Introduction
Morus alba, or white mulberry, is a deciduous tree with a versatile range of applications. Alongside species like Morus indica, Morus laevigata and Morus serrata, M. alba L. is a cornerstone of Indian agriculture, especially for sericulture (silk production) and fruit cultivation. This species holds a special place in horticulture and agriculture due to its significance in silk production as the primary food source for silkworms. M. alba is a Chinese native that was brought to North America in the 1600s (Dirr, Reference Dirr1998). M. alba is a small- to medium-sized shrub or tree that can reach a height up to 15 m. It features a dense canopy with spreading branches, a rounded crown and a short trunk. Leaves have three main veins that extend from the rounded or notched base and are alternating, simple, broadly oval and polymorphic. They are 6–18 cm long and 5–13 cm wide, with a lustrous green top and a lighter, slightly hairy underside. It has light brown to grey bark that is mostly smooth but furrowed at the edges. Male inflorescences are green and extremely small, appearing in long catkins, but female flowers are inconspicuous and packed in short spikes. It has white, purple or pinkish fruits that are 10–19 mm long and have a cylindrical aggregate (Little, Reference Little1980; Penskar, Reference Penskar2009; Invasive.org, 2010; USDA NRCS, 2010). It prefers a warm, moist, well-drained loamy soil in a sunny aspect. However, it is adapted to coarse, medium and fine soils and is described as intermediate shade tolerant and medium drought tolerant. Soil texture and depth are the important factors affecting growth. The tree cannot tolerate alkalinity and grows best on soils with pH ranging between 6.0 and 7.5 and rainfall between 30 and 60 in/year. It can survive temperatures down to −27°C and is quite salt tolerant once established (Burgess and Husband, Reference Burgess and Husband2004; Penskar, Reference Penskar2009; Invasive.org, 2010; USDA NRCS, 2010). The old leaves are shed in November–December and trees are leafless during winter season. Appearance of new leaves and flowering takes place in March–April and the fruits ripen from April to June (Brandis, Reference Brandis1906). Indian gene centre is very rich in Morus species. It is cultivated in northern India from Jammu and Kashmir to Assam. In some areas it ran wild and spread in suitable localities. In the Himalayas, it ascends to an elevation of about 1200 m (Dadhwal and Banerjee, Reference Dadhwal and Banerjee2023). It is extensively cultivated in Jammu and Kashmir, Uttarakhand, Haryana, Punjab, Uttar Pradesh, Karnataka, Tamil Nadu, West Bengal, Kerala and to a lesser extent in Madhya Pradesh, Bihar, Orissa, Assam, Manipur and Andhra Pradesh (Ghosh, Reference Ghosh1977). Wild relatives of genus Morus are reported to occur in India in the tropical and subtropical Himalayan belt.
Researchers are concentrating on the extraction, isolation, identification and characterization of organic compounds found in plants, animals and minerals since they are crucial to the study of medical chemistry (Chaachouay and Zidane, Reference Chaachouay and Zidane2024). Because natural goods are helpful, even though they may have a lot of negative consequences, conventional medicines use phytochemical-rich plant extracts to treat a variety of ailments (Dadhwal and Banerjee, Reference Dadhwal and Banerjee2023). Plants are therefore among the best sources for finding novel chemicals that act as building blocks for the creation of new drugs (Batiha et al., Reference Batiha, Al-Snafi, Thuwaini, Teibo, Shaheen, Akomolafe, Teibo, Al-kuraishy, Al-Garbeeb and Alexiou2023). Moraceae family is indeed a valuable source of medicinal plants with diverse applications in various industries. Because of sericulture techniques, mulberries, which are native to China, are grown all over the world (Yan et al., Reference Yan, Ruan, Huang, Sun, Zheng, Zhang and Wang2020). Its leaves have been used as cooling agents, perspiration inducers and antipyretics in addition to their nutritional importance as abundant sources of polysaccharides and proteins (Katsube et al., Reference Katsube, Imawaka, Kawano, Yamazaki, Shiwaku and Yamane2006). Flavonoids, amino acids, polysaccharides, vitamins and steroids are the main bioactive substances found in mulberry leaves. These substances can be utilized to treat a variety of internal illnesses and infections. Because of their possible biological qualities, such as anti-inflammatory, anti-ageing and antihyperglycaemic effects, flavonoids have become a significant focus of research in recent years (Katsube et al., Reference Katsube, Imawaka, Kawano, Yamazaki, Shiwaku and Yamane2006; Chen and Li, Reference Chen and Li2007). At least 6000 molecules make up the huge group known as flavonoids, which are divided into six groups: flavonols, flavones, phlobaphenes, anthocyanins, aurones and isoflavonoids (Winkel-Shirley, Reference Winkel-Shirley2001). Kuwanon (flavones), sangenon (flavanols), rutin (flavones), quercetin (flavanols) and catechins (flavanols) are among the flavonoid compounds that are frequently found in mulberries. Most flavonoids in plants are glycosylated, and this process moderate's flavonoids by making them more stable and soluble. They are essential to the metabolism of plants. The main chemical components of Morus plants with bioactive potentials are polyphenolic flavonoids, benzofurans, stilbenes and Diels–Alder adducts. These Morus plant-derived polyphenols exhibit a variety of bioactivities, such as cytotoxic, antibacterial, anti-inflammatory, antidiabetic, anti-oxidative and skin-whitening properties (Chaita et al., Reference Chaita, Lambrinidis, Cheimonidi, Agalou, Beis, Trougakos, Mikros, Skaltsounis and Aligiannis2017). Notably, several clinical investigations have recently confirmed the antidiabetic effects (Yan et al., Reference Yan, Ruan, Huang, Sun, Zheng, Zhang and Wang2020).
