Introduction
The effects of global warming are driving the poleward expansion of marine species’ distribution ranges (Bates et al., Reference Bates, Barrett, Stuart-Smith, Holbrook, Thompson and Edgar2014; Zarzyczny et al., Reference Zarzyczny, Rius, Williams and Fenberg2024). The changes in marine fishes can be exceptionally rapid, with numerous species markedly responding to recent sea temperature increases (Azzurro, Reference Azzurro2008). This process has been coined as ‘tropicalization’, and it has the potential to change community assemblages over decadal periods (Bianchi and Morri, Reference Bianchi and Morri2003; Verges et al., Reference Vergés, Steinberg, Hay, Poore, Campbell, Ballesteros, Heck, Booth, Coleman, Feary, Figueira, Langlois, Marzinelli, Mizerek, Mumby, Nakamura, Roughan, van Sebille, Gupta, Smale, Tomas, Wernberg and Wilson2014) and induce long-term ecological and evolutionary consequences, such as ecosystem phase shifts, phenotypic interactions, and economic effects on fisheries and tourism (Zarzyczny et al., Reference Zarzyczny, Rius, Williams and Fenberg2024).
The most well-known instances occurred in temperate seas, where the biota has adapted to the large seasonal temperature variability characteristic of mid-latitudes (Schuster et al., Reference Schuster, Stuart-Smith, Edgar and Bates2022). Since 1950, evidence of tropicalization in North-East Atlantic marine regions has been noted in several areas, initially described in Portuguese waters (Quéro, Reference Quéro1998; Quéro et al., Reference Quéro and Laborde1996), and subsequently in the North Sea (Beare et al., Reference Beare, Burns, Greig, Jones, Peach, Kienzle, McKenzie and Reid2004), British waters (Stebbing et al., Reference Stebbing, Turk, Wheeler and Clarke2002), the northwest of Spain (Bañón et al., Reference Bañón, Del Rio, Piñeiro and Casas2002), Macaronesia (Afonso et al., Reference Afonso, Porteiro, Fontes, Tempera, Morato, Cardigos and Santos2013; Brito et al., Reference Brito, Falcón and Herrera2005; Castro et al., Reference Castro, Schäfer, Parretti, Monteiro, Gizzi, Chebaane, Almada, Henriques, Freitas, Vasco-Rodrigues, Silva, Radeta, Freitas and Canning-Clode2021), and the Mediterranean Sea (Bianchi and Morri, Reference Bianchi and Morri2003), with the Gulf of Cadiz (GoC) being no exception.
The GoC, located on the Atlantic side adjacent to the Strait of Gibraltar, has been recognized as a bioinvasion hotspot due to the accelerated arrival of non-native species in recent years (Cuesta et al., Reference Cuesta, Acosta-Morillas, González, de Carvalho-Souza and González-Ortegón2024; de Carvalho-Souza et al., Reference de Carvalho-Souza, Gómez and González-Ortegón2024a; González-Ortegón et al., Reference González-Ortegón, García-Raso, Calado, López de la Rosa, Guerrero and Cuesta2020b, Reference González-Ortegón, Jenkins, Galil, Drake and Cuesta2020a). This process involves two primary components: the arrival of novel non-native species in southwestern Europe and a creeping spread from the Mediterranean and African coasts, the latter known as the ‘African Creep’ – the northward expansion of Atlantic native species with thermal affinities (Canning-Clode and Carlton, Reference Canning-Clode and Carlton2017). The occurrence and spread of thermophilic species from the African continent in the GoC are influenced by four main factors: human-mediated introductions, the influx of Mediterranean waters, Lessepsian migration, and ongoing sea warming. The first three processes provide the presence of these species outside their natural range, while the fourth also supplies the favourable conditions for their northward expansion to Europe from Africa.
Here, we present accelerated, unusual, and rare occurrences of 15 fish species that are expanding and potentially establishing their distribution range in the GoC due to ocean warming. We include additional data on the number of records, sizes, and distributions, as well as the possible pathways of their introduction. These findings improve our understanding of the tropicalization process in the region and highlight the critical need for ongoing monitoring and conservation efforts.
Material and methods
Data on unusual fish species reported in the GoC for the period 2008–2024 have been compiled. Since 2013, the Agencia de Gestión Agraria y Pesquera de Andalucía has carried out fishing monitoring of species that occur in areas of the GoC. Also, in the last decade, the Instituto de Ciencias Marinas de Andalucía has built a database of terminological and identification data for commercial fish species on the Andalusian coasts, called ‘ICTIOTERM’ (http://www.ictioterm.es). During these monitoring and projects, we also maintained close relationships with several stakeholders, such as fishers, staff of fish markets, and technicians from fishermen’s associations. Additional information was obtained from spearfishers, as citizen science has become a valuable tool for detecting and monitoring non-native species. Thus, reports of non-directed catches were also obtained opportunistically from professional and recreational fishermen and other users at several localities throughout the GoC. To ensure accuracy, records were included only if they contained specimen data and photographs, and each species was verified by ichthyologists with extensive expertise in fish research. The species classification is organized in taxonomic order, in accordance with ‘Catalog of Fishes’ (Van der Laan et al., Reference Van der Laan, Fricke and Eschmeyer2024). Species names are alphabetized within each family.
