Non-technical Summary
A new crinoid, Tuscumbiacrinus madisonensis n. gen. n. sp., is described from the Middle Mississippian (about 340 million years ago) of northern Alabama. It belongs to the enigmatic family Paragaricocrinidae, which is now known globally from the Middle Mississippian through the middle Permian. Tuscumbiacrinus n. gen. is the oldest known representative of this family. A re-examination of the entire family resulted in the recognition of four new genera, four new species, and one species is reassigned to a new genus. The Paragaricocrinidae is unusual because the anatomical construction of the body is more typical of morphologies characterizing Middle Paleozoic crinoids than Late Paleozoic forms. Further, very few specimens of this family are known, especially from the Permian. Following an abrupt drop in clade diversity, phylogenetic and macroevolutionary patterns indicate the Paragaricocrinidae exemplify patterns similar to a “dead clade walking,” in which a clade temporally persists after a decline at low taxonomic richness, abundance, and ecologic innovation before finally becoming extinct.
Introduction
Paleozoic crinoid faunas dominated by camerates with large, robust, many-plated calyxes ceased to exist during the middle Viséan (Middle Mississippian) transition from the middle Paleozoic Crinoid Evolutionary Fauna (CEF) to the late Paleozoic CEF (Ausich et al., Reference Ausich, Kammer and Baumiller1994, Reference Ausich, Kammer, Mirantsev, Lucas, Schneider, Wang and Nikolaeva2022; Baumiller, Reference Baumiller, David, Guille, Féral and Roux1994). More typically, the late Paleozoic CEF contained camerates with non-robust calyxes with relatively few plates, such as platycrinitids and dichocrinids. Exceptions always exist, such as Actinocrinites Miller, Reference Miller1821 (Tournaisian–Early Permian) and Thinocrinus (Tournaisian–Early Permian) (Rhenberg et al., Reference Rhenberg, Ausich and Kammer2015).
Another exception is the enigmatic Paragaricocrinidae that ranges from the Mississippian (middle Viséan) to the Permian (Wordian) with a nearly global distribution. Despite its long temporal duration, this family is known from relatively few species and from very few specimens. The dearth of specimens cannot solely be a sampling artifact because co-occurring echinoderm taxa form a natural taphonomic control (Bottjer and Jablonski, Reference Bottjer and Jablonski1988; Meyer et al., Reference Meyer, Ausich and Terry1989). Paragaricocrinids were typically more robustly constructed than other crinoids in the faunas in which they occur, a property that enhances the relative preservation probability in fossil crinoids (Meyer et al., Reference Meyer, Ausich and Terry1989; Ausich, Reference Ausich2021).
Prior to this study, 49 specimens were known in the Paragaricocrinidae, either named species or taxa left in open nomenclature (Fig. 1). Including new taxa described herein (Tuscumbiacrinus madisonensis n. gen. n. sp.; Palenciacrinus mudaensis n. gen. n. sp.; Nipponicrinus hashimotoi n. gen. n. sp.; Nipponicrinus akiyoshiensis n. gen. n. sp.; and Pulcheracrinus n. gen.), the Paragaricocrinidae has eleven described species confidently assigned to a genus based on 30 specimens. An additional 22 specimens remain questionably assigned to a genus of the Paragaricocrinidae or left in open nomenclature (Figs. 1, 2). These taxa are scattered globally through more than ca. 76 million years; and during the Late Pennsylvanian and Permian, the Paragaricocrinidae was a prime example of a “dead clade walking” (Jablonski, Reference Jablonski2002; Stillwell and Häkansson, Reference Stillwell, Häkansson and Talent2012).
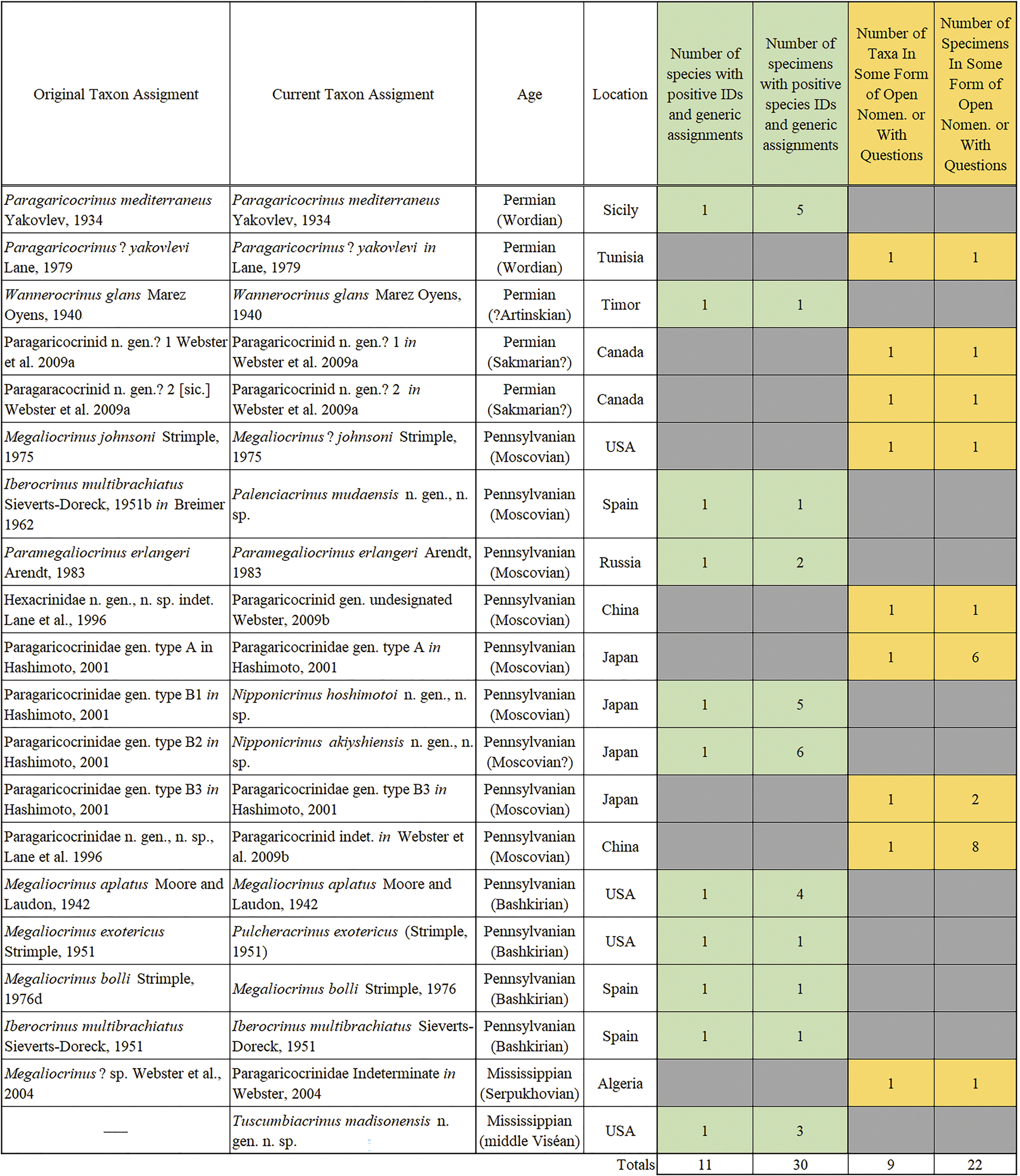
Figure 1. Listing of Paragaricocrinidae mentioned in the literature, in chronostratigraphic order with the oldest at the bottom. The diagram includes the original name in the literature, the name used in the present contribution, chronostratigraphic age, country of origin, and an accounting of the number of specimens in each category of species confidently assigned to a genus (green) and specimens questionably assigned to a genus or left in open nomenclature (yellow).
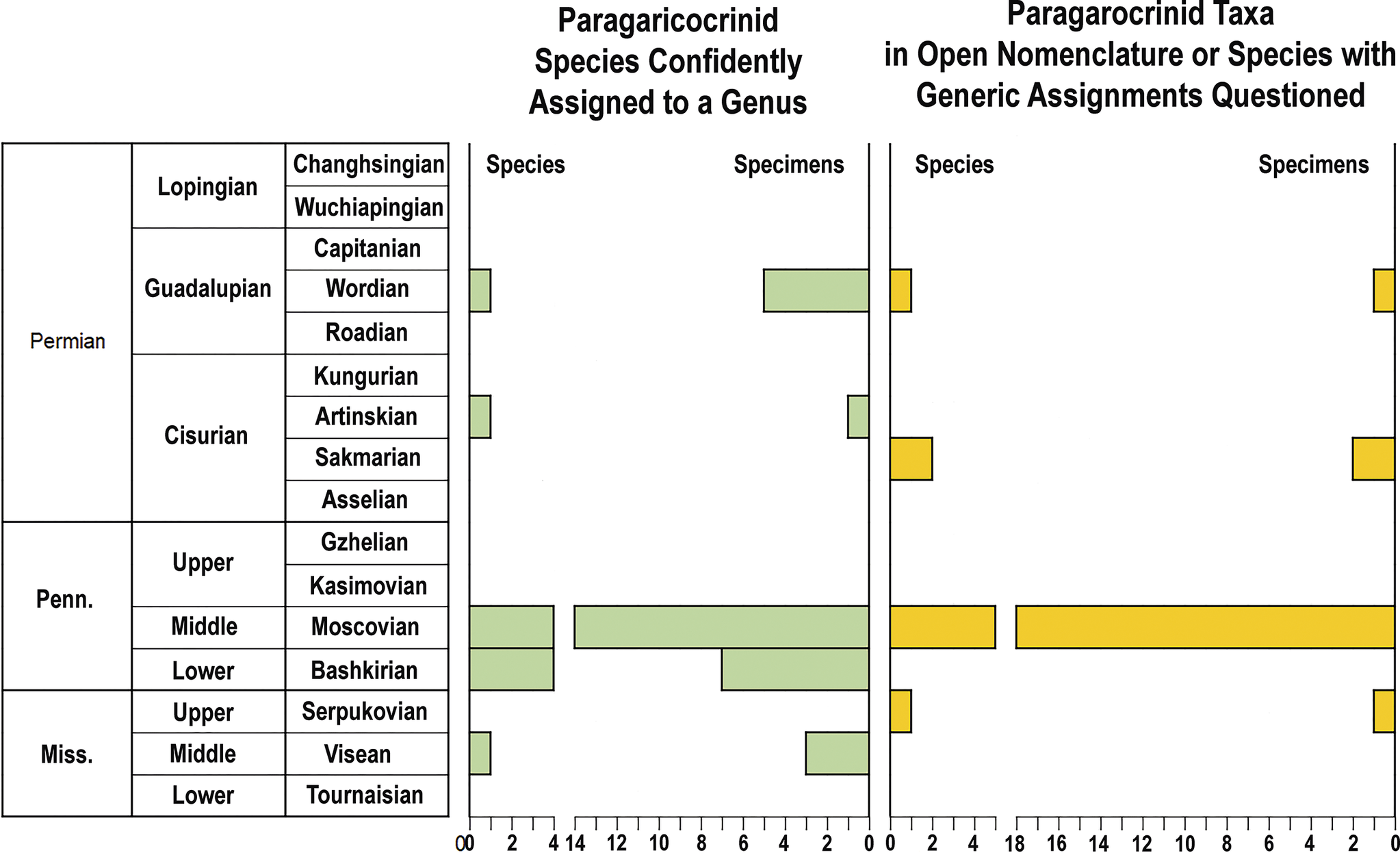
Figure 2. Numbers of paragaricocrinid species and specimens through the Upper Paleozoic for species confidently assigned to a genus (green) and for paragariocrinid specimens questionably assigned to a genus or left in open nomenclature (yellow).
The initial goal of this study was to describe the new paragaricocrinid, Tuscumbiacrinus madisonensis n. gen. n. sp. from the upper Tuscumbia Limestone (middle Viséan) on the East Warrior platform (Thomas, Reference Thomas1972) in northeastern Alabama (Figs. 3, 4). Typical of the family, this distinctive new crinoid is known from only three specimens from the upper Tuscumbia Limestone in northeastern Alabama. The characteristic tegmen spines and other calyx plates attributed to Tuscumbiacrinus n. gen. are commonly observed as disarticulated plates in weathered crinoidal limestones between the two colonial coral intervals in southern Madison County, Alabama.

Figure 3. Tuscumbiacrinus madisonensis n. gen. n. sp. (holotype, USNM PAL 781871). (1) Lateral view of theca with top of tegmen not preserved and matrix attached to the right. Note that the arm facets are the only portion of the calyx visible in this orientation. (2) Oblique basal view of theca; note deep concave base of calyx and the spines on tegmen plates. (3) Basal view of calyx; note low raised ridge around basal concavity in the center of the overall concave calyx base. Scale bar represents 5.0 mm for all specimens.