Millions of farming families worldwide, including those in India, rely on sericulture, a significant agro-based business, for their survival. Successful silkworm rearing is essential to the sericulture business's success. Mulberry silk is the most significant of the various silk manufacturing methods used in India, accounting for 75.6% of the country's silk output (Annual Report, 2022–23, CSB, Bengaluru). Since silkworms (Bombyx mori L.) primarily eat this plant, the viability and profitability of the sericulture sector are significantly impacted by the availability of high-quality leaves. The quality of cocoons, which is directly related to the quality of feed, such as mulberry leaves, is a major factor in increased silk output (Sarkar et al., Reference Sarkar, Roy, Gupta and Das1987). For taxonomists to categorize various mulberry species and variants and for breeders to create genotypes of mulberries that are drought-tolerant, pest- and disease-resistant and have large leaf yields, morphological studies are crucial. Establishing the taxonomic identity of germplasm can be aided by quantitative character analysis. The initial stage in characterizing and classifying the germplasm is phenotypic characterization. Additionally, estimations of genetic variation and the relationships between collections of germplasm are particularly helpful and make it easier to maintain and use the germplasm effectively (Rabbani et al., Reference Rabbani, Murakami, Suzuki and Takayangi1998).
Mulberry leaf yield is a complicated trait that is greatly impacted by genotypes and the environment and is simultaneously contributed by a considerable number of component features. Studying the genetics of yield components and the extent to which they are associated with yield is crucial since yield alone might not be the optimum selection criterion (Tikader and Rao, Reference Tikader and Rao2002).
The assessment of distinctness, uniformity and stability (DUS) characteristics for M. alba is crucial for proper identification and registration of its various cultivars. A useful method for identifying and preventing duplication is DUS characterization of crop genotypes (Das and Kumar, Reference Das and Kumar2012). This comprehensive examination will delve into the essential DUS characteristics of M. alba, exploring the distinct features that set its cultivars apart, the factors contributing to their uniformity and the stability of these characteristics over time. Understanding these traits is not only important for taxonomical purposes but also for agro-forestry practices, horticulturists, farmers, breeders and researchers, as they play a pivotal role in breeding, cultivation and conservation efforts. DUS testing of genotypes involves comparing the germplasm lines with respect to a set of morphological and physiological characters called descriptors (Anonymous, 2016) throughout the plant growth. The goal of this study is to provide a comprehensive understanding of the diverse and intriguing characteristics of M. alba, focusing on its distinct DUS traits. This species has played a crucial role in sericulture and the cultivation of mulberry trees, valued for both their nutritious and flavourful fruits.
Materials and methodology
Study area
The present study was conducted during 2019–2022 in the Department of Tree Improvement and Genetic Resources, College of Forestry, Dr Yashwant Singh Parmar University of Horticulture and Forestry, Nauni, Solan (India). The study site is situated at an altitude of 1250 m above mean sea level (Fig. 1). It lies at a latitude of 30°51′N and a longitude of 76°11′E. The region experiences subtropical climate with moderately hot summers and cold winters. Maximum temperature often exceeds 35°C in May–June and minimum temperature drops to as low as 2°C in January. Frost occurrence in winter is quite common. Mean annual rainfall of the site is 1000–1300 mm/year, with major during monsoon rainfall. Winter rains due to western disturbances are not uncommon. A clonal seed orchard of M. alba L. was established in January 2008 (Fig. 2) (online Supplementary Fig. S1). This orchard comprises 27 clones each with three replications at the spacing of 2 m × 2 m. The clones were collected from various states of north India including Uttarakhand and union territory of Jammu and Kashmir. Out of 27 clones, nine phenotypic superior parents were selected for the study. The hybridization experiment was conducted with selected parents and 20 crosses were obtained. The plant material (flowering branches) of selected five females and four male genotypes were used for hybridization with a total of nine parents (Table 1).
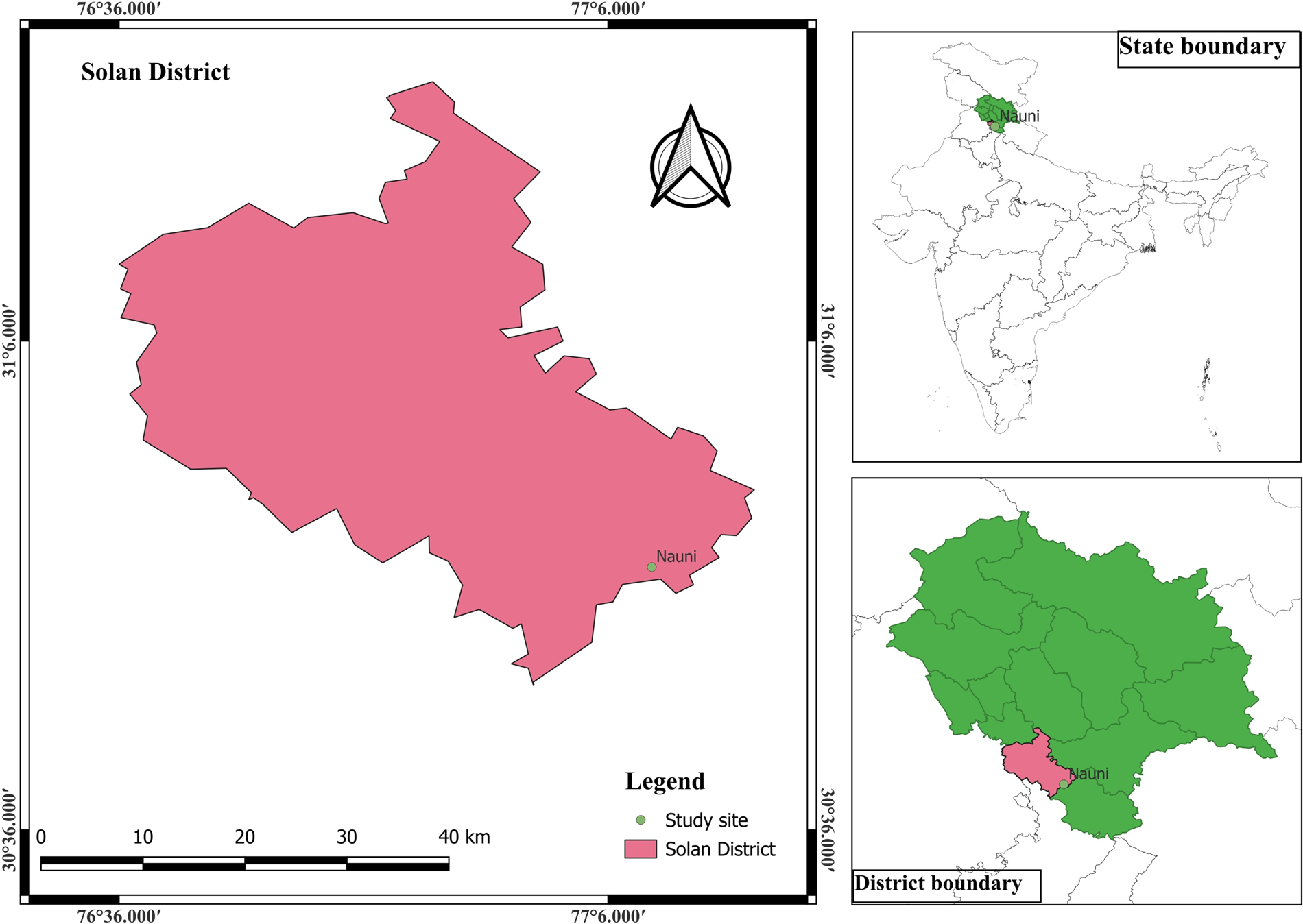
Figure 1. Map of study area.