To review the non-native occurrence records of fish species, we conducted comprehensive bibliographic research, compiling and updating records. The search encompassed literature published from 1970 to September 2024, sourced from the Web of Science database and Google Scholar. The search employed a variety of keywords for each species, including actual and previous scientific names, common English names, and others such as ‘tropicalisation/tropicalization’, ‘first record’, ‘occurrence’, ‘range expansion’, ‘non-native’, ‘exotic’, ‘alien’. Additional bibliographic sources were obtained by reviewing the reference lists of the localized publications. Furthermore, the information was cross-verified with the updated national checklist of marine fish in Spanish and Portuguese waters (Báez et al., Reference Báez, Rodríguez-Cabello, Bañón, Brito, Falcon, Maño, Baro, Macıas, Melendez, Camiñas, Arias-Garcıa, Gil, Farias, Artexe and Sanchez2019; Carneiro et al., Reference Carneiro, Martins, Landi and Costa2014), data obtained from the GBIF database (https://www.gbif.es), and citizen science platforms (e.g. iNaturalist.com and Observadoresdelmar.es).
Results
We present findings on 15 fish species across 12 families that are expanding and/or potentially establishing their distribution range in the GoC (Supplementary Table S1).
Carlarius parkii Günther, 1864, family: Ariidae, order: Siluriformes
In 2021, an individual of Carlarius parkii Günther, 1864 was captured in the GoC and subsequently commercialized in markets near the mouth of the Guadalquivir (Figure 1a). The individual was estimated to be ∼70 cm in total length. Typically found in the East Atlantic region from Cape Blanc (Mauritania) to Angola, C. parkii has been documented outside its native range only once in Israel (Golani and Ben-Tuvia, Reference Golani and Ben-Tuvia1986). Notably, this marks the first recorded instance of this species in European waters (Supplementary Figure S1). This species primarily inhabits coastal waters on muddy bottoms, with recurrent forays into the brackish waters of estuaries and coastal lagoons (Conand et al., Reference Conand, Camara and Domain1995). This species is a benthic predator that feeds mainly on fish and shrimp, displaying opportunistic feeding behaviour (Diouf, Reference Diouf1996). Males exhibit buccal incubation, carrying fertilized eggs in their mouths until hatching (Golani et al., Reference Golani, Orsi Relini, Massutí, Quignard and Fishes. F.2002), a reproductive strategy that may enhance offspring survival during dispersal.
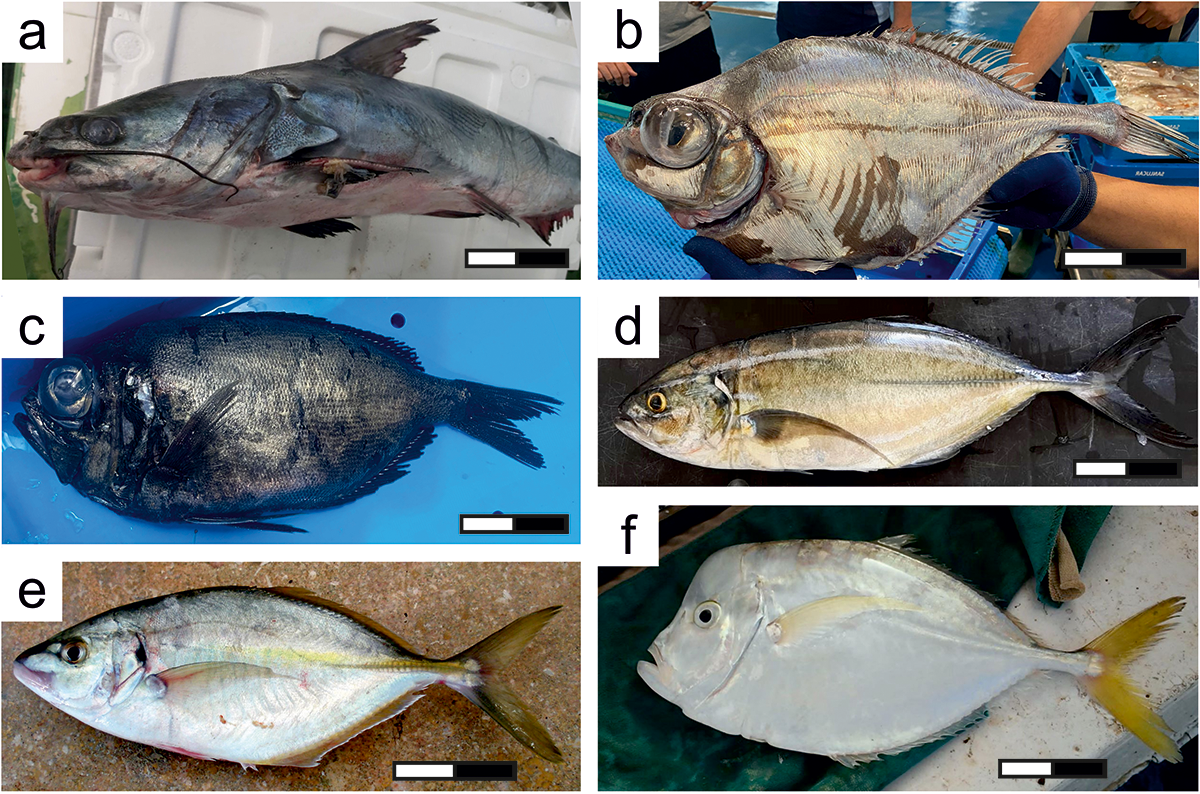
Figure 1. Newly observed species in the Gulf of Cadiz, ordered taxonomically: (a) Carlarius parkii; (b) Grammicolepis brachiusculus; (c) Diretmichthys parini; (d) Caranx crysos; (e) Pseudocaranx dentex; and (f) Selene dorsalis. Scale: 10 cm.
