Introduction
The common cockle Cerastoderma edule is an ecologically important species in tidal areas, coastal lagoons, and estuaries from Senegal to the Murmansk coast of the Barents Sea (Genelt-Yanovskiy et al., Reference Genelt-Yanovskiy, Poloskin, Granovitch, Nazarova and Strelkov2010; Tebble, Reference Tebble1976). Cockles play an important role as a link between producers (phytoplankton) and a multitude of consumers at higher trophic levels. They are prey to a range of invertebrate predators (crabs, starfish), many fish (e.g. flounder, plaice, and sea bream), and waterbirds (e.g. oystercatcher, seagull, and eider) (for an overview of consumers, see Malham et al., Reference Malham, Hutchinson and Longshaw2012). Cockles have also been a human resource since the Stone Age as seen by the many cockle shells found in kitchen middens (Andersen, Reference Andersen2014; Montgomery et al., Reference Montgomery, Beaumont, Jay, Keefe, Gledhill, Cook, Dockrill and Melton2013). Nowadays commercial cockle fishing takes place in many countries (Carss et al., Reference Carss, Brito, Chainho, Ciutat, de Montaudouin, Fernandez Otero, Filgueira, Garbutt, Goedknegt, Lynch, Mahony, Maire, Malham, Orvain, van der Schatte Olivier A and Jones2020) and the Limfjorden accounts for ca. 59% of European cockle landings (2017–2022, Eurostat; Freitas et al., Reference Freitas, Petersen, Madsen, Jensen, Nielsen, Joyce, Agüera, Olsen, Krause and Saurel2023). Cockle populations generally exhibit significant fluctuations in abundance, and given the commercial interest in cockles, much effort has been made to identify possible natural drivers controlling their dynamics (Burdon et al., Reference Burdon, Callaway, Elliott, Smith and Wither2014; Malham et al., Reference Malham, Hutchinson and Longshaw2012). Incidences of mass mortality in adult populations are a widespread phenomenon contributing to fluctuations. However, it is often difficult to identify the exact cause of a mass mortality event, unless due to adverse environmental conditions registered during the decay (e.g. oxygen depletion; temperature extremes). Predators, diseases, and macroparasites can all potentially decimate cockle populations (Burdon et al., Reference Burdon, Callaway, Elliott, Smith and Wither2014; Malham et al., Reference Malham, Hutchinson and Longshaw2012).
In connection with cockle fishing in the Limfjorden, a Danish estuary, the presence of unburied cockles has been reported repeatedly from subtidal sites based on observations from divers and through underwater cameras. These cockles that lie on the seabed are considered moribund, and to the extent that a high fraction of a cockle population exhibits such behaviour, uncovering the cause may contribute to the understanding of a mass mortality event. Incidences of surfacing and mass mortality in cockles have previously been associated with high prevalence of some trematode species (Jonsson and André, Reference Jonsson and André1992; Thieltges, Reference Thieltges2006; Villalba et al., Reference Villalba, Iglesias, Ramilo, Darriba, Parada, No, Abollo, Molares and Carballal2014). At least 16 species of trematodes use cockles as intermediate hosts and various fish or waterbird species as their final hosts (de Montaudouin et al., Reference de Montaudouin, Thieltges, Gam, Krakau, Pina, Bazairi, Dabouineau, Russell-Pinto and Jensen2009). Some of these parasites may have detrimental effects on their host individuals, such as deterred growth, tissue destruction, weakened tolerance of adverse environmental conditions, impaired burrowing ability, and reduced fecundity and lifespan (for an overview, see Longshaw and Malham, Reference Longshaw and Malham2013). Impaired burrowing ability of cockles could be due to muscle damage inflicted by parasites located in foot tissue (Jonsson and André, Reference Jonsson and André1992; Lauckner, Reference Lauckner and Kinne1983) or due to parasite-controlled behaviour. However, pathological effects of trematodes using an intermediate host as a transport vector are often limited, as their viability depends on the well-being of their host. Nonetheless, many trematode species depend on trophic transmission (capitalizing on prey–predator relationships) for completing their life cycle. Thus, it may have an adaptive value to manipulate their intermediate host to favour transmission to their final-host predators. Several examples of parasite-induced host manipulation across parasite taxa suggest that host manipulation is a widespread parasite strategy for enhancing transmission success (Lafferty and Shaw, Reference Lafferty and Shaw2013). To promote surfacing of host individuals could be a strategy for some cockle parasites to reach their vertebrate host.
Given that cockles from the Limfjorden are a human resource and knowing that some cockle trematodes may have detrimental effects on their host, our aim was to provide a survey of the trematodes in a subtidal cockle population in the Limfjorden, and to assess to what extent they might impact surfacing, growth, and viability of the studied cockles. Furthermore, it turned out to be important to clarify the life cycle of the dominant trematode in the studied cockle population to understand their success (high prevalence). We do not have any previous registration of the trematode fauna in cockles from subtidal sites in the Limfjorden, but screening of cockles from shallow water sites (<1 m depth) has revealed the presence of 4 of the 16 known trematode species using cockles as their intermediate host (own observation). To our surprise, a very high fraction of both buried and unburied cockles from our study site was infected by Monorchis parvus (Digenea) – a species not previously reported from Danish waters. Only a few species of Diplodus (Sparidae, sea breams) have been identified as hosts to M. parvus (Bartoli et al., Reference Bartoli, Jousson and Russell-Pinto2000). As they do not occur in the Limfjorden, the observation is enigmatic and triggered us to try to clarify its life cycle. Monorchis parvus is generally considered a trematode with an abbreviated life cycle with only two hosts, whereas most digenean trematodes have three hosts. For M. parvus, the cockle is both the first and the second intermediate host, and it has a cockle-eating fish as its final host. While digeneans are typically species-specific regarding their first intermediate hosts, they are less selective regarding their final host. So, we can expect that fish other than Diplodus species can be host to M. parvus. Apart from the consumption of infected cockles, transmission could also take place through a second intermediate host if this is an option. Whereas many have observed cercariae in sporocysts in M. parvus-infected cockles, they are generally considered an intermediate stage in the larval development of M. parvus that have lost their functionally as dispersal agents. However, there is still some doubt about this. Finally, transmission could potentially take place from dying cockles with their flesh accessible to carnivorous fish. We have observed that gobies are attracted by dying bivalves on the seabed in shallow water. This raised the question of whether gobies could host M. parvus and become infected by eating flesh from dying cockles with M. parvus sporocysts. To assess these transmission possibilities, we have examined infected cockles for the presence of active cercariae, and in a pilot experiment, we have fed juvenile gobies (Pomatoschistus microps) with infected cockle flesh. Based on this, we consider the importance of potential alternative M. parvus life cycles to understand its presence and success in the studied cockle population.
Materials and methods
Environmental data
From a monitoring station close to our field plot (location: 56°46′37.2″N and 8°53′18.0″E), we obtained monthly data (2012) on temperature, salinity, and oxygen content at a depth of 9–10 m (data provided by Svend Aage Bendtsen, the Danish Environmental Protection Agency [Miljøstyrelsen]). The salinity varied within a narrow range from 28.7 to 31.2, and water temperature ranged from 2.3°C (February) to 19.5°C (August). The oxygen content reached a minimum of 6.9 mg l−1 (91% saturation) on 28 August 2012 at a depth of 9–10 m.
Sampling sites and methods
We collected quantitative bottom samples in June (8 June 2012), August (17 August 2012), and November (7 November 2012) at a depth of about 10–12 m in the Sallingsund strait, the Limfjorden, at two nearby sites (St. 1: 56°44′46.9″N and 8°50′36.4″E and St. 2: 56°44′47.3″N and 8°50′33.2″E). Twenty Haps corers (surface area/corer: 145 cm2) were sampled at each station on each date. Given the fluidity of the surface substratum at our study site, we were often unable to distinguish between cockles on the surface and those partly buried. For practical reasons, we considered those with part of their shell visible on the sediment surface as unburied. Visible specimens were handpicked, counted, and kept in separate containers. Afterwards the sampled sediment was sieved through a 1-mm mesh, and cockle specimens remaining on the sieve were collected, counted, and stored for further processing. As our study of the parasite fauna in cockles started immediately after sampling in November, the collected cockles were kept alive in aerated seawater at 6°C with a salinity of 29, while those sampled earlier (in June and August) were kept in a freezer (−20°C) until being inspected for trematodes. In addition, cockles collected from a few other sites in the Limfjorden in 2012, July 2018, and August 2019 were also inspected for Monorchis-infected cockles.
Inspection for trematodes and other metazoans in cockles
To examine cockles for metazoans, flesh from each cockle was dissected from the shells and subsequently squeezed between two glass plates (‘compressorium’) divided into numbered cells. The ‘compressorium’ was placed under a stereomicroscope and systematically screened for trematodes in the various tissue fractions. The trematodes were identified to the lowest taxonomical level according to de Montaudouin et al. (Reference de Montaudouin, Thieltges, Gam, Krakau, Pina, Bazairi, Dabouineau, Russell-Pinto and Jensen2009) and Longshaw and Malham (Reference Longshaw and Malham2013). A few sporocysts identified as M. parvus were saved for DNA-analyses and preserved in 96% ethanol.
Shell growth of cockles
To test if the shell growth of cockles is affected by parasites, both their shell length and the length at the last winter ring were measured using Vernier calipers. We used the patterns of mean shell length over time (June, August, and November), position (unburied vs. buried), and condition (M. parvus-infected vs. uninfected) to provide an indication of the possible influence of these factors on the growth of cockles in the present environment. However, as the temporal patterns of mean length of a population can be a result of growth patterns, recruitment, and different size-selective mortality factors, we used individual length increments of uninfected and infected cockles from the ring indicating the winter of 2011–2012 until mid-November 2012 as a more unambiguous measure for assessing the growth impact of M. parvus. To minimize the influence of length-dependent growth, we used uninfected and infected cockles within the same size range during the winter of 2011–2012 (16–21 mm).
