General introduction to riboswitches
Riboswitches are RNA elements with a defined structure found in noncoding sections of genes that allow the direct control of gene expression by the binding of small molecules functionally related to the gene product. In most cases, this is a metabolite in the same (typically biosynthetic) pathway as an enzyme (or transporter) encoded by the gene that is controlled. Riboswitches allow the sensing of the concentration of a small molecule and switch the level of expression of the gene product at a particular threshold. Most riboswitches affect their genetic control either at the transcriptional level (where ligand binding can affect the relative stability of terminator or anti-terminator stem-loop structures for example) or at the translational level (where the RNA structure modulates the accessibility of the ribosome binding site). The riboswitches could operate either as an OFF or ON switch, increasing or decreasing the level of expression. For biosynthesis, expression needs to be turned off when the concentration of the metabolite has reached the required level. On the other hand, a transporter that is required to export a toxic metabolite (e.g., guanidine) would only be required when that compound has exceeded a predetermined level.
In principle, RNA folding might attain equilibrium with the ligand (thermodynamic control), or the ligand might bind folding intermediates during co-transcriptional folding (kinetic control). Early studies indicated that RNA folding led to a limited time during which ligand binding competed with continued transcription, that is, kinetic control (Wickiser et al., Reference Wickiser2005; Lemay et al., Reference Lemay2006), and this has been supported by more recent studies, at least in transcriptionally controlled riboswitches (Frieda and Block, Reference Frieda and Block2012; Widom et al., Reference Widom, Nedialkov, Rai, Hayes, Brooks, Artsimovitch and Walter2018; Hua et al., Reference Hua2020).
From the first discussions of riboswitches, the RNA was described in terms of two domains: the aptamer domain (that binds the ligand) and the expression domain that modulate gene expression, for example, by the formation of a transcriptional terminator. However, this may be more applicable to some riboswitches than others, and as we discuss below may not be appropriate for many translational riboswitches. The regulation of gene expression by riboswitches appears to be a largely prokaryotic phenomenon. Exceptions to this include the thiamine pyrophosphate (TPP) riboswitches found in fungi and plants (Winkler et al., Reference Winkler2002), where they control splicing (Cheah et al., Reference Cheah2007), and an archaeal fluoride riboswitch (Baker et al., Reference Baker2012). Perhaps other eukaryotic riboswitches await discovery.
There are over 55 riboswitch classes, sensing a diverse group of metabolites. These include coenzymes (such as SAM, TPP, NAD+, FMN); sugars (e.g., glucosamine-6-phosphate); elements of RNA (nucleobases, PRPP, etc.) and RNA derivatives (e.g., xanthine, pre-queuosine1); amino acids (e.g., glycine, glutamine); signaling molecules (e.g., cyclic AMP-GMP, cyclic diGMP, etc.); and even simple ions (cations like magnesium or anions like fluoride). Many of these have been discovered in the Breaker laboratory (Breaker, Reference Breaker2012; McCown et al., Reference Mccown2017; Kavita and Breaker, Reference Kavita and Breaker2023) using bioinformatics to identify structured inter-genic regions in RNA sequences. In many cases, a given ligand can be sensed by multiple riboswitches with different structures, modes of ligand binding, and, in some cases, different mechanisms of gene regulation. For example, there are six SAM-sensing riboswitches (seven if the SAM–S-adenosylhomocysteine [SAH] riboswitch is included).
Although according to their strict definition riboswitches are regulatory elements that respond to a small-molecule ligand, there are cis-acting elements that act in the same way in response to larger molecules, such as tRNA (Henkin et al., Reference Henkin1992; Zhang and Ferre-D’amare, Reference Zhang and Ferre-D’amare2013). The synthesis of the k-turn-binding protein L7Ae is subject to autoregulation by the binding of archaeal L7Ae to an element located in the 5′-UTR of its mRNA (Daume et al., Reference Daume2017), so stabilizing a k-turn-containing stem-loop and thereby preventing access to the ribosome binding site (Huang et al., Reference Huang2019a). In all respects other than the size of the ligand, this operates as a riboswitch.
We do not intend to survey the occurrence and function of riboswitches comprehensively here; there are several excellent reviews that do that (Kavita and Breaker, Reference Kavita and Breaker2023; Olenginski et al., Reference Olenginski2024). This is also surveyed in the web-based database https://riboswitch.ribocentre.org/riboswitches/ (Bu et al., Reference Bu2024). Rather, this review is written from our own structural perspective, and focuses on what can be learned from the large numbers of structures of riboswitches, as a valuable database of RNA structure and ligand binding to RNA.
Architectural principals of riboswitch structure
Riboswitches are autonomously folding sections of RNA that create small-molecule ligand binding sites. The structures of the majority of riboswitches have been determined at good resolution. The ensemble of these structures provides a rich source of structural data on RNA architecture.
Global structural architecture of riboswitches
In general, the majority of riboswitches are relatively small (typically between 50 and 80 nucleotides in length), and their architectures are often based around a single structural feature. These can be classified as helical junctions, pseudoknots (PKs), and loop–loop interactions. Moreover, these elements usually create the binding sites for the riboswitch ligands. In general, the helical junctions determine the trajectory of the helical arms, and contacts between them may be facilitated.
Helical junctions
Helical junctions are the association of multiple helical sections, connected through the exchange of covalently continuous strands, and can be classified according to a formal nomenclature (Lilley et al., Reference Lilley1995) (Figure 1). The most common junctions have three or four helical arms. These can be perfectly base paired (e.g., a 4H four-way junction has no unpaired nucleotides at the point of strand exchange between the helices), or they can have additional nucleotides on one or more sections that connect the helical arms (Figure 1a). There is a strong tendency for helical arms to undergo pairwise coaxial stacking, and junctions generally fold into a structure that maximizes stacking. The conformation of any junction can be influenced by additional tertiary contacts between remote elements within the helical arms; this is a very common occurrence in junctions found in riboswitches.
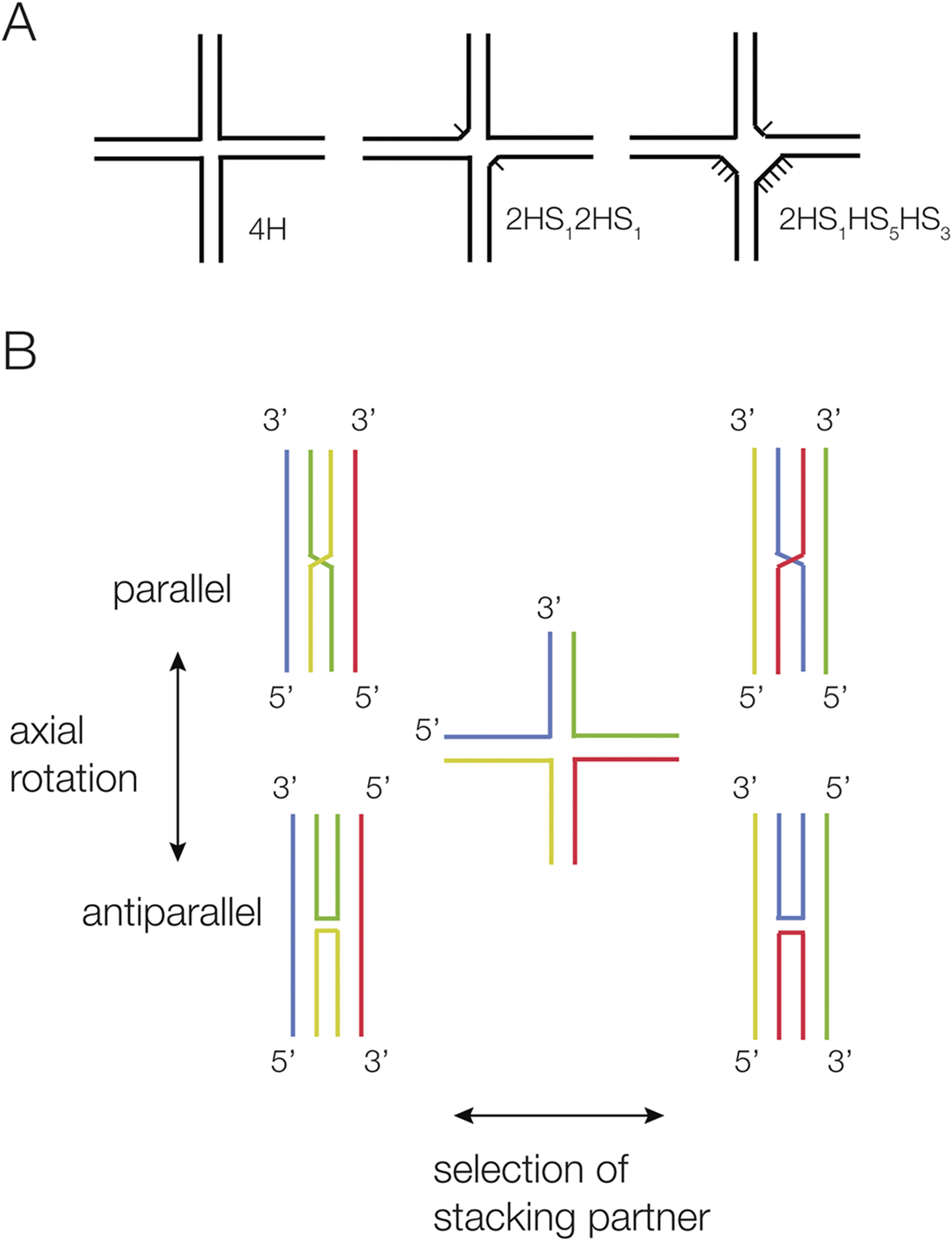
Figure 1. Scheme showing the nomenclature for four-way helical junctions, and their stacking conformations. (A) Junctions can vary according to the number of unpaired nucleotides between helical sections. A 4H junction has no unpaired nucleotides, whereas the 2HS12HS1 junction has two one-nucleotide single-strand sections diametrically opposed. These junctions are named according to the IUPAC nomenclature (Lilley et al., Reference Lilley1995). (B) Two conformers are possible when four-way junctions undergo pairwise coaxial stacking (left and right). The structures can rotate about their centers, forming parallel or antiparallel structures in the extreme (upper and lower). In the parallel structures, the continuous strands run in the same direction, and the exchanging strands cross.
Four-way helical junctions
A four-way junction with pairwise coaxial stacking of helical arms can adopt two possible conformers depending on the selection of stacking partners, and the coaxial pairs can adopt either parallel or antiparallel conformations (Figure 1b). Perfect four-way junctions in RNA are more structurally polymorphic than their DNA equivalents (Duckett et al., Reference Duckett1995; Hohng et al., Reference Hohng2004). DNA 4H junctions always adopt an antiparallel geometry (Duckett et al., Reference Duckett1988; Murchie et al., Reference Murchie, Clegg, Von Kitzing, Duckett, Diekmann and Lilley1989), and in the absence of branch migration, the only dynamic mode is the exchange of stacking conformers (McKinney et al., Reference Mckinney2003). By contrast, RNA 4H junctions can adopt both parallel and antiparallel conformations (Duckett et al., Reference Duckett1995), with the parallel geometry being more stable in most cases. Single-molecule FRET analysis has shown that a given junction can be quite dynamic in free solution, exchanging between parallel and antiparallel conformations as well as between stacking conformers (Hohng et al., Reference Hohng2004). If elements within the helical arms that are separated from the junction can interact (e.g., a loop–receptor interaction), this will naturally influence the angle between the axes between the coaxial pairs, and thus the geometry of the junction.
The NiCo (Furukawa et al., Reference Furukawa2015), yybP-ykoY manganese (Dambach et al., Reference Dambach2015), and PRPP (Nelson et al., Reference Nelson2017) riboswitches each contain a parallel four-way junction. The manganese riboswitch contains a perfect 4H junction (no unpaired additional nucleotides), with near-perfect pairwise coaxial stacking between the helical arms and within 20° of having parallel axes (Price et al., Reference Price2015) (Figure 2). Two of the helical arms interact via a loop extended from one of them, and this likely is responsible for the near side-by-side geometry of the helical arms. In contrast to the manganese riboswitch junction, the four-way helical junctions of the NiCo (Furukawa et al., Reference Furukawa2015) and PRPP riboswitches are not 4H junctions, but have a number of nucleotides within the sections that connect the helical arms. Despite this, the overall geometry of the junctions remains quite similar, and both adopt a parallel geometry. The NiCo riboswitch four-way junction (Furukawa et al., Reference Furukawa2015) has one extra nucleotide at the point of strand exchange on each of the exchanging strands. The junction is parallel, and the pairwise coaxial stacking across the exchange point is good, with the nucleobases spaced by ~3 Å for both. The PRPP riboswitch junction has more unpaired nucleotides, making a 2HS1HS5HS3 junction (Knappenberger et al., Reference Knappenberger2018). Two of the helices are perfectly coaxially stacked, with full base pairing and with the nucleobases spaced by 3.3 Å. In contrast, the other pair is separated by what is effectively an internal loop (five and three nucleotides on the two strands) and so that the helices are not truly coaxial. This loop forms the ligand binding site. The kinking directs one of the helices so that its terminal loop forms a terminal loop–internal loop interaction with the longer helix of the well-stacked pair.

Figure 2. The four-way RNA junction of the magnesium riboswitch (Price et al., Reference Price2015). This is a perfect 4H junction that adopts a parallel conformation. The structure is shown as a parallel-eye stereoscopic view (PDB ID 4YLI).
Three-way helical junctions
Three-way junctions are the most common helical junctions found in riboswitches, and have been analyzed in terms of their preferred structural conformers (Lescoute and Westhof, Reference Lescoute and Westhof2006; Ouellet et al., Reference Ouellet2010). The majority of three-way RNA junctions have additional nucleotides in the sections linking the helices; 3H junctions are very rare in RNA. As with four-way junctions, there is a strong tendency for three-way junctions to undergo pairwise coaxial stacking of helices, but with an odd number of helical arms, only two of the three can undergo coaxial stacking, and in general, a single stacking conformer is observed as the most stable. This is normally one that minimizes the number of unpaired nucleotides on the connecting strand. There are then two types of connection possible, according to whether the longest connecting section passes 5′ to 3′ from the coaxially stacked helices into the third helix (L ex) or from the third helix into the stacked pair of helices (L en) (Ouellet et al., Reference Ouellet2010) (Figure 3).