Figure 2. View of established clonal orchard of M. alba L.
Table 1. Source of parent material
Qualitative characteristics
The data outline various qualitative characteristics observed in the study of sexual forms and stigma types. Initially, the plants were assessed for their sex expression, revealing a diverse range of sexual forms on day 20, including gynoecious, androecious, bisexual, andromonoecious, gynomonoecious and androgynomonoecious. Concurrently, the stigma's nature was examined, identifying pubescent and papillate types. Additionally, the stigma type was categorized into four distinct forms: erect, spreading, divaricate and twisted, all observed on day 20. Collectively, these observations highlight the variability in sexual forms and stigma characteristics across different developmental stages.
Pseudo-qualitative characteristics
A comprehensive morphological study was conducted, focusing on various pseudo-qualitative characteristics. At 60 d, observations were made for plant growth habit (erect, semi-erect, spreading, drooping), shoot type (straight, slightly curved, curved), leaf shape (cordate, wide ovate, ovate, narrow ovate, lanceolate), leaf colour (light green, green, dark green), leaf margin (dentate, serrate), leaf base (acute, truncate, cordate, lobate), leaf apex (acute, acuminate, caudate, obtuse) and leaf type (unlobed, lobed, mixed type). Additionally, at 90 d, mature shoot colour (yellow-green group 147, greyed-green group 195, grey-brown group 199, brown group N200, grey group 201) and leaf hairiness (glabrous, sparsely hairy, hairy) were assessed. Finally, at 40 d, mature fruit colour was recorded, encompassing a wide range of hues, including black group 203, greyed-orange group 172, purple group 76, yellow-green group 145, white and green. This detailed analysis provides valuable insights into the morphological diversity of the plants under study.
Quantitative characteristics
A comprehensive analysis of quantitative morphological traits was conducted. At 20 d mature inflorescence length was recorded (short [<2 cm], medium [2–4 cm], long [>4 cm]). At 40 d, mature fruit length (short [<2 cm], medium [2–4 cm], long [4–8 cm], very long [>8 cm]) and width (narrow [<1 cm], medium [1–1.5 cm], broad [>1.5 cm]) were measured. The angle of leaf at 45 d (acute, horizontal and obtuse) was recorded. Additionally, at 60 d, shoot thickness (thin [<1 cm], medium [1–1.5 cm], thick [>1.5 cm]), petiole length (short [<3 cm], medium [3–5 cm], long [>5 cm]), petiole thickness (thin [<0.2 cm], medium [0.2–0.4 cm], thick [>0.4 cm]), leaf lamina length (short [<10 cm], medium [10–20 cm], long [>20 cm]) and leaf lamina width (narrow [<10 cm], medium [10–15 cm], broad [>15 cm]) were assessed. Finally, at 90 d, leaf size (small [<200 cm2], medium [200–400 and <200 cm2], large [>400 and <200 cm2]) and inter-nodal distance (short [<3 cm], medium [3–6 cm], long [>6 cm]) were recorded. This detailed analysis provides valuable insights into the morphological variability of the plants under study. Online Supplementary Fig. S2 shows the variation in fruit length and width.
Results
Characterization of parents
To enhance understanding of the various traits in F1 crosses, a graphical representation is provided in online Supplementary Fig. S3(a)–(e).
