Introduction
Physical activity (PA), which encompasses any sort of movement in which the contraction of skeletal muscles increases energy consumption, is recognised to provide several health advantages.(Reference Ortiz-Alvarez, Xu and Martinez-Tellez1) The main goal of physical exercise is to increase physical functionality through adaptation. Furthermore, physical exercise appears to boost microbial diversity in the gut, increase the Bacteroidetes:Firmicutes ratio, encourage the development of bacteria that can influence mucosal immunity, and improve intestinal barrier functions — all of which are advantageous to the host’s health.(Reference Choi, Eum, Rampersaud, Daunert, Abreu and Toborek2)
Athletes have very specific needs for nutrition to help performance, recovery, and health in general. Probiotics and prebiotics as dietary factors have been studied extensively for their contribution to gut health, immunity, and metabolic function.(Reference Jäger, Mohr and Pugh3,Reference Parker, Roy, D’Adamo and Wieland4) Research in Western populations has shown probiotic use to decrease exercise-induced gastrointestinal (GI) symptoms, increase nutrient absorption, and improve immune function.(Reference Clark and Mach5,Reference Jäger, Mohr and Carpenter6) GI disturbances are also common in this group due to intensive training, dehydration, and diet composition that detrimentally affect performance.(Reference Pugh, Fearn, Morton and Close7)
Nevertheless, there is a clear gap in current literature regarding the knowledge, consumption patterns, and perceived benefits of probiotics and prebiotics among Middle-Eastern athletes, including those in Jordan. Most existing studies focus on Western dietary patterns, where probiotic-rich foods are more commonly consumed.(Reference Tap, Furet and Bensaada8) In contrast, Jordanian athletes may have limited awareness and access to such foods due to different dietary habits, food environments, and cultural practices.
Understanding athletes’ knowledge and consumption of probiotics and prebiotics in Jordan may support the development of targeted nutritional interventions tailored to their specific needs.
Highly aerobic endurance athletes are at a higher risk of leaky gut and intestinal permeability because of exercise-induced stress, which reduces GI blood flow, causing inflammation and gut wall damage.(Reference Clark and Mach5,Reference Kahn9)
Short-chain fatty acids (SCFAs) produced by beneficial bacteria significantly contribute to the health of the intestinal wall and play a role as a barrier, preventing the passage of harmful substances.(Reference Peng, Tabashsum and Anderson10) According to supporting evidence, an increase in dietary fibre is also related to increased microbial diversity and richness in individuals who lack diversity of bacteria in their gut.(Reference Tap, Furet and Bensaada8) This research highlights how crucial it is for athletes to consume prebiotic and probiotic foods to keep a diverse and balanced gut microbiome.
In recent years, researchers have focused on the health of athletes to prevent or manage exercise-related health disorders through the use of dietary supplements.(Reference Maughan11) Additionally, studies have demonstrated that probiotics can decrease the likelihood of GI and respiratory infections as well as the severity of symptoms related to these conditions in athletes.(Reference Di Dio, Calella and Cerullo12) As a result, probiotic supplementation may benefit athletes’ health.(Reference Jäger, Mohr and Carpenter6)
Although several bacteria are utilised as probiotics, the most common strains are Lactobacillus, Enterococcus, Bifidobacterium, Propionibacterium, and yeasts such as Saccharomyces boulardii.(Reference McFarland, Evans and Goldstein13) Lactobacillus and Bifidobacterium strains show potential benefits for athletes and fitness enthusiasts by improving nutrient absorption, energy metabolism, and exercise performance while reducing oxidative stress and inflammation.(Reference Khan14)
Probiotic supplementation also boosts amino acid absorption from plant proteins and enhances glucose absorption and oxidation during exercise.(Reference Jäger, Mohr and Pugh3,Reference Pugh, Wagenmakers and Doran15) Microbes in the intestinal microbiota perform metabolic processes such as converting carbohydrates into SCFAs, lipid metabolism, and vitamin synthesis, promoting immune system maturation, and protecting against pathogens.(Reference Marttinen, Ala-Jaakkola, Laitila and Lehtinen16)
Limited knowledge of probiotics and prebiotics among athletes may hinder their ability to optimise gut health, which is important for digestion, immune function, and nutrient absorption, all of which are critical to training adaptation and recovery.(Reference Jäger, Mohr and Carpenter6,Reference Maughan11) In addition, probiotics and prebiotics are known to enhance immune function, reduce inflammation, and improve endurance performance; thus, they are important for an athlete’s diet.(Reference Clark and Mach5,Reference Shing, Peake and Lim17) This lack of awareness in this area indicates the need for specific educational interventions aimed at optimising gut health and performance in athletic populations.
Thus, the objective of this study is to evaluate the knowledge and consumption of probiotics and prebiotics among Jordanian athletes and to determine if a greater knowledge level is associated with higher dietary intake and fewer GI symptoms. It also examines the potential associations among knowledge, diet quality, and GI symptoms. The findings will contribute to developing targeted nutrition education programmes to enhance athletes’ gut health and overall well-being.
Methodology
Participant selection
The study was carried out in Jordan, using athletes as the target population. The study included athletes in Jordan who met the following inclusion criteria: (1) 18–22 years old, (2) trained physically in a structured manner at least three times a week, and (3) participated in recreational or competitive organised sports. This definition is consistent with previous literature that considers athletes as individuals who train regularly to improve performance.(Reference Araújo and Scharhag18) A screening question in the questionnaire compelled participants to acknowledge that they were eligible before continuing. Those who answered ‘Yes’ were the only ones permitted to move forward and take the survey to keep the study specific to the desired athlete population.
The 18–22 age bracket had been chosen to assess a relatively homogenous sample as it is known that athletes below 18 years old present different metabolic and nutritional requirements due to ongoing growth and development, whilst over 22 years old athletes will tend to undertake more independent feeding habits and training commitments will be more diverse.(19) This age range was selected to increase the homogeneity of the participants’ knowledge on probiotics and prebiotics within an organised sports setting. The survey was shared through Facebook, Instagram, and WhatsApp. This approach helped capture a broad and diverse group of athletes.
We used a cross-sectional approach to investigate particular parameters of interest in this demographic. To achieve adequate representation, the study used an online questionnaire that was disseminated using popular social media platforms like Facebook, Instagram, and WhatsApp. The high internet penetration rate in Jordan exceeds 90% of the population, and social media is used extensively among young adults, with university students and athletes being the most active users.(Reference Bank20) As social media is one of the primary tools of communication used by this demographic, its usage allowed for broad participation across sports. Moreover, the total number of samples collected (N=324) was more than N (279), which confirms the efficiency of the method of data collection used.
This study adhered to the ethical principles outlined in the Declaration of Helsinki and received approval from the Ethical Committee of the University of Petra, Jordan (approval number: S/6/10/2023).
The study questionnaire was anonymous and voluntary, with no requests for personal information. The participants were notified in writing about the anonymous collection and particular use of the data for the study’s purposes. Informed electronic consent was obtained from all participants before completing the questionnaire. Respondents had to confirm their consent to continue. As no personally identifiable or sensitive data were collected, the research ethics committee did not require a separate signed consent document.
