Introduction
The lichen genus Lecanora is one of the largest genera of lichenized fungi and shows large variation in thallus and apothecium morphology. Most species are crustose and develop apothecia with a thalline margin containing algae. The core part of the genus is the L. subfusca group, which is characterized by a crustose thallus containing atranorin, apothecia that are mainly brownish with a margin containing calcium oxalate crystals, and broadly ellipsoid simple spores. This group is very large and extends worldwide with many similar species, and includes the type species of the genus, L. allophana Nyl. One of the most enigmatic members of this group is L. hybocarpa (Tuck.) Brodo, which was described from Ohio, USA, by Tuckerman (Tuckerman in Lea (Reference Lea1849)) but for a long time was not distinguished from related species by American lichenologists (Brodo Reference Brodo1984). Zahlbruckner (Reference Zahlbruckner1928) overlooked it in his Catalogus and it continued to be disregarded until Brodo included it in his well-known work on the L. subfusca group in North America (Brodo Reference Brodo1984). The species was claimed to be endemic to North America by Brodo (Reference Brodo1984), but later listed from a number of European countries, such as France (Roux Reference Roux2020), Germany (Wirth et al. Reference Wirth, Hauck and Schultz2013), Great Britain (Edwards et al. Reference Edwards, Aptroot, Hawksworth, James, Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009), the Netherlands (Aptroot et al. Reference Aptroot, van Herk, Sparrius and Spier2004), Italy (Nimis Reference Nimis2016) and Spain (Llimona & Hladun Reference Llimona and Hladun2001). Recently, however, the identity of British specimens has been questioned, and it has been suggested that the British records could be forms of L. sinuosa Herk & Aptroot (Sanderson Reference Sanderson2020) and different from the North American taxon (Cannon et al. Reference Cannon, Malíček, Ivanovich, Printzen, Aptroot, Coppins, Sanderson, Simkin and Yahr2022).
In this study, we aimed to clarify the relationship between North American L. hybocarpa and European collections, including the morphologically similar L. sinuosa.
Materials and Methods
Material of the Lecanora group of interest was collected by the authors from a number of European countries and is deposited in LD, O, PRA, PRC, TRH and the personal herbaria of J. Malíček and U. Schiefelbein. We have also used material deposited in B, CANL, FH, GZU, L and NY or provided to us by B. Coppins, I. Garrido-Benavent, K. van Herk, Z. Palice, S. Pérez-Ortega, H. Sipman, L. Spier, J. Starosta, J. Steinová and J. Vondrák. We complemented our own sequence dataset with sequences determined as L. hybocarpa and mainly European sequences of the L. subfusca group from GenBank.
The specimens were examined by interference contrast and light microscopy. Anatomical features were measured on hand-cut sections or squash preparations mounted in water. Morphological characters were measured on dry material using a dissecting microscope (×40). The chemical reagents used were 10% potassium hydroxide (K), undiluted standard bleach (C), 50% nitric acid (N), and paraphenylenediamine in ethanol (Pd). Crystals were studied in polarized light (POL). Spore dimensions were calculated from ten measurements per specimen and are presented in the following way: (min. extremes) 85% of the variation with a mean in italics (max. extremes). Specimens were studied using thin-layer chromatography (TLC) according to standard methods (Orange et al. Reference Orange, James and White2001) or with a small number of minor modifications following Malíček et al. (Reference Malíček, Berger, Palice and Vondrák2017).
Molecular analyses
PCR amplification was carried out on DNA extracts or using direct PCR, either following Arup et al. (Reference Arup, Vondrák and Halıcı2015) or Malíček et al. (Reference Malíček, Berger, Palice and Vondrák2017, Reference Malíček, Coppins, Palice, Vančurová, Vondrák and Sanderson2023). Amplifications were made of the internal transcribed spacer regions (nrITS) and the small subunit of the mitochondrial ribosomal RNA gene (mtSSU). Primers used for amplification were ITS1F (Gardes & Bruns Reference Gardes and Bruns1993), ITS4 (White et al. Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990), mrSSU1, mrSSU2, mrSSU3R (Zoller et al. Reference Zoller, Scheidegger and Sperisen1999) and mrSSU7 (Zhou & Stanosz Reference Zhou and Stanosz2001). The PCR parameters included an initial hold at 94 °C for 5 min, then denaturation at 94 °C for 1 min, annealing at 50 or 54 °C (mrSSU) or 53–56 °C (nrITS) for 1 min, decreasing by 1 °C per cycle for the first six of the 39 cycles (touchdown), and an extension at 72 °C for 3 min. Sequencing was carried out by Macrogen Inc. (the Netherlands) and BIOCEV (Czech Republic) using the same primers as for the PCR and the resulting sequences were assembled using Geneious v. 11.1.5.
Sequence alignment
Two different datasets were prepared, one for a combined analysis consisting of 86 sequences each of the internal transcribed spacer region (nrITS) and the mitochondrial ribosomal RNA gene (mtSSU), and one dataset of 109 nrITS sequences, including one outgroup species. The combined analysis included Lecanora species in a wide sense and related genera in order to place the species of interest in a phylogenetic context. The nrITS dataset included only species of the L. subfusca group of interest for our study. For both analyses, Protoparmelia badia (Hoffm.) Hafellner from the family Parmeliaceae was used as outgroup; this has been shown to be an optimum distance from Lecanora (Arup et al. Reference Arup, Ekman, Grube, J-E and Wedin2007), and it is easy to align with the ingroup. Subsequent alignments were performed in Geneious v. 11.1.5 using the MAFFT option (auto) and adjusted manually. Unalignable ends, indels in all the aligned genes and ambiguously aligned parts were excluded from the alignment. Sequences were submitted to GenBank (Table 1). The alignments of the two different genes were first analyzed separately to check for incongruence between genes. A conflict between the datasets was assumed to be significant if two different relationships were both supported with posterior probabilities ≥ 0.95, but none were found. There were 15 additional mtSSU sequences that were not used in the analyses due to the absence of the accompanying ITS sequence (see Supplementary Material Table S1, available online). These sequences confirm the correct identification of the other specimens studied.
Table 1. Voucher information and GenBank Accession numbers of Lecanora species and related genera used in the phylogenetic analyses. Newly produced sequences are shown in bold.
Phylogenetic analysis
Phylogenetic relationships were inferred using maximum likelihood (ML) as implemented in IQ-TREE2 (Minh et al. Reference Minh, Schmidt, Chernomor, Schrempf, Woodhams, von Haeseler and Lanfear2020). Bayesian tree inference was carried out using Markov chain Monte Carlo (MCMC) as implemented in MrBayes v. 3.2 (Ronquist et al. Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012). In the combined analysis, the two included genes were treated as separate partitions. A suitable likelihood model for each of the genes was selected, using the Bayesian information criterion (BIC) as implemented in the software jModelTest v. 2.1.4 (Guindon & Gascuel Reference Guindon and Gascuel2003; Darriba et al. Reference Darriba, Taboada, Doallo and Posada2012), evaluating only the 24 models available in MrBayes (Ronquist et al. Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012). For the concatenated dataset, the SYM + I + G model was found to be optimal for the nrITS dataset and HKY + I + G for the mtSSU. For the pure nrITS dataset, the evolutionary model HKY + I + G was found to be optimal. The parameters used in the Bayesian analyses followed those of Arup et al. (Reference Arup, Søchting and Frödén2013), except for the branch length prior that was set to an exponential with mean 1/10. Three parallel runs with 20 000 000 generations starting with a random tree and employing six simultaneous chains were executed, five of which were incrementally heated with a temperature of 0.10. Analyses were diagnosed every 1000 generations in the last 50% of the tree sample and automatically halted when convergence was reached. Convergence was defined as an average standard deviation of splits (of frequency 0.1) between runs below 0.01. Every 2000th tree was sampled. A majority-rule consensus tree was constructed from the post-burn-in tree samples. The consensus trees were visualized using FigTree v. 1.4.4 (Rambaut Reference Rambaut2018) and redrawn in Adobe Illustrator. The maximum likelihood analyses used the same evolutionary models as in the Bayesian analyses. Branch support values were computed via 1000 non-parametric bootstrap replicates.