A pilot infection experiment
Based on our own observations of how dying bivalves on the sea bottom in shallow water attract scavengers such as small fish, we hypothesize that infected, dying cockles could be a transmission hotspot for M. parvus. This would enable fish other than those that can handle adult cockles to become hosts. It requires that sporocysts survive for a while after cockle death and that the cockle flesh is being ingested by a carnivorous fish or that the sporocyst worms themselves are prey items for fish. Potential fish exploiting dying cockles as a food resource in our system could be one of the goby species in the Limfjorden that are abundant on sandy bottoms in shallow water (Pomatoschistus minutus, P. microps). In a small pilot experiment, we used P. microps to test if they became infected by feeding on cockle tissue with M. parvus sporocysts. We collected juvenile specimens of P. microps (mean length 41.2 mm) in an estuary in Eastern Jutland (Norsminde Fjord), where we have studied cockles for a while and never found M. parvus-infected cockles, thus minimizing the risk of using already infected fish. We fed them individually with sporocysts with metacercariae and kept the fish (n = 16) in aerated water at 15°C. We picked specimens for dissection 3 (n = 4), 7 (n = 4), 9 (n = 2), and 20 (n = 4) days post-infection (p.i.), and two fish died. They were examined for the presence of stages of M. parvus throughout the digestive system by using a binocular microscope. To verify that the specimens observed in the goby were M. parvus, we did DNA analyses of both the sporocysts (food) and the adults found in gobies. The sampled specimens were preserved in alcohol (96%) for DNA extraction. The purpose of this pilot experiment was solely to test if M. parvus larvae could potentially survive and mature in P. microps before conducting a more comprehensive experiment.
Statistics
Differences in the prevalence of M. parvus were tested by using the Pearson chi-squared test (χ 2-test), and metric data were analysed by using Student’s t-test (length increment data) and ANOVA (length of cockles as a function of condition [uninfected vs. M. parvus-infected] and position [unburied vs. buried]). For metric data, normality was assumed, and prior to analyses, data were tested for homoscedasticity (Levene’s test of equality of error variances). As homoscedasticity was accepted, no data transformation was required. All statistical procedures were performed using the IBM SPSS program package (Statistics version 29.0.2.0).
Histology
Cockle tissue preserved in Davidson’s fixative was dehydrated and infiltrated with paraffin. Afterwards the samples were embedded in blocks of paraffin and later sectioned into 2–3 µm sections that were placed on glass slides. The tissue slides were coloured by traditional haematoxylin and eosin. The methods described in the SOP ‘Mollusc processing for diagnosis by histology’ (EURL, 2011) were followed.
DNA analyses
We used DNA barcoding to verify (1) that the trematode larvae identified as M. parvus based on morphological criteria were correctly identified and (2) that the adult stages of trematodes sampled from experimentally infected juvenile gobies (P. microps) were also M. parvus specimens (hatched from sporocysts with metacercariae added as food – see later). Four samples of the sporocysts fed to gobies and six samples of maturing adults from the stomach-intestine region of the gobies were used for DNA extraction. ‘AllGenetics & Biology SL’ (www.allgenetics.eu) performed the DNA barcoding analyses. The description of the applied methods (see later) is according to the internal report from ‘AllGenetics & Biology SL’.
DNA isolation
DNA was isolated using the Quick-DNA Microprep Kit (Zymo) according to the manufacturer’s instructions. DNA was resuspended in a final volume of 20 μL. An extraction blank (Bex) was included in every DNA extraction round and treated as a regular sample to check for cross-contamination.
PCR amplification and Sanger sequencing
A fragment of about 1040 base pairs (bp) of the 18S-ITS1 region (ribosomal 18S gene and internal transcribed spacer 1) was amplified using the primers S20T2 (5′ GGT AAG TGC AAG TCA TAA GC) and 5.8S1 (5′ GCT GCG CTC TTC ATC GAC A) (Bartoli et al., Reference Bartoli, Jousson and Russell-Pinto2000). PCRs were carried out in a final volume of 25 μL, containing 2.5 μL of template DNA, 0.5 μM of the primers, 12.5 μL of Supreme NZYTaq 2× Green Master Mix (NZYTech), and ultrapure water up to 25 μL. The reaction mixture was incubated as follows: an initial denaturation at 95°C for 5 min, followed by 35 cycles of 95°C for 30 s, 50°C (S20T2/5.8S1 primers) for 30 s, 72°C for 30 s, and a final extension step at 72°C for 10 min.
A negative control, which contained no DNA, was included to check for contamination during the experiments.
The amplicons were run on 2% agarose gels stained with GreenSafe (NZYTech) and imaged under UV light to verify the amplicon size. The amplicons were further purified using the Mag-Bind RXNPure Plus magnetic beads (Omega Biotek), following the instructions provided by the manufacturer.
The purified amplicons were bi-directionally sequenced using the same primers as those used for the PCR. Electropherogram analysis and overlapping were conducted in Geneious 8.1.9. During electropherogram analysis, the primer annealing regions and the low-quality regions at both ends of each electropherogram was trimmed (error probability limit of 0.03). Sequence reads were manually checked for sequencing errors or ambiguous base calls.
To check if the sequences obtained belonged to the target taxonomic group, they were compared to the 18S-ITS1 reference sequences available in DDBJ/ENA/GenBank by using the NCBI BLASTn tool against the Nucleotide database (nr/nt).
The results show that the primers S20T2/5.8S1 also amplified DNA from non-target species (fish host). Even though the primer pair S20T2/5.8S1 is presumably specific for digenean species (Bartoli et al., Reference Bartoli, Jousson and Russell-Pinto2000) and has been successfully used to amplify Monorchis spp. DNA from either sporocysts or living adult specimens isolated from fish digestive tracts (Jousson et al., Reference Jousson, Bartoli and Pawlowski2000) our analysis shows that they can also amplify the host DNA (i.e. P. microps).
Design of Monorchis-specific PCR primers
In order to avoid the amplification of P. microps or any other non-target species, a new primer pair was designed by AllGenetics using Primer3 in Geneious 8.9.1, based on the 18S-ITS1 reference sequences available in DDBJ/EMBL/GenBank for Monorchis monorchis (accession numbers: Y18937.1, Y18939.1, Y18940.1), M. parvus (Y18935.1, Y18936.1, Y18938.1, and AJ277374.1), and Monorchis sp. (AJ277375.1). The newly designed primer pair, Monorchis 18S F (5′ GGA TCG GTG CTA TTG TAG TT3′) and Monorchis ITS1 R (5′ GAA CAG AGC TTT GAT TGA CT 3′), amplifies a fragment of about 975 bp comprising part of the 18S gene and almost the complete ITS1 region. Primer specificity was checked using Primer-BLAST (Ye et al., Reference Ye, Coulouris, Zaretskaya, Cutcutache, Rozen and Madden2012) against the NCBI non-redundant nucleotide database (nr/nt) with the Organism field limited to (1) Trematoda, (2) Actinopteri, (3) Pomatoschistus, and (4) Homo sapiens. According to the results of Primer-BLAST, primers Monorchis 18S F/Monorchis ITS1 R are highly specific to the genus Monorchis, as no significant matches were found outside this group. Samples were then subjected to a new PCR round using primers Monorchis 18S F/Monorchis ITS1 R and the same conditions as above, but with an annealing temperature of 49°C.
Six of the 10 samples yielded an amplicon of the expected size. The PCR amplification products obtained for the six samples were bi-directionally sequenced using the PCR primers.
Electropherogram analysis and overlapping were conducted in Geneious 8.1.9, as described previously. The samples were identified at the species-level by comparing the consensus sequence obtained with the 18S/ITS1 reference sequences available in DDBJ/ENA/GenBank.
The 18S-ITS1 sequences retrieved from the samples are all identical across their overlapping regions. Nucleotide identities with other reference sequences assigned to M. monorchis are consistently lower (89.33–90.67%). Therefore, all the samples analysed can be reliably identified as M. parvus.
Results
Density and size of cockles
The cockle density (buried and unburied) at the Sallingsund sites varied from 558 to 1547 ind. m−2 from mid-June to August 2012 (Figure 1). In November, we did not find cockles at site 2, and in the following year (2013), cockles were also absent from station 1. In August and November, respectively, 85% and 68% of the cockles were unburied (located on the surface or in the upper 1 cm of the substratum and with part of their shell exposed). The population was strongly dominated by one cohort with a mean length (±95% CI) that increased from 17.9 (±0.26) mm in June to 22.5 (±0.35) mm in November.
