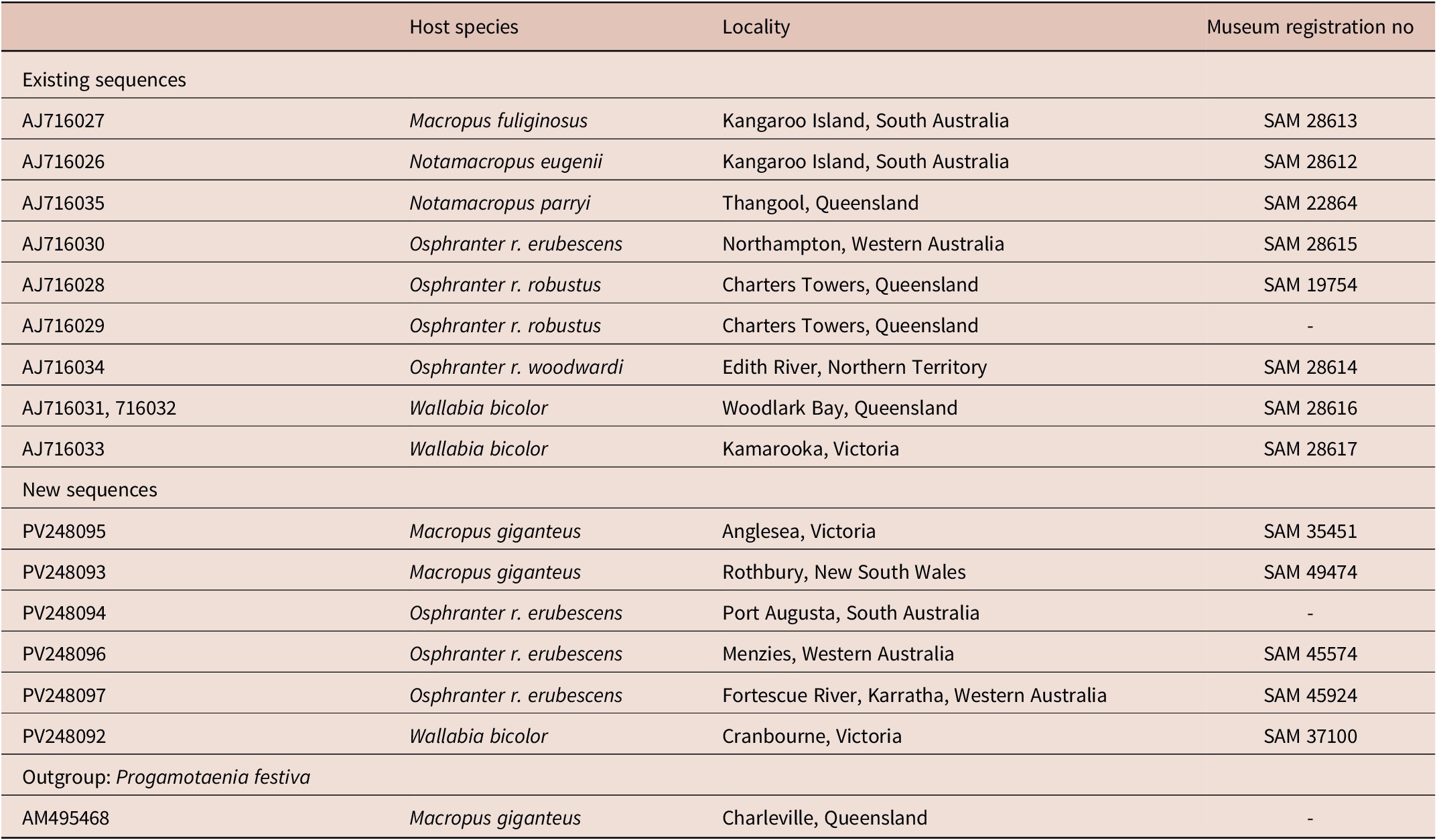
Table 1. Cox 1 DNA sequences of Progamotaenia macropodis used for phylogenetic analysis
Nucleotide sequences were aligned using the MUSCLE alignment program and adjusted manually by employing the Geneious Prime program. A dataset representing the cox1 sequences generated herein and the reference sequences from previous studies available through the GenBank database (Hu et al. Reference Hu, Gasser, Chilton and Beveridge2005) were aligned and adjusted manually. Progamotaenia festiva (sequence also from GenBank; Beveridge et al. Reference Beveridge2007) served as the outgroup. Phylogenetic analyses were performed using Bayesian Inference (BI) and Neighbor-Joining (NJ) methods. The BI was conducted using the Monte Carlo Markov Chain (MCMC) analysis in MrBayes 3.1.2 (Huelsenbeck and Ronquist Reference Huelsenbeck and Ronquist2001; Ronquist and Huelsenbeck Reference Ronquist and Huelsenbeck2003). The likelihood parameters for BI were based on the Akaike Information Criterion (AIC) test in jModeltest v2.1.10 (Darriba et al. Reference Darriba, Taboada, Doalla and Posada2012). AIC revealed the substitution model of evolution with equal sites with gamma distribution (HKY+G) as the ‘best’ model. Posterior probabilities (pp) were calculated using 2,000,000 generations, employing four simultaneous tree-building chains, with every 100th tree being saved. A consensus tree (50% majority rule) was constructed based upon the remaining trees generated by BI. The NJ analyses were performed using the software MEGA11.0.10 (Tamura et al. Reference Tamura, Stecher and Kumar2021), and the nodes were tested for robustness with 10,000 bootstrap replicates. The phylogenetic trees produced from the BI and NJ analyses were compared for concordance in their topologies.
Results
Material added to the molecular analysis of Hu et al. (Reference Hu, Gasser, Chilton and Beveridge2005) was as follows: O. robustus erubescens from Port Augusta in South Australia and Menzies and Karratha in Western Australia; W. bicolor from Cranbourne, Victoria; M. giganteus from Rothbury, New South Wales and Anglesea, Victoria (Table 1).
In the BI tree, there were two strongly supported clades (Figure 1) (1) including all specimens from O. robustus, N. eugenii, and N. parryi, and (2) specimens from W. bicolor, M. fuliginosus, and M. giganteus. Within the first clade, all specimens from O. robustus erubescens from South and Western Australia formed a strongly supported sub-clade, specimens from Notamacropus spp. formed a strongly supported second sub-clade, while one specimen from O. r. woodwardi and two from O. r. robustus were sisters to these two sub-clades. Within the second clade, specimens from M. giganteus and M. fuliginosus formed a well-supported sub-clade to the exclusion of specimens from W. bicolor. Within the latter sub-clade, the specimens from Victoria (Cranbourne, Kamarooka) formed a grouping distinct from the specimens from central Queensland (Woodlark Bay).