Sample size
To determine the sample size, we assumed a 50% prevalence of the factor of interest among the athletic population. Following the previously described approach, the required sample size was determined to be 279, taking into account a 90% confidence interval, an expected proportion of 0.5, and a margin of error (precision) of 0.5.(Reference Elsahoryi, Alathamneh, Mahmoud and Hammad21) This method created a solid foundation for statistical analysis, allowing us to safely state the presence of the factor in the research population with a 90% confidence level, as indicated by a confidence range spanning from 45% to 55%.
Data collection
The data collection involved distributing and sharing an online questionnaire across major social media channels during a 3-month period from the 21st of October 2023 to the 21st of January 2024 to ensure a varied and representative sample. In all, 324 responses were obtained from the athletes who actively participated in the study. Importantly, the actual sample size exceeded the needed sample size, demonstrating the reliability of our data collection method. Furthermore, participants in this study did not need to provide consent.
The study questionnaire
The questionnaire was developed in English following previous methods(Reference Kahn9) and translated into Arabic to facilitate effective communication between the data collectors and the respondents. This marked the inaugural translation of such a questionnaire into Arabic for use in Jordan, an Arabic-speaking nation.
The current study translated the questionnaire into Arabic using the forward-backward technique, which was recommended in a previous research.(Reference Degroot, Dannenburg and Vanhell22) The survey was originally translated from English to Arabic by a bilingual researcher who was fluent in both languages. After that, a second researcher who was multilingual translated the questionnaire backwards from Arabic to English. The original and back-translated English translations were then carefully reviewed to determine whether the item semantics had been accurately preserved.
The questionnaire included ten questions designed to assess athletes’ prior clinical history, types and frequencies of GI disturbances, dietary preferences, and supplementation practices. These questions aimed to capture relevant health and diet-related factors affecting athletes. The questionnaire was structured to ensure comprehensive data collection on these aspects. A pilot sample comprising twenty individuals was recruited to assess the Arabic version of the questionnaire to validate its psychometric qualities. The pilot research was carried out from 7 October 2023 to 20 October 2023. Ten working days after the initial tool submission, participants were recontacted and sent the questionnaire once again via the WhatsApp messaging application. Reliability was assessed through internal consistency (Cronbach’s alpha) for the knowledge (questions 7 and 10) and diet (questions 18 and 19) sections based on the original questionnaire scores. The content validity was calculated from the critical values for Lawshe’s content validity ratio(Reference Wilson, Pan and Schumsky23). The questionnaire needs approximately 10 min, and to safeguard participant anonymity, unique identifier codes were assigned to the questionnaires.
Assessment of knowledge and diet categories
Participants’ responses to specific items in the questionnaire were used to assess knowledge and diet categories. The knowledge scores were tracked based on responses to questions that assessed awareness of probiotics and prebiotics, including definitions, sources, and associated health benefits. Correct answers were scored with a total of 18 points. Participants were classified into the following categories: low knowledge (0–6 correct answers), moderate knowledge (7–12 correct answers), and high knowledge (13–18 correct answers).
Diet categories were directly assessed according to the consumption of probiotic- and prebiotic-containing foods in responses (questions 18 and 19). Participants chose foods that they consumed regularly, resulting in a maximum score of 12 points. It was classified as a low diet score (0–4 selected food items), a moderate diet score (5–8 selected food items), and a high diet score (9–12 selected food items).
These categorizations allowed for statistical analysis to determine associations between knowledge, dietary intake, and GI symptoms, using chi-square tests to evaluate significance at p < 0.05.(Reference Heaney, O’Connor, Michael, Gifford and Naughton24,Reference Spronk, Kullen, Burdon and O’Connor25)
Additional variables considered
Living arrangements were included as an additional variable, as they may affect dietary choices, meal regularity, and stress levels, all of which can influence GI symptoms and gut health.(Reference Clark and Mach5) Therefore, the relationship between living arrangements, GI symptoms, and intake of probiotic and prebiotic foods was analysed.
Additionally, dietary supplement usage was considered. The use of probiotics, prebiotics, and other performance-related supplements (e.g. multivitamins, protein powders, pre-workout formulas) was recorded to identify trends in gut health management and nutritional habits. Inclusion of these variables aimed to provide a more comprehensive overview of factors affecting athletes’ dietary behaviour and GI well-being beyond knowledge alone.
Statistical method
The data editing process involved thorough examination and validation of the completed questionnaires both at the conclusion of individual interviews and at the end of the entire survey, which were conducted prior to the subsequent analysis. Descriptive statistics were used to present the data, including frequencies (N) and percentages (%) for categorical variables, as well as means and standard deviations for continuous variables. Chi-square tests were performed to examine whether significant associations existed between levels of knowledge, dietary intake, and reported GI symptoms. The reliability of the questionnaire was assessed using Cronbach’s alpha, and validity was evaluated using the content validity ratio (CVR) and content validity index (CVI). Comparisons of probiotic and prebiotic food consumption across different living arrangements were analysed using cross-tabulations and percentage distributions to identify dietary patterns and variations. All analyses were conducted using SPSS version 25.0, with statistical significance set at p < 0.05.
Results
Reliability and psychometric validation of the study questionnaire
The questionnaire exhibited high reliability in terms of overall internal consistency, with a Cronbach’s alpha coefficient of 0.72 for the knowledge score and 0.76 for the diet score, indicating that the items are sufficiently consistent to indicate that the measure is reliable. To ensure the accuracy of the Arabic questionnaire, a panel consisting of ten food science lecturers was used to validate the questionnaire. In the content validity assessment, a panel of ten experts (Expert 1 to Expert 10) evaluated the various questionnaire items. All the experts, except for Expert 9, marked Item 1 as relevant, resulting in a CVR of 1. All the experts, including Expert 10, indicated the relevance of Item 2, yielding a CVR of 1. There was some variability in the opinions of the experts regarding the incorporation of specific foods into the diet. Experts 1 through 8 and Expert 10 marked the item as relevant, resulting in a CVR of 0.636. The experts’ evaluations for Item 4 were more consistent with all the experts, except for Expert 9, who indicated that the item was relevant. This led to a CVR of 0.818. The critical value for relevance for a panel size (N) of 11 is 0.636. The CVI for the overall assessment is 0.864, indicating a strong level of content validity. The results suggest a high degree of agreement among the experts regarding the relevance of most questionnaire items, as evidenced by the CVRs and the overall CVI.
Demographic characteristics
Tables 1–3 represent the sample count and percentage of the population for selection per questionnaire answer: demographic information, knowledge, and health and diet. Table 1 displays the responses to questions 1–3, emphasising demographic details. The age range of the individuals I included in the survey was 18–22 years, with an average age of 20.83 ± 1.20 years. Among the 324 participants, a significant portion engaged predominantly in cardio exercises (30.2%) and football (11.1%).