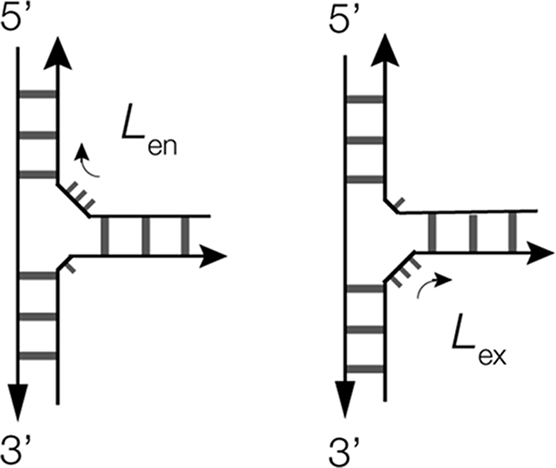
Figure 3. Scheme showing the possible conformations of three-way RNA junctions. The most stable conformer is generally the one that minimizes the number of unpaired nucleotides on the connecting strand. Two conformations are possible, that differ in the direction of the longest connecting section. We have defined these as L ex or L en, depending on whether the longest connecting section passes from the coaxially stacked helices into or out of the third helix (L ex), the third helix, respectively (Ouellet et al., Reference Ouellet2010).
Three-way junctions form the central structural motif of numerous riboswitches, including SAM-III (Lu et al., Reference Lu2008), tetrahydrofolate (THF) (Trausch et al., Reference Trausch2011), TPP (Thore et al., Reference Thore2006), 2′-deoxyguanosine-I (2′-dG-I) (Pikovskaya et al., Reference Pikovskaya2011), 3′,3’-cGAMP (Ren et al., Reference Ren2015a), magnesium ion (Ramesh et al., Reference Ramesh2011), glutamine-I (Ren et al., Reference Ren2015b), glycine (Huang et al., Reference Huang2010), adenine (Serganov et al., Reference Serganov2004), and guanine (Batey et al., Reference Batey2004) riboswitches (Figure 4). While most exhibit coaxial pairwise helical stacking, some do not, such as the three-way junctions found in the SAM-III and 3′,3′-cGMP riboswitch. In the majority of three-way junctions, the additional nucleotides linking the helices make specific interactions within the core of the junction, such as a U:G.A triple in the THF riboswitch (Trausch et al., Reference Trausch2011). Frequently base stacking is preserved within the formally single-stranded regions, helping to maintain coaxial alignment between the helical arms. In many riboswitches, three-way junctions, the longest linking region (typically ≥5 nucleotides) forms a distinct turn, behaving as a pseudo-forth helical arm. In these cases (both L ex and L en conformations), there is a sharp turn close to the 5′ end of the single-stranded region, after which the nucleotides at the 3′ end are mutually stacked (Figure 5). Good examples are found in the glycine (Huang et al., Reference Huang2010), THF (Trausch et al., Reference Trausch2011), and 2′-dG-I (Pikovskaya et al., Reference Pikovskaya2011) riboswitches. The most extreme versions of this conformation are found in the adenine (Serganov et al., Reference Serganov2004) and guanine (Batey et al., Reference Batey2004) riboswitches, where the linking section forms a complete turn terminated by a Watson–Crick G:C base pair (Figure 4c). Thus, these junctions are intermediate between three- and four-way helical junctions. The magnesium ion riboswitch contains two three-way junctions, where the third helix of the 5′ junction branches into the second junction, a 2HS2HS5 junction that is in the L ex conformation.
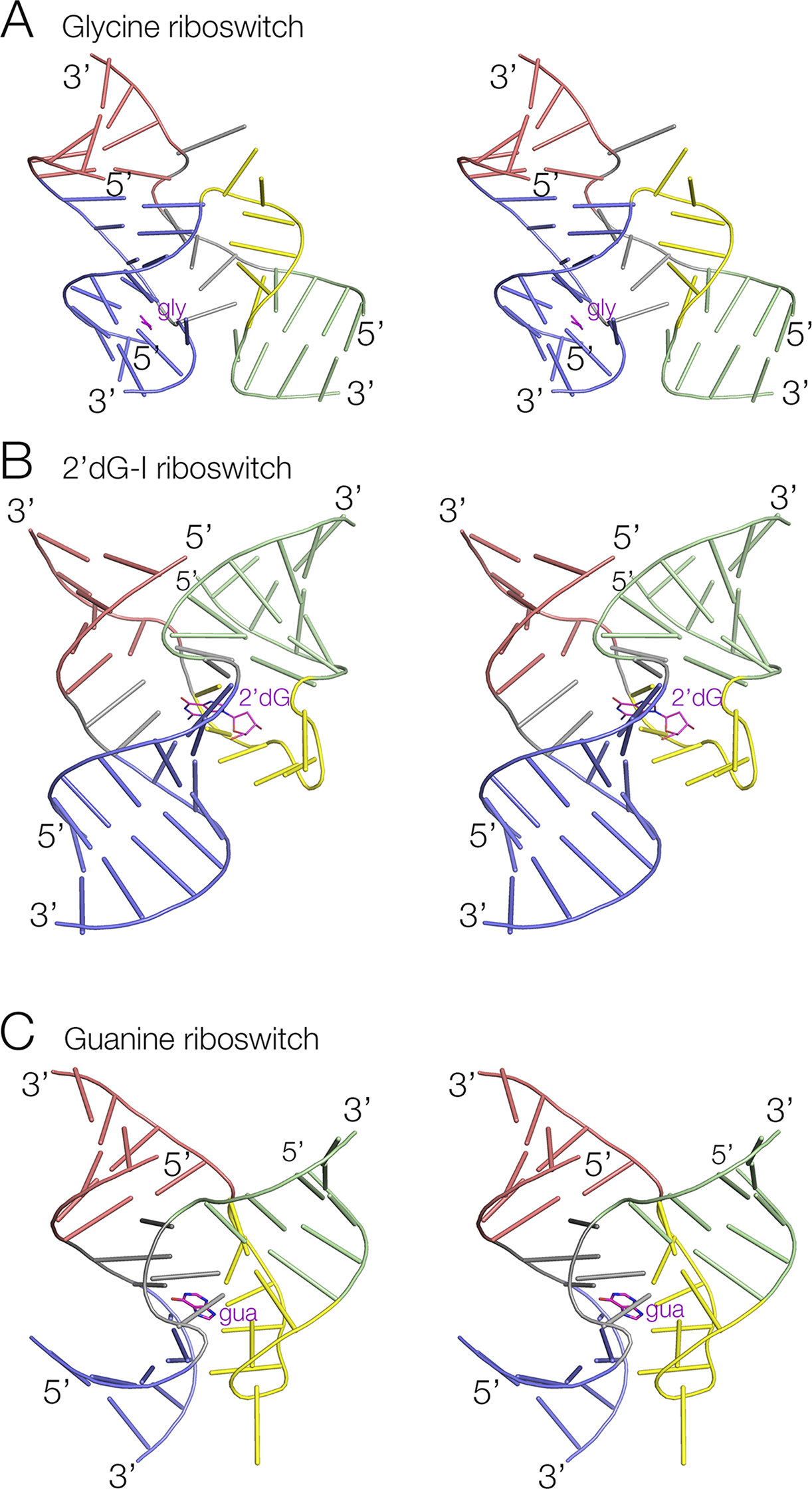
Figure 4. Three examples of three-way RNA junctions found in riboswitches. Parallel-eye stereoscopic views of the junctions found in (A) The glycine riboswitch (Huang et al., Reference Huang2010) (PDB ID 3OWW). (B) The 2′-deoxyguanine-I riboswitch (Pikovskaya et al., Reference Pikovskaya2011) (PDB ID 2SKI). (C) The guanine riboswitch (Batey et al., Reference Batey2004) (PDB ID 4FE5). In each case, the long unpaired loop region of RNA is colored yellow. The ligands are colored magenta and named gly, 2′dG, and gua, respectively.
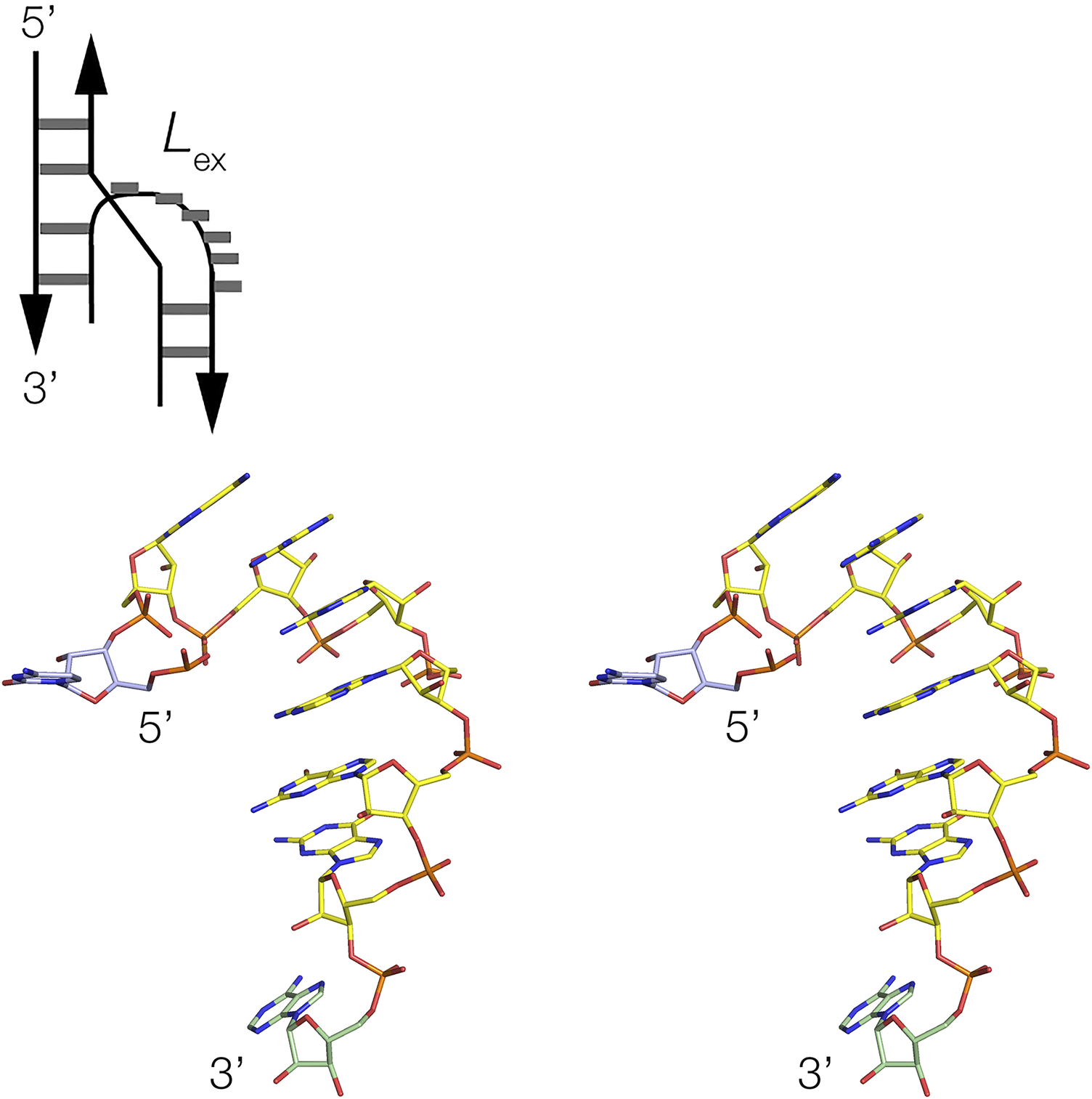
Figure 5. The unpaired loop of the glycine riboswitch (Huang et al., Reference Huang2010) three-way junction is shown schematically (top) and as a parallel-eye stereoscopic view (bottom). This is a detail taken from the three-way junction shown in Figure 4a (PDB ID 3OWW).
The three-way junctions of the TPP riboswitches (Serganov et al., Reference Serganov2006; Thore et al., Reference Thore2006) adopt a distinct conformation termed a k-junction, which is a combination of a three-way junction and a k-turn (see Section entitled “k-turns and k-junctions”) (Wang et al., Reference Wang2014; Li et al., Reference Li2023). The k-junction has all the features of a k-turn (Klein et al., Reference Klein2001; Goody et al., Reference Goody2004; Huang and Lilley, Reference Huang and Lilley2016), including the tandem sheared G•A base pairs, the cross-strand hydrogen bonds, and the bulge, but with an additional helix (thus creating a three-way junction) where the two helical arms of the k-turn connect on the non-bulged strand (Figure 6). Upon folding by addition of metal ions, the k-junction, like the k-turn, introduces a sharp kink into the C and NC helices, with an included angle of close to 50° (Li et al., Reference Li2023). In the Arabidopsis and Escherichia coli TPP riboswitches (Serganov et al., Reference Serganov2006; Thore et al., Reference Thore2006), the ligand binds into receptors formed between these helices, with a turn of helix remote from the junction. Although k-junctions were first identified in TPP riboswitches, they occur more widely and have been found in ribosomal RNA (Wang et al., Reference Wang2014) and in RNA sequences of unknown function (Li et al., Reference Li2023).
