Introduction
Texas panicum is a competitive warm-season annual grass that forms dense colonies or patches in fields and can grow up to 96 cm tall (Patterson Reference Patterson1990). Texas panicum reproduces primarily by seeds and is dispersed by wind, water, and animals. Each plant has the potential to produce more than 45,000 seeds (Schroeder et al. Reference Schroeder, Dowler and Stansell1990). Seeds can germinate and emerge from soil depths of 7.6 cm (Chandler and Santelmann 1969). Previous research has shown that Texas panicum seed can remain viable in the soil for several years after burial (Egley and Chandler Reference Egley and Chandler1978, Reference Egley and Chandler1983). It can grow and reproduce under a wide range of soil moisture conditions, including drought, partially explaining its ability to establish in extreme conditions and compete with the crop (Schroeder et al. Reference Schroeder, Dowler and Stansell1990). Patterson et al. (Reference Patterson, Russell, Mortensen, Coffin and Flint1986) found that greater Texas panicum height, tillers, leaf area, and dry weight occurred at a day/night temperature regime of 30/24 C than 24/18 C. The maximum Texas panicum growth rate in that study occurred at 34/26 C, which was the highest temperature used (Patterson Reference Patterson1990). These competitive characteristics along with a continuous emergence pattern throughout the summer make cost-effective, full-season control of Texas panicum extremely challenging (Chandler and Santelmann, Reference Chandler and Santelmann1969; Prostko et al. Reference Prostko, Johnson and Mullinix2001). Moreover, Texas panicum has become one of the most troublesome weed species in coarse-textured soils in South Carolina (M. Marshall, personal observation).
Clethodim is a foliar-applied herbicide that belongs to the cyclohexanedione chemical family and selectively inhibits lipid biosynthesis by targeting the acetyl-coenzyme A carboxylase (ACCase) enzyme in susceptible species (Burton et al. Reference Burton, Gronwald, Somers, Connelly, Gengenbach and Wyse1987). It is registered for use in cotton (Gossypium hirsutum L.), peanut (Arachis hypogaea L.), soybean [Glycine max (L.) Merr.], and various other broadleaf crops (Anonymous 2006, 2021). Clethodim is formulated as either a 0.24 kg ai L–1 or 0.12 kg ai L–1 emulsifiable concentrate (Anonymous 2006, 2021). The product label recommends the addition of crop oil concentrate (COC), either alone or in combination with a nitrogen source such as ammonium sulfate (AMS) for optimal efficacy (Anonymous 2006, 2021). Ammonium sulfate has been demonstrated to enhance the control of specific annual grasses by clethodim, even in the presence of other herbicides, such as 2,4-D, which are known to antagonize the efficacy of clethodim (Burke et al. Reference Burke, Price, Wilcut, Jordan, Culpepper and Tredaway-Ducar2004; Burke & Wilcut, Reference Burke and Wilcut2003).
Weed escapes following a clethodim application can result from several factors including very high weed densities, larger than optimum growth stage, and/or the use of reduced herbicide use rates (Grichar Reference Grichar1991; Prostko et al. Reference Prostko, Johnson and Mullinix2001). Several studies have shown that clethodim applied at the right timing or growth stage provided good to excellent control of Texas panicum (Grichar et al. Reference Grichar, Colburn and Kearney1994; Johnson et al. Reference Johnson, Prostko and Mullinix2002; Prostko et al. Reference Prostko, Johnson and Mullinix2001; Wilcut et al. Reference Wilcut, Wehtje and Hicks1990). Johnson et al. (Reference Johnson, Prostko and Mullinix2002) showed that clethodim provided 91% Texas panicum control under moderate (>10 plants m–2) to high (>20 plants m–2) weed infestations. In other studies, Prostko et al. (Reference Prostko, Johnson and Mullinix2001) found that a single clethodim application without preemergence herbicides provided 85% control of Texas panicum under low (3 plants m–2) to moderate (>10 plants m–2) population pressure. In addition, two applications of clethodim are often needed to control later emerging Texas panicum or escapes from heavy infestations (>20 plants m–2) or even to increase the control of Texas panicum when preemergence residual herbicides are not effective (Johnson et al. Reference Johnson, Prostko and Mullinix2002; Prostko et al. Reference Prostko, Johnson and Mullinix2001). For example, Prostko et al. (Reference Prostko, Johnson and Mullinix2001) showed that Texas panicum control with ethafluralin alone was less than 75%; however, a preemergence application of ethafluralin followed by a postemergence application of clethodim improved control by 19 percentage points. Grichar (Reference Grichar1991) found that clethodim at 0.11 kg ai ha–1 or above provided greater than 85% control of Texas panicum when applied to grasses less than 15 cm tall. However, clethodim at 0.07 kg ai ha–1 provided inconsistent control, particularly under low soil moisture and large plants, resulting in less translocation and herbicidal efficacy in Texas panicum (Chernicky et al. Reference Chernicky, Gossett and Murphy1984; Fawcett et al. Reference Fawcett, Harvey, Arnold, Bauman, Eberlein, Kells, Moshier, Slife and Wilson1987).
The efficacy of postemergence herbicides, such as clethodim, are influenced by several factors including the composition of weed species (Klingaman et al. Reference Klingaman, King and Oliver1992), size of the weeds (Klingaman et al. Reference Klingaman, King and Oliver1992; York et al. Reference York, Jordan and Wilcut1990), environmental conditions during application (Holshouser and Coble Reference Holshouser and Coble1990; Kent et al. Reference Kent, Wills and Shaw1991), product use rate (Klingaman et al. Reference Klingaman, King and Oliver1992), interactions with other agrichemicals (Hatzios and Penner Reference Hatzios and Penner1985), and the use of adjuvants (Bridges Reference Bridges1989; Wanamarta and Penner Reference Wanamarta and Penner1989; York et al. Reference York, Jordan and Wilcut1990). Adjuvants play a crucial role in enhancing the biological efficacy of herbicides by modifying various characteristics of the spray solution including surface tension, pH, viscosity, and droplet size and distribution (Green and Cahill Reference Green and Cahill2003). Their effectiveness lies predominantly in increasing herbicide absorption (McWhorter Reference McWhorter1982; Van Valkenburg Reference Van Valkenburg1982; Wanamarta and Penner, Reference Wanamarta and Penner1989). Adjuvants can be categorized into several types, including substances that reduce evaporation, penetrating agents, adhesives, spreaders, anti-foaming agents, drift reducers, and conditioners (Matthews et al. Reference Matthews, Bateman and Miller2014). Additionally, there are other functional categories identified in the literature (Urach Ferreira et al. Reference Urach Ferreira, Vinicius Thiesen, Pelegrini, Tavares Ramos, Dantas Pinto and da Costa Ferreira2020). Adjuvants enhance herbicide efficacy through two main mechanisms: one involves improving the physical properties of the carrier, whereas the other focuses on enhancing the movement of agrochemicals through the plant cuticles, especially waxy or dry ones (Hazen Reference Hazen2000). This improvement can occur by reducing the surface tension of spray solutions or by hydrating the leaf surface (Hazen Reference Hazen2000).
