Introduction
The interplay between cave and epigean environments becomes evident when considering that most cave fauna, especially in the tropics, consists of species adapted to living in both environments (troglophiles) and those dependent on the epigean compartment to complete their life cycle (trogloxenes) (Schiner Reference Schiner1854, Racovitza, Reference Racovitza1907, Sket Reference Sket2008). These species exhibit a closer relationship with epigean habitats than troglobites, which are restricted to subterranean habitats (Schiner Reference Schiner1854, Sket Reference Sket2008). Cave fauna often represents a subset of the regional species pool, highlighting the connection between underground and aboveground ecosystems, as seen in the neotropical region (Gibert & Deharveng Reference Gibert and Deharveng2002, Mendes-Rabelo et al. Reference Mendes-Rabelo, Souza-Silva and Ferreira2020).
The connection between epigean and cave environments is intricate and can be shaped by multiple factors (Prous et al. Reference Prous, Ferreira and Martin2004, Tobin et al. Reference Tobin, Hutchins and Schwartz2013, Prous et al. Reference Prous, Ferreira and Jacobi2015, Cardoso et al. Reference Cardoso, Ferreira and Souza-Silva2022). These factors encompass the diversity in the rock composition of the caves, the surrounding plant life, the level of conservation in the area, land utilisation practices, and the overall variety of aboveground species in the region (Souza-Silva et al. Reference Souza-Silva, Martins and Ferreira2011, Pellegrini et al. Reference Pellegrini, Sales, Aguiar and Ferreira2016, Mammola et al. Reference Mammola, Piano, Giachino and Isaia2017, Jaffé et al. Reference Jaffé, Prous, Calux, Gastauer, Nicacio, Zampaulo and Siqueira2018, Mendes-Rabelo et al. Reference Mendes-Rabelo, Souza-Silva and Ferreira2020, Cardoso et al. Reference Cardoso, Ferreira and Souza-Silva2022, Oliveira & Ferreira Reference Oliveira and Ferreira2024). Many of these factors significantly influence local microclimate patterns, and any changes in one or more of these factors may potentially disrupt the characteristic environmental equilibrium of caves (Barker & Genty Reference Baker and Genty1998). These variations, particularly those related to climate, can negatively affect cave-dwelling species, especially those restricted to this specific ecosystem, as they depend directly on stable temperatures and high humidity for their survival (Howarth Reference Howarth1993, Gillieson Reference Gillieson1996, Gibert & Deharveng Reference Gibert and Deharveng2002).
In cave ecosystems, the absence of light into deeper areas and the resulting absence of direct primary productivity through photosynthesis underscore the significance of input of organic resources from the epigean environment for sustaining the fauna (Schneider et al. Reference Schneider, Christman and Fagan2011, Souza-Silva et al. Reference Souza-Silva, Martins and Ferreira2011a, Smrž et al. Reference Smrž, Kováč, Mikeš, Šustr, Lukešová, Tajovsky, Nováková and Režňáková2015, Venarsky & Huntsman Reference Venarsky, Huntsman, Moldovan, Kováč and Halse2018). These organic resources encompass various plant components such as roots, leaves, trunks, and fruits, animal remains, including carcasses, faeces, and materials regurgitated by owls, and microorganisms, mainly sourced from the adjacent epigean environment (Ferreira et al. Reference Ferreira, Oliveira and Souza-Silva2015, Smrž et al. Reference Smrž, Kováč, Mikeš, Šustr, Lukešová, Tajovsky, Nováková and Režňáková2015).
It is essential to highlight that subterranean environments operate as ecosystems, providing refuge for a diverse array of invertebrate and vertebrate species, many of which play vital roles in delivering environmental services to both subterranean and epigean ecosystems (Kunz et al. Reference Kunz, Torrez, Bauer, Lobova and Fleming2011). Numerous studies seek to unravel the primary biotic and abiotic factors that significantly influence the species richness and composition of cave invertebrates (Christman & Culver Reference Christman and Culver2001, Simões et al. Reference Simões, Souza-Silva and Ferreira2015, Lunghi et al. Reference Lunghi, Manenti and Ficetola2017, Pacheco et al. Reference Pacheco, Souza-Silva, Cano and Ferreira2020, Souza-Silva et al. Reference Souza-Silva, Cerqueira, Pellegrini and Ferreira2021). However, our understanding of the invertebrate fauna in the epigean environments adjacent to caves and its relationship with cave invertebrates remains limited (Oliveira & Ferreira Reference Oliveira and Ferreira2024). It is imperative to understand the significance of epigean landscape structure in preserving cave-dwelling fauna to maintain environmental conditions and their sources of nourishment.