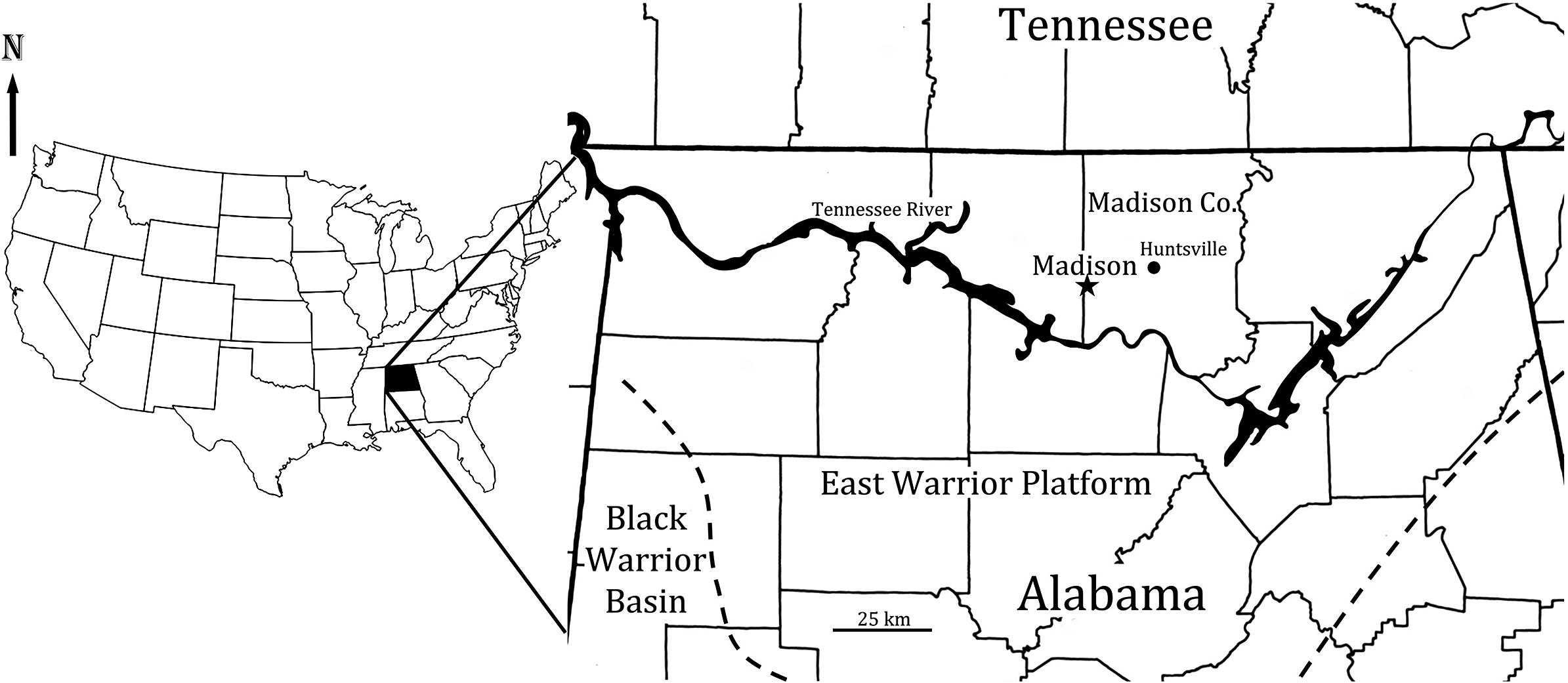
Figure 4. Black Warrior Basin and East Warrior Platform in northern Alabama. The dashed lines indicate the approximate boundary between the Black Warrior Basin and the East Warrior Platform. The star indicates locality where Tuscumbiacrinus madisonensis n. gen. n. sp. was collected (modified from Thomas, Reference Thomas1972).
Tuscumbiacrinus madisonensis n. gen. n. sp. is the oldest representative of the Paragaricocrinidae. However, the occurrence and morphology of Tuscumbiacrinus n. gen. raises broader questions concerning genus and species concepts within the Paragaricocrinidae. To determine the phylogenetic placement of Tuscumbiacrinus n. gen. and evaluate whether other taxonomic revisions were warranted, we applied maximum parsimony and Bayesian tip-dating phylogenetic methods to a character matrix of Mississippian to Permian paragaricocrinid taxa. Although phylogenetic results show conflict among optimal topologies, all results indicated that additional systematic revisions were necessary, therefore we describe four new genera, four new species, and reassign one existing species to one of the new genera. Using our phylogenetic results, we also comment on the macroevolutionary history and paleogeographic distribution of this unusual family.
Geologic setting
The Tuscumbia Limestone (Fig. 5) is a fossiliferous carbonate unit that is ~61 meters thick across the East Warrior platform in northern Alabama (Thomas, Reference Thomas1972, Reference Thomas, Thomas, Smith and Bicker1979) and thins westward into the Black Warrior basin (Fig. 4). It is a light gray bioclastic to micritic limestone, rarely oolitic, with irregular amounts of light gray chert scattered throughout the section and local coarse crinoidal cross-bedded limestones up to 3 m thick (Thomas, Reference Thomas, Thomas, Smith and Bicker1979; Kopaska-Merkel et al., Reference Kopaska-Merkel, Mann and Pashin2013). Dolostone and dolomitic limestone occur in northeastern Alabama. The lower Tuscumbia has a Warsaw–Salem fauna, and the upper Tuscumbia, which is present in the eastern part of the East Warrior platform (Butts, Reference Butts, Adams, Butts, Stephenson and Cooke1926, p. 175), has a St. Louis-associated fauna characterized by Acrocyathus floriformis d’Orbigny, Reference d’Orbigny1850, and Acrocyathus proliferus (Hall in Hall and Whitney, Reference Hall and Whitney1858) (Butts, Reference Butts, Adams, Butts, Stephenson and Cooke1926; Drahovzal, Reference Drahovzal and Smith1967) (Figs. 4, 5). Two peak coral zones occur in the upper Tuscumbia Limestone of northeastern Alabama. The upper peak coral zone of Acrocyathus floriformis is widespread in Madison County and northeastern Alabama and occurs ~6–7 m below the projected Tuscumbia Limestone–Monteagle Limestone contact. Approximately 17–20 m below the upper peak coral zone is the Acrocyathus proliferus peak coral zone (Fig. 5).
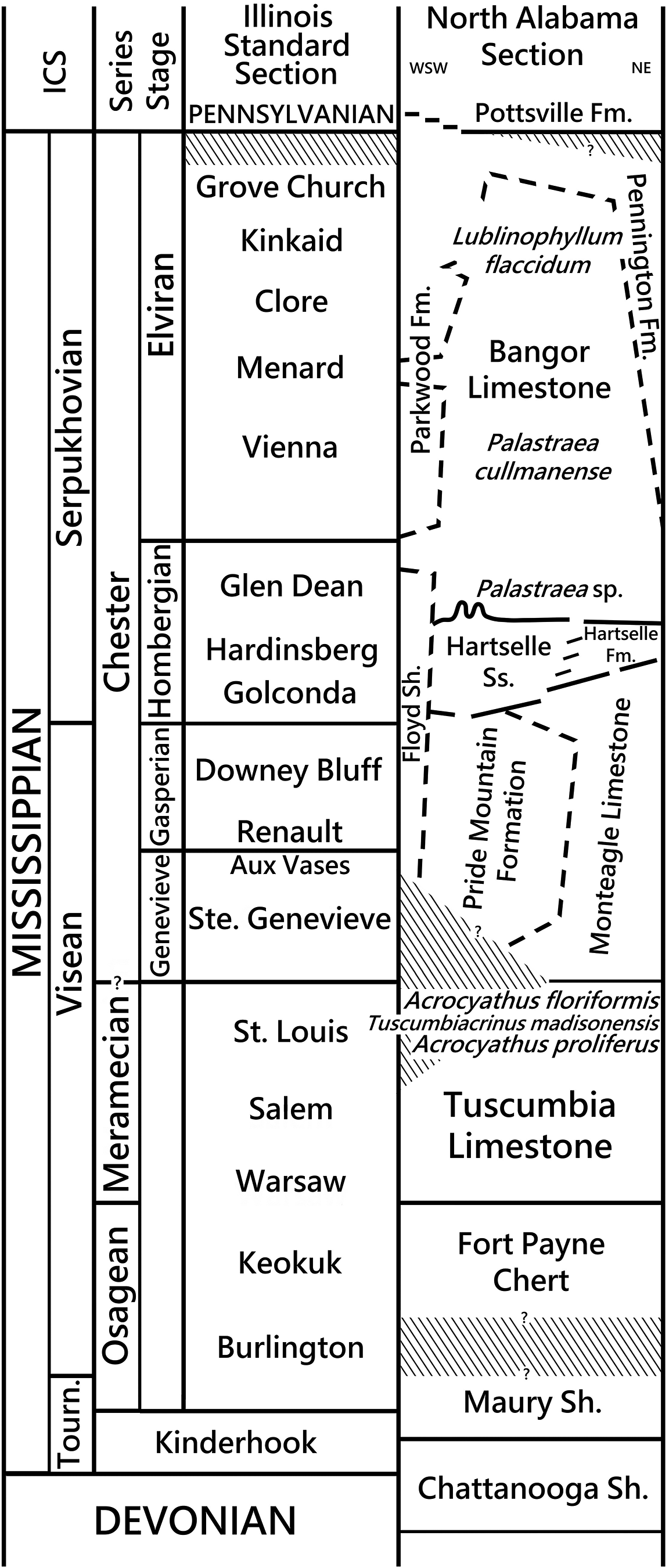
Figure 5. Mississippian stratigraphic section in northern Alabama compared to the Illinois Standard section. Position of Tuscumbiacrinus madisonensis n. gen. n. sp. is indicated as are key biostratigraphically important colonial coral intervals, including Acrocyathus proliferus (Hall in Hall and Whitney, Reference Hall and Whitney1858); Acrocyathus floriformis d’Orbigny, Reference d’Orbigny1850; Palastraea cullmanense Rodríguez and Kopaska-Merkel, Reference Rodríguez and Kopaska-Merkel2014; and Lublinophyllum flaccidum (Easton, Reference Easton1943) (coral).
The Tuscumbiacrinus madisonensis n. gen. n. sp. calyx (USNM PAL 781871) was collected from the top, southeastern end of the Madison, Alabama, quarry in southwestern Madison County (Figs. 3, 4). The specimen was on the bottom of a large thick block of crinoidal grainstone-packstone that was cross-bedded in part and overlying a thin (<1 m) colonial coral bioherm of Acrocyathus proliferus (Hall in Hall and Whitney, Reference Hall and Whitney1858). In addition to the holotype, two additional specimens of T. madisonensis n. gen. n. sp. were present on the underside of this large limestone block but were inaccessible for collection. The occurrence of Acrocyathus proliferus just below the specimen of T. madisonensis n. gen. n. sp. signifies a St. Louis age correlation for this Tuscumbia Limestone occurrence.
Below the top ledge of the quarry is ~3 m of partially cross-bedded crinoidal packstone–grainstone with some echinoid test plates, echinoid spines, and Acrocyathus proliferus coral fragments. Below this is an ~1 m limestone unit with multiple intervals of herringbone crossbedding, and below this is ~3 m of crossbedding in a dolomitic limestone, including some herringbone cross-bedding. Abundant displaced blocks of limestone in the quarry appear to be primarily crinoidal/fossiliferous packstones with some chondrichthyan teeth.
Materials and methods
Specimen collection and preparation
The Tuscumbiacrinus madisonensis n. gen. n. sp. specimen was removed from the underside of a large limestone block. Cleaning was done using dental picks, box cutter, water, and nylon brush.
Phylogenetic methods
Figure 1 is a list of currently recognized taxa in the Paragaricocrinidae. This list includes a specimen described in Breimer (Reference Breimer1962) as Iberocrinus multibrachiatus Sieverts-Doreck, Reference Sieverts-Doreck1951, that is reassigned herein to Palenciacrinus mudaensis n. gen. n. sp.; Megaliocrinus exotericus Strimple, Reference Strimple1951, that is reassigned herein to Pulcheracrinus n. gen.; and Nipponicrinus hashimotoi n. gen. n. sp. and Nipponicrinus akiyoshiensis n. gen. n. sp. are for two of the morphotypes delineated by Hashimoto (Reference Hashimoto2001).
Evolutionary relationships among camerate crinoids have received recent attention in the phylogenetic literature (e.g., Cole, Reference Cole2017, Reference Cole2018). However, phylogenetic relationships among middle to late Paleozoic monobathrid camerates remain largely unknown. Given the uncertainty in family-level phylogenetic relationships among middle Paleozoic monobathrids, we sampled four outgroup species from possible sister group taxa: Amphoracrinus gilbertsoni (Miller in Phillips, Reference Phillips1836) (Mississippian [Tournasian–Viséan]; China, Ireland, United Kingdom, United States); Athabascacrinus colemanensis Laudon, Parks, and Spreng, Reference Laudon, Parks and Spreng1952; Gennaeocrinus kentuckiensis (Shumard, Reference Shumard1868) (Devonian [Givetian]; United States); and Pimlicocrinus clitheroensis (Wright, Reference Wright1942) (Mississippian ([Viséan]; United Kingdom). In total, our phylogenetic character matrix includes 41 characters (Supplementary Tables 1 and 2). Of the 13 named species of Paragaricocrinidae, only 11 are complete enough to code characters for phylogenetic analysis (i.e., Megaliocrinus? johnsoni Strimple, Reference Strimple1975, and Paragaricocrinus? yakovlevi Lane, Reference Lane1979, were excluded). Note the species Iberocrinus multibrachiatus Sieverts-Doreck, Reference Sieverts-Doreck1951, represents two operational taxonomic units (OTUs) in our initial analyses. In our review of the taxonomic literature, we observed that the specimen referred to Iberocrinus multibrachiatus in Breimer (Reference Breimer1962) has considerable differences from the species concept in Sieverts-Doreck (Reference Sieverts-Doreck1951). Thus, we coded the holotype and the specimen described by Breimer (Reference Breimer1962) as two distinct OTUs.