Grammicolepis brachiusculus Poey, 1873, family: Grammicolepididae, order: Zeiformes
In 2022, a specimen of Grammicolepis brachiusculus Poey, 1873 was captured in the GoC and later sold in a local market (Figure 1b); in 2023, two additional specimens were captured in the same area and were also commercialized. The specimen was estimated to be ∼50 cm in total length. The Thorny tinselfish, G. brachiusculus is a rare deep ocean species distributed across the Pacific Ocean, Indian Ocean, and the western Atlantic (Froese and Pauly, Reference Froese and Pauly2024). In the northeastern Atlantic, this species has only previously been observed in rare reports (Quigley and Ní Churraidhin, Reference Quigley and Ní Churraidhin2021): off Sisargas Island, NW Spain (Quéro, Reference Quéro1998); off El Hierro, Canarias Island (González et al., Reference González, Rico and Santana2000); off Brittany, NW France (Quéro et al., Reference Quéro, Du Buit, Iglésias, Morizur, Soulier and Vayne2001); off the Algarve coast, southern Portugal (Vasconcelos et al., Reference Vasconcelos, Santos and Gaspar2003); on Pico Island and Terceira Island, Azores (Barreiros et al., Reference Barreiros, Machado, Vieira and Porteiro2011); in Grindavikurdjup, SW Iceland (Valdimarsson et al., Reference Valdimarsson, Astthorsson and Palsson2012); off Halinn, NW Iceland (Valdimarsson et al., Reference Valdimarsson, Astthorsson and Palsson2012); West of Hebridies, Scotland (Iglésias, Reference Iglésias2014); and on the Porcupine Bank, West Ireland (Quigley and Ní Churraidhin, Reference Quigley and Ní Churraidhin2021). Previously, in the GoC, two specimens were documented in technical reports (Sobrino and Burgos, Reference Sobrino and Burgos2012) during a long-term bottom trawl survey program. Alongside the specimens described here, these constitute the first five records of the species in this locality (Supplementary Figure S2). Currently, the available information on the biology and ecology of this species is sparse.
Diretmichthys parini Post & Quéro, 1981, family: Diretmidae, order: Trachichthyiformes
Two specimens of Diretmichthys parini Post & Quéro, 1981 were captured in 2022 and 2023 at the GoC and sold in fish markets (El Puerto de Santa Maria and Sanlúcar de Barrameda, respectively) (Figure 1c). The total length of the individual was estimated to be approximately 35–40 cm. The Parin’s spinyfish, D. parini is a deep-water species found in the Pacific Ocean, Indian Ocean, and the Atlantic Ocean (Smith-Vaniz, Reference Smith-Vaniz, Whitehead, Bauchot, Hureau, Nielsen and Tortonese1986) (Supplementary Figure S3). Within the northeastern Atlantic, this species has previously been recorded off northwestern Morocco and the Madeira Archipelago (Post, Reference Post, Whitehead, Bauchot, Hureau, Nielsen and Tortonese1986), off the Canary Islands (López-Abellán et al., Reference López-Abellán, Santamaría and Balguerías1994), off the southern coast of Portugal (Sanches and Pinto, Reference Sanches and Pinto1991), in the Bay of Biscay and Galician waters (Arronte and Heredia, Reference Arronte and Heredia2006; Bañon et al., Reference Bañón, Arronte, Rodriguez-Cabello, Piñeiro, Punzon and Serrano2016), off the northwest coast of Scotland and off the Faroe Islands, Northern Ireland (Quéro et al., Reference Quéro, Du Buit, Fonteneau, Laborde, Morandeau and Vayne1994, Reference Quéro1998), and off Iceland (Jónsson and Pálsson, Reference Jónsson and Pálsson2003), and more recently, various records have been compiled for the North Sea and Nordic Sea (Cresson et al., Reference Cresson, Rouquette, Mirallès, Dufour, Causse, Bouchoucha and Mahé2017, Reference Cresson, Iglésias, Jakobsdóttir and Lynghammar2021; Lynghammar et al., Reference Lynghammar, Byrkjedal, Bugjerde and Wienerroither2020). This is the first record of D. parini in the GoC. Limited data exist on the biology and ecology of this uncommon fish, but a 33-year-old specimen from the North Sea provided some insights (Cresson et al., Reference Cresson, Rouquette, Mirallès, Dufour, Causse, Bouchoucha and Mahé2017). These authors analysing stable isotopes revealed their vertical feeding migrations between deep, cold habitats and shallower, warmer epipelagic zones to eat zooplankton. The species produces pelagic eggs, which may increase larval dispersal potential (Post, Reference Post, Whitehead, Bauchot, Hureau, Nielsen and Tortonese1986).