Table 1. Demographic information (N =324)

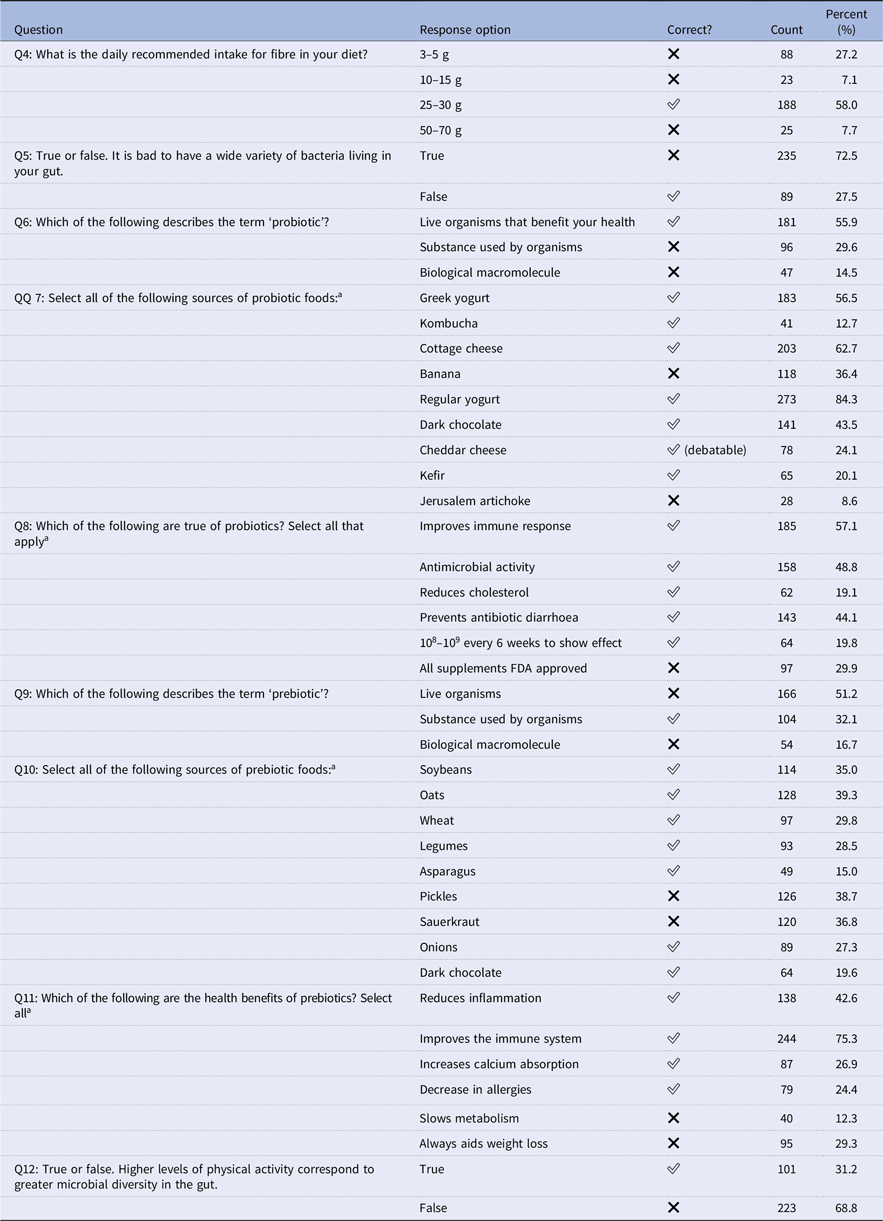
Table 2. Knowledge questions with correct response indication (N = 324)
a Multiple response.
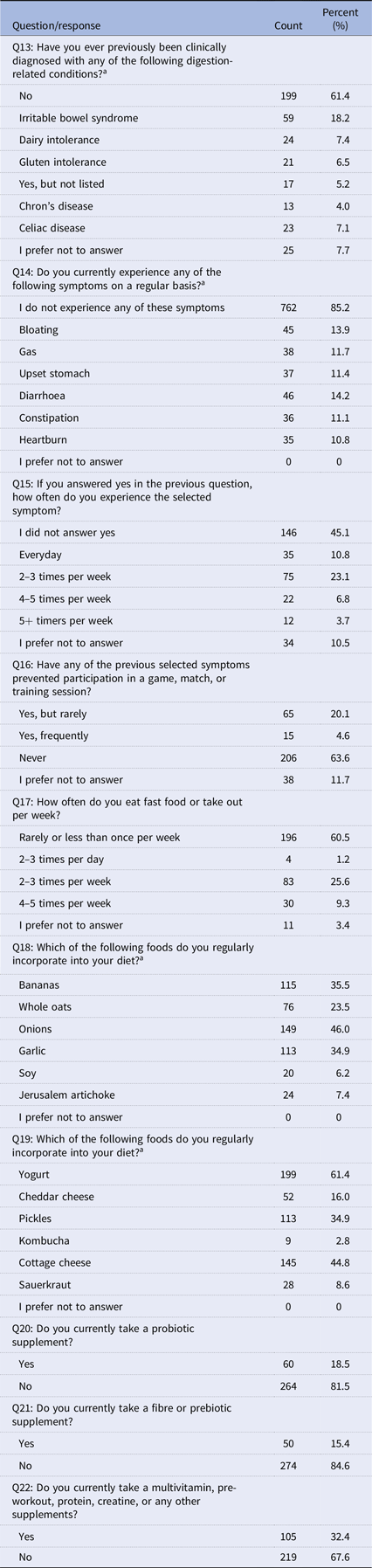
Table 3. Health and diet questions (N =324)
a Multiple response.
Knowledge of probiotics and prebiotics
This survey included nine questions specifically designed to assess participants’ knowledge of probiotics and prebiotics, focusing on their definitions, sources, and associated health benefits. The findings, outlined in Table 2, reveal uneven levels of understanding among the athletes. While 58.0% selected ‘25–30’ (the stated answer for fibre intake) as the daily recommendation, only 27.5% correctly recognised the significance of gut bacterial diversity. Knowledge regarding food sources was similarly variable: 84.3% identified regular yogurt as a probiotic source, yet only 8.6% recognised Jerusalem artichoke. In addition, dark chocolate was selected by 19.6% of respondents, whereas soybeans and pickles were chosen by 35.0% and 38.7%, respectively. Furthermore, when asked about the relationship between PA and microbial diversity, 68.8% erroneously responded that there was no association, with only 31.2% affirming the correct relationship.
