Non-technical Summary
Calyptosuchus wellesi is an aetosaur known exclusively from the southwestern United States. This species is collected from specific rock layers in northwestern Texas and northern Arizona that are approximately 223–218 million years old. Aetosaurs are characterized by their armor-covered bodies, similar to armadillos. This armor is formed by individual bony plates called osteoderms, which are the main way we identify a species of aetosaur. Calyptosuchus is an index fossil that allows us to understand the relationship of the rocks across a wider geographic area, including their ages. Because Calyptosuchus is an index taxon, it is important that we understand its anatomy to the best of our abilities so that way we can identify the species based on limited material.
Prior to our work, the skull anatomy of Calyptosuchus was based on a fragmentary dentary. Here we present new fossils that provide clarity on the skull of this animal, including new details on its teeth, which suggests that Calyptosuchus was likely an omnivorous animal. We present another individual that provides clarity on the variation of the osteoderms across the various divisions of the body, which allows us to identify isolated osteoderms of Calyptosuchus with a higher level of accuracy. This specimen preserves several complete vertebrae spanning most of the mid-section of the body, including the pelvis. This provides a better understanding of the variation in the vertebral column, which was previously not understood. Lastly, this new specimen also preserves a complete pelvic girdle. These new data resulted in the identification of a new pattern in the shape of the ilium and allowed us to reconstruct the pelvis of Calyptosuchus. Together, the two new specimens provide a better understanding of the anatomy of Calyptosuchus.
Introduction
Aetosaurs are a group of pseudosuchian archosaurs that were prevalent across terrestrial ecosystems during the Late Triassic epoch (Carnian–Rhaetian, ~237–201 Ma) (Desojo et al., Reference Desojo, Heckert, Martz, Parker, Schoch, Small, Sulej, Nesbitt, Desojo and Irmis2013). They are characterized by their osteoderm-covered bodies (Desojo et al., Reference Desojo, Heckert, Martz, Parker, Schoch, Small, Sulej, Nesbitt, Desojo and Irmis2013), similar to ankylosaurian dinosaurs (Burns and Currie, Reference Burns and Currie2014), extant armadillos (Hill, Reference Hill2006), and some extant squamates (e.g., cordylids, skinks, anguids; Williams et al., Reference Williams, Kirby, Marghoub, Kéver, Ostashevskaya-Gohstand, Bertazzo, Moazen, Abzhanov, Herrel, Evans and Vickaryous2022). Aetosaurs are documented from Upper Triassic strata within the United States, Argentina, Brazil, Greenland, western Europe, India, and northern Africa (Desojo et al., Reference Desojo, Heckert, Martz, Parker, Schoch, Small, Sulej, Nesbitt, Desojo and Irmis2013). Out of the 29 currently described species (Reyes et al., Reference Reyes, Martz and Small2024; Haldar et al., Reference Haldar, Ray and Bandyopadhyay2025), 21 taxa are documented exclusively from Upper Triassic strata of North America, particularly from the Chinle Formation and Dockum Group in the southwestern United States (Desojo et al., Reference Desojo, Heckert, Martz, Parker, Schoch, Small, Sulej, Nesbitt, Desojo and Irmis2013; Parker, Reference Parker2016a; Reyes et al., Reference Reyes, Martz and Small2024). There, the occurrence of aetosaurs provides a means to temporally constrain Upper Triassic strata through biostratigraphic and thus biochronologic correlation (e.g., Long and Murry, Reference Long and Murry1995; Heckert and Lucas, Reference Heckert and Lucas2000; Parker and Martz, Reference Parker and Martz2011; Parker, Reference Parker2016a; Martz and Parker, Reference Martz, Parker, Parker and Zeigler2017; Reyes et al., Reference Reyes, Parker and Heckert2023).
Aetosaurs are often documented based on their isolated dorsal osteoderms, which can be referred to a taxon based on the unique combination of character states that they exhibit (Long and Ballew, Reference Long, Ballew, Colbert and Johnson1985; Desojo et al., Reference Desojo, Heckert, Martz, Parker, Schoch, Small, Sulej, Nesbitt, Desojo and Irmis2013; Parker, Reference Parker2016a; Reyes et al., Reference Reyes, Parker and Heckert2023). However, it is becoming more apparent that dorsal osteoderms can exhibit a degree of convergence between distantly related taxa within the clade (Parker, Reference Parker2008b, Reference Parker2016a; Parker and Haldar, Reference Parker and Haldar2024; Reyes et al., Reference Reyes, Martz and Small2024). Aetosaurs exhibit an array of tooth morphotypes that can be generalized into either constricted at the base and apically bulbous (e.g., Desmatosuchus smalli Parker, Reference Parker2005; Small, Reference Small2002), or slightly labiolingually compressed, basally broad, and apically straight (e.g., Aetosaurus ferratus Fraas, Reference Fraas1877; Schoch, Reference Schoch2007) or recurved (e.g., Aetosauroides scagliai Casamiquela, Reference Casamiquela1960; Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021b). Thus, it is hypothesized that aetosaurs exhibited various feeding ecologies including herbivory, omnivory, and carnivory (Long and Murry, Reference Long and Murry1995; Desojo and Ezcurra, Reference Desojo and Ezcurra2011; von Baczko et al., Reference von Baczko, Taborda and Desojo2018, Reference von Baczko, Desojo, Gower, Ridgely, Bona and Witmer2021; Reyes et al., Reference Reyes, Parker and Marsh2020; Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021b). This may in part relate to the high species diversity, spatial distribution, and temporal longevity of the Aetosauria during the Late Triassic.
Partially or relatively complete aetosaur skeletons have been described or redescribed, thus allowing for a more holistic assessment of the interspecific relationships within the clade. Although these new data have become available, our understanding of the intraspecific variation within the clade remains limited. Historically, histological analyses of dorsal osteoderms have provided insight into intraspecific variation with relation to ontogeny and sexual maturity within aetosaurs (de Ricqlès et al., Reference de Ricqlès, Padian and Horner2003; Parker et al., Reference Parker, Stocker and Irmis2008; Werning, Reference Werning2013; Scheyer et al., Reference Scheyer, Desojo and Cerda2014; Taborda et al., Reference Taborda, Heckert and Desojo2015; Cerda et al., Reference Cerda, Desojo and Scheyer2018; Hoffman et al., Reference Hoffman, Heckert and Zanno2019; Ponce et al., Reference Ponce, Desojo and Cerda2023; Teschner et al., Reference Teschner, Konietzko-Meir, Desojo, Schoch and Klein2023). Recent studies on the vertebral anatomy of Aetosauroides scagliai by Paes-Neto et al. (Reference Paes-Neto, Desojo, Brust, Schultz, Da-Rosa and Soares2021a) resulted in the identification of ontogenetically variable character states that are often used to identify and diagnose taxa within the clade. This new understanding resulted in a taxonomic reassessment of Polesinosuchus aurelioi (Roberto-Da-Silva et al., Reference Roberto-Da-Silva, Desojo, Cabreira, Aires, Müller, Pacheco and Dias-Da-Silva2014) and indicated that our lack of understanding of intraspecific variation within aetosaurs is influencing our assessments of species diversity within the clade.
Aetosaur skeletons that preserve significant portions of their respective carapaces not only indicate that osteoderms are osteologically variable between species, but that there is also positional and osteological variation of the dorsal osteoderms across the various regions of the body within a single individual (Fig. 1; i.e., cervical, trunk, sacral, caudal; Parker and Martz, Reference Parker and Martz2010). Because of this, it is important that we understand the osteology and variation of osteoderms because they are fundamental in our assessments of interspecific relationships within the Aetosauria (Long and Ballew, Reference Long, Ballew, Colbert and Johnson1985; Long and Murry, Reference Long and Murry1995; Heckert and Lucas, Reference Heckert and Lucas1999; Parker, Reference Parker2016a) and directly influence our ability to use aetosaurs for temporally constraining strata across the southwestern United States through biostratigraphic correlation (Fig. 2; Lucas and Hunt, Reference Lucas, Hunt, Lucas and Morales1993; Heckert and Lucas, Reference Heckert and Lucas2000; Parker and Martz, Reference Parker and Martz2011; Martz and Parker, Reference Martz, Parker, Parker and Zeigler2017).
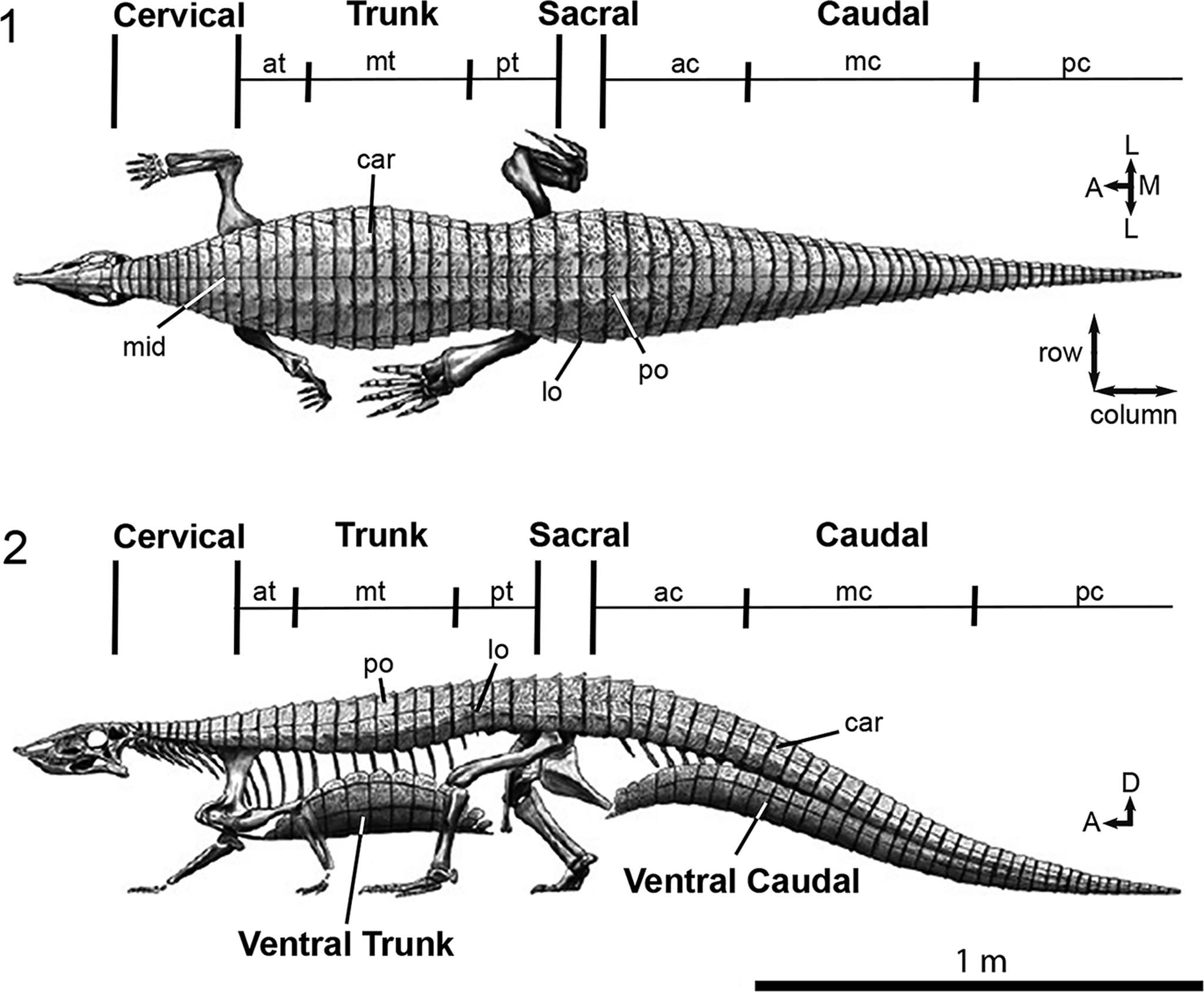
Figure 1. (1, 2) Generalized aetosaur body plan and osteoderm differentiation exemplified by Stagonolepis robertsoni in (1) dorsal, and (2) lateral views. Figure modified from Parker (Reference Parker2016b) and illustration by Jeffrey Martz. A = anterior; ac = anterior caudal region; at = anterior trunk region; car = carapace; D = dorsal; L = lateral; lo = lateral osteoderm; M = medial; mc = mid-caudal region; mid = midline; mt = mid-trunk region; pc = posterior-caudal region; po = paramedian osteoderm; pt = posterior trunk region. Arrows indicate anatomical direction.
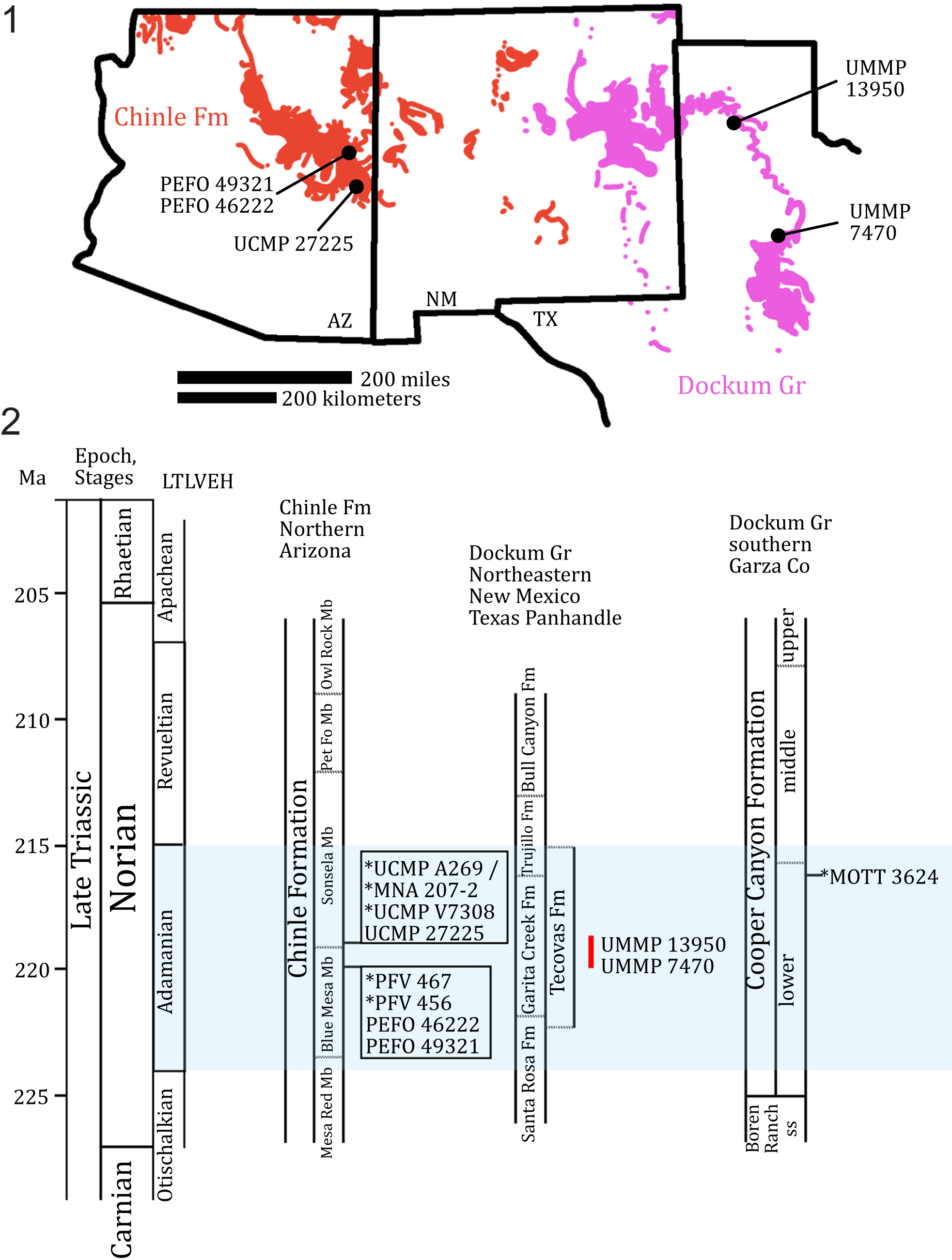
Figure 2. (1) Geographic and (2) stratigraphic occurrences of relevant specimens of Calyptosuchus wellesi and relevant localities (marked with *) across the Chinle Formation and Dockum Group in the southwestern United States. MOTT 3624 (the Post Quarry) is included because it is a fossil locality that provides important biostratigraphic context in the region. Red line marks hypothetical stratigraphical occurrence of UMMP 13950 and UMMP 7470 within the Tecovas Formation based on biostratigraphic range of Calyptosuchus wellesi within the Chinle Formation. Figure modified from Martz and Parker (Reference Martz, Parker, Parker and Zeigler2017), Lessner et al. (Reference Lessner, Parker, Marsh, Nesbitt, Irmis and Mueller2018), Nesbitt et al. (Reference Nesbitt, Stocker, Ezcurra, Fraser, Heckert, Parker and Mueller2021), and Reyes et al. (Reference Reyes, Parker and Heckert2023, Reference Reyes, Martz and Small2024). AZ = Arizona; Co = County; Fm = Formation; Gr = Group; LTLVEH = Late Triassic Land Vertebrate Estimated Holochronozones; Ma = millions of years; Mb = Member; MOTT = Museum of Texas Tech University vertebrate fossil locality; ss = sandstone; NM = New Mexico; Pet Fo = Petrified Forest; TX = Texas.
Calyptosuchus wellesi Long and Ballew, Reference Long, Ballew, Colbert and Johnson1985, is a stagonolepidoid aetosaur known from both the Dockum Group and the Chinle Formation in Texas and Arizona (Fig. 2; Case, Reference Case1932; Long and Murry, Reference Long and Murry1995; Parker, Reference Parker2016a). The paramedian, lateral, ventral, and appendicular osteoderms from the trunk through caudal region of Calyptosuchus wellesi have been described (Long and Ballew, Reference Long, Ballew, Colbert and Johnson1985; Parker, Reference Parker2018a) and are primarily based on the articulated carapace of the holotype specimen (UMMP 13950; Case, Reference Case1932), the referred individual UMMP 7470, and several disarticulated elements from UCMP A269 (the Placerias Quarry, Camp and Welles, Reference Camp and Welles1956; Long and Murry, Reference Long and Murry1995; Parker, Reference Parker2018a). However, documentation of the anatomical variation across the dorsal carapace was limited because of how the holotype bones were set in plaster for display and the loss of original association and/or non-documentation of the referred elements from UCMP A269 (Parker, Reference Parker2018a). Close re-examination of the holotype specimen of Calyptosuchus wellesi (UMMP 13950), now possible after its removal from exhibit, resulted in a new understanding of the postcrania, including the positional variation within the dorsal carapace and intraspecific variation of the vertebral column. Thus, a revision of the previous anatomical interpretations of the specimen (i.e., Case, Reference Case1932; Long and Murry, Reference Long and Murry1995; Parker, Reference Parker2018a) is merited.
We present our re-interpretations of the holotype specimen of Calyptosuchus wellesi (UMMP 13950), as well as two new specimens referable to C. wellesi (PEFO 49321, PEFO 46222) collected from the Chinle Formation of northern Arizona (Fig. 1). PEFO 49321 preserves several skull elements and provides new data about the interspecific variation of the skull in aetosaurs. Furthermore, PEFO 46222 preserves anatomy that is otherwise obscured, poorly preserved, or missing in UMMP 13950 and provides new anatomical understanding of the postcrania of Calyptosuchus wellesi, including portions of the skeleton subject to both positional and intraspecific variation. Understanding the degree of variation in Calyptosuchus wellesi is important because this taxon represents an index taxon of the Adamanian Land Vertebrate Estimated Holochronozone (~224–215 Ma; Parker and Martz, Reference Parker and Martz2011; Martz and Parker, Reference Martz, Parker, Parker and Zeigler2017).
Geological setting
Stratigraphic occurrence
Currently, Calyptosuchus wellesi has only been reported from the upper Blue Mesa Member and lower Sonsela Member of the Chinle Formation in northern Arizona (Fig. 2; Long and Murry, Reference Long and Murry1995; Parker and Martz, Reference Parker and Martz2011), and from the Tecovas Formation of the Dockum Group in northwestern Texas (Fig. 2; Martz, Reference Martz2008; Parker, Reference Parker2016a). Previously, a pair of paramedian osteoderms (TTU-P 9420) from the Post Quarry (MOTT 3624), which is located within the lower Tecovas Formation-equivalent part of the Cooper Canyon Formation, Dockum Group, Texas, were referred to Calyptosuchus wellesi (Martz, Reference Martz2008; Parker and Martz, Reference Parker and Martz2011; Martz et al., Reference Martz, Mueller, Nesbitt, Stocker, Parker, Atanassov, Fraser, Weinbaum and Lehane2013), however revision of these osteoderms indicate that they are actually referrable to Scutarx deltatylus Parker, Reference Parker2016a, based on the presence of a thick dorsal protuberance on the posteromedial corner of the dorsal surface (Parker, Reference Parker2016a, Reference Parkerb, Reference Parker2018a). UMMP 13950 (Case, Reference Case1932) and UMMP 7470 (Case, Reference Case1922, Reference Case1929) were recovered from the Tecovas Formation of the Dockum Group near Sierrita de la Cruz Creek, Oldham County, Amarillo, Texas, and Holmes Creek (sometimes referred to as ‘Home[s] Creek’, Gregory, Reference Gregory, Kelley and Trauger1972), Crosby County, Texas, respectively (Fig. 2.1; Parker, Reference Parker2016a). UCMP 27225, UCMP 25941, and UCMP 32148 were collected from the Chinle Formation near St. Johns in northern Arizona (Fig. 2.1; Long and Murry, Reference Long and Murry1995); UCMP 27225 from locality UCMP V7308 (the Blue Hills, Parker, Reference Parker2018a), and UCMP 25941 and UCMP 32148 were collected from locality UCMP A269/MNA locality 207 (the Placerias Quarry, Camp and Welles, Reference Camp and Welles1956) in Apache County, which is equivalent to the uppermost Blue Mesa Member or the lowermost Sonsela Member (Fig. 2.2; Parker, Reference Parker2018a).
PEFO 46222 was collected from PFV 456 (Thunderstorm Ridge), and PEFO 49321 was collected from PFV 467 (Metoposaur Genesis Supreme). Both localities are located in the upper Blue Mesa Member of the Chinle Formation (sensu Woody, Reference Woody, Parker, Ash and Irmis2006), near Blue Tank and Billings Gap, respectively, at Petrified Forest National Park, Arizona (Fig. 2.2). The Blue Mesa Member of the Chinle Formation within PEFO is divided into an upper and lower portion by the lithologically complex Newspaper Rock beds (Martz et al., Reference Martz, Parker, Skinner, Raucci, Umhoefer and Blakey2012). The strata of the upper Blue Mesa Member exhibit a pastel gray and blue color, and are composed primarily of mudstones, siltstones, and sandstones (Martz et al., Reference Martz, Parker, Skinner, Raucci, Umhoefer and Blakey2012). The mudstones and siltstones of the Blue Mesa Member were deposited by a large northwest-trending fluvial system on the western margin of Pangea at an equatorial paleolatitude of 5–15°N (Dubiel et al., Reference Dubiel, Parrish, Parrish and Good1991; Kent and Irving, Reference Kent and Irving2010; Martz et al., Reference Martz, Parker, Skinner, Raucci, Umhoefer and Blakey2012). Sedimentological evidence indicates deposition in a humid climatic regime with intense monsoonal influence (Nordt et al., Reference Nordt, Atchley and Dworkin2015).
PEFO 46222 was collected at PFV 456 from a medium light gray siltstone with a thickness of 60 cm characterized by large-scale slickensides. Additionally, a few elements of PEFO 46222 graded into the underlying unit, which is a 15-cm thick, highly fossiliferous, poorly sorted siltstone horizon characterized by coprolites and microvertebrate remains (Kligman et al., Reference Kligman, Marsh, Sues and Sidor2020, Reference Kligman, Gee, Marsh, Nesbitt, Smith, Parker and Stocker2023).
The stratigraphy of PFV 456 suggests that most of the vertebrate remains preserved at the site were initially deposited in a marginal lacustrine setting, likely a pond or lake bottom (Kligman, Reference Kligman2023). A subsequent episode of transport, likely a short-lived channel avulsion event, resulted in both the complete disarticulation and disassociation of these vertebrate remains and their redeposition at PFV 456 (Kligman, Reference Kligman2023). This is further supported by the well-preserved state of the delicate microvertebrate remains (Jenkins et al., Reference Jenkins, Pritchard, Marsh, Kligman, Sidor and Reed2020; Kligman et al., Reference Kligman, Marsh, Sues and Sidor2020, Reference Kligman, Gee, Marsh, Nesbitt, Smith, Parker and Stocker2023; Marsh et al., Reference Marsh, Smith, Parker, Irmis and Kligman2020) that would otherwise not survive extensive fluvial transport. Additionally, this same episode of transport likely also incorporated the associated skeleton of PEFO 46222 and re-deposited it at PFV 456 (Kligman, Reference Kligman2023), explaining why some of the skeletal elements of PEFO 46222 were also recovered from the underlying fossiliferous unit.
Preservation of the bones varies at the PFV 467 locality. Some are coated with a thick red layer of iron-rich mineralization making the bones dense, while this is not the case for other fossilized material occurring immediately adjacent. This variation in preservation is exemplified by PEFO 49321, in which the elements were found in close association, yet only some are coated with the iron-rich mineralization.
Age
Six localities are considered here: UCMP A269 (the Placerias Quarry), UCMP V7308 (the Blue Hills), PFV 456 (Thunderstorm Ridge), and PFV 467 (Metoposaur Genesis Supreme) within the Chinle Formation, and Sierrita de la Cruz Creek and Holmes (=Home[s], Gregory, Reference Gregory, Kelley and Trauger1972) Creek within the Dockum Group. U–Pb detrital zircon geochronology suggests an early-middle Norian (ca. 227–205 Ma; Kent et al., Reference Kent, Olsen, Lepre, Rasmussen, Mundil, Gehrels, Giesler, Irmis, Geissman and Parker2019) maximum depositional age (MDA) of ca. 219.39 ± 0.16 Ma for UCMP A269 (Fig. 2.2; Ramezani et al., Reference Ramezani, Fastovsky and Bowring2014), and by proxy UCMP V7308 because it occurs in a stratigraphically similar horizon. Chronostratigraphic correlation to the better age-calibrated Chinle Formation within PEFO suggests that UCMP A269 (the Placerias Quarry; Camp and Welles, Reference Camp and Welles1956) is potentially contemporaneous with the upper Blue Mesa Member or lower Sonsela Member (Fig. 2.2; Martz et al., Reference Martz, Parker, Skinner, Raucci, Umhoefer and Blakey2012; Rasmussen et al., Reference Rasmussen, Mundil, Irmis, Geisler, Gehrels, Olsen and Kent2020). This is based on the current geochronological understanding of the lower Chinle Formation within PEFO, where the upper Blue Mesa Member exhibits a maximum age of deposition (MDA) of ca. 223–218 Ma (Atchley et al., Reference Atchley, Nordt, Dworkin, Ramezani, Parker, Ash and Bowring2013; Rasmussen et al., Reference Rasmussen, Mundil, Irmis, Geisler, Gehrels, Olsen and Kent2020) and the lower Sonsela Member exhibits an MDA of ca. 219 Ma in the Lot’s Wife Beds (Parker, Reference Parker2018a; Marsh et al., Reference Marsh, Parker, Stockli and Martz2019). Additionally, recent hypotheses by Irmis et al. (Reference Irmis, Mundil, Martz and Parker2011) and Marsh et al. (Reference Marsh, Parker, Stockli and Martz2019) suggest that the fine-grained ‘upper Blue Mesa Member’ facies that characterize the Placerias Quarry in St. Johns, Arizona, are regionally diachronous with the course-grained lower Sonsela Member facies in PEFO (= Lot’s Wife Beds), as a result of the northeastward progradation of a massive fluvial fan (distributive fluvial system) that deposited the Blue Mesa Member and Sonsela Member in northern Arizona (Trendell et al., Reference Trendell, Atchley and Nordt2013).
Currently, there are no U–Pb maximum depositional ages for the Dockum Group (Riggs et al., Reference Riggs, Lehman, Gehrels and Dickinson1996). Rb–Sr ages bound the lower and most of the middle units of the Cooper Canyon Formation in Garza County, Texas, with an estimated age interval of ca. 225–211 Ma (Long and Lehman, Reference Long and Lehman1993, Reference Long, Lehman, Lanphere, Dalrymple and Turrin1994; Long, Reference Long2009; Marsh and Parker, Reference Marsh and Parker2020; Nesbitt et al., Reference Nesbitt, Stocker, Ezcurra, Fraser, Heckert, Parker and Mueller2021). The Tecovas Formation of the Dockum Group in eastern New Mexico and the Texas Panhandle is stratigraphically equivalent to the lower Cooper Canyon Formation in Garza County (Fig. 2.2; Martz, Reference Martz2008; Martz and Parker, Reference Martz, Parker, Parker and Zeigler2017, fig. 14; Martz et al., Reference Martz, Mueller, Nesbitt, Stocker, Parker, Atanassov, Fraser, Weinbaum and Lehane2013). Biochronological correlation of the Dockum Group to the better age-calibrated Chinle Formation exposed within PEFO places most of the Tecovas Formation and most of the lower Cooper Canyon Formation within the Adamanian Land Vertebrate Estimated Holochronozone, which currently has a temporal range of ca. 221–215 Ma (Fig. 2.2; Lucas and Hunt, Reference Lucas, Hunt, Lucas and Morales1993; Ramezani et al., Reference Ramezani, Hoke, Fastovsky, Bowring, Therrien, Dworkin, Atchely and Nordt2011, Reference Ramezani, Fastovsky and Bowring2014; Martz and Parker, Reference Martz, Parker, Parker and Zeigler2017, fig. 14; Nesbitt et al., Reference Nesbitt, Stocker, Ezcurra, Fraser, Heckert, Parker and Mueller2021, fig. 11).