Qualitative characteristics
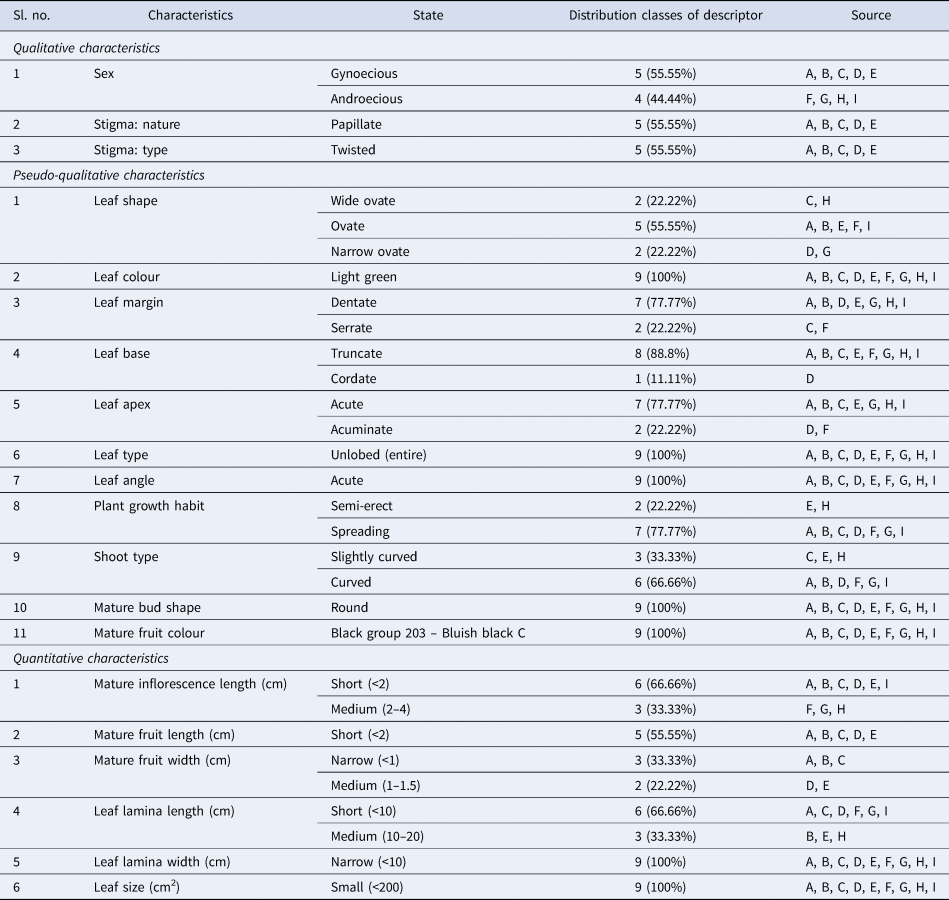
Parental characterization often begins with qualitative traits, which are visually observable characteristics. Visual observation was used to ascertain the sex, stigma type and stigma nature of a subset of clones. Table 2 lists the parents/clones including five gynoecious and four androecious. Among female parents, two types of stigmas were observed: twisted and papillate (Fig. 3). These traits are essential for identifying unique genetic markers within each parent, which aids in selecting suitable breeding pairs.
Table 2. Characterization of parents based on qualitative, pseudo-qualitative and quantitative characteristics
S30 = A, Phillipines = B, K2M5 = C, S41 = D, M5 = E, China white = F, S1531 = G, S799 = H and S1307 = I.
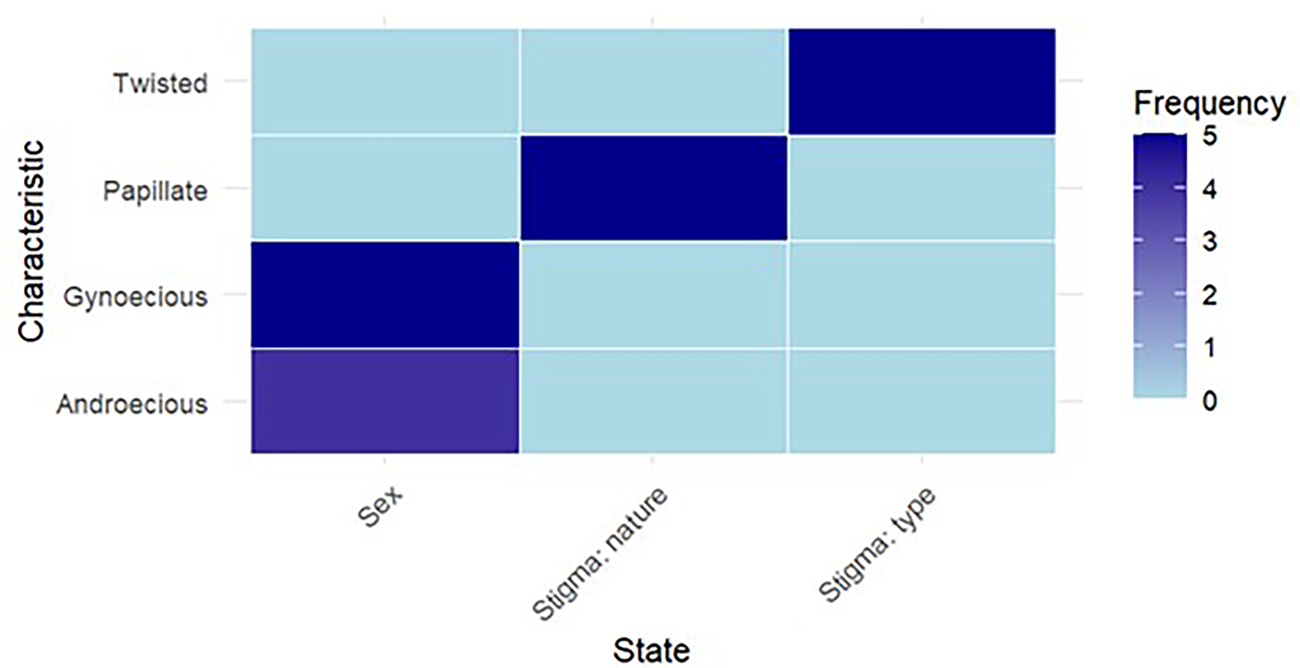
Figure 3. Characterization of parents based on qualitative characteristics.
Pseudo-qualitative characteristics for parents
These characteristics, which include plant growth style (erect, semi-erect, spreading), shoot type, leaf colour and margin type, offer more specific differentiations that integrate qualitative observation with quantitative evaluation (Fig. 4). By assessing these characteristics, scientists can learn more about the growth patterns and adaptability of each parent, which is essential for forecasting how well hybrids would thrive in different settings. From Table 2 it appears that clones K2M5 and S799 have wide ovate; clones S30, K2M5, M5, China white and S1307 have ovate and clones S41 and S1531 have narrow ovate leaf shape. Light green colour is predominant among clones. Clones S30, Phillipines, S41, M5, S1531, S799 and S1307 have the maximum dentate leaf margin, followed by serrate leaf margin in the remaining clones. All clones except for S41 and China white have the maximum acute leaf apex. All clones have spreading plant growth habits, except for M5 and S799, which have semi-erect growth habits. The mature buds are round, while mature fruits have a bluish black colour. Figures 5 and 6 elaborate the characterization of leaf and fruit in the present study.