Health and dietary patterns among athletes
In a comprehensive exploration of health and dietary habits among a sample of athletes (N = 324), Table 3 illuminates insights emerged from ten thoughtfully designed questions. The survey focused on prior clinical history, types and frequencies of GI disturbances, dietary preferences, and practices related to supplementation, presenting a vibrant mosaic of data. Regarding the history of digestive conditions (Q13), the majority (61.4%) reported no prior clinical diagnosis, with noteworthy cases including irritable bowel syndrome (18.2%), dairy intolerance (7.4%), and gluten intolerance (6.5%). The significant findings included 40.7% reporting no regular symptoms, while others highlighted bloating (24.7%), gas (18.8%), and upset stomach (24.7%) through Q14. The frequency of symptoms (Q15) ranged widely, from every day (10.8%) to 5+ times per week (3.7%), revealing the spectrum of experiences. A prevailing trend of consuming fast food rarely or less than once per week (60.5%) was observed. The answers to the supplemental practice questions (Q20, Q21, and Q22) indicated that 18.5% of the probiotic supplements were taken, while 81.5% were not taken. Fibre or prebiotic supplements were embraced by 15.4%, with 84.6% opting against them. In the broader supplement landscape, 32.4% of the participants used multivitamins, pre-workouts, protein, creatine, or other supplements, while 67.6% did not.
Knowledge and diet scores
Table 4 shows the distribution of participants across knowledge and diet categories. In terms of knowledge, 57.4% of the participants fell into the low category, 42% demonstrated a moderate level, and a substantial 0.6% exhibited a high level. In the realm of diet, 72.2% indicated a low score, 25.9% a moderate score, and an impressive 1.9% a high score
Table 4. Percentage of the knowledge and diet categories a N (%)
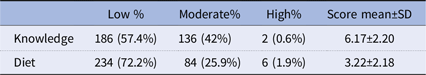
a Note: Knowledge categories were defined as follows: low = 0–6 correct answers, moderate = 7–12, high = 13–18. Diet categories were defined as follows: low = 0–4 prebiotic/probiotic foods consumed, moderate = 5–8, high = 9–12.
Cross-comparison between knowledge, diet, and GI Symptoms
Cross-comparison between knowledge, diet, and GI symptoms was explored to determine potential associations and help evaluate the initial hypotheses.
The association between knowledge and diet categories was first examined to determine whether knowledge about prebiotics and probiotics significantly influenced dietary choices. Table 5 presents the relationship between knowledge and diet categories as determined by the chi-square test. The distribution of participants in the low knowledge category was as follows: 131 participants reported a low diet, 102 participants a moderate diet, and 1 participant a high diet. For those with moderate knowledge, 53 participants had a low diet, 30 had a moderate diet, and 1 had a high diet. In the high knowledge category, 2 participants reported a low diet, 4 participants a moderate diet, and none reported a high diet. In total, there were 186 participants in the low knowledge category, 136 in the moderate knowledge category, and 2 in the high knowledge category, for a total of 324 participants.
Table 5. Chi-square of the knowledge versus diet
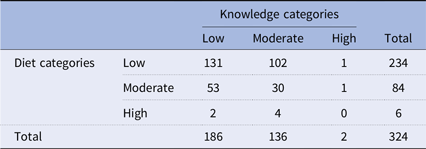
Chi-square = 3.58, p-value = 0.47.
The association between GI symptoms and diet categories was also analysed to assess whether dietary intake of prebiotic and probiotic foods correlated with the presence of GI issues. Table 6 illustrates the association between GI symptoms and different diet categories, as examined through the chi-square test. In the low diet category, out of 234 participants, 193 reported no GI symptoms, and 41 reported experiencing such symptoms. Among those in the moderate diet category, 84 participants were in total, 78 reported no GI symptoms, and 6 reported experiencing them. In the high diet category, which consisted of 6 participants, 5 reported no GI symptoms, and 1 reported experiencing them. The overall counts across all diet categories showed that out of 324 participants, 276 reported no GI symptoms, and 48 reported experiencing such symptoms. The chi-square analysis yields a value of 5.29 with a corresponding p-value of 0.07. While the association between GI symptoms and diet categories is not as pronounced as that observed with knowledge levels in Table 7. A p-value of 0.07 indicates that this association is not statistically significant at the conventional significance level of 0.05.
Table 6. Chi-square of the GI symptoms versus diet
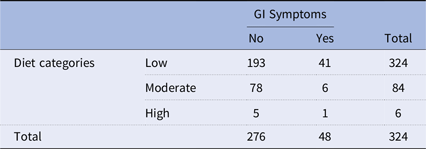
Chi-square = 5.29, p-value = 0.07.
Table 7. Chi-square of the GI symptoms versus knowledge

Chi-square = 2.11, p-value = 0.35.
The association between GI symptoms and knowledge levels was examined to determine whether awareness of probiotics and prebiotics correlates with digestive health status. Table 7 presents the analysis of the association between GI symptoms and knowledge levels using the chi-square test. In the low knowledge category, consisting of 186 participants, 160 reported no GI symptoms, while 26 reported experiencing such symptoms. Among those with moderate knowledge (136 participants in total), 115 reported no GI symptoms, and 21 reported experiencing them. In the high knowledge category, which included 2 participants, 1 participant reported no GI symptoms, and 1 reported experiencing them. Across all knowledge categories, out of 324 participants, 276 reported no GI symptoms, and 48 reported experiencing such symptoms.
The chi-square test yields a value of 2.11 with a corresponding p-value of 0.35. The results indicate that there is no statistically significant association between GI symptoms and knowledge levels at a conventional significance level of 0.05.
Supplement usage among athletes
Figure 1 indicates that incorporating prebiotic supplements was observed among 18.50% of participants, with the majority (81.50%) opting not to include them. Regarding probiotic supplements, 15.40% of participants integrated them into their routine, while 84.60% did not partake in probiotic supplementation. In the realm of other supplements (such as multivitamins, pre-workouts, proteins, creatine, etc.), 32.40% of participants included them in their regimen, whereas 67.60% did not use additional supplements regularly.