PFV 456 and PFV 467 are stratigraphically located within the upper Blue Mesa Member (Fig. 2.2; Jenkins et al., Reference Jenkins, Pritchard, Marsh, Kligman, Sidor and Reed2020; Kligman et al., Reference Kligman, Marsh, Sues and Sidor2020, Reference Kligman, Gee, Marsh, Nesbitt, Smith, Parker and Stocker2023; Marsh et al., Reference Marsh, Smith, Parker, Irmis and Kligman2020; Reyes et al., Reference Reyes, Parker and Heckert2023), which currently exhibits temporal bounds of ca. 223–218 Ma (discussed above; Atchley et al., Reference Atchley, Nordt, Dworkin, Ramezani, Parker, Ash and Bowring2013; Rasmussen et al., Reference Rasmussen, Mundil, Irmis, Geisler, Gehrels, Olsen and Kent2020), placing the localities within the Adamanian Land Vertebrate Estimated Holochronozone (Fig. 2.2; Martz and Parker, Reference Martz, Parker, Parker and Zeigler2017). These refined temporal constraints suggest a temporal range of ca. 223–218 Ma for Calyptosuchus wellesi (Parker and Martz, Reference Parker and Martz2011; Rasmussen et al., Reference Rasmussen, Mundil, Irmis, Geisler, Gehrels, Olsen and Kent2020).
Materials and methods
Collection of new referred specimens
PEFO 46222 was collected from PFV 456 across a 5 × 4 m area (Fig. S1). We employed a 1-m2 grid system to document element type, orientation, and collection numbers. A significant portion of the trunk and sacral region was collected from a single 1-m2 quadrant; those elements were semi-articulated and closely associated, so they were collected in a field jacket made with single-sheeted polyester air filter medium (MSC Industrial Supply) and Hydrocal plaster (United States Gypsum Co.). The remaining associated elements found across the larger area were collected using Gypsona plaster bandages (BSN Medical) or with aluminum foil. A combination of Butvar B-72 (Rhom and Hass) and Paraloid B-76 (Eastman Chemical Company) were used to stabilize the bones in the field and lab. A combination of water and toothbrush, pin vices with carbide steel needles, dental tools, acetone, and air scribes were used to remove the matrix from the bone under dissecting microscope magnification. The elements were individually prepared and separated, although a few elements were diagenetically cemented, which impeded separation.
PEFO 49321 was collected from PFV 467 using Gypsona plaster bandages (BSN Medical) and aluminum foil. The elements were found in close association in the field with some being discovered during preparation because they were overlapped by other elements. Both Butvar B-72 (Rhom and Hass) and Paraloid B-76 (Eastman Chemical Company) was used to stabilize the bones in the field and lab. Several of the elements of PEFO 49321 preserve a thick iron-rich mineral crust (Fig. S2), so preparators chose not to mechanically remove this coating because the anatomy is still discernable or better represented in other elements that lack this mineralization.
PEFO 46222 and PEFO 49321 are catalogued, stored in cushioned drawers, and reposited in the museum collections at Petrified Forest National Park and are available to future researchers. Most of the skeletal elements of PEFO 46222 were scanned with an Artec Space Spider high-resolution laser/optical scanner and 3D models were made from those data using Artec Studio 16 Professional (16.0.5.114). Surface meshes of PEFO 46222 are hosted on MorphoSource Project 609256 (https://www.morphosource.org/projects/000609256). High-resolution 3D surface scans of the pelvic girdles of UMMP 13950 and UMMP 7470 are available within the online repository of fossils of the UMMP at: https://umorf.ummp.lsa.umich.edu/wp/class-reptilia/.
Phylogenetic analyses
Our phylogenetic analyses build on those of Parker (Reference Parker2016a), Reyes et al. (Reference Reyes, Parker and Marsh2020, Reference Reyes, Martz and Small2024), Paes Neto et al. (Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021c), and Haldar et al. (Reference Haldar, Ray and Bandyopadhyay2023). We modified the definitions of characters 69 and 76 (Parker, Reference Parker2016a) to focus on the middle trunk region rather than the entire trunk region (see Supplemental Material). Additionally, we modified the scoring for Stagonolepis olenkae Sulej, Reference Sulej2010 (see Supplemental Material). We expanded a recent matrix of the Aetosauria by Reyes et al. (Reference Reyes, Martz and Small2024) by incorporating four new anatomical characters: [105] number of alveoli on the posterior process of the maxilla, starting ventral to the anteriormost margin of the antorbital fenestra; [106] presence of a pneumatic accessory cavity on the medial shelf of the maxilla; [107] co-ossification of the sacral vertebrae; and [108] position of anterior tip of preacetabular process in lateral view relative to the position of the pubic peduncle. We omitted the operational taxonomic unit (OTU) Aetobarbakinoides brasiliensis Desojo, Ezcurra, and Kischlat, Reference Desojo, Ezcurra and Kischlat2012, because it currently acts as a wildcard taxon (sensu Nixon and Wheeler, Reference Nixon, Wheeler, Novacek and Wheeler1992; Kearney, Reference Kearney2002; Kearney and Clark, Reference Kearney and Clark2003) as determined by Heckert et al. (Reference Heckert, Schneider, Fraser and Webb2015) and Parker (Reference Parker2016a). Additionally, we excluded Kryphioparma caerula Reyes, Parker, and Heckert, Reference Reyes, Parker and Heckert2023, following the discussion by Parker and Haldar (Reference Parker and Haldar2024), suggesting that the taxon should be omitted from future phylogenetic analyses as it is likely acting as a wildcard because of the lack of scorable characters (Nixon and Wheeler, Reference Nixon, Wheeler, Novacek and Wheeler1992; Kearney, Reference Kearney2002; Kearney and Clark, Reference Kearney and Clark2003). A recent study proposed that Polesinesuchus aurelioi Roberto-Da-Silva et al., Reference Roberto-Da-Silva, Desojo, Cabreira, Aires, Müller, Pacheco and Dias-Da-Silva2014, is a junior synonym of Aetosauroides scagliai based on the documentation of diagnostic characters being subjected to intraspecific variation due to ontogeny (Paes-Neto et al., Reference Paes-Neto, Desojo, Brust, Schultz, Da-Rosa and Soares2021a). Accordingly, we omitted Polesinesuchus aurelioi Roberto-Da-Silva et al., Reference Roberto-Da-Silva, Desojo, Cabreira, Aires, Müller, Pacheco and Dias-Da-Silva2014, from our analysis. Additionally, we excluded Garzapelta muelleri Reyes, Martz, and Small, Reference Reyes, Martz and Small2024, from the analyses because our study does not include new character information associated to the osteoderms that could assist in assessing the convergence exhibited by the trunk lateral osteoderms of this taxon (see discussion in Reyes et al., Reference Reyes, Martz and Small2024).
The modified matrix comprises 108 anatomical characters (49 cranial, 59 postcranial; see Supplemental Material). Two versions of the matrix (i.e., Run 1, Run 2) were each analyzed using both maximum parsimony and Bayesian inference in order to explore evolutionary hypotheses under two different models. Run 1 included 33 taxa with an ingroup composed of 31 aetosaur taxa, including the holotype specimen of Calyptosuchus wellesi and four referred specimens. We scored the holotype specimen of Calyptosuchus wellesi (UMMP 13950, Case, Reference Case1932) independently from other referred material with the goal of assessing the referral of UMMP 7470, UCMP 27225, PEFO 46222, and PEFO 49321 to C. wellesi through a phylogenetic analysis. The array of isolated specimens from UCMP A269, including braincases referred to Calyptosuchus wellesi (Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021c), were omitted because of both our inability to unambiguously refer them to a particular individual, and the ambiguity surrounding some of their taxonomic affinities (discussed below). Run 2 included 28 taxa with an ingroup composed of 26 aetosaur taxa. We coded all of the referred individuals listed above into a composite OTU of Calyptosuchus wellesi to assess its phylogenetic relationships within Aetosauria. The non-aetosaur aetosauriform Revueltosaurus callenderi Hunt, Reference Hunt, Lucas and Hunt1989, and rauisuchid Postosuchus kirkpatricki Chatterjee, Reference Chatterjee1985, served as the outgroup for all analyses (following Parker, Reference Parker2016a). The new and revised character scorings within this study were based on specimens that were studied firsthand, from figures and descriptions in the literature, personal communications, and/or shared photographs and 3D models. The supplemental material includes a detailed list of the taxa, specimens, and main references from which the new characters were scored.
Maximum parsimony
The matrix was analyzed via parsimony using the phylogenetic analysis software package TNT v1.5 (Goloboff et al., Reference Goloboff, Farris and Nixon2008). The analysis was performed using the traditional search option with 1,000 replications and tree bisection reconnection swapping while keeping 10 trees per replication and condensing zero-length branches (see Parker, Reference Parker2016a; Reyes et al., Reference Reyes, Parker and Marsh2020; Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021c). Fourteen characters (3, 4, 14, 20, 22, 23, 24, 28, 64, 70, 73, 76, 79, 83) were ordered. Our analysis resulted in 424 most-parsimonious trees (MPTs) with a length of 282 steps for Run 1 and 18 MPTs with a length of 281 steps for Run 2, a Consistency Index (C.I.) of 0.546 for Run 1 and 0.548 for Run 2, and a Retention Index (R.I.) of 0.721 for Run 1 and 0.723 for Run 2 (see Supplemental Material). The strict consensus of the MPTs for both runs is discussed below.
Bayesian inference
In addition to maximum parsimony, the matrix was analyzed via Bayesian inference to explore alternative hypotheses and methodologies. This was performed using the phylogenetic analysis software MrBayes v3.2.6 (Huelsenbeck and Ronquist, Reference Huelsenbeck and Ronquist2001) with the Mkv model and gamma rate variation under the following parameters: two runs with four Markov chain Monte Carlo (MCMC) chains each, sampled every 1000 generations, for five-million generations with a relative burn-in frequency of 0.25. Convergence of independent runs was assessed using Tracer v.1.7.1 (http://beast.bio.ed.ac.uk/Tracer). For consistency we ordered the same 14 characters listed above. The consensus cladogram and associated matrix for Run 2 were imported into PAUP*4.0 (Swofford, Reference Swofford2003) to extrapolate the synapomorphies of the consensus cladogram (see Supplemental Information).
Repositories and institutional abbreviations
AMNH, American Museum of Natural History, New York, New York, USA; DMNH V., Denver Museum of Nature and Science, Denver, Colorado, USA; ISI, Indian Statistical Institute, Kolkata, India; MCN, Museu de Ciências Naturais, Secretaria Estadual do Meo Ambiente e Infraestrutura, Porto Alegre, Brazil; MCP, Museu de Ciências e Tecnologia de Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, Brazil; MCZD, Marischal College, Zoology Department, University of Aberdeen, Scotland; MNA, Museum of Northern Arizona, Flagstaff, Arizona, USA; MOTT, refers to a fossil locality of TTU; NCSM, North Carolina Museum of Natural Sciences, Raleigh, North Carolina, USA; NHMUK, Natural History Museum, London, England, United Kingdom; NMT, National Museum of Tanzania, Dar es Salaam, Tanzania; NMMNH, New Mexico Museum of Natural History and Science, Albuquerque, New Mexico, USA; PEFO, Petrified Forest National Park, Arizona, USA; PFV, refers to a vertebrate locality number from PEFO; PULR, Paleontología Museo de Ciencias Naturales, Universidad de La Rioja, La Rioja, Argentina; PVL, Instituto Miguel Lillo, Paleontología de Vertebrados, Tucumán, Argentina; SMNS, Staatliches Museum für Naturkunde, Stuttgart, Germany; TMM, Texas Vertebrate Paleontology Collections, University of Texas at Austin, Austin, Texas, USA; TTU-P, Museum of Texas Tech University, Lubbock, Texas, USA; UCMP, University of California Museum of Paleontology, Berkeley, California, USA; ULBRA PVT, Universidade Luterana do Brasil, Coleção de Paleovertebrados, Canoas, Rio Grande do Sul, Brazil; UFSM, Laboratório de Estratigrafia e Paleobiologia of Universidade Federal de Santa Maria, Santa Maria, Rio Grande do Sul, Brazil; UMMP, University of Michigan Museum of Paleontology, Ann Arbor, Michigan, USA; UOPB, University of Opole, Palaeobiology Department, Opole, Poland; YPM, Yale Peabody Museum, New Haven Connecticut, USA; ZPAL, Institute of Paleobiology, Polish Academy of Sciences, Warsaw, Poland.
Systematic paleontology
Archosauria Cope, Reference Cope1869, sensu Gauthier and Padian, Reference Gauthier, Padian, Hecht, Ostrom, Viohl and Wellnhofer1985
Pseudosuchia Zittel, Reference Zittel1887–1890, sensu Gauthier and Padian, Reference Gauthier, Padian, Hecht, Ostrom, Viohl and Wellnhofer1985
Aetosauria Marsh, Reference Marsh1884, sensu Parker, Reference Parker2007
Stagonolepididae Lydekker, Reference Lydekker1887, sensu Heckert and Lucas, Reference Heckert and Lucas2000
Stagonolepidoidea Parker, Reference Parker2018a
Calyptosuchini new clade
Definition
The least inclusive clade containing Calyptosuchus wellesi, Scutarx deltatylus, and Adamanasuchus eisenhardtae Lucas et al., Reference Lucas, Hunt, Spielmann, Lucas and Spielmann2007a.
Unambiguous synapomorphy
Pubis exhibits two obturator foramina (character 50-1; convergent in Stagonolepis robertsoni Agassiz, Reference Agassiz1844, unknown in Adamanasuchus eisenhardtae).
Other possible synapomorphies
ACCTRAN: The basal tubera and basipterygoid processes are closely situated to each other (character 25-1); maxillary teeth are ovate, but not strongly mediolaterally compressed in occlusal view (character 34-1); maxillary teeth crown is bulbous and partly recurved, has a concave anterior edge, and straight posterior edge (character 35-1); co-ossified sacral vertebrae (character 106-1). DELTRAN: none.
Calyptosuchus Long and Ballew, Reference Long, Ballew, Colbert and Johnson1985
Type species
Calyptosuchus wellesi Long and Ballew, Reference Long, Ballew, Colbert and Johnson1985, by monotypy.
Diagnosis
As the monotypic species.
Occurrence
As the monotypic species.
Calyptosuchus wellesi Long and Ballew, Reference Long, Ballew, Colbert and Johnson1985
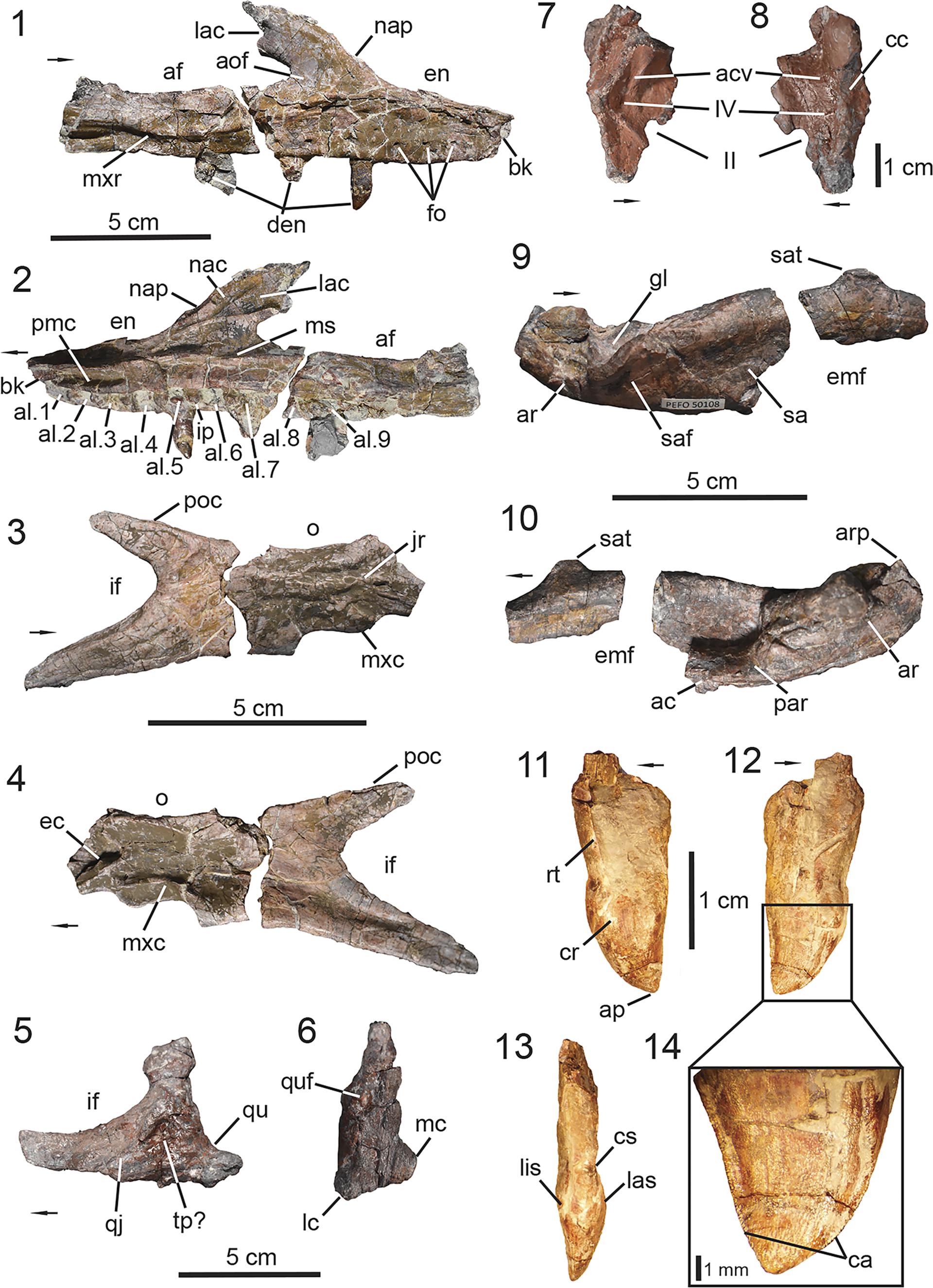
Figure 3. PEFO 49321, skull anatomy. (1, 2) Right maxilla; (3, 4) right jugal; (5, 6) left quadratojugal and quadrate; (7, 8) left laterosphenoid; (9, 10) right surangular, prearticular, and articular; (11–14) maxillary teeth. (1, 3, 5, 8, 9) Lateral views, (2, 4, 7, 10) medial views, (6) posterior view, (11) labial view, (12, 14) lingual view, (13) mesial view. ac = angular contact; acv = anterior cerebral vein; af = antorbital fenestra; al = alveoli; aof = antorbital fossa; ap = apex; ar = articular; arp = articular process; bk = break; ca = carinae; cc = cotylar crest; cr = crown; cs = constriction; den = dentition; ec = ectopterygoid contact; emf = external mandibular fenestra; en = external naris; fo = foramina; gl = glenoid; if = infraorbital foramen; ip = intradental plates; jr = jugal ridge; lac = lacrimal contact; las = labial surface; lc = lateral condyle; lis = lingual surface; mc = medial condyle; ms = medial shelf; mxc = maxilla contact; mxr = maxillary ridge; nac = nasal contact; nap = nasal process; o = orbit; poc = postorbital contact; par = prearticular; pmc = premaxilla contact; qj = quadratojugal; qu = quadrate; quf = quadrate foramen; rt = root; sa = surangular; saf = surangular foramen; sat = surangular tuber; tp = taphonomic pit; II = foramen or canal for optic nerve; IV = foramen or canal for trochlear nerve. Arrows indicate anterior or distal direction (dentition).
Holotype
UMMP 13950, a partially articulated skeleton that preserves the dorsal carapace from the mid-trunk through mid-caudal region with the associated vertebral column and pelvic girdle (Case, Reference Case1932).
Revised diagnosis
Calyptosuchus wellesi is a medium-sized aetosaur that currently lacks autapomorphies. It is differentiated from all other aetosaurs based on a unique combination of characters including a maxilla that lacks a pneumatic accessory cavity, unlike Desmatosuchus (Case, Reference Case1920; Small, Reference Small2002), Stagonolepis (Walker, Reference Walker1961; Sulej, Reference Sulej2010; Parker, Reference Parker2018b), Longosuchus meadei (Sawin, Reference Sawin1947) (Parrish, Reference Parrish1994), and Stenomyti huangae Small and Martz, Reference Small, Martz, Nesbitt, Desojo and Irmis2013; a preacetabular process of the ilium that is positioned dorsal to the pubic peduncle, a condition shared with most aetosaurs that preserve an ilium except for Neoaetosauroides engaeus Bonaparte, Reference Bonaparte1969, where the preacetabular process is positioned far anteriorly of the pubic peduncle (Desojo and Báez, Reference Desojo and Báez2005); a pubic apron that is perforated by two foramina as observed in Scutarx deltatylus (Parker, Reference Parker2016b) and Stagonolepis robertsoni (Walker, Reference Walker1961); and a posterior centrodiapophyseal lamina that becomes more discernable in the vertebrae from the middle trunk into the posterior-trunk region. Furthermore, Calyptosuchus wellesi exhibits two characters subject to intraspecific variation. These are the presence/absence of zygadiapophyseal laminae in the trunk vertebrae and co-ossification, or lack thereof, between the centra of sacral vertebrae #1–2.
Occurrence
Late Triassic, early-middle Norian, ca. 223–218 Ma, Adamanian Late Triassic Land Vertebrate Estimated Holochronozone (Atchley et al., Reference Atchley, Nordt, Dworkin, Ramezani, Parker, Ash and Bowring2013; Martz and Parker, Reference Martz, Parker, Parker and Zeigler2017; Marsh et al., Reference Marsh, Parker, Stockli and Martz2019; Rasmussen et al., Reference Rasmussen, Mundil, Irmis, Geisler, Gehrels, Olsen and Kent2020). Upper part of the Blue Mesa Member and lower part of the Sonsela Member (sensu Parker and Martz, Reference Parker and Martz2011), Chinle Formation, Arizona (Long and Murry, Reference Long and Murry1995; Parker, Reference Parker2018a); Tecovas Formation, Dockum Group, Texas (Case, Reference Case1932; Long and Ballew, Reference Long, Ballew, Colbert and Johnson1985).
Cranial description
PEFO 49321 preserves elements of the cranium and mandible, including the first dentition and unambiguous braincase elements referable to Calyptosuchus wellesi (Fig. 3). A dentary from another associated individual (UCMP 27225; Parker, Reference Parker2018a) represents the only other unambiguous skull material referable to this taxon. There are several braincases from UCMP A269 (the Placerias Quarry) that may be referrable to Calyptosuchus wellesi (Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021c), but their referral remains ambiguous due to the disarticulated nature and loss of original association of the elements collected from the site (Parker, Reference Parker2018a). The elements of PEFO 49321 are moderately well preserved with those associated with the left side of the skull being coated with an iron-rich mineralization. No elements from the skull roof or palate are preserved.
Maxilla
PEFO 49321 preserves both maxillae, but the anatomy of the maxilla is best observed on the right element (Fig. 3.1, 3.2). The right maxilla is well preserved, nearly complete, and not coated with the iron mineralization. The maxilla is triradiate in shape with a main body and three processes (Desojo et al., Reference Desojo, Heckert, Martz, Parker, Schoch, Small, Sulej, Nesbitt, Desojo and Irmis2013). In lateral view, the anterior process is dorsoventrally tall, and its dorsal margin forms the posterior half of the ventral margin of the external naris (Fig. 3.1). Anteriorly, the dorsolateral margin is crushed. It is evident that the anterior margin is not fully preserved in the maxillae (Figs. 3.1, S2), so it is likely that the anterior process tapers farther anteriorly, underlapping the lateral process of the premaxilla as typically observed in aetosaurs (e.g., Czepiński et al., Reference Czepiński, Dróżdż, Szcygielski, Tałanda, Pawlak, Lewczuk, Rytel and Sulej2021, fig. 6). The anteroventral margin of the dorsal process bears a posterodorsally inclined embayment in lateral view (Fig. 3.1, 3.2) at the junction between the anterior and dorsal processes. This embayment marks the lateral insertion point of the ventral process of the nasal, which comprises the posterior margin of the external naris as observed in most aetosaur taxa that preserve these elements (except Desmatosuchus smalli, TTU-P 9024, Small, Reference Small2002).
The dorsal process is acutely inclined posteriorly in relation to the main body of the element (Fig. 3.1, 3.2), but more so than the condition observed in Paratypothorax andressorum Long and Ballew, Reference Long, Ballew, Colbert and Johnson1985 (SMNS 19003, Schoch and Desojo, Reference Schoch and Desojo2016) where the orientation is more of a right angle. Overall, it resembles the condition observed in Stagonolepis spp. (S. robertsoni, NHMUK PV R 4787, Walker, Reference Walker1961; S. olenkae, ZPAL AbIII/1995, Sulej, Reference Sulej2010) and Aetosauroides scagliai (MCN-PV 2347, Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021b). Posterolaterally, the dorsal process is dorsoventrally tall and tapers into a posteriorly oriented triangular process near its dorsal border that wedges itself between the lacrimal and nasal in lateral view, resembling the condition in Stagonolepis robertsoni (Walker, Reference Walker1961) and Aetosaurus ferratus (SMNS 5770, Schoch, Reference Schoch2007). Additionally, the posteroventral margin of the dorsal process forms the anterodorsal border of the antorbital fenestra. The posterior process of the maxilla is nearly half the anteroposterior length of the entire element with a dorsal margin that is anteroposteriorly straight in lateral view and forms the ventral border of the antorbital fenestra. The anterior portion of the fenestra between the dorsal and posterior processes is not dorsoventrally tall (Fig. 3.1, 3.2), suggesting that the antorbital fenestra of Calyptosuchus wellesi is subtriangular in shape rather than semicircular or elliptical, resembling that of Aetosauroides scagliai (UFSM 11505, Biacchi Brust et al., Reference Biacchi Brust, Desojo, Schultz, Paes-Neto and Da-Rosa2018; MCN-PV 2347, Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021b) and Typothorax coccinarum Cope, Reference Cope and Wheeler1875 (NMMNH P-12964, Heckert et al., Reference Heckert, Lucas, Rinehart, Celeskey, Spielmann and Hunt2010; PEFO 38001/YPM VP.58121, Reyes et al., Reference Reyes, Parker and Marsh2020). The posterior extent of the maxilla is not preserved. Thus, the posterior process does not provide information regarding the contact between the maxilla, lacrimal, and jugal.
The ventral margin of the maxilla is anteroposteriorly straight in lateral view (Fig. 3.1, 3.2), resembling that of Coahomasuchus kahleorum Heckert and Lucas, Reference Heckert and Lucas1999 (TMM 31100-437) and Kocurypelta silvestris Czepiński et al., Reference Czepiński, Dróżdż, Szcygielski, Tałanda, Pawlak, Lewczuk, Rytel and Sulej2021 (ZPAL V.66/4). The antorbital fossa is deep and well demarcated on the lateral surface of the maxilla (Fig. 3.1). On the dorsal process the fossa expands onto most of the lateral surface of the maxilla, but it is evident that it does not continue onto the lateral extent of the nasal; instead, it continues posteriorly onto the lacrimal. On the posterior process, the fossa is restricted to the dorsal two-thirds of the lateral surface and is ventrally bordered by a well-developed transverse ridge (Fig. 3.1); a condition also described in the two maxillary fragments UCMP 195193 and UCMP 195194 referred to Calyptosuchus wellesi from UCMP A269 (the Placerias Quarry, Parker, Reference Parker2018a). In most aetosaurs with described maxillae this ridge is prominent except in both species of Desmatosuchus (D. spurensis Case, Reference Case1920, UMMP 7476, Case, Reference Case1922; D. smalli, Small, Reference Small2002), Longosuchus meadei (TMM 31185-98, Sawin, Reference Sawin1947; Parrish, Reference Parrish1994), Typothorax coccinarum (Heckert et al., Reference Heckert, Lucas, Rinehart, Celeskey, Spielmann and Hunt2010; Reyes et al., Reference Reyes, Parker and Marsh2020), and Kocurypelta sylvestris Czepiński et al., Reference Czepiński, Dróżdż, Szcygielski, Tałanda, Pawlak, Lewczuk, Rytel and Sulej2021, in which the ridge is poorly developed or absent. Additionally, the transverse ridge in PEFO 49321 continues anterodorsally, confining the antorbital fossa, and fades distally on the lateral surface of the dorsal process (Fig. 3.1) similar to the condition observed in Aetosauroides scagliai (Biacchi Brust et al., Reference Biacchi Brust, Desojo, Schultz, Paes-Neto and Da-Rosa2018; Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021b), Paratypothorax andressorum (Schoch and Desojo, Reference Schoch and Desojo2016), Aetosaurus ferratus (Schoch, Reference Schoch2007), and Stenomyti huangae Small and Martz, Reference Small, Martz, Nesbitt, Desojo and Irmis2013.