There are other factors that can influence the effectiveness of adjuvants including the type of adjuvant used, water quality, and prevailing weather conditions (Hatzios and Penner Reference Hatzios and Penner1985; Hull et al. Reference Hull, Davis and Stolzenberg1982; McWhorter Reference McWhorter1982). Shaner (Reference Shaner2014) noted that clethodim is susceptible to degradation by ultraviolet light. Falb et al. (Reference Falb, Bridges and Smith1990) also observed that clethodim degrades rapidly when exposed to ultraviolet light with a half-life ranging from 2.4 to 3.2 h. The addition of a petroleum-based adjuvant enhances herbicide efficacy by reducing exposure time on the leaf surface to ultraviolet light and preventing its breakdown into nonphytotoxic forms. Multiple studies have observed that the efficacy of adjuvants is influenced by the herbicide being applied and the characteristics of the target weed species (Culpepper et al. Reference Culpepper, Jordan, York, Corbin and Sheldon1999; Gugaa et al. Reference Gugaa, Zarzecka and Zadrozniak2010; Kammler et al. Reference Kammler, Walters and Young2010; Kieloch and Domaradzki Reference Kieloch and Domaradzki2008). Therefore, selecting an appropriate adjuvant for a specific herbicide and weed species is essential for optimizing herbicide performance (Wanamarta et al. Reference Wanamarta, Penner and Kells1989).
Limited research exists on the effect of weed size and adjuvants on clethodim efficacy on Texas panicum. Therefore, the objectives of research were (i) to evaluate the efficacy of single and sequential clethodim applications at different growth stages of Texas panicum and (ii) to evaluate the effect of selected adjuvant combinations on control of small and large Texas panicum. The overall goal of this study was to formulate recommendations for effective Texas panicum management in broadleaf crops by examining the interaction between application frequency, adjuvants, and weed size at the time of application.
Materials and Methods
Weed Size Field Study
Field experiments were conducted in 2023 and 2024 at the Clemson University Edisto Research and Education Center in Blackville, SC (33.36424° N, 81.33155° W; 100 m asl). The experimental design was a three (environment) by two (application, single or sequential) by four (Texas panicum growth stage) factorial arranged in a randomized complete block design with three or four replications. The three environments or study sites were assigned the following labels: 23-1, 24-2, and 24-3, which represented the one field site in 2023 and the two field sites in 2024. Plots were 2 m wide by 12 m long in 2023 and 4 m wide by 12 m long in 2024. The trial was established in a non-crop field with a natural Texas panicum infestation followed by supplemental overseeding by hand to ensure that adequate populations were present for the study. The average density of Texas panicum for the study sites in both years was 10 to 30 plants m– 2. Broadleaf weeds in the plots were removed using herbicides or hand weeding. In 2023, four plots were selected with varying Texas panicum sizes for the single application, and 10 plants were randomly assigned to each size group (10–15, 15–20, 20–30, and 30–60 cm) in the plot for the single clethodim application. Similarly, in the sequential application, three plots were selected with varying Texas panicum sizes, and 10 plants were randomly assigned in each size group (15–20, 20–30, and 30–60 cm). Each plot was then sprayed broadcast with clethodim. One plot was left untreated (i.e., no herbicide) during the study. The single application had four replications (four plots), sequential application had three replications (three plots), and the untreated had 10 replications (i.e., using individual plants at each different growth stage as experimental units). The trial was conducted at one field site. In 2024, each plot was assigned according to Texas panicum size. Therefore, 10 plots were randomly assigned by Texas panicum size or growth stage and replicated four times. An untreated check was included for the single and sequential application timings. The trial was conducted in two field sites. Clethodim was applied at 0.105 kg ai ha–1 at 10- to 15-cm, 15- to 20-cm, and 20- to 30-cm Texas panicum, with a higher rate of clethodim at 0.140 kg ai ha–1 applied to the 30- to 60-cm Texas panicum in both study years. The Texas panicum sizes represent a range recommended growth stage (10- to 15-cm) up to a salvage growth stage (30- to 60-cm). The salvage sizes represent an application timing where the grower could not treat Texas panicum at the recommended growth stage because of adverse field conditions. A sequential application of clethodim at 0.105 kg ai ha–1 was also sprayed in separate plots 2 wk after the first application for the 15- to 20-cm and 20- to 30-cm sizes, and clethodim at 0.140 kg ai ha–1 was applied to the 30- to 60-cm size.
Treatments were applied in water using a backpack sprayer calibrated to deliver 140 L ha–1 at a pressure of 276 kPa using TeeJet® XR8002 flat-fan nozzles (Spraying Systems Co., Glendale Heights, IL) with a spacing of 48 cm. Visible estimates of weed control ratings on a scale of 0 to 100% (0 indicates no control; 100 indicates complete control), and Texas panicum heights were collected 14 d after each application (DAA) timing. At 21 DAA, Texas panicum was clipped at the soil surface, dried in the oven at 41 C for 24 h, and weighed. In 2023, individual plant measurements were taken from the 10 preselected Texas panicum plants in each plot. In 2024, visible estimates of injury ratings were assessed for the entire plot, whereas plant height data were collected from six individual plants. Biomass samples were collected from two 0.25-m2 quadrats per plot. Weed height and biomass data were converted to a percentage of the nontreated for each weed size.