Parsimony analyses were run using the maximum parsimony criteria with heuristic searches using random addition repeated 1,000 times in PAUP (Swofford, Reference Swofford2015). All characters were unordered and identically weighted. Branch swapping was conducted using the tree bisection–reconnection algorithm. Equally most-parsimonious-trees (MPTs) recovered from analyses were summarized using strict consensus, 50% majority rule, and all-compatibility trees. The consistency index (ci), retention index (ri), and rescaled index (rc) were calculated for recovered MPTs. The all-compatibility tree illustrated in Supplementary Figure 1 is a parsimony analysis with all coded taxa (note that these figures list taxonomic names as known prior to this study; Webster and Webster, Reference Webster and Webster2014) (see Fig. 1). All revised taxonomic names are noted in the figure captions, on Figure 1, and in the systematic paleontology section. Figure 1 also includes the chronostratigraphic and geographic occurrence of each taxon.
We also conducted phylogenetic analyses using Bayesian methods incorporating the fossilized birth–death process (FBD) (Stadler, Reference Stadler2010; Gavryushkina et al., Reference Gavryushkina, Welch, Stadler and Drummon2014; Heath and Moore, Reference Heath, Moore, Chen, Kuo and Lewis2014; Wright, Reference Wright2017a; Warnock and Wright, Reference Warnock and Wright2020; Wright et al., Reference Wright, Wagner and Wright2021). We placed a broad, ~Uniform[0,10] prior on the FBD parameter for net diversification and flat ~Beta[1,1] priors on the relative extinction and fossil completeness parameters. Fossil ages were assigned uniform distributions based on their occurrences in geologic stages. Morphological character evolution was modelled using a variant of the simple Mk model (Lewis, Reference Lewis2001) that accounts for ascertainment bias and allows for morphological rates to vary among characters according to a lognormal distribution (Wagner, Reference Wagner2012; Wright, Reference Wright2017a). To account for rate variation among lineages, we applied an uncorrelated morphological clock where branch rates vary according to an independent gamma rates model (Lepage et al., Reference LePage, Bryant, Philippe and Lartillot2007).
Bayesian inference of phylogeny was conducted using Markov chain Monte Carlo (MCMC) simulation in MrBayes 3.2.6 (Ronquist et al., Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012a). Two MCMC runs with four chains were run for 5 million generations. Chains were sampled every 1000 generations and the first 50% of samples were discarded as burn-in. Chains reached an average deviation of split frequencies < 0.01. Convergence diagnostics were visually inspected in Tracer 1.7.1 (Rambaut et al., Reference Rambaut, Drummond, Xie, Baele and Suchard2018) and by examining the effective sample sizes and potential scale reduction factor values of model parameters (Ronquist et al., Reference Ronquist, Klopfstein, Vilhelmsen, Schulmeister, Murray and Rasnitsyn2012b). The character-by-taxon matrix and script to run the analysis are available in the Supplementary Materials. Node support was evaluated by examining their posterior probability (PP), which is calculated as the frequency of clades recovered across the posterior distribution of tip-dated phylogenies.
For macroevolutionary analysis, we used our Bayesian phylogenetic results to examine temporal patterns of morphological rate evolution and diversity through time. Using the uncorrelated, relaxed morphological clock model, median branch rates for clades recovered in the Bayesian tip-dated all-compatibility tree were calculated across the posterior distribution of phylogenies to document patterns of rate variation through time and among subclades. Per-branch rates are relative rates in the sense they represent independent draws from a gamma distribution with a mean of 1 and a variance parameter associated with the morphological clock. Thus, per-branch rates can be interpreted as a percent above or below the “background” average (i.e., a branch with a relative rate of 0.37 has a rate that’s 37% lower than average rate) (Wright, Reference Wright2017b; Thuy et al., Reference Thuy, Eriksson, Kutscher, Lindgren, Numberger-Thuy and Wright2022). Lineage diversity through time was calculated as the median number of phylogenetic lineages across the posterior distribution of Bayesian tip-dated phylogenies, with uncertainty quantified using the 95% quantile values of diversity for each interval.
Repositories and institutional abbreviations
The new specimen studied here is deposited in the Department of Paleobiology, U.S. Museum of Natural History, Smithsonian, Washington, D.C. (USNM PAL). Other paragaricocrinid specimens are deposited in the following: ASM, Akiyoshi-dai Museum of Natural History, Japan; CGRM, Central Geological Research Museum, St. Petersburg, Russia; GPI, Senckenberg Museum, Tübingen, Germany; MGMP, Geominero Museum (CN IGME-CISC), Madrid, Spain; MGUP, Gemmellaro Museum, Palmero, Italy; NIPG, Nanjing Institute of Geology, Chinese Academy of Sciences, Nanjing, China; PIN, Borissiak Paleontological Institute, Russian Academy of Sciences, Moscow, Russia; RGM, National Natuurhistorich Museum, Leiden, The Netherlands; SUI, Department of Earth and Environmental Sciences, University of Iowa, Iowa City, Iowa, USA; USNM PAL, The Department of Paleobiology at the National Museum of Natural History, Smithsonian Institution, Washington, D.C., USA.
Phylogenetic results
Results from an initial parsimony analysis of all nominal taxa did not result in a well-supported consensus tree (Supplementary Fig. 1). Few nodes are shared by > 50% of MPTs, and the position of Permian and Mississippian taxa among MPTs. In the 50% majority-rule tree, the Permian species Wannerocrinus glans Marez Oyens, Reference Marez Oyens1940 (Artinskian?, West Timor) is placed on a branch representing the earliest diverging lineage of the ingroup, whereas Paragaricocrinus mediterraneus Yakovlev, Reference Yakovlev1934 (Wordian, Sicily) is placed in a derived position as sister to Megaliocrinus bolli Strimple, Reference Strimple1976 (Bashkirian, Spain) (Supplementary Fig. 1). After the split with Wannerocrinus, another divergence occurs between Tuscumbiacrinus madisonensis n. gen. n. sp., the oldest known member of the Paragaricocrinidae (middle Viséan, United States), and all remaining ingroup taxa. The topological placement of Permian and Mississippian taxa among MPTs implies the Wannerocrinus lineage diverged by at least the Mississippian and is followed by a long, unbroken branch until Wannerocrinus glans occurs during the Permian. Given this unexpected result, we also conducted a parsimony analysis sampling only Pennsylvanian taxa, which yielded a single MPT (Supplementary Fig. 2).
In contrast with parsimony, the Bayesian tip-dating analysis resulted in a consensus tree featuring multiple, well-supported nodes (Fig. 6). Tuscumbiacrinus n. gen. is recovered as the oldest diverging member of the Paragaricocrinidae with strong support (PP = 0.97), with all other members of family forming a well-supported clade of geologically younger species (PP = 0.87). Similar to the parsimony analysis of Pennsylvanian taxa, Nipponicrinus hashimotoi n. gen. n. sp. and N. akiyoshiensis n. gen. n. sp. are placed as sister taxa with high posterior probability (PP = 0.98). Unlike the parsimony analysis sampling all paragaricocrinid taxa, Bayesian tip-dating strongly supports a sister-group relationship among the two Permian species (PP = 0.89).
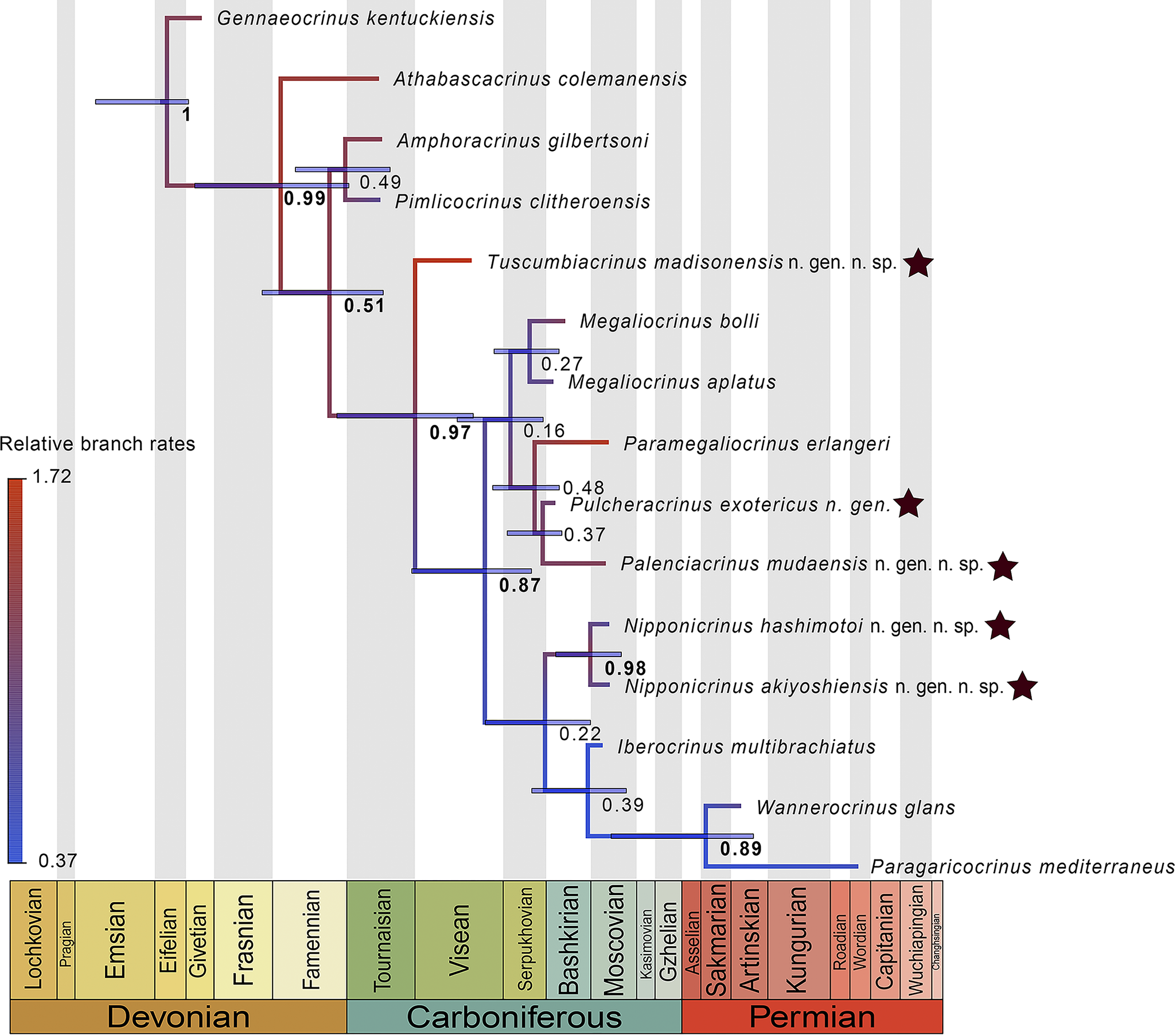
Figure 6. Paragaricocrinidae phylogeny from Bayesian tip-dating analysis. Stars indicate new and/or revised taxa; numbers correspond to the posterior probabilities of clades; branches are colored to show relative rates of morphological evolution. Taxonomic names are those from results of this study.
Results from the parsimony and Bayesian analyses show striking topological differences. Unlike parsimony, Bayesian phylogenetic methods using FBD models leverage a combination of morphologic and stratigraphic age data to infer phylogenetic hypotheses, which more fully take advantage of information provided by the fossil record (Barido-Sottani et al., Reference Barido-Sottani, van Tiel, Hopkins, Wright, Stadler and Warnock2020; Wright et al., Reference Wright, Wagner and Wright2021). Bayesian approaches using simple Mk models of morphologic evolution have been shown to outperform parsimony methods (Wright and Hillis, Reference Wright and Hillis2014), and tip-dating approaches using the FBD process have been shown to improve phylogenetic inferences compared to undated approaches in general (Barido-Sottani et al., Reference Barido-Sottani, van Tiel, Hopkins, Wright, Stadler and Warnock2020; Mongiardino Koch et al., Reference Mongiardino Koch, Garwood and Parry2021).
Although it is impossible to demonstrate the efficacy of a phylogenetic method using empirical data (i.e., the “true” tree is unknown), we nevertheless can use our results to further test specific phylogenetic hypotheses and compare their plausibility using basic sampling theory. For example, the Permian species Wannerocrinus glans is placed as the earliest diverging lineage in the Paragaricocrinidae in the parsimony analysis, which implies a minimum branch duration of ca. 56.6 million years. Notably, none of the taxa sampled in our analysis “breaks up” this temporally long branch duration. In contrast, the Bayesian tip-dating result places Wannerocrinus glans as sister to another Permian taxon, Paragaricocrinus mediterraneus, with high posterior probability (PP = 0.89). The number of occurrences (n) over a branch duration (t) follows a Poisson distribution with fossil sampling rate (Ψ) (Solow and Smith, Reference Solow and Smith1997). Given the mean sampling rate for paragaricocrinid taxa is 0.088 (median = 0.092), the Poisson probability of observing a branch duration of ca. 56.6 million years with 1 fossil sampling event (i.e., the topology implied by parsimony) is ≤ 0.03 (Supplementary Fig. 3). In fact, on average, one would expect ~5 (i.e., Ψt) occurrences breaking up the long branch and a 95% probability of there being 2–10 species sampled over such a long duration. Either this particular lineage is characterized by a fossil sampling rate ~5 times lower than expected (Supplementary Fig. 3), or the parsimony topology is incorrect. Although our initial parsimony analysis is inconsistent with expectations from sampling theory, the Bayesian tip-dated results are logically consistent with them. Thus, the differences between our parsimony and Bayesian tip-dating results corroborate simulation-based studies evaluating the efficacy of parsimony versus Bayesian approaches to inferring fossil phylogenies (Wright and Hillis, Reference Wright and Hillis2014; Barido-Sottani et al., Reference Barido-Sottani, van Tiel, Hopkins, Wright, Stadler and Warnock2020; Mongiardino Koch et al., Reference Mongiardino Koch, Garwood and Parry2021).