A row of five foramina is present on the ventrolateral surface of the maxilla that extends parallel to the ventral margin, just dorsal of the alveolar row (Fig. 3.1). This row of foramina begins 1 cm posterior of the anterior margin of the anterior process and terminates just posteroventral to the anterior margin of the antorbital fenestra, indicating that there is not a one-to-one correlation with the alveoli. In life, these foramina likely transmitted fibers of the superior alveolar nerve, an extension of the maxillary branch of the trigeminal nerve (CN V2) that innervates the alveoli and soft tissues surrounding the maxilla (George and Holliday, Reference George and Holliday2013; Lessner and Holliday, Reference Lessner and Holliday2020, fig. 10). As in Stenomyti huangae (DMNH V.34565; Small and Martz, Reference Small, Martz, Nesbitt, Desojo and Irmis2013), the alveolar row of the maxilla of PEFO 49321 contains nine alveoli with three teeth still in situ (Fig. 3.1, 3.2; described below). Based on current understanding, only Neoaetosauroides engaeus (PVL 4363, Desojo and Baez, Reference Desojo and Báez2007; Taborda et al., Reference Taborda, Desojo and Dvorkin2021), Aetosaurus ferratus (Schoch, Reference Schoch2007), Typothorax coccinarum (Reyes et al., Reference Reyes, Parker and Marsh2020), and Calyptosuchus wellesi (based on PEFO 49321) exhibit fewer than 10 tooth positions in their maxillae. The alveolar row extends far posteriorly onto the posterior process terminating at the midline of the antorbital fenestra, unlike Neoaetosauroides engaeus (Desojo and Báez, Reference Desojo and Báez2007) and Kocurypelta sylvestris (Czepiński et al., Reference Czepiński, Dróżdż, Szcygielski, Tałanda, Pawlak, Lewczuk, Rytel and Sulej2021), where the alveolar row terminates more anterior to it.
In medial view, the maxilla bears a medial shelf that runs the length of the element parallel to and dorsal to the alveolar row (Fig. 3.2). Anteriorly, on the anterior process, the shelf is bifurcated by a deep longitudinal groove that terminates dorsal to the third alveolus (from anterior to posterior). That groove marks the articulation point for the posteromedial process of the premaxilla (Fig. 3.2) as observed in Desmatosuchus smalli (Small, Reference Small2002) and Stagonolepis olenkae (Sulej, Reference Sulej2010). Dorsally, the medial ridge exhibits a shallow choanal recess (Witmer, Reference Witmer1997) at the junction of the anterior and dorsal processes. The medial surface of the dorsal process exhibits a triangular-shaped articular surface for its contact with the nasal and lacrimal dorsal to the choanal recess (Fig. 3.2). The anterior process of the lacrimal fits anteroventrally into a slot while the nasal truncates this process dorsally; this articulation is best observed in Longosuchus meadei (TMM 31185-97, Parrish, Reference Parrish1994, fig. 3). On the posterior process, the medial shelf is concave ventrally and expands mediolaterally just posterior to the last alveolus for the contact between the maxilla and palate.
PEFO 49321 shows no evidence of a pneumatic accessory cavity at the junction of the dorsal and posterior processes of the maxilla, posterior to the choanal recess (Fig. 3.2) (Witmer, Reference Witmer1997), similar to Aetosauroides scagliai (Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021b). A pneumatic accessory cavity is documented in Stenomyti huangae (Small and Martz, Reference Small, Martz, Nesbitt, Desojo and Irmis2013), Stagonolepis (Walker, Reference Walker1961; Sulej, Reference Sulej2010; Parker, Reference Parker2018b), Longosuchus meadei (Parrish, Reference Parrish1994), and Desmatosuchus (Case, Reference Case1920; Small, Reference Small2002), suggesting that this character may be phylogenetically informative, as originally proposed by Small (Reference Small2002). In medial view, the nine alveoli are equally spaced from each other and are divided by ventrally directed, well-spaced, sub-triangular interdental plates (Fig. 3.2), as also observed in Aetosauroides scagliai (Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021b). Additionally, the anterior wall of the first alveolus is not preserved (Fig. 3.2), further indicating that the anterior extent of the maxilla is not fully preserved.
Jugal
The right jugal of PEFO 49321 is only missing portions of the mid-body and its anterior margin (Fig. 3.3, 3.4). The element is ~60% the anteroposterior length of the maxilla, which is unlike Aetosaurus ferratus (Schoch, Reference Schoch2007) in which the jugal is shorter in length, being ~40% of the length of the maxilla. However, we note that the morphology of Aetosaurus ferratus is based on an aggregate of hatchling specimens (SMNS 5220; Teschner et al., Reference Teschner, Konietzko-Meir, Desojo, Schoch and Klein2023), suggesting that proportion between the jugal and maxilla may be influenced by its skeletal maturity. Anteriorly, the anterodorsal margin of the jugal is not preserved. Thus, we are unable to determine if the jugal separates the lacrimal and maxilla posteriorly and participates in the posterior margin of the antorbital fenestra, a variable character within the Aetosauria (Parker, Reference Parker2016a). The anteroventral margin of the jugal is partially preserved with a posteroventral incision (Fig. 3.3, 3.4). This indicates that the posterior process of the maxilla underlapped the jugal in lateral view as it would have tapered posteroventrally, exhibiting a sinuous, wedge-shaped contact with the jugal as described for Paratypothorax andressorum (Schoch and Desojo, Reference Schoch and Desojo2016). That condition differs from the prong-like bifurcating contact exhibited by the non-aetosaur aetosauriform Revueltosaurus callenderi (PEFO 34561, Parker et al., Reference Parker, Nesbitt, Irmis, Martz, Marsh, Brown, Stocker and Werning2021) and the early-branching aetosaur Aetosauroides scagliai (Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021b). Dorsally, the margin is concave and forms the ventral border of the orbit.
The main body of the jugal is anteroposteriorly oriented with a straight ventral margin (Fig. 3.3, 3.4), as observed in Stenomyti huangae (Small and Martz, Reference Small, Martz, Nesbitt, Desojo and Irmis2013) and both species of Coahomasuchus (C. chathamensis, Heckert, Fraser, and Schneider, Reference Heckert, Fraser and Schneider2017, NCSM 23618; C. kahleorum, TMM 31100-437). That condition differs from the strongly downturned jugal observed in Desmatosuchus (Case, Reference Case1920; Small, Reference Small2002) and Longosuchus meadei (Parrish, Reference Parrish1994). Posteriorly, the jugal bifurcates into two triangular-shaped processes that participate in the margins of the infratemporal fenestra. The posterodorsal process exhibits an anteroposterior length that is ~50% that of the posteroventral process. The posterodorsal process does not participate in the margins of the orbit, but its inclination indicates that the ventral process of the postorbital tapered anteroventrally along the posterior margin of the orbit (Fig. 3.3, 3.4), a condition commonly observed in aetosaurs except in both species of Desmatosuchus (D. spurensis, Case, Reference Case1922; D. smalli, Small, Reference Small2002). However, we are unable to ascertain if the postorbital contributes to the margin of the infratemporal fenestra as observed in Typothorax coccinarum (Reyes et al., Reference Reyes, Parker and Marsh2020), Paratypothorax andressorum (Schoch and Desojo, Reference Schoch and Desojo2016), and Aetosaurus ferratus (Schoch, Reference Schoch2007) but does not in Aetosauroides scagliai (Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021b). The posteroventral process is inclined posteroventrally indicating that the jugal wedged and underlapped the anterior process of the quadratojugal in lateral view (Fig. 3.3–3.5).
The lateral surface of the jugal is ornamented by a continuation of the transverse ridge described in the maxilla (Fig. 3.3). This condition is observed in several aetosaur taxa but not in Desmatosuchus smalli (TTU-P 9024, Small, Reference Small2002), Typothorax coccinarum (PEFO 38001/YPM VP.58121, Reyes et al., Reference Reyes, Parker and Marsh2020), or Paratypothorax andressorum (SMNS 19003, Schoch and Desojo, Reference Schoch and Desojo2016). The transverse ridge terminates just anterior to the posterior bifurcation of the jugal and does not extend onto the posteroventral process, in contrast to the condition observed in Stenomyti huangae (Small and Martz, Reference Small, Martz, Nesbitt, Desojo and Irmis2013) and Coahomasuchus chathamensis (Heckert et al., Reference Heckert, Fraser and Schneider2017). Additionally, there is no evidence of pitting or foramina on the external surface. In medial view, the anterior end of the jugal exhibits a posteriorly oriented, narrow, triangular surface for the medial reception of the ectopterygoid (Fig. 3.4). Ventral to the orbit, the surface is longitudinally depressed with well-delineated dorsal and ventral borders, as observed in Erpetosuchus (AMNH 29300, Foffa et al., Reference Foffa, Butler, Nesbitt, Walsh, Barrett, Brusatte and Fraser2021, fig. 5i), although there is no evidence of pneumatization (Fig. 3.4).
Quadratojugal
The left quadratojugal and quadrate of PEFO 49321 are still in articulation but are coated with an iron-rich mineralization, which inhibits our ability to fully differentiate the two elements and evaluate the nature of their articulation (Fig. 3.5, 3.6). The quadratojugal appears to exhibit an overall L-shape (Fig. 3.5), a condition that aetosaurs share with other pseudosuchians (Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021b). Anteriorly, it is evident that the quadratojugal overlaps the posteroventral process of the jugal, as observed in Stenomyti huangae (Small and Martz, Reference Small, Martz, Nesbitt, Desojo and Irmis2013), but unlike that taxon the quadratojugal inhibits the jugal from framing the entire posteroventral margin of the cranium. Thus, the contact observed in PEFO 49321 resembles that of Stagonolepis robertsoni (Walker, Reference Walker1961, fig. 2). As a result of the overlapping contact between the quadratojugal and jugal, the dorsal margin of the anterior process of the quadratojugal forms part of the ventral margin of the infratemporal fenestra, and the posterodorsal process of the jugal participates in the inclined anterior margin of the opening. Based on this information, the infratemporal fenestra most likely had an ovate shape in lateral view.
The lateral surface of the anterior process bears a shallow fossa around the margin of the infratemporal fenestra, which is separated from a deep pit by a ridge (Fig. 3.5). Because of the preservation of the element, it is difficult to ascertain whether the pit and ridge are true anatomical features or a result of taphonomic alteration. The dorsal process is dorsoventrally short, less than 50% of the anteroposterior length of the anterior process. The lateral surface is slightly depressed near the dorsal tip (Fig. 2.11, 2.12), marking the point of contact between the quadratojugal and ventral process of the squamosal. If this interpretation is accurate, then the squamosal would have participated in the margins of the infratemporal fenestra, a condition documented in most aetosaurs except Aetosauroides scagliai (Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021b), Coahomasuchus chathamensis (Heckert et al., Reference Heckert, Fraser and Schneider2017), Paratypothorax andressorum (Schoch and Desojo, Reference Schoch and Desojo2016), and Aetosaurus ferratus (Schoch, Reference Schoch2007).
Quadrate
The left quadrate is articulated with the left quadratojugal. The element is only represented by its ventral half, so the head of the quadrate is not preserved (Fig. 3.6). The lateral wing (sensu Walker, Reference Walker1961) is co-ossified to the medial surface of the quadratojugal, but the medial wing is not preserved. The quadrate crest, which divides both wings, is discernible but faint, unlike the well-developed strut in Aetosauroides scagliai (Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021b, fig. 12). The crest is situated medially, dorsal to the medial condyle of the quadrate. Lateral to the quadrate crest, there is a shallow pit marking the perforation of the quadrate foramen. The margins of the foramen appear to be formed by both the quadratojugal and the lateral wing of the quadrate (Fig. 3.6), a condition observed in most aetosaurs (Parker, Reference Parker2016a; Schoch and Desojo, Reference Schoch and Desojo2016) except Coahomasuchus kahleorum (TMM 31100-437) and Aetosaurus ferratus (Schoch, Reference Schoch2007) in which the foramen is restricted to the lateral wing of the quadrate. However, there is no evidence of a fossa located on the external surface of the lateral wing just posterior to the quadrate foramen. Ventrally, the convex quadrate condyles are separated by an anteroposteriorly oriented groove. The lateral condyle is larger than the medial condyle and expanded anteromedially, similar to that of Desmatosuchus smalli (Small, Reference Small2002). In posterior view, the medial condyle is positioned more dorsally than the lateral condyle (Fig. 3.6).
Laterosphenoid
PEFO 49321 preserves the first unambiguous braincase element that is referred to Calyptosuchus wellesi. The left laterosphenoid is partially preserved, missing its anterior extent, and is coated in the same iron-rich mineralization present on some of the cranial bones of PEFO 49321 (Fig. 3.7, 3.8). Laterally, the surface is smooth but exhibits a distinct dorsoventrally oriented cotylar crest (Fig. 3.8) similar to Stagonolepis olenkae (ZPAL ABIII/466/17, Sulej, Reference Sulej2010). Two shallow foramina are present anterior to the cotylar crest (Fig. 3.8). The dorsal foramen marks the exit for the anterior cerebral vein (Walker, Reference Walker1990; Sulej, Reference Sulej2010; = ophthalmic artery, Small, Reference Small2002; Clark et al., Reference Clark, Welman, Gauthier and Parrish2010; = transversotrigeminal vein, von Baczko et al., Reference von Baczko, Desojo, Gower, Ridgely, Bona and Witmer2021), and the ventral foramen marks the exit of the trochlear nerve (CN IV). The ventral margin of the laterosphenoid exhibits a well-incised notch for the exit of the of the optic nerve (CN II) anteroventral to the cotylar crest.
Medially, the laterosphenoid exhibits a rugose surface with shallow fossae that are separated by curved ridges (Fig. 3.7). This texture is reminiscent of the contact between the anterior wall of the braincase and the dural envelope, which encompasses the meninges, encephalon, associated nerves, bloods vessels, and sinuses (Witmer et al., Reference Witmer, Ridgely, Dufeau, Semones, Endo and Frey2008). The medial foramina for the exits of the trochlear nerve (CN IV) and anterior cerebral vein are positioned within a dorsoventrally oriented fossa just dorsal of the notch for the optic nerve (CN II) (Fig. 3.7). Dorsally, the capitate process of the laterosphenoid, which contacts the parietal anteroventrally, is short and mediolaterally compressed. The anterior and posterior portions of the laterosphenoid are not preserved. Thus, we are unable to determine if the element participated in the margin of the foramen marking the external exit of the trigeminal nerve (CN V), a feature that is variable across the Aetosauria. The external exit of the trigeminal nerve can be enclosed by both the prootic and laterosphenoid, as observed in the early-branching aetosaur Aetosauroides scagliai (MCP-3450-PV; Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021c) and Desmatosuchus smalli (TTU-P 9420, Small, Reference Small2002; UCMP 27410, von Baczko et al., Reference von Baczko, Desojo, Gower, Ridgely, Bona and Witmer2021), a condition that is shared with the aetosauromorphs Parringtonia gracilis von Huene, Reference von Huene1939 (NMT RB426, Nesbitt et al., Reference Nesbitt, Stocker, Parker, Wood, Sidor and Angielczyk2017, figs. 4, 5) and, hypothetically, Revueltosaurus callenderi (PEFO 34561, Parker et al., Reference Parker, Nesbitt, Irmis, Martz, Marsh, Brown, Stocker and Werning2021, fig. 6e). Alternatively, the foramen can be completely enclosed by the prootic as observed in Scutarx deltatylus (PEFO 34616, Parker, Reference Parker2016b) and Stagonolepis olenkae (ZPAL AbIII/466/17, Sulej, Reference Sulej2010), a condition shared with the rauisuchid Postosuchus kirkpatricki (TTU-P 9002, Weinbaum, Reference Weinbaum2011, fig. 24).
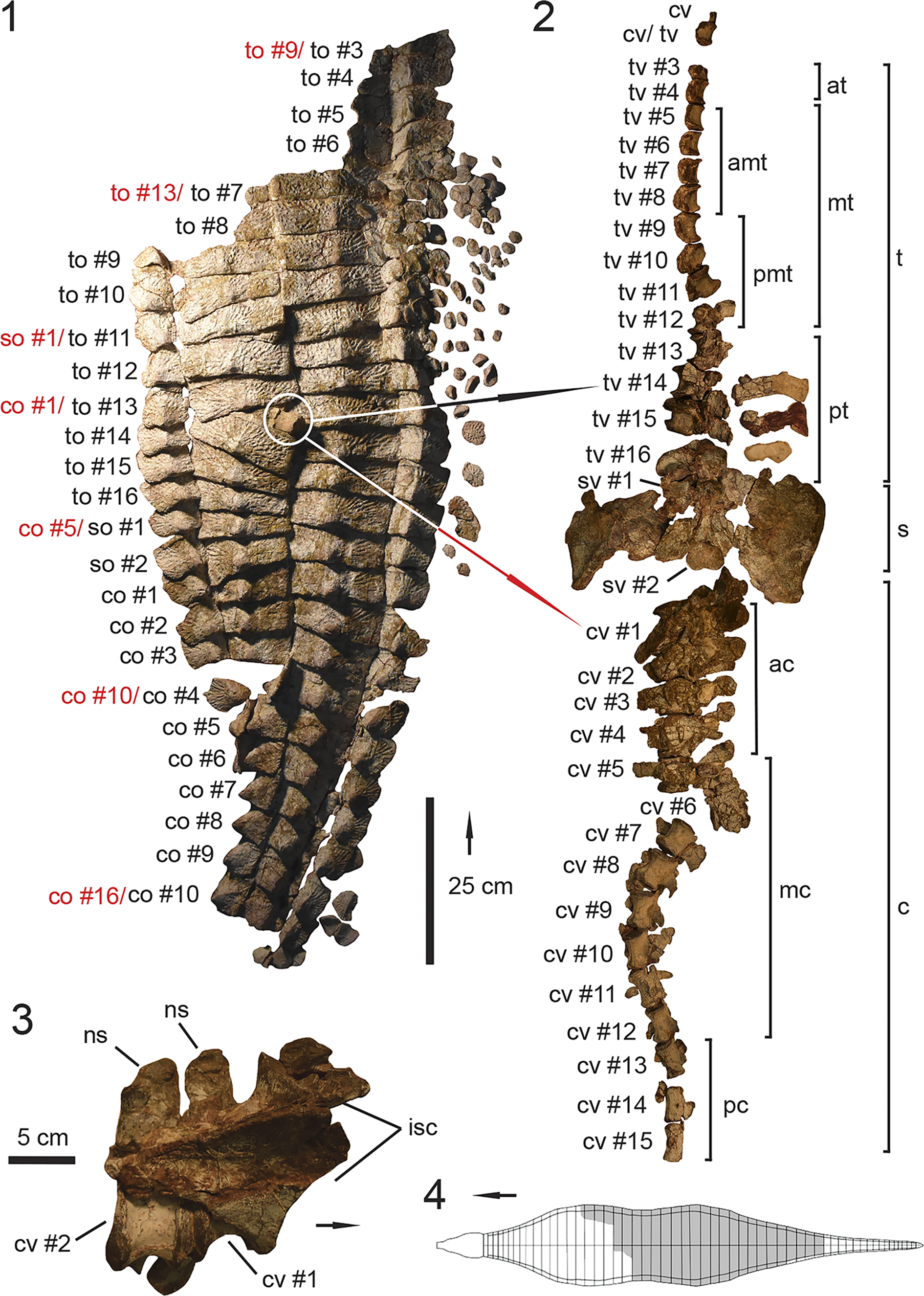
Figure 4. UMMP 13950, holotype specimen of Calyptosuchus wellesi. (1) Articulated dorsal carapace in dorsal view; (2) associated vertebral column with pelvis in dorsal view in approximate anatomical position to the dorsal carapace in (1); (3) anteriormost caudal vertebrae with articulated ischia; (4) schematic of the aetosaurian dorsal carapace in dorsal view, where gray indicates regions preserved in UMMP 13950. Anatomical interpretations based on this study are labeled in black and previous interpretations based on Parker (Reference Parker2018a), following those of Case (Reference Case1932), are labeled in red, arrows also follow color labels, accordingly. ac = anterior caudal region; amt = anterior mid-trunk region; at = anterior trunk region; c = caudal region; co = caudal osteoderm; cv = caudal vertebra; isc = ischia; mc = mid-caudal region; mt = mid-trunk region; ns = neural spine; pc = posterior-caudal region; pmt = posterior mid-trunk region; pt = posterior-trunk region; s = sacral region; so = sacral osteoderm; sv = sacral vertebra; t = trunk region; to = trunk osteoderm; tv = trunk vertebra. Arrows indicate anterior direction.
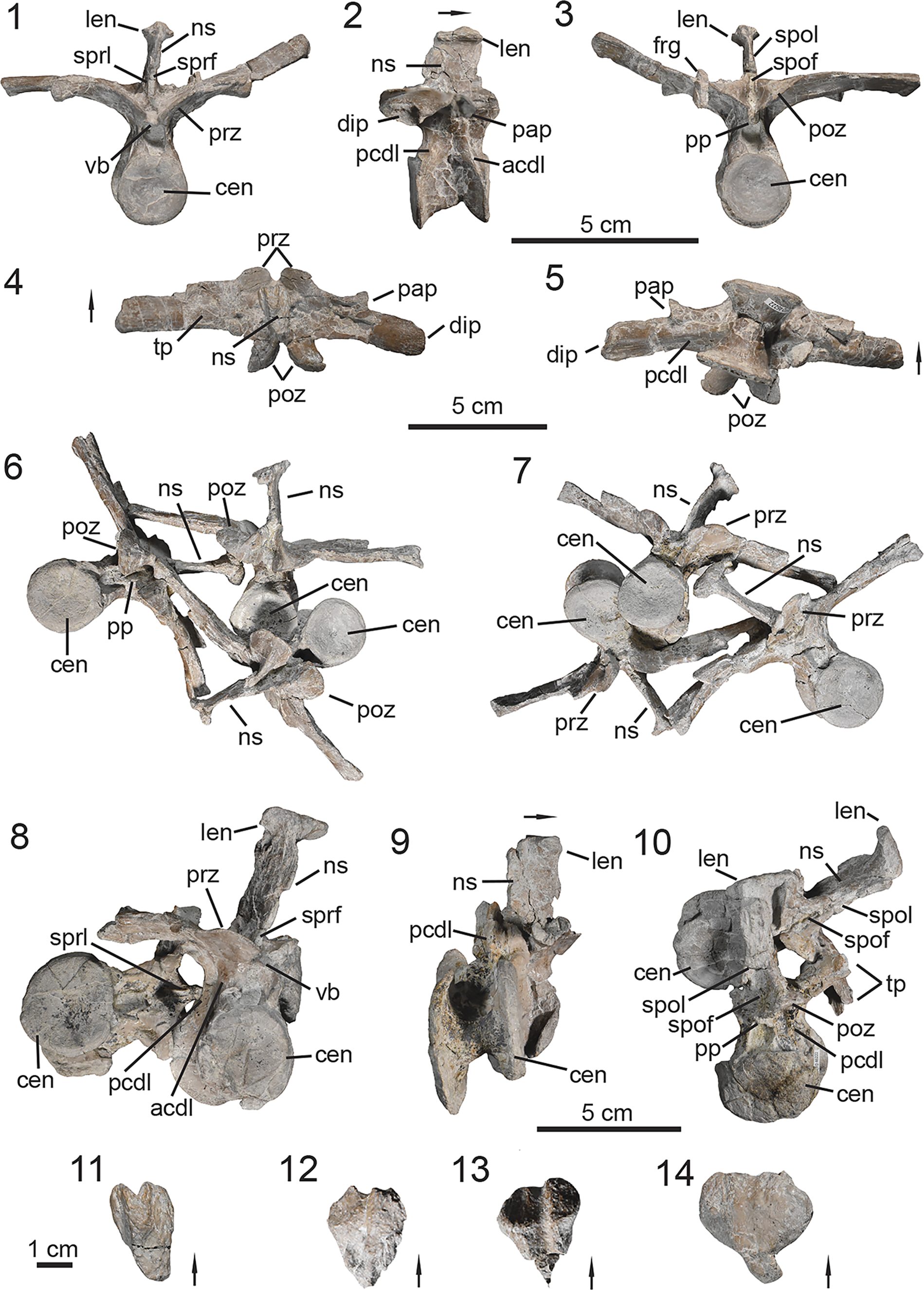
Figure 5. PEFO 46222, associated trunk vertebrae. (1–5, 11) Trunk vertebra #6; (6, 7, 12, 13) trunk vertebrae #10–12; (8–10, 14) trunk vertebrae #15–16; (11–14) lateral expansions of neural spines range. (1, 7, 8) Anterior views, (3, 6, 10) posterior views, (11–14) dorsal views, (5, 9) ventral views, (2, 9) lateral views. acdl = anterior centrodiapophyseal lamina; cen = centrum; dip = diapophysis; frg = bone fragment; len = lateral expansion of neural spine; ns = neural spine; pap = parapophysis; pcdl = posterior centrodiapophyseal lamina; pp = posteriorly projecting process; poz = postzygapophysis; prz = prezygapophysis; spof = spinopostzygapophyseal fossa; spol = spinopostzygapophyseal lamina; sprf = spinoprezygapophyseal fossa; sprl = spinoprezygapophyseal lamina; tp = transverse process; vb = venral bar. Arrows indicate anterior direction.
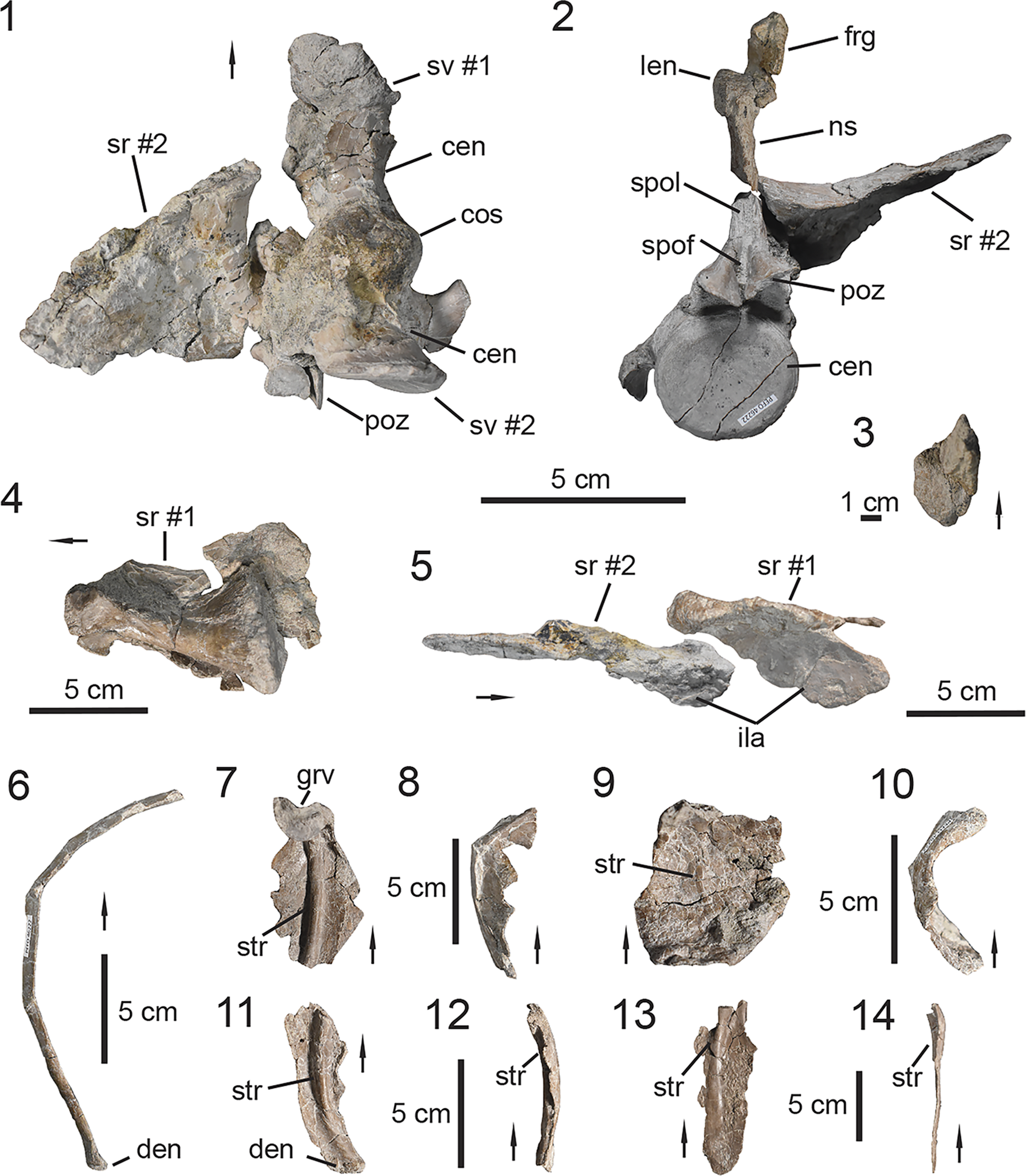
Figure 6. PEFO 46222, associated sacrum and ribs. (1, 2) Co-ossified sacral vertebrae; (3) lateral expansion of sacral vertebra #2 neural spine; (4, 5) sacral ribs; (6–14) trunk ribs. (1, 4) Ventral views, (2) posterior view, (3) dorsal view, (5) lateral/distal view, (7, 9, 11, 13) medial views, (6, 8, 10, 12, 14) anterior/posterior views. cen = centrum; cos = co-ossified sacral centra; den = distal end; frg = bone fragment; grv = groove; ila = ilium articulation; len = lateral expansion of neural spine; ns = neural spine; poz = postzygapophysis; spof = spinopostzygapophyseal fossa; spol = spinopostzygapophyseal lamina; sr = sacral rib; sv = sacral vertebra; str = strut. Arrows indicate anterior direction for vertebrae and proximal direction for ribs.
Mandible description
Prior to the discovery of PEFO 49321, our understanding of the mandible of Calyptosuchus wellesi was based solely on one partially preserved dentary that was associated with an individual of C. wellesi (UCMP 27225, Parker, Reference Parker2018a). PEFO 49321 preserves the posterior portion of the right mandible in articulation (Fig. 3.9, 3.10) and is not coated by the iron-rich mineralization that is present on several other elements from PFV 467, thus allowing the contacts of the elements to be differentiated.