Texas panicum percent control, height, and biomass data were subject to a three-way ANOVA model using the PROC MIXED procedure in SAS 9.4, considering the factorial treatment arrangement to assess the differences between single and sequential applications across different locations and years. Fixed factors were environment, herbicide application, and weed size. Random factors were replications nested inside of environments. A two-way ANOVA model was used to evaluate the efficacy of a single clethodim application across different locations and years. Data were analyzed as a four-by-three factorial in a randomized complete block design using the PROC MIXED procedure in SAS 9.4 software. Fixed factors were environment and weed size. Random factors were replications nested inside of environments. Normality and homogeneity of variances were evaluated for each model using Shapiro-Wilk and Anderson-Darling goodness-of- fit test and by visual assessment of the residual plots. The data fitted the assumptions of normality of residuals and homogeneity of variances. The global F-test was used to evaluate significance, and treatment means were separated using Fisher’s protected LSD with an alpha value of 0.05.
Adjuvant Field Study
Field experiments were conducted at the Clemson University Edisto Research and Education Center in Blackville, SC in 2023 and 2024. The experimental design was a three (environment) by two (growth stage) by five (adjuvant) factorial arranged in a randomized complete block design with plot dimensions of 2 m wide by 12 m long. A nontreated plot was included for each growth stage. The three environments or study sites were assigned the following labels: 23-1, 24-2, and 24-3, which represented the one field site in 2023 and the two field sites in 2024. The trial was established in a non-crop area with a natural Texas panicum infestation followed by supplemental overseeding by hand to ensure adequate populations for the study. The average density of Texas panicum for the study sites in both years was 12 to 20 plants m–2. Broadleaf weeds in the plots were removed by using herbicides or hand weeding. A standard clethodim application rate of 0.105 kg ha–1 was used across the treatments to assess the effectiveness of the various adjuvants. Nonionic surfactant (NIS) (Trademark®; Carolina Eastern, Denmark, SC), crop oil concentrate (COC) (Register®; Carolina Eastern, Denmark, SC), methylated seed oil (MSO) (SingeTM;, Carolina Eastern, Denmark, SC), and ammonium sulfate (AMS) (AS-34 Plus; Carolina Eastern, Denmark, SC) were mixed with clethodim. To evaluate the effect of weed size on adjuvant efficacy, treatments were applied at two different growth stages: (i) optimum product label application timing, 10 to 15 cm and (ii) above the optimum product label application timing, 20 to 30 cm. Treatments were applied in water using a backpack sprayer calibrated to deliver 140 L ha–1 at a pressure of 276 kPa using TeeJet® XR8002 flat-fan nozzles (Spraying Systems Co., Glendale Heights, IL) with a spacing of 48 cm. Visual estimates of percent Texas panicum control ratings on a scale of 0 to 100 % (0 indicates no control; 100 indicates complete control) and Texas panicum height were collected 14 DAA. At 21-DAA timing, Texas panicum plants were clipped at the soil surface, dried in the oven at 41 C for 24 h, and weighed. Weed height and biomass data were converted to a percentage of the nontreated for each weed size.
A three-way ANOVA model was used to evaluate adjuvant combinations at two Texas panicum growth stages across different locations and years. Visible Texas panicum control, height, and biomass data were analyzed using the PROC MIXED procedure in SAS 9.4. Fixed factors were environment, weed size, and adjuvant. Random factors were replications nested inside of environments. Normality and homogeneity of variances were evaluated for each model by the Shapiro-Wilk and Anderson-Darling goodness-of-fit test and by visual assessment of the residual plots. The data met the assumptions of normality of residuals and homogeneity of variances. The global F-test was used to evaluate significance, and treatment means were separated using Fisher’s protected LSD with an alpha value of 0.05.
Weed Size Greenhouse Study
Studies were conducted in 2023 and 2024 at the greenhouse complex at the Clemson University Edisto Research and Education Center. The experimental design was a randomized complete block with three replications. A nontreated check was included for comparison. The trial was repeated in time. Miracle Grow Moisture Control potting mix (Scotts, Columbus, OH) was placed into 48 pots, each 10 by 10 by 9 cm in size. Texas panicum (10 seeds per pot) was planted and then irrigated with water to ensure seed germination. Greenhouse conditions were maintained at 27/21 C day/night temperature with supplemental lighting (450 µmol m–2 s–1) on a 16-h day period. Pots were watered and fertilized as needed. Two weeks after planting, the pots were thinned to one Texas panicum per pot. Texas panicum was sprayed at five different growth stages ranging from 5 cm to greater than 30 cm. In addition, a sequential application of clethodim was applied 2 wk after the first application for the 15- to 20-cm, 20- to 30-cm, and 30- to 60-cm Texas panicum heights. At the 30- to 60-cm Texas panicum growth stage, the single and sequential clethodim use rate was 0.14 kg ai ha–1. Herbicide treatments were applied using a CO2-pressurized backpack sprayer using a 11002 nozzle (TeeJet Technologies, Spraying Systems Co., Glendale Heights, IL) calibrated to deliver 140 L ha–1 at 207 kPa. Visible estimates of Texas panicum control ratings on a scale of 0 to 100 % (0 indicates no control; 100 indicates complete control) and Texas panicum height were collected 14 DAA. At 21-DAA timing, Texas panicum was clipped at the soil surface, dried in the oven at 41 C for 24 h, and weighed. Texas panicum height and biomass data were converted to a percentage of the nontreated for each weed size.
A two-way ANOVA model was used to assess the differences between single and sequential applications at three Texas panicum growth stages across three trial replicates. Data were analyzed using PROC MIXED procedure in SAS 9.4 software. Fixed factors were herbicide application and weed size, whereas the random factor was run (replications in time). Normality and homogeneity of variances were evaluated for each model by the Shapiro-Wilk and Anderson-Darling goodness-of-fit test and by visible assessment of the residual plots. The data met the assumptions of normality of residuals and homogeneity of variances. The global F-test was used to evaluate significance, and treatment means were separated using Fisher’s protected LSD with an alpha value of 0.05.