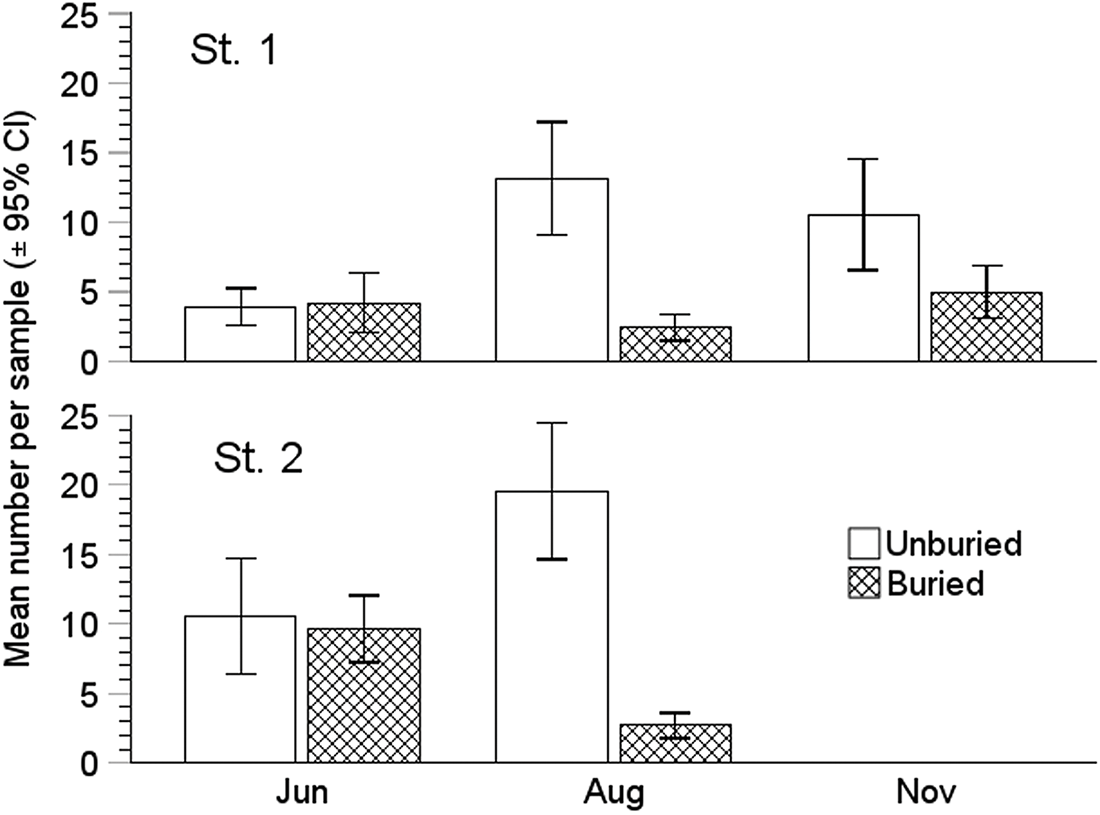
Figure 1. Mean number per sample (size: 145.2 cm2, n = 20) of unburied and buried individuals of Cerastoderma edule on the three sampling dates at the two sites at Sallingsund in 2012 (about 10–12 m depth, muddy substratum). Twenty individuals per sample (y-axis) correspond to 1377 ind. m−2.
Metazoans in cockles
The main objective of surveying a subtidal cockle population in the Limfjorden was to identify possible macroparasites that might trigger surfacing behaviour in cockles or otherwise interfere with the cockle’s well-being. We found only relatively few metazoan species in the cockles (Table 1). The copepod Hermanella rostrata and the turbellarian Paravortex cardii were present. They are considered relatively harmless commensals. Metacercariae of Himasthla trematodes that are common in cockles from shallow water sites (<1 m depth) in the Limfjorden (own unpublished observations, K. T. J.), were only seen in a few cockles. However, one trematode species was abundant. In August and November 2012, 19.2% and 18.1%, respectively (Table 1), of the examined cockles contained from hundreds to thousands of small worms with a length of up to 2 mm (Figure 2A–D). Based on the morphology of the sporocysts and the metacercariae, the trematode was identified as M. parvus – a trematode not previously reported in cockles from Danish waters. The observed worms are daughter sporocysts of M. parvus. In heavily infected specimens, they were located everywhere in the visceral mass, within gills and in the foot. The daughter sporocysts contained 2–26 metacercariae. The number of metacercariae is positively correlated with the length of sporocysts (r 2 = 67.2%) (Figure 3), and the median number (minimum to maximum) increased from August (8; 2–14) to November (12; 7–26). We did not observe cercariae inside the brood chamber of the daughter sporocysts in the cockles sampled in August and November 2012.
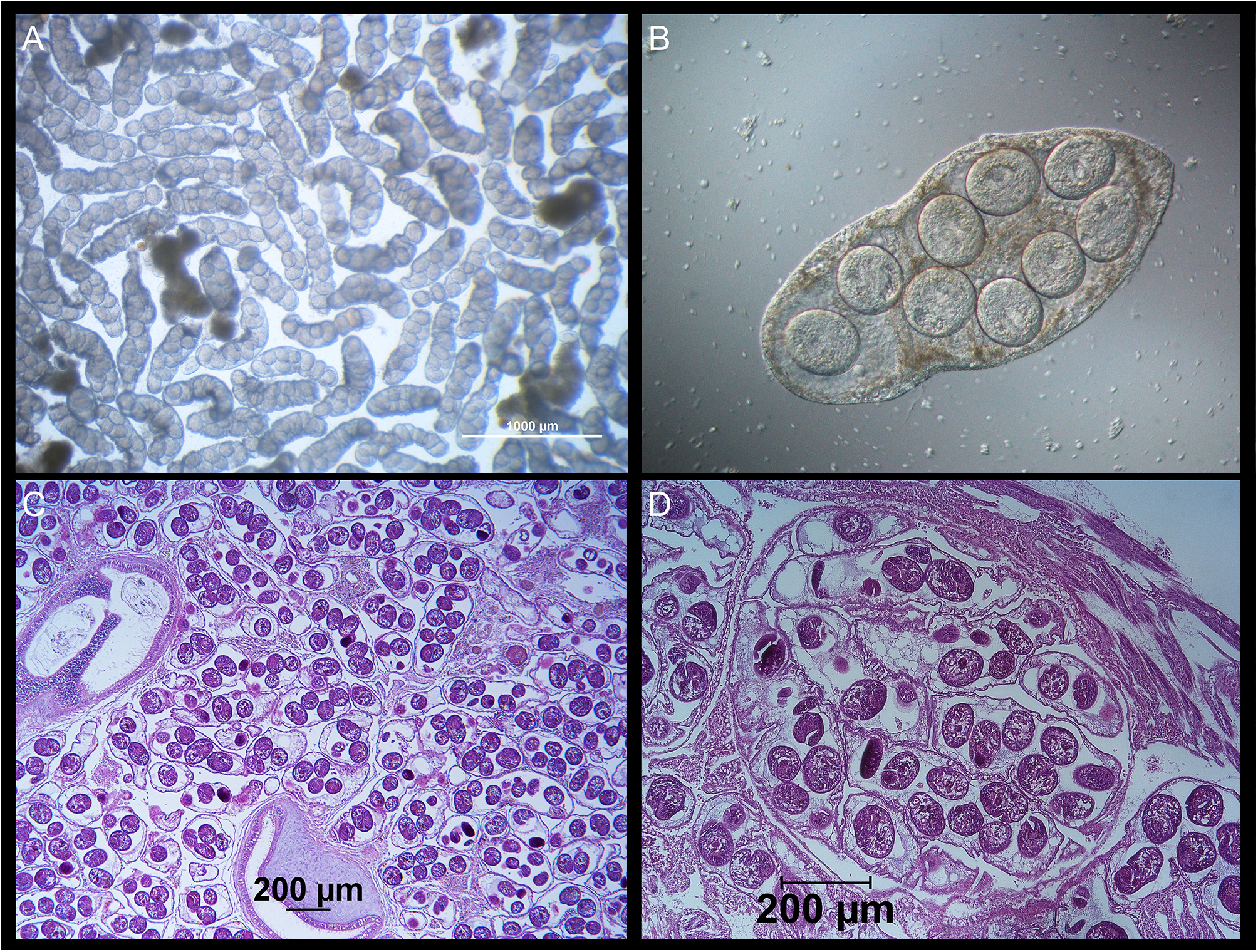
Figure 2. Monorchis parvus sporocysts from Cerastoderma edule collected in the Limfjorden. (A) Sporocysts from cockle tissue squeezed between two glass plates. The sporocysts exhibit vibrating movements from a buried specimen collected in November 2012 (photo taken through a stereomicroscope). (B) Close-up of one sporocyst with eight metacercariae from an unburied specimen collected in November 2012. (C) A histological slice of the visceral mass of an infected cockle collected in August 2019. While many sporocysts are spread throughout the visceral mass, a majority of the sporocyst mass is located between the intestinal tubules (cross-sections of two tubules are seen on the photo). (D) Close up of a group of sporocysts with metacercariae in different stages of maturity.