Results
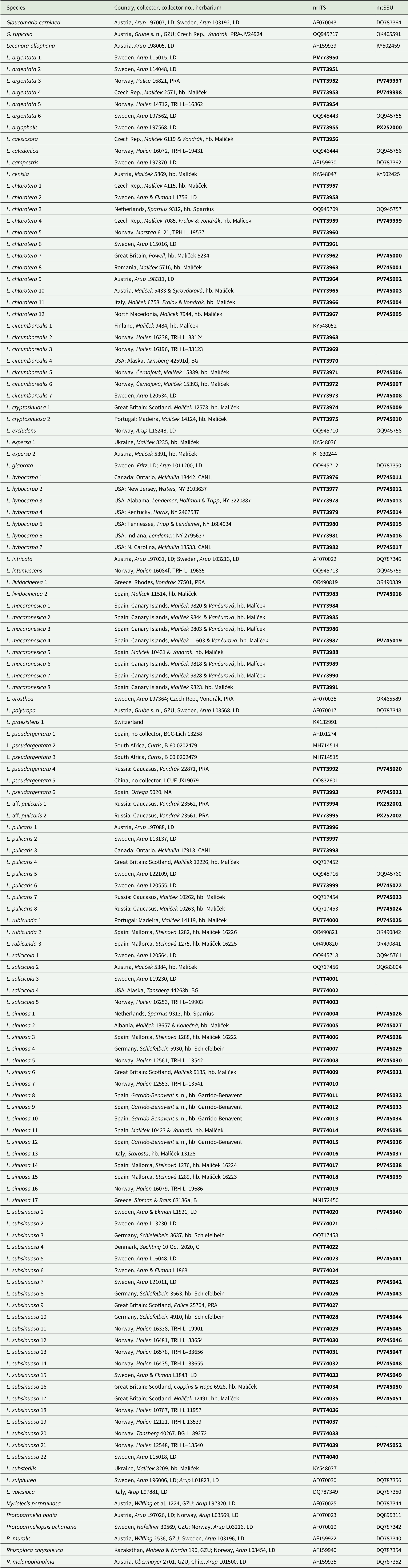
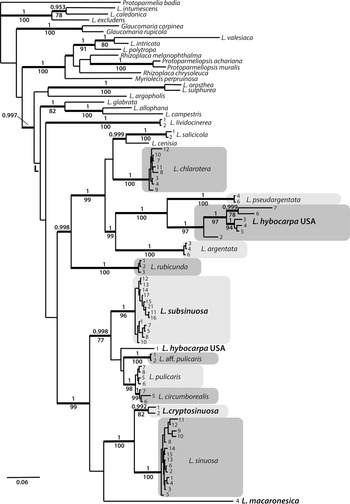
We generated 150 new sequences for this study. In the combined dataset of 86 OTUs, including the outgroup, the nrITS partition consisted of 586 sites (299 informative) and the mtSSU partition of 744 sites (95 informative). The Bayesian analysis halted after 2 400 000 generations and the resulting 50% majority-rule tree is shown in Fig. 1. The maximum likelihood analysis yielded a similar tree (not presented) and bootstrap values are shown in Fig. 1. The ITS dataset consisted of 588 sites (264 informative). The Bayesian analysis halted after 2 640 000 generations and a 50% majority-rule tree is shown in Fig. 2.
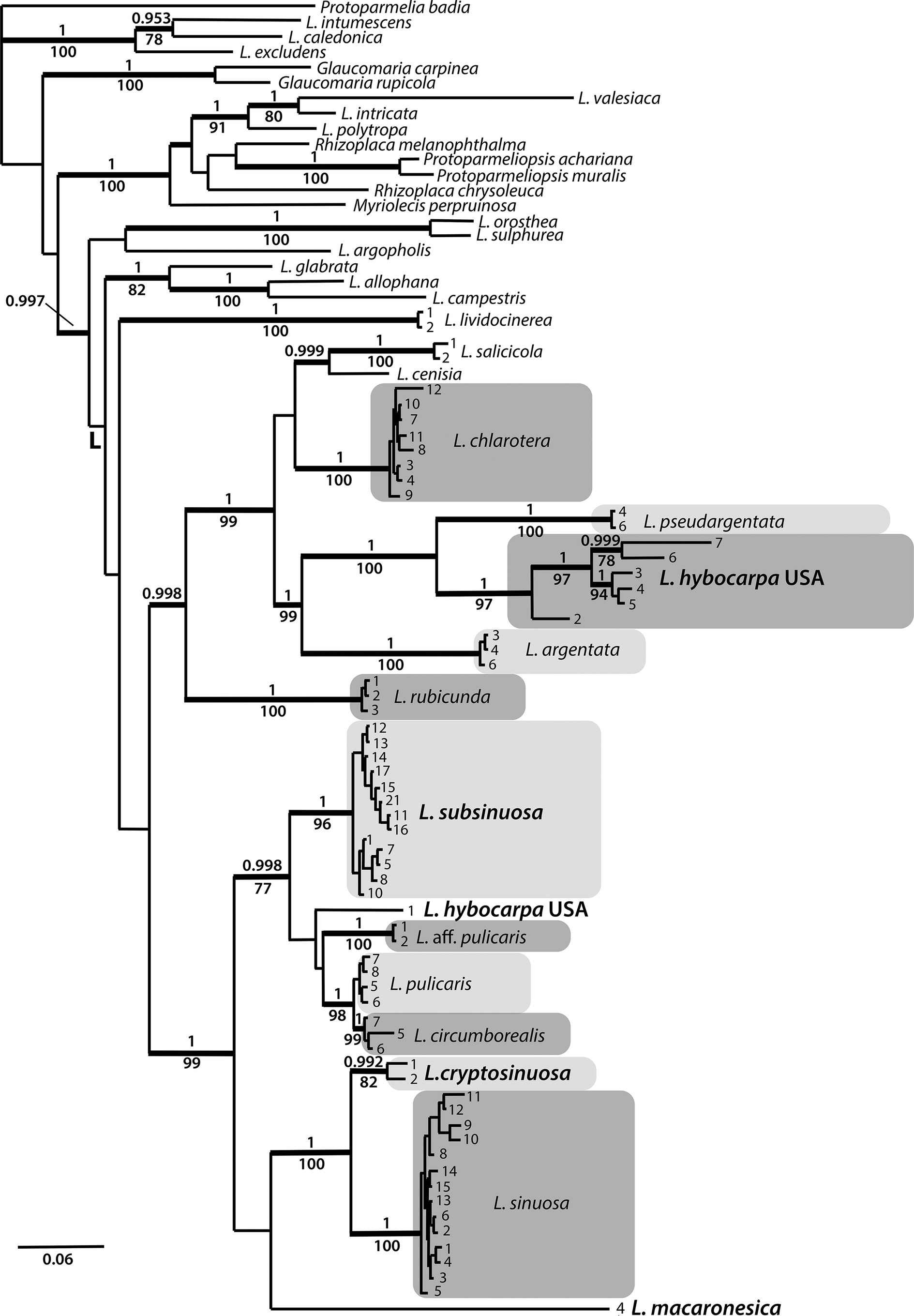
Figure 1. Majority-rule consensus tree based on a combined analysis of nrITS and mtSSU data using Bayesian MCMC. The tree shows the position of the new species of Lecanora in relation to other species in the genus and to related genera. Branches with posterior probabilities (PP) ≥ 0.95 are shown in bold. Bootstrap values and PP are presented below and above the branches, respectively. The letter L marks the clade of the Lecanora subfusca group and names in bold and larger font indicate species described in this paper or L. hybocarpa. Voucher information and GenBank Accession numbers are available in Table 1.
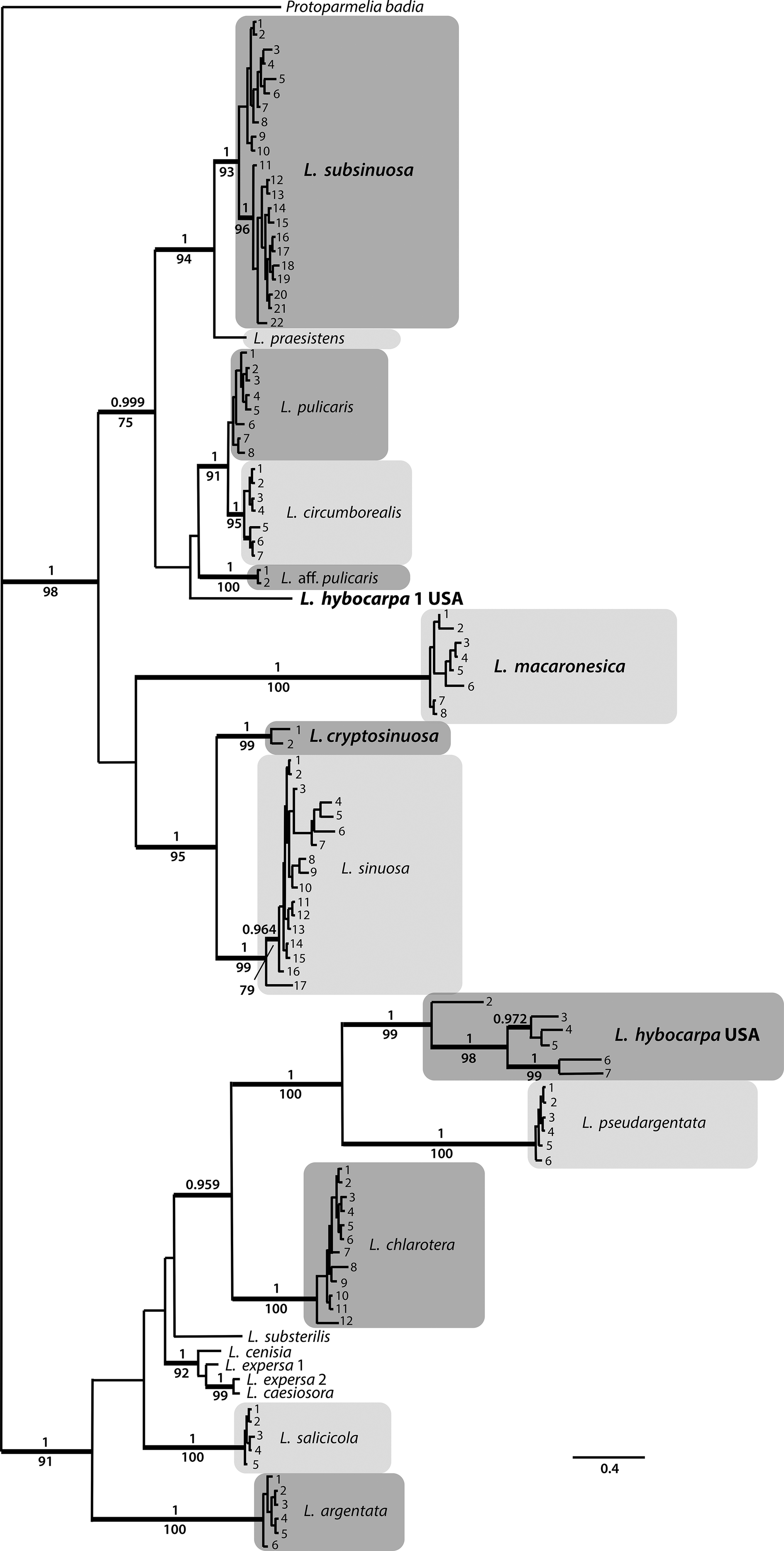
Figure 2. Majority-rule consensus tree based on an analysis of nrITS using Bayesian MCMC showing Lecanora species in a strict sense and their relationships. Names in bold and larger font indicate species described in this paper or L. hybocarpa. Branches with posterior probabilities (PP) ≥ 0.95 are shown in bold. Bootstrap values and PP are presented below and above the branches, respectively. Voucher information and GenBank Accession numbers are available in Table 1.