Despite these major differences in the recovered topologies between methodological approaches (Supplementary Figs. 1, 2; Fig. 6), the results of our maximum parsimony and Bayesian analyses do share a number of important similarities of taxonomic significance, especially comparing the Bayesian results with a parsimony analysis that sampled only Pennsylvanian taxa (i.e., eliminating the influence of taxa such as Wannerocrinus). For example, the OTU of Iberocrinus multibrachiatus Sieverts-Doreck, Reference Sieverts-Doreck1951, is phylogenetically distant from the OTU identified as I. multibrachiatus by Breimer (Reference Breimer1962) in both analyses, indicating the need for a taxonomic re-evaluation of the specimen in Breimer (Reference Breimer1962). Both results feature a major clade containing Megaliocrinus aplatus Moore and Laudon, Reference Moore and Laudon1942, M. bolli, Pulcheracrinus exotericus n. gen. n. comb., Paramegaliocrinus erlangeri Arendt, Reference Arendt1983, and Palenciacrinus mudaensis n. gen. n. sp. (=Iberocrinus multibrachiatus sensu Breimer, Reference Breimer1962), and indicate that the genus Megaliocrinus as previously conceived was not monophyletic. Finally, the two Paragaricocrinidae taxa from Japan are recovered as sister species in both analyses.
Together, the common features between our phylogenetic results justify the need for taxonomic revision of several paragaricocrinid taxa. We propose four new genera, recognize four new species, and one existing species is reassigned to a new genus. We emphasize our taxonomic revisions are not conditional on the results of a particular phylogenetic method or tree topology. Following our revision, the Paragaricocrinidae is understood to contain eleven species confidently assigned to eight genera, two species questionably assigned to a genus, and seven taxa left in open nomenclature (Fig. 1).
Evolutionary history of the Paragaricocrinidae: a dead clade staggering?
The diversity trajectory of paragaricocrinid lineages follows a unimodal waxing and waning pattern of clade diversification, with the majority of its duration characterized by relatively low taxonomic richness (Fig. 7). The middle Viséan Tuscumbiacrinus n. gen. is the oldest genus of the Paragaricocrinidae. It was derived from a Tournaisian or early Viséan genus that was one of many clades that were typical of the Middle Paleozoic Crinoid Evolutionary fauna (Ausich et al., Reference Ausich, Kammer and Baumiller1994, Reference Ausich, Kammer, Mirantsev, Lucas, Schneider, Wang and Nikolaeva2022; Baumiller, Reference Baumiller, David, Guille, Féral and Roux1994; Ausich and Kammer, Reference Ausich and Kammer2013). Although atypical for middle Viséan and younger crinoids, Paragaricocrinidae with robust calyxes reached peak taxonomic diversity during the early late Carboniferous and became a geographically cosmopolitan family despite the relatively small number of known specimens assigned to the family.
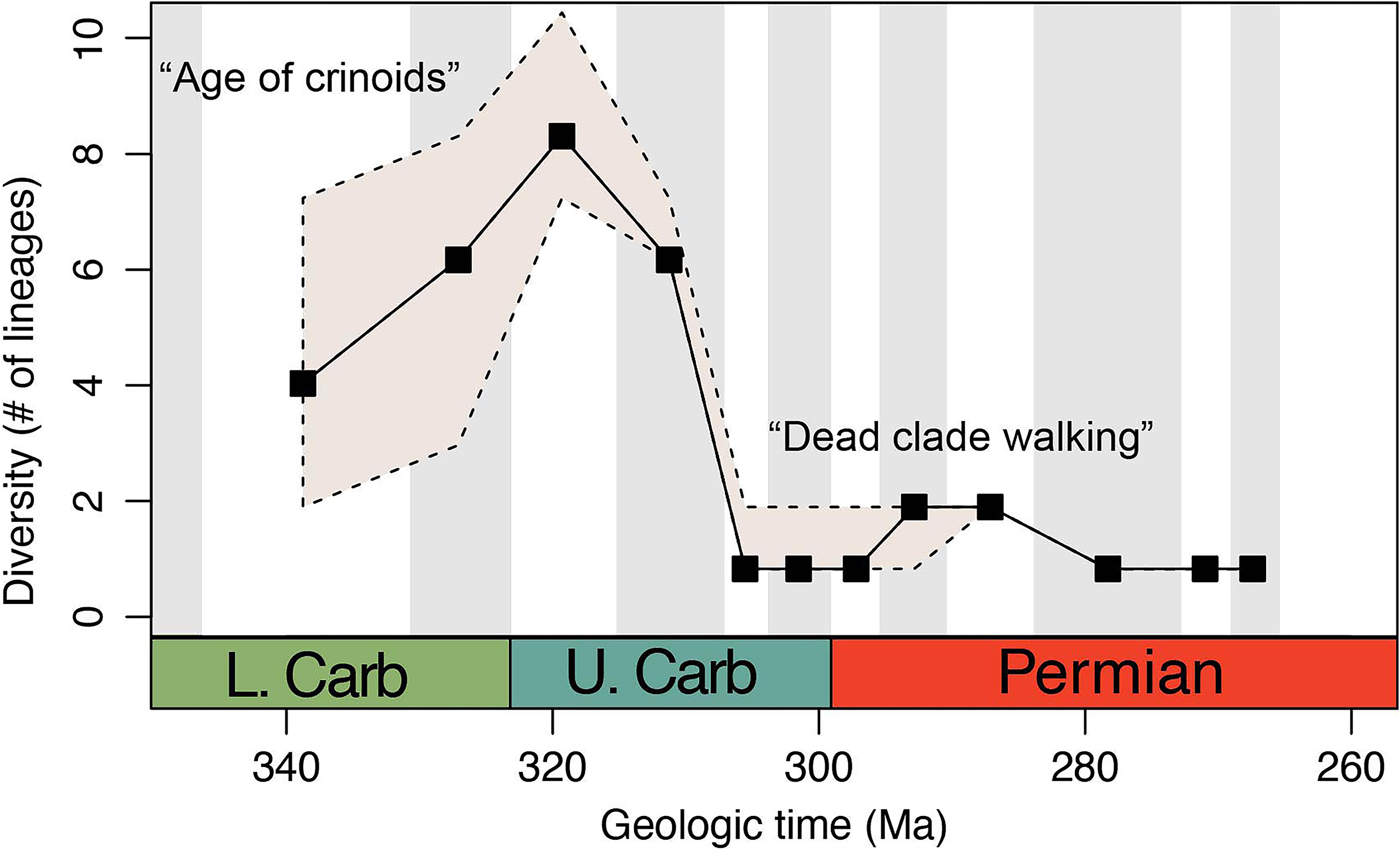
Figure 7. Diversity of lineages of the Paragaricocrinidae through the upper Paleozoic. Note sharp decline after the Middle Pennsylvanian.
Interestingly, the most diverse and abundant occurrence of paragaricocrinids is from the Akiyoshi Limestone Group of southwestern Japan. Hashimoto (Reference Hashimoto2001) reported 19 specimens that he assigned to four open-nomenclature groupings of Paragaricocrinidae. We place most of these specimens into Nipponicrinus n. gen. with two new species and two open-nomenclature groupings.
Taxonomic richness decreased through the Pennsylvanian (Figs. 2, 8), and the Paragaricocrinidae declined greatly in diversity after the Moscovian. On the basis of nine specimens, three Permian taxa have been described (Fig. 1). Wannerocrinus glans is known from a single specimen from the Artinskian? of West Timor, Paragaricocrinus? yakovlevi is known from a single specimen from the Wordian of Tunisia, Paragaricocrinus mediterraneus is known from six specimens the Wordian of Sicily, and possibly two specimens left in open nomenclature are from the early Permian of Vancouver Island, Canada (Webster et al., Reference Webster, Haggart, Saxifrage, Saxifrage, Gonau and Douglas2009a). These few specimens and taxa occurred in the western margin of the Paleotethys, Gondwana, and possibly the easternmost Panthalassia Sea.
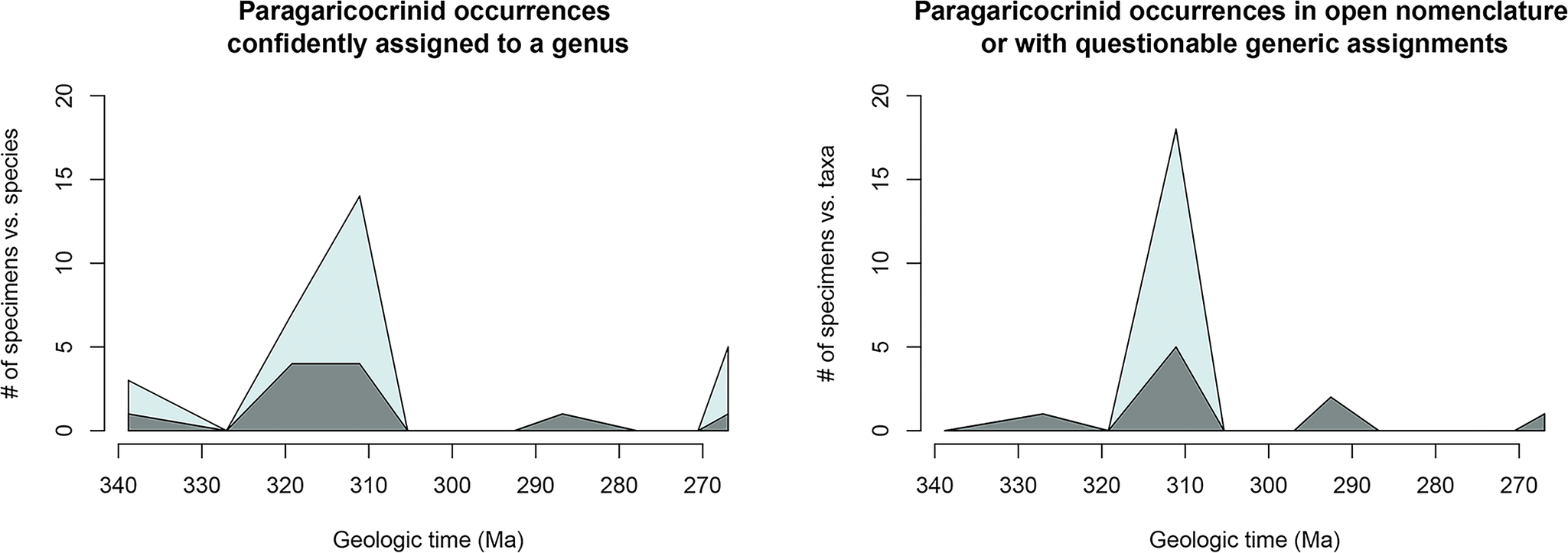
Figure 8. Comparison of the number of species (dark blue) to number of specimens (light blue) for species of paragaricocrinids (left) that are confidently assigned to a genus and (right) for species questionably assigned to a genus and those left in open nomenclature.
The term “Dead Clade Walking” (DCW) initially referred to clades that survived a mass extinction event but persisted at low levels of taxonomic richness for a protracted period before ultimately becoming extinct (Jablonski, Reference Jablonski2001, Reference Jablonski2002). Since Jablonski first coined the term, the concept of a DCW has occasionally been extended to describe any pattern in which a clade persists from tens to hundreds of millions of years at low taxonomic diversity (Barnes et al., Reference Barnes, Sclafani and Zaffos2021). For the Paragaricocrinidae, the early late Carboniferous drop in diversity corresponds to a “death sentence” (sensu Barnes et al., Reference Barnes, Sclafani and Zaffos2021) marking the initiation of a DCW pattern that lasted approximately 50.1 million years (Fig. 7), a duration nearly twice (~1.9) the median post-death sentence duration of DCWs (Barnes et al., Reference Barnes, Sclafani and Zaffos2021).
Interestingly, the Paragaricocrinidae seemingly exhibit a DCW pattern unrelated to a major mass extinction event but was potentially influenced by ecological changes surrounding a major turnover in crinoid evolutionary faunas (CEFs) (Ausich et al., Reference Ausich, Kammer and Baumiller1994; Kammer and Ausich, Reference Kammer and Ausich2006; Deline and Ausich, Reference Deline and Ausich2012; Cole and Wright, Reference Cole and Wright2022). The Paragaricocrinidae originated during the so-called “Age of Crinoids,” which is bracketed by the Late Devonian extinctions and the end-Serpukhovian event (Kammer and Ausich, Reference Kammer and Ausich2006). A major turnover in CEFs occurred during the late Mississippian (late Viséan), which resulted in a major shift in taxonomic diversity and abundance among crinoid higher taxa from a camerate-dominated fauna to a cladid-dominated fauna (Ausich et al., Reference Ausich, Kammer and Baumiller1994). The late Paleozoic CEF was comprised mostly of “advanced” cladids (“articuliforms” in Wright, Reference Wright2017b) that convergently evolved camerate-like feeding structures and diversified into regions of crinoid morphospace previously occupied only by camerates (Wright, Reference Wright2017b; Cole et al., Reference Cole, Wright and Ausich2019). This cladid-dominated CEF reached peak taxonomic diversity during the Bashkirian stage and persisted until the end-Permian mass extinction. Late Paleozoic cladids have previously been argued to have been better suited to changing ecological and environmental pressures than monobathrid camerates like paragaricocrinids (Ausich and Kammer, Reference Ausich and Kammer2013).