Caranx crysos Mitchill, 1815, family: Carangidae, order: Carangiformes
Between 2017 and 2024, several specimens (n = 7) of Caranx crysos Mitchill, 1815 were captured and sold in fish markets located in the GoC (Figure 1d). The total length of these specimens varied approximately between 30 and 35 cm. The blue runner, C. crysos is widely reported across the Atlantic Ocean, ranging from Brazil to Nova Scotia (Canada) in the western Atlantic and from Senegal to Angola in the east Atlantic, including the Mediterranean (Di Blasi et al., Reference Di Blasi, Bava, Desiderà, Merotto, Poli and Guidett2024; Psomadakis et al., Reference Psomadakis, Bentivegna, Giustino, Travaglini and Vacchi2011; Smith-Vaniz, Reference Smith-Vaniz and Carpenter2002). It is established in the Canary Islands (Brito et al., Reference Brito, Falcón and Herrera2005). Recent expansions in its distribution range have been documented in several regions, including the Adriatic Sea (Dulčić et al., Reference Dulčić, Pallaoro and Dragičević2009), the Bay of Biscay (Iglésias et al., Reference Iglésias, Bariche, Beau, Bérenger, Beucher, Chabrolle, Cottalorda, Cousin, Curd, Danet, Duhamel, Duval, Farque, Goascoz, Jadaud, Larnaud, Le Bouter, Le Bras, Le Bris, Lombard, Louisy, Mandine, Mas, Menut, Metral, Poussard, Quéro, Raybaud, Renoult, Richard, Spitz, Ternon, Thiriet and Tournier-Broer2021; Quéro et al., Reference Quéro, Du Buit, Fonteneau, Laborde, Morandeau and Vayne1994), Galician waters (Bañon and Casas-Sánchez, Reference Bañón-Díaz and Casas-Sánchez1997), the west coast of Ireland (Quigley, Reference Quigley2022), British and Newfoundland waters (Canada), and Argentine waters (Delpiani et al., Reference Delpiani, Lertora, Mabragaña and de Astarloa2011; Devine and Fisher, Reference Devine and Fisher2014; Swaby et al., Reference Swaby, Potts and Lees1996; Wheeler et al., Reference Wheeler, Merrett and Quigley2004), as well as in the present records. Although there have been several records, this species has not yet been documented in the Spanish Suratlantic waters (Supplementary Figure S4). The blue runner generally forms schools and is found inshore at depths of <100 m. Juveniles of C. crysos are frequently associated with jellyfish and floating debris (de Carvalho-Souza, Reference de Carvalho-Souza2015), while adults associate with rocky reefs (Smith-Vaniz, Reference Smith-Vaniz and Carpenter2002). The blue runner is an opportunistic predator that primarily preys on small fish and crustaceans. Its length at sexual maturity ranges from approximately 210 to 250 mm, typically reached at 2.4–2.8 years of age (Sley et al., Reference Sley, Jarboui, Ghorbel and Bouain2012). This species is oviparous, exhibiting high fecundity and producing pelagic eggs that disperse with ocean currents (Sley et al., Reference Sley, Jarboui, Ghorbel and Bouain2012).
Pseudocaranx dentex Bloch & Schneider, 1801, family: Carangidae, order: Carangiformes
A single specimen of Pseudocaranx dentex Bloch & Schneider, 1801 was captured in May 2010 and sold at the Rota fish market (Figure 1e). Later occurrences included one individual in October 2013, another in November 2016, two in October 2018, one in June 2023, and at least five more individuals in 2024, with their total lengths varying approximately from 40 to 60 cm. The white trevally, P. dentex is a tropical reef species widely distributed on continental and island shelves across the Atlantic, the Mediterranean, and the Indo-Pacific (Smith-Vaniz, Reference Smith-Vaniz and Carpenter2002) (Supplementary Figure S5). Only a few records of P. dentex are known from the Andalusian coast (ICTIOTERM, 2024; Rivas and Pasquier, Reference Rivas and Pasquier2003). In Galician waters, the species was recorded for the first time in May 1997 (Fernández-Cordero and Bañon, Reference Fernández-Cordeiro and Bañón1997). More recently, a northernmost occurrence was documented in the Istanbul Strait, which serves as the passage between the Sea of Marmara and the Black Sea (Keskin, Reference Keskin2023). The white trevally juveniles typically inhabit inshore waters, with young fish often seen in shoals following mullets, striped seabream, and other demersal fish on sandy bottoms, while adults live solitarily (Froese and Pauly, Reference Froese and Pauly2024). They commonly seek refuge from predators under floating structures (Keskin, Reference Keskin2023).
Selene dorsalis Gill, 1863, family: Carangidae, order: Carangiformes
In 2017, a specimen of Selene dorsalis Gill, 1863 was captured and sold in a local market in the GoC (Figure 1f). The estimated total length of the individual was approximately 30 cm. The African moonfish, S. dorsalis is a demersal species found in tropical and subtropical waters of the East Atlantic, from the Cape Verde Islands and Senegal to South Africa (Smith-Vaniz, Reference Smith-Vaniz, Whitehead, Bauchot, Hureau, Nielsen and Tortonese1986) (Supplementary Figure S6). Previously, it was recorded in Madeira (Wirtz et al., Reference Wirtz, Fricke and Biscoito2008) and Canary Islands (Castro-Hernández, Reference Castro-Hernandez2001), Moroccan waters (Baddyr and Guénette, Reference Baddyr, Guénette, Guénette, Christensen and Pauly2001), Mediterranean Sea (Vella and Deidun, Reference Vella and Deidun2009), and GoC (Juarez et al., Reference Juarez and Silva2006), this being the second record for this fish species in the GoC (Supplementary Figure S6). The African moonfish is a schooling species primarily found within a depth range of 20–100 m (Smith-Vaniz, Reference Smith-Vaniz, Whitehead, Bauchot, Hureau, Nielsen and Tortonese1986). It primarily feeds on small fish and planktonic crustaceans (Smith-Vaniz, Reference Smith-Vaniz, Whitehead, Bauchot, Hureau, Nielsen and Tortonese1986).
Seriola rivoliana Valenciennes, 1833, family: Carangidae, order: Carangiformes
Since 2016, several specimens (n = 170) of Seriola rivoliana Valenciennes, 1833 were captured and sold in fish markets in the GoC (Figure 2a). The total sizes of these individuals spanned approximately from 40 to 60 cm. The longfin yellowtail, S. rivoliana is frequently found in the Pacific Ocean, Indian Ocean, and the western Atlantic (Fischer, Reference Fischer1978), spanning at least 114 countries/territories (Froese and Pauly, Reference Froese and Pauly2024; Quigley, Reference Quigley2021) (Supplementary Figure S7). This species is also recorded in the Mediterranean Sea (Castriota et al., Reference Castriota, Greco, Marino and Andaloro2002, Reference Castriota, Falautano, Greco and Andaloro2004). Within the Iberian biogeographic regions, it has been recorded in Portugal (Fischer et al., Reference Fischer, Bianchi and Scott1981), as well as observed in the Balearic Islands (Valls et al., Reference Valls, Grau, Massutí, Tobaruela and Riera2011), Port of Cadiz in 1995 (ICTIOTERM, 2024), and in the Bay of Biscay and Galician waters (Bañon and Garazo, Reference Bañón and Garazo2006; Quéro, Reference Quéro1998). Adults of S. rivoliana are pelagic and epibenthic, while juveniles are typically found offshore, utilizing floating objects as shelter (Fischer, Reference Fischer1978).