Adjuvant Greenhouse Study
Studies were conducted during 2023 and 2024 at the greenhouse complex at the Clemson University Edisto Research and Education Center. The experimental design was a two (growth stage) by five (adjuvant) factorial arranged in a randomized complete block design with three replications per treatment. Trials were repeated in time. Miracle Grow Moisture Control potting mix (Scotts, Columbus, OH) was placed into 36 pots, each 10 by 10 by 9 cm in size. Texas panicum seed (10 seeds per pot) was planted and then irrigated with water to ensure seed germination. Greenhouse conditions were maintained at 27/21 C day/night temperature with supplemental lighting (450 µmol m–2 s–1) on a 16-h day period. Pots were watered and fertilized as needed. After 2 wk of planting, pots were thinned to one Texas panicum per pot. A single clethodim application rate of 0.105 kg ha–1 was used across the treatments to assess the effectiveness of the various adjuvants. Adjuvant, Texas panicum growth stage at the time of application, and application parameters in the greenhouse study were previously described in the adjuvant field study. Visible estimates of percent Texas panicum control ratings on a scale of 0 to 100% (0 indicates no control; 100% indicates complete control), and Texas panicum height were collected 14 DAA. At 21 DAA, Texas panicum plants were clipped at the soil surface, dried in the oven at 41 C for 24 h, and weighed.
Weed height and biomass data were converted to a percentage of the nontreated for each weed size and adjuvant combination. A two-way ANOVA model was used to assess adjuvant combinations at two Texas panicum growth stages across three trial replicates. Data were analyzed using PROC MIXED procedure in SAS 9.4 software. Fixed factors were weed size and adjuvant, and the random factor was run (replications in time). Normality and homogeneity of variances were evaluated by the Shapiro-Wilk and Anderson-Darling goodness-of-fit test and by visible assessment of the residual plots. The data fit the assumptions of normality of residuals and homogeneity of variances. The global F-test was used to evaluate significance, and treatment means were separated using Fisher’s protected LSD with an alpha value of 0.05.
Results and Discussion
Weed Size Field Study
Texas Panicum Control. Texas panicum percent control data were pooled across environments because of a nonsignificant environment-by-weed size-by-application (P = 0.2270). However, there was a significant application-by-environment (P < 0.0001) and application-by-weed size interaction (P < 0.0001). Therefore, the control data were combined across environments and presented by weed size and application. Clethodim provided 90% Texas panicum control when applied at a single application at the 10- to 15-cm growth stage (Table 1). Single clethodim application also provided 76% control at the 15- to 20-cm growth stage, but a sequential application improved Texas panicum control to 91% (Table 1). Texas panicum control decreased to 67% and 63% in the single application at 20- to 30-cm and 30- to 60-cm growth stages, respectively (Table 1). Previous research has shown that clethodim must be applied early to grasses less than 10 cm in height for adequate control (Grichar Reference Grichar1991). In this study, control declined as Texas panicum size increased in both the single and sequential applications (Table 1).
Table 1. The effect of single and sequential field applications on clethodim a efficacy on Texas panicum percent control and height reduction 14 d after treatment (DAT) and biomass 21 DAT in 2023 and 2024. b
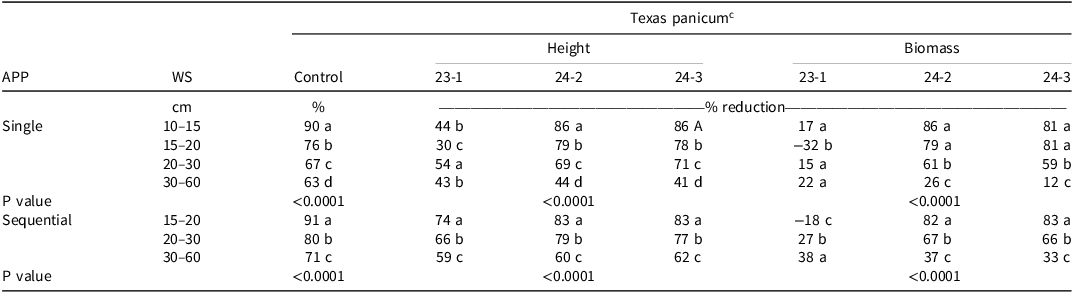
a Clethodim application rate was 0.105 kg ai ha–1 for the 10- to 15-cm, 15- to 20-cm, and 20- to 30-cm Texas panicum stages and 0.14 kg ai ha–1 for the 30- to 60-cm growth stages.
b Abbreviations: APP, application; WS, weed size; 23-1, site location 1 in 2023; 24-2, site location 2 in 2024; site location 3 in 2024, 24-3.
c Means within columns followed by the same letter are not significantly different according to Fisher’s protected LSD at α = 0.05.
Texas panicum control at the 20- to 30- and 30- to 60-cm growth stage was lower in the single applications (67% to 63%, respectively), but it was higher in the sequential applications (80% to 71%, respectively) (Table 1). Two applications of clethodim improved Texas panicum control with an average increase of 12% when comparing sequential to single applications. Texas panicum control was higher in 2023 (85%) compared to 2024 (78% at 24-2 and 80% at 24-3) with sequential applications, whereas it was lower in 2023 (66%) than in 2024 (70% at both sites) with a single clethodim application. These results confirm previous research observations where large grass sizes at the time of treatment have been a factor in reduced control with several postemergence herbicides (Grichar and Boswell Reference Grichar and Boswell1986; Grichar Reference Grichar1991).
Texas Panicum Height. There was a weed size-by-application-by-environment interaction (P < 0.0001); therefore, Texas panicum height data could not be pooled across environments. The Texas panicum height nontreated means 14 DAT following single application of clethodim were 24, 33, 64, and 78 cm for the 10- to 15-cm, 15- to 20-cm, 20- to 30-cm, and 30- to 60-cm growth stages, respectively, and following sequential application of clethodim was 69 cm, 77 cm, and 86 cm for 15- to 20-cm, 20- to 30-cm, and 30- to 60-cm growth stages, respectively, in 23-1. The Texas panicum height nontreated means 14 DAT were 94 cm (single) and 116 cm (sequential) in 24-2 and 99 cm (single) and 146 cm (sequential) in 24-3. Trends in Texas panicum height reduction were similar to the observed Texas panicum percent control, except for the single clethodim application at 23-1, which showed inconsistencies across the treatments (Table 1). The greatest reduction in Texas panicum height was 86% when clethodim was applied at the 10- to 15-cm growth stage in 2024 (Table 1). As expected, the reduction in height decreased as Texas panicum size increased in both the single and sequential clethodim treatments (Table 1). In contrast, clethodim efficacy on other larger grass species has been excellent. For example, clethodim provided 95% or better control of giant foxtail (Setaria faberi Herrm.) up to 60 cm in height (Krausz et al. Reference Krausz, Kapusta and Matthews1993). This indicates that clethodim efficacy on larger grasses is species dependent. A second application of clethodim 14 DAT resulted in a 15% increase in Texas panicum height reduction across the different Texas panicum growth stages.