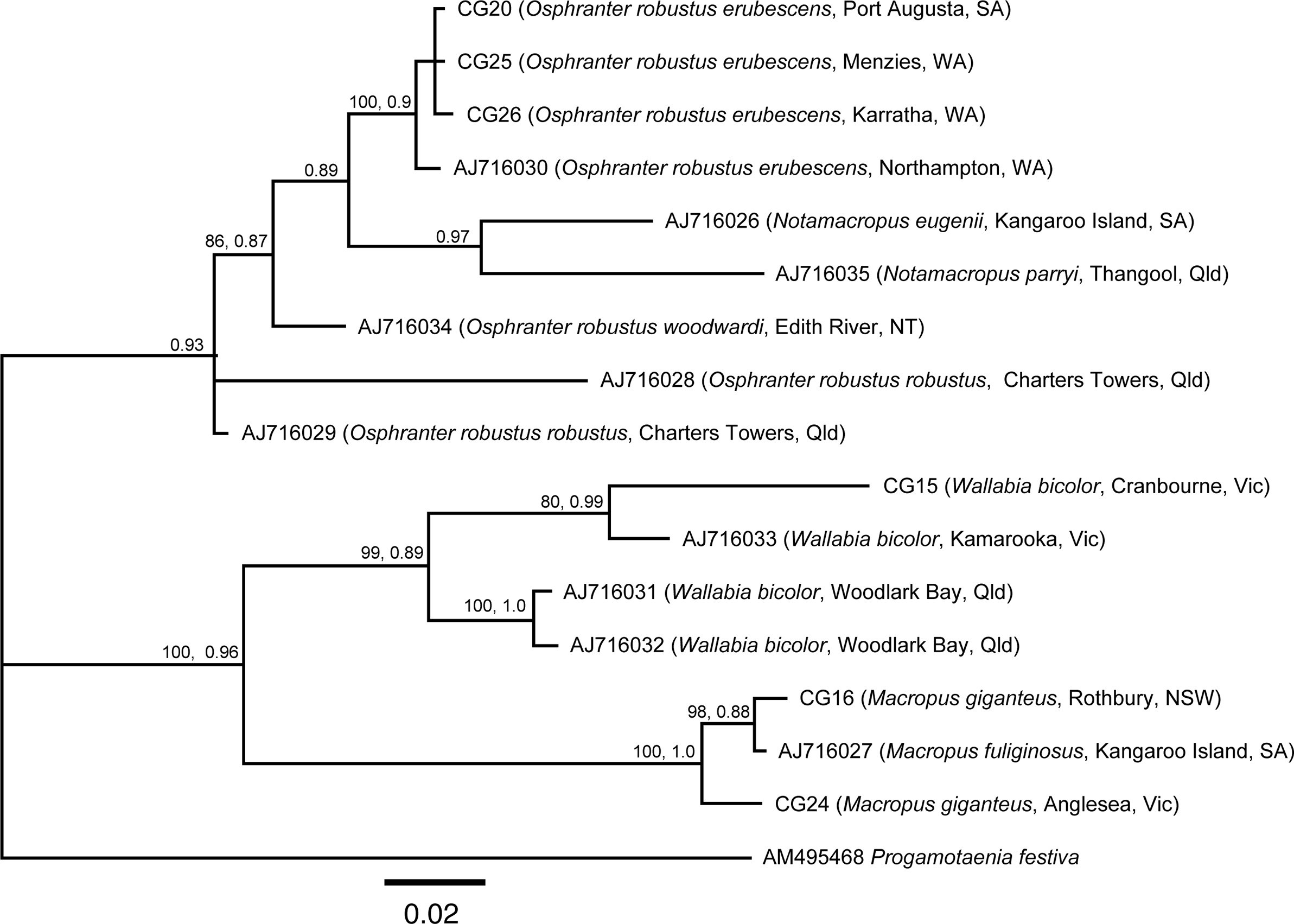
Figure 1. Bayesian Inference tree based on cox1 sequence data of Progamotaenia macropodis from various macropodid host species with Progamotaenia festiva as the outgroup. State name abbreviations and contractions: NSW, New South Wales; NT, Northern territory; Qld, Queensland; SA, South Australia; Vic, Victoria; WA, Western Australia. CG, laboratory code.
Based on a morphological study of all available material, the only genotype within the P. macropodis complex that was clearly differentiable from specimens in other host species was that found in N. eugenii. In this genotype, all specimens were shorter and more slender, and in immature and mature proglottids testes were invariably separated into two lateral groups. There was occasional fusion of the two groups of testes in post-mature proglottids. Therefore, based on both molecular and morphological data, this material is described below as a new species, P. mollicula sp. nov.
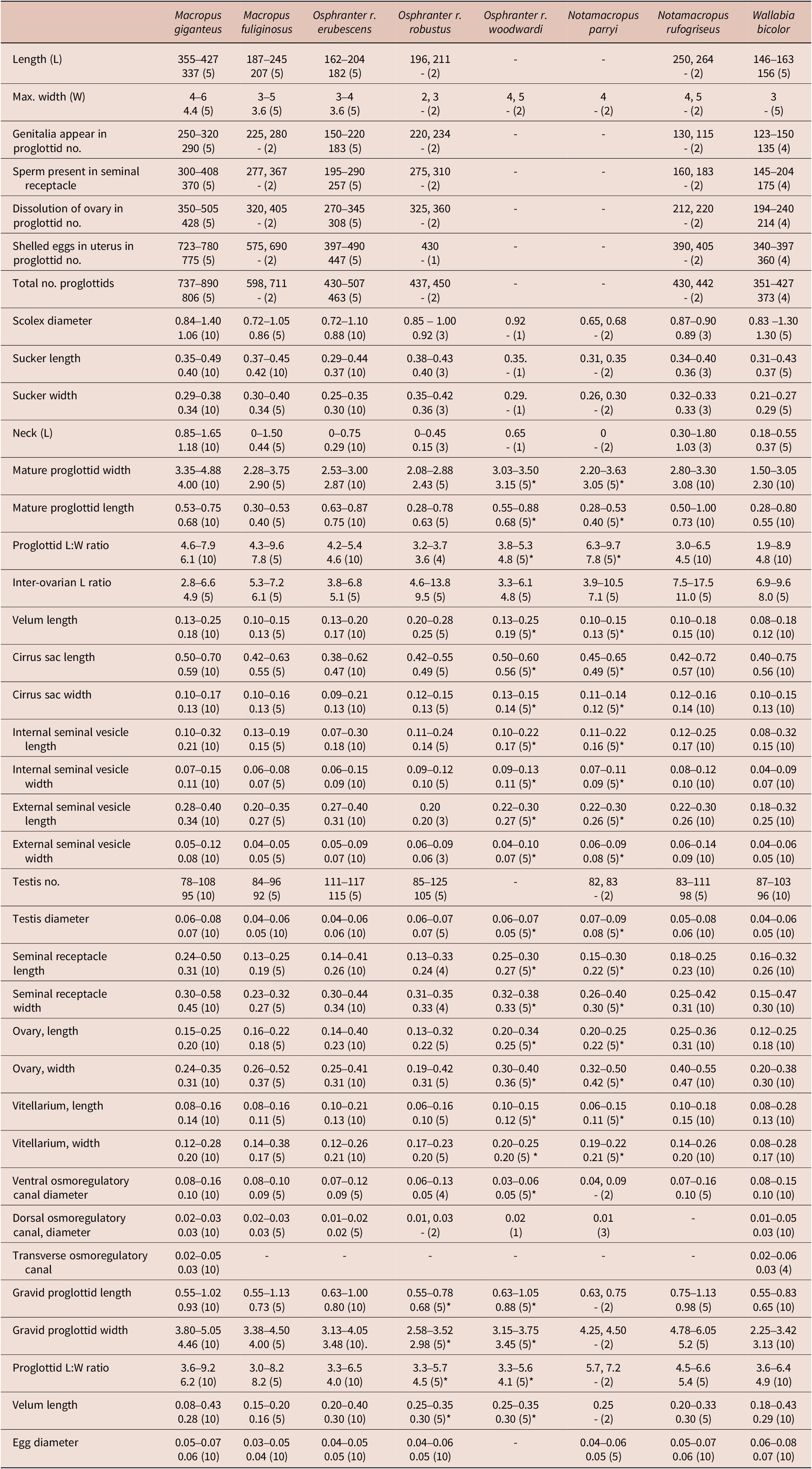
While some morphological differences were observed between the remaining genotypes, mainly in the size and shape of mature proglottids, there was considerable variation within genotypes leading to an overlap in characters and no clearly identifiable means of separating these genotypes morphologically. Extensive measurements of internal organs such as the cirrus sac, seminal vesicles, testes, seminal receptacle, ovary, and vitellarium (Table 2) failed to provide any means of reliably separating genotypes. The lack of obvious differences in mean values, the wide variation within measured parameters, and the variable and small numbers of observations that could be made on some genotypes due to the quality of the preserved material mitigated against a formal statistical analysis of the metrical data. Based on the genetic data, measurements of specimens from O. robustus were separated by host sub-species. In the case of W. bicolor, the genetically divergent material from Queensland was inadequate for morphological description, and as a consequence, the measurements and descriptions of material from this host species were based exclusively on the more abundant specimens from New South Wales and Victoria.