Figure 2. Newly observed species in the Gulf of Cadiz, ordered taxonomically: (a) Seriola rivoliana; (b) Kyphosus vaigiensis; (c) Sparisoma cretense; and (d) Pomadasys rogerii. Scale: 10 cm.
Kyphosus vaigiensis Quoy & Gaimard, 1825, family: Kyphosidae, order: Centrarchiformes
Two individuals of Kyphosus vaigiensis Quoy & Gaimard, 1825 were captured in July 2015 and landed at the Rota and Chipiona fish markets (Figure 2b). Afterward, one individual was reported in 2020, two in 2021, and several specimens in 2022 (Supplementary Figure S8). These individuals ranged from around 45 to 55 cm in total length, a common size relative to the maximum of 70 cm (Froese and Pauly, Reference Froese and Pauly2024). This circumtropical species has a wide distribution across the Pacific, Indian, and Atlantic Oceans, as well as the Red Sea and Mediterranean Sea (Azurro et al. Reference Azzurro, Pena-Rivas, Lloris and Bariche2013; Groud et al., Reference Groud, Chaoui and Kara2021; Knudsen and Clements, Reference Knudsen and Clements2013; Mannino et al., Reference Mannino, Balistreri, Iaciofano, Galil and Lo Brutto2015). It was first recorded in the waters of Galicia in 2014 with two specimens, marking the northernmost record in the eastern Atlantic (Bañon et al., Reference Bañón, Barros-García and de Carlos2017). Typically, this species is found as adults over hard bottoms near the shore and coastline, while juveniles are often associated with floating objects (Knudsen and Clements, Reference Knudsen and Clements2016).
Sparisoma cretense Linnaeus, 1758, family: Labridae, order: Labriformes
Since 2008, several individuals of Sparisoma cretense (n = 100) were captured and sold in fish markets along the Cadiz coast (Barbate, Algeciras, and Chipiona) (Figure 2c). These individuals ranged from around 20 to 30 cm in total length. The Mediterranean parrotfish, S. cretense, is distributed along the southern and eastern coasts of the Mediterranean, the northwest coast of Africa, and the Macaronesian archipelagos (Azores, Madeira, Canary Islands, and Cape Verde) (Froese and Pauly, Reference Froese and Pauly2024). Esposito et al. (Reference Esposito, Prearo, Menconi, Mugetti, Meloni, Tomasoni, Pizzul, Piras, Renzi, Gaspa and Pastorino2001) documented the northward expansion of this species in the Mediterranean Sea and its colonization process. Within the southwestern Iberian Peninsula, it has been recorded in the Ria Formosa lagoon, southern Portugal (Abecasis et al., Reference Abecasis, Bentes, Ribeiro, Machado, Oliveira, Veiga, Gonçalves and Erzini2008), and in Bolonia and Tarifa, southern coast of Spain (ICTIOTERM, 2024; Otero and Galeote, Reference Otero and Galeote1996) (Supplementary Figure S9). The Mediterranean parrotfish is a reef-associated species that generally occurs in shallow water to about 50 m (Pollard et al., Reference Pollard, Yokes, Francour, Rocha, Choat, Clements, Russell, Myers, Lazuardi, Muljadi, Pardede and Rahardjo2012). The species feeds on algae and small invertebrates by utilizing its fused beak-like jaws to excavate or scrape the surfaces of rocks and carbonate substrates (Hoey and Bonaldo, Reference Hoey, Bonaldo, Hoey and Bonaldo2018). The breeding season generally spans from July to September, and juveniles are recruited in late summer (Guidetti and Boero, Reference Guidetti and Boero2001).
Pomadasys rogerii Cuvier, 1830, family: Haemulidae, order: Acanthuriformes
A single Pomadasys rogerii was observed at GoC in 2014 (Figure 2d). The specimen’s total length was approximately 50 cm. The Pigsnout grunt, P. rogerii, is found in the Eastern Atlantic, from southern Mauritania to Angola (Froese and Pauly, Reference Froese and Pauly2024; Ly et al., Reference Ly, Diop and Girardin1996). This is the first record of P. rogerii in European waters (Supplementary Figure S10). This coastal pelagic is often found on the seabed up to 60 m, but it is also found in estuaries (Ly et al., Reference Ly, Diop and Girardin1996). The Pigsnout grunt is benthophagous, being one of the dominant species in the estuarine zones, especially during early life stages (Costa et al., Reference Costa, Santos and Cabral2002).
Lobotes surinamensis Bloch, 1790, family: Lobotidae, order: Acanthuriformes
More than 30 individuals of Lobotes surinamensis Bloch, 1790 were sold in fish markets in the GoC between 2015 and 2023 (Figure 3a). An individual was first reported in Chipiona (GoC, SW Spain) in 2015, followed by increasing reports in several other localities in subsequent years (Supplementary Figure S11 and Table S1). Previously, a unique specimen was reported in the locality of Rota in 1998 (ICTIOTERM, 2024). These individuals had a total size ranging approximately from 26.5 to 51 cm. The tripletail, L. surinamensis is a pelagic species found in the tropical and subtropical waters of all oceans (Fisher, Reference Fischer1978; Iglésias et al., Reference Iglésias, Bergot, Breton, Brunelle, Camusat, Causse, Charbonnel, Chevaldonné, Cordier, Cosquer, Cuillandre, Curd, Dubas, Duhau, Derrien-Courtel, Devique, Dixneuf, Duhamel, Farque, Francour, Fontana, Gamon, Gicqueau, Goascoz, Hassani, Jadaud, Kopp, Lamour, Le Bris, Lévèque, Liger, Lorance, Louisy, Maran, Méhault, Metral, Morin-Repinçay, Mouchel, Pere, Quéro, Renoult, Roche, Schweyer, Spitz, Thiriet and Thomas2020). Earlier, this species was considered exceptional or rare in the zone, as well as in the Azores (Afonso et al., Reference Afonso, Porteiro, Fontes, Tempera, Morato, Cardigos and Santos2013; Santos et al., Reference Santos, Porteiro and Barreiros1997), Madeira (Wirtz et al., Reference Wirtz, Fricke and Biscoito2008), and Canary Islands (Brito et al., Reference Brito, Pascual, Falcón, Sancho and González2002; Espino et al., Reference Espino, Falcón, Otero-Ferrer, Haroun and Brito2018). Tripletails often associated with channel markers, jetties, wrecks, flotsam, Sargassum algae, or other types of drift algae and anthropogenic floating debris (Baughman, Reference Baughman1941; Dooley, Reference Dooley1972).