Texas Panicum Biomass. Environment-by-weed size-by-herbicide application interaction was nonsignificant (P = 0.1098); however, there was significant interaction between environment and herbicide application (P = 0.0015), environment and herbicide application (P < 0.0001), and herbicide application and weed size (P < 0.0001). Therefore, Texas panicum biomass was presented separately by herbicide application, weed size, and environment. Nontreated means for Texas panicum biomass 21 DAT was 2 g, 4 g, 8 g, and 20 g plant–1 for the 10- to 15-cm, 15- to 20-cm, 20- to 30-cm, and 30- to 60-cm growth stages (single), respectively, and 7 g, 11 g, and 31 g plant–1 for the 15- to 20-cm, 20- to 30-cm, and 30- to 60-cm growth stages (sequential), respectively, in site 23-1. Nontreated Texas panicum biomass means 21 DAT were 138 g m–2 (single) and 175 g m–2 (sequential) in site 24-2 and 111 g m–2 (single) and 196 g m–2 (sequential) in site 24-3. The variability observed in the 23-1 Texas panicum biomass was a result of intra-plant Texas panicum competition in the untreated checks. The reduced growth and biomass led to untreated dry weights that were lower than the treated Texas panicum dry weights, resulting in minimal to negative percent reductions in biomass (Table 1). The decrease in Texas panicum biomass was greater in sites 24-2 and 24-3 than in 23-1 for both single and sequential clethodim applications (Table 1). The reduction in Texas panicum biomass following a single application of clethodim had a similar pattern to Texas panicum control and height data in 2024. Texas panicum biomass was reduced by 86% (24-2) and 81% (24-3) following a single clethodim application at 10 to 15 cm (Table 1). However, the reduction in biomass declined as Texas panicum size increased for both the single and sequential applications (Table 1). Metier et al. (Reference Metier, Lehnhoff, Mangold, Rinella and Rew2019) also observed that downy brome (Bromus tectorum L.) biomass reduction using clethodim was the highest at smaller plant sizes. Sequential clethodim application 14 d apart resulted in the largest Texas panicum biomass reduction compared to the single application with an average increase of 23% across the different Texas panicum growth stages.
Adjuvant Field Study
Texas Panicum Control. The interaction between environment, adjuvant, and weed size was not significant (P = 0.0534). However, the interaction between weed size and adjuvant was significant (P = 0.0013). Therefore, the data were pooled across environment and separated by weed size and adjuvant. Clethodim plus COC and COC + AMS provided 91% and 93% Texas panicum control, respectively, at the 10- to 15-cm growth stage, followed by clethodim plus MSO + AMS with 90% Texas panicum control (Table 2). The lowest Texas panicum control was observed with the NIS at 66% and 59% for the 10- to 15-cm and 20- to 30-cm growth stages, respectively. Culpepper et al. (Reference Culpepper, Jordan, York, Corbin and Sheldon1999) observed lower absorption of radiolabeled clethodim in barnyardgrass [Echinochloa crus-galli (L.) Beauv.] leaves with NIS compared to COC or MSO. Clethodim plus MSO provided 76% and 73% Texas panicum control at the 10- to 15-cm and 20- to 30-cm growth stages, respectively, which was significantly lower than clethodim plus COC or MSO + AMS. Jordan et al. (Reference Jordan, Vidrine, Griffen and Reynolds1996) observed similar results where COC or MSO plus clethodim resulted in higher Johnsongrass [Sorghum halepense (L.) Pers.], broadleaf signalgrass [Urochloa platyphylla (Nash) R.D. Webster], and barnyardgrass control compared to clethodim plus NIS. Bridges (Reference Bridges1989) also observed higher Johnsongrass control with clethodim tank mixed with a petroleum-based adjuvant, such as COC.
Table 2. The effect of adjuvant in the field on clethodim at 0.105 kg ai ha–1 efficacy on Texas panicum percent control and height reduction at two growth stages 14 d after treatment in 2023 and 2024. a
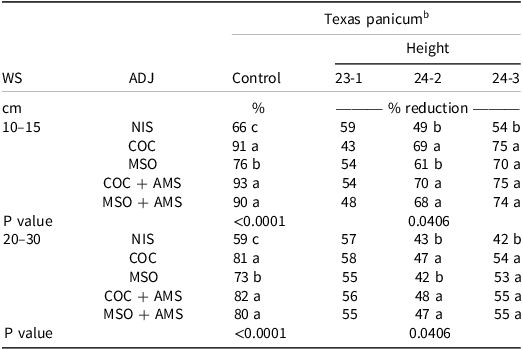
a Abbreviations: ADJ, adjuvant; AMS, ammonium sulfate; COC, crop oil concentrate; 23-1, site location 1 in 2023; 24-2, site location 2 in 2024; site location 3 in 2024, 24-3; MSO, methylated seed oil; NIS, nonionic surfactant; WS, weed size.
b Means within columns followed by the same letter are not significantly different according to Fisher’s protected LSD at α = 0.05.
Texas Panicum Height. Data were not pooled across environments because of a significant interaction between height, environment, and weed size (P = 0.0406). Nontreated means for Texas panicum height 21 DAT were 35 cm, 37 cm, and 47 cm for the 10- to 15-cm growth stage at the 23-1, 24-2, and 24-3 sites, respectively, and 57 cm, 66 cm, 75 cm for the 20- to 30-cm growth stage at the 23-1, 24-2, and 24-3 sites. No significant differences were observed in the height reduction across the different treatments in 2023 as a result of competition from other weeds in the plots. In 2024, clethodim plus COC, COC + AMS, and MSO + AMS provided the greatest reduction in plant height (68% to 70%) at the 10- to 15-cm growth stage at site 24-2 (Table 2). The addition of AMS improved MSO efficacy, resulting in an increase of 7% Texas panicum height reduction. In the 24-3 site, Texas panicum biomass was reduced (70% to 75%) in all treatments except for clethodim plus NIS (54%) (Table 2).