Surangular
The surangular is missing a large portion of its anterodorsal process (Fig. 3.9, 3.10). As typically observed in aetosaurs (Desojo et al., Reference Desojo, Heckert, Martz, Parker, Schoch, Small, Sulej, Nesbitt, Desojo and Irmis2013), the surangular arches posteriorly over the external mandibular fenestra and becomes dorsoventrally tall. The anterior process of the surangular is anteroposteriorly long, with a concave ventral margin that forms the dorsal border of the external mandibular fenestra. The process is mediolaterally compressed in contrast to the condition observed in Revueltosaurus callenderi (Parker et al., Reference Parker, Nesbitt, Irmis, Martz, Marsh, Brown, Stocker and Werning2021) and erpetosuchids (Benton and Walker, Reference Benton and Walker2002) in which it is expanded (= surangular shelf). A fragment of this process preserves a prominent anteroposteriorly broad tubercle on the dorsal margin of the element near the midline of the external mandibular fenestra (Fig. 3.9, 3.10), a feature document in most taxa with a preserved surangular except Aetosaurus ferratus (SMNS 5770, Schoch, Reference Schoch2007), Desmatosuchus smalli (TTU-P 9024, Small, Reference Small2002), and Coahomasuchus kahleorum (TMM 31100-437, Parker, Reference Parker2016a).
The lateral surface is smooth, unlike the ornamented surface observed in Acaenasuchus geoffreyi Long and Murry, Reference Long and Murry1995 (UCMP 293853, Marsh et al., Reference Marsh, Smith, Parker, Irmis and Kligman2020), and it lacks the surangular ridge that is present in Revueltosaurus callenderi (Parker et al., Reference Parker, Nesbitt, Irmis, Martz, Marsh, Brown, Stocker and Werning2021) and other archosauriforms (Ezcurra, Reference Ezcurra2016). As the surangular becomes dorsoventrally broad, a small process descends anteroventrally (Fig. 3.9). Although it is not fully preserved, it likely participated in forming the posteroventral margin of the external mandibular fenestra, as observed in Stagonolepis (Walker, Reference Walker1961; Sulej, Reference Sulej2010). The angular is not preserved, however the surangular indicates that these two elements exhibited an anteroposteriorly long (Fig. 3.10), straight contact as the angular tapered posteriorly similar to that of Longosuchus meadei (TMM 31185-97, Parrish, Reference Parrish1994). Ventrally, the contact wraps medially on the surangular and terminates at the retroarticular process. The posterior portion of the surangular bears a posteroventrally inclined elliptical surangular foramen. This foramen likely transmitted fibers of the inferior alveolar nerve, a derivative of the mandibular branch of the trigeminal nerve (CN V3) that innervates the lower jaw (Desojo and Báez, Reference Desojo and Báez2007; Lessner and Holliday, Reference Lessner and Holliday2020). The surangular foramen exits ventromedially on the medial surangular shelf. Medially, that shelf is anteroposteriorly oriented, dorsoventrally broad, and laterally concave. Posteriorly, the surangular exhibits a dorsoventrally curved contact with the articular in lateral view (Fig. 3.9). The surangular only contributes slightly to the retroarticular process, as observed in Typothorax coccinarum (Reyes et al., Reference Reyes, Parker and Marsh2020).
Articular and prearticular
The right articular and posterior portion of the prearticular are co-ossified in PEFO 49321 (Fig. 3.9, 3.10). The articular is complete. The posterior portion comprises most of the retroarticular process and is anteroposteriorly longer than dorsoventrally tall, as observed in Typothorax coccinarum (Reyes et al., Reference Reyes, Parker and Marsh2020) and Desmatosuchus spurensis (MNA V9300, Parker, Reference Parker2008a). Posterodorsally, the articular exhibits a small dorsally oriented triangular process (Fig. 3.10). The glenoid of the articular is mediolaterally broad for its articulation with the quadrate. The articulation surface for the lateral condyle of the quadrate is concave, mediolaterally compressed, and anteroposteriorly elongate, with a medial inclination in dorsal view. The medial articular surface of the glenoid is concave, circular in dorsal view, and is separated from the lateral articulation surface by a low ridge. Medially, the articular exhibits an anteroposteriorly oriented curved contact with the posterior process of the prearticular (Fig. 3.10). This contact terminates ventral to the glenoid, thus does not extend the entire length of the retroarticular process. Ventrally, the prearticular exhibits an elongate contact with the surangular and contacts the posteriorly tapering process of the angular medially, as observed in Longosuchus meadei (Parrish, Reference Parrish1994).
Dentition description
PEFO 49321 preserves several complete teeth, some of which are still in their respective alveoli within the maxillae (Fig. 3.1, 3.2). Because PEFO 49321 does not preserve the dentary, we interpret the isolated teeth as belonging to the preserved maxillae because they were found in proximity to each other. Additionally, if these teeth were derived from a dentary, they would likely be identical to those of the maxilla because most aetosaurs exhibit homodont dentition except Typothorax coccinarum (Reyes et al., Reference Reyes, Parker and Marsh2020). The isolated and in-situ teeth within the right maxilla are well preserved, being complete and relatively undistorted. The general anatomy of the dentition is best documented by an isolated left maxillary tooth (Fig. 3.11–3.14), which will serve as the main reference for the following description.
Maxillary teeth
The maxillary teeth of PEFO 49321 are bulbous, exhibit thecodont implantation (sensu Bertin et al., Reference Bertin, Thivichon-Prince, LeBlanc, Caldwell and Viriot2018), and are uniform in their anatomy, but become proportionately smaller distally in the tooth row. Unlike Coahoamasuchus kahleorum (TMM 31100-437, Parker, Reference Parker2016a), the base of the teeth are slightly constricted (Fig. 3.13) but not to the extent observed in Desmatosuchus smalli (TTU-P 9420, Small, Reference Small2002) or Neoaetosauroides engaeus (PULR 108, Desojo and Báez, Reference Desojo and Báez2007; Taborda et al., Reference Taborda, Desojo and Dvorkin2021). The mesiodistal width of the teeth is relatively unchanged until halfway between the base and apex. Apical to that point, the distal margin remains straight with a slight mesial inclination until it reaches the apex, while the medial margin curves distally (Fig. 3.11). This results in the crown exhibiting a curved, distally oriented apex. However, the teeth are not truly recurved like those of Aetosauroides scagliai (UFSM 11505, Biacchi Brust et al., Reference Biacchi Brust, Desojo, Schultz, Paes-Neto and Da-Rosa2018; MCN-PV 2347, MCP-3450-PV, Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021b) and Coahomasuchus kahleorum (TMM 31100-437, Parker, Reference Parker2016a) because the apex is not positioned distal to the distal basal margin; instead, it is positioned just mesial to it (Fig. 3.11, 3.12). The curvature of the teeth in PEFO 49321 is most similar to the condition observed in Aetosaurus ferratus (Schoch, Reference Schoch2007). There is no evidence of vertical fluting on the enamel surface of the teeth, which is observed in the dentition of Stagonolepis olenkae (ZPAL AbII/1995, Sulej, Reference Sulej2010) and Stenomyti huangae (DMNH V.60708, Small and Martz, Reference Small, Martz, Nesbitt, Desojo and Irmis2013). At the point that the mesial and distal margins become confluent, the margins exhibit fine serrations (Fig. 3.14) composed of small triangular-shaped denticles similar to those of Stagonolepis olenkae (ZPAL AbII/1995, Sulej, Reference Sulej2010), but contrary to those observed in Revueltosaurus callenderi (PEFO 34561, Parker et al., Reference Parker, Nesbitt, Irmis, Martz, Marsh, Brown, Stocker and Werning2021) and Acaenasuchus geoffreyi (PEFO 43699, Marsh et al., Reference Marsh, Smith, Parker, Irmis and Kligman2020) in which the denticles are large and round. The serrated edge forms the distal margin of the crown. However, on the mesial margin, the serrations originate on the lingual surface and curve onto the mesial margin of the crown. The crown exhibits a lingually oriented concave curvature near the apex similar to that of Aetosauroides scagliai (Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021b). The labial surface of the crown apex is relatively more curved than the lingual surface (Fig. 3.13). In cross-section the teeth are mesiodistally ovate, but not to the extent observed in ziphodont teeth, which are labiolingually compressed. There is no evidence of tooth replacement in this specimen.
Vertebrae description
PEFO 46222 preserves a total of 13 vertebrae. Most of the vertebrae were collected semi-articulated within a 1-m2 quadrant. The trunk vertebrae are poorly preserved in the holotype specimen UMMP 13950, thus the preserved vertebral column of PEFO 46222 provides new morphological understanding of the trunk series in Calyptosuchus wellesi,
Trunk vertebrae
PEFO 46222 preserves a total of 11 trunk vertebrae (= dorsal vertebrae; Parker, Reference Parker2008a; Heckert et al., Reference Heckert, Lucas, Rinehart, Celeskey, Spielmann and Hunt2010; Desojo et al., Reference Desojo, Ezcurra and Kischlat2012, Reference Desojo, Heckert, Martz, Parker, Schoch, Small, Sulej, Nesbitt, Desojo and Irmis2013). The discovery of partial or relatively complete vertebral columns of various aetosaur taxa indicates that on average the trunk series in aetosaurs is composed of 16 vertebrae and exhibits a 1:1 ratio with the dorsal carapace osteoderms (Parker, Reference Parker2008a). The holotype specimen (Fig. 4.2; UMMP 13950) preserves 14 trunk vertebrae (i.e., tv #3–16), but most of the vertebrae (i.e., tv #3–11) are poorly preserved and are only represented by their centra (Fig. 4.2). PEFO 46222 preserves most of the trunk vertebral series (Fig. 5); the most anterior vertebra represents tv #6 from the anterior middle trunk region (Fig. 5.1–5.5). The middle trunk vertebrae (i.e., tv #6–12) are well preserved with little taphonomic distortion, unlike those from the posterior trunk (i.e., tv #13–16), which are missing their respective transverse processes. Thus, PEFO 46222 allows us to assess the anatomy of trunk vertebrae that are missing or poorly preserved in UMMP 13950.
In general, the centra are amphicoelous with circular articular faces. The centra become proportionally larger towards the sacral region and exhibit a more ovate shape in the posteriormost trunk vertebrae (Fig. 5.8–5.10; tv #15–16). The faces of the centra exhibit well-developed rims and are positioned roughly in the same longitudinal plane (Fig. 5.2), unlike Stagonolepis robertsoni (Walker, Reference Walker1961) and Scutarx deltatylus (Parker, Reference Parker2016b) in which the posterior face is more dorsally offset than the anterior one in the more posterior vertebrae. As observed in UMMP 13950, the body of the centrum is transversely compressed and concave with a smooth surface lacking a ventral keel at the midline (Fig. 5.5, 5.9). These features give the centra a spool shape, which is characteristic among aetosaurs (Desojo et al., Reference Desojo, Heckert, Martz, Parker, Schoch, Small, Sulej, Nesbitt, Desojo and Irmis2013). Laterally, the body of the centra are smooth, (Fig. 5.2), as observed in UMMP 13950, UMMP 7470, and several referred vertebrae from UCMP A269 (UCMP 139694, UCMP 139796, UCMP 139702; Parker, Reference Parker2018a). There is no evidence of an ovate fossa on the lateral surface of the centrum in PEFO 46222, which is a feature that is subject to intraspecific variation and can become more developed through skeletal maturity, as observed in Aetosauroides scagliai (Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021c). As observed in UMMP 13950, the trunk centra become more anteroposteriorly compressed in lateral view from the middle trunk to the posterior trunk in which the transverse width of the centrum becomes proportionately larger than the anteroposterior length of the body of the centrum (Fig. 5.2, 5.9). Anteriorly, the neural canal is transversely wider than it is dorsoventrally tall, giving the opening a quadrangular shape (Fig. 5.1). The inverse condition is exhibited by the posterior end of the neural canal in which the opening is dorsoventrally taller than it is transversely wide, giving it an ovate shape (Fig. 5.3).
The trunk vertebrae of PEFO 46222 exhibit both anterior and posterior centrodiapophyseal lamina (sensu Wilson, Reference Wilson1999; = infradiapophyseal lamina, Desojo and Ezcurra, Reference Desojo and Ezcurra2011), as observed in UMMP 7470 (Parker, Reference Parker2018a), Aetosauroides scagliai (Desojo and Ezcurra, Reference Desojo and Ezcurra2011; Paes-Neto et al., Reference Paes-Neto, Desojo, Brust, Schultz, Da-Rosa and Soares2021a), Scutarx deltatylus (Parker, Reference Parker2016b), and Desmatosuchus spurensis (Parker, Reference Parker2008a). This condition is considered plesiomorphic within Archosauromorpha (Ezcurra, Reference Ezcurra2016). In PEFO 46222 the posterior centrodiapophyseal lamina is weakly developed in the middle trunk region (Fig. 5.2) and becomes more prominent in the posterior trunk vertebrae (Fig. 5.8), which also appears to be the case in UMMP 13950. The parapophyses are situated on the transverse process in all the known trunk vertebrae, indicating that no transitional cervical/trunk vertebrae are preserved in PEFO 46222 (Fig. 5.1–5.7). The positioning of the parapophyses on the transverse process is typical of aetosaurians (Desojo et al., Reference Desojo, Heckert, Martz, Parker, Schoch, Small, Sulej, Nesbitt, Desojo and Irmis2013), a condition shared with the trunk vertebrae in extant crocodyliformes such as Alligator mississippiensis (Daudin, Reference Daudin1802) Frey, Reference Frey1988, figs. 6, 7) and Alligator sinensis (Fauvel, Reference Fauvel1879) (Cong et al., Reference Cong, Hou, Wu and Hou1998, fig. 80). In general, the transverse processes project laterally, nearly perpendicular to the neural spine. They are anteroposteriorly broad with flat dorsal surfaces and transversely wide, around 2.0–2.5 times the transverse width of the centrum (Fig. 5.6, 5.7). The posterior trunk vertebrae #15–16 (Fig. 5.8–5.10) do not preserve enough of their transverse processes, so we are not able to confirm whether the ribs of PEFO 46222 are co-ossified to the transverse processes as described in Scutarx deltatylus (PEFO 34045, Parker, Reference Parker2016b), Desmatosuchus spurensis (MNA V9300, Parker, Reference Parker2008a), and the holotype of Calyptosuchus wellesi (UMMP 13950, Case, Reference Case1932; Long and Murry, Reference Long and Murry1995; Parker, Reference Parker2018a). The postzygadiapophyseal lamina has deep associated fossae on the dorsal and ventral surfaces of the transverse process in the middle trunk vertebrae of UMMP 7470 (Parker, Reference Parker2018a, fig. 7). However, postzygadiapophyseal lamina is absent in the trunk vertebrae of PEFO 46222 and there is no clear evidence of its presence in the holotype specimen (UMMP 13950) due to the poor preservation of the trunk vertebrae.
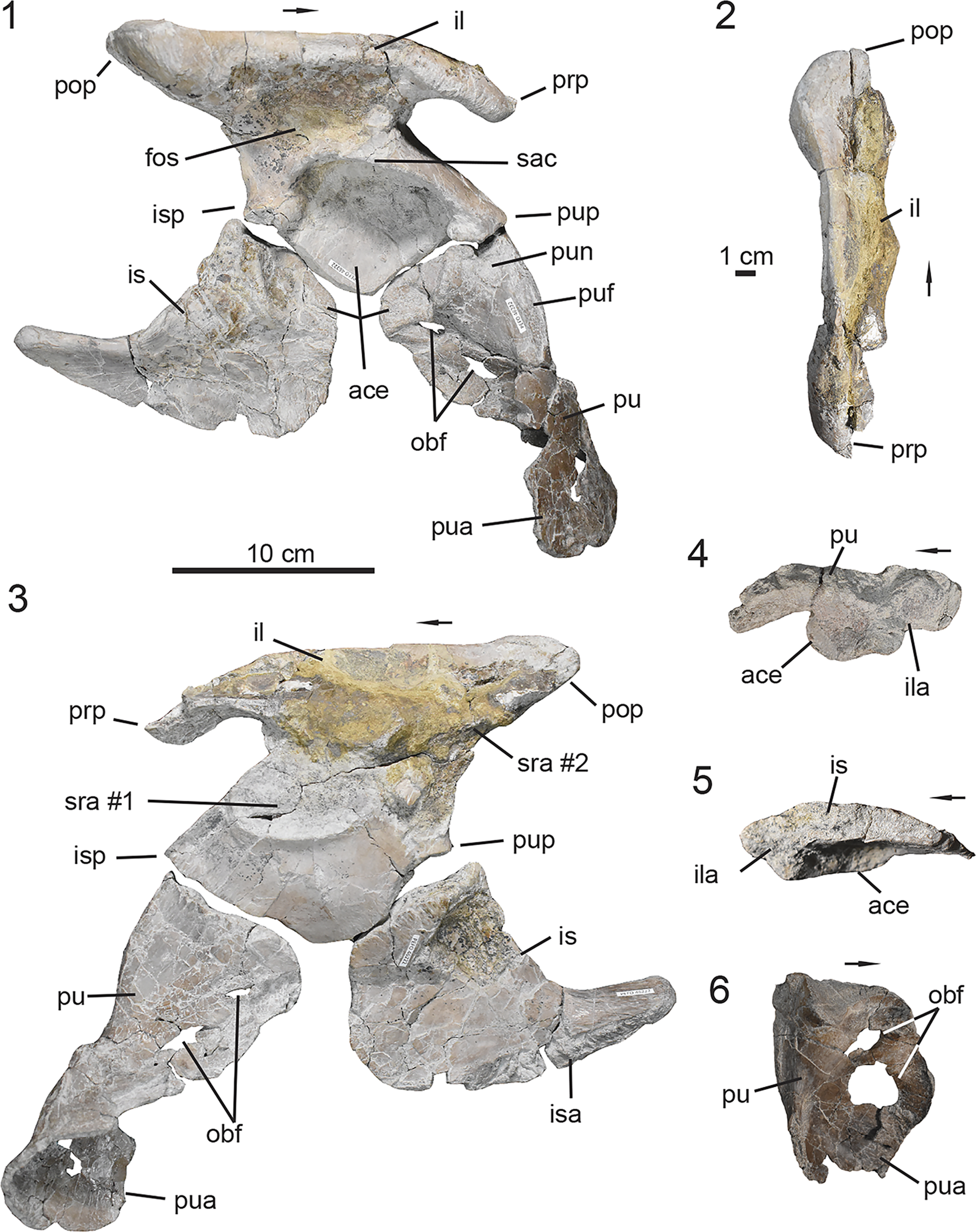
Figure 7. PEFO 46222, associated pelvic elements. (1–5) Right half of disarticulated pelvic girdle; (6) left pubis. (1, 6) Lateral views, (3) medial view, (2) dorsal view, (4, 5) proximal views. ace = acetabulum; fos = fossa; il = ilium; ila = ilium articulation; is = ischium; isa = ischium articulation; isp = ischiac peduncle; obf = obturator foramina; prp = preacetabular process; pop = postacetabular process; pu = pubis; pua = pubic apron; puf = pubic fossa; pun = pubic notch; pup = pubic peduncle; sac = supraacetabular crest; sra = sacral rib articulation. Arrows indicate anterior direction.
The vertebrae of PEFO 46222 lack hyposphene-hypantrum accessory articulations similar to other specimens of Calyptosuchus wellesi (Parker, Reference Parker2018a). Hyposphene-hypantrum accessory articulations are documented within Desmatosuchus (Parker, Reference Parker2005, Reference Parker2008a) and Aetobarbakinoides brasiliensis (Desojo et al., Reference Desojo, Ezcurra and Kischlat2012). The prezygapophyses are inclined ~40° from horizontal and are connected at the midline by a small transversely flat shelf (= ventral bar, Parker, Reference Parker2018a). This shelf marks the floor of the spinoprezygapophyseal fossa (sensu Wilson et al., Reference Wilson, D’Emic, Ikejiri, Moacdieh and Whitlock2011) at the base of the neural spine (Fig. 5.1). The postzygapophyses are also inclined ~40° from the horizontal and project slightly beyond the posterior face of the centrum. At the midline between the postzygapophyses there is a posteriorly oriented projection that rests on top of the ventral bar of the succeeding vertebra as in Scutarx deltatylus (Parker, Reference Parker2016b). Additionally, there is a deep spinopostzygapophyseal fossa (sensu Wilson et al., Reference Wilson, D’Emic, Ikejiri, Moacdieh and Whitlock2011) between the postzygapophyses at the base of the neural spine, dorsal to the posteriorly oriented process.
The main body of the neural spine is transversely compressed and anteroposteriorly broad. It becomes proportionally broader sequentially in the vertebral column until it surpasses the anteroposterior length of the centra in trunk vertebrae #15–16 (Fig. 5.8–5.10). In the middle trunk region, the neural spines are 1.41–1.68 times taller dorsoventrally than the centrum (Fig. 5.1–5.7), while in the posterior trunk region they are 1.30–1.33 times taller. The neural spine exhibits both spinoprezygapophyseal (Fig. 5.1, 5.8) and spinopostzygapophyseal laminae (Fig. 5.3, 5.10), as observed in UMMP 7470 (Parker, Reference Parker2018a) and UMMP 13950. Dorsally, the neural spine expands into a transversely broad and flat table-like structure. In dorsal view the ‘table’ exhibits a heart-shaped outline with the tapered end pointing posteriorly. The transverse width of the tops of the neural spines proportionately increase relative to the transverse width of the centrum down the vertebral column (Fig. 5.11–5.14). Overall, the trunk vertebrae become more robust towards the sacrum.
Sacral vertebrae
The previously known sacral anatomy of Calyptosuchus wellesi is based on UMMP 7470 and UMMP 13950, although the elements are distorted in the latter specimen (Case, Reference Case1922, Reference Case1929, Reference Case1932). The sacral vertebrae of PEFO 46222 are not well preserved (Fig. 6.1) and were recovered in series with the posterior trunk vertebrae (Figs. 5.8–5.10, S1). The body of the centrum is crushed and distorted anteriorly in sacral vertebra #1 (Fig. 6.1), but its anterior face is still mostly intact. The slightly ovate anterior face of sacral vertebra #1 is completely exposed and disarticulated from the posteriormost trunk vertebra (Fig. 5.8–5.10, S1). The neural arch of the anterior sacral vertebra is not preserved, and only a disarticulated sacral rib remains (Fig. 6.4, described below).
Sacral vertebra #2 of PEFO 46222 (Fig. 6.1, 6.2) is robust and well preserved, preserving the neural arch with an articulated sacral rib (described below) and a relatively uncrushed centrum with a slightly ovate articular face. Additionally, the centrum body is anteroposteriorly longer than the transverse width of the articular surface, unlike the more truncated posterior trunk vertebrae (Fig. 5.8, 5.9). The prezygapophyses are not preserved but the postzygapophyses are inclined approximately 30° from the horizontal and project slightly beyond the posterior face of the centrum (Fig. 6.1, 6.2). No posteriorly oriented ventral projection is discernable at the midline of the postzygapophyses. The neural spine is robust and exhibits a posterior spinopostzygapophyseal lamina and spinopostzygapophyseal fossa at its base (Fig. 6.2; sensu Wilson et al., Reference Wilson, D’Emic, Ikejiri, Moacdieh and Whitlock2011). Although the distal half of the neural spine is broken into a separate piece, when articulated the neural spine is 1.6 times taller than the centrum, a proportion similar to those of some middle trunk vertebrae. The dorsal transverse expansion of the neural spine (Fig. 6.3) is broad, robust, and more ovate than those in the trunk vertebrae, with a transverse width that is half that of the articular face of the centrum.
The centrum of sacral vertebra #1 (Fig. 6.1) is completely disarticulated from that of the posteriormost trunk vertebra (i.e., tv #16) (Fig. 5.8–5.10) in PEFO 46222, which is also observed in UMMP 13950 and UMMP 7470 (Case, Reference Case1932; Parker, Reference Parker2018a). This indicates that these two vertebrae were not co-ossified to each other in PEFO 46222. Co-ossification of the posterior trunk vertebra with the first sacral vertebra (= dorsosacral, Griffin et al., 2017) has only been reported in taxa within the more inclusive clade Desmatosuchini (Long and Murry, Reference Long and Murry1995; Parker, Reference Parker2008a, Reference Parker2016a; Nesbitt, Reference Nesbitt2011; Griffin et al., Reference Griffin, Stefanic, Parker, Hungerbühler and Stocker2017). Additionally, incorporation of the posteriormost trunk vertebra into the sacrum does not occur in the early-branching aetosaur Aetosauroides scagliai (Casamiquela, Reference Casamiquela1967; Heckert and Lucas, Reference Heckert and Lucas2002a), which suggests that the incorporation of the dorsosacral in desmatosuchins is a derived state. In PEFO 46222 the two sacral vertebrae are ankylosed at the centra faces (Fig. 6.1). Among aetosaurs, co-ossification of the sacral vertebrae has only been documented in Desmatosuchus smalli (TTU-P 9419, Parker, Reference Parker2005), Desmatosuchus spurensis (MNA V9300, TTU-P 10008, Long and Murry, Reference Long and Murry1995; Parker, Reference Parker2008a), and Longosuchus meadei (TMM 31100-236).
Rib description
PEFO 46222 preserves several ribs from the same anatomical regions as the preserved vertebrae. Most of the ribs are disarticulated, however no trunk ribs are complete.
Trunk ribs
Several trunk ribs were intermixed with the semi-articulated trunk vertebrae recovered with PEFO 46222. The ribs are neither complete nor do they preserve the capitulum or tuberculum. We describe the general anatomy of the trunk ribs of Calyptosuchus wellesi based on PEFO 46222 and UMMP 13950. Proximally, the capitulum and tuberculum are differentiated by a transversely oriented shallow groove in dorsolateral view (Fig. 6.7). In proximal cross-section, the bone thickens dorsoventrally just anterior to the groove. Based on comparisons to Scutarx deltatylus (PEFO 34045, Parker, Reference Parker2016b), this thickening is associated with the capitulum. The ribs flare out transversely from the transverse process, gradually descend ventrally, and arch more medially towards the distal end until they terminate at an ovate tip (Fig. 6.6). We note that there is one rib fragment with a strongly arched proximal end (Fig. 6.10). This suggests that the ribs may become strongly arched posteriorly in the trunk, as observed in Typothorax coccinarum (PEFO 42506, Parker et al., Reference Parker, Reyes and Marsh2023), however we cannot confirm this based on the preserved material of PEFO 46222.
The ribs are anteroposteriorly broad proximally with a compressed dorsolateral surface throughout the shaft. Ventromedially, there is variation between the preserved rib fragments near the proximal end. In the more complete fragment, the medial surface exhibits a well-developed strut near the proximal end with thin, gracile flanges extending towards the margins (Fig. 6.7). It is evident that the flanges and strut are confluent near the distal end of the rib, where the surface is flat, and the rib body becomes mediolaterally compressed (Fig. 6.11–6.14). This thin flaring of the bone near the proximal end of the rib is more pronounced in other fragments (Fig. 6.9, 6.10), suggesting that this happens sequentially towards the posterior region of the trunk. This condition is also observed in UMMP 13950 and is documented in Desmatosuchus spurensis, where the trunk (= dorsal) ribs become more expanded and thinner near the proximal end in the more posteriorly positioned trunk ribs (Parker, Reference Parker2008a).
Sacral ribs
The anatomy of the sacral ribs in Calyptosuchus wellesi was briefly described by Case (Reference Case1932) and Parker (Reference Parker2018a), however PEFO 46222 preserves both sacral ribs, allowing for a more thorough description of their anatomy. The right sacral rib of sacral vertebra #1 (= anterior) is preserved but not attached to the centrum (Fig. 6.4, 6.5), but the left sacral rib of sacral vertebra #2 (= posterior) is still in articulation. Based on the articulated sacral rib, the sacral ribs are not shared between the two sacral vertebrae, but rather they are each restricted to a single vertebra (Nesbitt, Reference Nesbitt2011, character 208). Because of poor preservation, we cannot determine how the anterior sacral rib contacts the main body of an anterior sacral vertebra. However, based on the in-situ articulated sacral rib and UMMP 7470, the ribs were attached dorsolaterally on the centrum and partially on the ventral portion of the neural spine (Fig. 6.1). The anterior sacral rib was damaged during excavation, resulting in partial loss of the thin flange near the proximal end (Fig. 6.4; PEFO 46222). In dorsal view, the surface is flat and dorsoventrally broad. In ventral view, there is a well-developed and robust strut running the transverse width of the rib (Fig. 6.4). The strut is concave, giving the rib a strongly bowed appearance that opens ventrally in anterior/posterior view. In lateral view there is a deep groove that separates the ventral strut from the anteroposteriorly expanded dorsal sheet of bone (Fig. 6.5). Distally, the anterior sacral rib (for sacral #1) exhibits a transversely ovate articulation surface that is dorsoventrally thickened (Fig. 6.5). Thus, the anterior sacral rib has a large, robust contact with the medial surface of the ilium. This contact occurs just dorsal to the pubic peduncle, ventral to the preacetabular process, and is slightly shared with the posterior sacral rib. Additionally, there is a thin sheet of bone that dorsally overhangs the articulation surface and contacts the ilium (Fig. 6.4).
The left posterior sacral rib is complete and well preserved. The rib is anteroposteriorly expanded distally giving it a subtriangular shape in dorsal/ventral view (Fig. 6.1). Additionally, it is dorsoventrally thickest anteriorly and gradually thins posteriorly (Fig. 6.5), as seen in UMMP 7470 (Case, Reference Case1929). In the distal cross-section, the articulation surface is dorsoventrally thick anteriorly, where it partially shares the articulation surface of the anterior sacral rib (Fig. 6.5). This was described and well figured by Walker (Reference Walker1961, fig. 9) for Stagonolepis robertsoni. Because the posterior sacral rib becomes anteroposteriorly expanded distally, it exhibits a broad articulation with the ilium. This articulation extends posterodorsally on the medial surface of the ilium, terminating ventral to the postacetabular process. The posterior sacral rib is not bowed like the anterior sacral rib, nor does it exhibit a well-developed strut in ventral view. Overall, the anatomy of the sacral ribs and their contact with the ilium in Calyptosuchus wellesi resembles that of Stagonolepis robertsoni (Walker, Reference Walker1961, fig. 9).