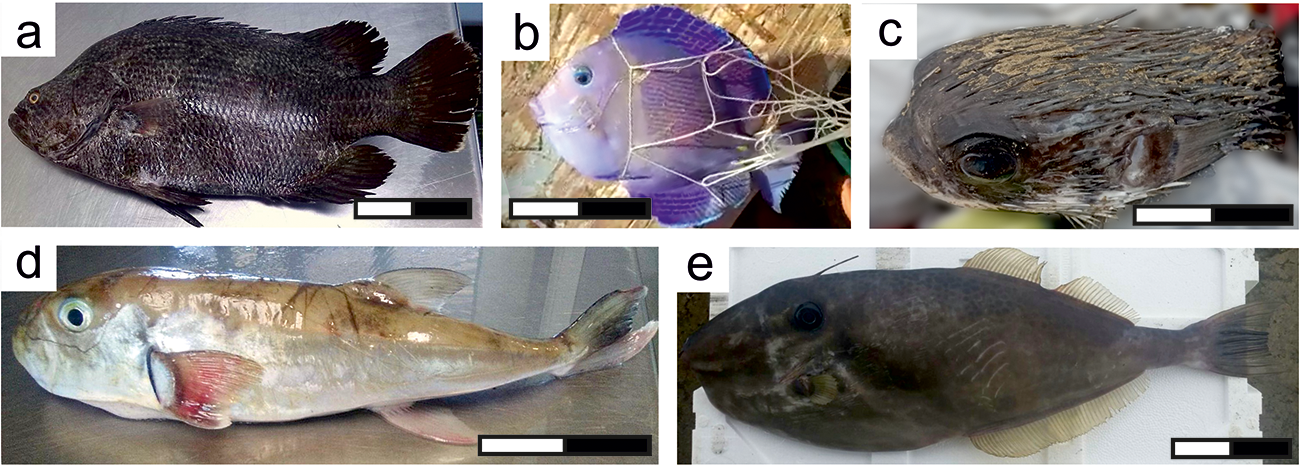
Figure 3. Newly observed species in the Gulf of Cadiz, ordered taxonomically: (a) Lobotes surinamensis; (b) Acanthurus coeruleus; (c) Diodon holocanthus; (d) Lagocephalus laevigatus; and (e) Aluterus monoceros. Scale: 10 cm.
Acanthurus coeruleus Bloch & Schneider, 1801, family: Acanthuridae, order: Acanthuriformes
In October 2021, an individual of Acanthurus coeruleus Bloch & Schneider, 1801 was captured in the GoC by an artisanal fisherman (Figure 3b). Known as the Blue Tang, this species is typically found in the Western Atlantic, ranging from New York and Bermuda to the Gulf of Mexico and Brazil, with additional records from Ascension Island in the Eastern Atlantic (Froese and Pauly, Reference Froese and Pauly2024). In the Mediterranean, A. coeruleus has been documented in Cyprus (Langeneck et al., Reference Langeneck, Marcelli and Simak2012), Israel (Golani et al., Reference Golani, Askarov and Appelbaum-Golani2015), Malta (Evans et al., Reference Evans, Tonna and Schembri2015), and the Canary Islands (Pajuelo et al., Reference Pajuelo, González, Triay-Portella, Martín, Ruiz-Díaz, Lorenzo and Luque2016), marking this recent record in the Eastern Atlantic as the first for the Iberian Peninsula (Supplementary Figure S12). This surgeonfish reaches up to 39 cm in total length and inhabits coral reefs as well as inshore grassy or rocky areas, forming small groups and displaying primarily diurnal behaviour while feeding on algae (Böhlke and Chaplin, Reference Böhlke and Chaplin1993).
Diodon holocanthus Linnaeus, 1758, family: Diodontidae, order: Tetraodontiformes
In November 2022, an individual of Diodon holocanthus Linnaeus, 1758 was found by a spearfisher in the coastal waters of the Cadiz Bay (Figure 3c). The total length of the individual was estimated to be approximately 30 cm. The long-spined porcupinefish, D. holocanthus, is a circumtropical species distributed along the Indian, Atlantic, and Pacific oceans (Froese and Pauly, Reference Froese and Pauly2024) (Supplementary Figure S13). In the eastern Atlantic, D. holacanthus has been known to inhabit southwestern Morocco and west-central Namibia (Duron and Queró, Reference Duron, Queró, Quero, Hureau, Karrer, Post and Saldanha1990), with records extending up to South Africa (Leis, Reference Leis, Carpenter and Niem2001). This species has also been documented in the Canary Islands (Brito and Falcón, Reference Brito and Falcón2006) and the Azores Islands (Afonso et al., Reference Afonso, Porteiro, Fontes, Tempera, Morato, Cardigos and Santos2013). Pelagic juveniles are often found under floating rafts, while demersal adults are found on all types of substrates, up to at least 100 m deep (Froese and Pauly, Reference Froese and Pauly2024). They feed on molluscs, urchins, and crustaceans at night (Leis, Reference Leis, Carpenter and Niem2001).