Table 2. Metric characters for specimens of Progamotaenia macropodis from different macropodid species (measurements are in mm with the range followed by the mean and the number of measurements made in parentheses; no mean is provided if fewer than 5 measurements were available) (* indicates few specimens available for measurement)
A more detailed redescription of P. macropodis is presented based on material from the type host, M. giganteus. This is followed by brief descriptions of specimens from each of the remaining known host species, including only key morphological features and noting differences from specimens from M. giganteus. Comprehensive sets of measurements are provided in Table 2 and, apart from the measurements from specimens from M. giganteus, are not repeated within the descriptions of material from the remaining host species.
Progamotaenia macropodis Beveridge, Reference Beveridge1976 (Figure 2, Table 2)
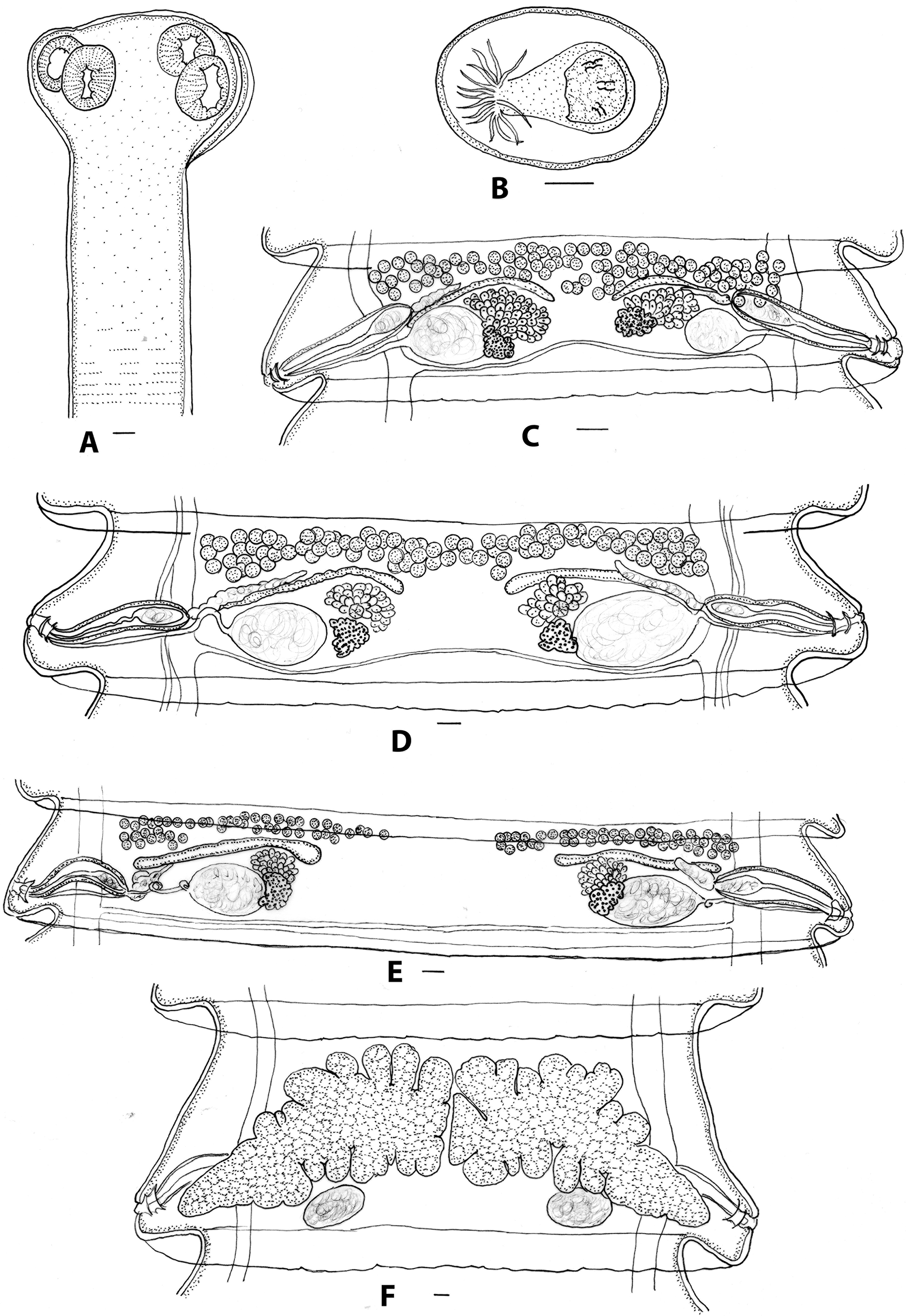
Figure 2. Progamotaenia macropodis, specimens from Macropus giganteus. A, Scolex; B, egg; C, mature proglottid from non-gravid specimen; D, mature proglottid from gravid specimen with testes in single band; E, mature proglottid from gravid specimen with testes in two separate groups; F, gravid proglottid. Figures E, F redrawn from Beveridge (Reference Beveridge1976). Scale-bars: A, C–F, 0.1 mm; B, 0.05 mm.
Progamotaenia. macropodis Beveridge, Reference Beveridge1976, pp. 58–60, figures 120–126; Beveridge and Arundel Reference Beveridge and Arundel1979, p. 73; Arundel et al. Reference Arundel, Dempster, Harrigan and Black1990, p. 43; Beveridge et al. Reference Beveridge, Chilton, Johnson, Smales, Speare and Spratt1998, p. 479; Webley et al. Reference Webley, Beveridge and Coulson2004, p. 629; Cripps et al. Reference Cripps, Beveridge, Martin, Borland and Coulson2015, p. 168; Beveridge Reference Beveridge2016, p. 210; Reference Beveridge2020, p. 4; 2023, p. 5.
Type data: holotype SAM 40776; paratypes SAM 40777-778, MV G2654-55, BMNH 1975.10.24.5-6.
Type locality: Yan Yean, Victoria (37° 33’S 145° 07’E).
Type host: Macropus giganteus Shaw.
Site in host: small intestine.
DNA sequence data: cox1: PV248093, PV248095.
Material examined:
From Macropus giganteus: Queensland: fragments, Townsville (SAM 21313 S); fragments, Bluewater (SAM 21335 S, 7653 W); New South Wales: 2 specimens, Armidale (SAM 20779 S, 10411 W); 4 specimens, Rothbury (SAM 37152 S, 49474 W); Australian Capital Territory: 2 specimens, Tidbinbilla (SAM 37106 S, 10941 W); Victoria: 1 specimen, Wodonga (SAM 37138 S, 47805 W); fragments, Dookie (SAM 37116 S, 18894 W); 1 specimen and fragments, Halls Gap (SAM 37129 S, 45642 W); fragments, Friarstown (SAM 37136 S, 46782 W); 7 specimens, Eildon (SAM 20917, 20932 S, 9507 W); 1 specimen, Yarra Glen (SAM 37113 S, 46170 W); types and 5 additional specimens, Yan Yean (SAM 20778, 20882, 20886, 20931, 21455, 2515 S); 1 specimen, Greswell Reserve, Bundoora (SAM 37137 S, 47802 W); 1 specimen, Anglesea, (SAM 35451 S, 46002 W).