This study seeks to investigate two main questions: (1) How similar is the composition and species richness of invertebrates between the epigean landscape and cave environments across quartzite and limestone rocks during dry and wet seasons? (2) What are the key environmental factors influencing the composition and species richness of invertebrate fauna in both epigean landscape and cave environments across quartzite and limestone rocks during dry and wet seasons? In addition, we will describe and compare the structure of invertebrate communities across epigean landscapes and cave environments and quartzite and limestone rocks during dry and wet seasons.
Based on the stated research questions, we proposed the following hypotheses: (a) the faunal similarities would be more significant within the same environment and lower when comparing the epigean and cave environments. Furthermore, the seasonality would have a different influence on the faunal similarities among the environments; (b) the variables that drive the invertebrate fauna composition would vary across environments, as well as across lithologies and seasons; and (c) strong differences will be observed in the number of species between epigean and cave environments.
Materials and methods
Study area
The six caves examined in this study are located in two State Parks, classified as Integral Protection Conservation Units (BRASIL, 2000), both in Minas Gerais. Lapa D’água, Lapa Grande, and Lapa da Santa caves were sampled at Lapa Grande State Park (LGSP – UTM 614287.29 E/8152224.37 S; 23k), situated in a significant Brazilian karst region (Auler & Farrant Reference Auler and Farrant1996, IEF 2014) in northern Minas Gerais, in the municipality of Montes Claros. The caves named Gruta das Bromélias, Gruta das Casas, and Gruta Moreiras were sampled in Ibitipoca State Park (ISP - UTM 613343.73 E/ 7598933.79 S; 23k), in the municipality of Lima Duarte, southern Minas Gerais, renowned for the presence of quartzitic caves (IEF 2007, Willems et al. Reference Willems, Rodet, Pouclet, Melo, Rodet, Compère and Auler2008) (Table 1, Figure 1).
Table 1. Cave name, conservation units (Park), type of rocks (lithology), locations, and length of the sampled caves. Lapa Grande State Park (LGSP), Ibitipoca State Park (ISP), UTM Coordinates (WGS 84, Zone, 23S)
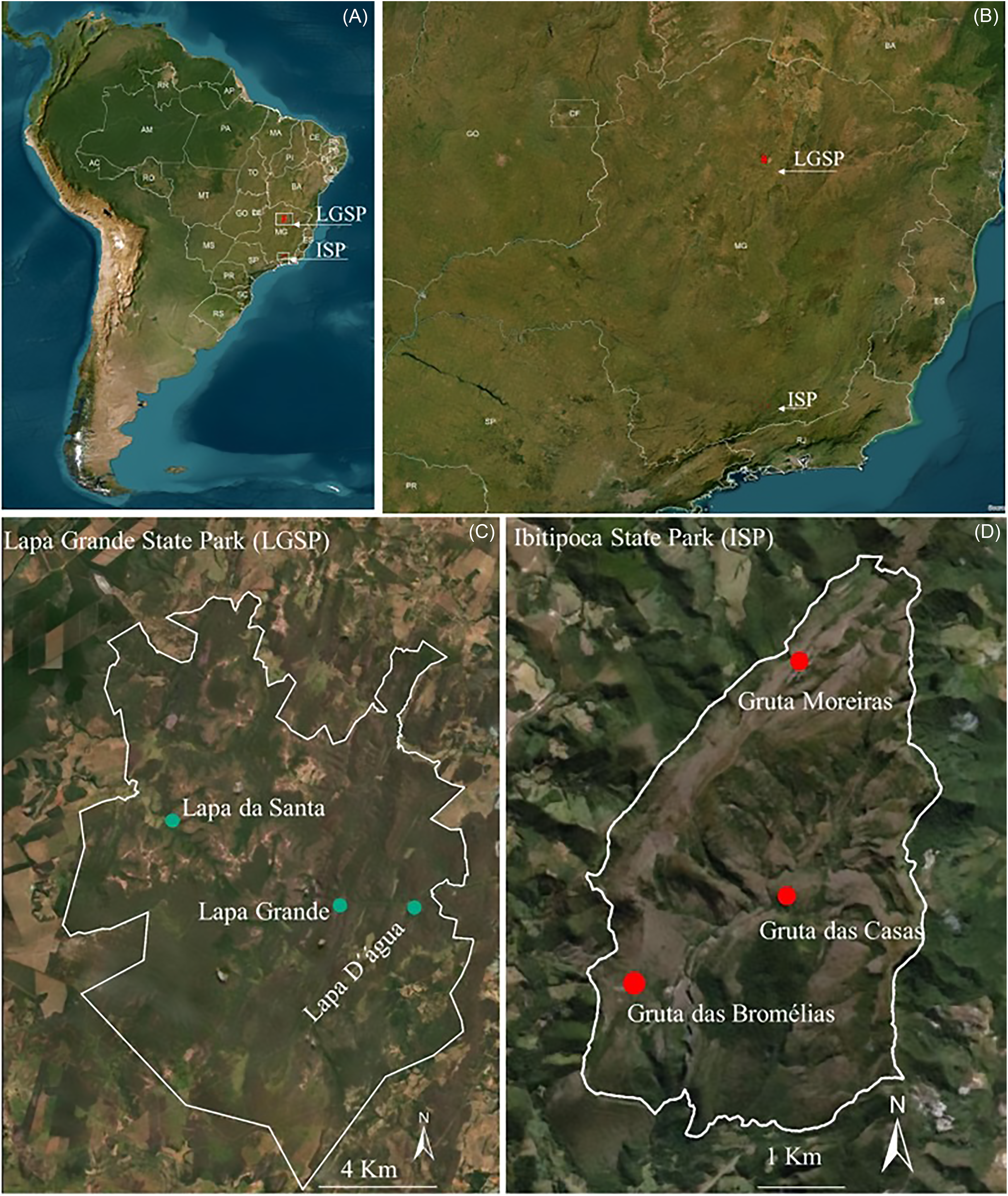