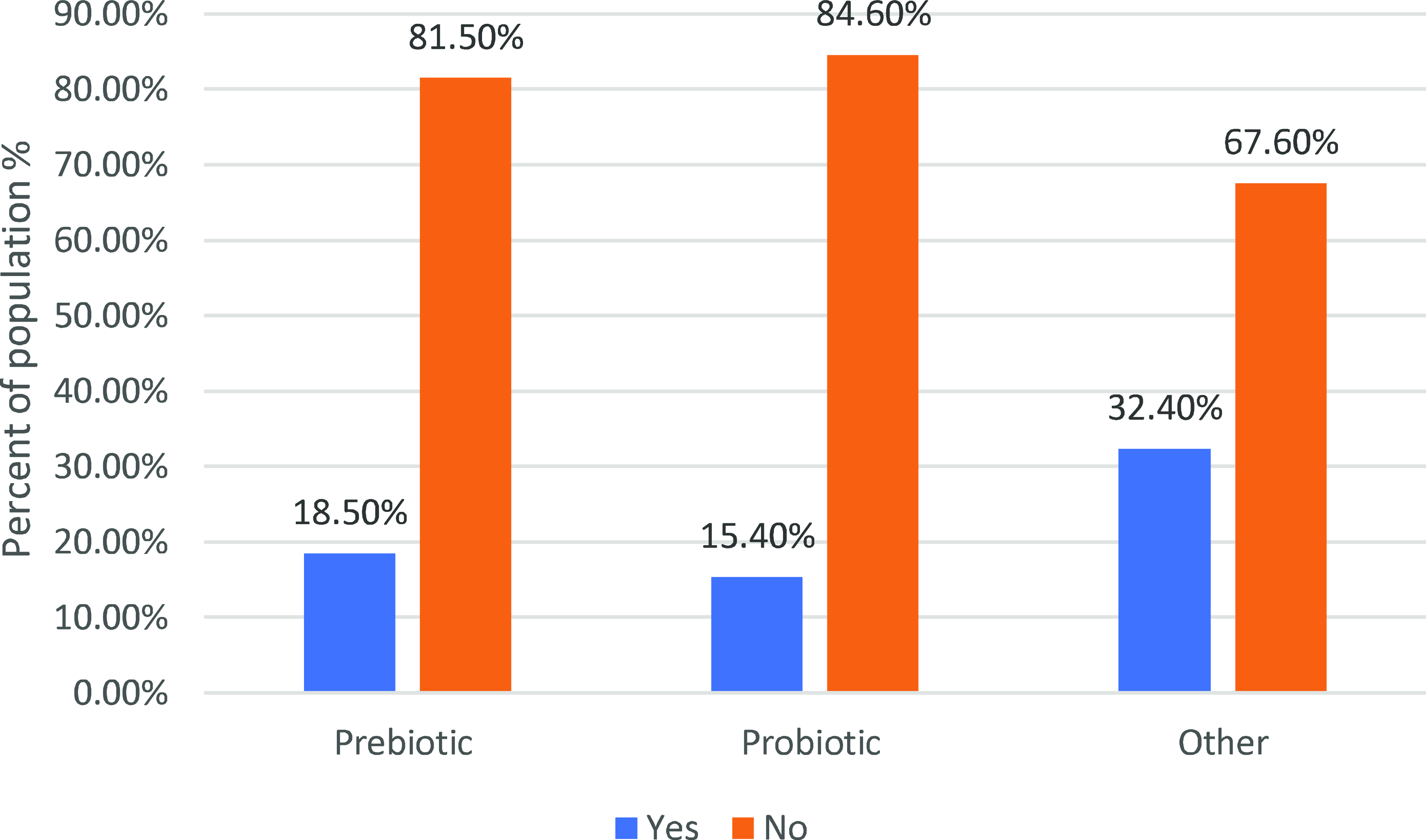
Fig. 1. Supplement usage among study participants.
Impact of living arrangements on GI symptoms, probiotic, and prebiotic intake
The impact of living arrangements on GI symptoms, probiotic, and prebiotic intake was evident across the study sample. Those who did not experience any of the listed symptoms constituted 14.8% of the total respondents (48 individuals). On the other hand, individuals reporting symptoms made up 85.2% of the total (276 individuals). Figure 2 displays the prevalence of GI symptoms across different living arrangements. Among the off-campus family homes, 86.20% of the individuals reported no symptoms, 13.10% experienced bloating, 11.00% reported gas, 10.00% had an upset stomach, 13.40% suffered from diarrhoea, 10.70% faced constipation, and 10.30% had heartburn. For the off-campus apartments, 75.00% of the patients had no symptoms, 20.80% had bloating, 25.00% had gas, 25.00% had an upset stomach, 20.80% had diarrhoea, 16.70% had constipation, and 16.70% had heartburn. According to the on-campus housing with a dining plan, 80.00% of the participants reported no symptoms, 20.00% experienced bloating, 0.00% reported gas, 20.00% had an upset stomach, 20.00% faced diarrhoea, 10.00% suffered from constipation, and 10.00% had heartburn. These figures highlight the variability in the prevalence of GI symptoms among individuals living in different arrangements.
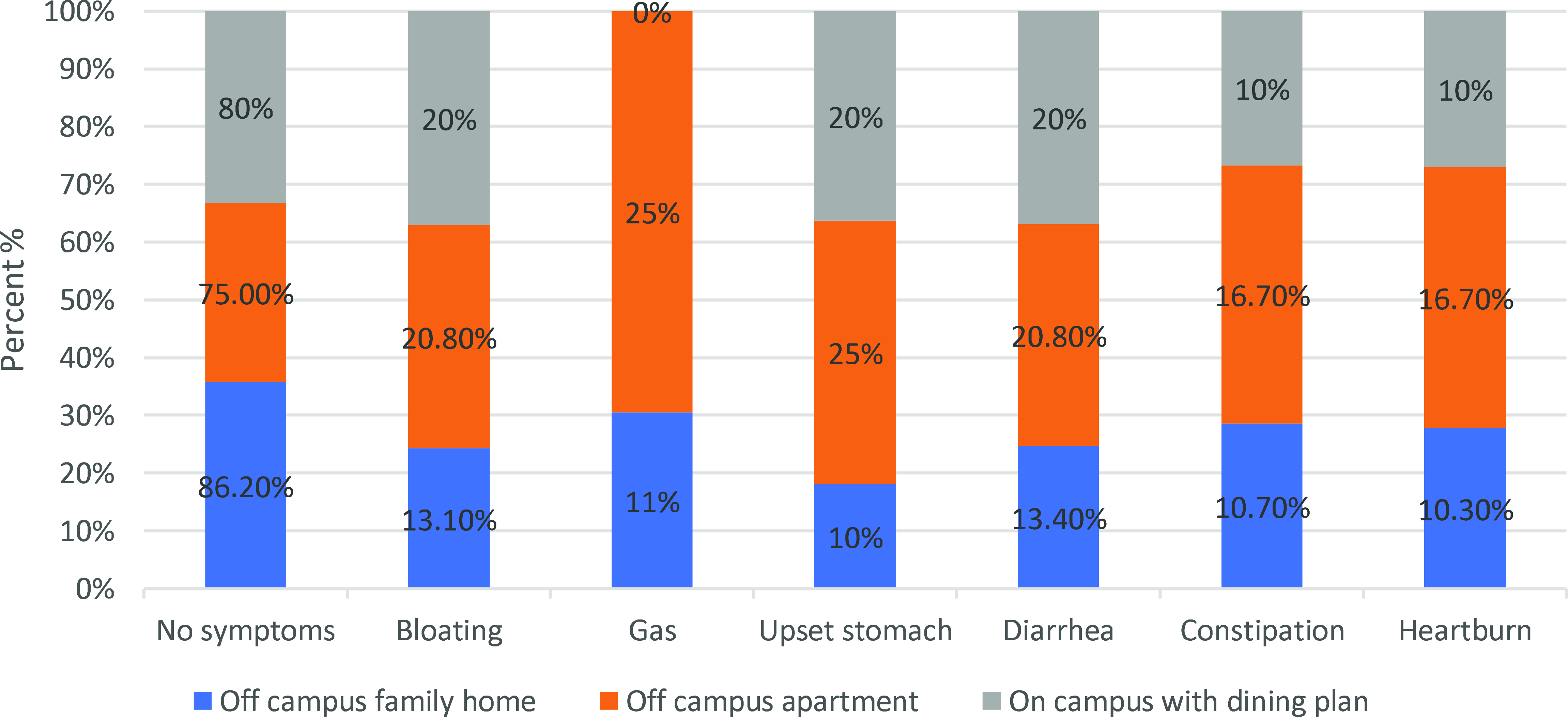
Fig. 2. Prevalence of gastrointestinal (GI) symptoms by living arrangement.