In the phylogenetic analyses of the combined dataset, the Lecanora subfusca group constitutes a major part of the tree, marked ‘L’ in Fig. 1. Outside of this clade there are several species belonging to different Lecanora morphogroups and genera that have been segregated from Lecanora s. lat. The L. subfusca clade is represented here by a number of well-known European species together with L. hybocarpa, L. pseudargentata, L. aff. pulicaris, and a small number of clades that will be described in this paper. All species or presumed species clades are well supported, except L. pulicaris (Pers.) Ach. which is in a supported sister relationship with L. circumborealis Brodo & Vitik. True Lecanora hybocarpa mainly appears as a variable clade sister to L. pseudargentata Lumbsch in a fully supported relationship, but one sequence from Ontario deviates greatly and is instead placed in a clade consisting of L. pulicaris, L. circumborealis and L. aff. pulicaris from the Caucasus. A new species, L. subsinuosa, appears in a supported position well separated from L. sinuosa that is situated in a clade together with L. cryptosinuosa and L. macaronesica, two further species described in this paper. The ITS tree (Fig. 2) agrees with the combined dataset.
Discussion
The name L. hybocarpa, as used by European authors, represents a number of different taxa that in several cases are not even closely related. True L. hybocarpa has so far not been recorded in Europe but is the most common member of the L. subfusca group in temperate eastern North America (Brodo Reference Brodo1984; Brodo et al. Reference Brodo, Duran Sharnoff and Sharnoff2001). The clade consisting of most of the American L. hybocarpa sequences is genetically variable with several long branches, indicating that it may consist of several closely related species. The single L. hybocarpa specimen from Ontario placed into the L. pulicaris/L. circumborealis clade is likely to represent yet another species.
The morphotype of L. hybocarpa is shared by L. pseudargentata, L. sinuosa, and the herein newly described L. cryptosinuosa, L. macaronesica and L. subsinuosa that have previously been treated as L. hybocarpa in Europe. Furthermore, difficulties in separating L. hybocarpa in the sense of European authors from L. chlarotera Nyl. have been noted (e.g. Sanderson Reference Sanderson2020; Nimis Reference Nimis2025). As confirmed by our phylogenies (Figs 1 & 2), L. chlarotera is distinct from the species with L. hybocarpa morphology, separated by epihymenium type (chlarotera-type in L. chlarotera vs pulicaris-type in the others except for L. pseudargentata) and secondary metabolites (e.g. Brodo Reference Brodo1984; Malíček Reference Malíček2014). Lecanora pseudargentata, the sister species to L. hybocarpa in our study, was described from Australia (Lumbsch Reference Lumbsch1994) and has been reported from Australasia, North America and South America (LaGreca & Lumbsch Reference LaGreca and Lumbsch2001). Here, we report this species as new to Europe. Lecanora sinuosa is the most common species sharing the L. hybocarpa morphotype, distributed mainly in the Atlantic and Mediterranean parts of Europe. The original material (van Herk & Aptroot Reference van Herk and Aptroot1999) represents an extreme morphological form sensu Malíček (Reference Malíček2014), which led to broad confusion in its identification. Lecanora cryptosinuosa, L. macaronesica and L. subsinuosa are described as new species from the Macaronesia, Western and Southern European regions. The group is taxonomically very difficult and identification without DNA is not reliable in most cases. Therefore, we suggest using the name Lecanora hybocarpa agg. for all species sharing the hybocarpa morphotype, which can be described as: apothecia with a crenulate margin, pale pink-brown to dark brown discs, amphithecium with large crystals, a pale brown to reddish brown epihymenium, with fine granules distributed between paraphyses tips.
Taxonomy
Lecanora cryptosinuosa Malíček sp. nov
MycoBank No.: MB 859465
Similar to Lecanora sinuosa but differs in the nrITS region. The new species is recognized by its unique barcode in ITS1, a 17-nucleotide motif CCAGTGGGGTCGCCCGC c. 110 bp after the start of ITS1.
Type: Great Britain, Scotland, V.C. 98, Argyll, Lochgilphead, Ardfern: Eilean Mhic Chrion, old-growth forest predominated by hazelwood on steep slopes of the island, 56.17329°N, 5.53974°W, elev. 20–40 m, on bark of Corylus avellana, 9 June 2018, J. Malíček 12573, A. Acton, Z. Palice, M. Powell & J. Vondrák (PRA—holotype). GenBank Accession nos: PV773974 (ITS) and PV745009 (mtSSU).
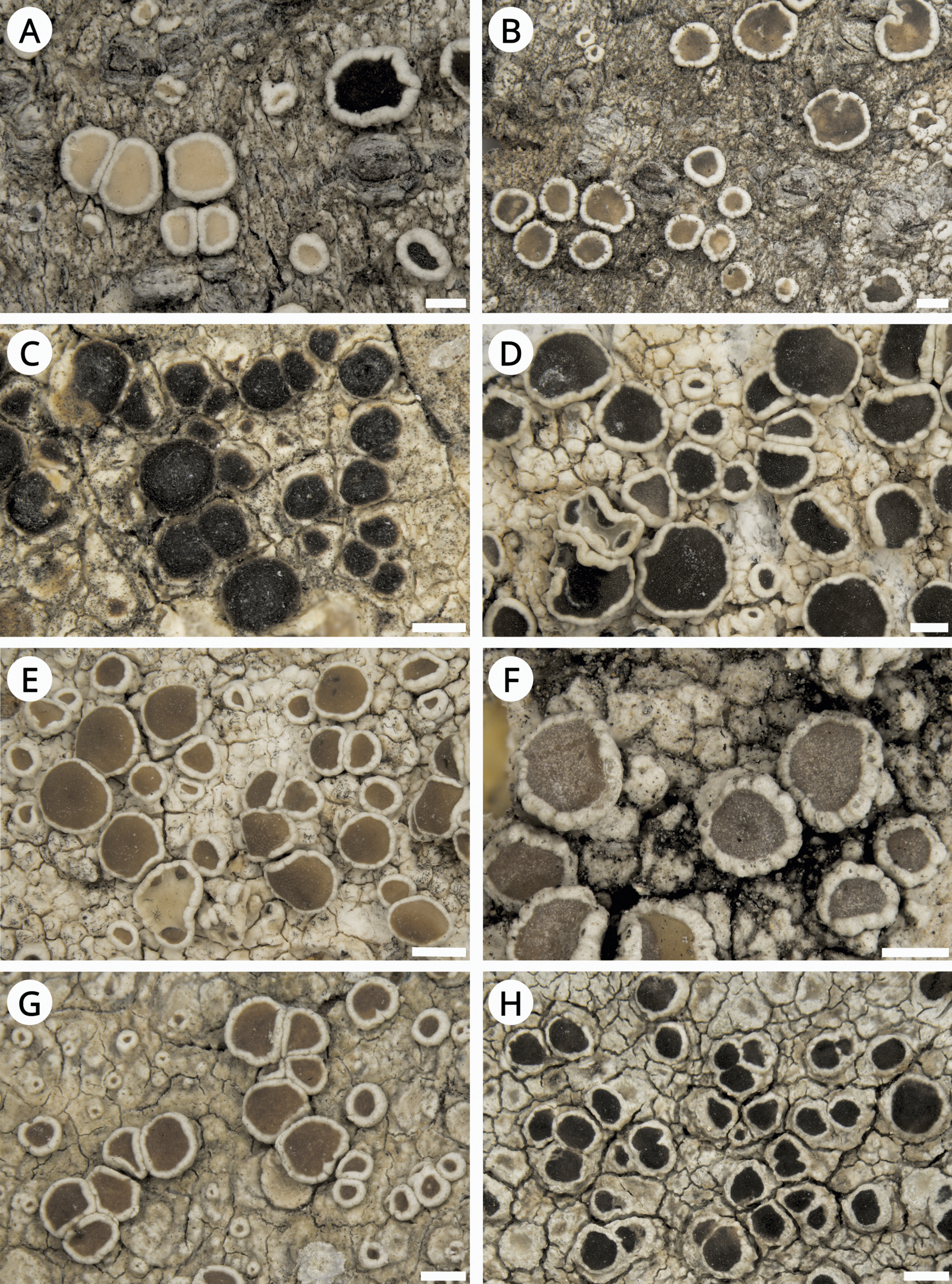
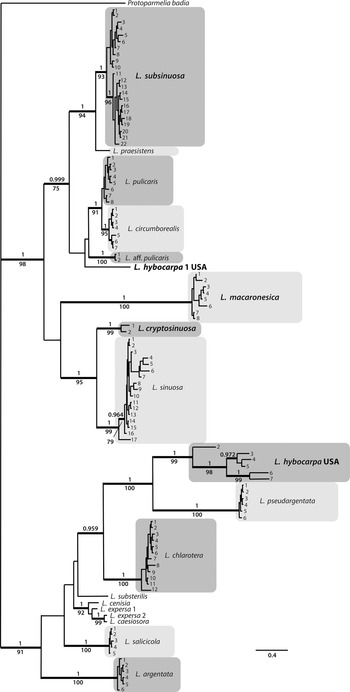
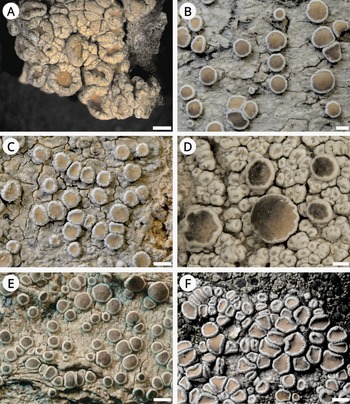
Figure 3. Habitus of Lecanora species. A & B, L. cryptosinuosa (Malíček 9838). C, L. hybocarpa, holotype (FH). D & E, L. macaronesica, holotype (PRA). F, L. pseudargentata, holotype (GZU). G, L. pseudargentata (Vondrák 22871, PRA). H, L. aff. pulicaris (Vondrák 23562, PRA). Scales: A–D, F–H = 0.5 mm; E = 1 mm. In colour online.