Figure 1. Brazilian map (A) and Minas Gerais state (B) with the location of the Lapa Grande State Park (Limestone) and Ibitipoca State Park in (Quartzite) Minas Gerais state, Brazil (A and B). Details of the park borders (C and D) and sampled cave distribution (green and red dots in C and D).
The region of LGSP is located within the São Francisco River basin, one of the major rivers in Brazil (CBHSF 2023). The climate is categorised as Hot Central Tropical and Semi-arid, with high average annual temperatures exceeding 18°C and a yearly dry period of at least six months (IDE-Sisema 2023). Furthermore, this area is part of the Cerrado biome, where the predominant phytophysiognomies include Cerrado Strictu Sensu, Deciduous Seasonal Forest (Dry Forest) associated with rocky outcrops, and Semideciduous Seasonal Forest along watercourses (Hoffman Reference Hoffmann2012). The region features elevations ranging from 600 to 1,000 metres above sea level. Its rugged terrain is noteworthy for its striking limestone outcrops and unique karst formations, adding to its distinctive natural character. The limestone rocks found in the park are part of the São Francisco Carbonate Supergroup, specifically the Bambuí Group, which includes the Lagoa da Jacaré and Serra da Saudade formations (Auler & Farrant Reference Auler and Farrant1996 CPRM/RIGEO 2014, IEF 2014, IDE-Sisema 2023).
On the other hand, the ISP region lies within the Paraíba do Sul River basin, and the Rio Grande River basin defines its western border (IDE-Sisema 2023). The climate is mild and humid mesothermal, characteristic of Central Brazil Tropical, with average annual temperatures between 10 and 15°C and up to two dry months per year (IDE-Sisema 2023). The local mountainous terrain significantly affects the park’s climate (IEF 2007). The park is part of the Atlantic Forest biome. However, the area has a variety of plant and tree types, including Altitude Grasslands, Rocky Grassland, Montane Semideciduous Seasonal Forest, and patches of Montane Rainforest (cloud forest) (IDE-Sisema 2023, IEF 2007). The Grassland phytophysiognomies dominate the park, while the forested phytophysiognomy occurs in smaller proportions, often linked to valley and drainage areas (IEF 2007). The Ibitipoca Ridge stands at one of the highest elevations in the region, with average elevations ranging from 1,000 to 1,400 m. Additionally, the terrain is rugged and marked by steep escarpments (IEF 2007). The geomorphological formation in which the park is located is a quartzitic unit referred to as the Andrelândia Plateau (IDE-Sisema 2023, IEF 2007).