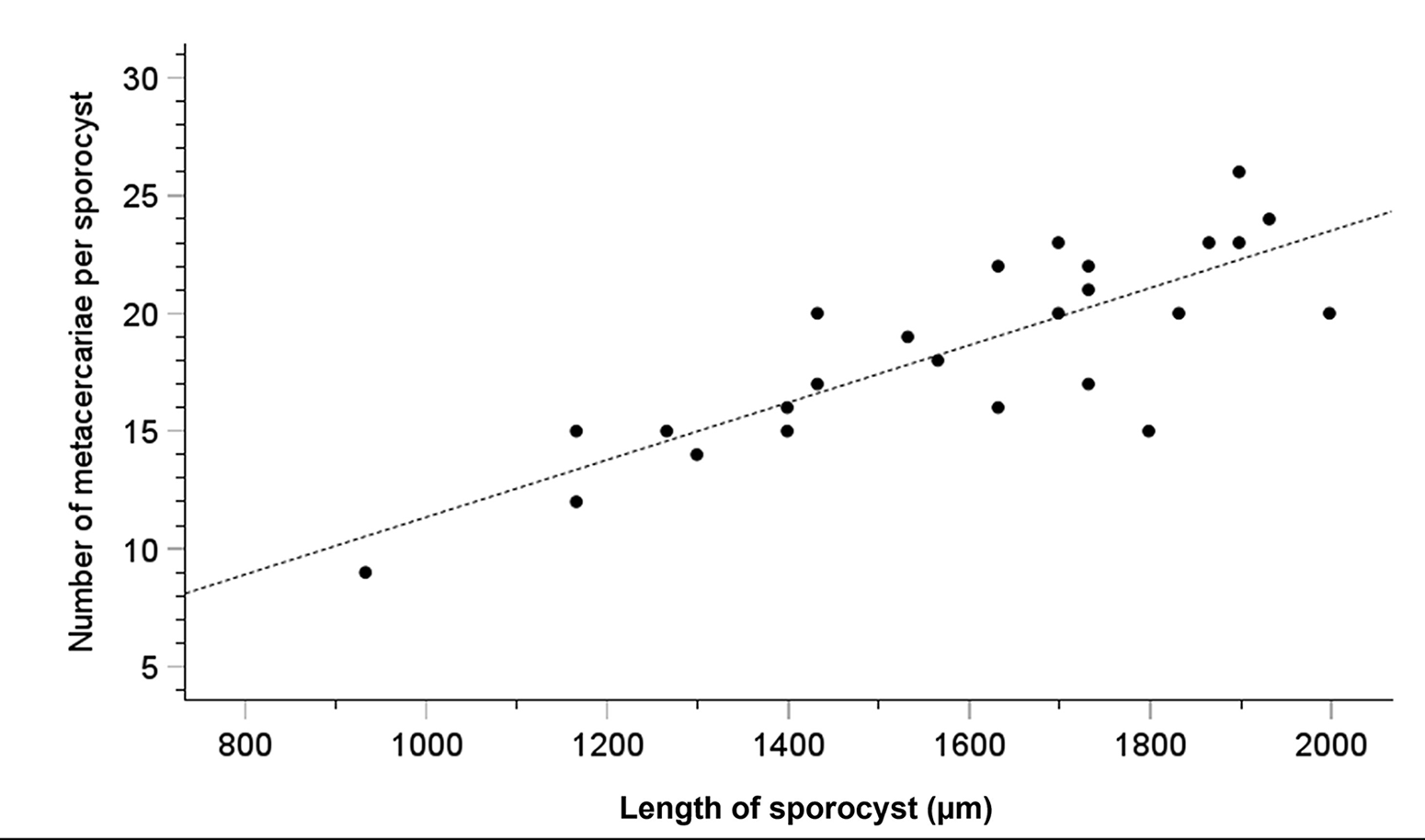
Figure 3. Relationship between sporocyst length (µm) (x-axis) and the number of metacercariae per sporocyst (y-axis) (correlation: r 2 = 0.672). Data are based on cockles collected in the Limfjorden in June, August, and November 2012. The mean number of metacercariae per sporocyst increased during autumn (from 8.4 to 13.9), and the maximum number of metacercariae per sporocyst was 26.
Table 1. Metazoans in Cerastoderma edule (August and November 2012) from Sallingsund, the Limfjorden (10 m depth)
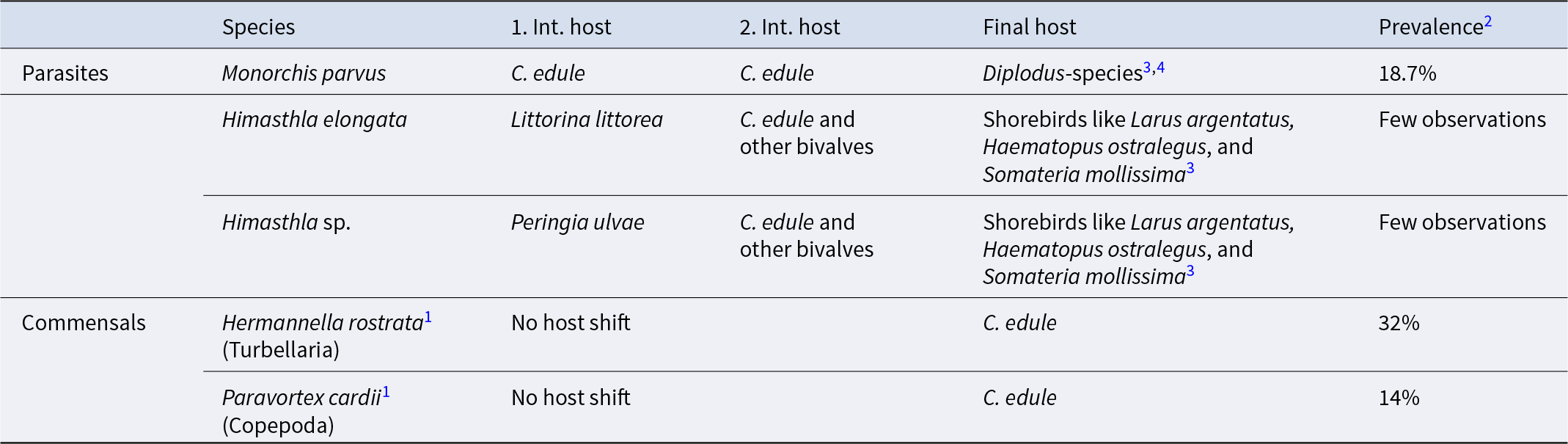
1 According to Longshaw and Malham (Reference Longshaw and Malham2013).
2 Mean prevalence in August and November and corrected for the different densities of buried and unburied
3 See de Montaudouin et al. (Reference de Montaudouin, Thieltges, Gam, Krakau, Pina, Bazairi, Dabouineau, Russell-Pinto and Jensen2009).
4 See Bartoli et al. (Reference Bartoli, Jousson and Russell-Pinto2000).
To verify the identification of M. parvus based on morphology, we further examined if diagnostic DNA sequences were in accordance with previously reported sequences from this species. Sequences of the 5.8S-ITS1 region were obtained from five individual sporocysts extracted from cockles collected at the Sallingsund site in August and November 2012. The sequences were identical. The longest of the obtained sequences showed high similarity (99.5% to 99.9%) with M. parvus from the GenBank database (AllGenetics). In conclusion, both DNA diagnostics and morphological characteristics confirm that the observed monorchid species is M. parvus.
Effect of M. parvus on cockles
Our data does not support that M. parvus should promote surfacing of cockles at our study site, as the differences in the prevalence of M. parvus in unburied and buried cockles in both August and November are insignificant (Pearson’s chi-squared test: χ 2Aug (1, 219) = .406, p > 5%; χ 2Aug (1, 118) = .506, p > 5%; Figure 4). We are aware that ‘unburied’ cockles may include individuals that are not truly unburied but have been assigned as such because of exposed shell parts. On the other hand, the category ‘buried’ cockles is well defined.
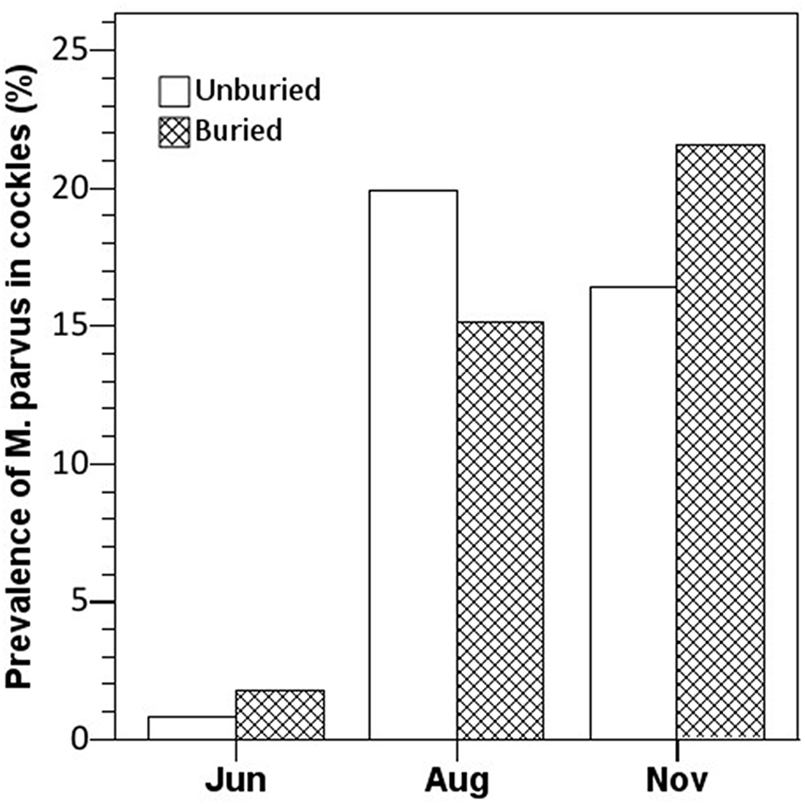
Figure 4. Prevalence of Monorchis parvus in buried and unburied specimens of Cerastoderma edule from Sallingsund in 2012. The number of examined cockles (unburied, buried): June (122, 172), August (186, 33), and November (67, 51).
The uninfected cockles show a nearly linear increase in mean shell-length from mid-June to mid-November (Figure 5). There are too few infected cockles in June to include them; otherwise, infected cockles show a similar increase in mean length from mid-August until mid-November as the uninfected, whether buried or unburied. The mean length (±95% CI) of uninfected cockles increased from 20.9 mm (±0.17) (mid-August) to 22.5 mm (±0.19) (mid-November) and infected cockles from 20.0 mm (±0.36) to 22.3 mm (±0.47 mm) (Figure 5). Two-way ANOVAs were performed to assess the effects of cockle position (buried and unburied) and condition (infected and uninfected) on the length of cockles in both August and November. In neither case was there a statistically significant interaction between the two factors (F Aug (1, 205) = 1.375, p = .29; F Nov (1, 114) = 1.208, p = .57). There were also no statistically significant effects of position or condition on the length of cockles in August and November (p > .10). Thus, both infected and unburied cockles appear to be unbiased fractions of the cockle population in terms of size.