Thallus up to 0.2 mm thick, 1–5 cm wide, grey-white, matt, ±continuous and cracked to areolate; areolae up to 1.0 mm diam., irregular in outline, sometimes verruculose, with large calcium oxalate (POL+) crystals; prothallus black, thin.
Apothecia abundant, scattered on thallus or in groups, ±round, sessile or with constricted bases, 0.5–1.2 mm diam.; discs flat, pale pink-brown to dark brown, the colour variable within a population, matt, young apothecia often with a very thin pruina; margin up to 0.2 mm thick, of the same colour as the thallus, crenulate to smooth, rarely flexuose, slightly elevated above the disc or the same level. Epihymenium red-brown, K+ pale (yellowish) brown or colourless, N−, with small, POL+ granules (< 1 μm) on top and between the paraphyses (pulicaris-type), dissolving in K but not in N; hymenium 50–80 μm high, hyaline; hypothecium hyaline, 100–200 μm high; paraphyses 1.0–2.0 μm wide, simple to occasionally branched, mainly straight, with tips widened up to 4.5 μm; amphithecium of pulicaris-type, filled with large POL+ crystals not dissolving in K and slowly dissolving in N, with groups of trebouxioid algae mainly in the margins; photobiont cells 5–13 μm diam.; cortex indistinct or up to 35 μm thick, composed of ±isodiametric cells, 5–9(–11) μm diam. (only seen after treatment with K), with fine POL+ crystals of atranorin dissolving in K but not in N; asci 50–75 × 12–20 μm, 8-spored; ascospores simple, hyaline, broadly ellipsoid, rarely ovoid, ends round, rarely slightly pointed, 11.0–12.9–15.0(–17.0) × (5.5–)6.5–7.8–9.0(–10.0) μm, Q = 1.3–1.7–2.7 [n = 30], spore wall 0.5–1.0 μm thick.
Pycnidia common, black, immersed in the thallus, globose to subglobose, 80–120 μm wide and 90–120 μm high in section, with reddish brown pigment in the upper part; conidia filiform, strongly curved, hyaline, 10–20 × 0.8 μm.
Chemistry
Atranorin and roccellic acid (n = 2). Thallus and apothecial margin K+ yellow, C−, KC−, Pd− or Pd+ yellowish, UV−.
Etymology
The new species is cryptic with L. sinuosa, differing only in the DNA sequences.
Ecology and distribution
Lecanora cryptosinuosa is so far known from three specimens collected on bark of deciduous trees (Corylus avellana, Malus domestica and an unknown tree). It occurs in very humid and light oceanic subtropical and temperate forests in Madeira, La Palma (the Canary Islands) and the Scottish coast. Arthonia atra (Pers.) A. Schneid., Bacidia laurocerasi (Delise ex Duby) Zahlbr., Buellia disciformis (Fr.) Mudd, Phaeographis smithii (Leight.) B. de Lesd., Pyrenula macrospora (Degel.) Coppins & P. James, and Thelotrema macrosporum P. M. Jørg. & P. James co-occurred with the new species.
Phylogeny
Lecanora cryptosinuosa is the supported sister species to L. sinuosa in both our DNA analyses (Figs 1 & 2).
Remarks
The new species falls into the variability of L. sinuosa and we observed only minor differences, such as typically crowded apothecia in L. sinuosa compared to scattered or distributed in groups in L. cryptosinuosa. So far pycnidia are unknown in L. sinuosa but common in L. cryptosinuosa. Nevertheless, without DNA sequence data L. cryptosinuosa cannot reliably be distinguished from L. hybocarpa, L. sinuosa and L. subsinuosa. Lecanora cryptosinuosa is similar to L. macaronesica, sharing a part of the distribution range. However, L. macaronesica differs in the constricted bases of apothecia, the photobiont layer under the hypothecium, and the presence of gangaleoidin and often also an unknown substance, forming colourless needle-like crystals in K.
Specimens examined
Portugal: Madeira: Laurisilva de Madeira, Ribeira da Janela, Levada da Ribeira da Janela, 32°49′27″N, 17°10′33″W, elev. 350 m, on bark of Malus domestica, 2019, J. Malíček 14124 (hb. Malíček).—Spain: Canary Islands: La Palma, Santo Domingo de Garafía, La Mata, crossroad near Parque Cultural La Zarza y La Zarcita, 28°48′29.6″N, 17°54′31.2″W, elev. 990 m, on bark of a deciduous tree, 2013, J. Malíček 9838 & L. Vančurová (hb. Malíček).
Lecanora macaronesica Malíček sp. nov
MycoBank No.: MB 859331
Thallus thick, areolate to rarely verrucose; apothecia with a constricted base, discs pale to dark (reddish)brown, occasionally with a scattered thin pruina, margin slightly crenulate, smooth in young apothecia. Epihymenium and amphithecium of pulicaris-type. Gangaleoidin and norgangaleodin present in most specimens. Epiphytic species in well-lit habitats of subtropical oceanic areas.
Type: Spain, Canary Islands, Fuerte Ventura, La Oliva, lava field 0.2 m NW of Montaňa de Molino, 28°37′33.1″N, 13°55′05.4″W, elev. 250 m, on bark of Ficus carica, 26 May 2013, J. Malíček 9818 & L. Vančurová (PRA—holotype; LD—isotype). GenBank Accession no.: PV773989 (ITS).
Thallus 0.1–0.3 mm thick, up to several cm wide, yellowish white, matt, continuous and cracked to areolate; areolae 0.1–0.8 mm diam., irregular in outline, rarely verrucose; delimited by a black prothallus when surrounded by other lichen thalli.
Apothecia abundant, scattered on thallus or in groups, round, old apothecia often flexuose, with distinctly constricted bases or sessile only when young, 0.6–1.8 mm diam.; discs flat, pale to dark (reddish)brown, the colour variable within a population or even a thallus, matt, locally pruinose, pruina thin; margin up to 0.2 mm thick, of the same colour as the thallus, slightly crenulate or smooth in young apothecia, rarely flexuose, slightly elevated above the disc or at the same level, conspicuously elevated in very young apothecia. Epihymenium red-brown, K+ pale brown to hyaline, N−, with small POL+ granules (mostly < 1 μm) on top and between the paraphyses (pulicaris-type), dissolving in K but not in N; hymenium 60–80(–100) μm high, hyaline; hypothecium hyaline to rarely very pale brown, 60–250 μm high; paraphyses 1.5–2 μm wide, simple to occasionally branched, mainly straight, with tips slightly widened up to 3 μm; amphithecium of pulicaris-type, filled with large POL+ crystals not dissolving in K and slowly dissolving in N, with a layer of trebouxioid algae just below the hypothecium; photobiont cells 5–11 μm diam.; cortex indistinct or up to 50 μm thick at the base, composed of ±isodiametric cells, 5–7 μm diam. (only seen after treatment with K), with fine POL+ crystals of atranorin dissolving in K but not in N; asci 40–60 × 13–18 μm, 8-spored, but often fewer spores present; ascospores simple, hyaline, broadly ellipsoid or ovoid, ends round, rarely slightly pointed, (11.0–)12.0–13.8–16.0(–18.0) × (6.0–)6.5–7.6–9.0 μm, Q = 1.37–1.83–2.66 [n = 52], spore wall 0.5–1.0 μm thick.