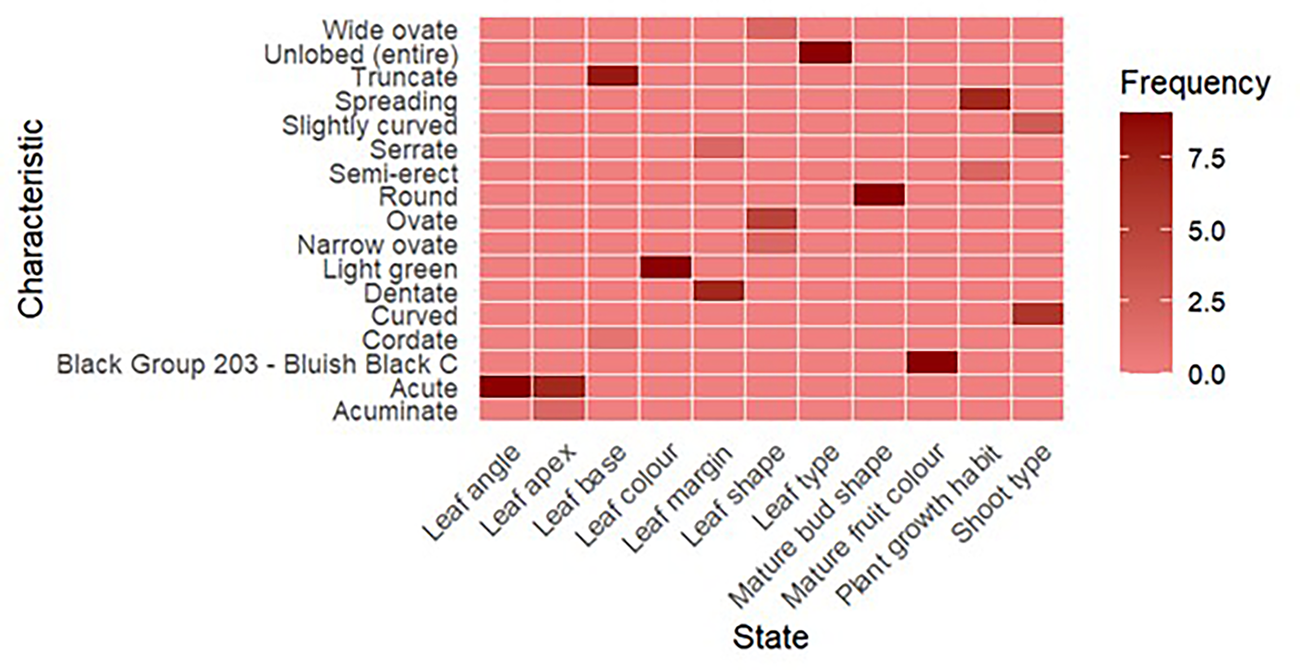
Figure 4. Characterization of parents based on pseudo-qualitative characteristics.

Figure 5. Characterization of pseudo-qualitative parameters (leaf).

Figure 6. Characterization of various pseudo-qualitative characters.
Quantitative characteristics for parents
These measurements provide accurate, numerical information that is useful for evaluating parent plant production, vigour and growth rates. For example, hybrids may have greater yield and structural resilience if their parents have longer internodes and thicker shoots. Table 2 shows that clones S30, Phillipines, K2M5, S41, M5 and S1307 have short mature inflorescence lengths, while China white, S1531 and S799 have medium lengths. All fruit-bearing female clones have short fruit lengths and narrow widths. Clones S30, K2M5, S41, China white, S1531 and S1307 have short leaf lamina lengths, while clones Phillipines, M5 and S799 have medium lengths and narrow widths. Leaf size is small (<200 cm) (Fig. 7).
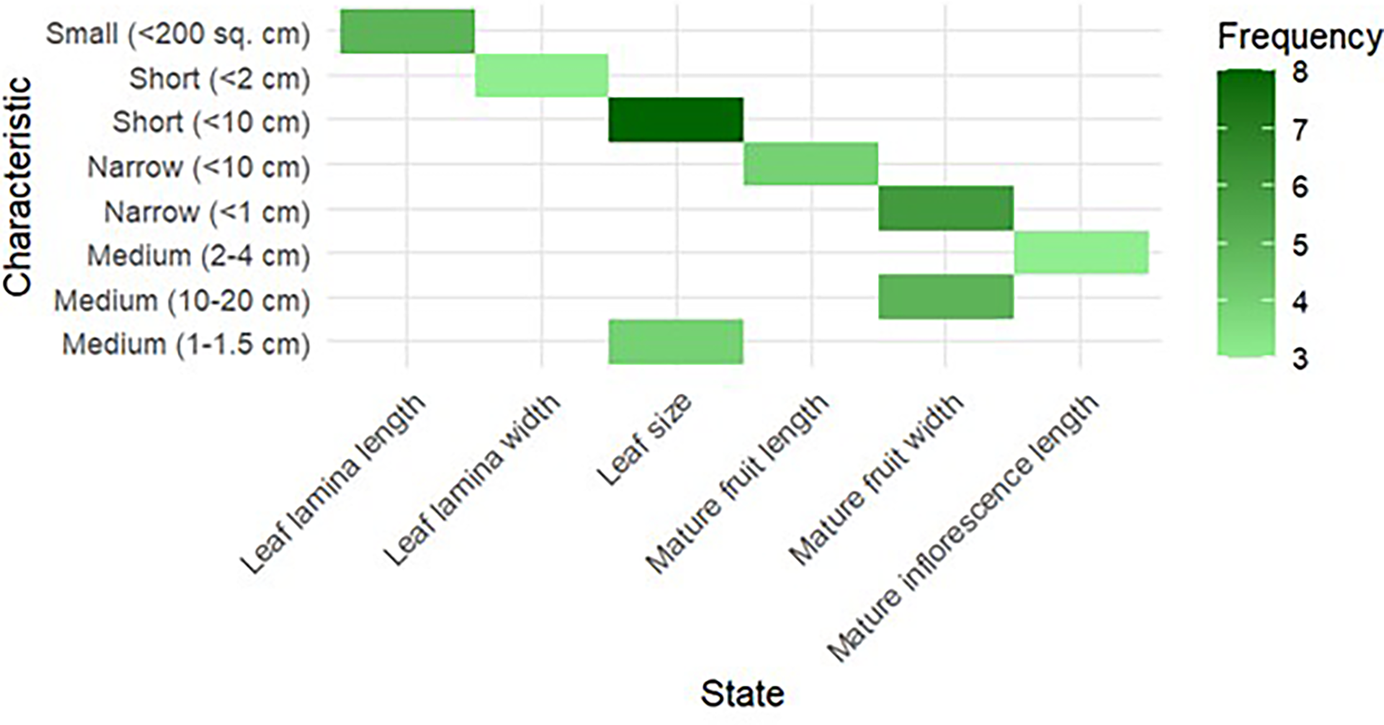
Figure 7. Characterization of parents based on quantitative characteristics.