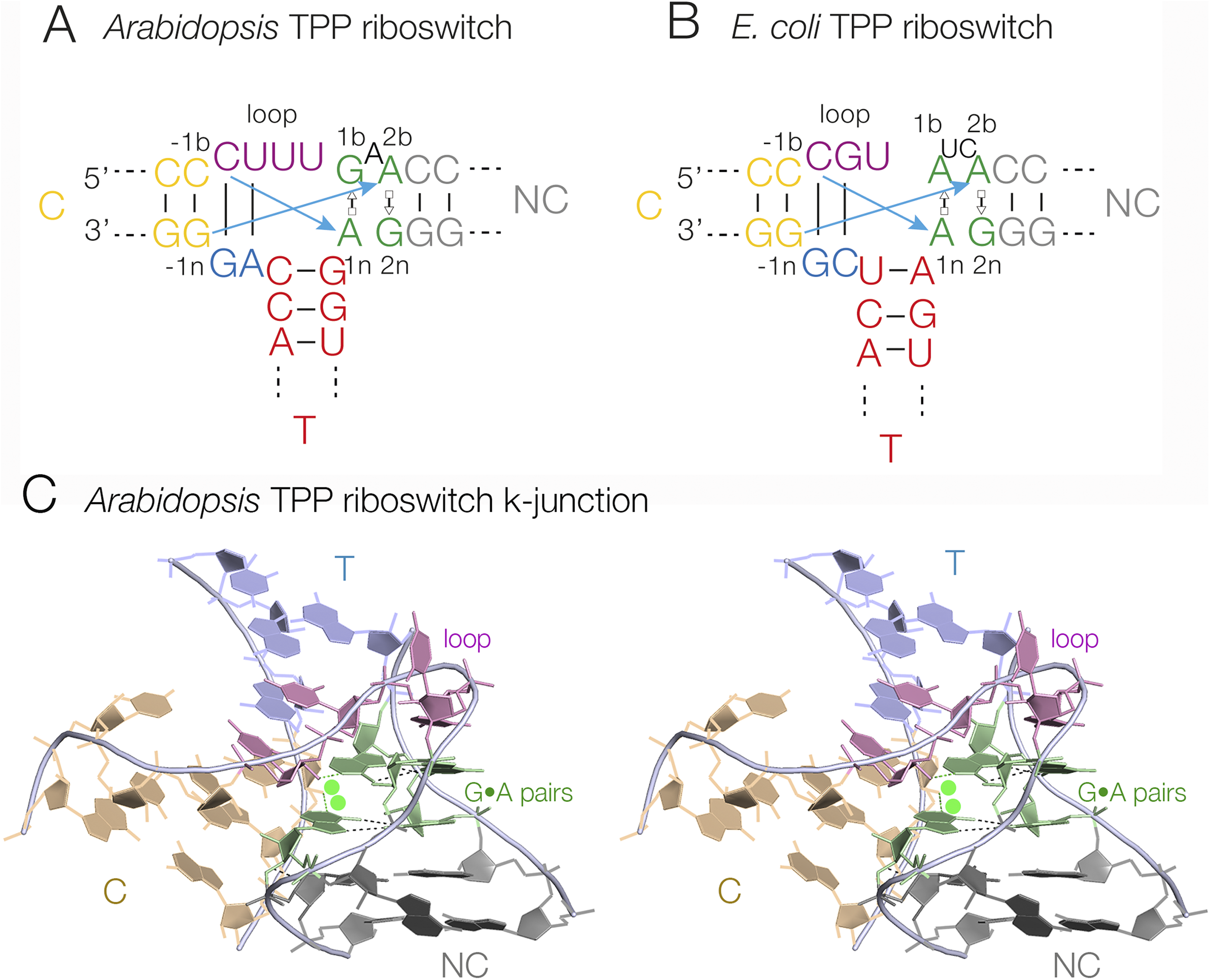
Figure 6. k-Junctions found in TPP riboswitches. (A) The sequence of the Arabidopsis thaliana TPP riboswitch. (B) The sequence of the E. coli TPP riboswitch. The standard cross-strand k-turn hydrogen bonds are indicated by the cyan arrows. (C) The crystal structure of the A. thaliana TPP riboswitch (Thore et al., Reference Thore2006) k-junction shown in parallel-eye stereoscopic view (PDB ID 3D2G).
Higher order helical junctions
Helical junctions of a higher order than four are possible, although they would be expected to experience some steric clash. The structure of the lysine riboswitch (Grundy et al., Reference Grundy2003; Sudarsan et al., Reference Sudarsan2003) is based upon a five-way helical junction (Figure 7) (Garst et al., Reference Garst2008; Serganov et al., Reference Serganov2008). Formally, the junction is 3HS2HS1HS2. The red, light green, pink, and blue helices form an antiparallel four-way junction in which the red and light green helices are perfectly coaxial. The fifth helix (dark green) is inserted at the center of the continuous strand linking the blue and pink helices. This is difficult to depict clearly in a two-dimensional image, but becomes significantly clearer when viewed in three dimensions. Interestingly, the lysine ligand is bound in the core of the five-way junction. The red, blue, and dark green helices form a three-helix bundle and are held together by loop–loop and loop–receptor interactions remote from the junction.

Figure 7. A five-way junction found in the Thermotoga maritima lysine riboswitch (Garst et al., Reference Garst2008). (A) Schematic showing the connectivity of the five-way RNA junction. (B) Front and Conura side views of the structure of the five-way junction shown in parallel-eye stereoscopic view (PDB ID 3DIL).
Pseudoknot-containing structures
The major structural feature of a number of riboswitches is a PK, including the three PreQ1 riboswitches (Liberman et al., Reference Liberman2013; Connelly et al., Reference Connelly2019; Schroeder et al., Reference Schroeder2023), SAM–SAH (Huang et al., Reference Huang2020a), guanidine-III (Huang et al., Reference Huang2017b), NAD+-II (Peng et al., Reference Peng2023; Xu et al., Reference Xu2023), and GlmS (Cochrane et al., Reference Cochrane2007; Klein and Ferré-D’amaré, Reference Klein and Ferré-D’amaré2006) riboswitches. The simplest form of PK is where the terminal loop of a stem-loop structure forms a helix with a remote complementary strand. The standard H-type PK structure (Figure 8) includes two such interactions formed from two inverted repeat sequences, each forming stem-loops such that the loop of each contains one strand of the stem of the other. The remaining part of the loop then acts as a linker. In general, the linker aligns with the major groove of the helix with an open 3′ end, while the other linker aligns with the minor groove of the helix with an open 5′ end. The basic PK structure can then be elaborated by the inclusion of additional helices.
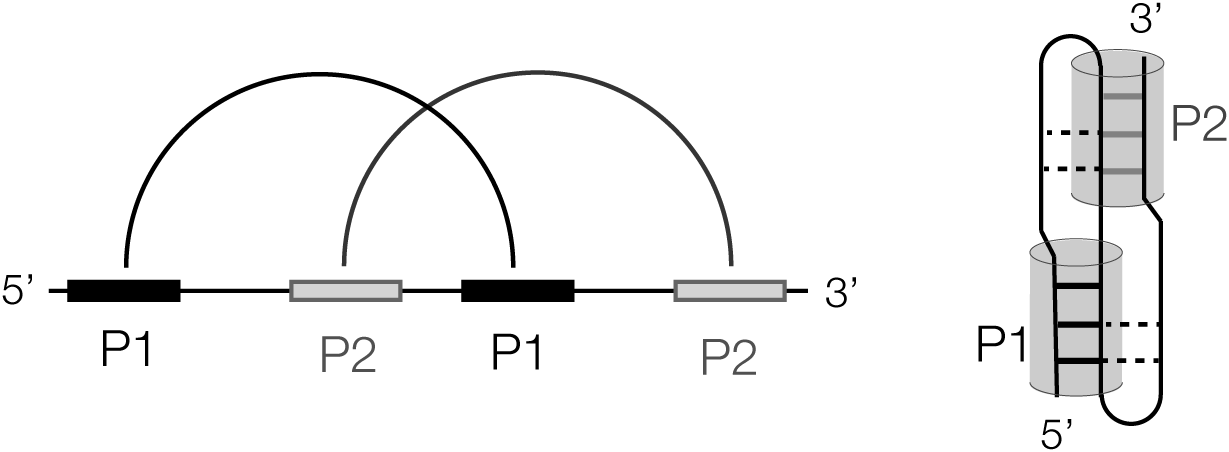
Figure 8. Scheme showing the formation of an H-type pseudoknot structure. In the linear form (left) paired regions are connected by the arcs. A cartoon of the folded form is shown (right) with the P1 and P2 helices shown as cylinders.
Some examples of riboswitch PK structures are shown in Figure 9. The organization and crystal structure of the SAM/SAH riboswitch are shown in Figure 9a. This is a standard H-type PK, although it was constructed from two RNA oligonucleotides so that the 11 nt linker connecting the two helices was omitted (Huang et al., Reference Huang2020a). The two helices are coaxial, and the linker lies in the major groove of the helix with an open 3′ end, making one triple interaction. The guanidine-III riboswitch is another standard H-type PK, and the complete structure is visible in the crystal structure (Figure 9b) (Huang et al., Reference Huang2017b). One linking segment lies in the major groove of the 3′ end helix and makes a series of triple interactions (see below), while the other is on the minor groove side of the 5′ end helix and makes fewer interactions. The PreQ1 riboswitches are all based upon a PK structure. The structure of the PreQ1-III riboswitch PK (Schroeder et al., Reference Schroeder2023) is shown in Figure 9c. Like that of the PreQ1-II riboswitch (Liberman et al., Reference Liberman2013), the PK is elaborated by an additional helix within a linking segment connecting the 3′ end helix. The NAD+-II riboswitch is based upon perhaps the most complicated structure, shown in Figure 9d (Peng et al., Reference Peng2023). The structure is an H-type PK with an additional helix inserted into the central linker that connects the two helices. It is further elaborated by two successive four-nucleotide interactions in the central region. Finally, the GlmS riboswitch, which has ribozyme activity mediated by its glucosamine-6-phosphate ligand (Winkler et al., Reference Winkler2004), is based upon a secondary structure with multiple PK structures (Cochrane et al., Reference Cochrane2007; Klein and Ferré-D’amaré, Reference Klein and Ferré-D’amaré2006).

Figure 9. Representative examples of ribozymes with structures that are based on pseudoknots. Each is shown as the schematic of the folded structure (left), and a cartoon representation of the three-dimensional structure shown in parallel-eye stereoscopic view (right). (A) The SAM/SAH riboswitch (Huang et al., Reference Huang2020a) (PDB ID 6YL5). (B) The guanidine-III riboswitch (Huang et al., Reference Huang2017b) (PDB ID 5NWQ). (C) The PreQ1-III riboswitch (Schroeder et al., Reference Schroeder2023) (PDB ID 6XKO). (D) The NAD+-II riboswitch (Peng et al., Reference Peng2023) (PDB ID 8HB8).
Loop–loop interacting structures
An interaction between two terminal loops is the principal structure feature in some riboswitches that creates the specific ligand binding site. Perhaps, the best example of this is the guanidine-II riboswitch (Sherlock et al., Reference Sherlock2017). This comprises two stem-loops of closely similar sequence connected by a short linker, and crystallographic studies have shown that the individual stem-loops dimerize in the crystal by an intimate interaction between the loops (Huang et al., Reference Huang2017a; Reiss and Strobel, Reference Reiss and Strobel2017) (Figure 10). The loop–loop interaction comprises the formation of two regular C:G base pairs, a triple interaction between the terminal C:G base pair of the loop and N1 and N6 of the 5′ adenine of the loop, and a stacking interaction between the two 3′ adenine nucleobases of the loop. The loop–loop interaction creates two symmetrically related guanidine binding sites, and the guanidine molecules contribute to the stability of the loop–loop interaction; this is discussed further below.

Figure 10. Loop–loop interaction in the guanidine-II riboswitch (Huang et al., Reference Huang2017a). (A) Schematic showing the interaction between the two loops, colored blue and green. (B) The crystal structure of the loop–loop interaction shown in parallel-eye stereoscopic view (PDB ID 5NOM).
Another riboswitch that can be considered to be based on two interacting loops is the glutamine-II riboswitch (Ames and Breaker, Reference Ames and Breaker2011), although semantically this might also be termed a kind of PK. The interface comprises six consecutive Watson–Crick base pairs with two major-groove triple base interactions (Huang et al., Reference Huang2019b). The L-glutamine ligand is bound on the major-groove face of the terminal loop. The lysine riboswitch has a loop–loop interaction comprising six uninterrupted Watson–Crick base pairs (Garst et al., Reference Garst2008; Serganov et al., Reference Serganov2008). This is important in achieving the overall fold of the RNA, but is not directly involved in the binding of the lysine ligand.
Local structural elements of riboswitches
Loop–loop and loop–receptor interactions
The guanidine-binding domain of the guanidine-II riboswitch is fundamentally based upon a loop–loop interaction between two stem-loops that is mediated by ligand binding. This is discussed in the previous section, and is shown in Figure 10. Loop–loop interaction also occurs in the lysine riboswitch (Garst et al., Reference Garst2008) and the various purine-binding riboswitches (Batey et al., Reference Batey2004; Serganov et al., Reference Serganov2004). Loop–receptor interactions are very common in folded RNA species, so unsurprisingly they occur in several riboswitches. Such interactions are structurally important in the guanidine-I riboswitch (Reiss et al., Reference Reiss2017), the TPP riboswitch (Thore et al., Reference Thore2006), and the THF riboswitch (Trausch et al., Reference Trausch2011).
Triple helical regions
Triple helices are formed where a third strand locates in the major or minor groove of a duplex, making hydrogen bonding interactions with the nucleobases and perhaps the backbone. These are relatively common in riboswitches, including the preQ1-III (Schroeder et al., Reference Schroeder2023), guanidine-III (Huang et al., Reference Huang2017b), SAM-II (Gilbert et al., Reference Gilbert2008), SAM-V (Huang and Lilley, Reference Huang and Lilley2018b), and NAD+-II (Peng et al., Reference Peng2023; Xu et al., Reference Xu2023) riboswitches. Riboswitches based upon PK structures will typically have a major groove triple helix, and in some cases, a minor groove triple helix too. In general, where the triple helix has an open 3′ end, this will form a major groove triplex. The guanidine-III riboswitch is an example, shown in Figure 11 (Huang et al., Reference Huang2017b). The triple helix forms four planes of base triple interactions, where the three nucleobases are approximately co-planar, and stacked on both sides with a separation of about 3.5 Å. The sequence of the third strand is 5′ AGGU, and in each case, the nucleobases are hydrogen bonded to the Hoogsteen edge of one or both base pairs of the duplex. The nucleotide 5′ to the third strand of the duplex (G7) plays a key role in bonding the guanidine ligand as we discuss below.