Pycnidia fairly common, black, semi-immersed, globose to pyriform, 80–130 μm wide and 120–220 μm high in section, with reddish brown pigment mainly in the upper part of the wall; conidia filiform, curved or rarely straight, hyaline, 12–20 × 0.8 μm.
Chemistry
Atranorin (constant), gangaleodin and norgangaleoidin in eight specimens (n = 9), roccellic acid in four specimens by TLC. Colourless needle-like crystals of an unknown substance formed close to apothecial sections in c. 50% of specimens in K, similar in shape and size to those of norstictic acid. Thallus and apothecial margin K+ yellow, C−, KC−, Pd− or Pd+ yellow, UV−.
Etymology
The new species is common on the Canary Islands, which are part of Macaronesia.
Ecology and distribution
The species occurs on trunks and twigs of various deciduous or more rarely coniferous trees and shrubs in open landscapes. It has been collected, for example, on Ficus carica in lava fields, in light Laurus forests, on pastures or in smaller settlements. Most of the localities known so far are reported from the Canary Islands: El Hierro, Fuerte Ventura, Gran Canaria, La Gomera, Lanzarote and Tenerife. One collection is known from Andalusia in Spain. The altitude ranges between 250 and 850 m a.s.l. Caloplaca aegatica Giralt et al., Lecidella elaeochroma (Ach.) M. Choisy, Pertusaria sp., Punctelia borreri (Sm.) Krog, Ramalina sp. and Tornabea scutellifera (With.) J. R. Laundon were accompanying species in herbaria specimens.
Phylogeny
Based on both our DNA analyses, Lecanora macaronesica forms a distinct and well-supported clade sister to L. sinuosa (Figs 1 & 2).
Remarks
Typical specimens of L. macaronesica are well recognizable due to the quite large apothecia with constricted bases and the finely crenulate margin. Additionally, the presence of gangaleoidin in species with the pulicaris-type epihymenium is generally rare. The colourless crystals forming in K seem to be unique within similar Lecanora species, but this is not a constant character for L. macaronesica. In the field, L. macaronesica can be misidentified, for example as L. chlarotera and L. rubicunda Bagl., which differ in the coarse epihymenial granules, usually sessile apothecia and in chemistry. It is difficult to distinguish poorly developed forms from L. hybocarpa, L. pulicaris, L. sinuosa and L. subsinuosa because of the very similar anatomy. Nevertheless, the latter species do not produce gangaleoidin.
Specimens examined
Spain: Canary Islands: El Hierro, Valverde, Los Jalares, garden close to village, 27°48′09.7″N, 17°58′34.9″W, elev. 730 m, on bark of Kleinia neriifolia, 2013, J. Malíček 9823 & L. Vančurová (hb. Malíček); El Hierro, Frontera, Sabinosa, chapel Ermita de Virgen de Los Reyes, 27°43′48.9″N, 18°07′13.6″W, elev. 710 m, on bark of Acacia, 2013, J. Malíček 9828 & L. Vančurová (hb. Malíček); ibid., crossroad 0.8 km SW of Mirador de Bascos, 27°44′53.2″N, 18°07′25.4″W, elev. 650 m, on twigs of Pinus nigra, 2013, J. Malíček 9856 & L. Vančurová (hb. Malíček); ibid., old Juniperus trees on W-facing wind-exposed slopes 3 km W of Sabinosa, 27°44′46″N, 18°7′39″W, elev. 600 m, on bark of Juniperus turbinata ssp. canariensis, 2013, J. Malíček 11914 & L. Vančurová (hb. Malíček); Fuerte Ventura, La Oliva, lava field 0.2 m NW of Montaňa de Molino, 28°37′33.1″N, 13°55′05.4″W, elev. 250 m, on bark of Ficus carica, 2013, J. Malíček 9820 & L. Vančurová (hb. Malíček); Gran Canaria, Moya, Los Tylos de Moya Nature Reserve, laurel forest 1 km W of San Fernando, 28°05′31.8″N, 15°35′42.1″W, elev. 510 m, on bark of Picconia excelsa, 2013, J. Malíček 9803 & L. Vančurová (hb. Malíček); La Gomera, Garajonay National Park, Alojera, surroundings of Ermita San Isidro chapel, 28°09′47″N, 17°17′58″W, elev. 850 m, on bark of Laurus sp., 2013, J. Malíček 12695, 12697 & L. Vančurová (hb. Malíček); Lanzarote, Haría, Ye, wine field SW of village, 29°11′28.8″N, 13°28′56.3″W, elev. 380 m, on bark of Ficus carica, 2013, J. Malíček 9844 & L. Vančurová (hb. Malíček); Tenerife, Parque rural de Anaga, Chamorga, rock outcrops 0.3 km NE of village, 28°34′18″N, 16°09′21″W, elev. 480 m, on twig of shrub, 2013, J. Malíček 11603 & L. Vančurová (hb. Malíček). Andalusia: Estrecho Natural Park, Tarifa, Betis, pastures on E-facing slopes of Loma de San Bartolomé, 36°05′10″N, 5°42′59″W, elev. 250 m, on bark of Salix?, 2017, J. Malíček 10431 & J. Vondrák (hb. Malíček).
Lecanora subsinuosa Arup, Malíček & Holien sp. nov
MycoBank No.: MB 859330
Similar to Lecanora sinuosa but differs in the ITS and mtSSU sequences, the darker apothecial discs with inconsistent pigmentation, occasional presence of a tiny pruina on young discs, and the usually thicker, and in the inner edge dentate, apothecial margin.
Type: Norway, Nordland, Gildeskål, Inndyr Lauvvatnet, 67.04031°N, 14.06149°E, elev. c. 107 m, corticolous on Alnus incana, 31 May 2022, H. Holien 16481 (TRH L-33654—holotype). GenBank Accession nos: PV774030 (nrITS), PV745046 (mtSSU).
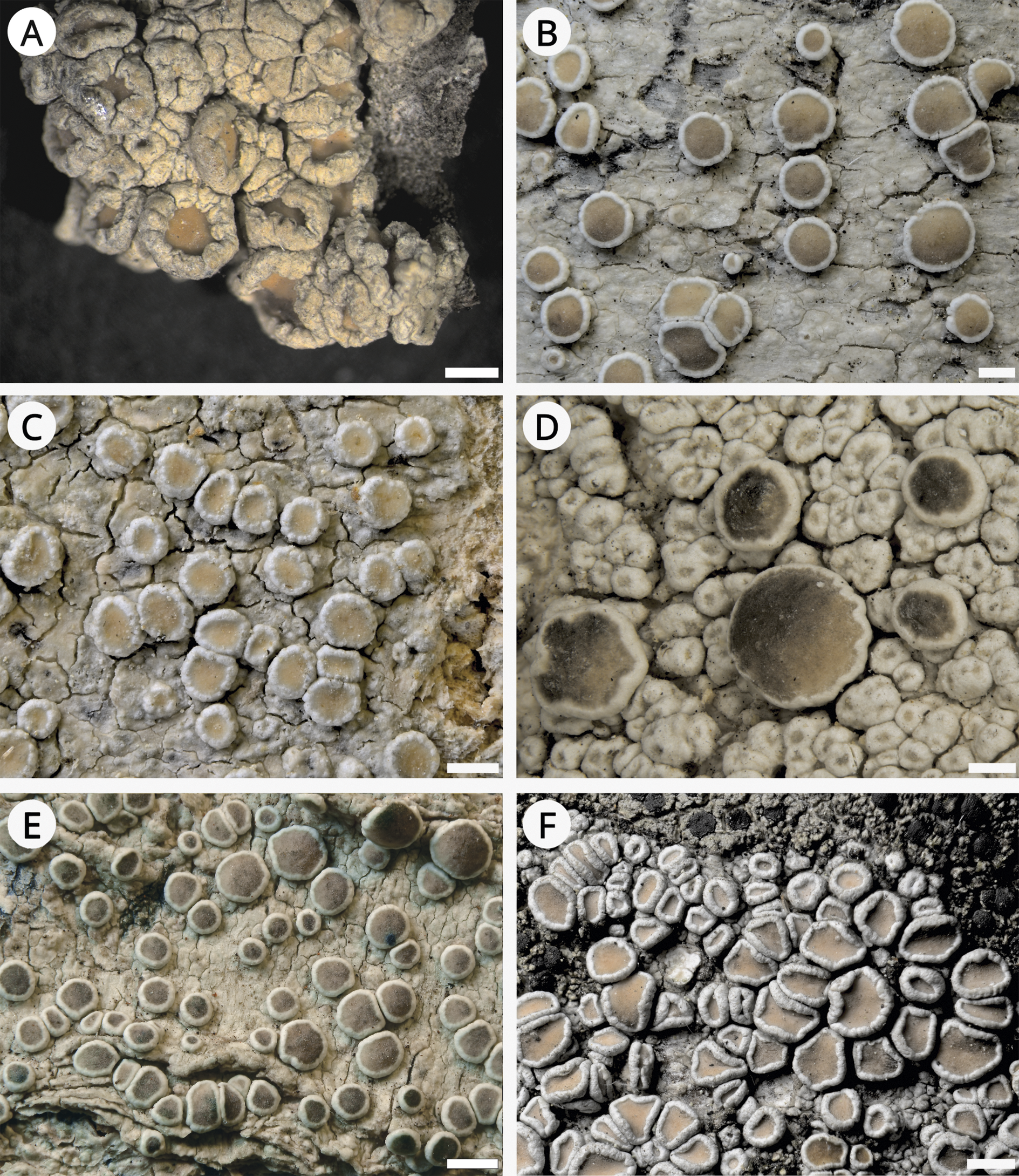
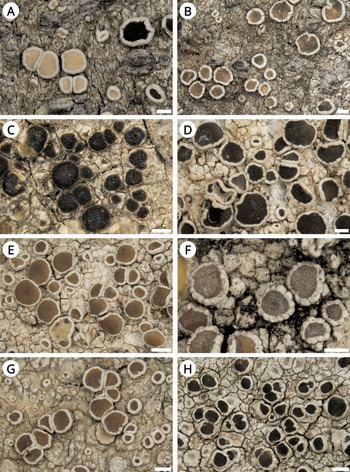
Figure 4. Habitus of Lecanora species. A, L. sinuosa, holotype (L). B, L. sinuosa (TRH L-19686). C, L. sinuosa (TRH L-13542). D, L. subsinuosa (Malíček 6928). E, L. subsinuosa, holotype (TRH L-33654). F, L. subsinuosa (TRH L-13230). Scales: A–F = 0.5 mm. In colour online.