Characterization of crosses
To enhance understanding of the various traits in F1 crosses, a graphical representation is provided in online Supplementary Fig. S4(a)–(c).
Pseudo-qualitative characteristics for crosses
These characteristics aid in hybrid differentiation and reveal which paternal qualities are more prominently shown. For example, specific leaf forms and development patterns may indicate enhanced environmental adaptation, which is advantageous for uses in sericulture and agroforestry. Table 3 shows that 14 crosses had ovate leaf shapes, three had wide-ovate and three had narrow-ovate shapes. Sixteen had green leaves, while four had light green. Out of 20 crosses, 17 had dentate leaf margins, while three had serrate margins. Among all 16 had truncate leaf bases, while four had cordate bases. Sixteen had an acute leaf apex, while four had an acuminate leaf apex. All 20 crosses had unlobed leaf types and acute leaf angles (Fig. 8).
Table 3. Characterization of crosses/F1 progenies on the basis of pseudo-qualitative characteristics
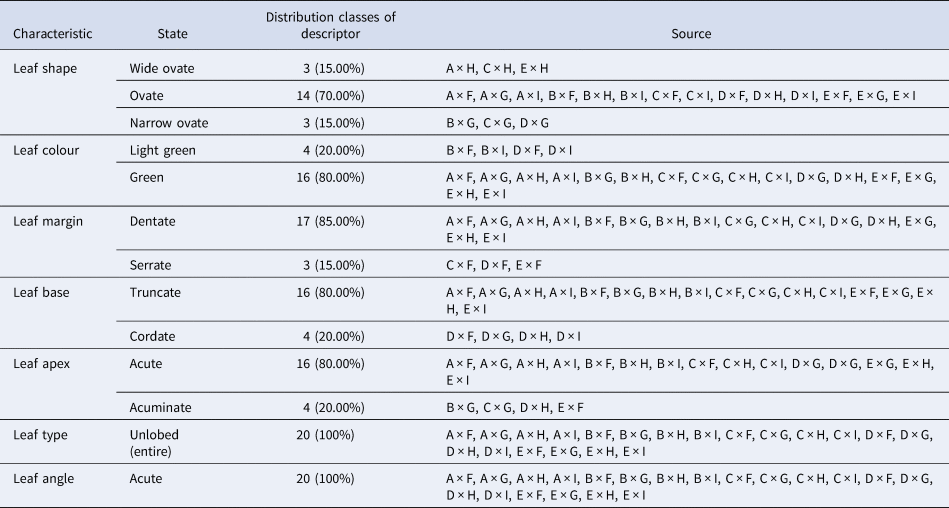
S30 = A, Phillipines = B, K2M5 = C, S41 = D, M5 = E, China white = F, S1531 = G, S799 = H and S1307 = I.
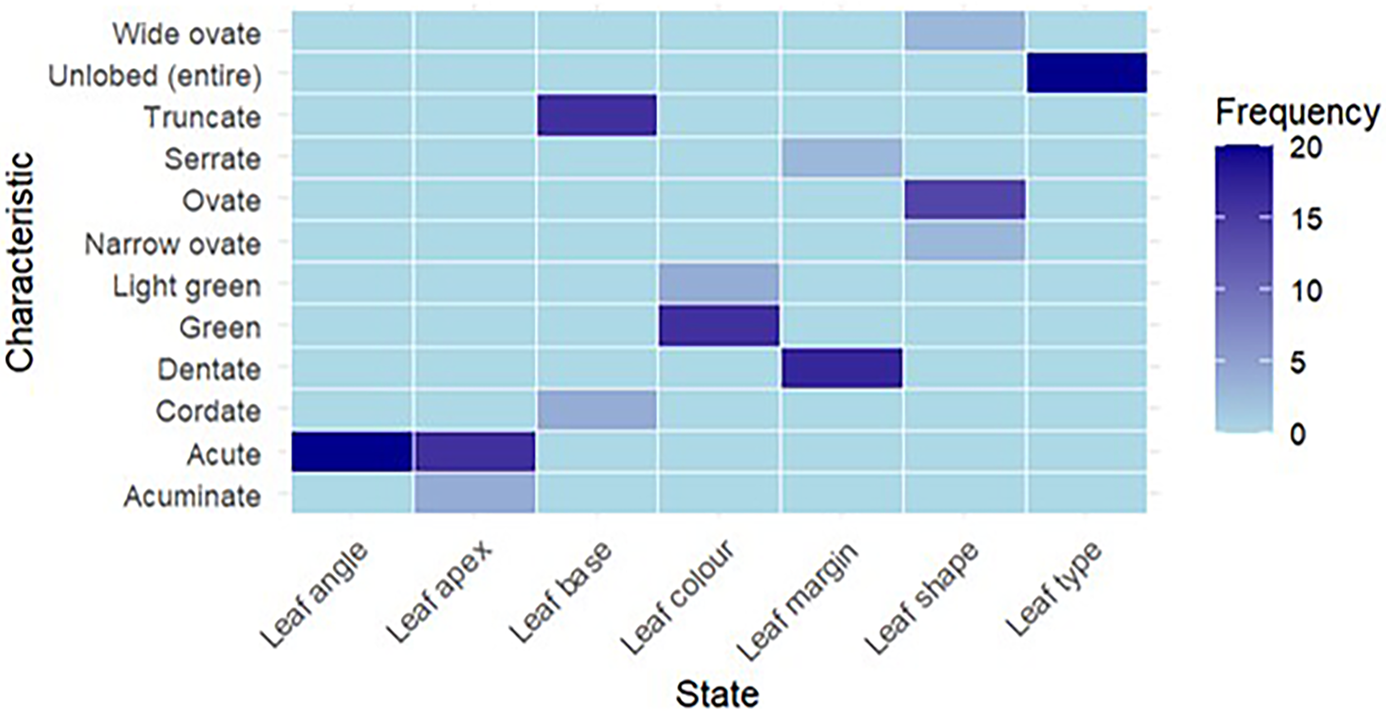
Figure 8. Characterization of crosses/F1 progenies based on pseudo-qualitative characteristics.