Probiotic intake and living arrangements also demonstrated notable differences. The distribution of probiotic food consumption according to each living arrangement is shown in Fig. 3. The prevalence of Greek yogurt, kombucha, cottage cheese, regular yogurt, dark chocolate, cheddar cheese, and kefir use among off-campus family home participants were 55.90%, 12.80%, 62.40%, 83.80% 43.10%, 24.10%, 19% respectively.
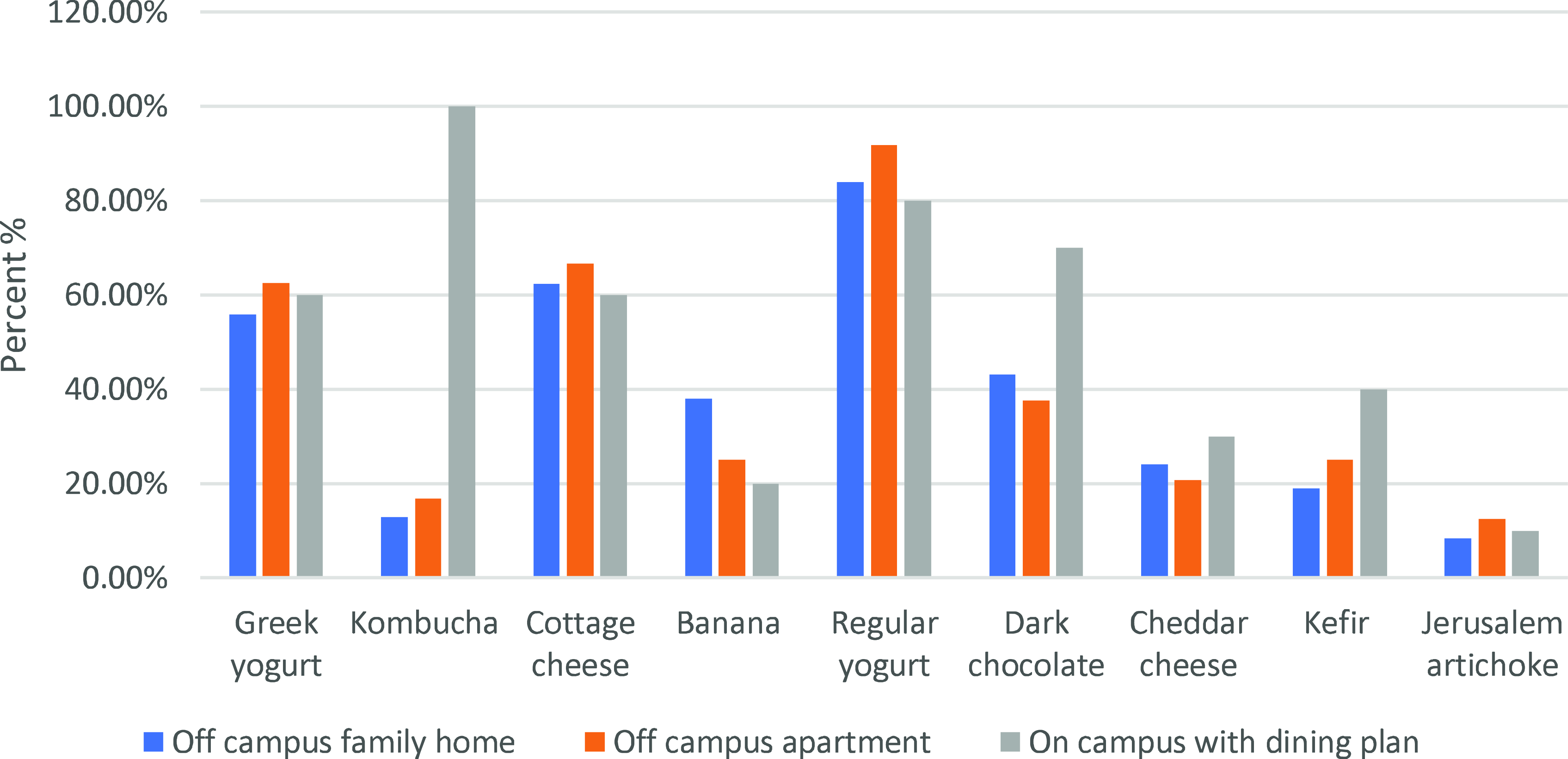
Fig. 3. Probiotic food consumption patterns across living arrangements.
According to off-campus apartment data, 62.50% of respondents reported consuming Greek yogurt, 16.70% reported consuming kombucha, 66.70% reported consuming cottage cheese, 91.70% reported consuming regular yogurt, 37.50% reported consuming dark chocolate, 20.80% reported consuming cheddar cheese, and 25% reported consuming kefir.
Of participants in on-campus housing with a dining plan: 60% Greek yogurt, 100% kombucha, 60% cottage cheese, 80% regular yogurt, 70% dark chocolate, 30% cheddar cheese, 40% kefir.
Prebiotic intake and living arrangements followed a similar trend. Figure 4 illustrates the correlation between the place of residence and the inclusion of prebiotic foods in the diet. The percentages represent the prevalence of various prebiotic-rich items among individuals in different living arrangements. In off-campus family homes, 35.90% had soya, 37.60% consumed oats, 27.60% had wheat, 27.60% had legumes, 15.50% had asparagus, 38.60% had pickles, 36.90% had sauerkraut, 28.30% had onions, and 19.70% had dark chocolate.