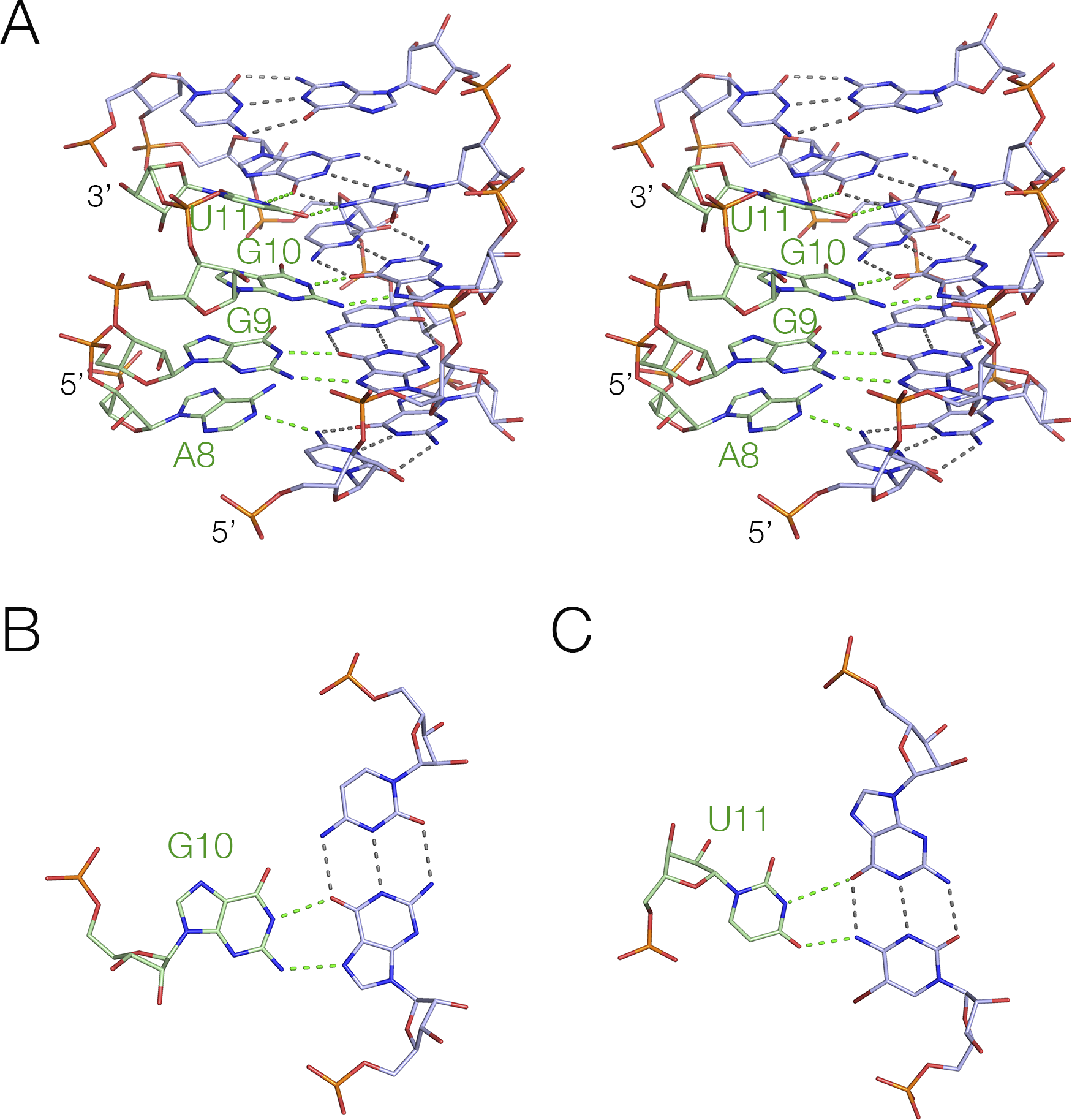
Figure 11. The major-groove triplex found in the guanidine-III riboswitch (Huang et al., Reference Huang2017b). (A) Crystal structure showing the triple interaction, where the third strand is shown in green, interacting with the major groove of the duplex shown blue (PDB ID 5NWQ). (B) and (C) Structures of two triple base interactions in the major groove.
As we have discussed above (see Figure 9b), the guanidine-III riboswitch forms a standard H-type PK, and the helix containing the 5′ end accommodates the connecting strand in its minor groove. However, this strand makes no triple-base interactions in the minor groove, and only two base pairs along its length, a cis-Watson–Crick and a trans-Watson–Crick base pair (Huang et al., Reference Huang2017b). The sequence requirements for a regular minor-groove triple helix are more stringent and essentially require an oligo-adenine sequence that can form A-minor interactions (Nissen et al., Reference Nissen2001). The NAD+-II riboswitch provides a good example of a minor groove triplex, where the third strand comprises five consecutive adenine nucleotides (A5, comprising A41 to A45). The structure of the triple helix (Peng et al., Reference Peng2023) is shown in Figure 12. Each adenine nucleobase makes hydrogen bonding interactions with the sugar edge of the base pairs of the duplex, either one or both nucleobases or the O2’ of the sugar. Starting from the 3′ end of the A5 sequence, the first three adenine nucleobases make hydrogen bonds from their Watson–Crick edge (N6, N1) to one or both nucleobases of the duplex plus an O2’, and the forth (A42) is hydrogen bonded from its Hoogsteen edge (N6, N7) to the sugar O2’ and nucleobase (cytosine O2) of the duplex. The final, that is, 5′ adenine (A41), is reoriented in making a turn, so that its sugar edge faces the duplex, whereupon it is hydrogen bonded to the nucleobase (guanine N2) and sugar (cytosine O2’) of the duplex.
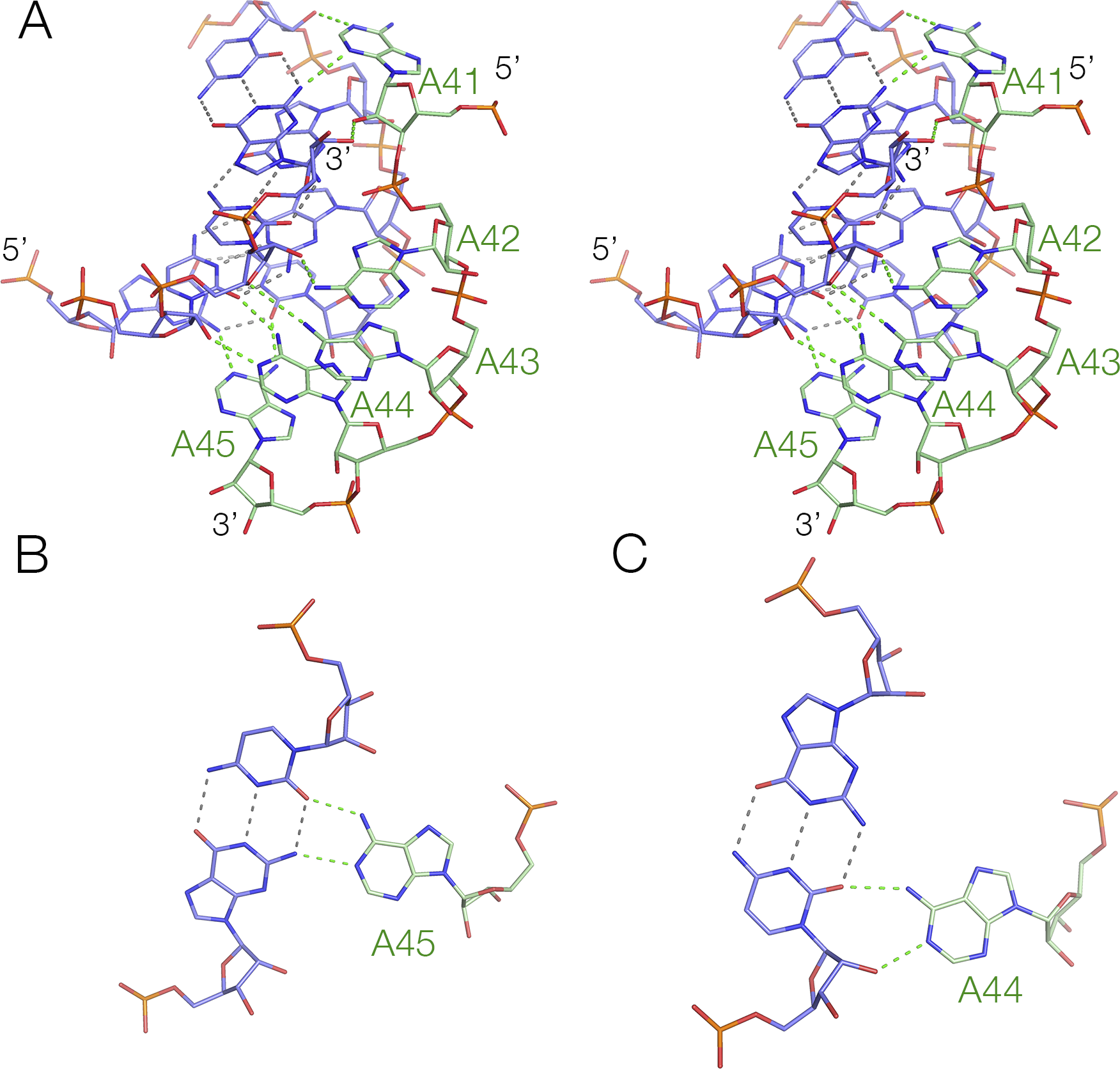
Figure 12. The minor-groove triplex found in the NAD+-II riboswitch (Peng et al., Reference Peng2023). (A) Crystal structure showing the triple interaction, where the An strand is shown in green, interacting with the minor groove of the duplex shown blue (PDB ID 8HB8). (B) and (C) Structures of two triple base interactions in the minor groove.
Tetraplex helices
While triple-base interactions have been found frequently in riboswitches, quadruple interactions are less common. Nevertheless, they exist. The NAD+-II riboswitch has a section of quadruple helix comprising two successive quadruplexes G:U:A:C and U:A:A:G (Figure 13) (Peng et al., Reference Peng2023). The nucleobases are all coplanar, and are connected by hydrogen bonding between successive nucleobases in a cyclic manner. The upper G:U:A:C plane forms a platform for the binding of the planar nicotinamide dinucleotide ligand. Base tetrads have also been found in purine riboswitches such as the 2′-dG-II riboswitch (Matyjasik and Batey, Reference Matyjasik and Batey2019). Base tetrads are relatively rare, and we are unaware of any examples of four-guanine tetraplex structures in riboswitches.
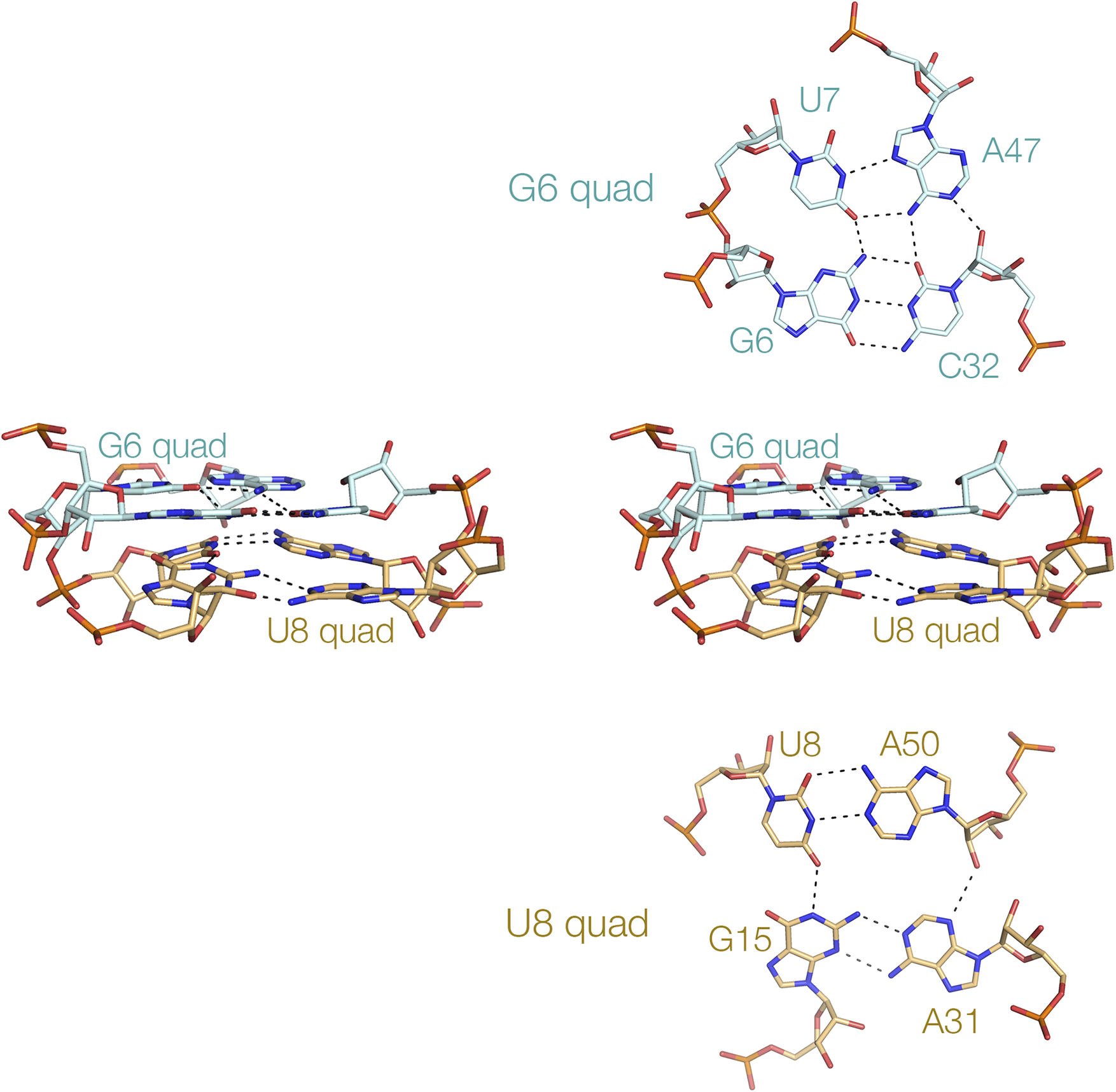
Figure 13. A tetraplex helix in the NAD+-II riboswitch (Peng et al., Reference Peng2023). This short four-stranded helix comprises two coaxial four-nucleotide tetrads. The structure is shown (center) in parallel-eye stereoscopic view, with the component G6 and U8 tetrads shown above and below, respectively (PDB ID 8HB1).
k-Turns and k-junctions
We have reviewed the structure and occurrence of kink turns (k-turns) at length previously (Huang and Lilley, Reference Huang and Lilley2018a). The motif is extremely widespread in RNA structure, being found in the ribosome (several examples) (Klein et al., Reference Klein2001), spliceosomal complexes (Vidovic et al., Reference Vidovic2000), and in snoRNA species such as box C/D (Moore et al., Reference Moore2004). The standard k-turn comprises two successive sheared G•A and A•G base pairs preceded by a three-nucleotide bulge. When folded in the presence of divalent cations, the RNA is tightly kinked, and stabilized by two key cross-strand A-minor interactions (Liu and Lilley, Reference Liu and Lilley2007; McPhee et al., Reference Mcphee2014; Huang et al., Reference Huang2016). The folded structure of the k-turn can also be stabilized by protein binding, particularly by proteins of the L7Ae class (Turner and Lilley, Reference Turner and Lilley2008; Wang et al., Reference Wang2012; Huang and Lilley, Reference Huang and Lilley2013). The SAM-I riboswitch contains a standard k-turn within its architecture (Montange and Batey, Reference Montange and Batey2006). It folds in response to the addition of divalent metal ions, and both folding and SAM binding are prevented by mutations that disrupt the standard A-minor interactions (Schroeder et al., Reference Schroeder2011). k-Turns also exist in the glycine (Baird and Ferre-D’amare, Reference Baird and Ferre-D’amare2013), lysine (Blouin and Lafontaine, Reference Blouin and Lafontaine2007), cobalamine (Johnson Jr et al., Reference Johnson2012), and cyclic diGMP (Smith et al., Reference Smith2009) riboswitches.