Morphological features: long, robust cestodes, gravid specimens up to 427 long, maximum width 4-6 with up to 890 proglottids. Scolex four-lobed, with four oval suckers; neck present (Figure 2A). Mature proglottids 0.53–0.75 (0.68, n=10) long, 3.45–4.88 (4.00, n=10) wide; width-to-length ratio 4.6–7.9 (6.1, n=10); velum entire. Genital atrium in posterior part of lateral proglottid margin; atrium develops initially as closed sub-spherical cavity; when patent, walls corrugated; in post-mature proglottids, genital atrium may be everted to form papilla. Cirrus sac thin-walled, extending dorsally just beyond osmoregulatory canals into medulla; distal cirrus armed; internal seminal vesicle ovoid to pyriform; external seminal vesicle elongate, leading medially and anteriorly; testes in anterior region of proglottid, arranged in single band extending across entire medulla or two lateral groups, extending from osmoregulatory canals to varying extent, either to just beyond medial margin of ovary or with two groups almost meeting in centre of proglottid. Vagina tubiform, slender, entering genital atrium posterior to cirrus sac, leading medially to ovoid seminal receptacle with space between osmoregulatory canals and seminal receptacle; distal vagina atrophies following insemination; ovary flabelliform, medial and anterior to seminal receptacle; vitellarium reniform, posterior to ovary, overlapping seminal receptacle; Mehlis’ gland between vitellarium and ovary. Uteri paired, arising anterior to ovary, becoming lobulate with prominent anterior and posterior diverticula, eventually meeting in mid-line, crossing osmoregulatory canals dorsally (Figure 2F). Gravid proglottids up to 1.02 long, 5.05 wide; width: length ratio 3.6–9.2 (6.2). Eggs spherical; pyriform apparatus conical with reflexed filaments (Figure 2B). Ventral osmoregulatory canals paired; dorsal osmoregulatory canals paired, lateral to ventral canals, single transverse osmoregulatory canal connects ventral canals at posterior margin of each proglottid.
Variation: in specimens from type locality (Yan Yean, Victoria), testes most commonly arranged in two groups, including in holotype and paratype (Figure 2E); at remaining localities, usually arranged in single band, sometimes with only single row in centre of proglottid (Figure 2D); in single immature specimen (SAM 20779), proglottids much narrower, with completely uninterupted band of testes (Figure 2C).
From Macropus fuliginosus: South Australia: 8 specimens and fragments, Parndana, Kangaroo Island (SAM 28613 S); fragments of one specimen, Cape Cassini, Kangaroo Island (SAM 20846 S); fragments, Macgillivray, Kangaroo Island (SAM 29221 S).
DNA sequence data: cox 1: AJ716027.
Morphological features: similar to those from M. giganteus; long, robust cestodes, gravid specimens to 245 long, maximum width 3–5 with up to 711 proglottids. Mature proglottids 0.30–0.53 long, 2.28–3.75 wide; width to length ratio 4.3–9.6. Testes in single band or in two groups; as with material from type host, some strobilae with predominantly single band of testes, other strobilae with predominantly two groups of testes. Gravid proglottids 0.55–1.13 long, 3.38–4.50 wide, width to length ratio 3.0–8.2.
From Notamacropus eugenii: South Australia: 2 specimens, Vivonne Bay, South Australia (SAM 37109 S, 13007 W).
Morphological features: robust cestodes, 127, 147 long, 4, 5 wide. Mature proglottids 0.70–1.00 (0.80) long, 3.28–3.75 (3.40) wide; width to length ratio 3.4–4.7. Testes in single band or more rarely in two groups. Gravid proglottids 0.80–1.00 long, 4.25–4.58 wide, width to length ratio 4.3–5.7, widest gravid proglottids contracted, excluded from measurements. Similar in features to material from type host.
From Notamacropus parryi: Queensland: 2 fragmented, incomplete specimens, Thangool (SAM 22864 S).
DNA sequence data: cox 1: AJ18035.
Morphological features: robust cestodes, to 4.5 wide; mature proglottids 0.28–0.53 long, 2.20–336 wide, width: length ratio 6.8–7.9; testes in single band or two groups (Figure 3D); gravid proglottids 0.63–0.75 long, 4.25–4.50 wide; width: length ratio 5.7–7.2.
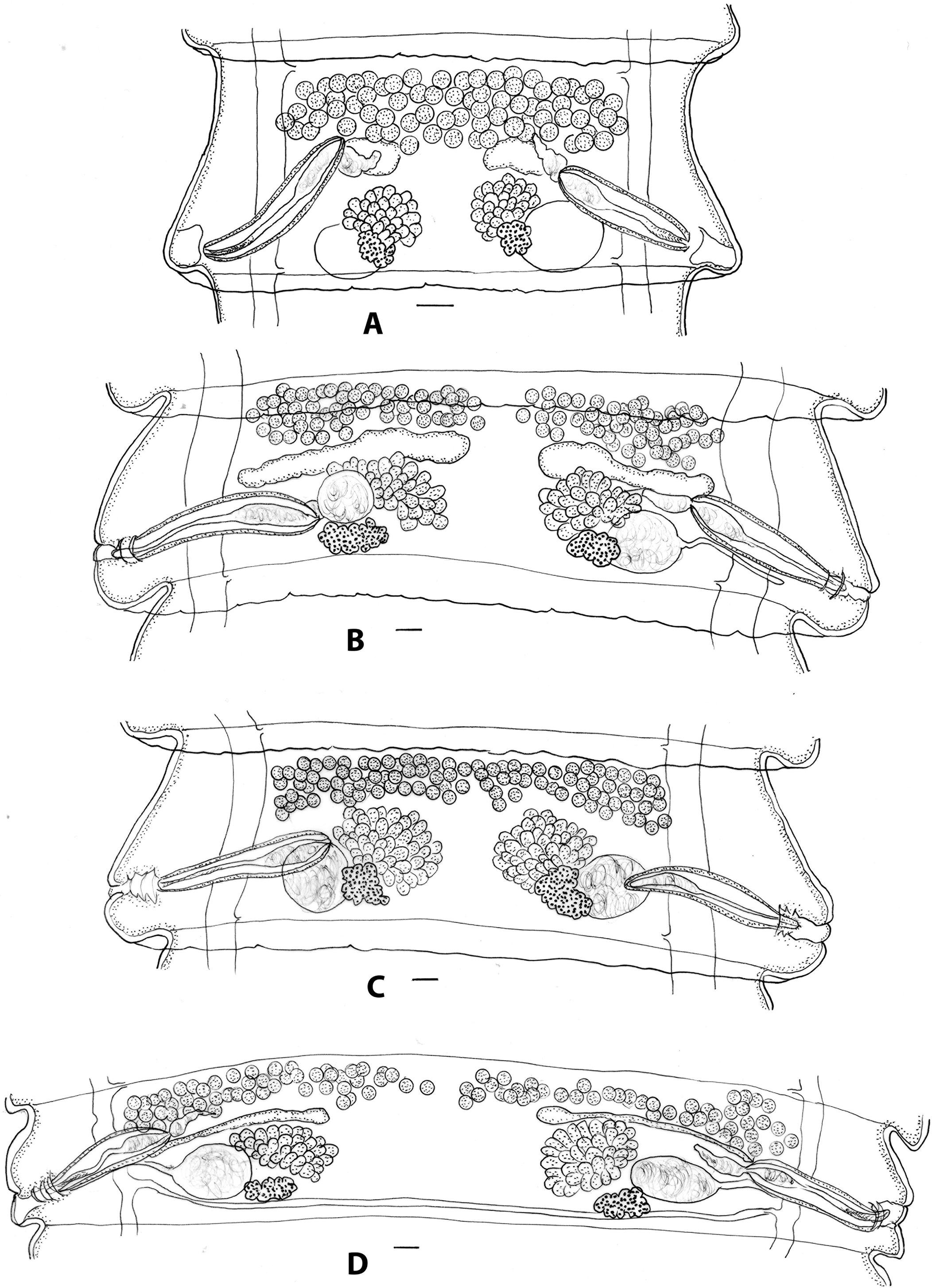
Figure 3. Progamotaenia macropodis, specimens from Notamacropus rufogriseus (A–C) and Notamacropus parryi (D). A, mature proglottid from non-gravid specimen; Figures B–C, two proglottids from same gravid strobila with testes either in two groups (B) or a single band (C); 3D, mature proglottid from gravid specimen. Scale-bars: 0.1 mm.