Ecological interactions, including competition, provide a possible mechanism of DCW patterns (Sepkoski et al., Reference Sepkoski, McKinney and Lidgard2000; Jablonski, Reference Jablonski2002; Barnes et al., Reference Barnes, Sclafani and Zaffos2021), and evidence for competition among crinoids is well documented in the fossil record (Ausich, Reference Ausich1980; Cole et al., Reference Cole, Wright and Ausich2019; Cole and Wright, Reference Cole and Wright2022). Examining rates of morphological evolution in paragaricocrinids provides an indirect test of whether or not ecological processes may have played a role in their diversification. For example, niche-filling models of morphological evolution exhibit decreases in rates of change when ecological interactions (e.g., competition) place limits on morphological diversification. Rates of morphological evolution among paragaricocrinid lineages show considerable variation through time and among lineages. The branch leading to Tuscumbiacrinus n. gen., the oldest member of the clade, is characterized by the highest rates of morphological evolution in the clade. In contrast, the lowest rates of morphological evolution follow the “death sentence” interval of the clade’s evolutionary history, and all post-Moscovian lineages are fractions of the “background” clade-wide average (Figs. 7, 8).
Although these results do not prove that competition with eucladids played a role in limiting paragaricocrinid diversification, they are nevertheless consistent with the hypothesis that ecologic drivers may have helped shape the DCW pattern in paragaricocrinids. If we restrict the concept of a DCW to Jablonski’s (Reference Jablonski2002) original definition, then a DCW pattern arising from long-term ecological interactions, and not related to a mass extinction, may be referred to as a “dead clade staggering.” Thus, Paragaricocrinidae may be better described as a dead clade staggering rather than a dead clade walking!
Systematic paleontology
Classification and terminology
The superordinal and ordinal classification of Camerata follows Cole (Reference Cole2017, Reference Cole2018), Wright (Reference Wright2017a, Reference Wrightb), and Wright et al. (Reference Wright, Ausich, Cole, Rhenberg and Peter2017). Family-level classification follows Moore and Teichert (Reference Moore and Teichert1978).
Morphologic terminology follows Ubaghs (Reference Ubaghs, Moore and Teichert1978a), Ausich et al. (Reference Ausich, Wright, Cole and Sevastopulo2020), and Ausich and Donovan (Reference Ausich, Donovan and Ausich2023). Plates in the interrays are the number of plates in each range from the proximalmost plate to the last range before the tegmen. The primanal is the proximalmost plate in the CD interray and is indicated by “P”; and the first interradial in regular interrays is indicated by “1.” Note, with a few exceptions, that synonymies include the first mention of a taxon in the literature and the citation in Webster and Webster (Reference Webster and Webster2014). Full synonymy listings are in Webster and Webster (Reference Webster and Webster2014). Abbreviations used in specimen measurements include the following: CaH, calyx height; CaMxW, calyx maximum width; CaMnW, calyx minimum width; TH, tegmen height. All measurements are in mm; * after a measurement indicates feature is incomplete or specimen is crushed. Supplemental Tables 3 and 4 list diagnostic characters for genera and species, respectively.
Class Crinoidea Miller, Reference Miller1821
Subclass Camerata Wachsmuth and Springer, Reference Wachsmuth and Springer1885
Infraclass Eucamerata Cole, Reference Cole2017
Order Monobathrida Moore and Laudon, Reference Moore and Laudon1943
Suborder Compsocrinina Ubaghs, Reference Ubaghs, Moore and Teichert1978b
Superfamily Periechocrinitoidea Bronn, Reference Bronn1848–Reference Bronn1849
Family Paragaricocrinidae Moore and Laudon, Reference Moore and Laudon1942
Included genera
Iberocrinus Sieverts-Doreck, Reference Sieverts-Doreck1951; Megaliocrinus Moore and Laudon, Reference Moore and Laudon1942; Nipponicrinus n. gen.; Palenciacrinus n. gen.; Paragaricocrinus Yakovlev, Reference Yakovlev1934; Paramegaliocrinus Arendt, Reference Arendt1983; Pulcheracrinus n. gen.; Tuscumbiacrinus n. gen.; and Wannerocrinus Marez Oyens, Reference Marez Oyens1940.
Remarks
The Paragaricocrinidae is a post-early Viséan camerate crinoid family with a large, robustly constructed thecae. Ubaghs (Reference Ubaghs, Moore and Teichert1978b) listed four genera in the Paragaricocrinidae: Iberocrinus, Megaliocrinus, Paragaricocrinus, and Wannerocrinus. Subsequently, Paramegaliocrinus Arendt, Reference Arendt1983, was named. Strimple (Reference Strimple1976, p. 639) reassigned the type species of Iberocrinus (I. multibrachiatus) to Megaliocrinus bolli. As noted by Webster and Webster (Reference Webster and Webster2014), if Strimple’s (Reference Strimple1976) reassignment is followed, Strimple’s new name is an objective junior synonym.
The five previously recognized genera are redefined, and four new genera are proposed herein (Supplementary Table 3). The Paragaricocrinidae range from the Mississippian (middle Viséan) through the Permian (Wordian). As discussed, given the diversity and duration of this family, it is represented by very few specimens, and many occurrences of the family are noted as either a questionable genus assignment or left in open nomenclature (Webster and Webster, Reference Webster and Webster2014).
Genus Paragaricocrinus Yakovlev, Reference Yakovlev1934
Reference Yakovlev1934 Paragaricocrinus Yakovlev, p. 271.
Reference Webster and Webster2014 Paragaricocrinus Yakovlev; Webster and Webster, p. 1628.
Type species
Paragaricocrinus mediterraneus Yakovlev, Reference Yakovlev1934.
Included species
Paragaricocrinus mediterraneus Yakovlev, Reference Yakovlev1934; Paragaricocrinus? yakovlevi Lane, Reference Lane1979.
Diagnosis
Overall calyx shape very low bowl, subcircular calyx outline in dorsal view, outer shape of calyx plates gently convex, overall shape of calyx base flat, basal concavity wide and deep, ridge around basal concavity; basal plates not hypertrophied, relative sizes of basal plates unknown; proximal plating in regular interrays 1-2, proximal plating in CD interray P-2-?, posterior interray not in contact with tegmen, posterior interray not depressed; tegmen flat inverted cone shape, presence of spines on side of tegmen unknown; 40 free arms, first primibrachial tetragonal, first primibrachials wider than high, one secundibrachial, intrabrachial plates absent, tertibrachials distalmost fixed brachials.
Occurrence
Permian, Wordian. Italy (Sicily), Tunisia?
Paragaricocrinus mediterraneus Yakovlev, Reference Yakovlev1934
Reference Yakovlev1934 Paragaricocrinus mediterraneus Yakovlev, p. 272, pl. 19, figs. 1–6; Figs. 1, 2.
Reference Webster and Webster2014 Paragaricocrinus mediterraneus; Webster and Webster, p. 1628.
Holotype
MGUP-001-C84001 is listed as the holotype (Yakovlev, Reference Yakovlev1934).
Diagnosis
As for genus.
Occurrence
Permian (Wordian); Italy (Sicily).
Other material
MGUP-001-C84002, MGUP-001-C84003.
Remarks
Yakovlev (Reference Yakovlev1934) listed the holotype as MGUP-001-C84001 and two paratypes. His specimens were split between the Gemmellaro Museum, Palmero, Italy (MGMP), and the National History Museum, Pisa, Italy. One additional specimen (CGRM 4/4349) is in the collections of the Central Geological Research Museum, St. Petersburg, Russia. Five species in total are thought to be available today in these museums, but the location of the holotype is not confirmed.
Paragaricocrinus ? yakovlevi Lane, Reference Lane1979
Reference Lane1979 Paragaricocrinus? yakovlevi Lane, p. 125, pl. 1, fig. 1.22, fig. 1H.
Reference Webster and Webster2014 Paragaricocrinus? yakovlevi; Webster and Webster, p. 1628.
Holotype
USNM PAL 251281.
Occurrence
Permian, Wordian; Tunisia, Djebel Tebaga area, Tunisia.
Remarks
The holotype and only specimen of this species is preserved such that only the interiors of calyx plates along the basal portion of the calyx are visible. Lane (Reference Lane1979) discussed the issues regarding a generic assignment, and his judgment on this specimen is retained herein.
Genus Wannerocrinus Marez Oyens, Reference Marez Oyens1940
Reference Marez Oyens1940 Wannerocrinus Marez Oyens, p. 294.
Reference Webster and Webster2014 Wannerocrinus; Webster and Webster, p. 2213.
Type species
Wannerocrinus glans Marez Oyens, Reference Marez Oyens1940.
Diagnosis
Overall calyx shape very low bowl, calyx outline in dorsal view subcircular, outer shape of calyx plates flat, overall shape of calyx base convex, basal concavity narrow and deep, ridge around basal concavity absent; basal plates not hypertrophied, relative sizes of basal plates unknown; proximal plating in regular interrays restricted to one, proximal plating in CD interray P-2-2, posterior interray not in contact with tegmen, posterior interray not depressed; tegmen medium inverted-bowl shape, spines on side of tegmen absent (but distal spine present); ~40 free arms, tetragonal first primibrachials, first primibrachials wider than high, one secundibrachial, intrabrachial plates absent, distalmost fixed brachials tertibrachials.
Occurrence
Permian (uncertain series); West Timor.
Wannerocrinus glans Marez Oyens, Reference Marez Oyens1940
Reference Marez Oyens1940 Wannerocrinus glans Marez Oyens, p. 295, pl. 1, fig. 1.
Reference Webster and Webster2014 Wannerocrinus glans; Webster and Webster, p. 2213.
Types
Holotype: RGM 893215.
Diagnosis
As for genus by monotypy.
Occurrence
Permian (uncertain); Basleo, West Timor.
Genus Megaliocrinus Moore and Laudon, Reference Moore and Laudon1942
Reference Moore and Laudon1942 Megaliocrinus Moore and Laudon, p. 68.
Reference Ubaghs, Moore and Teichert1978b Megaliocrinus Moore and Laudon (in part); Ubaghs, p. T450.
Reference Webster and Webster2014 Megaliocrinus Moore and Laudon (in part); Webster and Webster, p. 1453.
Type species
Megaliocrinus aplatus Moore and Laudon, Reference Moore and Laudon1942.
Included species
Megaliocrinus aplatus, M. bolli Strimple, Reference Strimple1976; M.? johnsoni Strimple, Reference Strimple1975.
Diagnosis
Overall calyx shape very low globe, calyx subcircular in dorsal view, outer shape of calyx plates from flat to very convex, overall shape of calyx base flat or shallow convex, basal concavity narrow to wide or shallow, ridge around basal concavity absent; basal plates not hypertrophied, basal plates subequal in size; proximal plating in regular interrays 1-2 or 1-2-1, proximal plating in CD interray P-3-3-1 or P-3-4-3, posterior interray in contact with tegmen, posterior interray not depressed; tegmen shape low inverted cone as known, spines present on side of tegmen, spines on side of tegmen short as known; 27–40 total free arm number, first primibrachial tetragonal or pentagonal, first primibrachials wider than high, 1 or 2 secundibrachials, intrabrachial plates absent, tertibrachials distalmost fixed brachials.
Occurrence
Pennsylvanian (Bashkirian); Spain, United States.
Megaliocrinus aplatus Moore and Laudon, Reference Moore and Laudon1942
Reference Moore and Laudon1942 Megaliocrinus aplatus Moore and Laudon, Reference Moore and Laudon1942, p. 68, figs. 1–3.
Reference Ubaghs, Moore and Teichert1978b Megaliocrinus aplatus; Ubaghs, p. T450, fig. 258.3.
Reference Webster and Webster2014 Megaliocrinus aplatus; Webster and Webster, p. 1454.
Holotype
USNM PAL 141190.
Diagnosis
Very low bowl- or globe-shaped calyx; outer shape of calyx plates very convex; basal concavity wide, shallow; posterior interray plating P-3-3-1; first primibrachial shape tetragonal.
Occurrence
Pennsylvanian (Bashkirian); Boyd Formation; southeast of Braggs, Oklahoma, United States.
Other material
SUI 33124, USNM PAL 141191, and USNM PAL 141192.
Remarks
As noted in the species diagnoses, Megaliocrinus species are distinguished on the basis of calyx shape, outer shape of the calyx plates, size and depth of the basal concavity, plating in the posterior interray, and shape of the first primibrachials.
Megaliocrinus bolli Strimple, Reference Strimple1976
Reference Strimple1976 Megaliocrinus bolli Strimple (in part), p. 636, figs. 1a, b, 3c–e.
Reference Webster and Webster2014 Megaliocrinus bolli; Webster and Webster, Reference Webster and Webster2014, p. 1454.
Holotype
GPI-PV-68524.
Diagnosis
Very low globe-shaped calyx; outer shape of calyx plates flat; basal concavity narrow, shallow; posterior interray plating P-3-4-3; first primibrachial shape pentagonal.
Occurrence
Pennsylvanian (Bashkirian); Puma Member, Perapertu Formation, and Cotarazzo Limestone; Spain.
Remarks
The species of Megaliocrinus are compared in the remarks of M. aplatus.
Megaliocrinus ? johnsoni Strimple, Reference Strimple1951
Reference Strimple1975 Megaliocrinus johnsoni Strimple, p. 119, fig. 1a–c.
Reference Webster and Webster2014 Megaliocrinus johnsoni Strimple; Webster and Webster, p. 1454.
Holotype
SUI 37949
Occurrence
Pennsylvanian (Moscovian); near Milan, Illinois, United States.