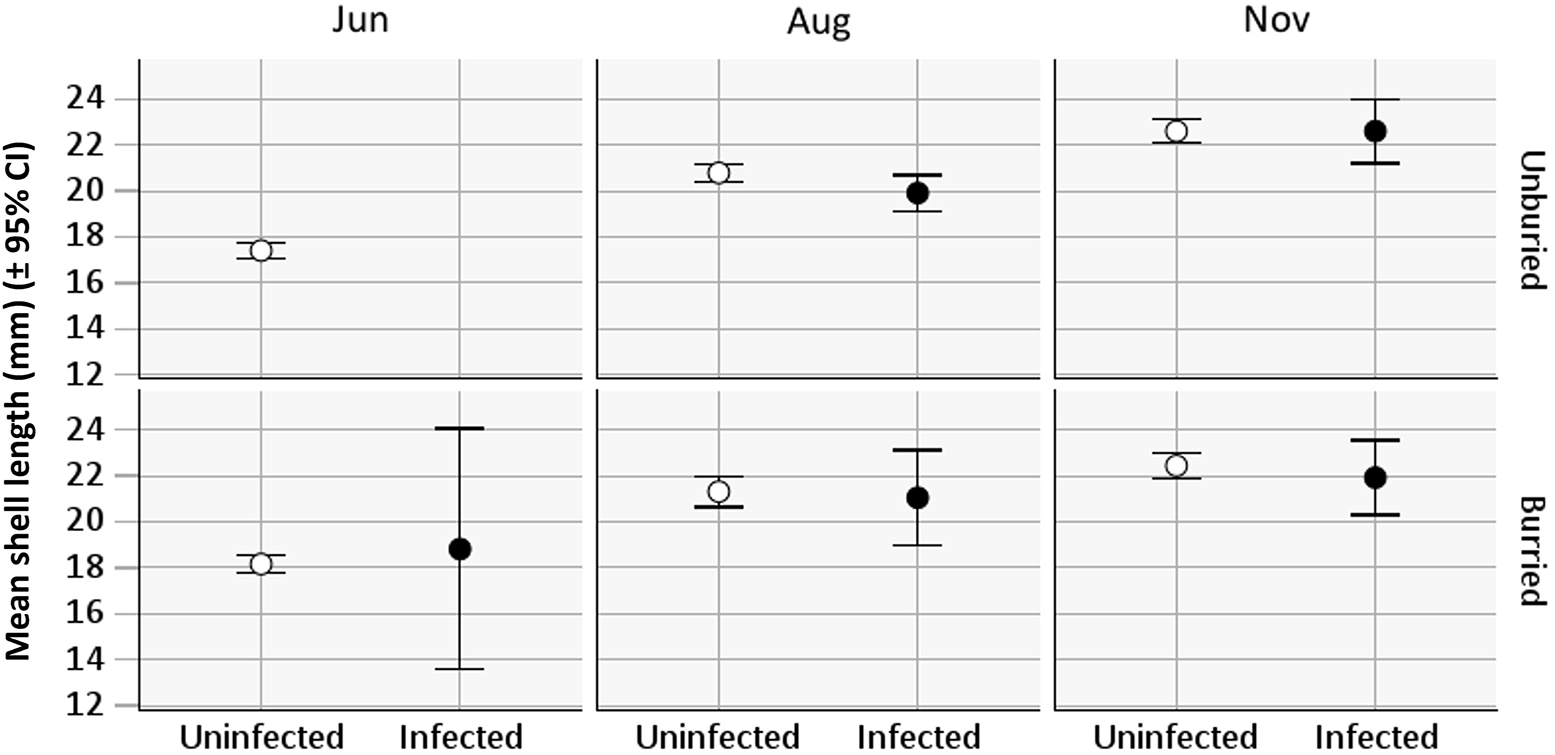
Figure 5. Shell length (mean ± 95% CI) of Cerastoderma edule from the field site at Sallingsund in 2012 for infected and uninfected and buried and unburied cockles in June, August, and November. All groups show the same increase in the mean length during the study period (the wide 95% CI of infected cockles in June is due to the few cockles present in this group).
A more direct measure of growth can be obtained from readings of annual length increments on individual shells. Based on winter rings on the cockle shells, we measured the length increments of uninfected and infected individuals from the winter ring indicating the winter of 2011–2012 until sampling in November 2012 (Figure 6). The mean length increments (±95% CI) of the uninfected and infected cockles were 4.76 mm (±0.28) and 4.05 mm (±0.67), respectively. This difference is statistically significant (t-test: t 86 = 2.27, p = .03). Thus, the annual mean length increment of an infected cockle is, on average, 85% of that of an uninfected cockle. However, it is important to note that the infected cockles only hosted the parasite from about mid-July to mid-November (one-third of a year).
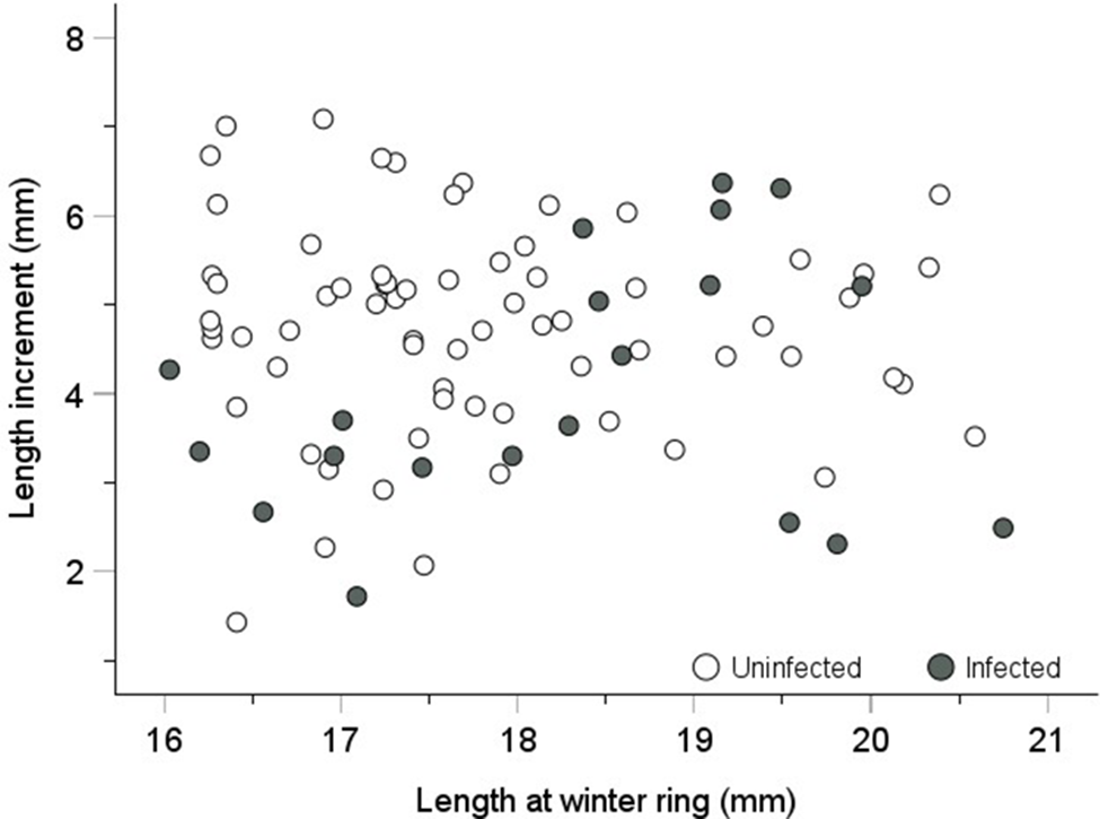
Figure 6. Shell length increments of uninfected and Monorchis parvus-infected cockles from the winter ring (formed in the winter of 2011–2012) until sampling in November 2012. Uninfected cockles were selected within the same length range as the infected cockles (16–21 mm) to minimize the effect of size-dependent growth. Mean length increments (±95% CL) of uninfected and infected cockles were 4.76 mm (±0.28) and 4.05 mm (±0.67), respectively (t-test, p = 2.6%).
Despite a heavy parasite load, where parasite tissue substituted cockle tissue, infected cockles remained viable under the present environmental conditions, as no selective mortality of the infected specimens took place.
Follow-up observations to clarify the life cycle mode and dispersal mechanisms of M. parvus in the Limfjord
If dispersion by cercariae is an option for M. parvus, we would expect such larvae to be present before the metacercarial stage is reached. As the prevalence of M. parvus increased significantly between early-June and mid-August, we expect cercariae to be present somewhere between early July and mid-August. To check this, we collected cockles from a site with a dense cockle stock in July 2018 (10 July, off Glyngøre at a depth of 4–5 m, 56°45′16.5″N and 8°51′49.0″E). However, at this time, the prevalence of M. parvus was low (2.8%). Nonetheless, we found active cercariae both inside and outside sporocysts in the Monorchis-infected cockles (Figure 7). We did also observe cercariae in cockles collected 8 August 2019 (near Trend at a depth of 0.5–1 m, 56°51′35.5″N and 9°12′39.1″E). Among the few infected cockles at this site, one specimen was double infected and filled with numerous larval stages of both M. parvus and Gymnophallus choledochus (Figure 8) – a very unlikely incidence.