From Notamacropus rufogriseus: Victoria: 1 specimen, Grampian Ranges (SAM 37117 S, 19042 W); Tasmania: 3 specimens, Cape Barren Island (SAM 20905, 20979 S, 8583, 9469, 9470, 9479 W); 3 specimens, Launceston (SAM 20910 S, 9462, 9472 W); 2 specimens and fragments, Waterhouse (SAM 37113 S, 16441 W).
Morphological features: robust cestodes, to 264 long, 6 wide with up to 442 proglottids; immature specimens (SAM 20910) slender, with testes forming thick, single band in mature proglottids (Figure 3A); mature segments 0.5–1.0 long, 2.80–3.30 wide; width length ratio 3.0–6.5; testes in single band (Figure 3C), occasionally divided in mid-line (Figure 3B); gravid proglottids 0.75–1.13 long, 4.78–6.05 wide, width: length ratio 4.5–6.6. Similar in overall features to material from type host.
From Osphranter robustus erubescens: Queensland: 3 specimens, Cloncurry (SAM 29215 S); Northern Territory: 8 specimens and fragments, Mulga Park Station via Alice Springs (SAM 35813, 37134, 37135 S, 46198, 46201 W); Western Australia: 4 specimens and fragments, Fortescue River Roadhouse (SAM 37130, 37131 S, 45923, 45924 W); 10 specimens and fragments, Northampton (SAM 28615 S, 34768 W); 5 specimens, Menzies (SAM 37128 S, 45574 W).
DNA sequence data: cox 1: AJ718030, PV248094, PV248096, PV248097.
Morphological features: robust cestodes, up to 204 long, 4 wide with up to 507 proglottids. Mature proglottids 0.63–0.87 long, 2.53–3.00 wide; width: length ratio 4.2–5.4; testes usually in single band (Figure 4B), with slight separation into two groups in few proglottids; inter-ovarian ratio high, 3.8–6.8; gravid proglottids 0.63–1.00 long, 3.13–4.05 wide, width: length ratio 3.2–6.5. Morphologically similar to material from type host.
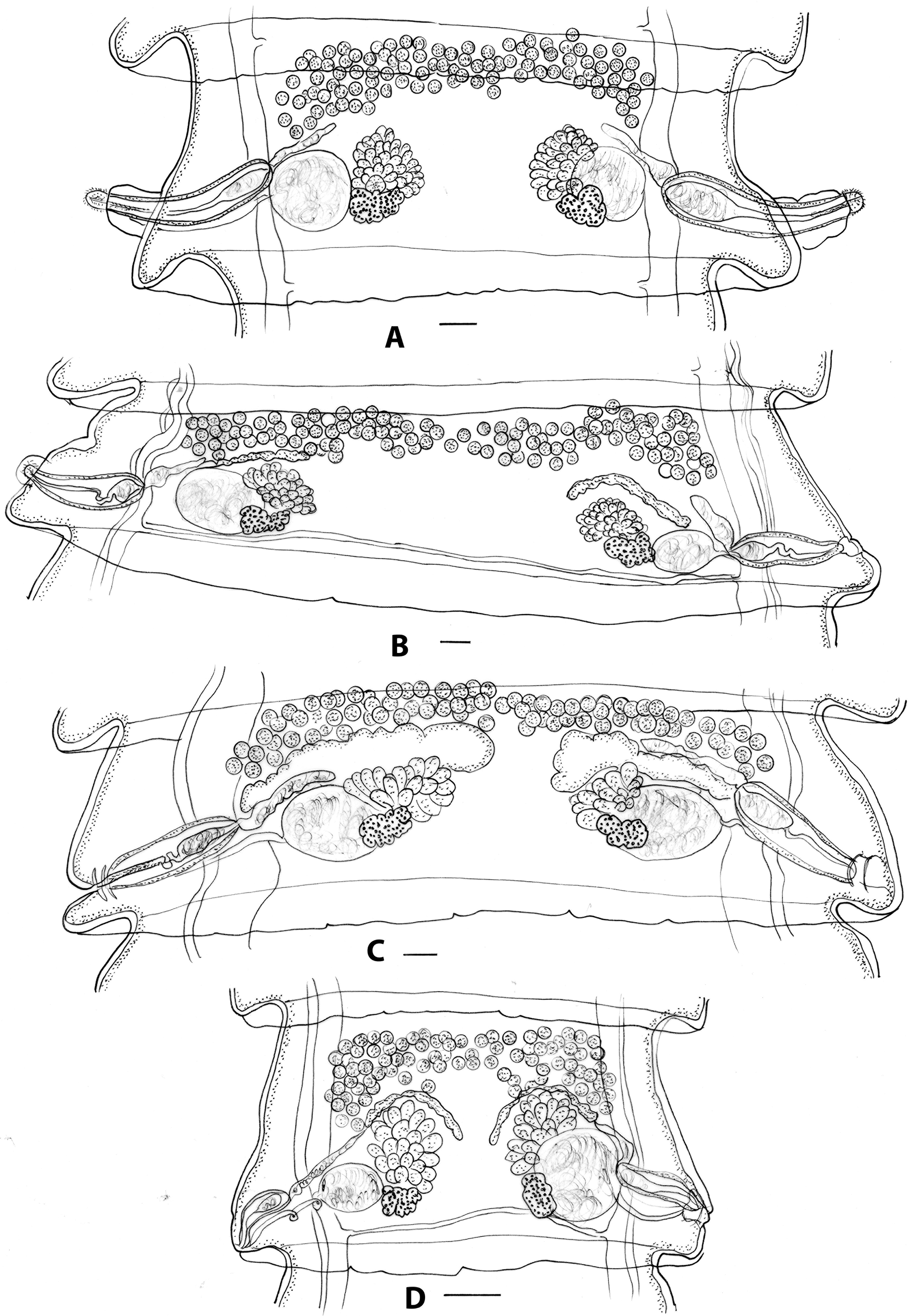
Figure 4. Progamotaenia macropodis, specimens from Osphranter robustus and Wallabia bicolor. A, mature proglottid from gravid specimen from O. r. robustus with slender strobila and shorter inter-ovarian distance; B, mature proglottid from gravid specimen from O. r. erubescens with broader strobila and larger inter-ovarian distance; C, mature proglottid from gravid specimen from W. bicolor; D, mature proglottid from non-gravid specimen from W. bicolor. Figure D redrawn from Beveridge (Reference Beveridge1976). Scale-bars: 0.1 mm.
From Osphranter robustus robustus: Queensland: fragments, Georgetown (SAM 29220 S); fragments, Mount Surprise (SAM 29219 S); fragments, Mingela (SAM 21328 S, 7599W); fragments, Charters Towers (SAM 21311, 21338 S, 7827 W); 2 specimens and fragments, Warrawee Station via Charters Towers (SAM 19754, 22082 S, 11751 W); fragments, Harvest Home Station via Charters Towers (SAM 22352 S); New South Wales: 1 specimen, Kingstown (SAM 29830, 35105 S, 44690 W).
DNA sequence data: cox 1: AJ718028, AJ718029.
Morphological features: (restricted to specimens from Charters Towers and Mingela) slender cestodes up to 211 long, 3 wide with up to 450 proglottids; mature segments 0.28–0.78 long, 2.08–2.88 wide, width: length ratio 3.2–3.7; testes in single band; inter-ovarian ratio 4.6–13.8 (Figure 4A); gravid proglottids 0.55–0.78 long, 2.58–3.52 wide; width: length ratio 3.3–5.7.
More slender than specimens from type host.
From Osphranter robustus woodwardi: Northern Territory: 1 specimen plus fragments, Edith River (SAM 28614 S, 34767 W).