Remarks
Megaliocrinus johnsoni was described based on a single specimen that is an internal mold of the lower portion of a calyx. Although Megaliocrinus is a reasonable generic assignment for this species, sufficient diagnostic characters are not preserved to place this taxon into Megaliocrinus with confidence or to prepare a diagnosis. Thus, the generic assignment remains questionable. The species of Megaliocrinus are compared in the remarks of M. aplatus.
Iberocrinus Sieverts-Doreck, Reference Sieverts-Doreck1951
Reference Sieverts-Doreck1951 Iberocrinus Sieverts-Doreck, p. 105.
Reference Strimple1976 Megaliocrinus; Strimple, p. 631.
Reference Ubaghs, Moore and Teichert1978b Iberocrinus; Ubaghs, p. T450.
Reference Webster and Webster2014 Megaliocrinus; Webster and Webster, p. 1453.
Type species
Iberocrinus multibrachiatus Sieverts-Doreck, Reference Sieverts-Doreck1951.
Diagnosis
Overall calyx shape very low bowl to cone, elliptical calyx outline in dorsal view, outer shape of calyx plates very convex, overall shape of calyx base shallow concave, basal concavity narrow and deep, ridge around basal concavity absent; basal plates not hypertrophied, relative size of basal plates unknown; proximal plating in regular interrays 1-2-1, proximal plating in CD interray P-3-5-3-3, posterior interray in contact with tegmen, posterior interray depressed; tegmen shape very low inverted cone, spines on side of tegmen absent, distal tegmen plate spinose; 32–36 free arms, first primibrachial shape tetragonal, first primibrachials wider than high, one secundibrachial, intrabrachial plates probably absent, distalmost fixed brachials tertibrachials or quartibrachials.
Occurrence
Pennsylvanian (Bashkirian); Spain.
Remarks
As recognized by Ubaghs (Reference Ubaghs, Moore and Teichert1978b), Iberocrinus is regarded herein as a valid genus and not a junior synonym of Megaliocrinus, as proposed by Strimple (Reference Strimple1976) (see Fig. 1, Supplemental Table 3). Further, I. multibrachiatus as described by Sieverts-Doreck (Reference Sieverts-Doreck1951) is the only known species of this genus.
Iberocrinus multibrachiatus Sieverts-Doreck, Reference Sieverts-Doreck1951
Reference Sieverts-Doreck1951 Iberocrinus multibrachiatus Sieverts-Doreck, p. 109, pl. 8, figs. 1, 2, Figs. 2d, 3.
Reference Strimple1976 Megaliocrinus bolli Strimple, Reference Strimple1976, p. 636.
Reference Ubaghs, Moore and Teichert1978b Iberocrinus multibrachiatus; Ubaghs, p. T450, Fig. 258.2a–d.
Reference Webster and Webster2014 Megaliocrinus bolli Strimple, Reference Strimple1976 (in part); Webster and Webster, p. 1454.
Holotype
The holotype could not be located.
Diagnosis
As for genus by monotypy.
Remarks
Iberocrinus multibrachiatus, as defined by Sieverts-Doreck (Reference Sieverts-Doreck1951), is a valid genus and species. It is neither the junior synonym of Megaliocrinus bolli nor is the specimen described by Breimer (Reference Breimer1962) another example of I. multibrachiatus (the latter of which is described below as Palenciacrinus mudaensis n. gen. n. sp.).
Genus Paramegaliocrinus Arendt, Reference Arendt1983
Reference Arendt1983 Paramegaliocrinus Arendt, Reference Arendt1983, p. 95.
Reference Webster and Webster2014 Paramegaliocrinus; Webster and Webster, p. 1634.
Type species
Paramegaliocrinus erlangeri Arendt, Reference Arendt1983.
Diagnosis
Overall calyx shape very low bowl, calyx outline subcircular in dorsal view, outer shape of calyx plates very convex, overall shape of calyx base shallow concave, basal concavity wide and deep, ridge around basal concavity; basal plates not hypertrophied, basal plates subequal in size, proximal plating in regular interrays 1-2-2-1, proximal plating in CD interray P-3-5-7, posterior interray in contact with tegmen, posterior interray not depressed; tegmen shape unknown, presence or absence of spines on side of tegmen unknown; 30 free arms, hexagonal first primibrachial shape, first primibrachials higher than wide, one secundibrachial, intrabrachial plates absent, tertibrachials distalmost fixed brachials.
Occurrence
Pennsylvanian (Moscovian); Russia.
Paramegaliocrinus erlangeri Arendt, Reference Arendt1983
Reference Arendt1983 Paramegaliocrinus erlangeri Arendt, p. 92, figs. 1, 2.
Reference Webster and Webster2014 Paramegaliocrinus erlangeri; Webster and Webster, p. 1634.
Types
Holotype: PIN N o 3678/67.
Diagnosis
As for genus by monotypy.
Occurrence
Pennsylvanian (Moscovian); Moscow Basin, Russia.
Other material
PIN N o 3678/68.
Remarks
Paramegaliocrinus erlangeri is the only paragaricocrinid known from Russia, and it further expands the geographic range of the peak of this family during the Moscovian.
Genus Tuscumbiacrinus new genus
Type species
Tuscumbiacrinus madisonensis n. gen. n. sp.
Diagnosis
As for the type species by monotypy.
Occurrence
Mississippian (middle Viséan); United States.
Etymology
The genus name recognizes the formation from which this new crinoid was found, the Tuscumbia Limestone.
Remarks
An overall deeply concave base of nearly the entire calyx, three subequal basal plates, low or very low bowl-shaped tegmen, and large spines on tegmen plates make Tuscumbiacrinus n. gen. unique among paragaricocrinids (Supplementary Table 3). Tuscumbiacrinus n. gen. is sister to all younger paragaricocrinids in tip-dating phylogenetic analysis (Fig. 5).
Tuscumbiacrinus madisonensis new species
Holotype
USNM PAL 781871.
Diagnosis
Shape of the calyx flat bowl, calyx outline elliptical in dorsal view, outer shape of the calyx plates flat, overall shape of the calyx base deeply concave, basal concavity narrow and shallow; three unequal basal plates, ridge around basal concavity, basal plates not hypertrophied; proximal plating in regular interrays 1-2-1, proximal plating in CD interray P-3-2, posterior interray not in contact with tegmen, posterior interray not depressed; tegmen shape low or very low inverted bowl, large spines on tegmen plates; ~40 free arms, tetragonal first primibrachial shape, first primibrachials wider than high, two secundibrachials, intrabrachial plates present, tertibrachials distalmost fixed brachials.
Occurrence
Mississippian (middle Viséan); Tuscumbia Limestone, southwestern Madison County, Alabama, United States.
Description
Calyx, large, flat bowl-shaped, overall shape of the calyx base deeply concave beginning along the proximal portion of the secundibrachials, subelliptical in outline (Fig. 3.3); smooth calyx plate sculpturing; calyx plates flat. Basal concavity narrow, shallow, short ridge around basal concavity.
Basal circlet very small; three subequal basal plates, basal plates not hypertrophied, primanal articulated to two basal plates (Fig. 9). Radial circlet completely in the broad concavity of the base of the calyx, interrupted in the CD interray; radial plates five, hexagonal or heptagonal, ~2 times wider than high.
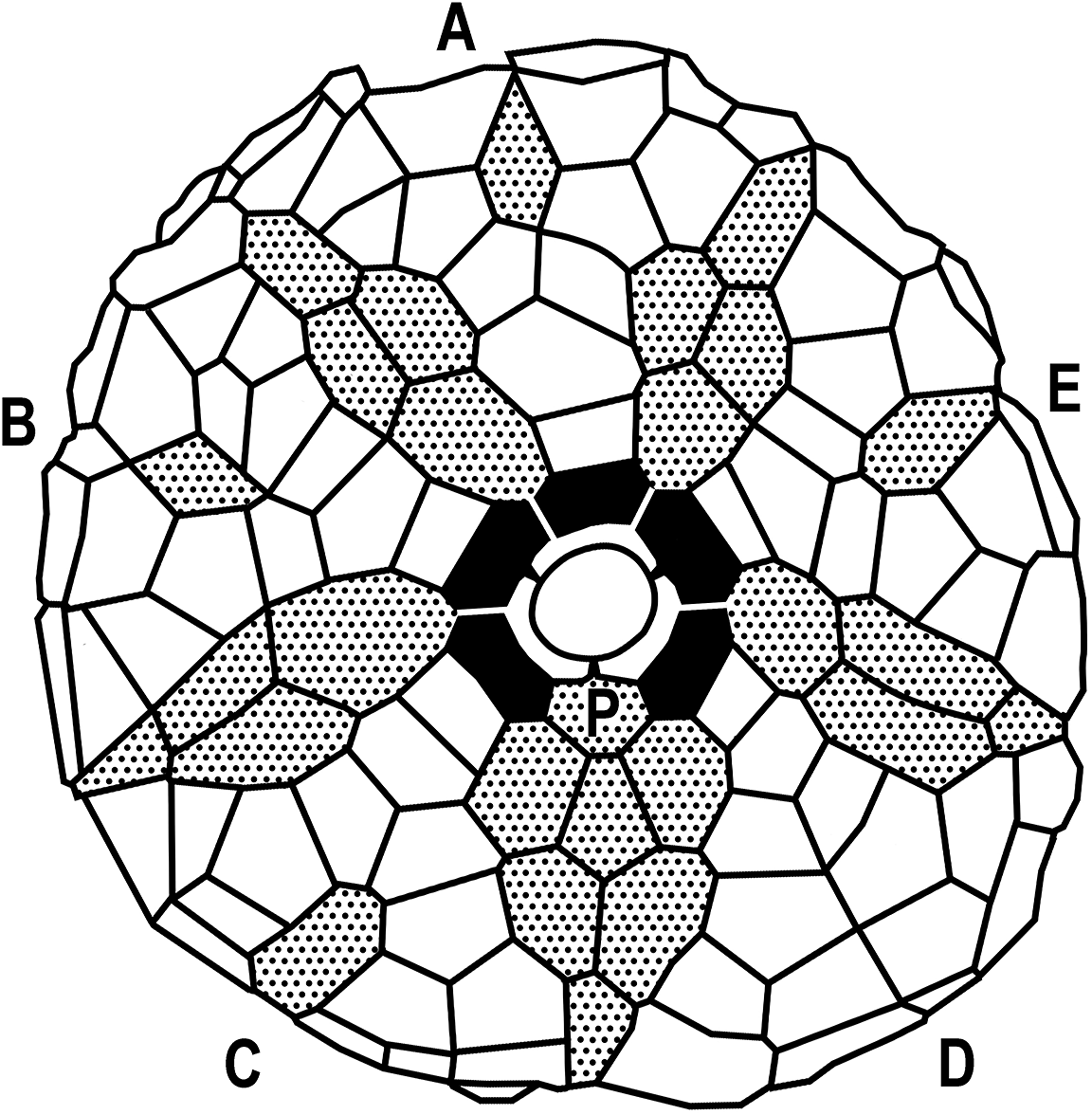
Figure 9. Plate diagram of Tuscumbiacrinus madisonensis n. gen. n. sp. Radial plates black, interradial and intraradial plates stippled, and P designates the primanal.
Regular interrays not in contact with tegmen, first interradial octagonal, as high as wide or higher than wide, larger than radial plates and primibrachial plates. Regular interray plating 1-2-1.
CD interray wider than regular interrays, not depressed. Primanal heptagonal, smaller than radial plates, interrupts the radial circlet; proximally in sutural contact with two basal plates below; three posterior interray plates above the primanal, fixed plating in CD interray P-3-2; CD interray not in contact with tegmen.
Primibrachials, secundibrachials, and tertibrachials fixed into calyx. First primibrachial tetragonal, wider than high, approximately same size or smaller than radial plates; second primibrachial axillary; three tertibrachials fixed into calyx; arm facets on three plates: third tertibrachial and two tegmen plates, circular to subelliptical in shape, directed outward. Intrabrachial plates present.
Tegmen low or very low inverted bowl shape; narrows immediately above radial facets, then widens distally (Fig. 3.1). First two to four ranges of tegmen plates small, flat; third to fifth range of tegmen plates large with prominent relatively long spines that expand the width of the tegmen to the same width as at the arm facets (Fig. 3.3). Distal tegmen plating not known.
Free arms ~40; other details of the arms and column unknown.
Etymology
The species name recognizes Madison, Alabama and Madison County, Alabama, where this crinoid was found.
Other material
Two additional specimens were identified in the field but could not be collected.
Measurements
CaH, 2.4; CaMaxW, 27.2; CaMinW, 22.4; TH, 7.3*.
Remarks
Tuscumbiacrinus madisonensis n. gen. n. sp. is described from only the holotype. The distal portion of the tegmen, arms, and column are not known. However, this is a distinctive crinoid unlike anything known from the middle Viséan. The base of this crinoid is deep and broadly concave, and this is slightly exaggerated by minor disarticulation, presumably from compaction. In addition to the overall shape of the calyx base, a narrow, shallow basal concavity is present that is surrounded by a subtle ridge.
Palenciacrinus new genus
Reference Breimer1962 Iberocrinus; Breimer, p. 75, pl. 8, figs. 1–8.
Reference Ubaghs, Moore and Teichert1978b Iberocrinus; Ubaghs, p. T450, figs. 2a–d.
Reference Webster and Webster2014 Iberocrinus; Webster and Webster, p. 1454.
Type species
Palenciacrinus mudaensis n. gen. n. sp.
Diagnosis
As for the type species by monotypy.
Occurrence
Pennsylvanian (Moscovian); Spain.
Etymology
The genus name recognizes the province of Palencia in Spain, where this crinoid was discovered.