Quantitative characteristics for crosses
These traits are essential for understanding the growth potential and productivity of each hybrid. For example, crosses with greater shoot thickness and larger leaf area may indicate a higher yield potential, making them suitable candidates for high-yield cultivation in sericulture. Table 4 reveals that all the 20 crosses had thin shoot thickness, medium internodal distance, short petiole length and thin petiole thickness. It also reveals that seven crosses have medium leaf lamina length (10–20 cm), 13 have short lamina length (<10 cm) and all 20 crosses have small leaf sizes (<200 cm2) and thin lamina widths (<10 cm) (Fig. 9).
Table 4. Characterization of crosses/F1 progenies on the basis of quantitative characteristics
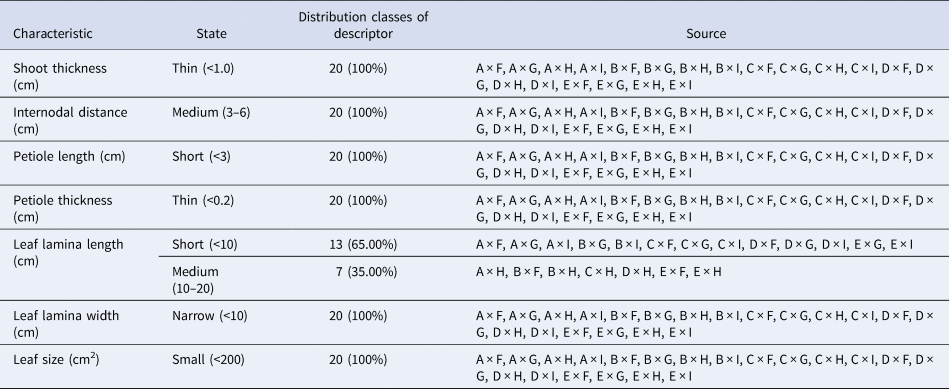
S30 = A, Phillipines = B, K2M5 = C, S41 = D, M5 = E, China white = F, S1531 = G, S799 = H and S1307 = I.
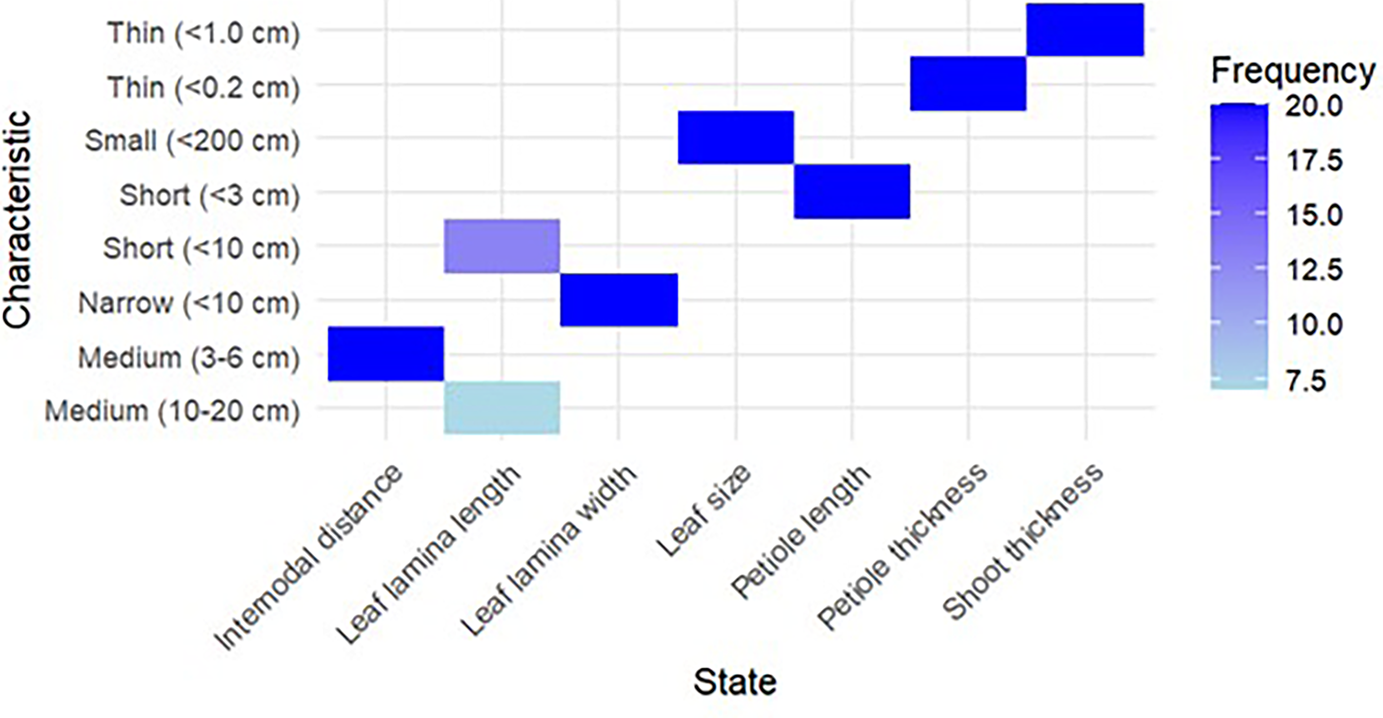
Figure 9. Characterization of crosses/F1 progenies based on quantitative characteristics.