DNA sequence data: cox 1: AJ718034
Morphological features: robust cestodes, up to 3.75 wide; mature segments 0.55–0.88 long, 3.03–3.50 wide; width: length ratio 3.8–5.3; testes in single band; gravid proglottids 0.63–1.05 long, 3.15–3.75 wide, width: length ratio 3.3–3.6. Specimens too poorly stained to assess testis numbers.
From Wallabia bicolor: Queensland: fragments, Dingo Beach (SAM 21298 S); fragments, Woodlark Bay, Airlie Beach (SAM 26679, 28616 S, 34769 W); New South Wales: 1 specimen, Dorrigo (SAM 20789 S, 10404 W); 1 specimen, Nowra (SAM 21509 S); Victoria: 1 specimen, Warby Range (SAM 20845 S, 10123 W); fragments, Kamarooka (SAM 28617 S, 30502 W); 2 specimens, Dartmouth (SAM 20921 S); 1 specimen, Mitta Mitta (SAM 22124 S, 12094 W); 2 specimens, fragments, Zumsteins (SAM 29871 S); fragments, Woodend (SAM 29216 S); 1 specimen, Buangor (SAM 21386 S, 6467 W); 1 specimen, Beaufort (SAM 29605 S, 44661 W); 1 specimen, Healesville (SAM 29217 S); 2 specimens, Bonang (SAM 20844 S, 10507 W); 2 specimens, Orbost (SAM 37148 S, 10099 W); fragments, Sunday Island (SAM 37115 S, 17494 W); fragments, Camperdown (SAM 37119 S, 19290 W); 3 specimens Phillip Island (SAM 37141 S, 48943 W).
DNA sequence data: cox 1: AJ718031, AJ718032, AJ718033, PV248092.
Morphological features: slender cestodes, up to 156 long, up to 3 wide with up to 427 proglottids in gravid strobilae; mature segments 0.28–0.80 long, 1.50–3.05 wide; width: length ratio 1.9–8.9; testes invariably in single band (Figure 4C); mature proglottids of non-gravid specimens slender, with shorter inter-ovarian distance (Figure 4D); gravid proglottids 0.55–0.83 long, 2.25–3.42 wide, width: length ratio 3.6–6.4.
Progamotaenia mollicula sp. nov. (Figure 5)
Type data: holotype SAM 37085; paratypes SAM 37086- 37087.
Type locality: Kangaroo Island, South Australia (35° 48’S 137° 13’E).
Type host: Notamacropus eugenii (Desmarest) (Marsupialia: Macropodidae).
Site in host: small intestine.
DNA sequence data: cox 1: AJ718026.
Material examined: from N. eugenii, Kangaroo Island, South Australia: types; 1 specimen (SAM 20776 S); 2 specimens (SAM 22817 S); Parndana, 2 specimens (SAM 20879 S); 1 specimen (SAM 28612 S, 10078 W); Karratta, 1 specimen (SAM 20877 S, 8595 W); American River, incomplete specimens (SAM 37105 S, 10075 W).
Zoobank registration number: urn:lsid:zoobank.org:act:D21C5BC6-DBF8-46D7-B43F-98BD4023D059.
Etymology: from molliculus, Latin, meaning ‘dainty’.
Description: elongate, slender cestodes, gravid specimens 150–294 (218, n=5) long, 3–4 (3.6, n=5) wide, with 254–360 (298, n=5) proglottids. Scolex diameter 0.74–0.90 (0.81, n=10); suckers 0.28–0.38 (0.32, n=10) long, 0.22–0.32 (0.25, n=10) wide; neck: 0–1.45 (0.68, n=10) long (Figure 5A). Mature proglottids 0.88–1.13 (1.00, n=10) long, 2.18–3.13 (2.53, n=10) wide; width: length ratio 2.11–3.57 (2.55, n=10); mid-point of ovary to proglottid margin 0.80–1.00 (0.90, n=10); velum entire, narrow, 0.05–0.11 (0.08, n=10) wide; genital atrium in posterior region of lateral proglottid margin, develops initially as closed sub-spherical cavity (Figure 5C); when patent, walls corrugated (Figure 5D). Cirrus sac thin-walled, 0.53–0.80 (0.61, n=10) long, 0.10–0.13 (0.12, n=10) wide, extending dorsally beyond osmoregulatory canals into medulla; distal cirrus armed; internal seminal vesicle ovoid to pyriform, 0.10–0.20 (0.13, n=10) long, 0.08–0.11 (0.09 n=10) wide; external seminal vesicle elongate, 0.11–0.25 (0.16, n=10) long, 0.04–0.07 (0.06, n=10) wide, leading medially and anteriorly; testes arranged in two lateral groups in all premature and mature proglottids, extending just beyond medial margin of ovary (Figures 5C–E), 28–42 (34, n=10) testes per group; testis diameter: 0.07–0.11 (0.08, n=10); in post-mature proglottids, testis fields may extend medially to almost unite or unite (Figure 5E), but are invariably separate in mature proglottids. Vagina tubiform, slender, entering genital atrium posterior to cirrus sac, leading to ovoid seminal receptacle immediately adjacent to osmoregulatory canals, 0.20–0.35 (0.28, n=10) long, 0.21–0.38 (0.32, n=10) wide; ovary flabelliform, medial and anterior to seminal receptacle, 0.22–0.34 (0.29, n=10) long, 0.35–0.45 (0.39, n=10) wide; asymmetry ratio 2.5–3.1 (2.8, n=10); vitellarium reniform, posterior to ovary, overlapping seminal receptacle 0.08–0.18 (0.13, n=10) long, 0.12–0.23 (0.20, n=10) wide; Mehlis’ gland between vitellarium and ovary, 0.08–0.14 (0.11, n=5) in diameter. Uteri paired, arising anterior to ovary, obliquely arranged with medial ends anterior to lateral ends; becoming lobulate with prominent anterior and posterior diverticula, eventually meeting in mid-line; crossing osmoregulatory canals dorsally. Gravid proglottids 1.00–1.70 (1.23, n=10) long, 2.65–3.90 (3.25, n=10) wide; width: length ratio 2.11–4.00 (2.74, n=10); velum 0.15–0.30 (0.19, n=10) wide (Figure 5F); cirrus sac 0.55–0.80 (0.66, n=10) long, 0.10–0.15 (0.12, n=10) wide. Eggs ovoid, 0.045–0.058 (0.051, n=10) long, 0.028–0.038 (0.035, n=10) wide; pyriform apparatus conical, 0.025–0.033 (0.028, n=10) long; oncosphere 0.010–0.017 (0.013, n=10) in diameter (Figure 5B). Ventral osmoregulatory canals 0.04–0.10 (0.07, n=10) in diameter; dorsal osmoregulatory canal lateral to ventral canal, 0.01–0.02 (0.014, n=10) in diameter; single transverse osmoregulatory canal connects ventral canals at posterior margin of each proglottid, 0.02–0.04 (0.03, n=10) in diameter.