Remarks
Phylogenetic analyses (Fig. 5, Supplementary Fig. 1) demonstrate that Palenciacrinus mudaensis n. gen. n. sp. is distinct from Iberocrinus multibrachiatus Sieverts-Doreck, Reference Sieverts-Doreck1951, and not conspecific, as suggested by Breimer (Reference Breimer1962). The only unique genus-diagnostic character for Palenciacrinus n. gen. is the very low globe or subcylindrical calyx, although other genera have a calyx with a very low bowl shape. In addition, the more distinctive aspects of its morphology are a slightly depressed posterior interray, tetragonal or pentagonal first primibrachials, and the presence of intrabrachial plates. This combination of genus-level characters is unique among the Paragaricocrinidae.
Palenciacrinus mudaensis new species
Reference Breimer1962 Iberocrinus multibrachiatus; Breimer, p. 75–77, fig. 13, pl. 8.1–8.4.
Reference Strimple1976 Megaliocrinus bolli Strimple (in part), p. 636.
Reference Webster and Webster2014 Megaliocrinus bolli; Webster and Webster, p. 1454.
Holotype
MGMP-30H.
Diagnosis
Overall calyx shape low globe to subcylindrical, subcircular calyx outline in dorsal view, outer shape of calyx plates very convex, overall shape of calyx base flat, basal concavity wide and deep, a ridge around the basal concavity is absent, basal plates are not hypertrophied, relative size of basal plates unknown, proximal plating in regular interrays 1-2, proximal plating in CD interray P-3-5-5, posterior interray in contact with tegmen, posterior interray slightly depressed, tegmen shape very low inverted cone, spines on side of tegmen absent, anal tube absent, 26 free arms, first primibrachial tetragonal or pentagonal, first primibrachials wider than high, one secundibrachial, intrabrachial plates present, tertibrachials distalmost fixed brachials.
Occurrence
Pennsylvanian (Moscovian); Palencia Province, Muda, Spain.
Description
Calyx small, low globe to subcylindrical shape, overall shape of base of calyx flat, subcircular in outline; smooth calyx plate sculpturing; calyx plates very convex with depressed plate sutures. Basal concavity wide, deep.
Basal circlet small, confined to basal concavity, ridge around basal concavity absent; basal plates not hypertrophied (relative sizes of basal plates unknown). Radial circlet on flat portion of calyx base, interrupted in only the CD interray; radial plates five, hexagonal or heptagonal, ~2.0 times wider than high.
Regular interrays not in contact with tegmen, first interradial plate hexagonal or heptagonal, wider than high, larger than radial plates and primibrachial plates. Regular interray plating 1-2-2-1 or 1-2-2-2.
CD interray wider than regular interrays, slightly depressed. Primanal hexagonal, larger than radial plates, interrupts the radial circlet; proximally in sutural contact with basal plates below; three posterior interray plates above the primanal, fixed plating in CD interray P-3-5-5-; CD interray in contact with tegmen.
Primibrachials, secundibrachials, and tertibrachials fixed into calyx. First primibrachial tetragonal or pentagonal, wider than high, approximately the same size as radial plates; second primibrachial axillary; two or four tertibrachials fixed into calyx; arm facets circular to subelliptical shape, directed downward. Intrabrachial plates or tegmen plates between some adjacent arm openings.
Tegmen very low inverted cone shape; tegmen plates convex with depressed plate sutures, spines on tegmen plates absent. Anal tube absent.
Free arms ~27; other details of the arms and column unknown.
Etymology
The species name recognizes the village of Muda, Spain, which is near the type locality of this taxon.
Measurements
CaH, 9.0; CaW, 17.4; TH, 5.0.
Remarks
Breimer (Reference Breimer1962) acknowledged that this specimen differed from the type of Iberocrinus multibrachiatus Sieverts-Doreck, Reference Sieverts-Doreck1951; however, he placed it in that species because it was only the second paragaricocrinid specimen known from Spain, and it resembled I. multibrachiatus. However, the morphology of Breimer’s specimen is substantially different from that of Sieverts-Doreck (Reference Sieverts-Doreck1951), and we place it in Palenciacrinus n. gen. By association given the name I. multibrachiatus Sieverts-Doreck, Reference Sieverts-Doreck1951, note that Strimple placed the Breimer specimen in Megaliocrinus bolli Strimple, Reference Strimple1976, but Palenciacrinus mudaensis n. gen. n. sp. differs in many ways from M. bolli, as well as I. multibrachiatus Sieverts-Doreck, Reference Sieverts-Doreck1951 (Fig. 5; Supplemental Tables 3, 4).
Genus Pulcheracrinus new genus
Type species
Megaliocrinus exotericus Strimple, Reference Strimple1951.
Diagnosis
As for the type species by monotypy.
Occurrence
Pennsylvanian (Bashkirian); United States.
Etymology
The genus is from pulchera, beautiful, fine (Latin).
Remarks
As is typical for paragaricocrinid genera, Pulcheracrinus n. gen. lacks any unique characters (Supplementary Table 3). A flat bowl-shaped calyx is present in Tuscumbiacrinus n. gen. and Pulcheracrinus n. gen. and not in other paragaricocrinids. Also, having a depressed posterior interray and spinose tegmen plates, and the distalmost fixed brachials either the tertibrachials or quartibrachials are not typical for this family (Supplementary Table 3). Tip-dating analysis has M. exotericus in a separate clade from both M. aplatus and M. bolli, and designation of a new genus for M. exotericus is needed.
Pulcheracrinus exotericus (Strimple, Reference Strimple1951) n. gen. n. comb.

Figure 10. Paragaricocrinids. (1, 2) Nipponicrinus akiyoshiensis n. gen. n. sp. (ASM 50058), holotype, scale bar represents 10.0 mm; images from Hashimoto (Reference Hashimoto2001), used with permission; (1) basal view of calyx, (2) lateral view of calyx. (3–5) Pulcheracrinus exotericus (Strimple, Reference Strimple1951) n. gen. n. comb. (USNM PAL 4717), scale bar represents 5.0 mm; images courtesy of the Smithsonian Institution, GUID: http://n2t.net/ark:/65665/39b179562-3e0a-4c14-b245-c827fdb712d1; (3) basal view of calyx, (4) A-ray lateral view of theca, (5) CD-interray lateral view of calyx. (6, 7) Nipponicrinus hashimotoi n. gen. n. sp. (ASM 50053), holotype, scale bar represents 10.0 mm; images from Hashimoto (Reference Hashimoto2001), used with permission; (6) basal view of calyx, (7) lateral view of calyx.
Reference Strimple1951 Megaliocrinus exotericus Strimple, p. 14, pl. 14, figs. 5–8.
Reference Webster and Webster2014 Megaliocrinus exotericus; Webster and Webster, p. 1454.
Holotype
USNM PAL 4717.
Diagnosis
Overall calyx shape flat bowl, calyx outline subcircular in dorsal view, outer shape of calyx plates very convex, overall shape of calyx base flat; shape of basal concavity shallow and narrow, ridge around the basal concavity absent, hypertrophied basal plates absent, basal plates equal in size; proximal plating in regular interrays 1-2, proximal plating in CD interray P-3-3, posterior interray in contact with tegmen, posterior interray depressed; tegmen shape very low inverted cone, small spines on side of tegmen; 20–40 arms, first primibrachials tetragonal, first primibrachials wider than high, one secundibrachial, intrabrachial plates absent, distalmost fixed brachials tertibrachials or quartibrachials.
Occurrence
Pennsylvanian (Bashkirian); Brentwood Limestone; southeast of Fort Gibson, Oklahoma, United States.
Description
Calyx small, flat bowl shaped, overall shape of calyx base flat, subcircular in outline; smooth calyx plate sculpturing with depressed sutures; calyx plates very convex. Basal concavity narrow, shallow (Fig. 10.3).
Basal circlet very small, confined to basal concavity, ridge absent; three equal-sized basal plates, basal plates not hypertrophied, primanal articulated proximally with two basal plates. Radial circlet along flat base of the calyx, interrupted in only the CD interray; radial plates five, hexagonal or heptagonal, 0.72–0.89 times wider than high.
Regular interrays not in contact with tegmen, first interradial plate octagonal, as high as wide, smaller than radial plates, larger than pimibrachial plates. Regular interray plating 1-2-2.
CD interray wider than radial interrays, depressed (Fig. 10.5). Primanal heptagonal, narrower and higher than radial plates, interrupts the radial circlet; proximally in sutural contact with two basal plates below; three posterior plates above the primanal, fixed plating in CD interray P-3-3; CD interray in contact with tegmen.
Primibrachials, secundibrachials, and tertibrachials fixed into calyx. First primibrachial tetragonal or axillary and pentagonal, wider than high, smaller than radial plates; one or two secundibrachials, tertibrachials fixed into calyx in most rays; arm facets directed upward and outward. Intrabrachial plates absent.
Tegmen low or very low inverted cone shape. All tegmen plates spinose; distal, central tegmen plate with large spine (Fig. 10.4), anal tube absent.
Free arms ~20; other details of arms and column unknown.
Remarks
Pulcheracrinus exotericus n. gen. n. comb. was originally placed in Megaliocrinus by Strimple (Reference Strimple1975). However, with a flat bowl-shaped calyx, the posterior interray depressed, a very low cone-shaped tegmen, and tertibrachials or quartibrachials as the highest fixed brachials, this species is distinct from other members of Megaliocrinus. It is also in a separate clade from other Megaliocrinus in parsimony analysis of all taxa (Supplementary Fig. 1), in parsimony analysis of only Pennsylvanian taxa (Supplementary Fig. 2), and in the Bayesian tip-dating cladogram (Fig. 6). It is sister to the clade with Palenciacrinus mudaensis n. gen. n. sp. and Paramegaliocrinus erlangeri.
Nipponicrinus new genus
Type species
Nipponicrinus hashimotoi n. gen. n. sp.
Included species
Nipponicrinus hashimotoi n. gen. n. sp. and Nipponicrinus akiyoshiensis n. gen. n. sp.
Diagnosis
Overall calyx shape very low bowl, subcircular calyx outline in dorsal view, outer shape of calyx plates gently convex, overall shape of calyx base flat, basal concavity wide and shallow; relative sizes of basal plates unknown, ridge around the basal concavity, basal plates hypertrophied; proximal plating in regular interrays 1-1 or 2-2, proximal plating in CD interray P-2-?, posterior interray not in contact with tegmen, posterior interray not depressed; tegmen shape low or very low inverted bowl, spines on side of tegmen present or absent; ~24–28 free arms (as known), first primibrachial tetragonal, first primibrachials wider than high, 1–3 secundibrachials, intrabrachial plates absent, tertibrachials distalmost fixed brachials.
Occurrence
Pennsylvanian (Moscovian); Japan.
Etymology
The genus name recognizes the Japanese name for Japan, Nippon.
Remarks
Hashimoto (Reference Hashimoto2001) described several Pennsylvanian camerate crinoids from the Akiyoshi Limestone Group of southwestern Japan, including four open-nomenclature taxa in the Paragaricocrinidae. These include Paragaricocrinidae gen. type A, Paragaricocrinidae gen. type B1, Paragaricocrinidae gen. type B2, and Paragaricocrinidae gen. type B3. Only Paragaricocrinidae gen. type B1 and Paragaricocrinidae gen. type B2 are sufficiently known to describe as new species, and they are recognized herein as Nipponicrinus hashimotoi n. gen. n. sp. and Nipponicrinus akiyoshiensis n. gen. n. sp. Paragaricocrinidae gen. type A and Paragaricocrinidae gen. type B3 are retained in open nomenclature as Paragaricocrinidae indeterminate A and Paragaricocrinidae indeterminate B, respectively.
As discussed in Hashimoto (Reference Hashimoto2001), the hypertrophied basal circlet in these Japanese specimens is distinctive and unique among paragaricocrinids. This distinctive character is a rare paragaricocrinid character. Other important characters are gently convex outer surfaces of calyx plates, a wide shallow basal concavity, P-2 plating in the CD interray, the posterior not in contact with the tegmen, and two primibrachials. These characters all differentiate Nipponicrinus n. gen. (Supplementary Table 3). In Figure 6, Nipponicrinus n. gen. is a more derived Pennsylvanian clade as a sister to the clade with Iberocrinus and Permian taxa.
Nipponicrinus hashimotoi new species
Reference Hashimoto2001 Paragaricocrinidae gen. type B1 Hashimoto, p. 9, pl. 1, figs. 4–6; pl. 2, figs. 5, 6; Fig. 4.1.
Reference Webster and Webster2014 Paragaricocrinidae gen. type B1 Hashimoto; Webster and Webster, p. 1628.
Types
Holotype: ASM 50053; paratypes: ASM 50054–50056.
Diagnosis
Nipponicrinus n. gen. with a bowl-shaped tegmen, tegmen plates without spines, and two secundibrachials.
Occurrence
Pennsylvanian (Moscovian); Akiyoshi Limestone Group, Japan.
Description
Calyx large, low bowl shape, overall shape of the calyx base flat, subcircular in outline (Fig. 10.6); smooth calyx plate sculpturing; calyx plates gently convex. Basal concavity wide, shallow, prominent ridge around basal concavity.
Basal circlet large, hypertrophied and covering radial circlet; three subequal basal plates, hypertrophied.
Regular interrays in contact with tegmen, first interradial plate octagonal, higher than wide, much larger than radial plates and primibrachial plates. Regular interray plating 1-2 or 1-1.
CD interray wider than regular interrays, not depressed. Primanal heptagonal, covered by hypertrophied basal plates, interrupts the radial circlet; proximally in sutural contact with two basal plates; two posterior interray plates above primanal, fixed plating in CD interray P-2-3-?; CD interray in contact with tegmen.