Pelvis description
PEFO 46222 preserves most of the pelvis, except the proximal portion of the left pubis. All the elements are disarticulated. Most of them were collected next to the associated sacral vertebrae.
Ilium
PEFO 46222 preserves both ilia. The right ilium is well preserved and serves as the main reference for the anatomical description of the element (Fig. 7). The left ilium is partly preserved and was accidently damaged during excavation (i.e., found unexpectedly with a pickaxe while removing overburden). This resulted in the acetabular rim, ischiac peduncle, and part of the postacetabular process to be broken, and the breaks are delineated by fresh surfaces. The preacetabular process extends anteriorly but not beyond the anterior margin of the pubic peduncle, similar to the condition in Scutarx deltatylus (Parker, Reference Parker2016b) and UMMP 7470 (Case, Reference Case1929). In the referred ilium UCMP 25941 from UCMP A269, the preacetabular process is positioned just dorsal to the pubic peduncle (Long and Murry, Reference Long and Murry1995; Parker, Reference Parker2018a), unlike the preacetabular process of Neoaetosauroides engaeus, which extends far anteriorly of the pubic peduncle (Desojo and Báez, Reference Desojo and Báez2005). The preacetabular process is a finger-like projection that hooks anteroventrally (Fig. 7.1). This resembles that of UMMP 7470 (Case, Reference Case1929) but is unlike that of UCMP 25941, the specimen from UCMP A269 (Long and Murry, Reference Long and Murry1995; Parker, Reference Parker2018a), which is more triangular and robust in lateral view. The finger-like anatomy of PEFO 46222 is also shared with the early-branching Aetosauroides scagliai (PVL 2073, Casamiquela, Reference Casamiquela1967; Heckert and Lucas, Reference Heckert and Lucas2002a), Aetosaurus ferratus (Schoch, Reference Schoch2007), and ?Lucasuchus hunti Long and Murry, Reference Long and Murry1995 (TMM 31100-1). In lateral view, the outline of the ilium between the preacetabular process and pubic peduncle is U-shaped (Fig. 7.1), as seen in UMMP 7470 (Case, Reference Case1929), but unlike that of UCMP 25941, which is more V-shaped. Although crushed, the ilium in the holotype specimen of Calyptosuchus wellesi (UMMP 13950, Case, Reference Case1932; Parker, Reference Parker2018a) exhibits a reminiscent U-shape outline between the pubic peduncle and preacetabular process (Parker, Reference Parker2018a, fig. 9).
Anteroposteriorly, the dorsal margin of the iliac blade ascends gradually from the preacetabular process to the postacetabular process and becomes slightly concave dorsal to the iliac body. The dorsal surface of the blade is rugose (Fig. 7.1, 7.2), presumably marking the attachment of the M. iliotibialis (Romer, Reference Romer1956; Hutchinson, Reference Hutchinson2001; Schachner et al., Reference Schachner, Manning and Dodson2011, Reference Schachner, Irmis, Huttenlocker, Sanders, Cieri and Nesbitt2019), although it is not as well defined as seen in UCMP 25941 (Long and Murry, Reference Long and Murry1995; Parker, Reference Parker2018a). In dorsal view, the iliac blade is mediolaterally compressed and becomes expanded posteriorly (= squared off, Parker, Reference Parker2016a, Reference Parker2018a) at the postacetabular process (Fig. 7.2), as observed in UMMP 13950 and UMMP 7470. As typically seen in aetosaurs, the postacetabular process extends well beyond the posterior margin of the ishiac peduncle. In lateral view, the postacetabular process is knob-like with the ‘neck’ exhibiting a concave posterior margin between it and the acetabulum, a condition shared with UMMP 7470 (Case, Reference Case1929) and UCMP 25941 (Long and Murry, Reference Long and Murry1995; Parker, Reference Parker2018a). The acetabulum is deeply concave and broad, being anteroposteriorly longer than it is dorsoventrally tall (Fig. 7.1), but not to the extent observed in UCMP 25941 (Parker, Reference Parker2018a). As observed in UMMP 7470, the acetabulum of PEFO 46222 would have faced ventrolaterally due to the articulation between the ilium and sacral vertebrae (Parker, Reference Parker2018a).
The ilium exhibits a well-developed supraacetabular crest, similar to that of Scutarx deltatylus (PEFO 31217, Parker, Reference Parker2016b), Stagonolepis (NHMUK PV R4789, Walker, Reference Walker1961; UOPB00150, Desmet et al., Reference Desmet, Antczak and Bodzioch2022), and Aetosauroides scagliai (PVL 2073, Casamiquela, Reference Casamiquela1967; Heckert and Lucas, Reference Heckert and Lucas2002a). Just dorsal to the supraacetabular crest, the body of the ilium exhibits a shallow fossa (Fig. 7.1), as observed in UMMP 13950. However, it is not deep as described in Scutarx deltatylus (Parker, Reference Parker2016b). This region is partially distorted in UMMP 7470. Ventrally, the margins of the ilium form a V-shaped ventrally projecting point at the midline of the acetabulum. The pubic peduncle is mediolaterally expanded with a flat surface in anterior view and exhibits a comma-shaped outline in ventral view. In contrast, the ishiac peduncle is also comma-shaped in ventral view but exhibits a sharp ridge in posterior view due to it being mediolaterally compressed. The medial surface exhibits prominent scars/attachment sites for the sacral ribs (Figs. 6.5, 7.3). There is a large ovate surface medial to the acetabulum, just dorsal to the pubic peduncle, which represents the attachment site of the anterior sacral rib. Posterodorsal to this ovate surface there is a crest that terminates at the postacetabular process. This serves as the main attachment site for the transversely expanded posterior sacral rib.
Pubis
The right pubis of PEFO 46222 is mostly complete although it is slightly crushed (Fig. 7.1). In contrast, the left pubis preserves the proximal two-thirds of the element (Fig. 7.6) but is not as crushed as the right pubis. Together, the preservation of the pubes allows for a description of the entire element, which is only proximally preserved in UMMP 7470 and partially obscured in UMMP 13950. The pubis of PEFO 46222 exhibits a dorsoventral height that is less than the anteroposterior length of the iliac blade as observed in UMMP 13950, but unlike the condition exhibited by Desmatosuchus spurensis (MNA V9300, Parker, Reference Parker2008a) and ?Lucasuchus hunti (TMM 31100-313, Long and Murry, Reference Long and Murry1995), in which the height of the pubis exceeds the length of the ilium. Proximally, the pubis exhibits a comma-shaped surface that matches the contact surface of the pubic peduncle on the ilium (Fig. 7.4). This is also the case in the referred pubis of Calyptosuchus wellesi (UCMP 23195, Parker, Reference Parker2018a) from UCMP A269. More posteriorly, this surface expands ventrally for its contact with the anterior margin of the ischium. The acetabular portion of the pubis is strongly concave with a well-developed rim (Fig. 7.1), as seen in UCMP 23195 (Parker, Reference Parker2018a) and UMMP 7470 (Case, Reference Case1929). Proximally, the shaft of the pubis is robust anteriorly and becomes more gracile posteriorly where it composes part of the pubic apron, as observed in UMMP 13950. On the lateral surface, the pubis exhibits a deep notch that originates just anterodorsal to the acetabular rim and runs anteroventrally on the anterior surface of the shaft as described in UMMP 7470 (Case, Reference Case1929; Parker, Reference Parker2018a). There is a shallow fossa on the shaft posterior to that groove that extends parallel to the anterior margin.
The surface is smooth on the pubic apron portion of the element and exhibits faint rugosities near its medial margin (Fig. 7.3). This rugose surface presumably marks the attachment site of the M. puboischiofemoralis externus 1–3 (Hutchinson, Reference Hutchinson2001; Schachner et al., Reference Schachner, Manning and Dodson2011). The perforate region of the pubic apron is often poorly preserved or obscured from view in taxa with a preserved pubis (i.e., Aetosauroides scagliai, Casamiquela, Reference Casamiquela1967; Heckert and Lucas, Reference Heckert and Lucas2002a; ?Lucasuchus hunti Long and Murry, Reference Long and Murry1995; Aetosaurus ferratus, Schoch, Reference Schoch2007; Desmatosuchus spurensis, Parker, Reference Parker2008a; Typothorax coccinarum, Heckert et al., Reference Heckert, Lucas, Rinehart, Celeskey, Spielmann and Hunt2010; Stenomyti huangae Small and Martz, Reference Small, Martz, Nesbitt, Desojo and Irmis2013), fortunately this region is well preserved in PEFO 46222. It is evident that the pubis in PEFO 46222 is perforated by two foramina (Fig. 7.1, 7.2, 7.6).
Currently, the presence of two foramina on the pubic apron in aetosaurs has only been unambiguously described in Scutarx deltatylus (PEFO 31217, Parker, Reference Parker2016b) and Stagonolepis robertsoni (NHMUK R4793, MCZD 4, Walker, Reference Walker1961). This is unlike the condition observed in the aetosaurs Neoaetosauroides engaeus (PVL 3525, Desojo and Báez, Reference Desojo and Báez2005) and Desmatosuchus smalli (TTU-P 9419, Martz, Reference Martz2008) where the pubis is perforated by only one foramen, the obturator foramen. In life, the single obturator foramen conveys the obturator nerve and associated blood vessels that partially innervate and vascularize the hindlimb (Romer, Reference Romer1956; Hutchinson, Reference Hutchinson2001; Claessens and Vickaryous, Reference Claessens and Vickaryous2012).
In PEFO 46222 the dorsally positioned foramen is ovate with a dorsomedial inclination, whereas the ventral foramen is significantly larger and semicircular (Fig. 7.6). The two foramina are separated by a thin plate of bone. Ventral to the foramina, the pubic shaft twists medially, and becomes broad and mediolaterally compressed with a similar thickness as the dorsal pubic apron portion (Fig. 7.1). Distally, the pubis exhibits a curved, mediolaterally compressed ventral margin. It lacks the distal expansion (= ‘pubic boot’, Gauthier, Reference Gauthier1986; Nesbitt, Reference Nesbitt2011) present in Desmatosuchus spurensis (MNA V9300, Parker, Reference Parker2008a), Typothorax coccinarum (TTU P-9214, Martz, Reference Martz2002), and Aetosauroides scagliai (PVL 2073, Casamiquela, Reference Casamiquela1967; Heckert and Lucas, Reference Heckert and Lucas2002a).
Ischium
The left ischium of PEFO 46222 is well preserved with few signs of taphonomic distortion, unlike its right counterpart, which is slightly crushed with a distal process that is preserved as a separate piece. The element is anteroposteriorly longer than it is dorsoventrally tall. The main body of the ischium is broad and thickest mediolaterally near the acetabulum. Proximally, the ischium exhibits a comma-shaped cross-section (Fig. 7.5) matching that of the ishiac peduncle on the ilium. Unlike the pubis, the acetabulum is concave and shallow (Fig. 7.1). The acetabular rim is prominent near the proximal contact with the ilium (Fig. 7.5). Posteroventrally, the ischium tapers to a blunt, posteriorly oriented process unlike that of Desmatosuchus spurensis (Parker, Reference Parker2008a) in which the posterior process hooks ventrally. In lateral view, it exhibits a concave posterior margin between its contact with the ilium and posterior process. The ischium exhibits a uniform mediolateral thickness posteroventral to the acetabulum. This is unlike the referred ischia of Calyptosuchus wellesi (UCMP 32153, UCMP 32148, Long and Murry, Reference Long and Murry1995; Parker, Reference Parker2018a) from UCMP A269, which exhibit a ‘rod-like’ body posteriorly and fan out ventrally becoming more gracile.
Laterally, the expanded surface is generally flat and smooth but becomes more rugose near the anteroposteriorly straight ventral margin (Fig. 7.1). This rugose texture presumably marks the attachment site of M. puboischiofemoralis externus 3 (Hutchinson, Reference Hutchinson2001; Schachner et al., Reference Schachner, Manning and Dodson2011). The medial contact between the ischia is well exposed; this contact runs the length of the ventral margin and is composed of a series of inclined ridges and grooves, indicating a strong interlocking contact between the ischia (Fig. 7.3). Previously, the presence of a notch was described on the anterior margin of the ischium in the referred specimens UCMP 32148 and UMMP 7470 (Parker, Reference Parker2018a), however those notches are artifacts of preservation.
Carapace description
PEFO 46222 preserves approximately 30 osteoderms that are from various regions of the body. The majority of the osteoderms are well preserved, although some exhibit taphonomic distortion because of crushing or diagenetic cementation at PFV 456. The articulated carapace of the holotype specimen of Calyptosuchus wellesi (Fig. 4.1; UMMP 13950, Case, Reference Case1932) allows us to determine the relative anatomical position of the osteoderms recovered with PEFO 46222. Our recent examination of the carapace of UMMP 13950 indicates that previous interpretations by Parker (Reference Parker2018a), which followed those by Case (Reference Case1932), are offset. Originally, Case (Reference Case1932, p. 64) interpreted the neural spine protruding through the dorsal carapace as belonging to that of the first caudal vertebra (cv #1) and designated the carapacial row sequence based on that landmark (Fig. 4.1, 4.2). Our re-examination of the associated vertebral column indicates that this is not the case because the neural spines are complete and articulated from the 15th trunk vertebra through the mid-caudal region (Fig. 4.1–4.3). These observations, as well as the morphology and measurements (i.e., anteroposterior length, transverse width) of the lateral projections on the top of the neural spines, indicate that the neural spine protruding through the carapace belongs to the 14th trunk vertebra (Fig. 4.1–4.3). Thus, the carapace of UMMP 13950 preserves more of the anterior trunk region than previously recognized (Fig. 4.1, 4.4), which correlates with the associated vertebral column (Fig. 4.2).
Thanks to this new understanding we can compare PEFO 46222 to UMMP 13950 and assess the morphological variation of the osteoderms across the dorsal carapace with a higher fidelity. Previous studies (e.g., Case, Reference Case1932; Long and Ballew, Reference Long, Ballew, Colbert and Johnson1985; Long and Murry, Reference Long and Murry1995; Parker, Reference Parker2018a) were limited in their interpretations of the morphological variation of the dorsal carapace because of the articulated state of the holotype specimen, which obscures several anatomical features (i.e., anterior bar, ventral surface, lateral edge, flexure), or because of the ambiguity in determining which disarticulated elements were associated with specific individuals, which is the case for the referred elements from UCMP A269 (Parker, Reference Parker2018a). The associated carapace of PEFO 46222 provides important insight into the positional morphological variation of the osteoderms within a single individual of Calyptosuchus wellesi.
Paramedian osteoderm description
PEFO 46222 preserves several complete paramedian osteoderms. This includes four individual right paramedian osteoderms that are imbricated and diagenetically cemented to each other in an articulated sequence. These imbricated osteoderms were collected adjacent to the posterior trunk vertebrae and partially diagenetically cemented to the medial surface of the right ilium. Counting from the 14th trunk osteoderm row, as indicated by the protruding 14th vertebra, it is evident that the articulated carapace of the holotype specimen UMMP 13950 (Fig. 4.1; Case, Reference Case1932) preserves osteoderms in anatomical sequence (Fig. 4.1) from the anterior trunk (to #3) through middle caudal (co #10) region. Case (Reference Case1932, p. 61) noted that the transverse width of the paramedian osteoderms decreases posteriorly on the carapace of UMMP 13950, and we agree with that observation. As noted by Case (Reference Case1932) and Parker (Reference Parker2018a), the dorsal eminence becomes more robust and well developed posteriorly along the dorsal carapace. We note that the anatomy of the lateral margin becomes less sigmoidal into the caudal region. Because PEFO 46222 preserves a similar region of the body as the holotype, we use these noted features of the carapace in UMMP 13950 as proxies to position the paramedian osteoderms of PEFO 46222 in a relative sequence. Additionally, several paramedian osteoderms articulate with some of the isolated lateral osteoderms. The holotype specimen of Calyptosuchus wellesi (UMMP 13950) indicates that the anatomy of the lateral osteoderms varies significantly between the regions of the dorsal carapace (Figs. 1, 4; Parker, Reference Parker2018a). Thus, we used the anatomy of the lateral osteoderms as another proxy for determining the regional differentiation to which the isolated paramedian osteoderms are referable.
Middle trunk region
The middle trunk region encompasses trunk vertebrae and osteoderm rows numbering 5–12 (Figs. 1, 4). It is further subdivided into the anterior middle trunk region, encompassing rows 5–8 (Figs. 1, 4), and the posterior middle trunk region, which encompasses rows 9–12 (Figs. 1, 4). In PEFO 46222 the middle trunk region is composed of several complete paramedian osteoderms (Fig. 8) with three associated lateral osteoderms, which are characterized by a ‘wing’-like lateral flange. In dorsal view, the plates are rectangular with some exhibiting a slight anterolateral curvature. The paramedian osteoderms of this region exhibit the largest width-to-length ratios (W:L) and anteroposterior length of any of the preserved paramedian osteoderms, with an average W:L of 2.7 and anteroposterior length of 8.5 cm. In posterior view and lateral to the dorsal eminence, the paramedian osteoderms exhibit varying degrees of ventral arching at the center of ossification, causing the bone surface to be dorsally convex (Fig. 8.3, 8.4). This is exemplified by a right paramedian osteoderm that is strongly arched (Fig. 8.4), similar to an isolated osteoderm of Adamanasuchus eisenhardtae (PEFO 34638, Lucas et al., Reference Lucas, Hunt, Spielmann, Lucas and Spielmann2007a, fig. 3). This is likely associated with constriction of the trunk into a gracile ‘waist’ (Desojo et al., Reference Desojo, Heckert, Martz, Parker, Schoch, Small, Sulej, Nesbitt, Desojo and Irmis2013), which occurs anterior to the sacral region in the middle to posterior trunk transition and has been documented in aetosaurs such as Coahomasuchus kahleorum (Heckert and Lucas, Reference Heckert and Lucas1999).
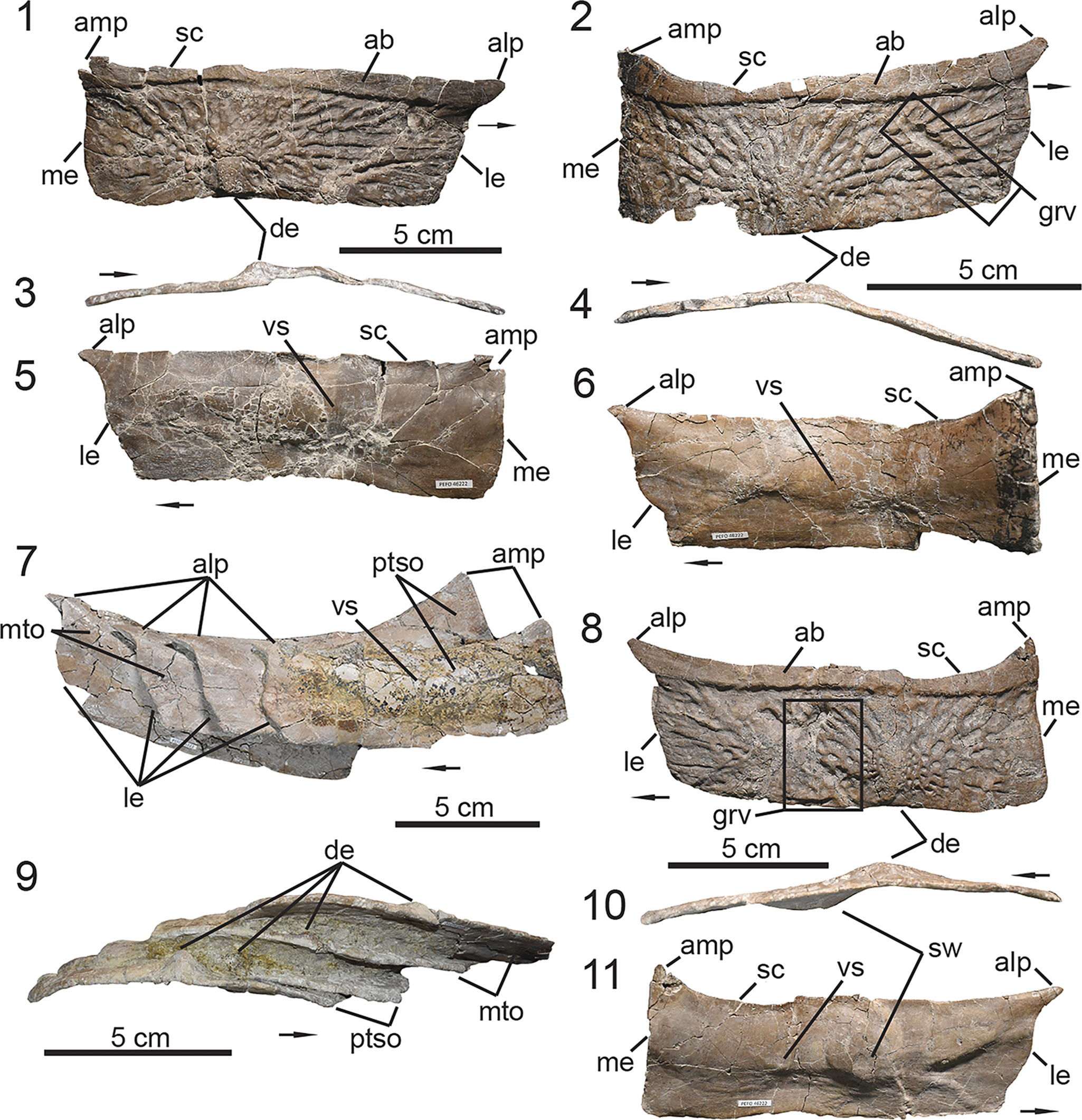
Figure 8. PEFO 46222, associated mid-trunk paramedian osteoderms. (1–6) Isolated osteoderms showing the variation in anatomy within the mid-trunk region; (7, 9) four imbricated paramedian osteoderms that include osteoderms derived from the posterior trunk and/or sacral regions; (8, 10, 11) isolated paramedian osteoderm with possible pathology that is observed across the carapace of PEFO 46222. (1, 2, 8) Dorsal views, (5–7, 11) ventral views, (3, 4, 9, 10) posterior views. ab = anterior bar; alp = anterolateral process; amp = anteromedial process; de = dorsal eminence; grv = groove; le = lateral edge; me = medial edge; mto = mid-trunk osteoderm; ptso = posterior-trunk / sacral osteoderm; sc = scalloped; sw = swelling; vs = ventral strut. Arrows indicate lateral direction.
The paramedian osteoderms of the middle trunk region exhibit a pronounced anterolateral triangular process of the anterior bar (Parker, Reference Parker2018a), however this process varies in its inclination in dorsal view throughout this region. In the anterior middle trunk region, the process projects parallel to the horizontal plane in dorsal view (Fig. 8.1) and becomes anterolaterally inclined in the posterior middle trunk region (Fig. 8.2). The sigmoidal nature of the lateral margin of the paramedian osteoderms vary in the middle trunk region; it ranges from being weakly to moderately sigmoidal (Fig. 8.1, 8.2, 8.7, 8.8). Additionally, the lateral margin exhibits an embayed surface positioned just posterior to the anterolateral process for overlap of the adjacent lateral osteoderm. The anterior bar also exhibits an anteromedial process that projects anteriorly. It has been noted that the anterior margin of the anterior bar is ‘scalloped’ in the paramedian osteoderms of Calyptosuchus wellesi (UMMP 7470, Parker, Reference Parker2018a). PEFO 46222 indicates that this scalloped margin is not consistent across all the paramedian osteoderms of this region; it is weak in the anterior trunk region (Fig. 8.1), whereas in the more posteriorly positioned osteoderms it is moderately to well incised (Fig. 8.2, 8.8). The medial edge is roughly anteroposteriorly straight, although on some of the osteoderms the edge curves slightly medially, just posterior to the anterior bar. The medial edge lacks the interlocking tongue-and-groove articulation surface that is seen in Desmatosuchus (Long and Ballew, Reference Long, Ballew, Colbert and Johnson1985; MNA V9300, Parker, Reference Parker2008a). In the middle trunk region, the dorsal eminence is positioned more medially on the posterior border of the plate. It is weakly developed, being a relatively low boss with a slight keel that originates from the posterior margin of the anterior bar (Fig. 8.1, 8.2). In posterior view, the eminence exhibits a mound-like curved outline (Fig. 8.4, 8.10).
The dorsal ornamentation is composed of well-incised pits and grooves radiating from the eminence (Fig. 8.1). However, in several of the preserved paramedian osteoderms, the dorsal ornamentation is broken by large, deep, anteroposteriorly oriented grooves with straight margins (Fig. 8.2, 8.8). Ventrally, there is a ridge and rounded swelling of the bone associated with the groove on the dorsal surface (Fig. 8.10, 8.11). It is possible that this is associated with predation or scavenging (i.e., bite traces), which has also been documented in the osteoderms of Typothorax coccinarum (PEFO 34869, Drymala et al., Reference Drymala, Bader and Parker2021). Alternatively, this damage may be pathological in origin, although testing that hypothesis requires further analysis outside the scope of the present study. In the absence of this abnormal swelling and damage of the bone, the ventral surface is smooth with a weak, transversely oriented ventral strut (sensu Long and Ballew, Reference Long, Ballew, Colbert and Johnson1985; Fig. 6.5) and an embayed posterior margin (Fig. 8.5, 8.6), which overlaps the anterior bar of the succeeding paramedian osteoderm, as described by Parker (Reference Parker2018a).
Posterior trunk and sacral region
The posterior trunk region encompasses trunk osteoderm rows #13–16 and the succeeding sacral region is composed of two sacral osteoderm rows (Figs. 1, 4). UMMP 13950 indicates that the paramedian osteoderms in the posterior trunk and sacral regions are similar in anatomy to those of the mid trunk region. These regions can be differentiated from each other based on the anatomy of the respective lateral osteoderms. The articulated carapace of UMMP 13950 indicates that the lateral osteoderms in the posterior trunk and sacral regions lack the ‘wing’-like lateral flange that is characteristic of the middle trunk region (Fig. 4.1), instead they exhibit a square-like outline in dorsal view with a quadrangular lateral flange.
Two paramedian osteoderms referable to the posterior trunk region are imbricated and diagenetically cemented to two other osteoderms from the middle trunk region (Fig. 8.7, 8.9). Linear measurements of these imbricated osteoderms indicate that they exhibit an average W:L of 2.5 and an anteroposterior length of 7.9 cm. These osteoderms are slightly arched from the medial edge to the dorsal eminence and transversely flat lateral to the eminence in posterior view (Fig. 9.3). Additionally, they exhibit a slight anterolateral curvature in dorsal view (Fig. 9.1), as observed in the posterior middle trunk region (Fig. 8.8). The preserved elements indicate that the triangular anterolateral process of the anterior bar gradually becomes less pronounced moving posterior in the trunk series (Fig. 8.7). The orientation of this process is predominantly inclined anterolaterally. The anterior margin of the anterior bar is moderately ‘scalloped’, unlike those of the posterior middle trunk region where the scalloping of the anterior bar is well developed. A complete left paramedian osteoderm articulates with an associated square-like lateral osteoderm, indicating that the lateral margin is moderately sigmoidal in the posterior trunk and sacral regions (Fig. 9.1), a condition also observed in those osteoderms that are imbricated and diagenetically cemented (8.7). This particular osteoderm exhibits a slightly lower W:L of 2.3 and an anteroposterior length of 7.5 cm, indicating that it succeeds the imbricated osteoderms described above. The medial margins of the paramedian osteoderms are as described for those of the middle trunk region (Fig. 8). The dorsal eminence in the posterior trunk and sacral region is taller and more transversely expanded than those of the middle trunk region. The eminence is a more prominent boss that has a weak keel anteriorly and is rounded or ‘mound-shaped’ in posterior view (Fig. 9.3). The eminence itself is not medially situated; instead, it is positioned at the midline of the posterior border (Fig. 9.1). This aligns with the sequential transverse shortening of the paramedian osteoderms in the carapace (Case, Reference Case1932), which gradually positions the dorsal eminence closer to the medial edge of the osteoderm. The gradual change of the dorsal eminence from the mid trunk to sacral region in PEFO 46222 is best observed in the imbricated osteoderms (Fig. 8.9). The dorsal ornamentation is characterized by well-incised grooves and ridges radiating from the eminence. The ventral surface is smooth and exhibits a weak, transversely oriented ventral strut.
Caudal region
PEFO 46222 preserves three caudal paramedian osteoderms from the right side (Fig. 9.2, 9.6, 9.8). Two of these elements are not fully preserved, missing the lateral extent of the element, which prohibits calculation of their respective width-to-length ratios. However, it is evident that caudal osteoderms are transversely shorter than the paramedian osteoderms of the trunk and sacral regions. The key feature that supports their referral to the caudal region is the presence of a tall, robust, blunt pyramidal dorsal eminence with an anterior keel (Fig. 9.4, 9.9, 9.10). The holotype specimen of Calyptosuchus wellesi (UMMP 13950, Case, Reference Case1932) preserves most of the caudal carapace, and it is evident that only in this region do the dorsal eminences become this prominent (Fig. 4.1; Parker, Reference Parker2018a). Additionally, the caudal paramedian osteoderms become more flexed distally on the tail. Thus, the anatomy of the eminence along with the flexure of the osteoderm provide the best proxy in determining the anatomical position of these caudal elements. The paramedian caudal osteoderms are referable to middle caudal rows #5–10 (Figs. 1, 4). Two of these elements are rectangular in dorsal view (Fig. 9.2, 9.6), whereas the other exhibits a more square-like outline (Fig. 9.8). Comparison with UMMP 13950 indicates that the square-like osteoderm is positioned more posteriorly in the caudal region near caudal row #10 (Figs. 1, 4).