Discussion
This study effectively illustrates how to use DUS testing to distinguish between distinct cultivars of M. alba based on morphological traits, which are essential for plant variety registration and breeding initiatives (Chakrabarty and Choudhury, Reference Chakrabarty and Choudhury2019). The study identifies key elements that support the uniqueness, consistency and stability of M. alba genotypes by comparing qualitative and quantitative characteristics across different clones. Assessing qualitative characteristics including fruit colour, apex, border and leaf form offers a fundamental way to differentiate M. alba clones. Their function in guaranteeing the genetic purity and identification of various cultivars is highlighted by consistent observations in characteristics such as leaf colour and shape across clones. In this study, superior features that are anticipated to improve hybrid vigour, and productivity in future generations are identified by describing parents. To ensure that valuable qualities are handed down and conserved, breeding can favour certain parents that exhibit desirable traits, such as optimal leaf lamina length or high fruit yield. Based on a statistical assessment, parents with desired traits are selected and control hybridization is affected.
Notably, as these traits were seen in most clones, the preponderance of the dentate border and sharp leaf apex indicates genetic markers of relevance among the examined germplasms. Despite environmental impacts, the variance in shoot thickness, leaf lamina length and petiole thickness supports the use of quantitative features to identify and classify genotypes of M. alba. Similarly, Suresh et al. (Reference Suresh, Ghosh, Chakravarty and Trivedy2018) conducted a multivariate analysis of indigenous and exotic mulberry germplasm and identified diverse genotypes as promising donors in improving foliage production. According to the study's findings, breeders that prioritize quality and production can use these quantitative features as trustworthy indications. In sericulture, where mulberry leaves are the main food source for silkworms, characteristics like leaf size and leaf lamina length are very important because they directly affect leaf productivity.
With observed variances among F1 progenies indicating the possibility of choosing hybrids with desired agronomic traits, the study also demonstrates the importance of hybridization in boosting genetic variety. Breeding strategies aiming at maximizing leaf output, and other desirable qualities in M. alba can benefit from this genetic variety, which is emphasized by differences in leaf size and form between crossings. Similarly, seedlings raised in a nursery are transplanted to the field in progeny row trials for initial screening based on selected traits like growth, branching, leaf texture and disease susceptibility. Since almost all mulberry accessions are highly heterozygous and have a long gestation period, traditional breeding methodologies mostly rely on the production of F1 hybrids (Das, Reference Das1984). The possibility of DUS testing to support the defence of farmer and breeder rights under the PPV&FR Act further emphasizes its importance. DUS testing makes it easier to register and conserve genetic resources by defining quantifiable, unambiguous requirements for every cultivar. This promotes sustainable agriculture and increases the value of M. alba in agroforestry and sericulture.
Thus, the study's findings are in line with the necessity of cultivar protection, guaranteeing that growers and breeders will continue to have access to high-quality plant materials and take use of the intellectual property protection that variety registration provides. The study's overall findings support the significance of DUS characterization in plant breeding, particularly for valuable species like M. alba. This study promotes more effective breeding methods, preserves genetic variety and permits the sustained development of M. alba for agricultural and industrial applications by offering a framework for differentiating cultivars according to both qualitative and quantitative traits. Likewise, Tikader and Kamble (Reference Tikader and Kamble2008) have studied the variation in indigenous mulberry germplasm on growth and yield and identified better performing accessions. This draft discusses the wider agricultural importance of DUS characterization in M. alba, highlights the results' implications for breeding and cultivar protection and summarizes the findings.
Conclusion
This study offers precise standards for distinguishing many M. alba varieties. This makes each cultivar distinct and recognizable by aiding in the precise classification and distinction of different cultivars. By adhering to DUS guidelines, breeders and growers can maintain the quality standards of M. alba varieties. This includes characteristics such as leaf shape, fruit colour, size and other agronomic traits that contribute to the overall quality of the plant. This kind of study ensures uniformity within a particular variety of M. alba. This means that plants within the same variety should exhibit consistent traits, making it easier for farmers to predict crop performance and manage cultivation practices effectively. Hybrids which have proved to be out yields for leaf characteristics such as S30 × S799, S30 × China white, Phillipines × China white, Phillipines × S799, K2M5 × S799, S41 × S799, M5 × China white and M5 × S799 can be registered under PPV&FR Act 2001, NBPGR, New Delhi and CSGRC, Hosur.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1479262125000085.
Acknowledgements
The authors are grateful to the Department of Tree Improvement and Genetic Resources, Dr Yashwant Singh Parmar University of Horticulture and Forestry, Nauni, Solan (Himachal Pradesh), India for providing the necessary facilities during the study.
Author contributions
Experimentation including conception and design: A. N. and S. T.; data collection and analysis: A. N. and R. T.; writing and original draft preparation: A. N.; final review and editing: A. N. and R. T. All authors have read and approved the final manuscript.
Funding statement
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests
None.