Sampling design
Three caves were sampled in each of the study areas (LGSP and ISP), and biotic data collection occurred during two consecutive seasons, dry and rainy, resulting in two field campaigns for each region. As data collection extended to the epigean environment, we considered intrinsic cave characteristics and the surrounding landscape characteristics for the cave selection process (Oliveira & Ferreira Reference Oliveira and Ferreira2024). Consequently, caves with less than 300m of linear projection and those in excessively rugged terrain were not included.
A linear transect was marked out, running from inside each cave to the surrounding epigean area. This path covered 300 metres in each direction. We marked 20 sampling points along the path—10 inside the cave and 10 in the epigean area. Each sampling point was 30 metres apart. We drew a 10-metre line perpendicular to the transect at each collection point and gathered three samples from one-square-metre quadrants. This method ensured the samples were at least 5 metres apart (Figure 2).
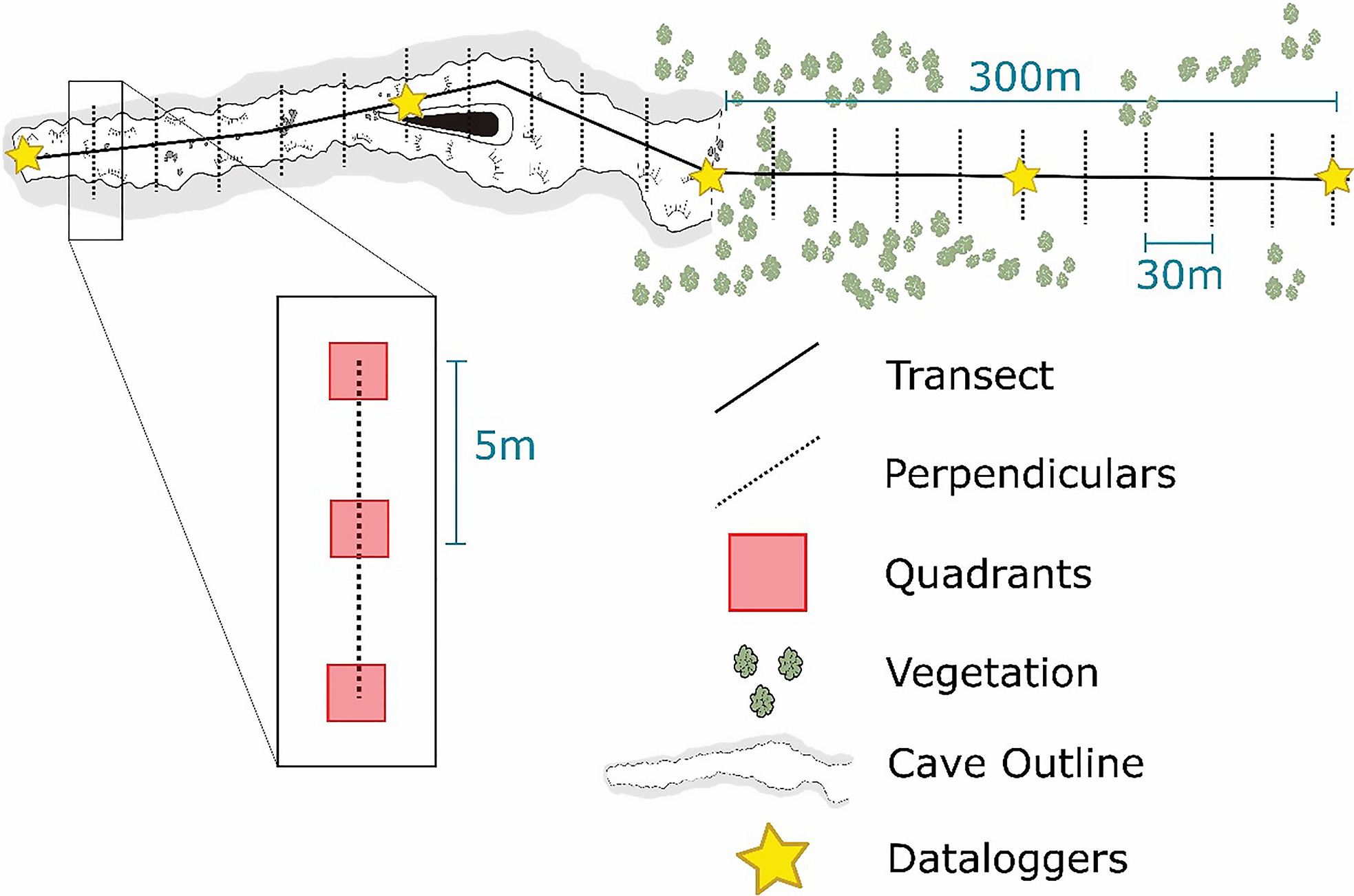
Figure 2. Infographic representing sampling design used to analyse the relationship between terrestrial invertebrates’ fauna and environmental characteristics in caves and surrounding landscapes (epigean habitats).