Thallus 0.05–0.20 mm thick, up to several cm wide, continuous, smooth without cracks to finely cracked or rarely areolate; areolae 0.2–1.0 mm diam., irregular in outline, finely to strongly verruculose or uneven, verruculae 0.1–0.4(–0.8) diam., whitish to medium grey, often with a yellowish tinge, sometimes with primordia of apothecia; margin clearly delimited, sometimes by a thin black line against other thalli or fading gradually.
Apothecia normally present and abundant, 0.4–1.5(–2.5) mm diam., adnate to sessile, round to irregular and flexuose, scattered or sometimes angular from compression in dense aggregates; disc pale beige to dark greyish brown, often appearing dirty, frequently mottled, normally matt, without pruina, but young apothecia may look slightly pruinose, slightly concave to ±flat; thalline margin prominent and persistent, (25–)50–150(–300) μm thick, level with or slightly raised above disc, smooth to uneven, somewhat ridged or flexuose, white or of thallus colour; epihymenium brownish, with small POL+ granules on top and between the paraphyses (pulicaris-type), dissolving in K but not in N; hymenium 70–100(–115 μm) μm thick; hypothecium hyaline, 125–250(–300) μm thick; paraphyses 1.5–2.0 μm wide, simple to occasionally slightly branched, mainly straight, with tips not widened or only slightly so, to 2.5 μm; amphithecium of pulicaris-type, usually filled with large POL+ crystals not dissolving in K and slowly dissolving in N, plus small POL+ crystals dissolving in K, but not or very slowly in N, with a layer of trebouxioid algae just below the hypothecium; cortex fairly distinctly delimited but occasionally not, 15–60 μm thick, fairly uniform in thickness, composed of ±isodiametric cells, 5–6 μm diam. (seen only after treatment with K), with fine POL+ crystals of atranorin dissolving in K but not in N; asci 50–70 × 11–18 μm, with eight spores; ascospores simple, hyaline, broadly ellipsoid or ovoid, ends round, rarely slightly pointed, (10.5–)12.3–13.2–14.4(–17.0) × (6.0–)6.7–7.9–8.8(–11.0) μm, Q = 1.14–[1.70]–2.50 [n = 100], spore wall 0.8–1.2 μm, often thicker in younger spores.
Pycnidia uncommon, immersed, as dark brown to blackish dots, up to 200 μm wide in microscope preparations, containing brown, olive and blue-green pigments in the cell-walls; conidia filiform, curved to almost straight, hyaline, 12–18 × 0.8 μm.
Chemistry
Atranorin and roccellic acid (n = 15), sometimes with an additional unknown fatty acid, possibly nephrosteranic acid. Thallus and apothecial margin K+ yellow, C−, KC+ yellow, Pd+ pale yellow, UV−.
Etymology
The name subsinuosa refers to the similarity to L. sinuosa.
Ecology and distribution
Lecanora subsinuosa is found on deciduous trees and bushes of various kinds, such as Acer platanoides, Alnus incana, Carpinus betulus, Corylus avellana, Fagus sylvatica, Fraxinus excelsior, Prunus spinosa, Quercus robur/petraea, Sorbus aucuparia and Tilia sp. It grows both on the trunk and on small twigs and branches in exposed situations, quite often together with L. chlarotera or L. argentata. With a small number of exceptions, the collections come from oceanic lowland forests, and solitary and roadside trees. This new species is so far known from Denmark, Germany, Norway, Romania, Scotland and Sweden, which implies a mainly north-western distribution in Europe.
Phylogeny
Based on both our DNA analyses, Lecanora subsinuosa forms a distinct and well-supported clade sister to a clade consisting of L. circumborealis, L. pulicaris and two related taxa with unclear identifications (Fig. 1). The clade of L. subsinuosa is split into two subclades, suggesting that a further division of the species could be possible. However, the branches are very short and none of the subclades are supported. In addition, there are no characters that support a further split and we therefore consider it one species without any subtaxa.
Remarks
See under L. sinuosa for differences from that species.
Specimens examined
Denmark: Århus: Bispelundvej, c. 300 m S of Mosegard Museum, on Fraxinus, 10 x 2020, R. Jensen (C).—Germany: Mecklenburg-Vorpommern: Vorpommern-Rügen, Darss Peninsula, dunes near the Tiefenstück-Weg, 54°27′04″N, 12°29′08″E, elev. 5 m, Fagus sylvatica, 2013, U. Schiefelbein 3557, 3563 (hb. Schiefelbein); Rostock-Land, Nienhagen, Nienhäger Holz, mesophytic beech forest, 54°09′45″N, 11°56′18″E, elev. 15 m, 2012, U. Schiefelbein 3637 (hb. Schiefelbein); Rostock, Gartenstadt/Stadtweide, Kopernikusstrasse, E of the junction with Asternweg, 54°05′12.1″N, 12°04′46.9″E, elev. c. 30 m, roadside trees, Tilia sp., 2018, U. Schiefelbein 4910 (hb. Schiefelbein).—Great Britain: Scotland: V.C. 86, Stirling, Airth, The Pineapple, on Fraxinus at N edge of wood, adjacent to arable field, 56°04′39″N, 3°47′03″W, elev. 10 m, on Fraxinus, 2014, B. J. Coppins 24410 & J. C. E. Hope (hb. Malíček 6928); V.C. 98, Argyll, Oban, Port Appin, Glen Stockdale, grazed old-growth mixed deciduous forest with predominant Corylus, steep W-facing slope, 56°34′23″N, 5°21′35″W, elev. 65–90 m, on twig of Corylus avellana and Sorbus aucuparia, 2018, J. Malíček 12491, A. Acton, Z. Palice 25704, M. Powell & J. Vondrák (hb. Malíček, PRA).—Norway: Nordland: Bindal, N of Bindalseidet along river Fiskaroselva, 65.1755°N, 12.1274°E, elev. c. 50 m, on trunk of Alnus incana in humid spruce forest, 2007, H. Holien 11413b (TRH L-12849); Gildeskål, Finnsetvatnet N, by the road, 67.13061°N, 14.23742°E, elev. c. 80 m, corticolous on Sorbus aucuparia, 2022, H. Holien 16435 (TRH L-33655); ibid., Grimstad, Vassbekkmyra, 66.94382°N, 13.69475°E, elev. c. 13 m, corticolous on Alnus incana in mixed forest, 2022, Holien 16578 (TRH L-33656); Leirfjord, NE of Kviting, 66.04017°N, 12.9765°E, elev. c. 40 m, on trunk of Alnus incana in swampy forest, 2006, H. Holien 10767 (TRH L-11957). Trøndelag: Nord-Trøndelag, Leka, W of Ramtindmoen, 65.08°N, 11.6811°E, elev. c. 80 m, on trunk of Alnus incana, 2009, H. Holien 12121 (TRH L-13539); ibid., Flatanger, NE of lake Honnavatnet, 64.41262°N, 10.92026°E, elev. c. 160 m, corticolous on dead trunk of Alnus incana in mixed forest, 2021, H. Holien 16338 (TRH L-19901); Sør-Trøndelag, Ørland, Storfosna, Holmen, 63.6579°N, 9.3727°E, elev. c. 5 m, on trunk of Betula pubescens, 2009, Holien 12548 (TRH L-13540). Troms: Tranøy, Senja, W of fjord Tranøybotn, just SE of Road 860, 69°10.49′N, 17°25.25′E, elev. 1–10 m, corticolous on trunk of Populus tremula in a Populus grove just uphill from sea, 2010, T. Tønsberg 40267 (BG L-89272).—Romania: Transylvania: Southern Carpathians, Făgăraș Mts, Vâlcea County, Mt Stârpele [1480], open beech-dominated forest on S-facing steep slope above the valley of Boia Mică, 16–17 km SE of Tălmaciu, 45°33′21.9″N, 24°24′33.9″E, elev. 1135 m, on bark of Fagus sylvatica, 2024, J. Halda & Z. Palice 37822 (PRA).