Lagocephalus laevigatus Linnaeus, 1766, family: Tetraodontidae, order: Tetraodontiformes
An individual of Lagocephalus laevigatus Linnaeus, 1766 was collected in 2017 at the GoC (Figure 3d). The total length of the individual was estimated to be approximately 35 cm. The smooth puffer, L. laevigatus is an amphi-Atlantic species distributed in the western Atlantic Ocean from Bermuda and New England, USA, to Argentina, whereas in the eastern Atlantic Ocean, it is recorded from Mauritania to Namibia (Froese and Pauly, Reference Froese and Pauly2024). Previously, this species has had other records north of its distribution, in the Cies Islands in Galician waters (Bañon and Santas, Reference Bañon and Santás2011) and in the lower Guadiana estuary (Encarnação et al., Reference Encarnação, Morais, Baptista, Cruz and Teodósio2019), being the first report of this species for the GoC and the second for the Iberian Peninsula (Supplementary Figure S14). The smooth puffer typically inhabits shallow waters down to depths of 180 m, primarily on mud or silt substrates, where it is commonly encountered either solitary or in small groups (Shipp, Reference Shipp, Fischer, Bianchi and Scott1981).
Aluterus monoceros Linnaeus, 1758, family: Monacanthidae, order: Tetraodontiformes
An individual of Aluterus monoceros Linnaeus, 1758 was captured in 2017 at the GoC (Figure 3e). The total length of the specimen was estimated to be approximately 50 cm. The Unicorn leatherjacket filefish, A. monoceros, is a circumtropical species distributed in the Western Atlantic Ocean from Massachusetts, USA, to Argentina; in the Eastern Atlantic Ocean along the west coast of tropical Africa; in the Eastern Pacific Ocean from Guatemala to Chile, and likely in Mexico; and in the Western Indian Ocean from Mozambique to South Africa (Froese and Pauly, Reference Froese and Pauly2024). This species has previously has been reported in the Azores (Santos et al., Reference Santos, Porteiro and Barreiros1997), Madeira (Freitas and Biscoito, Reference Freitas and Biscoito2002), and the Canary Islands (Brito, 2002), as well as in the Mediterranean Sea (Guallart and Vicent, Reference Guallart and Vicent2009), off Arcachon, Bay of Biscay (Quéro and Laborde, Reference Quéro and Laborde1996) and two specimens (1995 and 2008) in the coastal waters off Chipiona in the GoC (Galeote et al., Reference Galeote, Oter and Arias1996; ICTIOTERM, 2024), making this the third specimen recorded occurrence in the studied area (Supplementary Figure S15). While A. monoceros adult are demersal, juveniles are pelagic and often associate with floating objects (Sommer et al., Reference Sommer, Schneider and Poutiers1996), a behaviour that may facilitate the dispersal of individuals from the main distribution area when conditions are favourable.
Discussion
This study reports new, unusual, and rare occurrences of 15 fish species in the GoC ecosystem, confirming a significant range expansion and the potential establishment of species previously observed only occasionally or regarded as uncertain within this region. Despite the uncertainty surrounding the introduction vector in many bioinvasion cases, the ecological interpretation of these occurrences can be divided into three main pathways.
First, two coastal fishes with similar habitats and distribution, P. rogerii and C. parkii, are reported for the first time in European waters, possibly as a result of the climate-induced range expansion of West African biota northwards into European waters (‘African Creep’; Canning-Clode and Carlton, Reference Canning-Clode and Carlton2017), although accidental introduction via ballast waters cannot be completely ruled out. This process has been documented in recent years for species such as Lysmata uncicornis Holthuis & Maurin, 1952, and Penaeus notialis Pérez Farfante, 1967 (González-Ortegón et al., Reference González-Ortegón, de Carvalho-Souza, Muñoz, Gómez, Arana and Cuesta2024, Reference González-Ortegón, Jenkins, Galil, Drake and Cuesta2020a). The movement of fish species during their pelagic phase (eggs, larvae, and juveniles) can occur via oceanic currents from the northwest African coast to the southwestern Iberian Peninsula. The near-surface circulation in the GoC is characterized by upper-thermocline North Atlantic Central Waters (Machín et al., Reference Machín, Pelegrí, Marrero-Díaz, Laiz and Ratsimandresy2006). Intense mixing with South Atlantic Central Water occurs in the coastal waters of Northwest Africa, where the northward advection along the inshore side is largely driven by the poleward undercurrent associated with coastal upwelling (Arístegui et al., Reference Arístegui, Alvarez-Salgado, Barton, Figueiras, Hernández-León, Roy, Santos, Robinson and Brink2006). On the other hand, the past four decades have seen a general increase in sea surface temperatures and a weakening of upwelling intensity in the Iberian/Canary and Northwest African regions (Pardo et al., Reference Pardo, Padín, Gilcoto, Farina‐Busto and Pérez2011). Notably, sea temperatures in the Northeast Atlantic have risen by at least 0.4°C per decade since the late 1980s (Brander et al., Reference Brander, Blom, Borges, Erzini, Henderson, MacKenzie, Mendes, Ribeiro, Santos and Toresen2003) and continue to rise (Supplementary Figure S16). As these trends persist, the warming waters of the GoC create increasingly suitable conditions for these species.