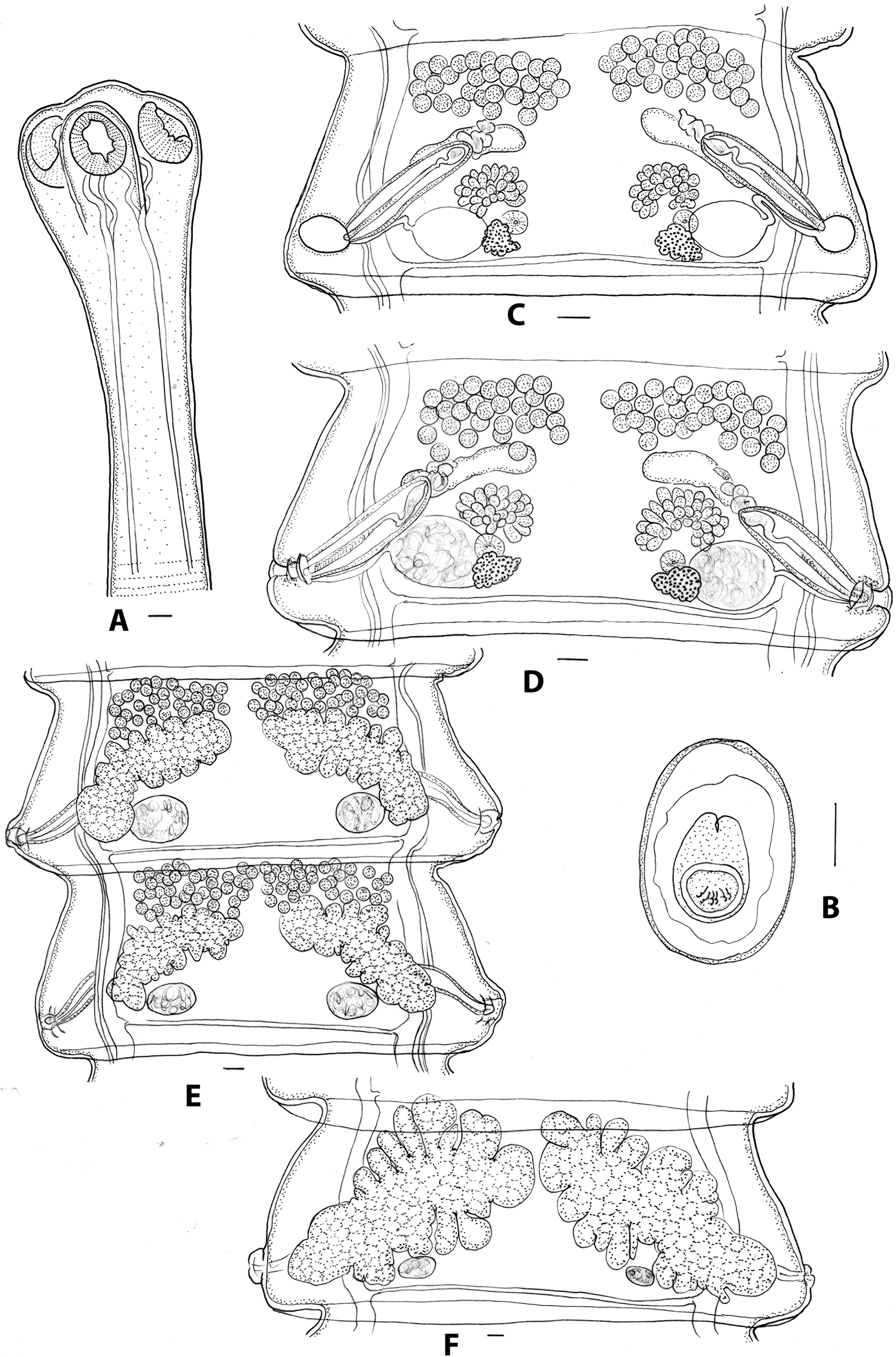
Figure 5. Progamotaenia mollicula sp. nov. from Notamacropus eugenii. A, scolex; B, egg; C, pre-mature proglottid with non-patent genital atrium and empty seminal receptacles; D, mature proglottid with patent genital atrium and sperm-filled seminal receptacles; E, adjacent, post-mature proglottids with testes in two separate groups or joined in midline; F, gravid proglottid. Scale-bars: A, C–F, 0.1 mm; B, 0.01 mm.
Individual elements of genitalia first evident in proglottids 60–80 (71, n=4); all genitalia fully formed in proglottids110–150 (130, n=5); genital atrium remains closed following full development of genitalia; genital atrium becomes patent and sperm appears in seminal receptacle in proglottids146–190 (166, n=5); female genitalia disappear by proglottids170–222 (199, n=5); eggs first appear in proglottids 248–290 (269, n=2); total number of proglottids 254–346 (298, n=5).
Remarks: The most obvious differences between the new species and P. macropodis sensu stricto are that the new species is shorter (355–427 in M. giganteus; 150–294 in N. eugenii) and more slender (4–6 in M. giganteus; 3–4 in N. eugenii), there are fewer proglottids (737–890 in M. giganteus; 254–360 in N. eugenii) with the proglottids maturing earlier (250–320 in M. giganteus; 110–150 in N. eugenii). In addition, the testes invariably occur in two lateral groups in mature proglottids of the new species. This distinction in testis distribution is partially lost in post-mature proglottids in which the enlargement of the uteri and the involuting testes results in the testis fields approaching one another medially and sometimes becoming confluent. However, based on the origins of the testis fields and their positions in mature proglottids, they are primarily arranged in two lateral groups. The oblique orientation of the uteri also differentiates the new species from related genotypes. The new species is found exclusively in N. eugenii on Kangaroo Island in South Australia.
Discussion
Although only a small number of additional molecular samples have been added to those of Hu et al. (Reference Hu, Gasser, Chilton and Beveridge2005), they provide additional resolution within this species complex. The addition of material from the type host, M. giganteus, from two localities, forming a clade with material from the closely related host species M. fuliginosus, suggests the existence of single genotype in the two species of grey kangaroos. The existence of distinct genotypes in N. eugenii and N. parryi in the study of Hu et al. (Reference Hu, Gasser, Chilton and Beveridge2005) is also evident in the present study as well as the existence of two genotypes in W. bicolor, one occurring in Victoria and the other in central Queensland. The addition of sequences from O. robustus supports the occurrence of different genotypes in each of the three subspecies of host, O. r. erubescens, O. r. robustus, and O. r. woodwardi. The two genotypes present in O. r. robustus in northern Queensland, first reported by Hu et al. (Reference Hu, Gasser, Chilton and Beveridge2005), provide a result similar to that found in P. festiva in which several genotypes are found in kangaroo species in this same geographical region (Beveridge et al. Reference Beveridge2007).
In spite of a detailed examination of all of the available morphological material, consistent differential morphological features were found only in the genotype from N. eugenii on Kangaroo Island, South Australia, being shorter and more slender and with the testes in immature and mature proglottids invariably separated into two groups. On this basis, a new species, together with molecular and host data, P. mollicula sp. nov. has been erected. The rate of development of the genital systems has not been used frequently in cestode systematics but was considered to be useful by Hoberg and Soudachanh (Reference Hoberg and Soudachanh2021) in separating cryptic species within the Tetrabothrius jagerskioeldi Nybelin 1916 species complex in Arctic birds and appears to provide some difference among members of the P. macropodis complex.
While some morphological differences between remaining genotypes were found, they were highly variable and did not permit the reliable separation of specimens from different host species. Consequently, they are all retained provisionally under the name P. macropodis. Differences were noted in the total lengths of cestodes, the number of proglottids, and the rate of development of the genitalia. Differences in the size and shape of mature proglottids also had some effect on the distribution of testes and female genitalia within the proglottid. In narrower mature proglottids, the seminal receptacle was often adjacent to the osmoregulatory canals, while in broader proglottids, there was a distinct space between them, and in addition, the inter-ovarian space appeared to be shorter in narrower proglottids. However, no major differences were noted in the measurements of major internal organs (Table 2).
Given the differences in the shape of mature proglottids, attempts were made to quantify them using the width-length ratio of proglottids, the asymmetry index of Haukisalmi and Henttonen (Reference Haukisalmi and Henttonen2007), and measuring the inter-ovarian distance ratio. The asymmetry index provided no obvious differences between genotypes (Table 2). The mean inter-ovarian distance ratio was higher in the genotypes with narrower strobilae. It was also higher in specimens from N. rufogriseus with broader strobilae, and the ranges of the ratio within each genotype were frequently large. The failure of these methods to provide criteria for separating genotypes may be because they are essentially allometric indices, with narrower proglottids invariably having female genitalia closer to the proglottid margin and closer to one another in the medulla.