—Sweden: Bohuslän: Kärra par., IKEA at Bäckebol, on young Carpinus betulus in the parking area in front of the entrance, 57.77427°N, 11.99987°E, 2013, U. Arup L13230 (LD); Lycke par., Älgön, S side of the island, S side of the peninsula N of Lammholmen, 57.91800°N, 11.68817°E, elev. 1.5 m, on twigs of Prunus spinosa on the shore, 2021, U. Arup L21011 (LD). Skåne: Brunnby par., Kullaberg, at the parking area NW of Kullagården, 56.29978°N, 12.46726°E, elev. 52 m, on young Acer, exposed, 2016, U. Arup L16048 (LD); ibid., on young Sorbus, Arup L19034 (LD); Torekov par., Hallands Väderö, c. 120 m SW of Skogvaktarbostället, 56.43386°N, 12.56376°E, elev. 4 m, on twigs of Malus sylvestris, exposed, 2021, U. Arup & S. Ekman L1821 (LD); ibid., 115 m E of the lighthouse, 56.45062°N, 12.54438°E, elev. 5 m, on Quercus, exposed, 2022, U. Arup & S. Ekman L1840 (LD); ibid., c. 170 m SSE of Fyrbostäderna, 56.44812°N, 12.55748°E, elev. 9 m, on twigs of Rhamnus cathartica, exposed, 2022, U. Arup & S. Ekman L1836 (LD); ibid., SE of Oakärret, just N of the wall to Söndre skog, 56.44213°N, 12.56199°E, elev. 10 m, on twigs of tall stump of Populus, exposed, 2022, U. Arup & S. Ekman L1843 (LD); ibid., NE part of Stora Gröning, 56.44563°N, 12.55967°E, elev. 10 m, on twigs of solitary Tilia, 2022, U. Arup & S. Ekman L1848 (LD); ibid., c. 430 m ENE of Fyrbostäderna, 56.45118°N, 12.55274°E, elev. 6 m, on solitary Fraxinus, exposed, 2022, U. Arup & S. Ekman L1868 (LD); ibid., 120 m E of the lighthouse, E of trail to Tjuvelthamn, 56.45064°N, 12.54441°E, elev. 5 m, on branch of Quercus in open situation, 2024, U. Arup & S. Ekman L2043 (LD). Östergötland: Västra Tollstad par., Omberg, N of Storpissan nature reserve, 58.33805°N, 14.65328°E, elev. 140 m, on rather young Quercus in open wooded meadow on W-facing slope with scattered, very old oaks, exposed, 2015, U. Arup L15018 (LD).
Lecanora hybocarpa (Tuck.) Brodo
Beih. Nova Hedwigia 79, 134 (1984).—Parmelia hybocarpa Tuck. Cat. Pl. Cincinnati, 45 (1849); type: Ohio, in Fago, 1848, T. G. Lea (FH 01006011!—holotype).
This species is very well described in Brodo (Reference Brodo1984). He reported it as one of the most abundant representatives of the Lecanora subfusca group in North America. Brodo (Reference Brodo1984) also highlighted the great variability of the species and considered the holotype (Fig. 3C) quite atypical due to having rather dark, convex apothecia with thin, almost excluded margins, much resembling L. glabrata. We can fully confirm his observations, including the presence of atranorin as the only lichen substance detected by TLC.
Based on our results, the occurrence of L. hybocarpa has not been confirmed in Europe. We have sequenced 13 specimens (13 mtSSU and 5 ITS) of the species from the USA. They form a genetically variable but distinct clade very close to L. pseudargentata. One sample clusters with the L. pulicaris/circumborealis clade (Fig. 1) and most probably represents a different taxon. Without DNA, L. hybocarpa is indistinguishable from L. cryptosinuosa, L. sinuosa and L. subsinuosa.
Lecanora pseudargentata Lumbsch
J. Hattori Bot. Lab. 77, 127 (1994); type: Australia, Queensland, NE of Gunalda, c. 59 km S of Maryborough, E of Hwy 1, c. 180 m, 25°58′S, 152°36′E, on trees in a grazing ground, Ficus, 22 August 1986, J. Hafellner 18604 & R. Rogers (GZU46250!—holotype).
This species is macroscopically very similar to L. argentata but differs mainly in the presence of POL+ granules on the paraphyses tips (chlarotera-type). However, the granules are often sparse and fine, resembling those in L. hybocarpa. Additionally, the epihymenial pigment of L. pseudargentata is red-brown ( Fig. 3F & G) and only partly soluble in K (also observed by LaGreca & Lumbsch (Reference LaGreca and Lumbsch2001)), more resembling the epihymenium of L. hybocarpa or L. sinuosa than of, for example, L. chlarotera with a usually pale brown epihymenium (Malíček Reference Malíček2014). In our phylogenies (Figs 1 & 2), L. pseudargentata forms a sister clade to L. hybocarpa, confirming their close relationship. For a detailed description of L. pseudargentata see Lumbsch (Reference Lumbsch1994) and LaGreca & Lumbsch (Reference LaGreca and Lumbsch2001).
The species is distributed worldwide, but records from Europe have until now been lacking. Here, we report L. pseudargentata from Austria, Russia (Black Sea coast near Sochi) and Spain. All three records come from very humid localities.
Specimens examined
Austria: Tirol: Alps Mts, Brandenberg, on right bank of Brandenberger Ache brook, NW of Kaiserklamm Gorge, 47°32′48″N, 11°54′39″E, elev. 750 m, on bark of Salix elaeagnos, 2012, J. Malíček 5539 (hb. Malíček, PRC).—Russia: Krasnodar Region: Adler, Khosta, protected area Tiso-Samshitovaya Roshcha, 43.53116°N, 39.87683°E, elev. 120 m, on bark of Staphylea pinnata, 2019, J. Vondrák 22871 (PRA).—Spain: [locality data not available], 2017, S. Pérez-Ortega 5020 (?).—USA: Michigan: Cheboygan County, Gaylord State Forest, E of Waveland Rd, 3.4 mi N of jct w/ MI-68, 45°24′19″N, 84°20′58″W, 715 ft, mixed hardwood (Acer, Alnus, Betula, Fraxinus, Populus)–conifer (Abies, Thuja) swamp forest, on Abies branch, 2015, J. Lendemer 45013 (NY02439461).
Lecanora aff. pulicaris (Pers.) Ach
This is probably an undescribed Lecanora species, most similar to L. pulicaris. It differs from its most common morphotype by the semi-immersed apothecia with an unevenly thick but generally thick and slightly verrucose margin (Fig. 3E); in this it also resembles L. hybocarpa. Fumarprotocetraric acid has not been detected. According to both phylogenies (Figs 1 & 2), it is sister to L. circumborealis and L. pulicaris. The species is so far known from a single locality in the Russian part of the Caucasus, not far from Sochi, and requires further study.
Specimens examined
Russia: Krasnodar Region: Adler, Kazachiy Brod, forest on limestone cliff above Mzymta River, 43.52826°N, 40.00270°E, elev. 290 m, on bark of Carpinus orientalis, 2019, J. Vondrák 23561, 23562 (PRA).
Lecanora sinuosa Herk & Aptroot
Lichenologist 31, 548 (1999); type: The Netherlands, Drenthe, Hoogeveen, Nieuweroord, Verlengde Hoogeveensche Vaart, on Quercus robur along road near canal, 5 March 1998, C. M. van Herk 2232–1, 6 & A. Aptroot 41891 (L!—holotype; hb. van Herk!—isotype).
Thallus 0.1–0.3(–1.0) mm thick, up to several cm wide, greyish to yellowish white, areolate to verrucose, very rarely ±smooth or rimose, delimited by a black prothallus when surrounded by other lichen thalli. Apothecia abundant, often crowded, sessile, only old apothecia rarely with a constricted base, 0.4–1.8 mm diam., round to flexuose; discs pale to medium brown or pinkish brown, matt, epruinose, flat; margin 0.05–0.3 mm thick, persistent, of the same colour as the thallus, matt, smooth or slightly crenulate, at the same level as discs to distinctly raised. Pycnidia unknown. For anatomical characters see van Herk & Aptroot (Reference van Herk and Aptroot1999).
Chemistry
Atranorin and roccellic acid by TLC (n = 16).
Ecology and distribution
The species grows on various deciduous trees in open landscape and light forests. It is widely distributed across the Mediterranean and Atlantic parts of Europe. Here we report this species as new for Albania, Greece, Italy, Norway and Spain.
Phylogeny
Based on both our DNA analyses, Lecanora sinuosa forms a distinct and well-supported clade sister to L. cryptosinuosa and is closely related to L. macaronesica (Fig. 1).