Additionally, species such as A. coeruleus, A. monoceros, D. holocanthus, P. dentex, and S. dorsalis, previously observed in the Macaronesia zone (Azores, Canary Islands, and Madeira Islands), could potentially expand their range across these islands, which might serve as a stepping-stone for further expansion, together with the favourable oceanographic conditions mentioned earlier. The Macaronesia islands likely served as a stepping-stone for other tropicalization cases of fishes and decapod crustaceans (Castro et al., Reference Castro, Schäfer, Parretti, Monteiro, Gizzi, Chebaane, Almada, Henriques, Freitas, Vasco-Rodrigues, Silva, Radeta, Freitas and Canning-Clode2021; Schäfer et al., Reference Schäfer, Monteiro, Castro, Rilov and Canning-Clode2019). This expansion pattern seems similar to what is happening in the Mediterranean, where several Lessepsian migrants, such as the lionfish, Pterois miles Bennett 1828 and the blue swimming crab, Portunus segnis Forskål 1775, start in the Levantine Sea and gradually spread into the western Mediterranean, with the latter arriving at the GoC (Bernardi et al., Reference Bernardi, Azzurro, Bariche, Jimenez, Kalogirou and Kleitou2024; de Carvalho-Souza et al., Reference de Carvalho-Souza, Cuesta, Arana, Lobato and González-Ortegón2023).
Some of the records appear to be well established in the GoC ecosystem: S. rivoliana, L. surinamensis, and S. cretense. In these cases, due to the ecological traits of these species, there are multiple dispersal mechanisms, both natural and anthropogenic. For the first two species, currents and ballast water in its pelagic phase (eggs, larvae, and juveniles) or by rafting underneath floating debris or Sargassum spp. may facilitate, under suitable conditions, the dispersal and settlement of individuals from the main occurrence area (Baughman, Reference Baughman1941; Dooley, Reference Dooley1972; Keskin, Reference Keskin2023). For S. cretense, considering its Mediterranean origin and expansion patterns (Esposito et al., Reference Esposito, Prearo, Menconi, Mugetti, Meloni, Tomasoni, Pizzul, Piras, Renzi, Gaspa and Pastorino2001), we can also hypothesize its arrival in the GoC through the Strait of Gibraltar.
The records of D. parini and G. brachiusculus demand a more thorough approach, considering their broad distribution in tropical and temperate waters of the Atlantic, Pacific, and Indian Oceans. Various studies have indicated that the apparent increase in the occurrence of these species in Northern European Atlantic waters might be associated with ocean warming (Barreiros et al., Reference Barreiros, Machado, Vieira and Porteiro2011; Cresson et al., Reference Cresson, Rouquette, Mirallès, Dufour, Causse, Bouchoucha and Mahé2017; Quéro et al., Reference Quéro, Du Buit, Fonteneau, Laborde, Morandeau and Vayne1994, Reference Quéro1998; Valdimarsson et al., Reference Valdimarsson, Astthorsson and Palsson2012). On the other hand, a review of Icelandic fishery records shows that several unpublished observations for D. parini were overlooked (Cresson et al., Reference Cresson, Iglésias, Jakobsdóttir and Lynghammar2021), as they were mainly collected by fishery observers and occasionally by the fishermen themselves. Consequently, the frequency of these records is significantly affected by individual motivations driven by the discovery of a rare species or a personal interest in biodiversity. Furthermore, the complexity increases for deep-water species, since these habitats are poorly monitored, and their fish fauna is not as well documented as that of coastal regions (de Carvalho-Souza et al., Reference de Carvalho-Souza, Rodríguez-Ruiz, Gómez and González-Ortegón2024b). Thus, the occurrence of new fish species alone cannot definitively indicate whether recent faunal shifts are a result of ocean warming or the outcome of intensified fishing, sampling efforts, or citizen science activities.
Therefore, these findings emphasize the necessity for ongoing monitoring studies, alongside early detection and rapid response strategies in the GoC (due to its strategic location), using fish species as indicators to predict the effects of climate change. Initiatives of this nature are essential given the significant alterations observed in the ichthyofauna of the southwestern Iberian Peninsula and their habitats in recent decades. These alterations are driven by well-documented factors that go beyond warming: overfishing and regime shifts; human activities in essential fish habitats; the introduction of species for aquaculture and aquarium trade; intensification of maritime traffic, which consequently increases propagule pressure; and the transportation of larvae and juveniles via ballast water (de Carvalho-Souza et al., Reference de Carvalho-Souza, González-Ortegón, Baldó, Vilas, Drake and Llope2019, Reference de Carvalho-Souza, Torres, Farias, Acosta, Tornero, Sobrino and Llope2021; González-Ortegón et al., Reference González-Ortegón, de Carvalho-Souza, Muñoz, Gómez, Arana and Cuesta2024; González-Ortegón and Moreno-Andrés, Reference González-Ortegón and Moreno-Andrés2021; Torres et al., Reference Torres, Coll, Heymans, Christensen and Sobrino2013).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315425100143.
Acknowledgements
The authors would like to thank the fishermen who provided records and fishing information. Moreover, we thank Antonio Moreno from the ICMAN Field Operations Unit (OPECAM) for his technical support.
Author contributions
G.F.d.C.-S. and E.G.-O. conceived the study and contributed to the study design. G.F.d.C.-S. and E.G.-O. analysed the data and wrote the paper. All authors contributed to drafting and editing. All authors read and approved the final manuscript.
Funding
G.F.d.C.-S. acknowledges the support received from the Spanish State Research Agency (PTA2022-021378-I) and the postdoctoral fellowship from the Junta de Andalucía (DGP_POST_2024_00956). Financial support was given by CSIC through Intramural Research program under grant number 2024ICT027.
Data availability statement
All data generated or analysed during this study are included in this published article.
Ethics statement
In this study, no experiments with animals were performed. Specimens were collected as part of fishery-dependent surveys. Therefore, all examinations were made upon deceased animals that were captured during legal commercial fishing operations.