The k-junction is a combination of a three-way junction and a k-turn. This is found in the TPP riboswitches of Arabidopsis (Thore et al., Reference Thore2006) and E. coli (Serganov et al., Reference Serganov2006), and is discussed above in the Section entitled “Three-way helical junctions”. Like standard k-turns, k-junctions are also induced to fold by the addition of divalent metal ions (Li et al., Reference Li2023).
Ligand binding in riboswitches
Binding of a small-molecule ligand to a riboswitch leads to a conformational change in the RNA that in some manner modulates gene expression. As discussed earlier, in many riboswitches, the binding occurs during co-transcriptional folding of the RNA. Some riboswitches bind their ligands with high affinity (e.g., the cyclic-di-GMP-II riboswitch binds c-di-GMP with a K d of 2 nM (Smith et al., Reference Smith2011)), while many exhibit affinities that are lower, such as 10–100 μM range. The affinity needs to be in the range determined by the required biological response so that gene expression is altered in the required range of ligand concentration. RNA is often capable of binding ligands with higher affinities. For example, the guanidine-II riboswitch binds two guanidine molecules in separate sites with an affinity of 68 μM; when these molecules are covalently tethered, the affinity is lowered by an order of magnitude to 5 μM (Huang et al., Reference Huang2019c). However, evidently in the biological context, the riboswitch needs to be sensitive to changes in guanidine concentration around 70 μM, and that is what the riboswitch has evolved to respond to.
In most cases, ultrahigh affinity is not required. Rather, binding specificity is key, and ribozymes must discriminate the biological ligand from other, potentially similar, molecules. RNA is extremely good at selective binding. RNA is a charged polymer, with hydrogen bond donors and acceptors held in a fairly rigid frame. The majority of riboswitches make multiple hydrogen bonds with their ligands. In many cases, the ligands can stack with nucleobases of the RNA. Inner-sphere metal ion interactions are found, along with cation–π interactions. All of these interactions can generate great selectivity in ligand binding.
Multiple ways a given ligand can be bound by RNA in different riboswitches
Many ligands are bound by different riboswitches, providing an opportunity to compare the manner of binding to different RNA structures. We discuss some examples of these in the following sections.
Binding of adenine-containing coenzymes to riboswitches
A number of riboswitches bind coenzymes that include nucleobases, nucleosides, or nucleotides, particularly adenosine derivatives. These often become incorporated into the riboswitch structure very much as an integral part of the RNA, base pairing with nucleobases of the RNA and stacking with nucleobases on one or both faces. This manner of binding should provide significant stabilization of the bound conformation of the RNA.
Several riboswitches bind molecules that include nucleobases, nucleosides, or nucleotides (e.g., SAM or NAD+), and these can both hydrogen bond and stack with nucleobases of the RNA. However, the manner of base pairing varies widely. For example, the adenine of SAM base pairs as a cis Hoogsteen:Watson–Crick base pair with U in the SAM-I riboswitch (Montange and Batey, Reference Montange and Batey2006), as a trans Watson–Crick:sugar base pair with G in the SAM-III riboswitch (Lu et al., Reference Lu2008), and as a trans Hoogsteen:Watson–Crick base pair with U in the SAM-V riboswitch (Huang and Lilley, Reference Huang and Lilley2018b). The adenine of NAD+ forms a Watson–Crick:sugar base pair with G in the NAD+-I riboswitch, where it is only stacked on one face (Huang et al., Reference Huang2020b), while it forms a trans Hoogsteen:Watson–Crick with A in the NAD+-II riboswitch (Peng et al., Reference Peng2023). The adenine of cobalamine forms a trans Watson–Crick:Hoogsteen base pair with A in the adenosylcobalamin (B12) riboswitch (Peselis and Serganov, Reference Peselis and Serganov2012).
The conformation of SAM differs greatly between different SAM-binding riboswitches (Figure 14). In SAM-V, the SAM ligand is full extended along the axis of an RNA triple helix, with the adenine adopting an anti-conformation (Huang and Lilley, Reference Huang and Lilley2018b). The distance between adenine N1 and the methionyl carboxylate C (N1-C) is 9.5 Å. At the other extreme, in the SAM-I riboswitch, SAM adopts an overall C-shaped conformation where the adenine adopts a syn conformation and the methionyl chain curves around so that the N1-C distance is 5.2 Å (Montange and Batey, Reference Montange and Batey2006). This is bound in a constrained pocket formed by the minor grooves of two juxtaposed helices. In the SAM-III riboswitch, the methionyl chain of SAM is relatively extended but the adenine is in the syn conformation, with an N1-C distance of 7.1 Å (Lu et al., Reference Lu2008).
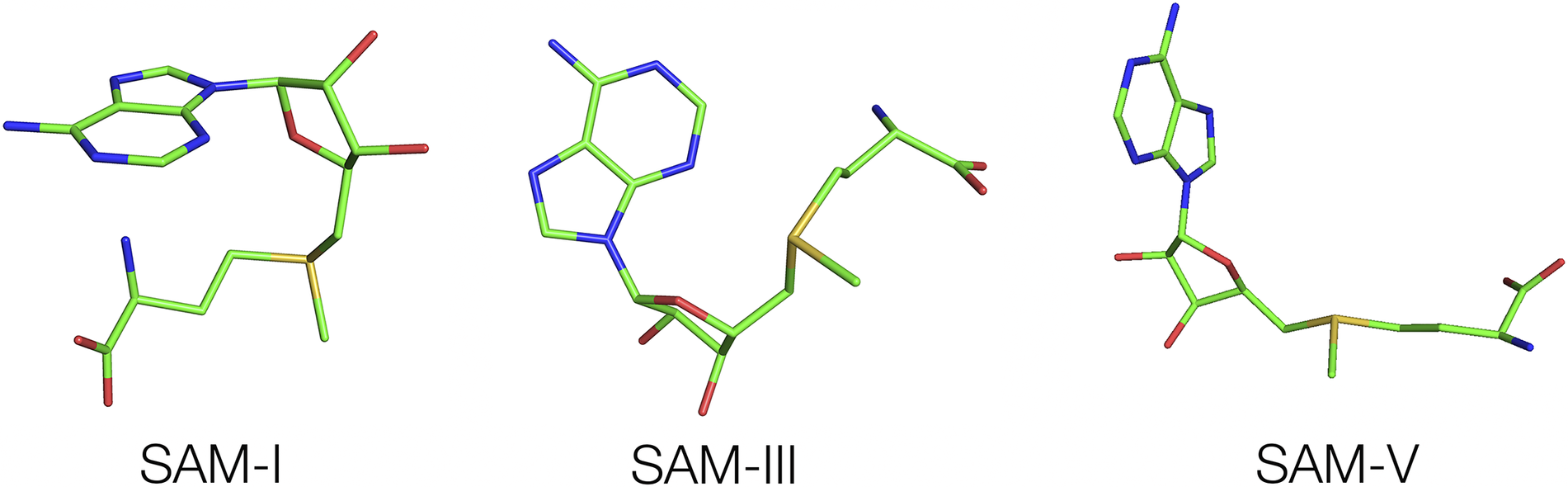
Figure 14. Various conformations of SAM observed when bound to different SAM-binding riboswitches. Left—Bound to the SAM-I riboswitch (Montange and Batey, Reference Montange and Batey2006) (PDB ID 3GX5), center—bound to the SAM-III riboswitch (Lu et al., Reference Lu2008) (PDB ID 3E5C), and right—bound to the SAM-V riboswitch (Huang and Lilley, Reference Huang and Lilley2018b) (PDB ID 6FZ0).
These comparisons demonstrate how the different classes of SAM-binding riboswitches have evolved completely different modes of ligand binding, although SAM-II and V riboswitches are clearly very similar (Gilbert et al., Reference Gilbert2008; Huang and Lilley, Reference Huang and Lilley2018b). The SAM-III and SAM-VI riboswitches were also proposed to be similar (Arachchilage et al., Reference Arachchilage2018), but this was not supported by subsequent structural analysis (Lu et al., Reference Lu2008; Sun, Reference Sun2019).
Binding of guanidine to riboswitches
There are three classes of guanidine-binding riboswitches. These differ in their mode of genetic control (type I exerts its control over transcriptional initiation, while types II and III control the initiation of translation) and have totally different structures (Huang et al., Reference Huang2017a, Reference Huang2017b; Reiss and Strobel, Reference Reiss and Strobel2017; Reiss et al., Reference Reiss2017). For example, while the guanidine-II riboswitch generates two ligand binding sites by loop–loop interaction (Huang et al., Reference Huang2017a; Reiss and Strobel, Reference Reiss and Strobel2017) (see Section entitled “Loop–loop interacting structures”; Figure 10), the guanidine-III riboswitch contains a complex triple helix that includes a guanidine binding site (see Section title “PK-containing structures”; Figures 9b and 11) (Huang et al., Reference Huang2017b). Yet the three riboswitches exhibit significantly common modes of ligand binding. In all three cases, the guanidine donates hydrogen bonds from two nitrogen atoms to O6 and N7 of a guanine in the RNA (Figure 15). In the guanidine-I riboswitch (Reiss et al., Reference Reiss2017) it is G90 that binds, while in the guanidine-II riboswitch, G9 fulfills the same role. This is the same mode of binding as some zinc finger proteins that use an arginine guanidino side chain to bind guanine in G-rich DNA (Pavletich and Pabo, Reference Pavletich and Pabo1991). In the case of the guanidine-III riboswitch, it does this twice (Huang et al., Reference Huang2017b), using two different guanine nucleobases (G7 and G17). Guanidine has six protons that can be donated, and at least four are involved in hydrogen bonding to the RNA. In each case, the guanidine is stacked on a nucleobase – we return to this in Section title “Electrostatic interactions and the direct involvement of metal ions”.
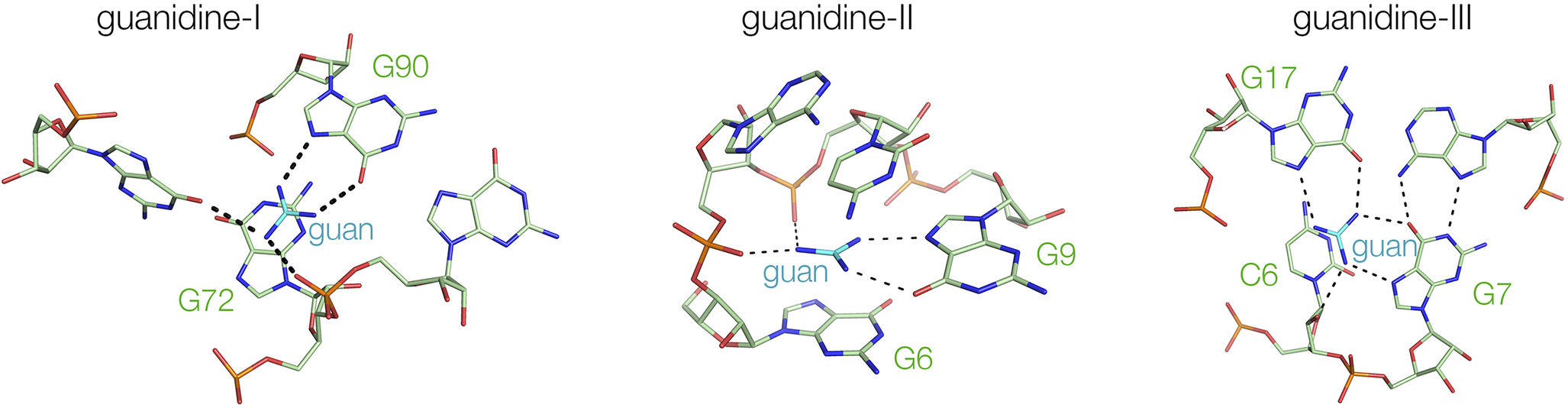
Figure 15. Comparison of the manner of guanidine binding in the guanidine I, II and III riboswitches. Left—bound to the guanidine-I riboswitch (Reiss et al., Reference Reiss2017) (PDB ID 5T83), center—bound to the guanidine-II riboswitch (Huang et al., Reference Huang2017a) (PDB ID 5NOM) and right—bound to the guanidine-III riboswitch (Huang et al., Reference Huang2017b) (PDB ID 5NWQ).
Few structures have been determined for riboswitches in the absence of a ligand. In a sense, this was achieved for the guanidine-II riboswitch. However, it transpired that the binding site was not really empty, but rather was occupied by three ammonium ions that took the place of the three nitrogen atoms of the guanidine ligand (Huang et al., Reference Huang2017a).