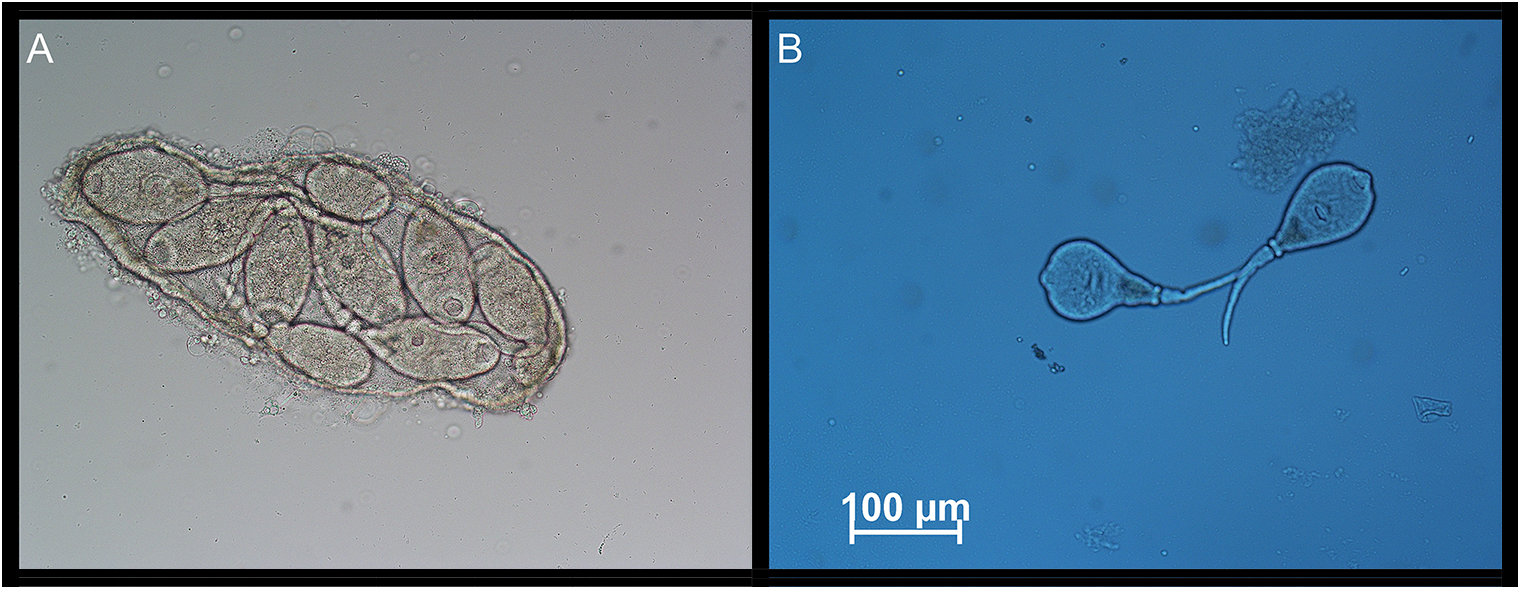
Figure 7. (A) Monorchis parvus sporocyst filled with mature cercariae from a specimen of Cerastoderma edule collected in the Limfjorden on 10 July 2018. (B) Free-moving M. parvus cercariae from the same cockle specimen. The cercariae may have been artificially released from sporocysts due to our preparation of the cockle tissue.
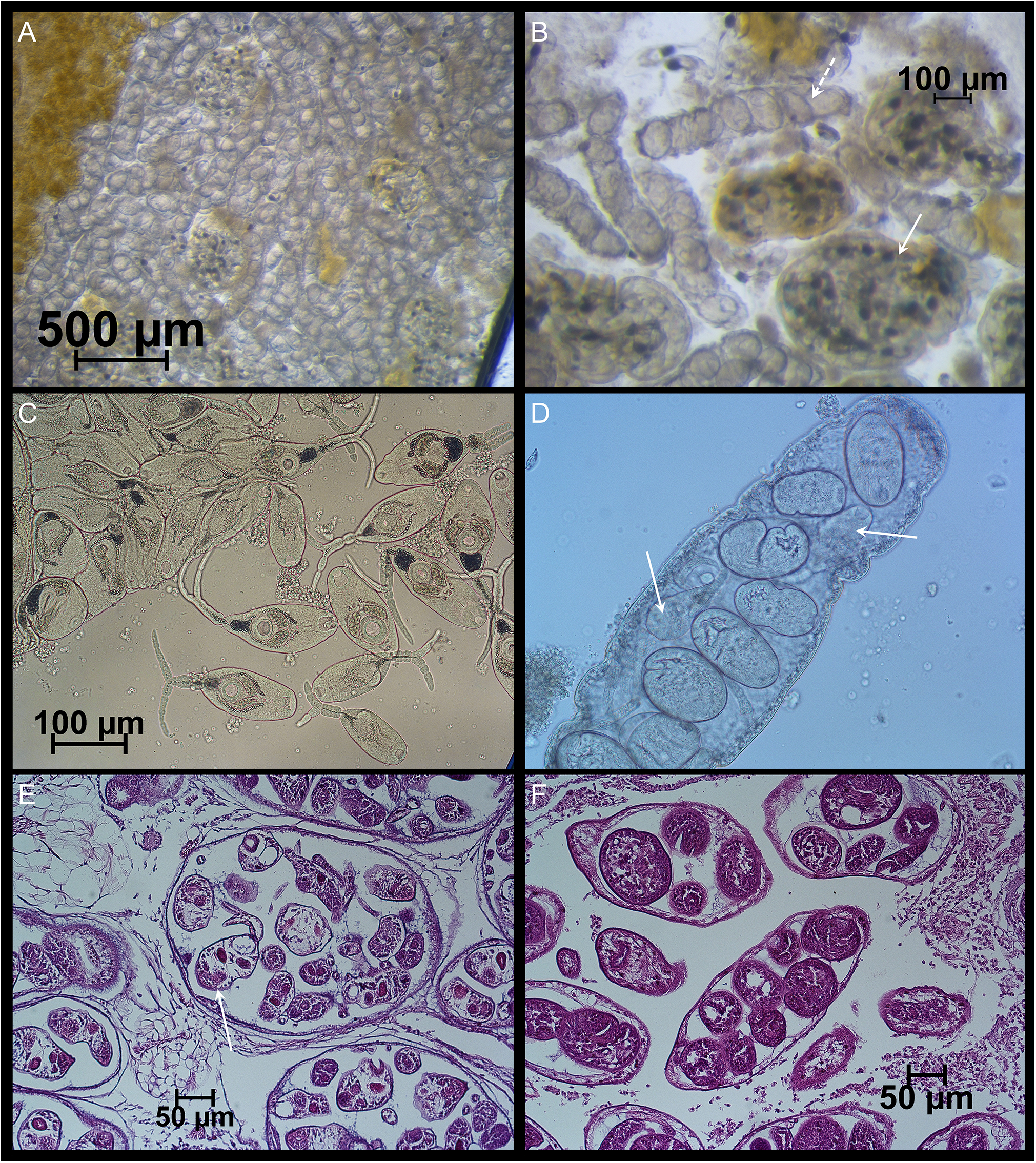
Figure 8. (A), (B), (C), and (D) show images from a double-infected cockle with larval stages of both Monorchis parvus and Gymnophallus choledochus (collected at trend, the Limfjorden, 24 August 2019). (A) Mixture of sporocysts of the two species; (B) close-up of the sporocysts (white arrow: G. choledochus; dashed arrow: M. parvus); (C) free cercariae of G. choledochus; (D) close-up of an M. parvus sporocyst – note a few cercariae (white arrow) partially hidden behind the metacercariae. (E, F) Haematoxylin and eosin-coloured tissue slices with G. choledochus (E) and M. parvus (F) from two different cockle specimens (each mono-infected) from the Limfjorden, 8 august 2019. Note the presence of a typical G. choledochus cercaria with a split tail in one of the sporocysts (white arrow).
In the infection experiment with P. microps, we found M. parvus individuals attached to the intestinal wall of the P. microps individuals fed with M. parvus sporocysts from 3 to 9 days p.i. in 8 of the 14 examined fish (Figure 9). In one fish at 7 days p.i., there were more than hundred living individuals, and in another at 9 days p.i., there were many apparently dead specimens (no sign of movement), whereas the last four dissected at 20 days p.i. were still infected. DNA extracted from the sporocysts fed to the fish and from adult M. parvus specimens picked from the experimentally infected gobies verified the identity between these and reported DNA-sequences of M. parvus. All 18S-ITS1 sequences retrieved from the samples were 100% identical across their overlapping regions. Nucleotide identities with other reference sequences assigned to M. monorchis were consistently lower (89.33–90.67%). Therefore, all the samples analysed were reliably identified as M. parvus.