The preserved osteoderms are anteroposteriorly shorter than those from the trunk and sacral region with an average anteroposterior length of 7.15 cm. This shortening occurs gradually from the mid trunk to mid caudal region, as observed in UMMP 13950. The general anatomy of the dorsal eminence is the same in the preserved elements, only varying in their respective heights. The height of the eminence is directly correlative to the transverse shortening of the paramedian osteoderm in the caudal region, thus the eminence becomes taller and more robust in the more caudally positioned elements within the middle caudal region of Calyptosuchus wellesi (Figs. 4.1, 6.4, 9.9, 9.10). In the posteriormost caudal paramedian osteoderms, the eminence exhibits a height that is equivalent to the anteroposterior length of the osteoderm (Fig. 9.8, 9.9). Additionally, the dorsal eminence is positioned closer to the medial edge than those in the trunk and sacral regions. The dorsal ornamentation is composed of grooves and ridges radiating from the eminence. However, in the more posteriorly positioned osteoderm the dorsal surface is dominated by the eminence rather than the ornamentation (Fig. 9.8). Thus, the ornamentation is not as well incised as those from the trunk and sacral regions. One of the caudal paramedian osteoderms exhibits several shallow channels incised faintly onto the bone surface forming irregular loops that are inconsistent with the ornamentation on the dorsal surface (Fig. 9.6, 9.7). We hypothesize that these looped channels may represent osteophagous invertebrate traces similar to those described from other Triassic assemblages (Paes Neto et al., Reference Paes Neto, Parkinson, Pretto, Soares, Schwanke, Schultz and Kellner2016). Alternatively, they may represent evidence of root etching on the bone surface. However, those patterns tend to exhibit a randomized and/or irregular pattern with channels that are interconnected on the bone surface (Francischini et al., Reference Francischini, Lucas, Dentzien-Dias, Schultz, Martínez, Rojas and Cabrera2020). Ventrally, the preserved elements lack a ventral strut and exhibit a smooth surface with a more deeply embayed posterior margin for the overlap of the succeeding osteoderm.
Lateral osteoderm description
PEFO 46222 preserves several lateral osteoderms that act as the main anatomical landmarks for our divisions of the preserved carapace. Aetosaur lateral osteoderms are composed of dorsal and lateral flanges divided by a pronounced flexure (Parker, Reference Parker2007; Desojo et al., Reference Desojo, Heckert, Martz, Parker, Schoch, Small, Sulej, Nesbitt, Desojo and Irmis2013). At the point of this flexure, the dorsal surface exhibits an eminence, usually in the form of a keel or spine (Desojo et al., Reference Desojo, Heckert, Martz, Parker, Schoch, Small, Sulej, Nesbitt, Desojo and Irmis2013). The lateral osteoderms of PEFO 46222 exhibit a predominantly obtuse flexure between the dorsal and lateral flanges that is laterally convex in posterior view (Fig. 10.4–10.6, 10.8), similar to other taxa within the Stagonolepidoidea (Parker, Reference Parker2016a, Reference Parker2018a; Reyes et al., Reference Reyes, Parker and Marsh2020). The lateral osteoderms are overlapped anteriorly by the anterolateral projection of the adjacent paramedian osteoderm, unlike the condition exhibited by desmatosuchin taxa where the overlapping projection is on the lateral osteoderm (Parker, Reference Parker2007, Reference Parker2016a). They exhibit a well-developed anterior bar, pyramidal-shaped dorsal eminences that contact the posterior margin, and a surface ornamentation consisting of grooves and ridges radiating from the eminence (Fig. 10.1–10.3). Together the anatomy of the lateral and paramedian osteoderms of PEFO 46222 support its referral to Calyptosuchus wellesi (Long and Ballew, Reference Long, Ballew, Colbert and Johnson1985).
Middle trunk region
PEFO 46222 preserves several lateral osteoderms that are associated with paramedian osteoderms of the mid trunk region. These osteoderms exhibit a significantly larger lateral flange compared to the dorsal flange (Fig. 10.1, 10.2). The outline of the dorsal flange is triangular in dorsal view. The medial edge of the dorsal flange is sigmoidal along its contact with the lateral margin of the adjacent paramedian osteoderm. The anteromedial edge of the anterior bar is incised for reception of the anterolateral process of the anterior bar in the adjacent paramedian osteoderm. The angle of incision varies between the lateral osteoderms (Fig. 10.1, 10.2), which matches the variation observed in the projection of the anterolateral process of the paramedian osteoderms throughout the dorsal carapace. The lateral flange of these lateral osteoderms is triangular in dorsal view and exhibits an anterolateral ‘wing-like’ appearance (sensu Parker, Reference Parker2018a), in which the posterior margin curves anteriorly until it meets the anterior margin at a rounded tip (Fig. 10.2; Parker, Reference Parker2018a). This anatomy is associated with lateral osteoderms positioned in the middle trunk region of UMMP 13950 (Figs. 1, 4). When measuring from the dorsal eminence, the transverse width of the lateral wing varies between these lateral osteoderms. The lateral flange exhibits a larger width the more anteriorly located it is in the middle trunk region and becomes gradually reduced posteriorly in the sequence.
There is also variation in the dorsal eminence on the middle trunk lateral osteoderms. The more anteriorly located elements exhibit a low pyramidal eminence with an anteroposteriorly elongate keel at the midline (Fig. 10.4, 10.5; Parker, Reference Parker2018a). However, unlike the condition observed in an isolated lateral osteoderm (UCMP 136744) referred to Calyptosuchus wellesi from UCMP A269, the dorsal eminence does not project beyond the posterior margin (Parker, Reference Parker2018a). The flexure between the dorsal and lateral flange varies between these lateral osteoderms. The more anteriorly positioned lateral osteoderms exhibit a flexure of nearly 90° between the dorsal and lateral flanges in posterior view (Fig. 10.4), whereas the succeeding lateral osteoderms exhibit a more obtuse flexure in comparison (Fig. 10.5). This variation in the flexure of the lateral osteoderm is also well documented in Desmatosuchus spurensis (MNA V9300, Parker, Reference Parker2008a, fig. 27), in which the osteoderms become more acutely flexed in the middle trunk region.
Posterior trunk and sacral regions
PEFO 46222 preserves a left lateral osteoderm (Fig. 10.3, 10.6) that articulates with a paramedian osteoderm referable to the posterior trunk or sacral region of the dorsal carapace. The element exhibits a sigmoidal medial edge similar to the middle trunk lateral osteoderms, although the anterior bar is not well incised due to the decreased prominence of the anterolateral process of the adjacent paramedian osteoderm (Fig. 10.3). The dorsal flange no longer exhibits a triangular outline in dorsal view, instead it is more rectangular. The lateral flange lacks the triangular-shaped ‘wing’ (sensu Parker, Reference Parker2018a) that is characteristic of the middle trunk laterals. Instead, it exhibits a transverse width similar to the dorsal flange, giving the element a squared outline in dorsal view with a wide obtuse flexure in posterior view (Fig. 10.3, 10.6). The dorsal eminence is partially eroded near the apex, but it is evident that it was moderately developed and exhibited an anteroposteriorly oriented keel on the dorsal surface similar to those from the trunk.
Caudal region
PEFO 46222 preserves a left lateral caudal osteoderm (Fig. 10.7, 10.8) that is referable to the anterior caudal region based on comparisons to UMMP 13950 (Fig. 4.1). It exhibits well-incised radial ornamentation, a low pyramidal eminence that is anteriorly keeled, and a transversely short triangular lateral flange that is slightly wider than the dorsal flange with an anterolateral inclination (Fig. 10.7). Although the lateral flange is similar to those from the middle trunk region, the osteoderm exhibits a wide, obtuse flexure of nearly 180° (Fig. 10.8). The dorsal flange exhibits a rectangular outline in dorsal view similar to those in the posterior trunk and sacral regions. However, the medial edge is weakly sigmoidal in comparison to the more anteriorly positioned lateral osteoderms.
Referred specimens
UMMP 7470, trunk and sacral vertebrae, paramedian osteoderms, and a relatively complete pelvic girdle preserving the right ilium, right pubis, and co-ossified ischia (Case, Reference Case1922, Reference Case1929); UCMP 27225, dentary fragment, cervical centra, paramedian, lateral, and ventral osteoderms (Parker, Reference Parker2018a); UCMP 25941 and UCMP 32148, associated left ilium and ischium, respectively (Long and Murry, Reference Long and Murry1995); and approximately 400 catalogued elements from UCMP A269 (the Placerias Quarry, Parker, Reference Parker2018a, supplemental material).
New referred PEFO specimens
PEFO 46222, an associated skeleton preserving elements from the trunk through caudal region including paramedian and lateral osteoderms, vertebrae, ribs, and a disarticulated pelvic girdle; PEFO 49321, an associated skeleton preserving paramedian and lateral osteoderms as well as skull elements including the left and right maxillae with teeth, the right jugal, the left quadratojugal, the left quadrate, the left laterosphenoid, the right surangular, the right prearticular, and the right articular.
Rationale for referral of PEFO specimens
PEFO 46222 and PEFO 49321 preserve osteoderms that are rectangular in dorsal view, indicating they are paramedian osteoderms derived from two medial anteroposterior columns of the dorsal carapace (Parker and Martz, Reference Parker and Martz2010). The paramedian osteoderms lack a beveled edge on the posterodorsal margin, unlike Tecovasuchus chatterjeei Martz and Small, Reference Martz and Small2006 (TTU-P 545), Paratypothorax sp. (TTU-P 09169, Martz et al., Reference Martz, Mueller, Nesbitt, Stocker, Parker, Atanassov, Fraser, Weinbaum and Lehane2013; PEFO 3004, Lucas et al., Reference Lucas, Heckert, Rinehart, Harris, Lucas, Spielmann, Lockley, Milner and Kirkland2006), Kocurypelta silvestris (ZPAL V.66/1, Czepiński et al., Reference Czepiński, Dróżdż, Szcygielski, Tałanda, Pawlak, Lewczuk, Rytel and Sulej2021), and Venkatasuchus armatum Haldar, Ray, and Bandyopadhyay, Reference Haldar, Ray and Bandyopadhyay2023 (ISIR267/1-7), which exhibit this anatomy in the trunk and caudal regions. The dorsal ornamentation of the paramedian osteoderms in PEFO 46222 and PEFO 49321 is composed of a strong radial pattern of ridges and grooves radiating from the eminence, unlike Typothorax coccinarum (TTU-P 9214, Martz, Reference Martz2002; NMMNH P-12964, Heckert et al., Reference Heckert, Lucas, Rinehart, Celeskey, Spielmann and Hunt2010), Redondasuchus rineharti Spielmann et al., Reference Spielmann, Hunt, Lucas and Heckert2006 (NMMNH P-43311), Kryphioparma caerula (UCMP 165173, Reyes et al., Reference Reyes, Parker and Heckert2023), and Sierritasuchus macalpini Parker, Stocker, and Irmis, Reference Parker, Stocker and Irmis2008 (UMMP 60817), which exhibit well-incised oblong to circular pits across the dorsal surface, or Apachesuchus heckerti Spielman and Lucas, Reference Spielmann and Lucas2012, which is primarily devoid of ornamentation. Anteriorly, the trunk paramedian osteoderms exhibit a thickened well-developed anterior bar unlike paratypothoracins (e.g., Rioarribasuchus chamaensis Zeigler, Heckert, and Lucas, Reference Zeigler, Heckert and Lucas2003), which exhibit a weakly developed anterior bar (Parker, Reference Parker2007; Martz et al., Reference Martz, Mueller, Nesbitt, Stocker, Parker, Atanassov, Fraser, Weinbaum and Lehane2013; Czepiński et al., Reference Czepiński, Dróżdż, Szcygielski, Tałanda, Pawlak, Lewczuk, Rytel and Sulej2021; Haldar et al., Reference Haldar, Ray and Bandyopadhyay2023; Reyes et al., Reference Reyes, Martz and Small2024), Desmatosuchus spurensis (UMMP 7476, Case, Reference Case1922; MNA V9300, Parker, Reference Parker2007, Reference Parker2008a), which exhibits a depressed surface (= anterior lamina, sensu Long and Ballew, Reference Long, Ballew, Colbert and Johnson1985; Parker, Reference Parker2005, Reference Parker2008a), or Garzapelta muelleri (Reyes et al., Reference Reyes, Martz and Small2024), which exhibits presacral osteoderms with a flat and smooth anterior margin. The anterior bar exhibits both strongly projecting anterolateral and anteromedial projections, similar to Scutarx deltatylus (Parker, Reference Parker2016b). The anterolateral process on the paramedian osteoderm overlaps the anterior surface of the adjacent lateral osteoderm, unlike desmatosuchins (Parker, Reference Parker2007). More specifically, the trunk paramedian osteoderms of PEFO 46222 and PEFO 49321 exhibit a ‘scalloped’ anterior margin of the anterior bar, a weakly developed ventral strut, and a low, rounded, anteriorly keeled eminence in the trunk series that becomes more pronounced, tall, robust, and pyramidal caudally; a condition shared with Scutarx deltatylus (Parker, Reference Parker2016b) and Adamanasuchus eisenhardtae (Lucas et al., Reference Lucas, Hunt, Spielmann, Lucas and Spielmann2007a). The dorsal eminence contacts the posterior margin, a condition shared with most taxa except Desmatosuchus (Parker, Reference Parker2005, Reference Parker2008a), Lucasuchus hunti (Long and Murry, Reference Long and Murry1995), Neoaetosauroides engaeus (Desojo and Báez, Reference Desojo and Báez2005), and paratypothoracins (Parker, Reference Parker2007). Additionally, PEFO 46222 and PEFO 49321 preserve asymmetrical osteoderms with dorsal and lateral flanges, flexed at the center of ossification, and articulate with paramedian osteoderms, indicating they are lateral osteoderms from the two outer columns of the dorsal carapace (Parker and Martz, Reference Parker and Martz2010). The lateral osteoderms are broadly flexed, similar to those of non-desmatosuchin and non-typothoracine taxa (Parker, Reference Parker2007). The dorsal eminence is a low keeled triangular boss, unlike desmatosuchins, which exhibit a conical spike (Parker and Martz, Reference Parker and Martz2010), or paratypothoracins, which exhibit a dorsoventrally flattened horn (Parker, Reference Parker2007).
The suite of characters described above supports the referral of PEFO 46222 and PEFO 49321 to the more inclusive clade Calyptosuchini n. clade, which includes Calyptosuchus wellesi, Scutarx deltatylus, and Adamanasuchus eisenhardtae (Parker, Reference Parker2016a). Those three taxa are further differentiated from each other based on a combination of characters and autapomorphies of the trunk paramedian osteoderms. Scutarx deltatylus exhibits a strong dorsal protuberance on the posteromedial dorsal surface, which is an autapomorphic condition of the taxon (Parker, Reference Parker2016b). In Adamanasuchus eisenhardtae (Lucas et al., Reference Lucas, Hunt, Spielmann, Lucas and Spielmann2007a) the dorsal surface exhibits an unornamented triangular area on the dorsal surface of the posteromedial corner, which is an autapomorphy of the taxon (Parker, Reference Parker2016a, Reference Parkerb), as well as a strongly incised ‘cut-off’ posterolateral corner for its contact with the adjacent lateral osteoderm shared with paratypothoracins (e.g., PEFO 3004). In Calyptosuchus wellesi (Parker, Reference Parker2018a) the posteromedial corner is flat and exhibits radial ornamentation instead of a protuberance or unornamented area and lacks a strongly incised (‘cut-off’ corner) posterolateral margin. Thus, the osteoderm anatomy of PEFO 46222 and PEFO 49321 aligns with that of Calyptosuchus wellesi (Long and Ballew, Reference Long, Ballew, Colbert and Johnson1985) following the revision by Parker (Reference Parker2018a).
Ontogenetic assessment
PEFO 46222, UCMP 27225, UMMP 7470, and UMMP 13950 are considered skeletally mature, based on the complete co-ossification of the neurocentral sutures of the preserved cervical and trunk vertebrae and the fusion of the sacral centra (see Griffin et al., Reference Griffin, Stocker, Colleary, Stefanic, Lessner, Riegler, Formoso, Koeller and Nesbitt2021). PEFO 49321 does not preserve any vertebrae, however the proportional size of its paramedian osteoderms (Fig. S2) suggests that it was of similar size to PEFO 46222, and possibly skeletal maturity.
Remarks
The diagnosis for Calyptosuchus wellesi (Long and Ballew, Reference Long, Ballew, Colbert and Johnson1985) by Parker (Reference Parker2018a) is mostly retained. We modified it based on our reassessment of the holotype specimen UMMP 13950 and referred pelvis UMMP 7470, which corrects some of the original interpretations by Case (Reference Case1932), and the new morphological understanding provided by PEFO 49321 and PEFO 46222 (this study). Calyptosuchus wellesi remains a biostratigraphically informative taxon because it is currently restricted to the Adamanian Late Triassic Land Vertebrate Estimated Holochronozone (Martz and Parker, Reference Martz, Parker, Parker and Zeigler2017).
Results
Phylogenetic analyses
Maximum parsimony
The strict consensus of Run 1 (Fig. 14.1) recovered UCMP 27225, PEFO 49321, PEFO 46222, UMMP 7470, and UMMP 13950 in a polytomy at the base of the Stagonolepididae. Because of the inconclusive nature of this quantitative analysis, we incorporated these specimens into a composite OTU of Calyptosuchus wellesi, assuming that they are indeed referrable to the taxon, in order to explore the influence these specimens would have on the topological position of C. wellesi and the topology of the Aetosauria. Run 2 (Fig. 14.2) resulted in a strict consensus tree that recovers Aetosauroides scagliai as the earliest branching aetosaur, as seen in the strict consensus trees presented by Parker (Reference Parker2016a) and Reyes et al. (Reference Reyes, Parker and Marsh2020, Reference Reyes, Martz and Small2024). The Stagonolepidoidea (Parker, Reference Parker2016a) is apparent (Fig. 14.2) but is missing Calyptosuchus wellesi (composite OTU) and Neoaetosauroides engaeus, which are taxa traditionally recovered within this clade (Parker, Reference Parker2016a; Reyes et al., Reference Reyes, Parker and Marsh2020, Reference Reyes, Martz and Small2024; Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021c). In our analysis (Fig. 14.2) they are recovered in a large polytomy at the base of the Stagonolepididea, which is predominantly composed of taxa from the Aetosaurinae. The Desmatosuchini remains stable and composed of Gorgetosuchus pekinensis Heckert et al., Reference Heckert, Schneider, Fraser and Webb2015, Sierritasuchus macalpini, Longosuchus meadei, Lucasuchus hunti, Desmatosuchus smalli, and Desmatosuchus spurensis, as observed in previous studies (Parker, Reference Parker2016a; Reyes et al., Reference Reyes, Parker and Marsh2020, Reference Reyes, Martz and Small2024; Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021c; Haldar et al., Reference Haldar, Ray and Bandyopadhyay2023).
Bayesian inference
The Bayesian consensus of Run 1 (Fig. 15.1) recovered UCMP 27225, PEFO 49321, PEFO 46222, UMMP 7470, and UMMP 13950 within an inclusive clade that includes Scutarx deltatylus, Adamanasuchus eisenhardtae, Neoaetosauroides engaeus, and Stenomyti huangae. In this clade PEFO 46222 and PEFO 49321 are sister taxa. These specimens are recovered basal to UMMP 7470 and UCMP 27225, where the latter is directly basal to Scutarx deltatylus and Adamanasuchus eisenhardtae, two taxa that exhibit a similar osteoderm anatomy to Calyptosuchus wellesi and often are recovered in an inclusive clade (= Calyptosuchini n. clade; Parker, Reference Parker2016a, Reference Parkerb; Reyes et al., Reference Reyes, Parker and Marsh2020; Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021c). UMMP 13950 is positioned basally within the clade and separated from the PEFO 46222, PEFO 49321, UMMP 7470, and UCMP 27225 by Neoaetosauroides engaeus and Stenomyti huangae, which are recovered as sister taxa. The recovery of these two taxa within this clade does make sense qualitatively because they exhibit a very similar dorsal osteoderm anatomy to Calyptosuchus wellesi (UMMP 13950). UCMP 27225 preserves a dentary, cervical vertebrae, and cervical osteoderms, which are elements that are absent in UMMP 7470, PEFO 46222, and PEFO 49321. UMMP 13950 preserves fragmentary cervical vertebrae, however these elements are represented by partial centra. The topological position of UCMP 27225 is due to this specimen preserving anatomy of the cervical region, which cannot be compared quantitatively to the UMMP or PEFO specimens because they do not preserve that anatomy, but they are comparable with those of Scutarx deltatylus (Parker, Reference Parker2016b). UMMP 7470 preserves a limited number of paramedian osteoderms and trunk vertebrae along with an articulated pelvic girdle— elements that are preserved in UMMP 13950, PEFO 46222, and PEFO 4932. However, the topological position of UMMP 7470 is influenced by the lack of data of its lateral osteoderms and the presence of zygadiapophyseal laminae on the trunk vertebrae, a condition shared with Scutarx deltatylus (Parker, Reference Parker2016b).
We reject the referral of UCMP 27225, PEFO 46222, PEFO 49321, and UMMP 7470 to Scutarx deltatylus and Adamanasuchus eisenhardtae because the paramedian osteoderms of these specimens lack the unornamented triangular protuberance on the posteromedial corner that is autopomorphic to S. deltatylus or the ‘cut-off’ corner observed in A. eisenhardtae (Lucas et al., Reference Lucas, Hunt, Spielmann, Lucas and Spielmann2007a, Parker, Reference Parker2016a, Reference Parkerb). Although they exhibit similarities to the dorsal osteoderms of Neoaetosauroides engaeus and Stenomyti huangae, the stratigraphic and geographical occurrences of the specimens in question do not support their referral to either of these taxa. Neoaetosauroides engaeus is known exclusively from the Los Colorados Formation of Argentina (Desojo and Báez, Reference Desojo and Báez2007), while S. huangae is only documented from outcrops of the Chinle Formation (or its equivalent) in northwestern Colorado (Small and Martz, Reference Small, Martz, Nesbitt, Desojo and Irmis2013). Additionally, Neoaetosauroides engaeus and Stenomyti huangae are both restricted to the Revueltian estimated holochron (215–207 Ma, sensu Martz and Parker, Reference Martz, Parker, Parker and Zeigler2017) (Small and Martz, Reference Small, Martz, Nesbitt, Desojo and Irmis2013; Kent et al., Reference Kent, Santi Malnis, Columbi, Alcober and Martinez2014; Parker, Reference Parker2016a), unlike the UCMP, PEFO, and UMMP specimens in question, which are restricted to the Adamanian estimated holochron (224–215 Ma, sensu Martz and Parker, Reference Martz, Parker, Parker and Zeigler2017). Based on the topological position of UCMP 27225, PEFO 46222, PEFO 49321, and UMMP 7470, their anatomical similarities to UMMP 13950, the stratigraphic relationship between UCMP 27225 and the PEFO specimens within the Chinle Formation as well as that of the UMMP specimens within the Dockum Group, and our rationale for rejecting their referral to Neoaetosauroides engaeus, Stenomyti huangae, Scutarx deltatylus, and Adamanasuchus eisenhardtae, we support the referral of UCMP 27225 and UMMP 7470 to Calyptosuchus wellesi, as originally proposed by Parker (Reference Parker2018a), and refer the new PEFO specimens to this taxon.
The Bayesian consensus of Run 2 (Fig. 15.2) resulted in a fully resolved tree with a topology that is similar to the strict consensus trees presented by Parker (Reference Parker2016a, fig. 7), Reyes et al. (Reference Reyes, Parker and Marsh2020, fig. 8), Paes Neto et al. (Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021c, fig. 8), and the Bayesian consensus tree presented by Reyes et al. (Reference Reyes, Martz and Small2024, fig. 11d). The Aetosauria is composed of two major clades, Aetosaurinae and Stagonolepidoidea (Parker, Reference Parker2016a), however several taxa shifted in their topological position. Aetosauroides scagliai is no longer recovered as a non-stagonoloepidid aetosaur; instead, it is recovered as a sister taxon of the Stagonolepidoidea (Fig. 15.2). The topological position of Stenomyti huangae remains unstable as it is recovered within the Stagonolepidoidea in our analysis but has also been recovered at the base of the Aetosaurinae (Parker, Reference Parker2016a; Reyes et al., Reference Reyes, Parker and Marsh2020, Reference Reyes, Martz and Small2024) or in a polytomy at the base of the Aetosauria (Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021c; Haldar et al., Reference Haldar, Ray and Bandyopadhyay2023). Although the anatomy of Stagonolepis robertsoni is most similar to that of Calyptosuchus wellesi, our Bayesian analysis recovers it basal to the Desmatosuchini alongside Stagonolepis olenkae as observed in Reyes et al. (Reference Reyes, Martz and Small2024). The inclusive clades Desmatosuchini, Typothoracinae, and Paratypothoracini are recovered as seen in previous studies (Parker, Reference Parker2016a; Reyes et al., Reference Reyes, Parker and Marsh2020, Reference Reyes, Martz and Small2024; Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021c; Haldar et al., Reference Haldar, Ray and Bandyopadhyay2023). It is evident that the addition of four new characters and rescoring did not influence the topological position of Calyptosuchus wellesi because it still forms an inclusive clade (= Calyptosuchini n. clade) with Scutarx deltatylus and Adamanasuchus eisenhardtae.
Discussion
Obturator foramina within the Aetosauria
Prior to the discovery of PEFO 46222, the presence of two foramina had only been documented on the pubic apron within the pubis of Scutarx deltatylus (PEFO 31217, Parker, Reference Parker2016b) and Stagonolepis robertsoni (NHMUK R4793, MCZD 4, Walker, Reference Walker1961). Small and Martz (Reference Small, Martz, Nesbitt, Desojo and Irmis2013, p. 407) described “two shallow notches” in the broken pubic apron of a pubis (DMNH V.34565) referred to Stenomyti huangae, suggesting that the pubic apron of this taxon may have also been perforated by two foramina as observed in Scutarx deltatylus (Parker, Reference Parker2016b), Stagonolepis robertsoni (Walker, Reference Walker1961), and Calyptosuchus wellesi (PEFO 46222, Fig. 7). Recently, Desmet et al. (Reference Desmet, Antczak and Bodzioch2022) suggested that the pubis of Stagonolepis olenkae also exhibits two ‘obturator foramina’ based on a right pubis (UOPB01143) that is part of an associated pelvis collected from the Upper Triassic strata in Krasiejów, Poland (Desmet et al., Reference Desmet, Antczak and Bodzioch2022). This is a region where S. olenkae is the only documented aetosaurian within the Upper Triassic (Keuper) strata (Dzik and Sulej, Reference Dzik and Sulej2007; Sulej, Reference Sulej2010; Antczak, Reference Antczak2015; Parker, Reference Parker2016a; Teschner et al., Reference Teschner, Konietzko-Meir and Klein2022). However, pelvic material described from the same region by Drożdż (Reference Dróżdż2022) suggests otherwise. Drożdż (Reference Dróżdż2022) described several pelvic elements, including several pubes, that were recovered in association with dorsal paramedian osteoderms, which support their referral to Stagonolepis olenkae. This new material indicates the presence of only one obturator foramen within a well-preserved right pubis (ZPAL AbIII/3266) of Stagonolepis olenkae.
Desmet et al. (Reference Desmet, Antczak and Bodzioch2022, fig. 11) described the presence of two ‘obturator foramina’ in UOPB01143, however the associated figures of this element do not support their interpretation; the ‘obturator foramina’ are labeled in lateral view, however in medial view there is no evidence of perforation on the bone surface. Instead, the bone surface is smooth and well preserved. Based on this, we interpret the two ‘obturator foramina’ described in UOPB01143 by Desmet et al. (Reference Desmet, Antczak and Bodzioch2022) as taphonomic indentations of the bone surface, and the actual obturator foramen, or foramina, are not preserved. Thus, we follow the description of Drożdż (Reference Dróżdż2022) and interpret Stagonolepis olenkae as exhibiting one obturator foramen similar to Neoaetosauroides engaeus (PVL 3525, Desojo and Báez, Reference Desojo and Báez2005) and Desmatosuchus smalli (TTU-P 9419, Martz, Reference Martz2008; Martz et al., Reference Martz, Mueller, Nesbitt, Stocker, Parker, Atanassov, Fraser, Weinbaum and Lehane2013). However, in recent years, several researchers (e.g., Lucas et al., Reference Lucas, Spielmann, Hunt, Lucas, Spielmann and Lockley2007b; Antczak, Reference Antczak2015; Górnicki et al., Reference Górnicki, Antczak and Bodzioch2021; Desmet et al., Reference Desmet, Antczak and Bodzioch2022) have suggested that Stagonolepis olenkae (Sulej, Reference Sulej2010) may be a junior synonym of Stagonolepis robertsoni (Walker, Reference Walker1961) and attributed the few morphological differences of the skull and postcrania to intraspecific variation (i.e., sexual dimorphism, ontogenetic variation). Under this interpretation, the number of foramina within the pubis of Stagonolepis robertsoni would be considered variable. Further study is required to assess the taxonomic status of Stagonolepis olenkae (Sulej, Reference Sulej2010) and its relationship to Stagonolepis robertsoni (Walker, Reference Walker1961).
It is evident that the number of foramina that perforate the pubic apron is variable within the Aetosauria (Parker, Reference Parker2016b). The perforated region of the pubis is not preserved within the early-branching aetosuaur Aetosauroides scagliai (PFV 2073, Casamiquela, Reference Casamiquela1967; Heckert and Lucas, Reference Heckert and Lucas2002a), thus inhibiting our ability to determine the plesiomorphic state of this condition within the clade. However, the presence of one obturator foramen within the non-aetosaur aetosauriformes Acaenasuchus geoffreyi (UCMP 285841, Marsh et al., Reference Marsh, Smith, Parker, Irmis and Kligman2020) and Revueltosaurus callenderi (PEFO 36875, Parker et al., Reference Parker, Nesbitt, Irmis, Martz, Marsh, Brown, Stocker and Werning2021) suggests that this is the plesiomorphic condition within the Aetosauria and the presence of two foramina in Scutarx deltatylus (Parker, Reference Parker2016b), Stagonolepis robertsoni (Walker, Reference Walker1961), and Calyptosuchus wellesi (Fig. 7; PEFO 46222) is a derived condition. Outside of the Aetosauria, the presence of two foramina is also documented within the non-archosaur archosauriform Euparkeria capensis Broom, Reference Broom1913, and the sauropodomorph Sarahsaurus aurifontanalis Rowe, Sues, and Reisz, Reference Rowe, Sues and Reisz2011 (Marsh and Rowe, Reference Marsh and Rowe2018), indicating that this condition convergently evolved within aetosaurs.