Binding of adenine and nicotinamide to the NAD+ riboswitches
The two known NAD+-riboswitches (Malkowski et al., Reference Malkowski2019; Panchapakesan et al., Reference Panchapakesan2021) bind their NAD+ and NADH ligands in very different ways. NAD+ comprises adenosine and nicotinamide linked head-to-head by diphosphate (Figure 16a). In principle, either element or both could be recognized by RNA. The NAD+-I riboswitch has two domains, with similar secondary structures composed of a long stem-loop with internal loops (Figure 16). The crystal structure of the 5′ domain (Figure 16b,c) was solved bound to AMP, ADP, ATP, NADH, NAD+, and other derivatives (Huang et al., Reference Huang2020b). In the crystal, the three helical sections are coaxially stacked, with an extended section formed by eight bulged-out nucleotides (Figure 16d). All the adenine nucleotides were observed bound to the riboswitch, but in the NADH- and NAD+-bound forms only the adenine part was visible in the electron density. The nicotinamide was not observable, and we suspect that its binding site lies in the second domain, although no evidence in support of that has been obtained to date. The adenine moiety was observed bound under the extended bulge in an anti-conformation, and the whole binding site is strongly conserved. The nucleoside was coplanar with the G46:C6 base pair on its sugar edge, forming four hydrogen bonds (Figure 16e). It was stacked on one face to A8 in the bulge, while the other side was free. The binding of the diphosphate domain is metal ion mediated – this is discussed below (Section title “Electrostatic interactions and the direct involvement of metal ions”).
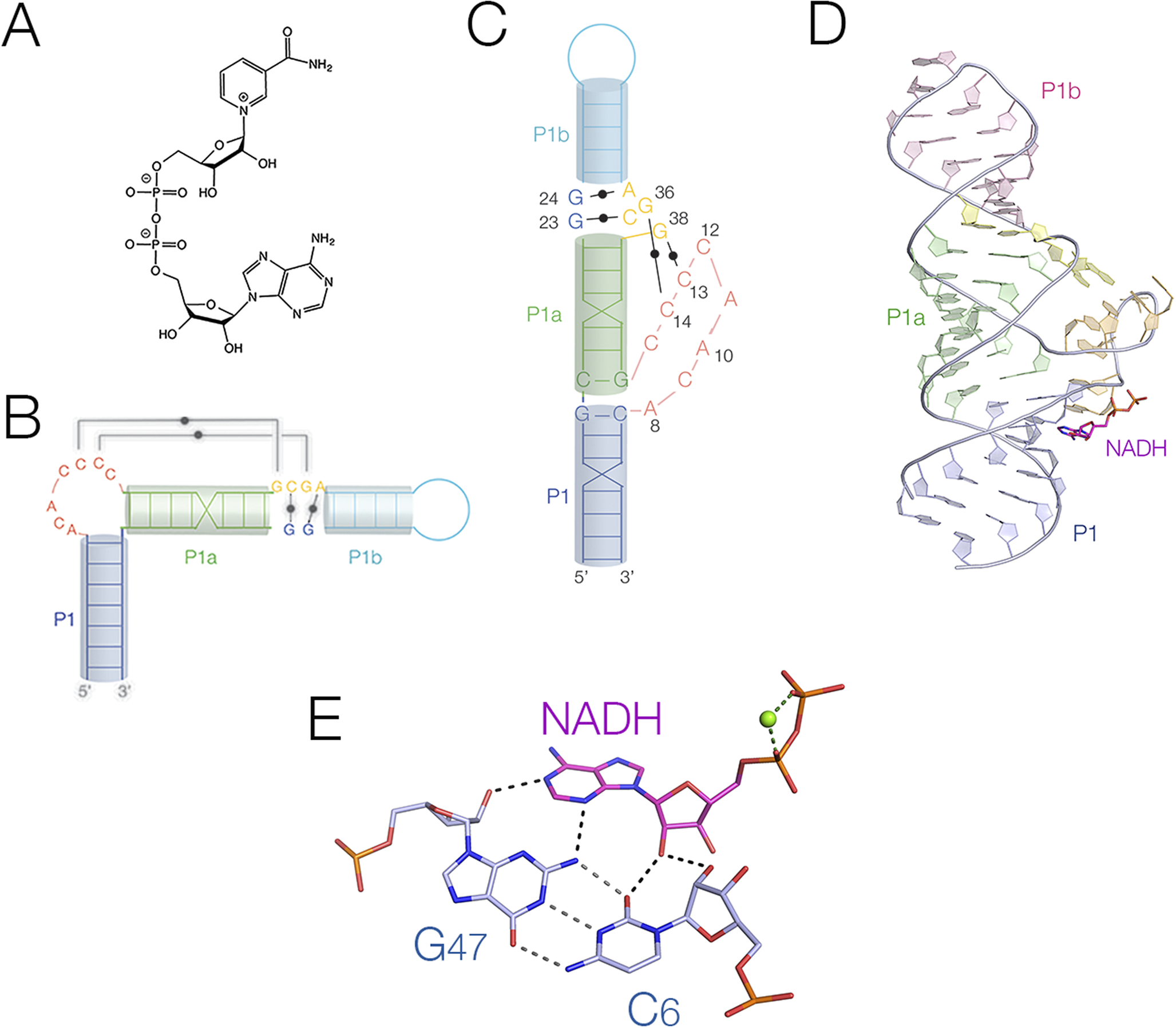
Figure 16. The binding of NADH to the NAD+-I riboswitch. (A) The chemical structure of NAD+. (B) Cartoon showing the secondary structure of the NAD+-I riboswitch. (C) Cartoon showing the folded structure of the NAD+-I riboswitch. (D) The crystal structure of the NAD+-I riboswitch bound to NADH (Huang et al., Reference Huang2020b) (PDB ID 6TF0). (E) The interaction between the NADH ligand and the C6:G47 base pair of the NAD+-I riboswitch.
The second NAD+-binding riboswitch (NAD+-II riboswitch) (Panchapakesan et al., Reference Panchapakesan2021) binds principally through the nicotinamide moiety, and crystallographic structures have been determined (Peng et al., Reference Peng2023; Srivastava et al., Reference Srivastava2023). We determined the structure of the riboswitch bound to NAD+, nicotinamide mononucleotide (NMN), and nicotinamide riboside (Peng et al., Reference Peng2023). The secondary structure comprises a bulged stem-loop, with a PK formed by the ribosome binding site of the RNA (Figure 17a–c). The PK forms a triple helix (see the Section entitled “Triple helical regions” and Figure 12) that sits atop the tetraplex discussed in the Section entitled “Tetraplex helices”. This forms the principal binding site for the ligand (Figure 17D). However, in contrast to the NAD+-I riboswitch, it is the nicotinamide moiety that is bound at this site, coplanar with and sharing three hydrogen bonds to the C45:G33 base pair on its Hoogsteen edge (Figure 17e). Nicotinamide alone (as NMN) was bound at this site. NAD+ was observed bound in an extended conformation, with the adenine bound remotely in a pocket where its nucleobase forms a single hydrogen bond to the RNA. A second binding site for NAD+ was found in the PK helix, in which the nicotinamide was bound in pocket while the adenine end of the ligand made no specific contacts. A second structure of the NAD+-II riboswitch was solved for a slightly truncated form of the RNA, where binding at a second site was not observed (Xu et al., Reference Xu2023).
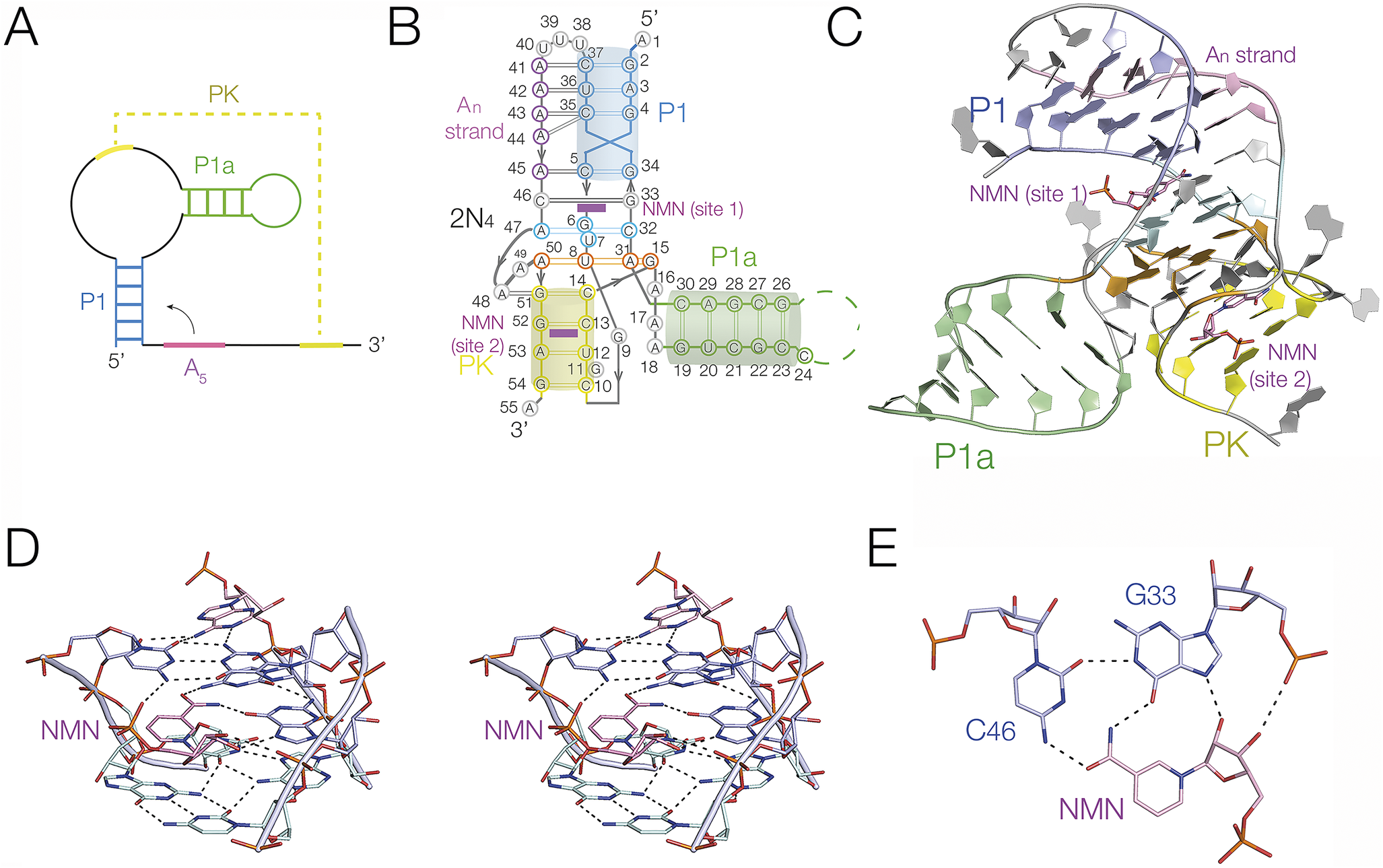
Figure 17. The binding of NMN to the NAD+II riboswitch. (A) Scheme showing the secondary structure of the NAD+-II riboswitch. The pseudoknot (PK, shown in yellow) forms between the internal loop and the 3′ end of the riboswitch RNA. Note that the coloring is the same in parts (a)–(c). (B) Cartoon showing the structure of the NAD+-II riboswitch. Note that there are two molecules of NMN (shown magenta) bound at two different sites. 2N4 depicts the two quadruple base interactions; their structure is shown in Figure 13. (C) The crystal structure of the NAD+-II riboswitch (Peng et al., Reference Peng2023) (PDB ID 8HB1). (D) Detail of the structure showing the binding of NMN at site 1 shown in parallel-eye stereoscopic view. (E) The bonding interactions between the NMN ligand at site 1 and G33 and C46 of the RNA.
Binding of single versus multiple ligands by riboswitches
The majority of riboswitches bind a single ligand molecule. However, some bind two copies, either within the same RNA domain or individually to two tandem binding domains. The glycine riboswitch is an example of a tandem double riboswitch (Mandal et al., Reference Mandal2004). It was found that the riboswitch comprised two structured domains, and bound glycine cooperatively, in order to achieve a sharper response to rising glycine concentration by the riboswitch. The structure was determined by crystallography in the Patel and Strobel laboratories (Huang et al., Reference Huang2010; Butler et al., Reference Butler2011). Butler et al.’s (Reference Butler2011) structure confirmed that the ribozyme folded in two domains of similar structure, with inter-domain connections. These were shown to be important for the cooperative binding of glycine (Baird and Ferre-D’amare, Reference Baird and Ferre-D’amare2013; Erion and Strobel, Reference Erion and Strobel2011).
The NAD+-I riboswitch also appears to comprise tandemly connected domains (Malkowski et al., Reference Malkowski2019). We solved the crystal structure of the 5′ domain, bound to a series of ligands based upon ADP, NADH and similar (Huang et al., Reference Huang2020b). As discussed above (see Section entitled “Binding of adenine and nicotinamide to the NAD+ riboswitches”) only the ADP moiety was observed in these structures. It was tempting to speculate that the 3′ domain might bind the nicotinamide domain, yet when Ren and coworkers (Chen et al., Reference Chen2020) solved the structure of the 3′ domain they found that this also predominantly bound the ADP moiety. Nevertheless, we speculate that the tandem nature of the NAD+-I riboswitch suggests a modular nature, so that by exchanging domains a different ligand might be recognized. Such as coenzyme-A, for example.
Some riboswitches can bind two ligand molecules within the same RNA. We have discussed the NAD+-II riboswitch above (Section entitled “Binding of adenine and nicotinamide to the NAD+ riboswitches”). Two molecules of NADH bind at different sites within the single riboswitch domain (Peng et al., Reference Peng2023), although it is not clear whether or not binding at the second site is functionally important. The guanidine-II riboswitch also binds two molecules of guanidine within its functional unit, which is a loop–loop interaction between either closely similar tandem stem-loops, or identical ones used in crystallization (Huang et al., Reference Huang2017a; Reiss and Strobel, Reference Reiss and Strobel2017). Each of the two interacting stem-loops binds guanidine in the same manner, stabilizing the interaction. In addition, they can be covalently linked, so increasing binding affinity (Huang et al., Reference Huang2019c).
Electrostatic interactions and the direct involvement of metal ions
Unlike proteins, RNA has a charged phosphodiester backbone; thus, metal ions are always involved in RNA folding processes. Most of these will be bound atmospherically without exchange of water from their inner coordination sphere, and such ions will be in rapid exchange. However, a few will undergo site binding, whereby groups from the RNA will displace inner-sphere water molecules with direct metal-RNA bonding. Such ions will be bound for much longer periods, and we frequently observe these crystallographically.