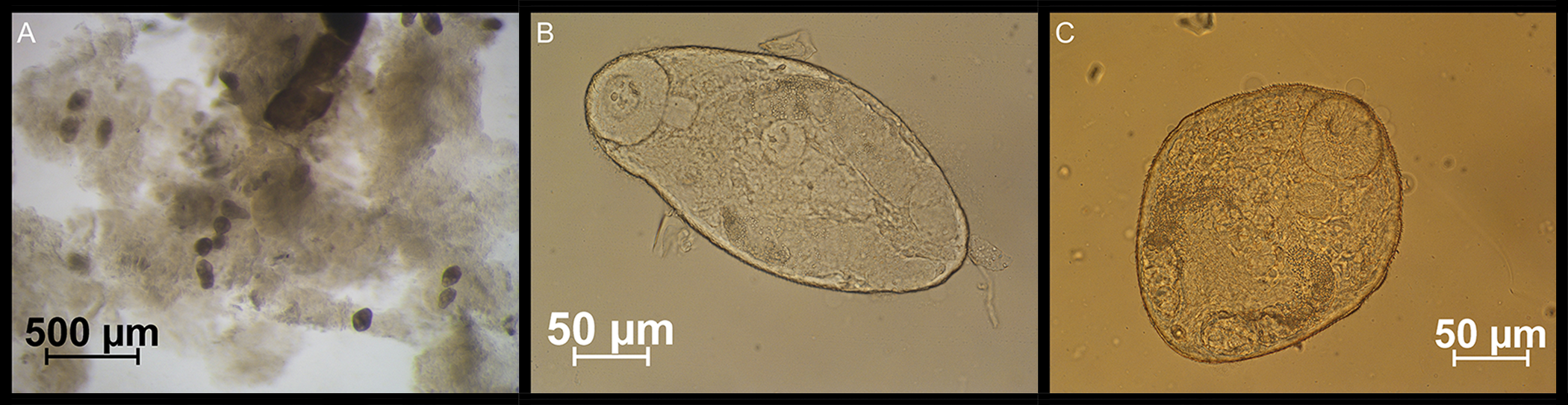
Figure 9. (A) Developing adult Monorchis parvus specimens in the intestine of experimentally infected juvenile Pomatoschistus microps. (B, C) Ventral and oral suckers are seen on the isolated adult specimens. DNA-analyses have demonstrated the identity of the M. parvus sporocysts fed to the fish and the excysted adults picked from the intestine.
We also observed that some of the sporocysts released from dying cockles could survive up to 48 h in a Petri dish incubated at room temperature (ca. 20°C).
Discussion
Cockle population
We do not have any specific explanation for the tempo-spatial variation of cockle density at the sites in Sallingsund where we sampled cockles, and nothing in the environmental data suggests unfavourable temperature, salinity, or oxygen conditions as triggering factors for the local cockle elimination. Whether potential predators, such as crabs (Carcinus maenas) or starfish (Asterias rubens), both quite common in the Limfjorden, could be the causative agents remains unresolved. Commercial cockle fishery is practiced in the Sallingsund area, but to the best of our knowledge, not at our study sites.
Monorchis parvus identification and prevalence patterns
The present digenean trematode M. parvus was named Cercaria cerastodermae until its phylogeny and life cycle were clarified (Bartoli et al., Reference Bartoli, Jousson and Russell-Pinto2000). Based on molecular data, Bartoli et al. (Reference Bartoli, Jousson and Russell-Pinto2000) demonstrated that trematode larvae identified as C. cerastodermae in cockles (C. edule) from an intertidal site in the Aveiro Lagoon (Portugal) were genetically identical to the adult monorchid M. parvus found in Diplodus annularis (Sparidae) in the Gulf of Marseille, France.
As the ITS1-sequences of Monorchis larvae from the present study and those described from C. edule from Aveiro (Portugal) and from adult stages of Monorchis from the sparid fish Diplodus annularis caught at Marseille (Bartoli et al., Reference Bartoli, Jousson and Russell-Pinto2000) showed high identity (99.5% to 99.9%), we conclude that these genotypes are from the same species: M. parvus. The same ITS1 genotype has also been found in infected C. edule from Arcachon (Southwestern France) (Magalhaes et al., Reference Magalhaes, Daffe, Freitas and de Montaudouin X2020) and in C. glaucum and D. annularis from the Gulf of Gabès, Tunisia (see figure 2 in Youssef-Dridi et al., Reference Youssef-Dridi, Antar, Gey, Justine and Gargouri2023). So far, adult M. parvus with the present ITS1-genotype has only been observed from wild host populations of D. annularis. The reported Monorchis specimens from Diplodus sargus and D. vulgaris segregate distinctly from the M. parvus type (Monorchis sp. in figure 2 in Youssef-Dridi et al., Reference Youssef-Dridi, Antar, Gey, Justine and Gargouri2023), and given a divergence between the M. parvus group and the Monorchis sp. group of 19.28%, it is justified to consider the latter genotypes as belonging to another yet unnamed Monorchis species. This ITS1-genotype has been observed in the two mentioned Diplodus species from Marseille, Corsica, and Tunisia. Even if Bartoli et al. (Reference Bartoli, Jousson and Russell-Pinto2000) showed that metacercariae from the M. parvus genotype from Portuguese C. edule fed to D. sargus individuals could mature to egg-carrying adults, this is not proof that the fish is a host to M. parvus in nature. The life cycle of the Monorchis sp. is not known.
So far, there have not been any reports on the helminth fauna in cockles from the Limfjorden. But we have screened samples of cockles for parasites a few times from a shallow water site (<1 m depth at Trend), where we found M. parvus for the first time in July 2012 (prevalence: 8.3%).
There are two previous reports of M. parvus in cockles from Scandinavian waters: (1) Jonsson and André (Reference Jonsson and André1992) and (2) Krakau (Reference Krakau2008). Jonsson and André (Reference Jonsson and André1992) observed plenty of surfacing cockles on the seabed at two subtidal sites in the Kattegat near Tjärnö, Sweden (Tenskär and Saltö, <1 m water depth at both sites) in June 1991 and examined buried and unburied cockles from the two sites for parasites. At Tenskär, 20% (n = 40) of the buried cockles and 50% (n = 20) of the unburied cockles were infected by M. parvus, and at Saltö the corresponding figures were 17% (n = 46) and 81% (n = 41), respectively. Krakau (Reference Krakau2008) examined the trematode fauna of cockles collected from various sites along the eastern Atlantic shoreline and found 20% M. parvus-infected cockles at an intertidal site on the Norwegian Skagerrak coast (near Arendal) in August 2005.
Monorchis parvus has been found in C. edule within most of its biogeographical range from Northern Africa (Morocco) to Scandinavia (de Montaudouin et al., Reference de Montaudouin, Thieltges, Gam, Krakau, Pina, Bazairi, Dabouineau, Russell-Pinto and Jensen2009; Magalhães et al., Reference Magalhaes, Daffe, Freitas and de Montaudouin X2020) and has also been reported in C. glaucum from the Mediterranean coast (Bartoli et al., Reference Bartoli, Jousson and Russell-Pinto2000; Youssef-Dridi et al., Reference Youssef-Dridi, Antar, Gey, Justine and Gargouri2023) and from UK sites (Boyden, Reference Boyden1970).
Except for the high prevalence of M. parvus reported from the Scandinavian sites and from a site in Albufeira Lagoon, Portugal (prevalence 40%, n = 20, Correia et al., Reference Correia, Magalhães, Freitas, Bazairi, Gam and de Montaudouin X2020), many reports show very low prevalences (less than 1% in 28% of the reported cases from 1902 to 2020; Magalhães et al., Reference Magalhaes, Daffe, Freitas and de Montaudouin X2020). Therefore, several authors consider M. parvus a relatively rare trematode species in C. edule. This is also the case for its occurrence in C. glaucum, according to the few reported studies of parasites in C. glaucum. For an overview of reported prevalences of M. parvus in cockles until 2020, see Magalhães et al. (Reference Magalhaes, Daffe, Freitas and de Montaudouin X2020).
However, the present study, together with the previous Scandinavian studies, challenges the perception that M. parvus is a relatively rare trematode in cockle populations. But it is remarkable that we have found high prevalenceof M. parvus in cockles without known final hosts being present. How can this be explained despite an apparently missing life cycle component?
While the study by Jonsson and André (Reference Jonsson and André1992) was triggered by an observation of unburied cockles, the study site selected by Krakau (Reference Krakau2008) was unrelated to the in-situ conditions of the cockles. The field site in our study was chosen as an area where the surfacing of cockles had been observed previously, but it was not triggered by an observation of a sudden event. So, given that two of the three Scandinavian observations of M. parvus-infected cockles were based on a random choice of sampling site and time for examining cockles for helminths, we expect that M. parvus is quite common in Scandinavian waters. Furthermore, the high prevalence of M. parvus in cockles must be a relatively common incident.
Possible life cycle options of M. parvus
The life cycle options of M. parvus are not yet completely clarified, although the experimentally verified two-host life cycle demonstrated by Bartoli et al. (Reference Bartoli, Jousson and Russell-Pinto2000) has so far been considered the typical life cycle of M. parvus. In their study, sporocysts with metacercariae were fed directly to juvenile, laboratory-reared Diplodus sargus, and they matured to adults within 5 days (Bartoli et al., Reference Bartoli, Jousson and Russell-Pinto2000). Mature parasites (egg-producing adults) were observed until 37 days post-infection in the fish host. Both C. glaucum (Mediterranean) and C. edule (Atlantic area) can be the first and second intermediate hosts to larval stages of M. parvus according to Bartoli et al. (Reference Bartoli, Jousson and Russell-Pinto2000). Diplodus annularis is the only natural final host to M. parvus reported so far (Bartoli et al., Reference Bartoli, Jousson and Russell-Pinto2000), and the fish becomes infected by M. parvus by eating cockles (Jousson et al., Reference Jousson, Bartoli and Pawlowski2000).
However, not all M. parvus larvae in cockles mature into metacercariae. Most of the studies dealing with Monorchis infections in cockles have reported that cercariae were present among the metacercariae inside sporocysts (Bartoli et al., Reference Bartoli, Jousson and Russell-Pinto2000; Jonsson and André, Reference Jonsson and André1992; Sannia and James, Reference Sannia and James1978; Sannia et al., Reference Sannia, James and Bowers1978). While Sannia et al. (Reference Sannia, James and Bowers1978) observed sporocysts filled with cercariae ready for being emitted, Bartoli et al. (Reference Bartoli, Jousson and Russell-Pinto2000) considered the observed free cercariae as an artefact caused by rupture of sporocysts during necropsy of the cockle host. As the cercariae in cockles from the Aveiro Lagoon were smaller (and presumed less viable) than those observed by Sannia and James (Reference Sannia and James1978) in cockles from the Thames Estuary, a difference in life cycle (two versus three hosts) related to different environments or temperature regimes cannot be excluded, but differences in phenology could also be a possibility.