Remarks
Lecanora sinuosa has been described as having a thick verrucose thallus, an often incurved thick apothecial margin raised above pale to medium brown discs (Fig. 4A–C), and producing atranorin and also gangaleoidin in part of the collections (van Herk & Aptroot Reference van Herk and Aptroot1999). Based on our observations, the species is morphologically very variable and the described phenotype mainly represents its extreme form (sensu Malíček Reference Malíček2014). In addition, we did not confirm gangaleoidin in sequenced specimens or the holotype and four paratypes. We hypothesize that the report of gangaleoidin is based on a mistake, such as misidentification with the similar L. chlarotera. This species occurs in the same localities in the Netherlands and some forms are very similar. The main difference, epihymenial granules, are sometimes of a similar size and their distribution in the epihymenium could be difficult to see in thick sections.
Lecanora sinuosa is very similar to L. cryptosinuosa and L. subsinuosa, both described here as new species. We found only minor differences from L. subsinuosa, which are not entirely constant between populations. Lecanora sinuosa has paler discs than those in L. subsinuosa which are often medium to ±dark or greyish brown. Additionally, the disc pigmentation in L. subsinuosa is often not uniform and shades of brown vary within a single apothecium. Young apothecia in L. subsinuosa are sometimes very thinly pruinose and their margin is often dentate in the inner edge. Macroscopically, the thalline margin is thicker in L. sinuosa. Finally, the apothecial cortex in L. sinuosa is usually somewhat thinner than in L. subsinuosa. Nevertheless, for accurate identification, a DNA barcode is recommended and, in the case of L. cryptosinuosa, even necessary.
Specimens examined
Albania: Vlorë: Llogara National Park, Orikum, grazed pine-fir forests in central part of the park, c. 40°12′20″N, 19°34′59″E, elev. 890 m, on twigs of Crataegus sp., 2019, J. Malíček 13657 & E. Konečná (hb. Malíček).—Germany: Mecklenburg-Vorpommern: Vorpommern-Rügen, Rügen Island, Stubnitz, S of Stubbenkammer, calcareous beech forest, 54°34′09″N, 13°40′06″E, elev. c. 100 m, Fagus sylvatica, 2018, U. Schiefelbein 5011 (hb. Schiefelbein); ibid., Jasmund peninsula, coast between ‘Kieler Bach’ and Sassnitz, S of Karin-Christiane-Sicht, calcareous beech forest, 54°27′26″N, 12°29′35″E, elev. 70 m, Fagus sylvatica, 2020, U. Schiefelbein 5930 (hb. Schiefelbein). Niedersachsen: Vesterstede, Wiefelstede, near Garnholterdamm, old tree near farm, Quercus robur, 1998, C. M. van Herk (hb. Herk).—Great Britain: Scotland: V.C. 98, Argyll, Lochgilphead, Kilmartin, old-growth forest Old Poltalloch 3.5 km S of Ardfern, in valley of a brook close to coast, 56°08′43″N, 5°32′04″W, elev. 50 m, on twig of Fraxinus excelsior, 2014, J. Malíček 9135, B. J. Coppins & J. Vondrák (hb. Malíček) .—Greece: Chios (East Aegean Islands): 1 km from Aghios Irini beach on road to Elata, steep N-facing slope, epiphytic, 25°58.4′N, 38°17.3′E, elev. c. 50 m, 15 ix 2013, H. Sipman & T. Raus (B 600192735). Lakonia: E of Neapoli, Paradisi spring above Lachio, 36°30.6′N, 23°05.4′E, elev. 375 m, epiphyte on dead Quercus coccifera twigs in gorge, 25 ix 2011, H. Sipman & T. Raus (B 600189808). Cyclades: Milos Island, W part, Koutsounorachi, antenna on hilltop, 36°40.9′N, 24°23.8′E, elev. 360 m, volcanic rock outcrops and cliffs on hilltop, N-facing, on Pistacia branches, 21 ix 2011, H. Sipman & T. Raus (B 600189589). Corfu: Krimi, castle Angelokastro, 39.67835°N, 19.68634°E, elev. 270 m, on bark, 17 iv 2011, L. Syrovátková (PRA).—Italy: Liguria: Alassio, Garlenda, bank of Torrente Lerrone brook, 44°01′57.2″N, 8°05′54.2″E, elev. 55 m, on bark of Tilia, 3 vii 2019, J. Starosta (hb. Malíček 13128).—The Netherlands: Drenthe: Hoogeveen, Nieuweroord, oaks along road, 52°43′28″N, 6°34′30″E, elev. 0–50 m, on bark of Quercus robur, 2014, J. Malíček 6959 & L. Syrovátková (hb. Malíček ); Meppel, Nijeveen, old oaks along road in village, 52°44′09″N, 6°10′41″E, elev. 0–50 m, on bark of Quercus robur, 2014, J. Malíček 6969 & L. Syrovátková (hb. Malíček ); Edlde, De Punt, cor. 236,1/571,3, old tree at crossing of unsurfaced roads, Quercus robur, 1998, C. M. van Herk (hb. Herk); between Peize and Foxwolde, 227,6/574,0, row of trees along country road, Quercus robur, 1998, C. M. van Herk (hb. Herk); Nyeveen, 208,2/527,7, old tree along road in village, Quercus robur, 1997, C. M. van Herk (hb. Herk). Utrecht: Amersfoort, Hooglanderveen, Heideweg, mature wayside tree in suburban area, on bark of Quercus robur, 2021, L. Sparrius 9313 (hb. Sparrius).—Norway: Trøndelag: Nord-Trøndelag, Flatanger, Lauvsnes, N end of lake Lauvsnesvatnet, corticolous on trunk of Sorbus aucuparia in mixed forest, 64.49762°N, 10.91779°E, elev. c. 25 m, 2021, H. Holien 16079 (TRH L-19686); Sør-Trøndelag, Ørland, Storfosna, between Melskardet and Vebergodden, on trunk of Salix caprea in deciduous forest, 63.6668°N, 9.4071°E, elev. c. 20 m, 2009, H. Holien 12533 (TRH L-13541); ibid., on trunk of Fraxinus excelsior, elev. c. 20 m, 2009, H. Holien 12561 (TRH L-13542).—Spain: Andalusia: Estrecho Natural Park, Tarifa, Betis, pastures with single houses on E-facing slopes of Loma de San Bartolomé, 36°04′51″N, 5°43′07″W, elev. 210 m, on bark of Quercus suber, 2017, J. Malíček 10423 & J. Vondrák (hb. Malíček) . Mallorca: c. 1 km E of Ebbassament de Cúber, between the hiking path and the road Ma-10, 39.78885°N, 2.80123°E, elev. 760 m, on Pinus sp. and Quercus sp., 2023, J. Steinová 1288, 1289 (hb. Malíček 16222 & 16223); ibid., c. 2 km S of Port de Canonge, 39.68391°N, 2.56168°E, elev. 350 m, on the bark of an olive tree, 2023, J. Steinová 1276 (hb. Malíček 16224); Gasalnera/Estewsude servicio, La Pobla de Tornesa (Castellos), on Prunus dulcis bark, 16 viii 2016, I. Garrido-Benavent (hb. Garrido-Benavent).
Key to epiphytic esorediate species of the L. subfusca group in Europe and northern Africa
As we have mentioned several times in this article, identifying many of the species in this group is extremely difficult and accurate identification without DNA is in several cases not possible. This applies primarily to species with the L. hybocarpa morphotype, which share ±the same diagnostic characters and have high intraspecific variability. One generally problematic character is the epihymenium; it is often difficult to distinguish between the chlarotera- and pulicaris-types (for differences see Brodo (Reference Brodo1984) and Malíček (Reference Malíček2014)). The epihymenium type was even erroneously reported in the description of L. paramerae I. Martínez et al. (Martínez et al. Reference Martínez, Aragón and Lumbsch1999). Both its holotype and isotype have an epihymenium of the chlarotera-type, and not the pulicaris-type as written in the protologue (Martínez et al. Reference Martínez, Aragón and Lumbsch1999). In this key, we place L. paramerae among the taxa with a chlarotera-type epihymenium.

Supplementary Material
The Supplementary Material for this article can be found at http://doi.org/10.1017/S0024282925101291.
Acknowledgements
Brian J. Coppins provided valuable comments on the manuscript and one important collection. Isaac Garrido-Benavent, Ivana Černajová, Kok van Herk, Zdeněk Palice, Harrie Sipman, Laurens Sparrius, Jakub Starosta, Jana Steinová, Ulrik Søchting and Jan Vondrák kindly provided their collections for our study. The curators of herbaria CANL, FH, GZU, L and NY loaned us the (type) specimens important for this study. Jiří Machač provided images of several specimens. Lucie Vančurová and Lada Syrovátková helped with the fieldwork. JM has been supported by the long-term research development project RVO 67985939 and UA by the project dha 150/2011 from the Swedish Taxonomy Initiative.
Author ORCIDs
Ulf Arup, 0000-0001-6612-8099; Jiří Malíček, 0000-0002-3119-8967; Ulf Schiefelbein, 0009-0004-6366-6069; Håkon Holien, 0000-0003-3913-4746.
Competing Interests
The authors declare none.
Data Accessibility
Newly generated sequences have been deposited in GenBank. Nomenclatural novelties have been deposited in MycoBank.