Abiotic and biotic data sampling
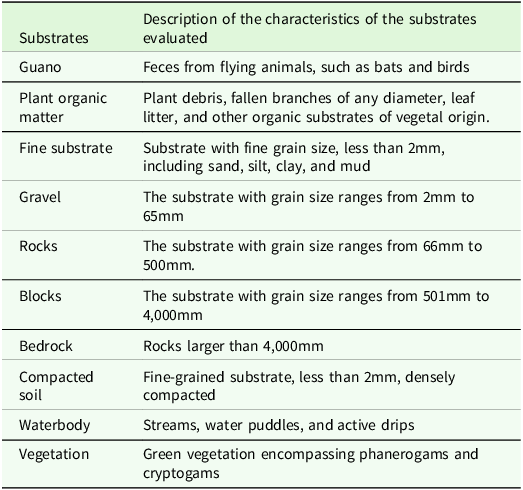
We measured ground-level temperature and humidity at each sampling point using a digital thermo-hygrometer (AKSO AK-625, with an accuracy of ±0.8°C and ±4% relative humidity). A photograph was taken at chest height in each quadrant, at a right angle to the ground, to analyse substrate types and organic resources quantitatively. These photographs underwent analysis using Image-J software (Schneider et al. Reference Schneider, Rasband and Eliceiri2012, Souza-Silva et al. Reference Souza-Silva, Cerqueira, Pellegrini and Ferreira2021), where the areas occupied by each type of substrate were measured and recorded. The specific substrate types and organic resources assessed in the quadrants are outlined in Table 2. Subsequently, substrate diversity was evaluated using the Shannon index (Peet Reference Peet1974, Magurran Reference Magurran2013).
Table 2. Substrate type categories measured inside the quadrants
Invertebrates within the quadrants were collected using the Direct Intuitive Search method (Wynne et al. Reference Wynne, Howarth, Sommer and Dickson2019). This approach involved gathering the invertebrate fauna from the leaf litter and other potential microhabitats, including areas beneath stones, blocks, and logs, utilising a tray, forceps, and brush. The sampling effort was standardised as 10 minutes per square metre (10min/m2) or, in other words, 10 minutes per quadrant.
The collected invertebrates were then preserved in a 70% alcohol solution and subsequently categorised into morphospecies in the laboratory, with the assistance of a stereomicroscope, following the protocols outlined in Oliver and Beattie (Reference Oliver and Beattie1996) and Derraik et al. (Reference Derraik, Closs, Dickinson, Sirvid, Barratt and Patrick2002). This study employed a morphospecies-based approach rather than identifying all specimens to the lowest possible taxonomic level. This decision was made due to the high richness and abundance of morphospecies observed. Further taxonomic identification would not significantly affect the statistical analyses or substantially enhance the discussion. The specimens were deposited in a scientific collection by the Center of Studies in Subterranean Biology (https://www.biologiasubterranea.com.br).
Data analysis
Species richness and similarity comparisons
We performed the Wilcoxon-Mann-Whitney tests to compare the average species richness between the epigean landscape and cave environments across quartzite and limestone rocks during dry and wet seasons. This non-parametric test is well suited for comparing data distributions from two independent sample groups, making it a preferred tool when the data does not adhere to the assumptions of normality. For the creation of the graph and the execution of this analysis, we utilised the R software (R Core Team 2022).