Whereas a three-host life cycle is the most common among flukes in marine hosts, several have abbreviated life cycles. Many flukes also have optional life cycle patterns where an abbreviated life cycle is a possibility. As an example, the trematode Gymnophallus choledochus using waterbirds as the final host and cockles as the first intermediate host alternates between a two-host and three-host life cycle. During the summer period, cercariae produced in G. choledochus sporocysts are shed from the cockle host and infect polychaetes, where they encyst and await excystment until the host worm is eaten by a waterbird (such as oystercatchers or seagulls). In wintertime, cercariae encyst in the cockle host and remain there as metacercariae until the host is consumed by a waterbird (Loos-Frank, Reference Loos-Frank1969).
Based on our study, we must consider three life cycle possibilities for M. parvus: (i) a two-host cycle with the final host as a direct consumer of cockles, (ii) a two-host cycle with the final host as a scavenger on dying/dead infected cockles, or (iii) a three-host system with an unknown intermediate host involved.
All infected cockles from our August and November samples from 2012 were filled with daughter sporocysts stocked with metacercariae. So, this would suggest direct transmission of M. parvus to a cockle-eating fish. However, there are no species belonging to Sparidae (sea breams) in the Limfjorden. Sea breams have not been registered in the Limfjorden area since the early Stone Age. Bones from at least one species of Sparidae (Spondyliosoma cantharus) have been found in kitchen middens from the Limfjorden area (Enghoff et al., Reference Enghoff, MacKenzie and Nielsen2007). During that period, the water temperature was a few degrees higher, and the fish fauna was more diverse than it is nowadays (Enghoff et al., Reference Enghoff, MacKenzie and Nielsen2007). Still, if we assume that other cockle-eating fish besides sea breams could host M. parvus, it would require an abundant and widespread cockle-eating fish in the Limfjorden to explain the high prevalence observed. The potential fish agents are limited. Generally, the demersal fish fauna is impoverished in the Limfjorden (Hoffman, Reference Hoffmann2005) and there is no base for commercial fishery on demersal fish. One candidate could perhaps be Myoxocephalus scorpius, although it is doubtful if it can eat cockles above 13–14 mm in shell length. Fish, crustaceans, polychaetes, and amphipods are reported as their prey items (Froese and Pauly, Reference Froese and Pauly2025).
Sannia et al. (Reference Sannia, James and Bowers1978) tried to infect Scyliorhinus caniculus, Blennius pholis, Gobius minutus, and Pleuronectes platessa by feeding them sporocysts containing metacercariae, but in all cases, the parasites were digested.
We cannot exclude that cercariae have been shed by infected cockles in the Limfjorden before mid-August, as we observed active cercariae outside sporocysts in cockles collected in July and early August. If another second intermediate host (an invertebrate) is involved in the life cycle of M. parvus, the final fish host could become infected by eating such an intermediate host. This would extend the possible fish host spectrum to a variety of smaller fish that normally do not eat cockles, such as sticklebacks, gobies, eelpouts, and others. However, this remains speculative until evidence is provided. Anyway, we will consider this as only a potential minor transmission route, given the limited numbers of cercariae observed.
Our preliminary infection experiment suggests that fish consumption of either sporocysts released from dead cockle tissue or a mixture of decaying cockle tissue and sporocysts can be a transmission point of M. parvus between its intermediate and final host in the Limfjorden. Our observations indicate that M. parvus metacercariae can hatch and attach themselves to the intestinal wall of P. microps and that they can survive in this environment for several days without being digested. As we did not observe egg-producing M. parvus specimens, we do not have sufficient evidence supporting small gobies as potential hosts. Nonetheless, the observations in our pilot experiment provide good reasons to further explore the idea that dying cockles are the focus point for transmission and that one of the goby species living in the Limfjorden (P. microps, P. minutus, and G. niger) might be the missing host. Identification of the natural final host(s) for M. parvus in the Limfjorden requires that fish be screened for parasites at the right time and place. Another trematode species known from cockles, Asymphylodora demeli, is taxonomically related to M. parvus, as their two families Lissorchiidae and Monorchidae, respectively, belong to the superfamily Monorchioidea (Suborder Monorchiata) (WoRMS, 2025). Asymphylodora demeli has P. microps as a fish host (Montaudouin et al., Reference de Montaudouin, Thieltges, Gam, Krakau, Pina, Bazairi, Dabouineau, Russell-Pinto and Jensen2009; Zander et al., Reference Zander, Ö, Skroblies and Strohbach2002). The question is whether their taxonomic relatedness also includes traits that enable them to infect P. microps. At least, this might further justify the focus on gobies as potential hosts for M. parvus.
Before the ecological conditions for the mass occurrence of infections in a cockle population can be clarified, it is necessary to know how cockles become infected. Presently, it is unknown how the first larval stage reaches a cockle. Knowing all life cycle components is not only scientifically interesting but may also have importance for the management of cockle fisheries. With a parasite that can significantly reduce the spawning stock, knowledge of factors promoting the mass occurrence of the parasite, including factors controlling the transmission of the parasite to cockles, is vital.
Host effect of M. parvus
The frequency of infected cockles was not different between buried and unburied cockles in the present study. But there could still be a difference in the intensity of the infections, and we have not been able to quantify if the number of sporocysts differed between unburied and buried cockles. Furthermore, it can be a challenge to make a sharp distinction between buried and unburied cockles on a muddy subtidal seabed. Based on prevalence, cockles on the surface seem to be a random subset of the cockles present at the study sites and are not overrepresented by infected cockles. Neither could we observe any increased mortality of infected cockles over uninfected cockles between mid-August and mid-November. Otherwise, M. parvus might be a good case for a trematode that would benefit from the surfacing behaviour of its host to get exposed to potential fish consumers and thus capitalize on a prey–predator link. Based on Bartoli et al. (Reference Bartoli, Jousson and Russell-Pinto2000), the right fish host will easily be infected after consumption of M. parvus sporocysts with mature metacercariae. In contrast to our observations, Jonsson and André (Reference Jonsson and André1992) observed that M. parvus (C. cerastodermae)-infected cockles were overrepresented among surface-dwelling cockles at a shallow water locality (<1 m). A preliminary experiment showed that significantly fewer infected than uninfected cockles managed to burrow. The latter could be explained by the impaired burrowing ability of infected cockles and not necessarily due to parasite control of host behaviour.
With our present understanding, we expect that an M. parvus-infected cockle will eventually reach a stage where the load of the growing sporocyst population compromises the basic life functions of the host. Our data indicate that one effect of a growing sporocyst population in a cockle is reduced shell growth. On average, the annual increase in shell length of a cockle infected for 4–5 months (from early July to mid-November) was 85% of the length increase of an uninfected cockle. Presumably, an increasing proportion of an infected cockle’s food intake is lost to the growing sporocyst population. Starvation, functional oxygen deficiency, or deterred muscle function could all be possible results of a dense population of sporocysts. According to Sannia and James (Reference Sannia and James1978), infected cockles die within a year after infection due to excessive destruction of tissues in the visceral mass. Jonsson and André (Reference Jonsson and André1992) observed that the muscular tissue of the foot of some infected individuals was almost absent. Anyway, the effect might be ‘surfacing’ of an infected cockle or an inability to reburrow if brought to the surface for other reasons. Muscle decay may result in failure to keep shells closed, and this would be the moment for the parasitic worms to escape cockle flesh and act as a lure to fish (or be eaten together with cockle flesh). The conclusion is that the parasites do not necessarily affect the cockles’ burrowing behaviour before a final point is reached, and this may be an explanation for not seeing infected cockles being overrepresented among surface-dwelling cockles in our study. The continued production of sporocysts inside an infected cockle may finally pass the capacity of a cockle individual to accommodate sporocysts and a point of no return will be reached. Food supply and temperature are probably the most important determinants of this.
At our study site (around 10 m depth), the summer temperatures are lower than at intertidal or shallow water sites within the Southern distributional area of M. parvus. This implies potentially a longer lifespan of the cockle-M. parvus association, a slower growth rate of M. parvus sporocysts, and probably a more favourable balance between energetic costs of living and food supplies for the host.
Do environmental settings determine the life cycle options for M. parvus? Not necessarily. If infected cockles are eaten by fish to an extent that no cockles survive until being otherwise overexploited by the sporocyst population, we have the Southern life cycle model. But if, as in the Limfjorden, cockle-eating fish are missing in the ecosystem, small fish that eat dead, infected cockles may be the main route for the parasite to reach a final host.
Acknowledgements
Senior Researcher Lone Madsen (DTU Aqua) is acknowledged for preparing the histological slices, and thanks are extended to Laboratory Technician Susanne Vase Petersen (AU) for laboratory work. Two anonymous referees are also acknowledged for their valuable comments and suggestions.
Author contributions
Study concept: K.T.J. and C.F. Data collection and processing: K.T.J., J.L., P.S.d.F., C.S., and C.F. Analysis and interpretation of results: K.T.J. First draft: K.T.J. Writing – review, & editing: K.T.J., J.L., P.S.d.F., C.S., and C.F.
Funding
The present study was financially supported by a grant to K.T.J. from Elisabeth og Knud Petersens Fond and by the project ‘Sustainable Cockle Fishery in the Limfjorden’ (co-financed by ‘The European Maritime and Fisheries Fund’ and ‘The Danish Foreign Ministry’s development programme for fisheries,’ ref.: 33113-B-17-109).
Competing interests
The authors declare none.