Texas Panicum Biomass. There was no interaction among environment, weed size, and adjuvant (P = 0.9559), and environment and weed size interaction was not significant (P = 0.3032); however, environment-by-adjuvant (P = 0.0074) interaction and weed size (P = 0.0172) main effect were significant. Therefore, Texas panicum biomass data were separated by environment and adjuvant and combined across environment and adjuvant for weed size. Nontreated means for Texas panicum biomass 21 DAT were 209 g m–2, 357 g m–2, and 420 g m–2 in the 23-1, 24-2, and 24-3 sites, respectively. The biomass reduction was 6% lower at the 20- to 30-cm Texas panicum growth stage when averaged across the different adjuvant treatments (Table 3). In 2023, differences in Texas panicum biomass reduction were not observed as a result of competition from other weeds, which reduced the growth of the nontreated Texas panicum resulting in lower biomass relative to the treated plants (Table 3). The dry-biomass reduction followed a similar pattern to Texas panicum control and height across the two sites during 2024. Clethodim plus COC, COC + AMS, or MSO + AMS provided the greatest Texas panicum biomass reduction (74% to 76%, 74% to 75%, and 73% to 74%, respectively) (Table 3). The clethodim plus AMS + MSO provided 17% increase in biomass reduction compared to clethodim plus MSO across the two sites in 2024 (Table 3).
Table 3. The effect of adjuvant on clethodim at 0.105 kg ai ha–1 in the field on Texas panicum biomass reduction at 21 d after treatment in 2023 and 2024. a
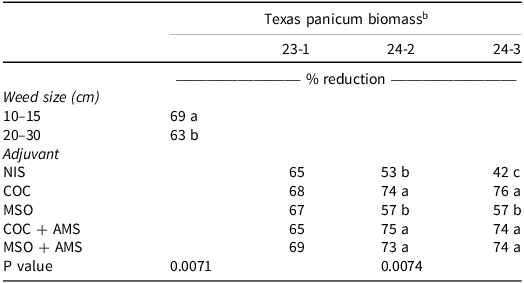
a Abbreviations: 23-1, site location 1 in 2023; 24-2, site location 2 in 2024; site location 3 in 2024, 24-3; AMS, ammonium sulfate; COC, crop oil concentrate; MSO, methylated seed oil; NIS, nonionic surfactant.
b Means within columns followed by the same letter are not significantly different according to Fisher’s protected LSD at α = 0.05.
Congreve and Cameron (Reference Congreve and Cameron2019) observed that lipophilic herbicides, such as clethodim, are best complemented by a lipophilic adjuvant, such as COC, which increases leaf penetration. The use of petroleum oil concentrates and esterified vegetable oils is common with clethodim and other Group 1 herbicides. However, Texas panicum control was reduced at the 20- to 30-cm growth stage, regardless of the adjuvant. Previous studies have shown that clethodim applied to larger grasses was generally less effective, and higher rates were required for adequate control (Grichar Reference Grichar1991). Ammonium sulfate did improve clethodim plus MSO efficacy on Texas panicum but not with the mixture of clethodim plus COC. Ammonium sulfate has been demonstrated to enhance the control of specific annual grasses by clethodim, even in the presence of herbicides targeting broadleaf and sedge control, which are known to antagonize the efficacy of clethodim (Burke et al. Reference Burke, Price, Wilcut, Jordan, Culpepper and Tredaway-Ducar2004; Burke & Wilcut, Reference Burke and Wilcut2003).
Greenhouse Weed Size Study
Texas Panicum Control. There was a significant interaction between weed size and application (P < 0.0001). Therefore, data were reported separately. Clethodim applied in a single application provided 97% and 98% Texas panicum control at the 5- to 10-cm and 10- to 15-cm growth stages, respectively (Table 4). Texas panicum control was 76% at the 15- to 20-cm growth stage; however, a sequential clethodim application increased Texas panicum control by 15% (Table 4). The 20- to 30-cm and 30- to 60-cm Texas panicum with a single clethodim application had control of 63% to 73%, respectively; However, control increased by 12% and 13% for the 20- to 30-cm and 30- to 60-cm Texas panicum, respectively, when a sequential application of clethodim was used (Table 4). Similar to the weed size field studies, clethodim efficacy on Texas panicum declined as weed size increased for both the single and sequential applications. When averaged over weed size, two applications of clethodim 14 d apart provided a higher level of Texas panicum control with an average increase of 13%.
Table 4. The effect of single and sequential applications of clethodim a at 0.105 kg ai ha–1 in the greenhouse on Texas panicum percent control and height reduction 14 d after treatment in 2023 and 2024. b
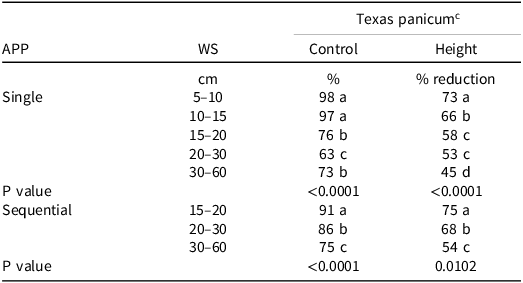
a Clethodim application rate was 0.105 kg ai ha–1 for the 10- to 15-cm, 15- to 20-cm, 20- to 30-cm Texas panicum growth stages and 0.14 kg ai ha–1 for the 30- to 60-cm growth stages.
b Abbreviations: APP, application; WS, weed size.
c Means within columns followed by the same letter are not significantly different according to Fisher’s Protected LSD Test at P ≤ 0.05.