Then, we built non-metric Multidimensional Scaling (nMDS) graphs along with an Analysis of Similarities (ANOSIM) to investigate the similarities of the fauna composition from the different lithologies (limestone and quartzite) during both seasons (dry and rainy) in the different environments (epigean and cave). The resemblance matrix was built from squared root transformed abundance data and the Bray-Curtis resemblance measure. The nMDS graphs visually represented the differences between groups, while the ANOSIM determined the statistical significance of these differences (Somerfield et al. Reference Somerfield, Clarke and Gorley2021).
Influence of environmental variables on the invertebrates’ composition
Distance-based linear models (DistLM) were developed to evaluate the relative significance of environmental variables in explaining variations in species composition across different habitats (epigean and caves), lithologies (limestone and quartzite), and seasons (wet and dry) (Legendre & Anderson Reference Legendre and Anderson1999). Bray Curtis resemblance matrices, squared root transformed abundance, were used in model construction, as they are considered one of the most suitable methods for examining species composition data and comparing biological communities (Ricotta & Podani Reference Ricotta and Podani2017). Additionally, this approach is sensitive to relative abundances and remains robust even with a substantial amount of missing data (Ricotta & Podani Reference Ricotta and Podani2017).
The Forward approach was chosen for model selection. This approach starts with an empty model and progressively incorporates predictor variables, significantly enhancing model fit (Andersen & Bro Reference Andersen and Bro2010). The Adjusted R-squared coefficient was used as the model selection criterion. This coefficient quantifies the proportion of total variability in the response variable that the model accounts for while also considering model complexity by excluding variables that do not substantially contribute to explaining the data. A higher Adjusted R-squared value indicates a better-fitting model, balancing fit with parsimony (Johnson & Omland Reference Johnson and Omland2004).
The environmental variables incorporated into the models included all sampled inorganic substrates and organic debris (detailed in Table 1), as well as substrate diversity, temperature, humidity at the time of sampling, and the distance from the cave entrance (considered only for sampling spots within the cave environment). Substrate variables were aggregated into indicators in the DistLM models to enhance model fitting and aid interpretation. Specifically, the substrate variables ‘fine substrate’, ‘bedrock’, and ‘compacted substrate’ were consolidated under ‘homogeneous substrates’. Furthermore, the substrate variables ‘gravel’, ‘rocks’, and ‘blocks’ were combined as ‘shelter availability’.