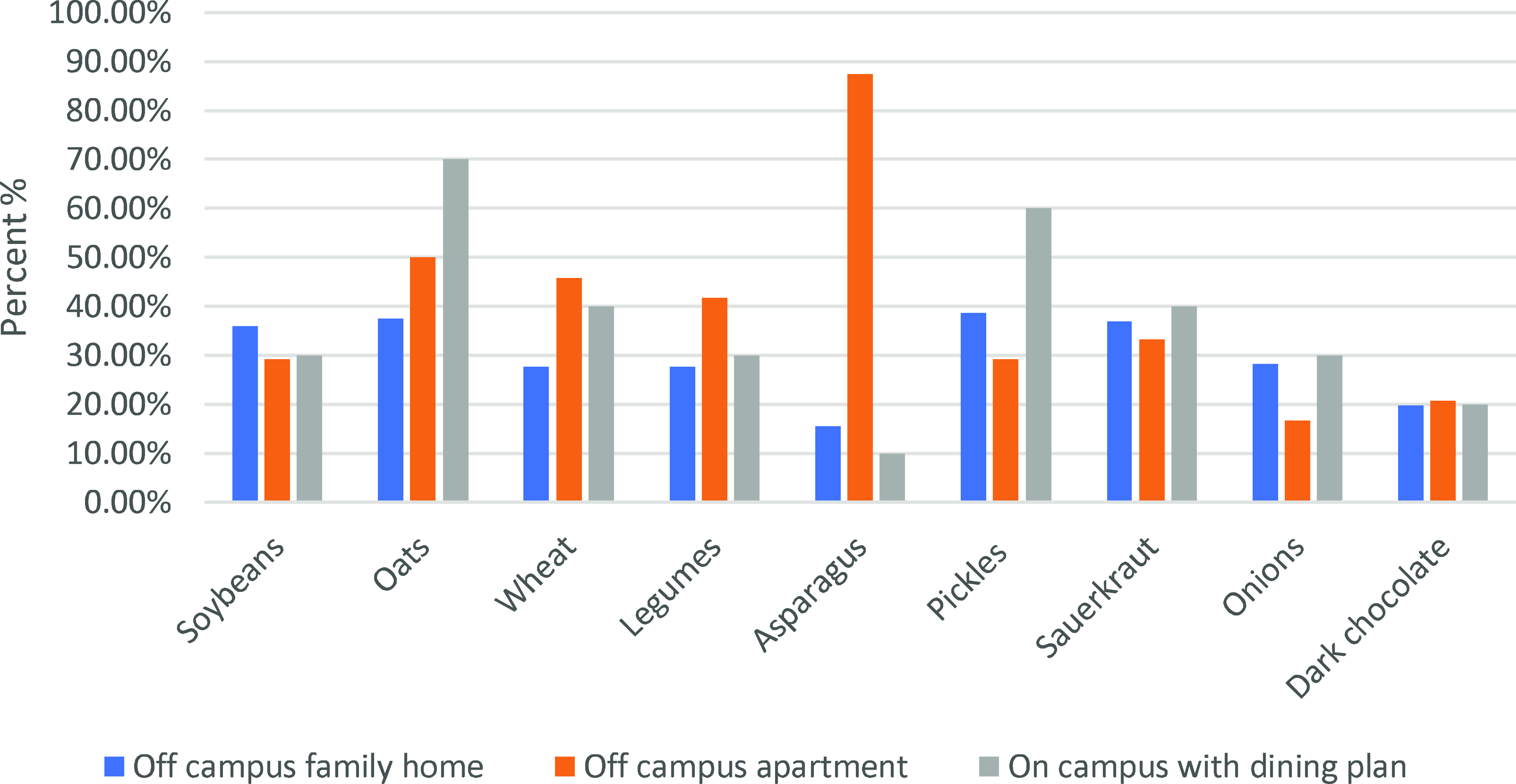
Fig. 4. Prebiotic food intake by residential living arrangement.
Among off-campus apartments individuals, soybeans, oats, wheat, legumes, asparagus, pickles, sauerkraut, onions, and dark chocolate intake were 29.20%, 50%, 45.80%, 41.70%, 87.50%, 29.20%, 33.30%, 16.70%, and 20.80%.
Of those who were on-campus housing with a dining plan, 30% ate soybeans, 70% ate oats, 40% ate wheat, 30% ate legumes, 10% ate asparagus, 60% ate pickles, 40% ate sauerkraut, 30% ate onion, and 20% ate dark chocolate.
Discussion
This study aimed to assess the level of knowledge, dietary habits, and GI health among Jordanian athletes about probiotics and prebiotics. The findings revealed a clear knowledge gap, as many athletes were unfamiliar with key terms and concepts. No significant associations were observed between knowledge levels and dietary choices or between knowledge and GI symptoms. However, a potential trend suggested that dietary habits may influence the occurrence of GI symptoms, warranting further investigation. Although GI symptoms were frequently reported, most athletes did not perceive these symptoms as affecting their athletic performance.
Understanding of probiotics and prebiotics among athletes remains an area of global concern, reflecting gaps in nutritional education and awareness. Our results align with previously published studies indicating limited knowledge of probiotics and prebiotics among athletes globally. For instance, a survey found that 85% of participants reported knowing very little or nothing about probiotics.(Reference Betz, Uzueta, Rasmussen, Gregoire, Vanderwall and Witowich26). In the United States, one report indicated that college athletes lacked even basic nutritional education, which led to poor dietary choices.(Reference Jacobson, Sobonya and Ransone27) In Europe, awareness of probiotics was reported to be between 60% and 70%, but actual incorporation into diets remained low.(Reference Jäger, Mohr and Pugh3)
Such a difference stresses the importance of educational strategies to improve athletes’ knowledge regarding probiotics and prebiotics. While the research is in its infancy, a disconnect between the recent advances of the gut biome and practical implementation will stifle the adoption of dietary approaches that support gut health, immune response, and overall performance.(Reference Jäger, Mohr and Carpenter6)
GI health is a prevalent concern among athletes and can significantly impact both training and performance. Previous studies have reported that a notable proportion of athletes experience GI disturbances during training or competition.(Reference Heaney, O’Connor, Michael, Gifford and Naughton24)
Previous research has shown that 30–70% of athletes — particularly those involved in endurance sports and weightlifting — experience some form of GI disruption.(Reference Coleman28) The current results affirm the high prevalence of GI symptoms among Jordanian athletes and suggest that these symptoms are not solely dependent on sport type. Instead, they likely reflect a combination of physiological and lifestyle factors. This aligns with prior literature indicating that GI disturbances in athletes are multifactorial in nature, influenced by training intensity, psychological stress, hydration habits, and dietary patterns(Reference Jeukendrup29–Reference de Oliveira, Burini and Jeukendrup31). As dietary and gut health continue to gain attention in the context of athletic performance, a more coordinated and individualised approach to managing GI symptoms is warranted. Therefore, sports nutritionists are encouraged to incorporate tailored interventions that support digestive health, such as the inclusion of probiotic and prebiotic foods, hydration strategies, and scheduled meal timing. Further research is recommended to assess the effectiveness of these personalised nutritional approaches in reducing GI symptoms and enhancing performance outcomes among athletes.
This study aimed to explore whether GI symptoms in Jordanian athletes are influenced by their knowledge and intake of prebiotic- and probiotic-rich foods. While no significant association was found, the findings underscore the importance of targeted nutritional education and intervention strategies to enhance athletes’ understanding of gut-friendly foods and improve dietary patterns, which in turn could support better digestive health and athletic performance.
Cross-comparison between knowledge, diet, and GI symptoms reveals important behavioural insights. In this sample, knowledge of probiotics and prebiotics did not appear to directly influence athletes’ dietary habits or digestive health. However, a possible link was observed between dietary patterns and GI symptoms, suggesting that diet may play a role in managing digestive issues, even when knowledge is present.
The absence of a clear relationship between knowledge and behaviour suggests that other factors may influence food choices more strongly — such as access to probiotic-rich foods, cultural eating habits, or affordability. Previous research has shown that nutritional knowledge alone often fails to change behaviour unless accompanied by environmental support or structured intervention programmes.(Reference Heaney, O’Connor, Michael, Gifford and Naughton24,Reference Spronk, Kullen, Burdon and O’Connor25)
These findings align with previous studies reporting limited awareness of prebiotic and probiotic sources and benefits among athletes, and they confirm that GI issues remain common within this population. Despite moderate to high knowledge levels and good dietary adherence in many participants, improvements in digestive health were not clearly observed. This suggests that knowledge alone may not lead to meaningful behaviour change or symptom relief.