In some riboswitch-ligand co-crystal structures metal ions are observed playing a direct role in the specific binding of the ligand. In the glutamine-II riboswitch, the glutamine ligand is bound to a region of the RNA that is partially a major-groove triple helix (Huang et al., Reference Huang2019b) (Figure 18A). The amide end of the glutamine forms two hydrogen bonds to the C1:G40 base pair, while the carboxylate end forms a hydrogen bond to N4 of C39. However, in addition, the other oxygen atom of the carboxylate group bonds directly to a metal ion, displacing an inner sphere water of hydration (Figure 18B). The metal ion is held in place by hydrogen bonding between other inner-sphere water molecules with the RNA. A metal ion-mediated contact of the methionyl carboxylate of SAM was found in the SAM-V riboswitch (Huang and Lilley, Reference Huang and Lilley2018b). Both carboxylate oxygen atoms were hydrogen bonded to the inner-sphere water molecules of a magnesium ion that was directly bonded to a backbone phosphate non-bridging oxygen atom.

Figure 18. Metal ion-mediated binding of glutamine to the glutamine-II riboswitch. (A) The glutamine-binding domain observed in the crystal structure of the glutamine-II riboswitch (Huang et al., Reference Huang2019b) shown in parallel-eye stereoscopic view. The glutamine (gln) is shown in magenta, and the metal ion is shown yellow, with red water molecules in its inner sphere of hydration (PDB ID 6QN3). (B) The bonding interactions between glutamine and the riboswitch RNA together with a hydrated magnesium ion. Note that the metal ion is directly bonded to a carboxylate oxygen of the glutamine, and that two of the inner-sphere water molecule are hydrogen bonded to G18 in the binding site.
Another striking example of metal ion mediated ligand binding is found in the NAD+-I riboswitch (Huang et al., Reference Huang2020b). In the structure of the NAD+-I riboswitch (see Section entitled “Binding of adenine and nicotinamide to the NAD+ riboswitches” and Figure 16), the neck of the loop emerging from the stacked helices is very narrow, so that the backbones approach very closely. This should generate a high local electrostatic potential, and the repulsion is diminished by binding two tightly-held divalent metal ions between the backbone phosphodiester linkages (Figure 19). Both ions are extensively dehydrated, each exchanging three inner-sphere water molecules for RNA ligands. Most are phosphate non-bridging oxygen atoms, but ion m1 bonds directly to N7 of A10 – such direct Mg2+-N bonding is rare (Leonarski et al., Reference Leonarski2017). Each phosphate of the diphosphate linkage of the NADH ligand makes a direct interaction with ion m2, thus making a significant contribution to the binding of the ligand to the riboswitch. In the Arabidopsis thaliana TPP riboswitch, the ligand diphosphate interacts with one arm of the three-way junction, with direct contacts between the non-bridging oxygen atoms of the two phosphates and magnesium ions (Thore et al., Reference Thore2006).
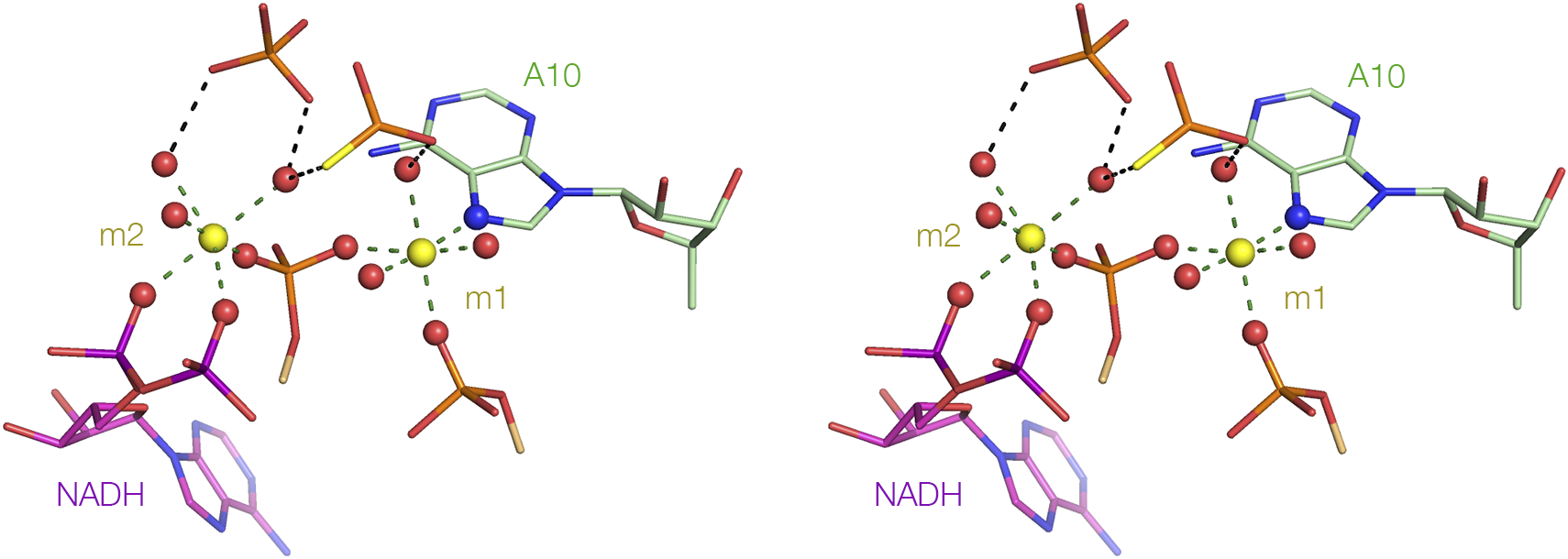
Figure 19. Metal ion-mediated binding of the diphosphate of NADH to RNA in the NAD+-I riboswitch. The NADH is bound at the narrow neck of the extruded loop of the NAD+-I riboswitch (refer back to Figure 16D) (Huang et al., Reference Huang2020b) (PDB ID 6TF0). Two metal ions bridge the two strands of the loop at this point, shown here in parallel-eye stereoscopic view. Both ions are extensively dehydrated, forming direct bonds to the RNA or NADH ligand. In particular, we see that two non-bridging oxygen atoms are directly bonded to non-bridging oxygen atoms of the m2 metal ion.
For some riboswitches, a metal ion IS the ligand. Winkler and colleagues (Dann III et al., Reference Dann2007) identified an Mg2+-sensing riboswitch they termed the M-box RNA. This undergoes an Mg2+-induced folding in the millimolar range. The structure was solved by X-ray crystallography, and the structure was found to coordinate a number of Mg2+-ions, with direct Mg2+-O coordination with RNA phosphate groups. Perhaps surprisingly, anion-sensing riboswitches are also known (Baker et al., Reference Baker2012). Given the electronegativity of RNA, it is not immediately obvious how it would bind an anion. The crystal structure (Ren et al., Reference Ren2012) showed that the folding of the riboswitch creates a pocket that binds three Mg2+ ions, in the middle of which binds the fluoride ion. So locally in the center of the RNA an electropositive binding pocket is created that can bind an anion. This is perhaps similar to the binding of charged phosphate groups within NAD+ (Huang et al., Reference Huang2020b) and TPP (Thore et al., Reference Thore2006) discussed above, where magnesium ions mediate the interaction.
Electrostatic interactions not involving metal ions are also important. In the three guanidine riboswitch structures (Huang et al., Reference Huang2017a, Reference Huang2017b; Reiss and Strobel, Reference Reiss and Strobel2017; Reiss et al., Reference Reiss2017), in each case, the guanidine ligand is stacked over the face of a guanine or cytosine nucleobase (Figure 15). The pK a of guanidine is relatively high, so that at neutral pH it exists as the positively charged guanidino cation. Here, ligand binding is stabilized by a cation–π interaction. Electrostatic interactions can also be important in discriminating one ligand over another similar ligand as discussed in the following section.
Discrimination of similar ligands by riboswitches
In order for riboswitches to regulate gene expression precisely, it is vital that they discriminate their ligand from other chemically-similar molecules.
Guanidine differs from urea in that one amine of the former is replaced by a carbonyl in the latter (Figure 20a). Guanidine is highly toxic, and guanidine riboswitches control the expression of a guanidine efflux pump that detoxifies the cell (Breaker et al., Reference Breaker2017; Nelson et al., Reference Nelson2017). The regulation needs to respond to guanidine, not urea. Guanidine has three amine groups, each of which can donate two protons to hydrogen bond acceptors. The binding pocket in each guanidine riboswitch contains only hydrogen bond acceptors. There are no donors that could hydrogen bond to the carbonyl group of urea. Moreover, urea is electrically neutral at physiological pH, so there is no possibility for a cation–π interaction. This provides good discrimination between guanidine and urea in these riboswitches.
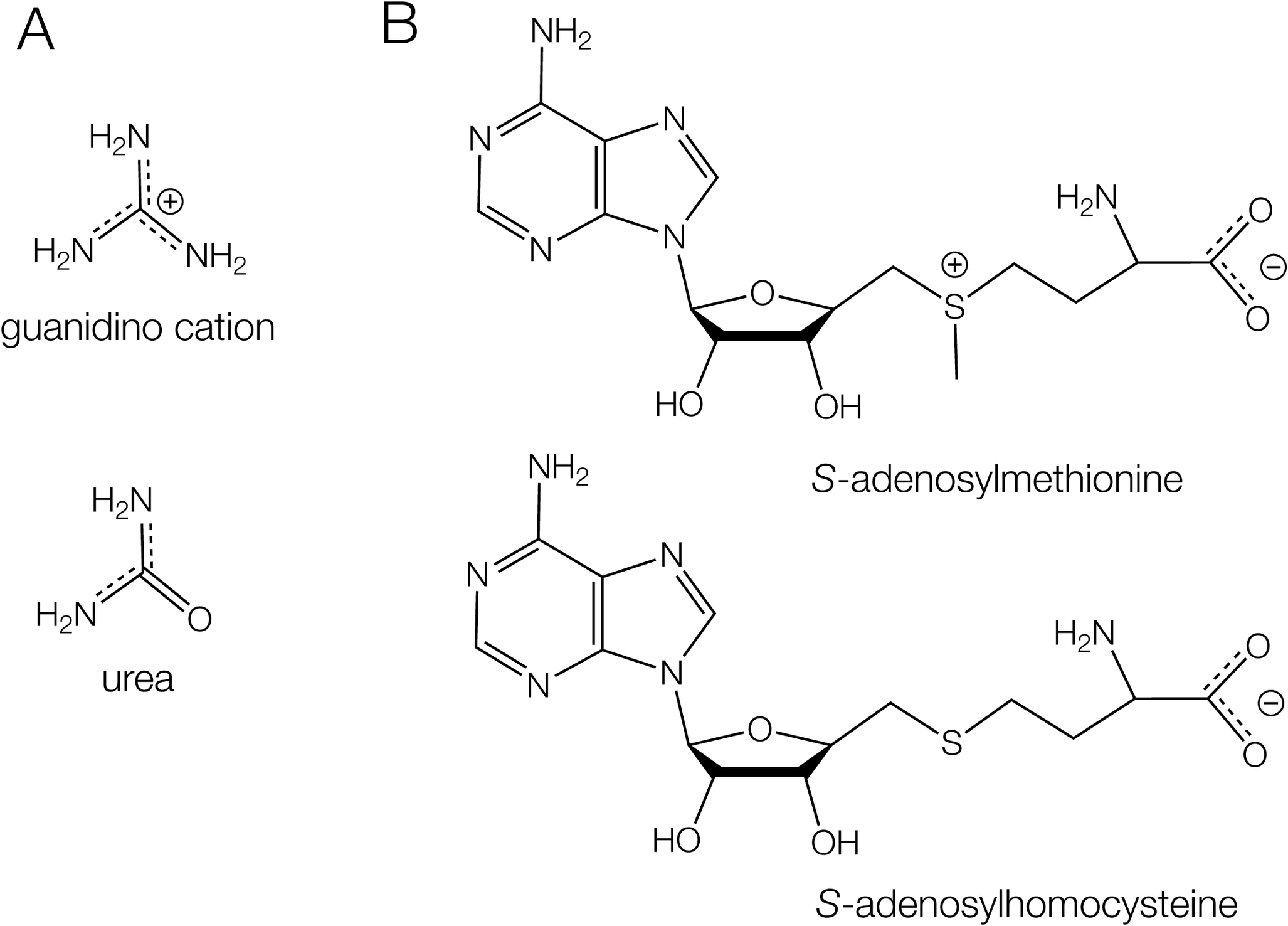
Figure 20. Comparisons of two riboswitch ligands with similar compounds that must be distinguished. (A) Guanidine (as the guanidino cation at neutral pH) compared with urea. In the latter one amine is replaced by a carbonyl, exchanging two potential hydrogen bond donors for an acceptor, and lacking the positive charge. (B) S-adenosylmethionine compared with S-adenosylhomocysteine.
In general, the SAM-responsive riboswitches should discriminate between SAM and SAH (Figure 20b). SAH has lost the methyl group from the sulfur, which has therefore converts the positively charged sulfonium of SAM to an electrically neutral thioether. Both SAM-II and SAM-V riboswitches distinguish between SAM and SAH; for example, no evolution of heat is detectable upon the addition of SAH to the SAM-V riboswitch (Huang and Lilley, Reference Huang and Lilley2018b). The structure of the SAM-V riboswitch has been determined (Huang and Lilley, Reference Huang and Lilley2018b), which reveals the manner of SAM binding and the way it discriminates against SAH. The methyl group experiences no steric clash, so the only plausible way of distinguishing SAM from SAH is by the charge on the sulfonium group. In the riboswitch process, the SAM is bound to the triple helix (Figure 21a), and its elongated chain is extended along the axis of the triplex (Figure 21b). This places the sulfur adjacent to the U20:A48:U9 triple (Figure 21c). The C4 carbonyl groups of the two uracil nucleobases are directed toward the sulfur atom at a distance of 3.1 Å. The carbonyl oxygen atoms have a significant negative charge, and there will be an electrostatic interaction with the sulfonium in this region of low dielectric. This will not occur with a neutral SAH bound, thus providing an electrostatic mechanism for ligand discrimination. A similar mechanism of discrimination has been proposed for a similar SAM-II riboswitch (Doshi et al., Reference Doshi2012).