Results
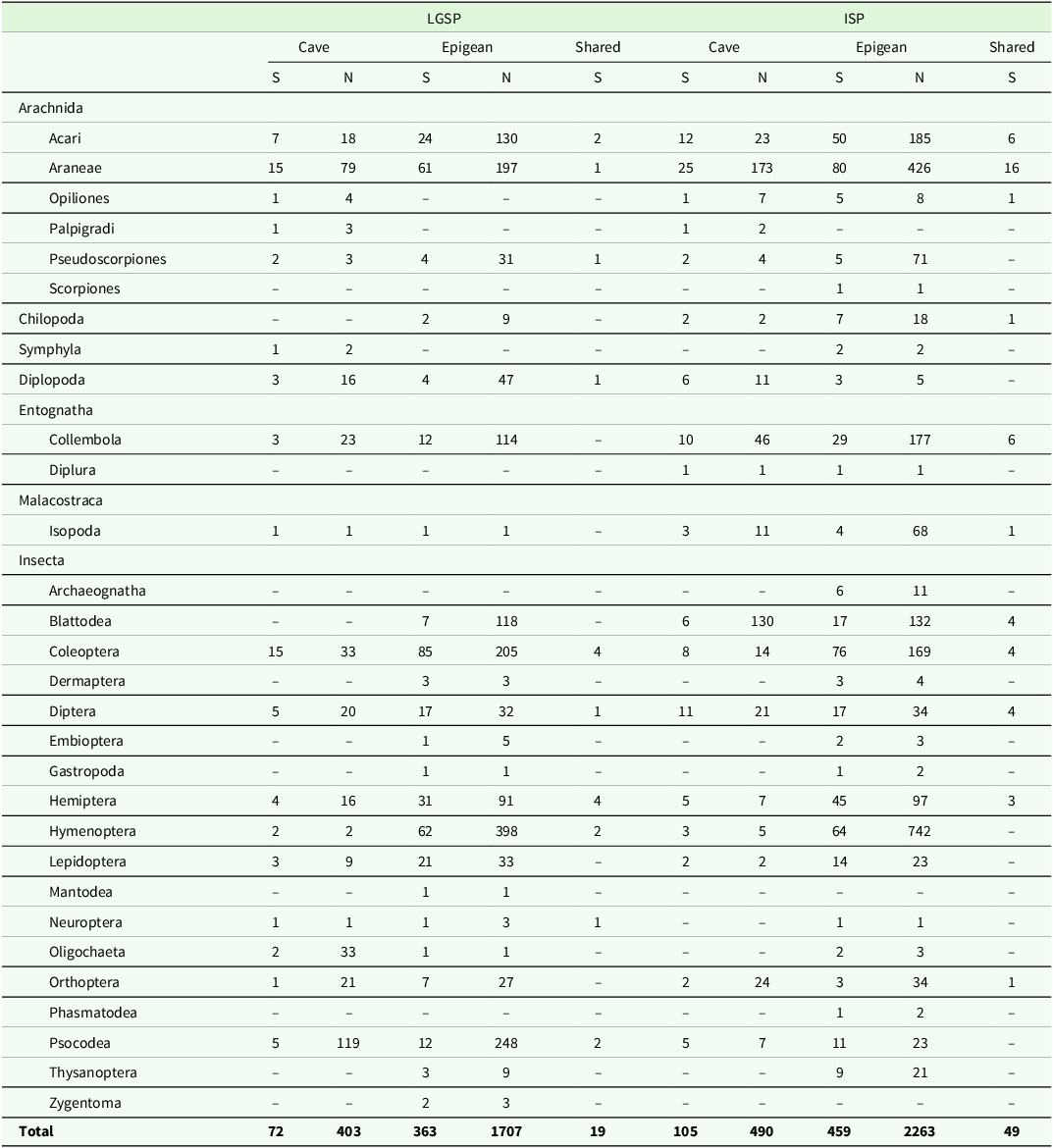
In total, 4,863 invertebrates were recorded, comprising 2,110 from LGSP and 2,753 from ISP. We identified 31 invertebrate orders, with 27 present in LGSP and 29 in ISP. LGSP yielded 415 invertebrate species (72 inside the caves, 363 from epigean, and 19 shared), while ISP revealed 553 species (105 inside the caves, 469 from epigean, and 49 shared). In LGSP, the most diverse invertebrate orders were Coleoptera, Araneae, and Hymenoptera, with respectively 96, 75, and 62 species. In ISP, on the other hand, the most diverse orders were Araneae, Coleoptera, and Acari, with species richness of 89, 80, and 56, respectively (Table 3).
Table 3. Species richness and abundance of terrestrial invertebrates recorded in epigean and cave habitats from Lapa Grande State Park (LGSP) and Ibitipoca State Park (ISP) during dry and rainy seasons. Shared = Species shared between both environments; S Species richness; N = number of individuals
Spatial and temporal variations in species richness
Differences in total species richness between the two state parks were not statistically significant during either the dry or rainy periods (rainy: W = 1,885; p = 0.657; dry: W = 1,470; p = 0.083). However, species richness was significantly higher in epigean areas than in cave habitats for both lithologies during dry and rainy periods (rainy: W = 55.5; p < 0.01; dry: W = 183; p < 0.01). The average species richness in epigean habitats was 160 (SD = 20.52) for the limestone area and 223 (SD = 65.09) for the quartzite area. In limestone cave habitats, the average species richness was 29 (SD = 13.57), and in quartzite caves, it was 42 (SD = 17.61).
Furthermore, when considering the different lithologies separately, we observed that for limestone, the average total species richness varied significantly between seasons (W=2,359; p = 0.003), whereas for quartzite, it did not (W = 1,880.5; p = 0.674). Additionally, when comparing fauna from different environments across seasons, species richness was higher during the rainy season (RS) in the epigean habitats compared to the dry season (DS) (W = 2,590; p < 0.01), while no significant difference was observed for the cave environment (W = 1,994; p = 0.305) (Figure 3).