Among taxa that exhibit two foramina within the pubis, the dorsal foramen is considered homologous with the single obturator foramen observed in the pubis of most archosauromorphs (Ezcurra, Reference Ezcurra2016; Parker, Reference Parker2016a). However, the obturator foramen is lost within crocodyliforms (Claessens and Vickaryous, Reference Claessens and Vickaryous2012), turtles, and some dinosaurs (Hutchinson, Reference Hutchinson2001). In the literature, the ventral foramen has been referred to as a ‘pubic foramen’ (Rowe et al., Reference Rowe, Sues and Reisz2011), ‘secondary pubic foramen’ (Hutchinson, Reference Hutchinson2001), or ‘thyroid fenestra’ (Walker, Reference Walker1961). However, a thyroid fenestra is actually an opening that develops within the puboischiadic plate and is completely or partially enclosed by the pubis and ischium (Romer, Reference Romer1956; Ezcurra, Reference Ezcurra2016); among saurian reptiles this condition is observed in lepidosauromorphs, tanystropheids, and trilophosaurids (Ezcurra, Reference Ezcurra2016; Pritchard and Sues, Reference Pritchard and Sues2019). It is possible that this ventral foramen is homologous with the thyroid fenestra, however this requires further study. The function of the ventral foramen is presently not understood but it likely served a similar function as the single obturator foramen. It is possible that the obturator nerve and associated vessels split into two separate branches before exiting through the pubic apron, a feature that typically happens external to the pubis (Romer, Reference Romer1956; Hutchinson, Reference Hutchinson2001; Claessens and Vickaryous, Reference Claessens and Vickaryous2012).
Intraspecific variation within the Aetosauria
The influence of ontogeny is poorly understood among extinct archosaurs, particularly within the Aetosauria (Nesbitt et al., Reference Nesbitt, Stocker, Parker, Wood, Sidor and Angielczyk2017; Griffin et al., Reference Griffin, Stocker, Colleary, Stefanic, Lessner, Riegler, Formoso, Koeller and Nesbitt2021; Paes-Neto et al., Reference Paes-Neto, Desojo, Brust, Schultz, Da-Rosa and Soares2021a). In recent years, histological analyses have provided insight into the intraspecific variation within the clade. Authors who studied skeletal growth documented a shift from highly vascularized, woven-fibered tissue to less-vascularized, lamellar tissue in the cortex of several taxa, indicating a shift in the rate of skeletal growth early in ontogeny (e.g., de Ricqlès et al., Reference de Ricqlès, Padian and Horner2003; Werning, Reference Werning2013; Scheyer et al., Reference Scheyer, Desojo and Cerda2014; Taborda et al., Reference Taborda, Heckert and Desojo2015; Cerda et al., Reference Cerda, Desojo and Scheyer2018; Hoffman et al., Reference Hoffman, Heckert and Zanno2019; Ponce et al., Reference Ponce, Desojo and Cerda2023; Teschner et al., Reference Teschner, Konietzko-Meir, Desojo, Schoch and Klein2023). This new understanding has led to a new hypothesis in which the complexity in the dorsal ornamentation of paramedian osteoderms correlates with the transition in tissue vascularization (Hoffman et al., Reference Hoffman, Heckert and Zanno2019).
Aetosauroides scagliai has been used as a sample taxon to explore the relationship between body size and growth rate within the Aetosauria because the taxon is known from several specimens that cover an array of body sizes (Taborda et al., Reference Taborda, Cerda and Desojo2013, Reference Taborda, Heckert and Desojo2015; Cerda et al., Reference Cerda, Desojo and Scheyer2018). These authors determined that the lines of arrested growth (LAG) count varied significantly between similar-sized individuals and hypothesized that this disparity could be attributed to sexual dimorphism. Additionally, histological studies on Typothorax coccinarum indicate a discordance between skeletal maturity and body size, which is related to the variation in the timing of the co-ossification of the neural arch and centrum across the vertebral column (Parker et al., Reference Parker, Reyes and Marsh2023).
Although aetosaur elements, particularly their osteoderms, are commonly reported from Late Triassic strata (Desojo et al., Reference Desojo, Heckert, Martz, Parker, Schoch, Small, Sulej, Nesbitt, Desojo and Irmis2013), most taxa are not represented by a large enough sample size that includes individuals at various ontogenetic stages and/or share homologous skeletal elements; the exception being the early-diverging aetosaur Aetosauroides scagliai (Desojo and Ezcurra, Reference Desojo and Ezcurra2011). The ontogenetic series of Aetosauroides scagliai indicates that phylogenetically informative characters such as the lateral fossae on the vertebral centra and centrodiapophyseal laminae are ontogenetically variable within the trunk vertebrae of A. scagliai (Paes-Neto et al., Reference Paes-Neto, Desojo, Brust, Schultz, Da-Rosa and Soares2021a). This understanding resulted in a new hypothesis in which Polesinesuchus aurelioi Roberto-Da-Silva et al., Reference Roberto-Da-Silva, Desojo, Cabreira, Aires, Müller, Pacheco and Dias-Da-Silva2014, a taxon documented exclusively through a skeletally immature specimen (ULBRA PVT003), represents a skeletally immature individual of Aetosauroides scagliai (Paes-Neto et al., Reference Paes-Neto, Desojo, Brust, Schultz, Da-Rosa and Soares2021a). Thus, this brings to question the taxonomic status of small-bodied ‘dwarf’ aetosaur taxa that are based on skeletally immature specimens (e.g., Coahomasuchus chathamensis, Heckert et al., Reference Heckert, Fraser and Schneider2017, Hoffman et al., Reference Hoffman, Heckert and Zanno2019; Aetosaurus ferratus, Schoch, Reference Schoch2007, Schoch and Desojo, Reference Schoch and Desojo2016, Scheyer et al., Reference Scheyer, Desojo and Cerda2014; Polesinesuchus aurelioi, Roberto-Da-Silva et al., Reference Roberto-Da-Silva, Desojo, Cabreira, Aires, Müller, Pacheco and Dias-Da-Silva2014, Paes-Neto et al., Reference Paes-Neto, Desojo, Brust, Schultz, Da-Rosa and Soares2021a). Small-bodied taxa are present within the Aetosauria (Desojo et al., Reference Desojo, Heckert, Martz, Parker, Schoch, Small, Sulej, Nesbitt, Desojo and Irmis2013), as exemplified by Sierritasuchus macalpini (Parker et al., Reference Parker, Stocker and Irmis2008). Historically, this taxon was hypothesized to either represent a juvenile of Desmatosuchus (Long and Murry, Reference Long and Murry1995) or Longosuchus meadei (Parker, Reference Parker, McCord and Boaz2002) due to the small body size of the type specimen UMMP V6081. However, histological analysis of UMMP V60817 indicated that the individual was not skeletally immature (Parker et al., Reference Parker, Stocker and Irmis2008; Parker, Reference Parker2016a). Similarly, Heckert and Lucas (Reference Heckert and Lucas2002b) hypothesized that small (< 25 mm) dorsal osteoderms collected from UCMP V7308 (Blue Hills locality) represented juvenile individuals of Calyptosuchus (= ‘Stagonolepis’) wellesi, which is also documented from this locality based on UCMP 27225 (Long and Murry, Reference Long and Murry1995; Parker, Reference Parker2018a), because of their size. However, revision of these osteoderms indicated these elements are referable to the aetosauriform Revueltosaurus callenderi (Parker et al., Reference Parker, Nesbitt, Irmis, Martz, Marsh, Brown, Stocker and Werning2021).
Our new understanding of the anatomy of Calyptosuchus wellesi provides new insight into the intraspecific variation of the postcranial skeleton within the Aetosauria. Based on skeletal proxies including the proportional size of the ilia and complete co-ossification of the centra with their respective neural arches (Brochu, Reference Brochu1996), we hypothesize that PEFO 46222, UMMP 7470, and UMMP 13950 are of similar skeletal maturity. However, to independently assess that hypothesis, further histological sampling is needed because recent studies indicate that there is a disparity in the timing of the co-ossification of the neurocentral suture across archosaurs (Irmis, Reference Irmis2007; Griffin et al., Reference Griffin, Stocker, Colleary, Stefanic, Lessner, Riegler, Formoso, Koeller and Nesbitt2021; Parker et al., Reference Parker, Reyes and Marsh2023). PEFO 46222 records the only occurrence of co-ossification of the centra in the sacral vertebrae of Calyptosuchus wellesi (Fig. 6.1), a condition that was previously only documented in the desmatosuchins Desmatosuchus (Parker, Reference Parker2005, Reference Parker2007, Reference Parker2008a) and Longosuchus meadei (TMM 31100-236). However, co-ossification of the sacral vertebrae is absent in UMMP 13950 (Fig. 12.3) and UMMP 7470 (Fig. 12.2) indicating that this is an area of intraspecific variation that may be attributed to ontogeny or sexual dimorphism. Although the sacral vertebrae in PEFO 46222 are co-ossified to each other, the posteriormost trunk vertebra is not co-ossified with the first sacral vertebra (Figs. 5, 6; = dorsosacral, Griffin et al., Reference Griffin, Stefanic, Parker, Hungerbühler and Stocker2017). Among aetosaurs, incorporation of the dorsosacral into the sacrum is documented within Desmatosuchus (MNA V9300, Parker, Reference Parker2008a), Longosuchus meadei (TMM 31100-236, Griffin et al., Reference Griffin, Stefanic, Parker, Hungerbühler and Stocker2017), and ?Lucasuchus hunti (TMM 31100-313, Long and Murry, Reference Long and Murry1995). Thus, the absence of a dorsosacral in PEFO 46222 suggests that its incorporation is a derived condition of desmatosuchins.
PEFO 46222 preserves relatively complete vertebrae for most of the trunk series, a region that is poorly preserved in the holotype specimen UMMP 13950 (Fig. 4.2). Similar to Aetosauroides scagliai (Paes-Neto et al., Reference Paes-Neto, Desojo, Brust, Schultz, Da-Rosa and Soares2021a), we document variation of the laminae within the trunk vertebrae of a single specimen of Calyptosuchus wellesi. In PEFO 46222, the posterior centrodiapophyseal lamina is weakly developed in the middle trunk region (Fig. 5.2) and becomes more prominent in the posterior trunk region (Fig. 5.8). Based on this observation, we hypothesize that the posterior centrodiapophyseal lamina may be more incipient in the anterior trunk region, but we cannot confirm this due to limited specimens and preservation. A well-defined postzygadiapophyseal lamina has been described in the middle trunk vertebrae of Calyptosuchus wellesi based on UMMP 7470 (Fig. 12.1; Parker, Reference Parker2018a). However, that morphological feature is absent in PEFO 46222 (Fig. 5.3, 5.7), indicating that this state is variable in the taxon. Due to the poor preservation of the middle trunk vertebrae in UMMP 13950, we are unable to assess the presence of postzygadiapophyseal laminae in that specimen.
In addition to the noted variation in the vertebral column of Calyptosuchus wellesi, PEFO 46222 also provides more clarity on the variation of the osteoderms across the dorsal carapace of the taxon (Figs. 8, 9, 15). We document variation associated with morphological characters of the anterior bar in paramedian osteoderms including the presence of a scalloped edge, anterior triangular projection, and the anterolateral process in the trunk region (Parker, Reference Parker2016a). The scalloped edge and anterior triangular projection vary in how prominent or well incised they are across the same region of the carapace. Additionally, the anterolateral process varies in its lateral inclination with no real consistent pattern, however, as noted by Parker (Reference Parker2018a), it does reduce in its prominence/size caudally in the carapace. Our re-examination of the articulated carapace in UMMP 13950 indicates that the lateral edge of the paramedian osteoderms is weakly sigmoidal in the mid- through posterior-trunk region and is more straight in the caudal region, a feature that is also reflected in the adjacent lateral osteoderms.
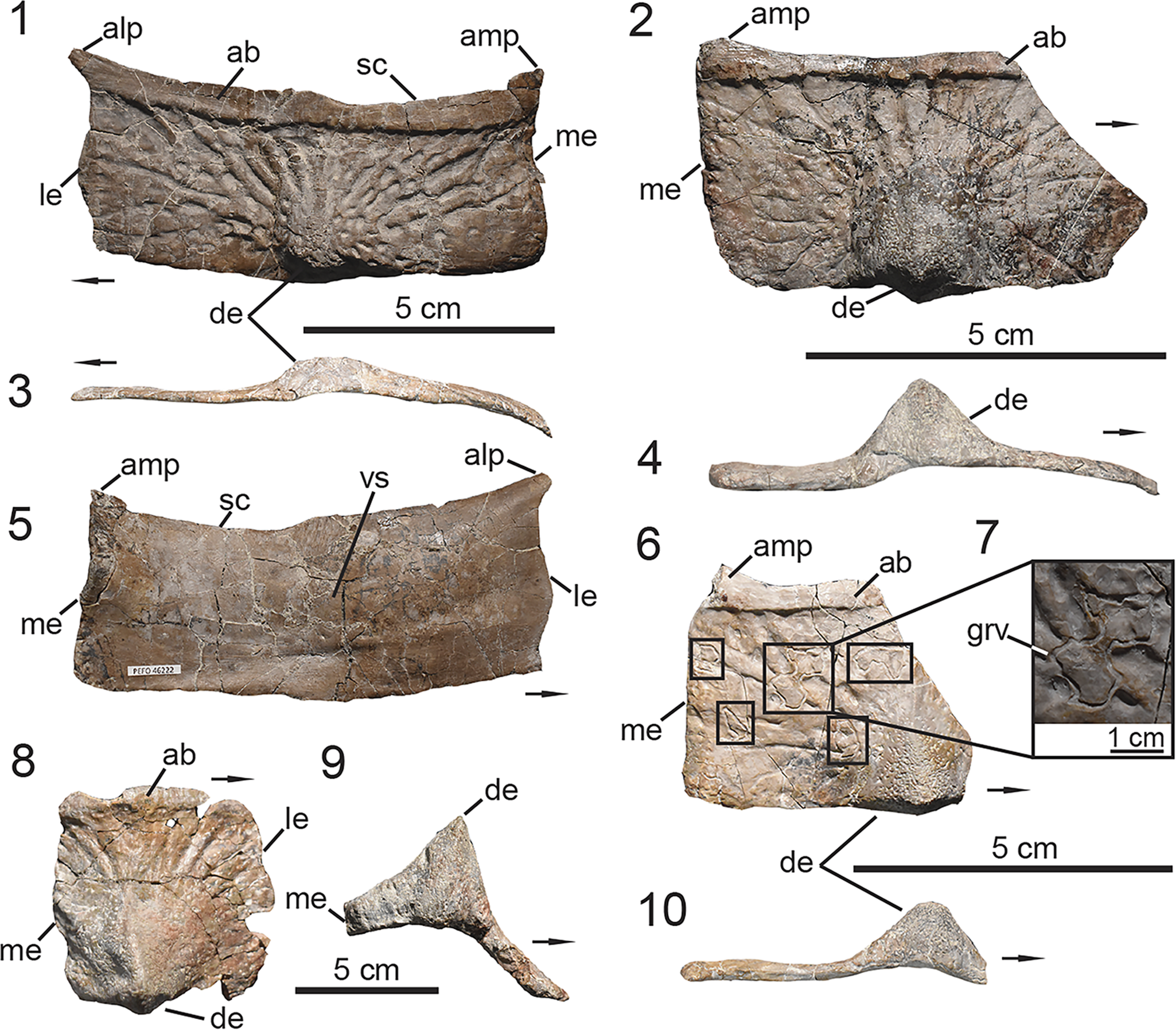
Figure 9. PEFO 46222, associated posterior trunk/sacral and caudal paramedian osteoderms. (1, 3, 5) Isolated paramedian osteoderm derived from the posterior trunk or sacral region; (2, 4, 6–10) caudal paramedian osteoderms; (6, 7, 10) caudal osteoderm showing possible invertebrate traces on the dorsal surface. (1, 2, 6–8) Dorsal views, (5) ventral view, (3, 4, 9, 10) posterior views. ab = anterior bar; alp = anterolateral process; amp = anteromedial process; de = dorsal eminence; grv = groove; le = lateral edge; me = medial edge; sc = scalloped; vs = ventral strut. Arrows indicate lateral direction.
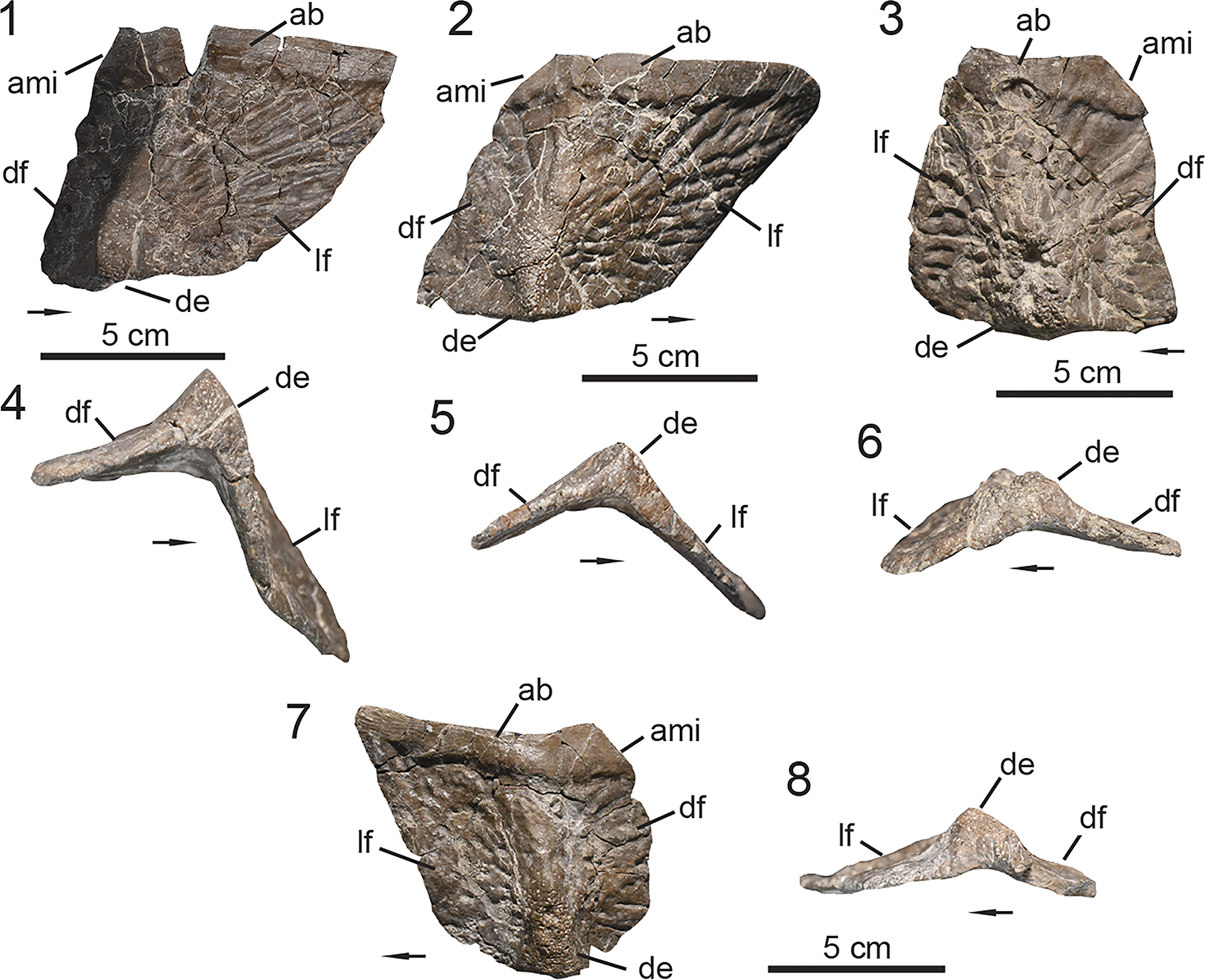
Figure 10. PEFO 46222, associated lateral osteoderms. (1, 2, 4, 5) Mid-trunk lateral osteoderms; (3, 6) posterior trunk/sacral lateral osteoderms; (7, 8) caudal lateral osteoderms. (1–3, 7) Dorsal views, (4–6, 8) posterior views. ami = anteromedial incision; ab = anterior bar; de = dorsal eminence; df = dorsal flange; lf = lateral flange. Arrows indicate lateral direction.
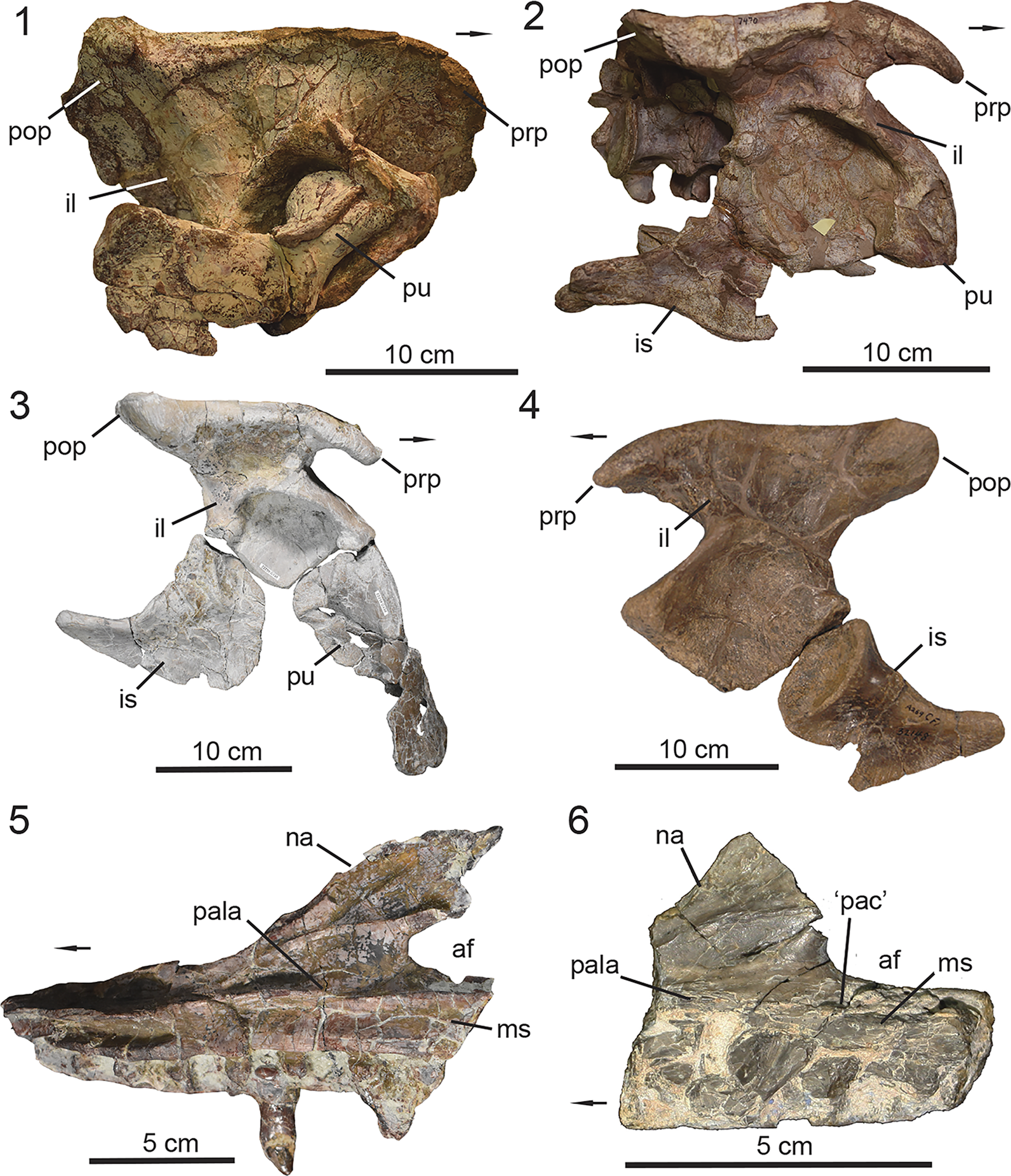
Figure 11. Taxonomic assessment of elements referred to Calyptosuchus wellesi from A269. (1) UMMP 13950, partial right portion of pelvis; (2) UMMP 7470, right aspect of partial pelvic girdle; (3) PEFO 46222, right half of disarticulated pelvis; (4) UCMP 25941 and UCMP 32148, associated pelvis from UCMP A269; (5) PEFO 49321, fragment of right maxilla; (6) UCMP 195193, fragmentary right maxilla. (1–4) Lateral views, (5, 6) medial views. af = antorbital fenestra; il = ilium; is = ischium; ms = medial shelf; na = nasal articulation; ‘pac’ = misidentified ‘pneumatic accessory cavity’; pala = palate articulation; pu = pubis; prp = preacetabular process; pop = postacetabular process. Arrows indicate anterior direction.
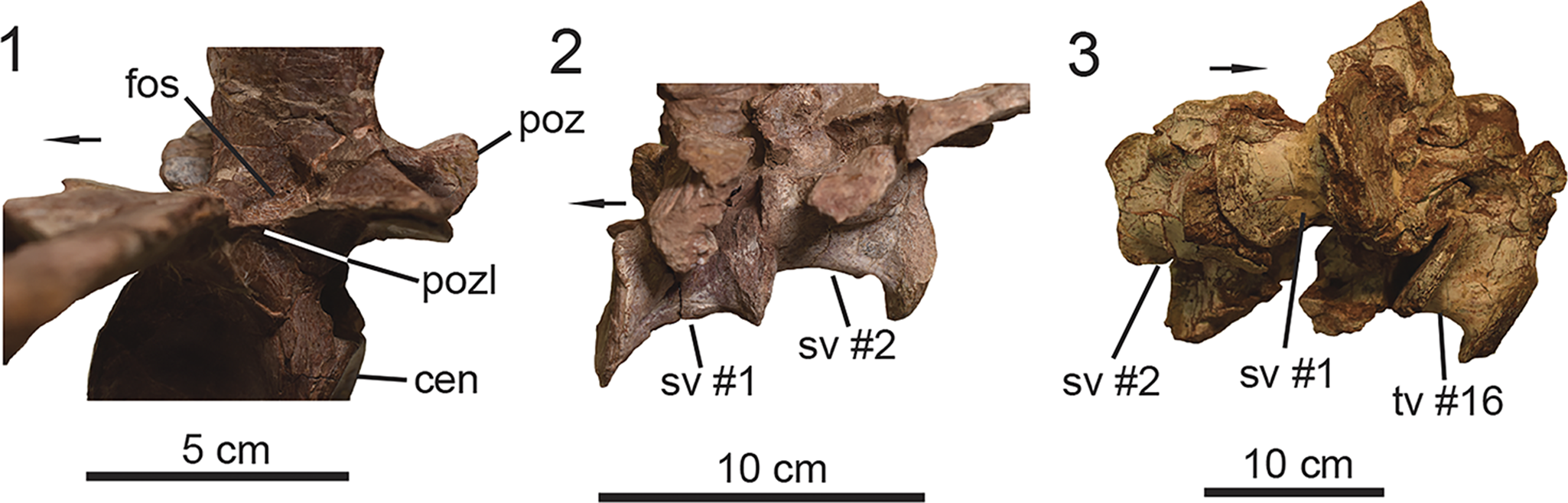
Figure 12. Noted areas of intraspecific variation within Calyptosuchus wellesi. (1) Postzygapophyseal lamina present in the middle trunk vertebra of UMMP 7470; un-co-ossified sacral vertebrae in (2) UMMP 7470 and (3) UMMP 13950. (1, 2) Lateral views, (3) ventral view. cen = centrum; fos = fossa; poz = postzygapophysis; pozl = postzygapophyseal lamina; sv = sacral vertebra; tv = trunk vertebra. Arrows indicate anterior direction.