Texas Panicum Height. There was a significant interaction between weed size and application (P = 0.0102). Therefore, weed size and application were reported separately. Nontreated Texas panicum height means 14 DAT were 26 cm, 35 cm, 53 cm, 71 cm, and 81 cm for the 5- to 10-cm, 10- to 15-cm, 15- to 20-cm, 20- to 30-cm, and 30- to 60-cm growth stages (single), respectively, and 81, 83, and 86 cm for the 15- to 20-cm, 20- to 30-cm, and 30- to 60-cm growth stages (sequential), respectively. The reduction in Texas panicum height showed a similar trend to Texas panicum control. Clethodim provided the highest reduction in Texas panicum height when applied at a single application at the 5- to 10-cm and 10- to 15-cm growth stage (73% and 66%, respectively) (Table 4). However, height reduction declined at greater than 15- to 20-cm Texas panicum. Clethodim applied at the 20- to 30-cm and 30- to 60-cm Texas panicum resulted in the lowest Texas panicum height reduction. Texas panicum height reduction following a sequential application was 75% and 68% at 15- to 20-cm and 20- to 30-cm, respectively (Table 4). Similar to Texas panicum control, height reduction declined as Texas panicum size increased even with a sequential application of clethodim. Two applications of clethodim 14 d apart reduced Texas panicum height compared to the nontreated at the 15- to 20-cm, 20- to 30-cm, and 30- to 60-cm growth stages with an average increase of 14% over a single application.
Texas Panicum Biomass. There was a nonsignificant interaction between weed size and clethodim application (P = 0.7074). However, weed size (P = 0.0010) and clethodim application (P < 0.001) were significant interactions; therefore, data were presented separately by weed size and clethodim application. Nontreated means for Texas panicum biomass at 21 DAT were 11 g plant–1 and 4 g plant–1 for the sequential and single applications, respectively. Nontreated means for Texas panicum biomass at 21 DAT were 7, 8, and 10 g plant–1 for the 15- to 20-cm, 20- to 30-cm, and 30- to 60-cm growth stages, respectively. The dry-biomass reduction followed a similar pattern to Texas panicum control. The highest Texas panicum dry-biomass reduction (88% and 81%, respectively) was observed at the 5- to 10-cm and 10- to 15-cm growth stages (Table 5). However, biomass reduction declined as Texas panicum size increased. The “late application” at the 30- to 60-cm Texas panicum growth stage had the smallest relative dry-biomass reduction. Two applications of clethodim 14 d apart increased Texas panicum dry-biomass reduction by 22% when averaged across Texas panicum growth stages compared to a single application (Table 5). Texas panicum biomass reduction declined as weed size increased regardless of single or sequential application of clethodim.
Table 5. The effect of single and sequential applications of clethodim a in the greenhouse on Texas panicum biomass reduction as affected by different applications and weed sizes 21 d after treatment in 2023 and 2024.

a Clethodim application rate was 0.105 kg ai ha–1 for the 10- to 15-cm, 15- to 20-cm, 20- to 30-cm Texas panicum growth stages and 0.14 kg ai ha–1 for the 30- to 60-cm growth stages.
b Means within columns followed by the same letter are not significantly different according to Fisher’s protected LSD Test at α = 0.05.
Greenhouse Adjuvant Study
Texas Panicum Control. The Texas panicum control data had no significant interaction between weed size and adjuvant (P = 0.9277). However, weed size (P < 0.0001) and adjuvant (P < 0.0001) were significant; therefore, data were presented separately by weed size and adjuvant. Clethodim plus COC, COC + AMS, and MSO + AMS provided the highest level of Texas panicum control of 92%, 96%, and 93%, respectively (Table 6). Control was reduced for adjuvant treatments at the 20- to 30-cm Texas panicum size. Texas panicum control was 6% lower at the 20- to 30-cm growth stage (Table 6). Ammonium sulfate plus clethodim plus MSO increased Texas panicum control 6% over clethodim plus MSO alone. However, adding AMS to clethodim plus COC did not increase the level of control compared to clethodim plus COC (Table 6).
Table 6. The effect of adjuvant in the greenhouse on clethodim at 0.105 kg ai ha–1 on Texas panicum percent control and height 14 d after treatment (DAT) and biomass 21 DAT at two growth stages. a
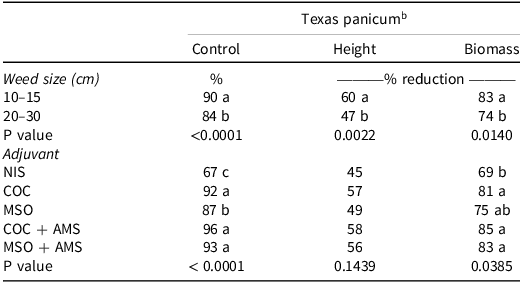
a Abbreviations: AMS, ammonium sulfate; COC, crop oil concentrate; MSO, methylated seed oil; NIS, nonionic surfactant.
b Means within columns followed by the same letter are not significantly different according to Fisher’s protected LSD test at α = 0.05.
Texas Panicum Height. The data showed no significant interaction between weed size and adjuvant (P = 0.9314). Therefore, the data were reported by weed size (P = 0.0022) and adjuvant (P = 0.1439). Nontreated means for Texas panicum height 14 DAT were 42 cm and 53 cm for the 10- to 15-cm and 20- to 30-cm growth stages, respectively. There were no differences in Texas panicum height reduction among the adjuvants (NIS, COC, MSO, COC + AMS, and MSO + AMS) (Table 6). However, when averaged across adjuvants, Texas panicum height reduction was 13% higher at the 10- to 15-cm Texas panicum growth stage compared to the 20- to 30-cm growth stage. In contrast to the field study, clethodim plus COC + AMS or MSO + AMS did not improve Texas panicum height reduction compared to clethodim plus MSO or COC (Table 6).
Texas Panicum Biomass. There was no significant interaction between weed size and adjuvant (P = 0.9086). Therefore, data were separated by weed size (P = 0.0140) and adjuvant (P = 0.0385). Nontreated means for Texas panicum biomass 21 DAT were 3 g plant–1 and 12 g plant–1 for the 10- to 15-cm and 20- to 30-cm growth stages, respectively. The dry-biomass reduction followed a similar pattern to Texas panicum control. Texas panicum dry-biomass reduction was higher at the 10- to 15-cm Texas panicum growth stage compared to the larger ones when averaged across adjuvants. Clethodim plus NIS had the lowest Texas panicum dry-biomass reduction (69%) when combined across weed sizes. Clethodim plus COC, MSO, COC + AMS, and MSO + AMS provided 81%, 75%, 85%, and 83% Texas panicum dry-biomass reduction, respectively, averaged over the Texas panicum growth stages (Table 6). No differences in Texas panicum dry-biomass reduction were observed between clethodim plus COC or MSO and clethodim plus COC + AMS or MSO + AMS (Table 6).