A significant limitation in the morphological study was the lack of complete gravid specimens representing some of the genotypes. Because of variation seen in immature cestodes, it appears that reliable comparisons can only be made between gravid specimens, and because the cestodes are large, many collections are fragmented, entire cestodes have not been mounted on slides, or such examples are few or have stained poorly. Despite these limitations, it has been possible to provide additional data on morphological variation within this species complex.
The redescription presented above is based primarily on material from the type host, M. giganteus. Beveridge (Reference Beveridge1976) provided measurements for the holotype and paratypes only while the current redescription includes material from the type locality as well as from other localities and therefore provides a better indication of variation within the genotype, although additional complete, gravid-mounted specimens are likely to indicate that variation within the genotype is even more extensive than that described here. Additional sequence data have been added from sites in Victoria and New South Wales, but material from the type locality has not yet been sequenced. Additional molecular data would be useful to understand the variation in testis distribution noted in specimens collected from different but geographically proximal sites from which the species was collected from M. giganteus in Victoria. Of particular note is the observation of mature proglottids in non-gravid specimens (Figure 2C) in which the inter-ovarian space is much shorter and the testes are arranged in a greater number of rows.
Specimens from M. fuliginosus, which were genetically similar to those from M. giganteus, were shorter but had a similar number of proglottids, although only two complete, gravid specimens were available for comparison. Otherwise, they were indistinguishable from specimens found in M. giganteus. Progamotaenia macropodis has been found in M. fuliginosus only on Kangaroo Island despite extensive sampling of this host on the mainland (Beveridge Reference Beveridge2023, supplementary Table 1). Macropus giganteus occurred on Kangaroo Island in the past (Seershol et al. Reference Seershol, Grealy, McDowell, Cole, Arnold, Prideaux and Bunce2021), but this does not explain why the cestode has not been found in M. fuliginosus in areas on the mainland where it is currently sympatric with M. giganteus. A single instance was found of specimens clearly identifiable as P. macropodis in a single N. eugenii on Kangaroo Island where N. eugenii and M. fuliginosus occur in sympatry. This finding suggests that host specificity between genotypes may not be absolute, but the observation requires confirmation using molecular methods.
Although the material from N. parryi was genetically distinct, the morphological material is represented by only two fragmented and slightly contracted specimens from a single host animal. Only limited morphological observations were possible, but there were no obvious differences when compared with specimens from M. giganteus. No molecular data are available for specimens from N. rufogriseus, but the gravid specimens are large and broad and indistinguishable from material from the type host (Figures 3A–C). The illustrations of mature proglottids of this material show the differences in the distribution of the testes, either in a single band or in two groups occurring in the same strobila (Figures 3B, C). As with specimens from M. giganteus, immature cestodes have a much narrower strobila with a different width: length ratio for mature proglottids resulting in a shorter inter-ovarian distance and the testes arranged in a greater number of rows (Figure 3A). The material available for examination came principally from Tasmania (including Cape Barren Island), with a single specimen from Victoria. The species has not previously been reported from Victoria (Aussavy et al. Reference Aussavy, Bernardin, Corrigan, Hufschmid and Beveridge2011; unpublished observations). Spratt et al. (Reference Spratt, Walter and Haycock2017) reported the gastric and oesophageal parasites of N. rufogriseus from various sites in south-eastern New South Wales. The intestinal tracts were also examined, but no cestodes were found (D.M. Spratt, personal communication). The parasite therefore appears to be uncommon in mainland populations of N. rufogriseus.
Based on the genetic data, the cestodes from O. robustus were subdivided into three sub-clades related to the sub-species of the host for morphological comparisons. Material from O. r. erubescens was most abundant and proved to be morphologically indistinguishable from specimens of P. macropodis from the type host, M. giganteus. Only a single specimen plus fragments from a single locality were available from O.r. woodwardi, and the limited available material was again indistinguishable from typical P. macropodis, although the specimens were too poorly stained to allow assessments of testis numbers (Table 2). An illustration of the mature proglottid of these specimens has not been provided, as they appear to be indistinguishable from those found in M. giganteus. Genetic data indicated two different genotypes among the specimens of P. macropodis from O. r. robustus, being differentiated grossly based on width, with slender specimens from all localities around Charters Towers and Mingela (40 km north-east of Charters Towers) and much broader specimens from Mount Surprise and Georgetown (both c. 320 km north-west of Charters Towers). Unfortunately, the latter specimens were very poorly fixed and fragmented. The mean length-to-width ratio of mature proglottids of the Charters Towers specimens was 3.6 compared with 4.6 and 4.8 in specimens from O. r. erubescens and O. r. woodwardi, respectively, and 6.1 and 7.8 in specimens from M. giganteus and M. fuliginosus, respectively (Table 2), leading to a marked difference in proglottid shape. As indicated above, the presence of two genotypes in this region mirrors the finding of Beveridge et al. (Reference Beveridge2007) of different genotypes of P. festiva in these hosts in the same region. Hu et al. (Reference Hu, Gasser, Chilton and Beveridge2005) examined seven specimens from this host and region but only deposited a single voucher specimen (SAM 19754) representing the morphotype with a narrow strobila. Additional collections are needed from this region to resolve the apparent differences between the morphological and molecular data currently available.
Specimens from W. bicolor were abundant from southern hosts and were also particularly slender in shape, relatively short, and with fewer proglottids in gravid specimens compared with other genotypes (Table 2). Specimens from Queensland, which were genetically distinct from those collected in Victoria, consisted of poorly preserved fragments only and were not included in the morphological analyses. Proglottid shape varied considerably, and unlike material from M. giganteus and N. rufogriseus in which narrow proglottids were found in immature cestodes only, in specimens from W. bicolor, narrow gravid segments were also present. Although comprehensive morphological data were available for this genotype and there were clear differences in the size of gravid specimens and the numbers of proglottids (Table 2), other differences were limited. The typical size and shape of a mature proglottid from a gravid strobila is shown in Figure 4C. The illustration of Beveridge (Reference Beveridge1976, Figure 124) (erroneously attributed to M. giganteus as the host), reproduced here in Figure 4D, is from a non-gravid specimen (SAM 20921) and is therefore not representative of mature proglottids of this genotype.
Overall, although there were only minor features that separated some genotypes such as length, number of proglottids, rate of development of the genitalia and length:width ratios of mature proglottids, these features were variable and frequent overlap occurred. The morphology of the genitalia provided no distinguishing features. Therefore, a single new species has been described based on genetic and morphological characteristics, but the remaining genotypes remain poorly distinguishable or not distinguishable at all morphologically. These results are similar to studies of the P. festiva and W. ewersi complexes in macropodids, with one or a small number of genotypes being distinguishable morphologically, but with a residual group of genotypes remaining indistinguishable (Beveridge Reference Beveridge2009; Beveridge and Shamsi Reference Beveridge and Shamsi2009). For similar problems with morphologically cryptic species in trematodes, Bray et al. (Reference Bray, Cutmore and Cribb2022) have suggested the use of an integrative approach wherein reciprocal monophyly of the chosen genetic marker, host range, and geographical data are included in the recognition of cryptic species. In the case of P. macropodis, this will need to include much wider genetic sampling as well as the availability of more well-preserved, gravid, entire specimens for morphological studies. For P. festiva, a case can be made for recognising certain genotypes as species based on host specificity and geographical distribution. However, the molecular basis for this is much sounder than that currently available for P. macropodis, with only limited molecular evidence. In addition, it has not been established whether the genotypes found in P. macropodis are completely host-specific, with at least one possible instance of its occasional occurrence in N. eugenii, a host occurring in close sympatry with M. fuliginosus on Kangaroo Island.
Acknowledgements
Thanks are due to Dr Leslie Chisholm and Jo Wood for access to material in the collections of the South Australian Museum.
Financial support
This project was funded by the Australian Biological Resources Study (NTRGI000050).
Competing interests
The authors declare no conflicts of interest.
Ethical standard
This study was conducted entirely on museum specimens.