Figure 21. Electrostatic discrimination between S-adenosylmethionine and S-adenosylhomocysteine in the SAM-V riboswitch. Parallel-eye stereoscopic views are shown. (A) Side and (B) axial views of S-adenosylmethionine binding to the triple helical region observed in the crystal structure of the SAM-V riboswitch (Huang and Lilley, Reference Huang and Lilley2018b) (PDB ID 6FZ0) Note that the elongated SAM (see Figure 14) runs along the triple-helical axis. (C) The local environment of the S-adenosylmethionine bound to the SAM-V riboswitch. The chain extending along the axis of the triplex locates the positively charged sulfonium adjacent to the U20:A48:U9 triple such that the C4-O vectors of U20 and U9 are directed toward the sulfur. The oxygen atoms have a significant negative charge, generating an electrostatic interaction with the sulfonium ion.
Control of translation by riboswitches
Riboswitches are ligand-responsive genetic control elements that act in cis in the 5′ region of mRNA to modulate gene expression. This is intimately related to RNA conformation, and changes induced by the binding of the ligand. Riboswitches can act as ON or OFF switches, although the majority act as OFF switches, downregulating gene expression when the metabolite concentration has exceeded a threshold. Riboswitches that modulate translation generally do so by alteration in RNA structure that can potentially occlude the ribosome binding site. We shall not attempt to address this topic in a comprehensive way, but rather take two examples that illustrate how this can occur, using SAM-binding riboswitches. These illustrate how a refolding of the RNA creates the ligand binding site and so becomes stabilized by the binding, and this refolding makes the ribosome binding site less accessible.
Control of translation by the SAM-V riboswitch – formation of a triple helix
The crystal structure of the SAM-V riboswitch shows the structure of the ligand-bound RNA (Huang and Lilley, Reference Huang and Lilley2018b). The structure is schematically depicted in Figure 22, showing that SAM binds within the triplex that forms with helix P2 (shown in Figure 21A), and thus binding of SAM should stabilize the triple helix. From bioinformatic analysis, we further proposed (Huang and Lilley, Reference Huang and Lilley2018b) that SAM binding leads to the stabilization of a short additional helix (P2a) located immediately 3′ to the riboswitch, and this includes part of the Shine–Dalgarno sequence. The consequence of stabilization of the triplex plus the P2a helix would be to occlude the ribosome binding site and so prevent translational initiation. Although there is no crystal structure for the ligand-free state, the structural transition suggested in Figure 22 involving the release of the third strand (colored yellow) in the absence of bound SAM is fully consistent with the change in the pattern of in-line probing data in the presence and absence of SAM observed by Poiata et al. (Reference Poiata2009).
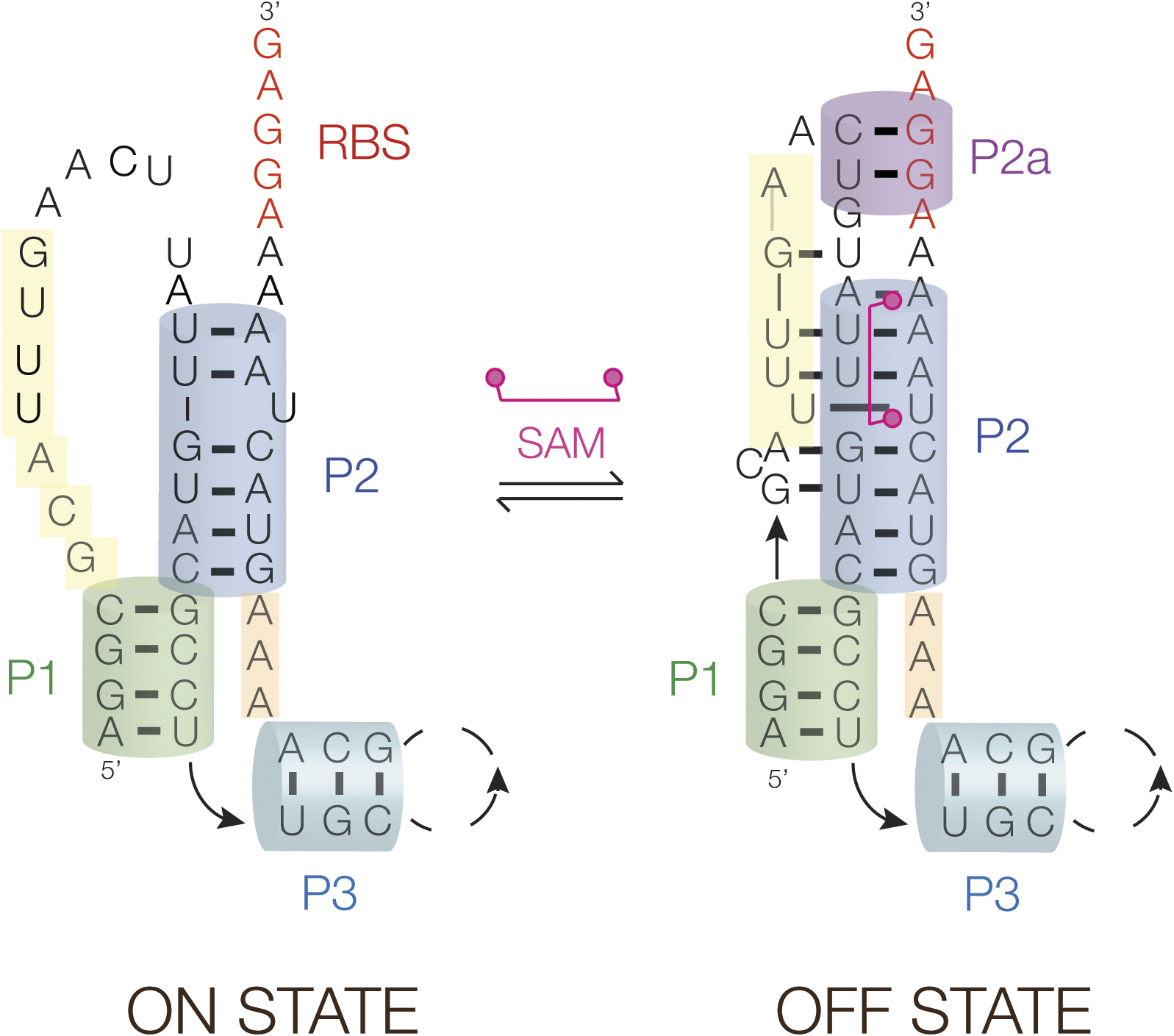
Figure 22. Translational regulation by the SAM-V riboswitch. Schematic showing the proposed mechanism for regulating the accessibility of the ribosome binding site (RBS). In the OFF state, that has the structure observed in the crystal structure (see Figure 21) (Huang and Lilley, Reference Huang and Lilley2018b), the bound SAM stabilizes the triple helical region plus a short duplex region (P2a) so sequestering the ribosome binding site (shown red). In-line probing data (Poiata et al., Reference Poiata2009) indicate that the third strand (shown yellow) disengages from the triplex with the lowering of SAM concentration, thus allowing access to the ribosome binding site and the initiation of translation.
Control of translation by the SAM–SAH riboswitch – formation of a PK helix
The SAM/SAH riboswitch is overall quite similar, undergoing an SAM (or SAH)-ligand-induced conformational change that occludes the ribosome binding site in the mRNA. However, it differs in the nature of the structural change induced. In this case, the ON and OFF states differ by the formation of a ligand-stabilized PK structure (Figure 23). The ligand-induced folded structure differs in the formation of two elements, an extension of the 5′ helix (P1) by three base pairs, and the formation of the PK helix. In the crystal structure of the ligand-bound structure (Huang et al., Reference Huang2020a), the PK helix is coaxial with the extended P1 helix, and the SAM or SAH ligand is bound at the interface between the two. Single molecule experiments showed that either SAM or SAH stabilizes the folded form with the PK helix stabilized (Huang et al., Reference Huang2020a; Liao et al., Reference Liao2023). Since the ribosome binding site is contained within the PK helix it is clear that this would be occluded by ligand-induced folding. This was demonstrated by studying the accessibility to oligonucleotides that should mimic the ribosome binding site (Liao et al., Reference Liao2023).
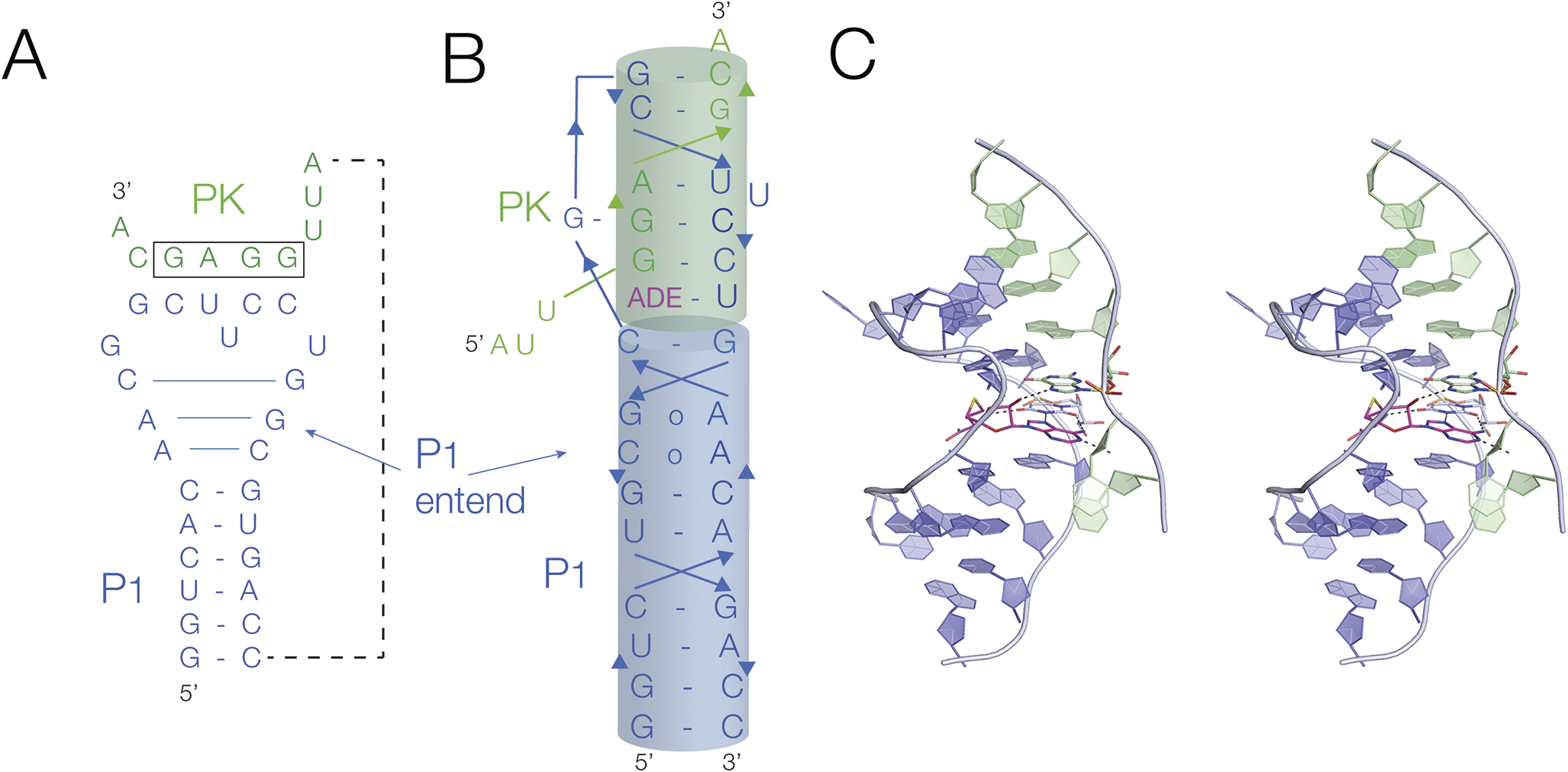
Figure 23. Translational regulation by the SAM–SAH riboswitch. (A) The secondary structure of the SAM–SAH riboswitch. In the presence of the ligand (SAM or SAH), there are two changes in conformation. The P1 helix becomes extended by three base pairs, and the pseudoknot is stabilized. The sequence forming the pseudoknot contains the ribosome binding site (boxed). (B) Cartoon of the folded structure, showing the coaxial alignment of the extended P1 and PK helices. (C) Parallel-eye stereoscopic view of the crystal structure of the SAM–SAH riboswitch with bound SAH (magenta) (Huang et al., Reference Huang2020a) (PDB ID 6YL5).
These two examples show how intimately connected ligand binding and structural rearrangement into a conformation that prevents access to the ribosome binding site are. Therefore, in these riboswitches at least, the commonly used division of riboswitches into an aptamer (ligand binding) domain, and an expression platform (where the genetic control is mediated) is not really applicable.
Conclusion
Near-atomic resolution structures have been determined for most classes of riboswitch, and this has provided a valuable database of RNA structure that can be mined for general insights into RNA conformation and folding. Moreover, the riboswitches specifically bind a wide variety of a small-molecule ligand, thus yielding a general understanding of RNA–ligand interactions.
For the most part, riboswitches are relatively small RNA molecules, folding as a single domain. We see that frequently these are based upon a single structural element such as a helical junction or PK. In addition, some structural elements recur through the ensemble of riboswitch structures, particularly elements such as triple helical sections, loop–loop and loop–receptor interactions, and k-turns and junctions.
In general, it is these elements that create ligand binding sites in the RNA, binding with great specificity, and discriminating against similar related molecules. The riboswitches exhibit multiple ways to bind their ligands, and the same ligand (e.g., SAM) can be bound in different ways in different riboswitches. The ligand can become intimately assimilated into the RNA structure, almost to become part of the RNA. Adenosine-containing ligands form base pairs with the RNA, being both hydrogen bonded and stacked just like a section of the RNA itself. Electrostatic interactions can be important, and metal-ion-mediated contacts are frequently found.
The binding of a ligand into local structural elements stabilizes the structure, and that is often the key to how the riboswitch functions. In the translational riboswitches, the ligand-bound structure generally occludes the ribosome binding site, so preventing ribosomal access and, thus, the initiation of translation.
Study of the riboswitches teaches us much about the RNA structure, ligand binding, and how these combine to regulate gene expression.
Acknowledgments
The authors would like to thank Dr Timothy Wilson for discussion.
Financial support
Riboswitch work has been funded in Dundee by Cancer Research UK (program grant A18604) and in Guangzhou by the National Natural Science Foundation of China (32171191) and Guangdong Science and Technology Department (2024A1515012594, 2023B1212060013, and 2020B1212030004).
Competing interests
The authors declare no conflict of interest.
