Future research should investigate other potential contributing factors that may play a more influential role in the prevalence of GI symptoms among athletes, such as nutrient timing — whether athletes take probiotics or prebiotics may play a role in digestion; training intensity and stress, as high-stress training environments may exacerbate GI issues; and the gut microbiome, where analysis could discover if certain bacterial strains enhance digestion in athletes.
Furthermore, targeted interventions of nutrition education should be conducted in order to establish if increasing the probiotic/prebiotic awareness can contribute to quantifiable changes in dietary practices through the years. A comprehensive assessment may also offer a more accurate representation of the amount of understanding of prebiotic and probiotic foods.
Supplement usage among athletes appears to prioritize performance over digestive health. In this study, athletes showed a greater tendency to use general supplements, which aligns with previous studies reporting that athletes generally give more preference to supplements that directly enhance muscle recovery, energy metabolism, and performance compared to those that specifically support gut health.(Reference Clark and Mach5,Reference Maughan11) This pattern may reflect limited awareness among athletes of the role of gut microbiota in exercise adaptation, immune function, and nutrient absorption, which could explain the preference for performance-oriented supplements over those targeting digestive health.(Reference Jäger, Mohr and Carpenter6,Reference Mohr, Basile, Meli’sa, Sweazea and Carpenter32)
Living arrangements may influence dietary habits and digestive health among athletes. Variability in GI symptoms and the intake of probiotic and prebiotic foods appears to be linked to living conditions. These differences in symptoms and dietary practices may be influenced by several contextual factors, including access to food, availability of cooking facilities, and stress associated with independent living or residence hall environments. Studies have shown that athletes living in stable home environments are more likely to eat healthy meals than those in independent living conditions, which may affect gut health and dietary behaviours.(Reference Parker, Roy, D’Adamo and Wieland4) Furthermore, the relationship between living arrangements and dietary habits may be altered by socioeconomic status, cooking skills, and access to fermented, probiotic-rich, fresh foods, which warrants further investigation in future studies. This trend needs further assessment with a greater number of participants for a more robust outcome.(Reference Parker, Roy, D’Adamo and Wieland4,Reference Cheifetz, Gianotti, Luber and Gibson33)
GI symptoms across different living arrangements were found to be more common among athletes who live off-campus or by themselves, showing an increase in GI symptoms, likely a result of spontaneous meal patterns, heavy consumption of processed foods, and heightened stress levels. These issues may be further exacerbated by limited fibre intake and suboptimal food safety practices.(Reference Clark and Mach5) In contrast, those who live with family enjoy structured meals abundant in gut-supporting nutrients with lower GI discomfort.
Probiotic intake and living arrangements showed that athletes living with their family tend to consume more probiotics as fermented foods such as yogurt and laban (a type of fermented drink) are often used in traditional meals. Conversely, meals provided by parents—whether for students on campus dining plans or those studying independently—may lack probiotic-rich options due to limited awareness.(Reference Parker, Roy, D’Adamo and Wieland4)
Prebiotic intake and living arrangements followed a similar trend, where athletes living in family homes also report higher consumption of fibre-rich foods — whole grains and vegetables. Those who are living alone or in dormitories may depend on processed meals with a decreased prebiotic content, which in turn reduces the gut microbiota diversity.(Reference Chudzik, Orzyłowska, Rola and Stanisz34)
Implications
These findings highlight the need for targeted nutrition education tailored to athletes’ living arrangements, as dietary patterns and supplement use vary significantly between those living independently and those in family homes. Future research should explore how factors such as meal frequency, food accessibility, cooking skills, and the availability of probiotic- and prebiotic-rich foods influence gut health in student athletes. This is particularly relevant for athletes in dormitories or off-campus apartments, who may face greater challenges in maintaining gut-friendly dietary habits compared to those living in structured family environments. Educational programmes, particularly within university athletic departments, could be tailored to enhance knowledge and access to probiotic and prebiotic-rich foods.
Strengths and limitations
This study provides valuable insight into the knowledge and dietary practices related to probiotics and prebiotics among a relatively large and diverse sample of Jordanian athletes, using a culturally adapted and psychometrically validated questionnaire. A key strength is its focus on an under-researched population in the Middle East, contributing to the global understanding of nutrition behaviours in athletic settings.
However, certain limitations should be acknowledged. The cross-sectional design precludes causal inference, and reliance on self-reported data may introduce recall or social desirability bias. Furthermore, the absence of objective clinical measurements — such as biomarkers of gut health or stool microbiota analysis — limits the ability to validate reported GI symptoms.
The lack of a control group or intervention component also restricts the ability to test the effectiveness of specific nutrition strategies. Finally, the sample was limited to athletes aged 18–22, which may affect the generalisability of the findings to older or professional athletic populations.
Conclusion
This study aimed to assess the knowledge, dietary habits, and GI health of Jordanian athletes about probiotics and prebiotics. The findings revealed that Jordanian athletes had a limited understanding of probiotics and prebiotics. However, differences in certain knowledge areas and eating patterns were discovered. GI problems were common but not significantly linked with knowledge levels or diet categories. Symptoms and nutritional choices varied depending on the living situation.
These results underscore the need for tailored educational interventions in sports nutrition and emphasise the role of policymakers in collaborating with sports organisations to improve access to probiotic-rich foods and evidence-based dietary guidelines to support the health and performance of Jordanian athletes. Future studies should investigate the longer-term effects of these interventions and evaluate the effect of conditions such as training volume, hydration and stress on the occurrence of GI symptoms in this population.
Availability of data and materials
The data and materials supporting the findings of this study are available upon reasonable request.
Acknowledgements
The author wishes to state that there are no acknowledgements for this manuscript.
Authors’ contributions
O.D.A. and N.E. contributed to the conceptualisation of the research. O.D.A., L.M., N.E., and L.A. prepared the original draft of the manuscript. O.D.A., N.E., and L.A. were responsible for the writing, review, and editing. O.D.A., L.A., N.E., and L.M. managed data acquisition. O.D.A. and N.E. conducted the analysis and interpretation of the data. O.D.A. supervised the project. O.D.A., N.E., and L.M. critically revised the manuscript. All authors contributed to the article and approved the final submitted version.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests
The author(s) declare(s) that there are no competing interests regarding the publication of this paper.
Ethical consideration statement
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving humans were approved by the Ethical Committee Decision Research under the ethical approval number S/6/10/2023 from the Ethical Committee of the University of Petra, Jordan.
Participation in this study was voluntary and anonymous. Written informed consent was obtained from all participants through an electronic consent form embedded within the online questionnaire. Participants were required to acknowledge their agreement before proceeding. Since no personally identifiable or sensitive data were collected, a separate, signed consent document was not required by the research ethics committee.
Consent for publication
The authors of the manuscript grant permission to publish the aforementioned manuscript in the journal.