Texas panicum response at various growth stages to clethodim in the greenhouse was higher than in the field, likely as a result of the plants being grown under optimal environmental conditions along with regular irrigation and fertilization. These favorable conditions enhanced clethodim efficacy on Texas panicum, particularly at smaller weed sizes, with control reaching up to 97% in the greenhouse at the 10- to 15-cm growth stage. In contrast, Texas panicum plants in the field often grew under challenging environmental conditions, such as water stress and high temperatures, which can negatively affect clethodim efficacy by reducing its absorption and translocation in the target plant. The field results showed reduced control, particularly on larger Texas panicum plants, where the thicker cuticles, exacerbated by water stress, limited herbicide uptake. This was consistent with previous studies, where water stress led to increased cuticle thickness, reducing the effectiveness of foliar-applied herbicides (Shaner Reference Shaner1989). Additionally, sequential applications improved Texas panicum control, but the improvement was more pronounced under greenhouse conditions because of the absence of environmental stresses. These results suggest that clethodim efficacy declines as weed size increases and that harsh field conditions further limit herbicide performance, especially on larger Texas panicum plants. Therefore, although sequential applications did improve Texas panicum control, the impact of environmental factors in the field may have reduced overall efficacy compared to the observations in the greenhouse.
In the adjuvant study, clethodim plus COC, COC + AMS, or MSO + AMS provided the highest Texas panicum control. However, the differences among adjuvants were more pronounced in the field, where environmental stress, such as water stress and temperature, likely reduced herbicide performance, especially at the larger Texas panicum growth stages. Whereas adjuvant enhanced clethodim efficacy in both the field and greenhouse, the controlled conditions of the greenhouse resulted in consistent and higher levels of Texas panicum control. For instance, under greenhouse conditions, the average efficacy of clethodim across weed sizes was 92%, 96%, and 93% for COC, COC + AMS, and MSO + AMS, respectively. In contrast, the same adjuvants only provided 86%, 88%, and 85% Texas panicum efficacy in the field. Across both environments, AMS addition improved clethodim plus MSO efficacy on Texas panicum, but its effect on clethodim plus COC was less pronounced. Overall, although adjuvants enhanced clethodim efficacy across environments, the optimum growing conditions in the greenhouse allowed for more consistent and higher levels of Texas panicum control compared to the field, where environmental variability posed challenges.
The findings from this study highlight the importance of growth stage, application timing, and proper adjuvant selection for effective control of Texas panicum using clethodim. Two applications 14 d apart significantly improved Texas panicum control across the weed sizes evaluated. A single clethodim application provided adequate Texas panicum control at the 10- to 15-cm or less growth stage. However, a second application is needed for effective management of Texas panicum exceeding 15 cm. This study showed that clethodim efficacy decreases as Texas panicum size increases, emphasizing the need for timely applications. Both Texas panicum size and the choice of adjuvant play crucial roles in determining clethodim effectiveness. Among the adjuvants tested, clethodim combined with COC, COC + AMS, and MSO + AMS provided the highest level of Texas panicum control at the 10- to 15-cm growth stage. However, control efficacy was notably reduced for Texas panicum sizes in the 20- to 30-cm range, regardless of the adjuvant used. The addition of AMS improved the efficacy of MSO on Texas panicum but did not enhance the performance of COC. Based on these findings, growers are advised to target clethodim applications when Texas panicum is within the 5- to 15-cm growth stage and to use either COC or MSO in combination with AMS for optimal results. This study underscores the need for herbicide application timing at the optimum growth stage and the selection of an adjuvant that maximizes control of Texas panicum. If Texas panicum size is larger than 15 cm, a second application will be needed for effective control. Growers can improve their Texas panicum management strategies, which can ultimately contribute to higher crop yields from less weed competition.
Practical Implications
Texas panicum is a challenging weed to manage in broadleaf crops, especially in non-glyphosate-resistant crops. Texas panicum, a large-seeded grass, is only suppressed by Group 15 residual herbicides, such as acetochlor, dimethanemid-p, pyroxasulfone, and S-metolachlor; therefore, clethodim is often used to manage escapes during the growing season. Challenging environmental conditions including drought and high temperature can reduce the efficacy of clethodim on Texas panicum. In addition, applications of clethodim to larger Texas panicum often result in reduced control with regrowth often occurring. As farm size increases, growers face greater challenges to spray clethodim before Texas panicum grows too large for optimum control. Clethodim applications made after Texas panicum exceeds 15 cm in height resulted in a significant decrease in control and reductions in height and biomass, confirming that timely clethodim applications are important. The clethodim product label recommends tank-mixing an oil-based adjuvant for optimum efficacy on grass weeds. Our results showed that the addition of COC or MSO + AMS to clethodim provided the best Texas panicum control, regardless of growth stage. However, this synergism was not as apparent at the larger Texas panicum growth stages. In the greenhouse environment, clethodim control of Texas panicum was higher, regardless of size or adjuvant, compared to studies conducted in the field. These results confirm that clethodim is still an efficacious herbicide on Texas panicum; however, the use of a single herbicide, such as clethodim, for Texas panicum management is a concern because there are numerous other confirmed ACCase-resistant populations of grasses across the southern United States. This research demonstrates the importance of applying clethodim in mixture with an oil-based adjuvant at the optimum Texas panicum growth stage. Larger Texas panicum may require a sequential application and/or a higher use rate of clethodim.
Acknowledgments
This paper is Technical Contribution No. 7402 of the Clemson University Experiment Station. This material is based upon work supported by the U.S. Department of Agriculture–National Institute of Food and Agriculture under Project SC-1700592. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Department of Agriculture.
Funding
Partial funding for this project was provided by the South Carolina Peanut Board.
Competing of Interests
The authors declare they have no competing interests.