Primibrachials, secundibrachials, and tertibrachials fixed into calyx. First primibrachial tetragonal, wider than high, first or second primibrachial axillary; two or three tertibrachials fixed into calyx; arm facets directed upward and outward. Intrabrachial plates absent.
Tegmen very low inverted bowl shape (Fig. 10.7). First several ranges of tegmen plates very small, convex; central tegmen plates large, convex; distal tegmen plate spinose.
Free arms ~30; other details of the free arms and column unknown.
Etymology
The species name recognizes Kyoichi Hashimoto, who provided the initial description of these Pennsylvanian crinoids from Japan (Hashimoto, Reference Hashimoto2001).
Additional material
ASM 50057.
Remarks
The morphology of two of the four paragaricocrinid morphotypes (type B1 and B2) identified by Hashimoto (Reference Hashimoto2001) is sufficiently understood to name as two species. Nipponicrinus hashimotoi n. gen. n. sp. is distinguished by a bowl-shaped tegmen, a tegmen without spines, and two secundibrachials. In contrast, N. akiyoshiensis n. gen. n. sp. has a cone-shaped tegmen, a tegmen with spines, and one to three secundibrachials.
Nipponicrinus akiyoshiensis new species
Reference Hashimoto2001 Paragaricocrinidae gen. type B2 Hashimoto, p. 9, pl., 2, figs. 1–4, 7; pl. 3, fig. 3; Fig. 4.2.
Reference Webster and Webster2014 Paragaricocrinidae gen. type B2; Webster and Webster, p. 1628.
Types
Holotype: ASM 50058; paratype: ASM 50059.
Diagnosis
Nipponicrinus n. gen. with a cone-shaped tegmen, tegmen plates with spines, and one to three secundibrachials.
Occurrence
Pennsylvanian (Moscovian?); Akiyoshi Limestone Group, Japan.
Description
Calyx small, very low bowl shape, overall shape of calyx base convex, subcircular in outline (Fig. 10.1); smooth calyx plate sculpturing; calyx plates very convex with deep depressions along sutures. Basal concavity wide, shallow; short ridge around basal concavity.
Basal circlet large, confined to basal concavity; three basal plates, hypertrophied. Radial circlet covered by hypertrophied basal plates, interrupted in only the CD interray; radial plates five.
Regular interrays in contact with tegmen, first interray ten-sided, as high as wide, larger than radial plates and primibrachial plates. Regular interray plating 1-2.
CD interray wider than regular interrays, not depressed. Primanal covered by hypertrophied basal plates, interrupts the radial circlet; fixed plating in CD interray P-2-3; CD interray in contact with tegmen.
Primibrachials, secundibrachials, and tertibrachials fixed into calyx. First primibrachial (if visible) tetragonal, wider than high; second primibrachial axillary; two or three tertibrachials fixed into calyx; arm facets directed outward. Intrabrachial plates absent.
Tegmen low or very low inverted bowl shape. Tegmen relatively large, gently convex or spinose; long spine on terminal tegmen plate (Fig. 10.2).
Free arms ~20; other details of free arms and column unknown.
Etymology
The species name recognizes the Akiyoshi Terraine in southeastern Japan, where this crinoid was found.
Remarks
The species is distinguished from its congener in the remarks of Nipponicrinus hashimotoi n. gen. n. sp.
Paragaricocrinidae indeterminant
Remarks
Paragaricocrinids have robustly constructed calyxes that resist disarticulation. This is the reason that paragaricocrinids are enigmatic post-Mississippian crinoids and the reason that many partially disarticulated post-Mississippian crinoids have been described as Paragaricocrinidae left in open nomenclature. The taxonomic positions of some of these taxa have been refined in the present study, but others must remain in open nomenclature pending more complete specimens. Below is an accounting of paragaricocrinids left in open nomenclature.
?Paragaricocrinidae
Reference Lane, Waters, Maples, Marcus and Liao1996 Paragaricocrinidae new genus and species, indeterminate Lane et al., p. 119, figs. 5.3–5.5. 6.11–6.12. 6.14.
Reference Webster and Webster2014 Paragaricocrinidae new genus and species, indeterminate; Webster and Webster, p. 1628.
Occurrence
Pennsylvanian (Moscovian); Qijiagou Formation, Xinjiang–Uygar Region, China.
Material
USNM PAL 483313–483316, plus two specimens in lot NIPG 148867.
Remarks
Specimens assigned to Paragaricocrinidae new genus and species, indeterminate by Lane et al., Reference Lane, Waters, Maples, Marcus and Liao1996, are very poorly preserved specimens composed of the basal and radial circlets and a few proximal columnals. These may be paragaricocrinids, but major features characteristic of the Paragaricocrinidae are not preserved. Also, the shape of the proximal calyx and the radial plate sculpturing on Lane et al. (Reference Lane, Waters, Maples, Marcus and Liao1996, fig. 5.4) is not typical for the Paragaricocrinidae. Enough is unknown about the morphology of these crinoids that leaving them in open nomenclature is appropriate.
Paragaricocrinidae indeterminate A
Reference Hashimoto2001 Paragaricocrinidae gen. type A Hashimoto, p. 8, pl. 1, figs. 1–3; Fig. 3.2.
Reference Webster and Webster2014 Paragaricocrinidae gen. type A; Webster and Webster, p. 1628.
Occurrence
Pennsylvanian (Moscovian); Akiyoshi Limestone Group, Japan.
Material
Specimens 1–6 from Hashimoto (Reference Hashimoto2001).
Remarks
Hashimoto (Reference Hashimoto2001, fig. 3, pl. 1, figs. 1–3) illustrated three specimens that he assigned to Paragaricocrinidae gen. type A. Similar to other paragaricocrinids reported by Hashimoto (Reference Hashimoto2001), these specimens have hypertrophied basal plates and large first interradial plates in regular interrays. However, other characters differ, such as the overall concave shape of the calyx base. Not enough morphological detail is preserved to place this taxon in a genus or species with confidence.
Paragaricocrinidae indeterminate B
Reference Hashimoto2001 Paragaricocrinidae gen. type B3 Hashimoto, p. 10, pl. 3, figs. 1, 2.
Reference Webster and Webster2014 Paragaricocrinidae gen. type B3; Webster and Webster, p. 1628.
Occurrence
Pennsylvanian (Moscovian); Akiyoshi Limestone Group, Japan.
Material
Specimens 18 and 19 from Hashimoto (Reference Hashimoto2001).
Remarks
Hashimoto (Reference Hashimoto2001, pl. 3, figs. 1, 2) assigned two incompletely preserved specimens to Paragaricocrinidae gen. type B3. These specimens are similar to Nipponicrinus n. gen., but enough differences exist that a confident assignment cannot be made until more complete specimens are available.
Paragaricocrinidae indeterminate C
Reference Webster, Maples, Sevastopulo, Frest and Waters2004 Megaliocrinus? sp. Webster et al., p. 19, pl. 1, fig. 13.
Reference Webster and Webster2014 Megaliocrinus? sp.; Webster and Webster, p. 1454.
Occurrence
Mississippian (Serpukhovian); Mouizeb el Atchane Member, Aïn el Mizab Formation; Maderel Mahjib, Algeria.
Material
RGM 361 175.
Remarks
Webster et al. (Reference Webster, Maples, Sevastopulo, Frest and Waters2004) recognized Megaliocrinus? sp. based on a single, partial, crushed theca, which was described as Amphoracrinus nov. sp. by Pareyn (Reference Pareyn1961). Webster et al. (Reference Webster, Maples, Sevastopulo, Frest and Waters2004) identified this as Megaliocrinus? because it has numerous ungrouped arm openings, numerous convex tegmen plates, and a large distal tegmen plate. Although similar to Megaliocrinus, these characteristics are also similar to other crinoids, including other Paragaricocrinidae. Because the overall shape of the tegmen and plating of the calyx are unknown, we reassign this specimen to Paragaricocrinidae indeterminate C.
Paragaricocrinidae indeterminate D
Reference Lane, Waters, Maples, Marcus and Liao1996 Hexacrinidae new genus and species indeterminate Lane et al., p. 121, figs. 4.20, 5.6.
Reference Webster, Waters, Liao and Maples2009b Paragaricocrinid gen. undesignated Webster et al., p. 46, fig. 2R.
Reference Webster and Webster2014 Paragaricocrinid gen. undesignated; Webster and Webster, p. 1627.
Occurrence
Pennsylvanian (Moscovian); Qijiagou Formation, Xinjiang, China.
Material
NIPG 148866, USNM PAL 483317.
Remarks
Two specimens were assigned to Hexacrinidae new genus and species indeterminate by Lane et al. (Reference Lane, Waters, Maples, Marcus and Liao1996) and later to Paragaricocrinid gen. undesignated in Webster et al., Reference Webster, Waters, Liao and Maples2009b. These specimens consist of a basal circlet with three basal plates and a radial circlet with five radial plates and one primanal. The characters of the very incomplete specimens are consistent with the Paragaricocrinidae, but the specimens are too poorly preserved to speculate on a genus assignment.
Paragaricocrinidae indeterminate E
Reference Lane, Waters, Maples, Marcus and Liao1996 Paragaricocrinidae new genus and species, Lane et al., p. 119, figs. 5.3–5.5, 6.11, 6.12.
Reference Webster, Waters, Liao and Maples2009b Paragaricocrinidae indet. Webster et al. p. 46, not illustrated.
Reference Webster and Webster2014 Paragaricocrinidae indet.; Webster and Webster, p. 1628.
Occurrence
Pennsylvanian (Moscovian); Qijiagou Formation, Xinjiang, China.
Material
USNM PAL 48331–48336.
Remarks
The specimens assigned to Paragaricocrinidae new genus and species by Lane et al. (Reference Lane, Waters, Maples, Marcus and Liao1996) were reassigned to Paragaricocrinidae indeterminate by Webster et al. (Reference Webster, Waters, Liao and Maples2009b). As noted by Lane et al. (Reference Lane, Waters, Maples, Marcus and Liao1996), three basal plates and six plates in the radial circlet (five radial plates and one primanal) align these specimens with the Paragaricocrinidae. Further, the basal plates extend beyond the proximal columnal. However, other important morphological characters are lacking, which make comparisons to other members of the Paragaricocrinidae inconclusive. Rather than Paragaricocrinidae new genus and species, we follow Webster et al. (Reference Webster, Waters, Liao and Maples2009b) and refer these specimens to Paragaricocrinidae indeterminate E.
Paragaricocrinidae indeterminate F
Reference Webster, Haggart, Saxifrage, Saxifrage, Gonau and Douglas2009a Paragaricocrinid n. gen.? 1 Webster et al., p. 668, fig. 6a, b.
Reference Webster and Webster2014 Paragaricocrinid n. gen.? 1; Webster and Webster, p. 1627.
Occurrence
Permian (Sakmarian?); Mount Mark Formation, Vancouver Island, British Columbia, Canada.
Material
Specimens illustrated by Webster et al. (Reference Webster, Haggart, Saxifrage, Saxifrage, Gonau and Douglas2009a) were not collected and remain in the field.
Remarks
The specimen assigned to Paragaricocrinid n. gen.? 1 by Webster et al. (Reference Webster, Waters, Liao and Maples2009b) is essentially a longitudinal cross section through a crown with an attached proximal column. If this is, indeed, a paragaricocrinid, it is the only known specimen with arms and a length of column preserved. Unfortunately, no details of the calyx plating are known, which precludes a generic assignment. The nearly vertical side of the calyx wall is similar to Palenciacrinus mudaensis n. gen. n. sp. from the Pennsylvanian of Spain.
Paragaricocrinidae indeterminate G
Reference Webster, Haggart, Saxifrage, Saxifrage, Gonau and Douglas2009a Paragaracocrinid [sic.] n. gen.? 2 Webster et al., p. 669, fig. 6f.
Reference Webster and Webster2014 Paragaracocrinid [sic.] n. gen.? 2; Webster and Webster, p. 1628.
Occurrence
Permian (Sakmarian?); Mount Mark Formation, Vancouver Island, British Columbia, Canada.
Material
Specimens illustrated by Webster et al. (Reference Webster, Haggart, Saxifrage, Saxifrage, Gonau and Douglas2009a) were not collected and remain in the field.
Remarks
The specimen described by Webster et al. (Reference Webster, Haggart, Saxifrage, Saxifrage, Gonau and Douglas2009a) is a partial specimen that exposes the inner surface of the tegmen. A series of small plates adjacent to the tegmen form a conical structure and were interpreted by Webster et al. (Reference Webster, Haggart, Saxifrage, Saxifrage, Gonau and Douglas2009a) as the distal portion of an anal tube. The complete morphology of a paragaricocrinid tegmen is only known in five species (see Supplementary Table 1). Unfortunately, it is impossible to assign this partial specimen to a genus.
Data availability statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.280gb5n03.
Acknowledgments
We thank T. Adrain, C. d’Arpa, A. Ferretti, Dr. Fujikawa, K. Hashimoto, K. Hollis, S. Menédez, G. Mirantsev, T. Oji, N. den Ouden, and C. Sorbni for helping us clarify the locations and specimen numbers for Paragaricocrinidae material; and the following allowed permission to reproduce figures: H. Little and B.T. Huber, Smithsonian; M. Fujikawa and K. Hashimoto, Akiyoshi-dai, Museum of Natural History, Japan. We also thank the following reviewers and the editorial staff who greatly improved this manuscript: D.L. Meyer, P. Gorzelak, S. Zamora, and one anonymous reviewer.
Competing interests
The authors declare none.