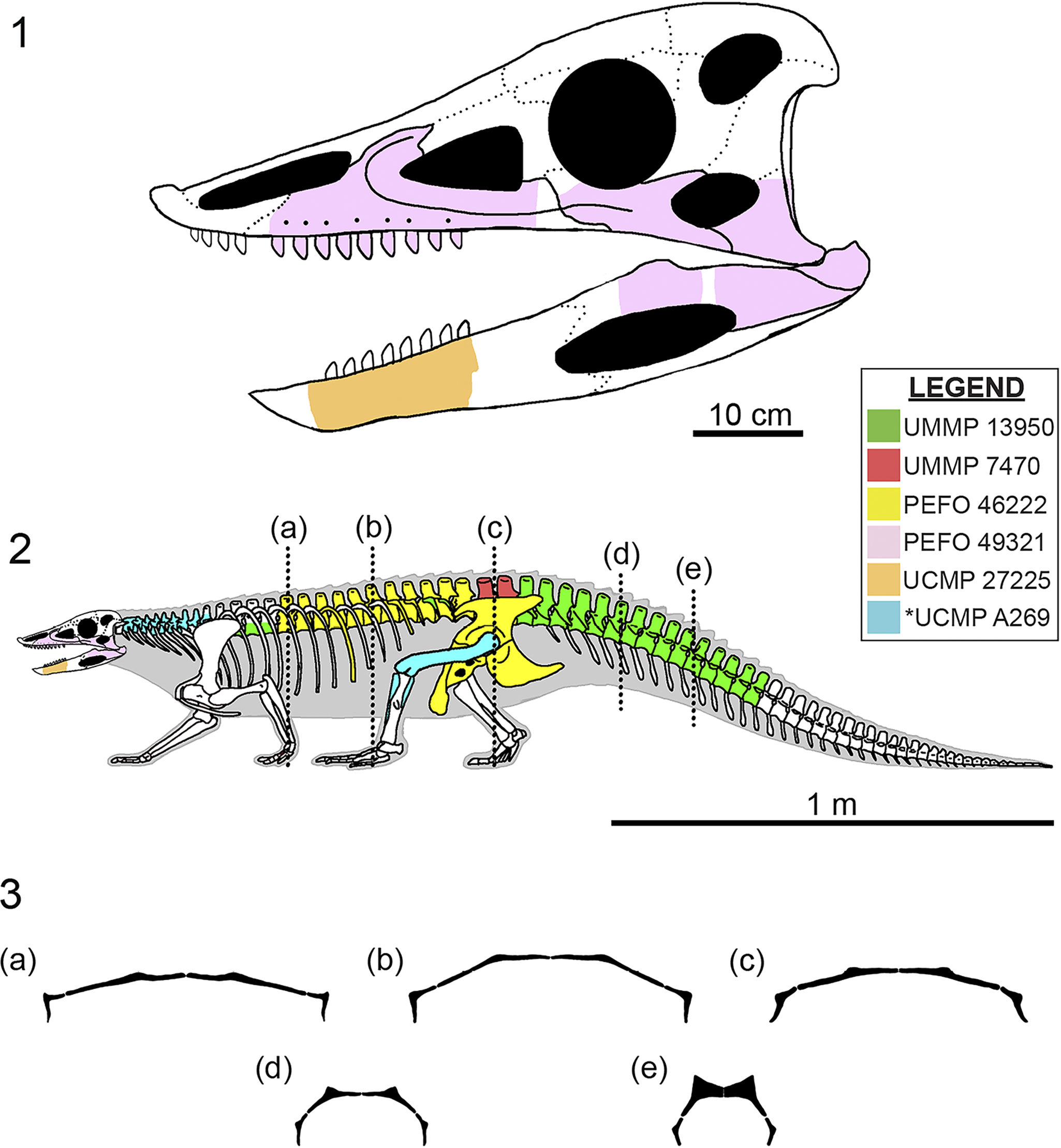
Figure 13. Hypothetical reconstructive illustrations of Calyptosuchus wellesi. (1) Skull reconstruction in left lateral view based on PEFO 49321 and UCMP 27225, and the closely related taxa Neoaetosauroides engaeus (Desojo and Báez, Reference Desojo and Báez2007) and Scutarx deltatylus (Parker, Reference Parker2016b). Dotted lines indicate hypothetical contacts. Colored regions indicate anatomy preserved. (2) Skeletal reconstruction in left lateral view. The osteoderms are excluded, but the carapace is incorporated into the silhouette of the body. The reconstruction of Stagonolepis robertsoni (Walker, Reference Walker1961) served as the template, but was modified accordingly based on the anatomy of UCMP 27225, PEFO 46222, PEFO 49321, UMMP 7470, UMMP 13950, and specimens referred to C. wellesi from UCMP A269 (the Placerias Quarry) by Parker (Reference Parker2018a). Colored regions based on specimen(s) that best exemplify the anatomy. The specimens from UCMP A269 are incorporated into a single label (* = fossil locality). (3) Transverse cross-sections of the dorsal carapace through (a) anterior middle trunk, (b) posterior middle trunk, (c) sacral, and (d) anterior portion and (e) middle portion of the middle caudal regions. Cross-sections (not to scale) of the dorsal carapace are based on PEFO 46222; sectioned regions are represented by vertical dashes in (2).
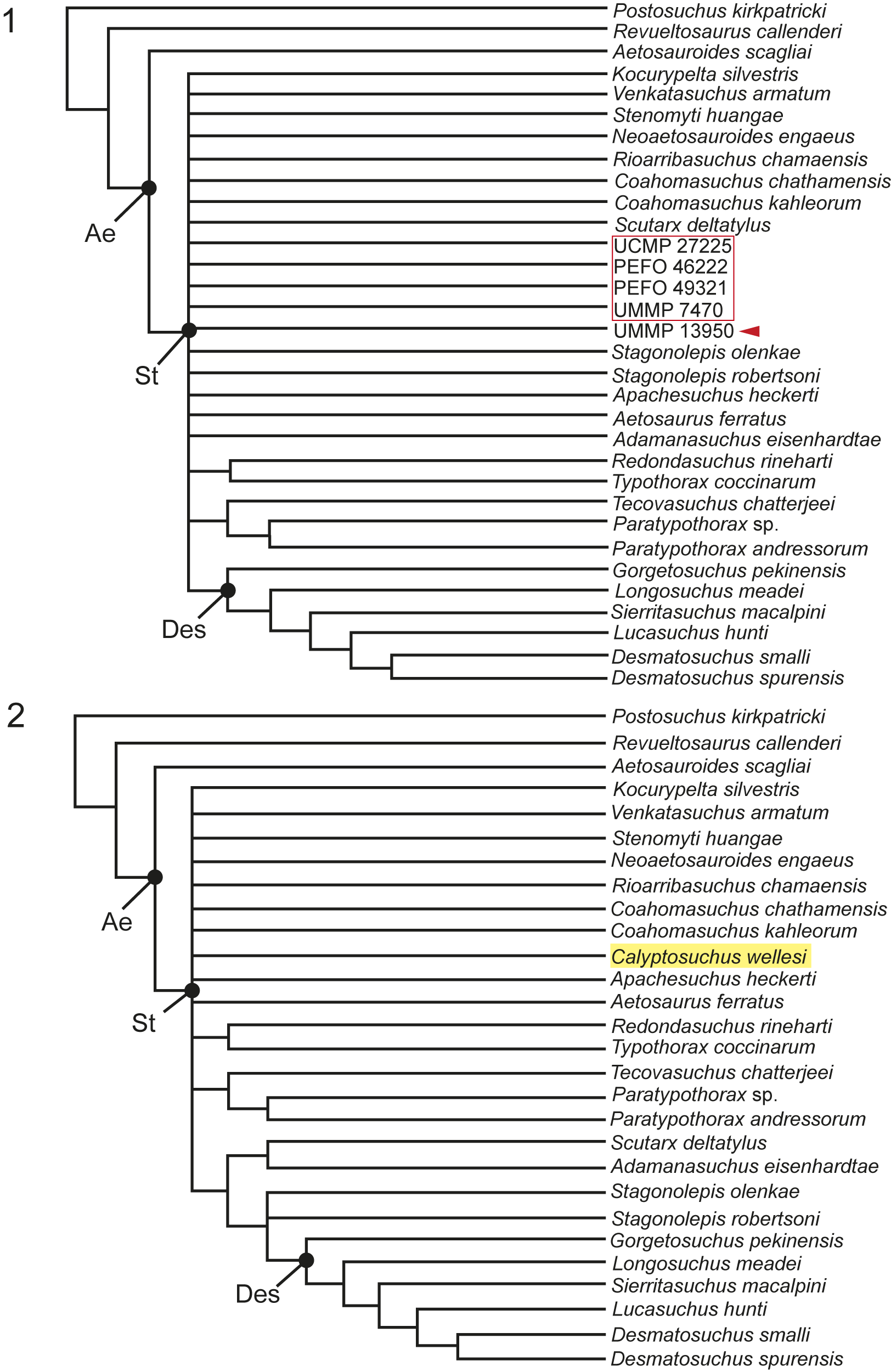
Figure 14. Maximum parsimony phylogenetic analyses. (1) Run 1: Strict consensus of the holotype specimen of Calyptosuchus wellesi, UMMP 13950 (indicated by red arrow), with other referred individuals (indicated by red-bounded box). (2) Run 2: Strict consensus of composite OTU Calyptosuchus wellesi (indicated by yellow highlight). Ae = Aetosauria; Des = Desmatosuchini; St = Stagonolepididae.
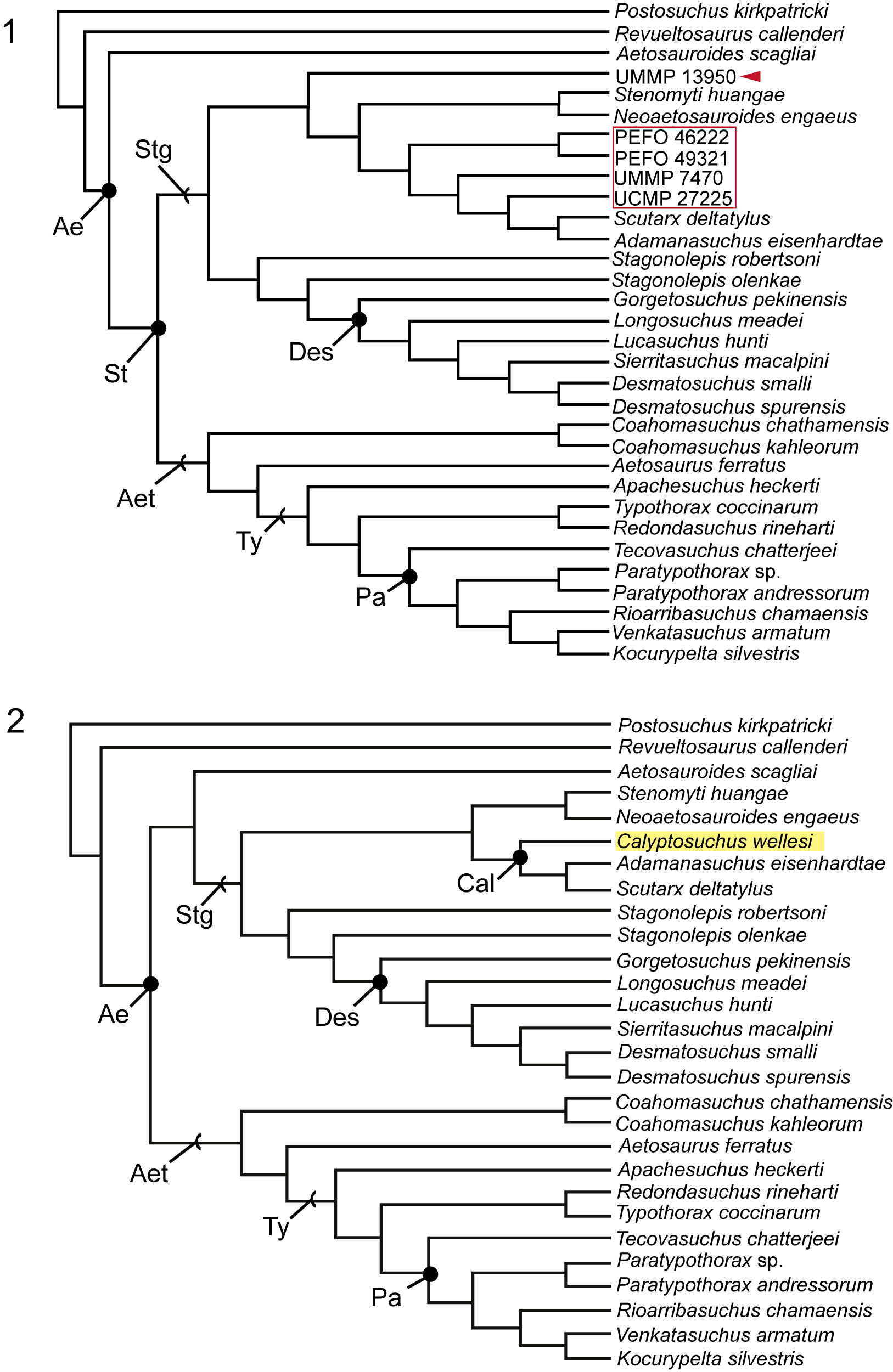
Figure 15. Bayesian inference phylogenetic analyses. (1) Run 1: Bayesian consensus cladogram of the holotype specimen of Calyptosuchus wellesi, UMMP 13950 (indicated by red arrow), with other referred individuals (indicated by red-bounded box). (2) Run 2: Bayesian consensus cladogram of composite OTU Calyptosuchus wellesi (indicated by yellow highlight). Ae = Aetosauria; Aet = Aetosaurinae; Cal = Calyptosuchini n. clade; Des = Desmatosuchini; Pa = Paratypothoracini; St = Stagonolepididae; Stg = Stagonolepidoidea; Ty = Typothoracinae.
Status of elements referred to Calyptosuchus wellesi from the Placerias Quarry
The new specimens from PEFO bring to question the taxonomic affinities of elements from the Placerias Quarry (UCMP A269) referred by previous authors to Calyptosuchus wellesi (e.g., Long and Murry, Reference Long and Murry1995; Parker, Reference Parker2018a). UCMP 195193 is a partial right maxilla referred to Calyptosuchus wellesi by Parker (Reference Parker2018a) and is characterized by the presence of a ‘pneumatic accessory cavity’ (Fig. 11.6). However, when comparing this anatomical feature to that of Stenomyti huangae (Small and Martz, Reference Small, Martz, Nesbitt, Desojo and Irmis2013), Desmatosuchus smalli (Small, Reference Small2002), and Stagonolepis (Walker, Reference Walker1961; Sulej, Reference Sulej2010; Parker, Reference Parker2018b), it is evident that the ‘pneumatic accessory cavity’ described within UCMP 195193 is not homologous to those observed within the mentioned taxa because it is not characterized as an anteroposteriorly oriented pit positioned anteroventral to the junction of the anterior and ventral margins of the antorbital fenestra. Thus, the ‘pneumatic accessory cavity’ within UCMP 195193 is likely a taphonomic feature and is absent in this specimen as observed in PEFO 46222. However, this reinterpretation and the presence of a deep antorbital fossa that is delineated ventrally by a ridge on the lateral surface do support the referral of UCMP 195193 to Calyptosuchus wellesi, as originally proposed by Parker (Reference Parker2018a).
An isolated sacral vertebra (UCMP 139785) was originally referred to Calyptosuchus wellesi because it was not co-ossified to another trunk or sacral vertebra (Parker, Reference Parker2018a), a condition characteristic of Desmatosuchus (Parker, Reference Parker2005, Reference Parker2008a), which is also present at UCMP A269 (Parker, Reference Parker2018a; von Baczko et al., Reference von Baczko, Desojo, Gower, Ridgely, Bona and Witmer2021). However, PEFO 46222 indicates that co-ossification of the sacral vertebrae, albeit variable, is a condition also exhibited by Calyptosuchus wellesi (Fig. 9.1). Thus, referral of the isolated sacral vertebra UCMP 139785 remains tentative.
Historically, the pelvic anatomy of Calyptosuchus wellesi was based on an associated left ilium and ischium (UCMP 25941 and UCMP 32148, respectively) from UCMP A269 (Fig. 11.4; Long and Murry, Reference Long and Murry1995; Parker, Reference Parker2018a) due to the poor preservation of UMMP 13950 (Fig. 11.1) and distortion of UMMP 7470 (Fig. 11.2). Together, UMMP 13950, UMMP 7470, and PEFO 46222 (Fig. 12.1–12.3) provide a composite picture of the pelvic anatomy in Calyptosuchus wellesi, thus allowing us to assess the referral of UCMP 25941 and UCMP 32148 to C. wellesi. The ilia are similar in size across these individuals, yet they vary in the shape of the preacetabular process, inclination of the iliac blade, roundness of the postacetabular process, the outline of the ilium between the preacetabular process and pubic peduncle in lateral view, and the width of the acetabulum (Fig. 11.1–11.4). Additionally, the ischia do not share a rod-like body that becomes ventrally gracile near the ischiadic symphysis.
It is unlikely that UCMP 25941 and UCMP 32148 are referrable to Desmatosuchus. The pelvic anatomy of Desmatosuchus is currently exemplified by that of MNA V9300, a specimen of D. spurensis that preserves a complete pelvic girdle that is heavily distorted and exhibits crushed preacetabular processes of the ilia (Parker, Reference Parker2008a), thus, limiting the utility of MNA V9300 in our comparison. However, the acetabular region of UCMP 25941 does bear a resemblance to that of a partial ilium preserved within the holotype of Desmatosuchus spurensis (UMMP 7476, Parker, Reference Parker2008a), which exhibits a poorly preserved preacetabular process and body, and is missing the postacetabular process. A recently prepared pelvic girdle (TTU-P 9419) from MOTT 3624 (the Post Quarry) that is referrable to Desmatosuchus smalli, which is also reported from UCMP A269 (von Baczko et al., Reference von Baczko, Desojo, Gower, Ridgely, Bona and Witmer2021), preserves nearly complete ilia that are undistorted, only missing their preacetabular processes. Based on TTU-P 9419, the postacetabular process of Desmatosuchus is not expanded and exhibits a posterodorsally inclined iliac blade in lateral view, which are conditions unlike that of UCMP 25941.
Although the anatomy of UCMP 25941 and UCMP 32148 from UCMP A269 show variation from the pelvic girdles of UMMP 13950, UMMP 7470, and PEFO 46222 (Fig. 11), their referral to Calyptosuchus wellesi remains contentious because they were collected in a quadrant dominated by osteoderms and vertebrae of that taxon (see Parker, Reference Parker2018a). It is possible that the morphological variation observed in the pelvic girdles of these specimens is attributable to intraspecific variation, similar to the observed variation in the co-ossification of their sacral vertebrae (Fig. 12). Alternatively, although UCMP 25941 and UCMP 32148 are unlikely to be referable to Desmatosuchus (see above), they could be referrable to either the typothoracine Kryphioparma caerula (Reyes et al., Reference Reyes, Parker and Heckert2023) or ambiguous paratypothoracin (Parker, Reference Parker2005, Reference Parker2007) that are also documented within UCMP A269 (Parker, Reference Parker2007; Reyes et al., Reference Reyes, Parker and Heckert2023; Parker and Haldar, Reference Parker and Haldar2024). Unfortunately, those taxa are known solely from their osteoderms and the loss of original association within UCMP A269 (Parker, Reference Parker2018a) inhibits our ability to refer the pelvis in question to either taxon. Thus, the referrals of UCMP 25941 and UCMP 32148 to Calyptosuchus wellesi remain tentative. A better understanding of the intraspecific variation of the pelvic girdle in aetosaurians is needed to unambiguously reject their referral to Calyptosuchus wellesi.
Aetosaur ecology
Since the beginning of the twenty-first century, the ecology of aetosaurs has been a topic of high interest among aetosaur researchers (e.g., Small, Reference Small2002; Desojo and Báez, Reference Desojo and Báez2007; Desojo and Vizcaíno, Reference Desojo and Vizcaíno2009; Heckert et al., Reference Heckert, Lucas, Rinehart, Celeskey, Spielmann and Hunt2010; Sulej, Reference Sulej2010; Desojo and Ezcurra, Reference Desojo and Ezcurra2011; Desojo et al., Reference Desojo, Heckert, Martz, Parker, Schoch, Small, Sulej, Nesbitt, Desojo and Irmis2013; Biacchi Brust et al., Reference Biacchi Brust, Desojo, Schultz, Paes-Neto and Da-Rosa2018; von Baczko et al., Reference von Baczko, Taborda and Desojo2018, Reference von Baczko, Desojo, Gower, Ridgely, Bona and Witmer2021; Reyes et al., Reference Reyes, Parker and Marsh2020; Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021b; Taborda et al., Reference Taborda, Desojo and Dvorkin2021). This is due to the discoveries and/or reports of new specimens preserving dentigerous material for a variety of aetosaur taxa (e.g., Aetosauroides scagliai, Calyptosuchus wellesi, Coahomasuchus kahleorum, C. chathamensis, Paratypothorax andressorum, Stenomyti huangae, Stagonolepis olenkae, Typothorax coccinarum), which have brought to light the extent of the anatomical disparity in their dentition across the clade (Reyes et al., Reference Reyes, Parker and Marsh2020; Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021b). Based on this new understanding, their dentition can be generalized into three morphotypes: (1) a basally constricted, peg-like tooth with a bulbous, spade-like crown with fine serrations, as exemplified by Desmatosuchus (Small, Reference Small2002), Stagonolepis (Walker, Reference Walker1961; Sulej, Reference Sulej2010; Antzack, Reference Antczak2015), and Neoaetosauroides engaeus (Desojo and Báez, Reference Desojo and Báez2007); (2) a slightly basally constricted tooth with an apicobasally straight distal margin and curved mesial margin that meet at an apex that does not project distally beyond the basal margin, as exemplified by Aetosaurus ferratus (Schoch, Reference Schoch2007), Stenomyti huangae (Small and Martz, Reference Small, Martz, Nesbitt, Desojo and Irmis2013), Paratypothorax andressorum (Schoch and Desojo, Reference Schoch and Desojo2016), and Calyptosuchus wellesi (Fig. 3.11–3.14); and (3) a non-basally constricted tooth with a serrated and recurved crown with an apex that projects distally beyond the basal margin, as exemplified by Aetosauroides scagliai (Biacchi Brust et al., Reference Biacchi Brust, Desojo, Schultz, Paes-Neto and Da-Rosa2018; Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021b) and Coahomasuchus (Parker, Reference Parker2016a; Heckert et al., Reference Heckert, Fraser and Schneider2017). Currently, only Typothorax coccinarum exhibits a heterodont dentition that is composed of two of the mentioned morphotypes (Reyes et al., Reference Reyes, Parker and Marsh2020).
Historically, it was hypothesized that aetosaurs represented an herbivorous group of archosaurs (Walker, Reference Walker1961). However, because of the recognized disparity in their dentition, it is now hypothesized that aetosaurs were likely an omnivorous/faunivorous group (Small, Reference Small2002; Desojo and Báez, Reference Desojo and Báez2007; Desojo et al., Reference Desojo, Heckert, Martz, Parker, Schoch, Small, Sulej, Nesbitt, Desojo and Irmis2013; von Baczko et al., Reference von Baczko, Taborda and Desojo2018; Reyes et al., Reference Reyes, Parker and Marsh2020; Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021b). This hypothesis is further supported by the presence of recurved, serrated teeth in the clade (e.g., Aetosauroides scagliai, Biacchi Brust et al., Reference Biacchi Brust, Desojo, Schultz, Paes-Neto and Da-Rosa2018; Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021b; Coahomasuchus, Parker, Reference Parker2016a; Heckert et al., Reference Heckert, Fraser and Schneider2017; Parker et al., Reference Parker, Stocker, Reyes and Werning2025), which is suggestive of a faunivorous ecology and rejects the previous hypothesis that they were strictly herbivorous animals (e.g., Walker, Reference Walker1961). Additionally, the absence of taxa with ‘herbivorous-like’ teeth that are basally constricted and exhibit large denticles further weakens the hypothesis that aetosaurs were a herbivorous clade because this morphotype is typically associated with a herbivorous ecology (Parker et al., Reference Parker, Irmis, Nesbitt, Martz and Browne2005) and is observed in other Late Triassic archosaurs such as the aetosauriforms Revueltosaurus callenderi (Parker et al., Reference Parker, Nesbitt, Irmis, Martz, Marsh, Brown, Stocker and Werning2021) and Acaenasuchus geoffreyi (Marsh et al., Reference Marsh, Smith, Parker, Irmis and Kligman2020), as well as silesaurid dinosauriforms (e.g., Martz and Small, Reference Martz and Small2019). Thus, the dental morphology of Calyptosuchus wellesi (Fig. 3.11–3.14) does not align with that associated with a strictly herbivorous ecology, further bolstering the hypothesis that aetosaurs were likely an omnivorous/faunivorous group.
Calyptosuchus wellesi is recovered as a relative of Neoaetosauroides engaeus, Stagonolepis, and desmatosuchins within the Stagonolepidoidea (Fig. 15). However, its dentition is unlike that of those taxa, suggesting that the dentition among calyptosuchins may have diverged from the peg-like tooth exhibited by other stagonolepidoids. By proxy, this would suggest that calyptosuchins may have exhibited a different ecology from those taxa. If so, the variation in dentition among stagonolepidoids may correlate with why calyptosuchins and Desmatosuchus are often recovered together within fossiliferous localities (e.g., PFV 456, Reyes et al., Reference Reyes, Parker and Heckert2023; UCMP A269, Parker, Reference Parker2018a; MOTT 3624, Martz et al., Reference Martz, Mueller, Nesbitt, Stocker, Parker, Atanassov, Fraser, Weinbaum and Lehane2013) in Norian-age strata of the southwestern United States (Parker and Martz, Reference Parker and Martz2011). Their coexistence may indicate niche partitioning because these taxa likely would not have been in direct competition for resources (i.e., food), as suggested by the dental variation between Calyptosuchus wellesi (Fig. 3.11–3.14) and Desmatosuchus smalli (Small, Reference Small2002).
Conclusions
The discovery of two new specimens referable to Calyptosuchus wellesi from Petrified Forest National Park prompted re-interpretation of the holotype specimen (UMMP 13950) and provided new data on the cranial anatomy, ecology, positional variation, and intraspecific variation of the taxon (Fig. 13). The first unambiguous cranial material of Calyptosuchus wellesi (PEFO 49321) indicates that its skull anatomy (Fig. 13.1) is similar to that of Stagonolepis olenkae and Neoaetosauroides engaeus. Additionally, it highlights the disparate anatomy of the dentition within the Aetosauria, providing more support for the hypothesis that some members of the clade were more omnivorous/faunivorous than previously recognized (Desojo and Ezcurra, Reference Desojo and Ezcurra2011; von Baczko et al., Reference von Baczko, Taborda and Desojo2018, Reference von Baczko, Desojo, Gower, Ridgely, Bona and Witmer2021; Reyes et al., Reference Reyes, Parker and Marsh2020; Paes Neto et al., Reference Paes Neto, Desojo, Brust, Ribeiro, Schultz and Soares2021b).
Aetosaurs, particularly their osteoderms, are considered biostratigraphically informative across the southwestern USA because they provide a means of temporally constraining Upper Triassic strata in the region (Long and Murry, Reference Long and Murry1995; Heckert and Lucas, Reference Heckert and Lucas2000; Parker and Martz, Reference Parker and Martz2011; Parker, Reference Parker2016a; Martz and Parker, Reference Martz, Parker, Parker and Zeigler2017), and their dorsal and lateral osteoderms play a key role in our ability to assess taxonomic affinities (Parker and Martz, Reference Parker and Martz2010, Reference Parker and Martz2011; Parker, Reference Parker2016a). Thus, it is important that we understand the degree of variation within the dorsal carapace to improve phylogenetic and biostratigraphic hypotheses (Reyes et al., Reference Reyes, Martz and Small2024). The associated skeleton of PEFO 46222 adds new understanding of the osteoderm anatomy and positional variation within the dorsal carapace of Calyptosuchus wellesi (Fig. 13.2, 13.3), thus refining our ability to document isolated osteoderms of Calyptosuchus wellesi and utilize them as a biostratigraphic marker to correlate Late Triassic strata across the southwestern USA (Parker and Martz, Reference Parker and Martz2011).
PEFO 46222 also adds new insight into both the positional and intraspecific variation within the vertebral column of Calyptosuchus wellesi, particularly the documentation of co-ossified sacral vertebrae. This indicates that the co-ossification of the sacral vertebrae is more widely shared across the Aetosauria than previously recognized and suggests that it is likely associated with ontogeny or sexual dimorphism. This new anatomical understanding of Calyptosuchus wellesi expands our understanding of the inter- and intraspecific variation within the postcranial skeleton of aetosaurs.
The coding of polymorphic characters for Calyptosuchus wellesi did not influence its hypothesized topological position within the Aetosauria. The documentation of two foramina within the pubic apron of Calyptosuchus wellesi indicates that this state is more widely distributed across the Aetosauria than previously recognized and is a synapomorphy of the new clade Calyptosuchini. The recovery of Stagonolepis robertsoni as a non-calyptosuchin stagonolepidoid suggests that our current matrix needs to be further expanded to account for more morphological patterns within the clade because S. robertsoni exhibits a strong resemblance to Calyptosuchus wellesi as opposed to desmatosuchins. Our taxonomic reassessment of specimens previously referred to Calyptosuchus wellesi highlights the issue surrounding our ability to identify isolated elements from UCMP A269. The loss of original association inhibits our ability to refer many specimens, most of which remain taxonomically ambiguous, to known taxa or determine if they represent new, previously unrecognized species with a high degree of confidence (Parker, Reference Parker2018a; Reyes et al., Reference Reyes, Parker and Heckert2023).
Acknowledgments
We thank the Youth Conservation Corp, members of the 2019 Virginia Tech Paleobiology & Geobiology Research Group, and PEFO vertebrate paleontology interns X. Jenkins, E. Patellos, and E. Armour Smith. M. Smith, D. Wagner, D. Boudreau, and P. Varela prepared and curated the PEFO specimens. We thank K. Mackenzie at the DMNH, K. Dean-Wallace and J.-H. Voss at TTU-P, N. Volden at the NMMNH, A. Rountrey at the UMMP, F.J. Prevosti and D. Ruiz-Ramoni at the PULR, and P. Ortiz at the PVL, for providing access to comparative specimens used in this study. Thanks to V. Paes-Neto and D. Dróżdż for sharing specimen photographs and 3D models. We thank A. Turner for helping us troubleshoot our phylogenetic analysis in TNT. This study was supported by the United States National Park Service (PMIS 209814 to W. Parker, A. Marsh, and B. Kligman), the Petrified Forest Museum Association (to W. Reyes), Jackson School of Geosciences Off-Campus Research Grant (to W. Reyes), UT-Austin Graduate School Mentoring Fellowship (to W. Reyes) and the National Science Foundation (No. 2137420 to W. Reyes). C. Bell, M. Stocker, and J. Clarke provided insightful discussion and constructive reviews during the development of the manuscript. We thank H-D, Sues, T. Sulej, V. Paes-Neto, and an anonymous reviewer for their peer reviews. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation or of the United States Government. This is Petrified Forest National Park paleontological contribution no. 93. This paper is dedicated to the memory of Robert A. Long (1946–2023).
Competing interests
The authors declare none.
Data availability statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.w0vt4b91s.


