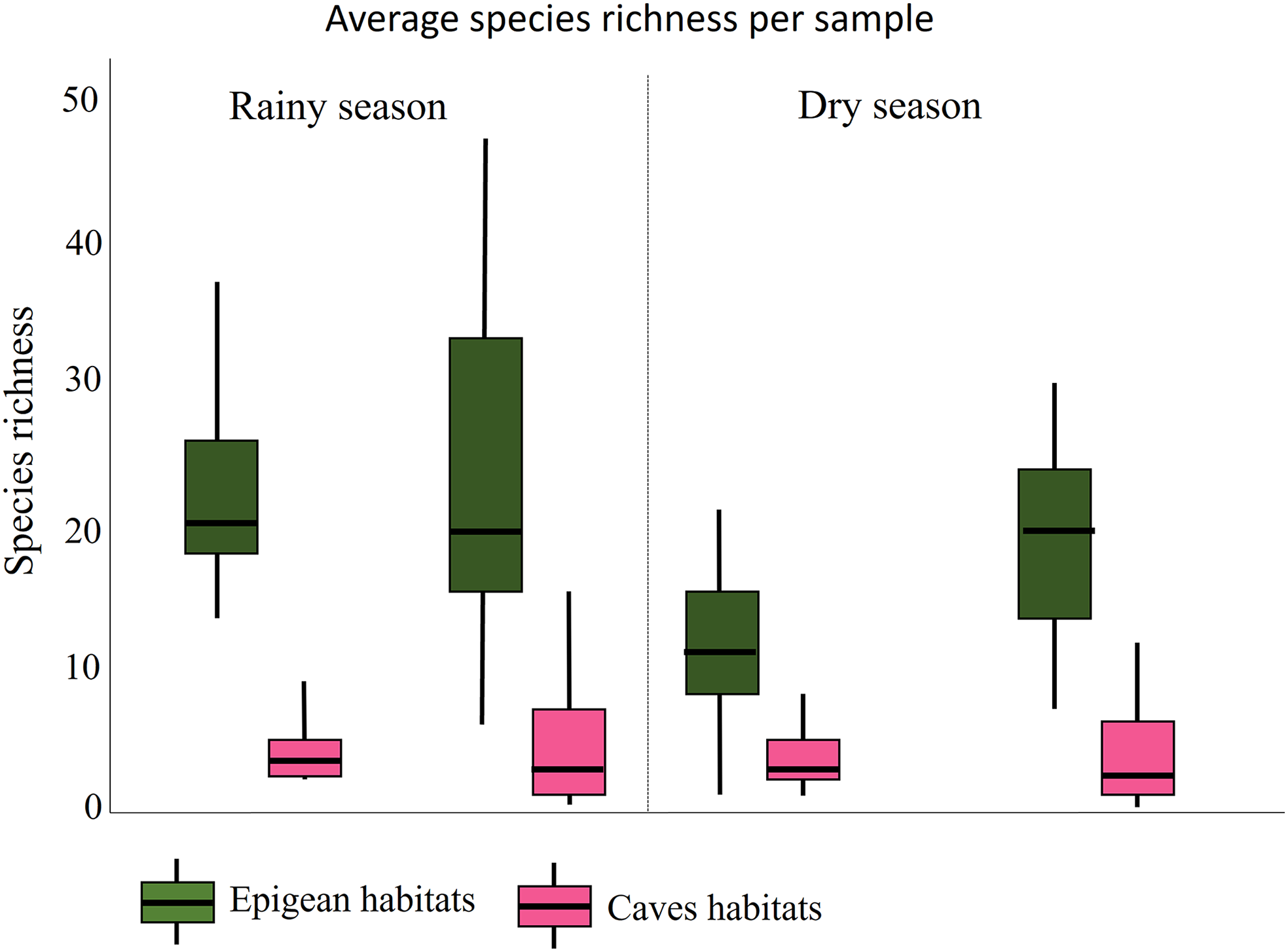
Figure 3. Boxplot illustrating the average species richness recorded in caves and their adjacent surrounding areas (epigean habitats) from Lapa Grande State Park and Ibitipoca State Park, Brazil, during rainy and dry seasons. The central line within each box represents the median value. The lower and upper edges of the box indicate the first (Q1) and third (Q3) quartiles, respectively. The whiskers extend to the smallest and largest values within 1.5 times the interquartile range from the first and third quartiles.
Spatial and temporal variations in species composition
Limestone caves (LGSP)
The nMDS plots illustrate the faunal similarities among caves and epigean sampling points (Figure 4). While these differences are not visually distinct in the nMDS plots, they are statistically significant according to the ANOSIM (Table 3). Additionally, the epigean fauna exhibited seasonal differences, whereas the cave fauna did not (Figure 4; Table 4).
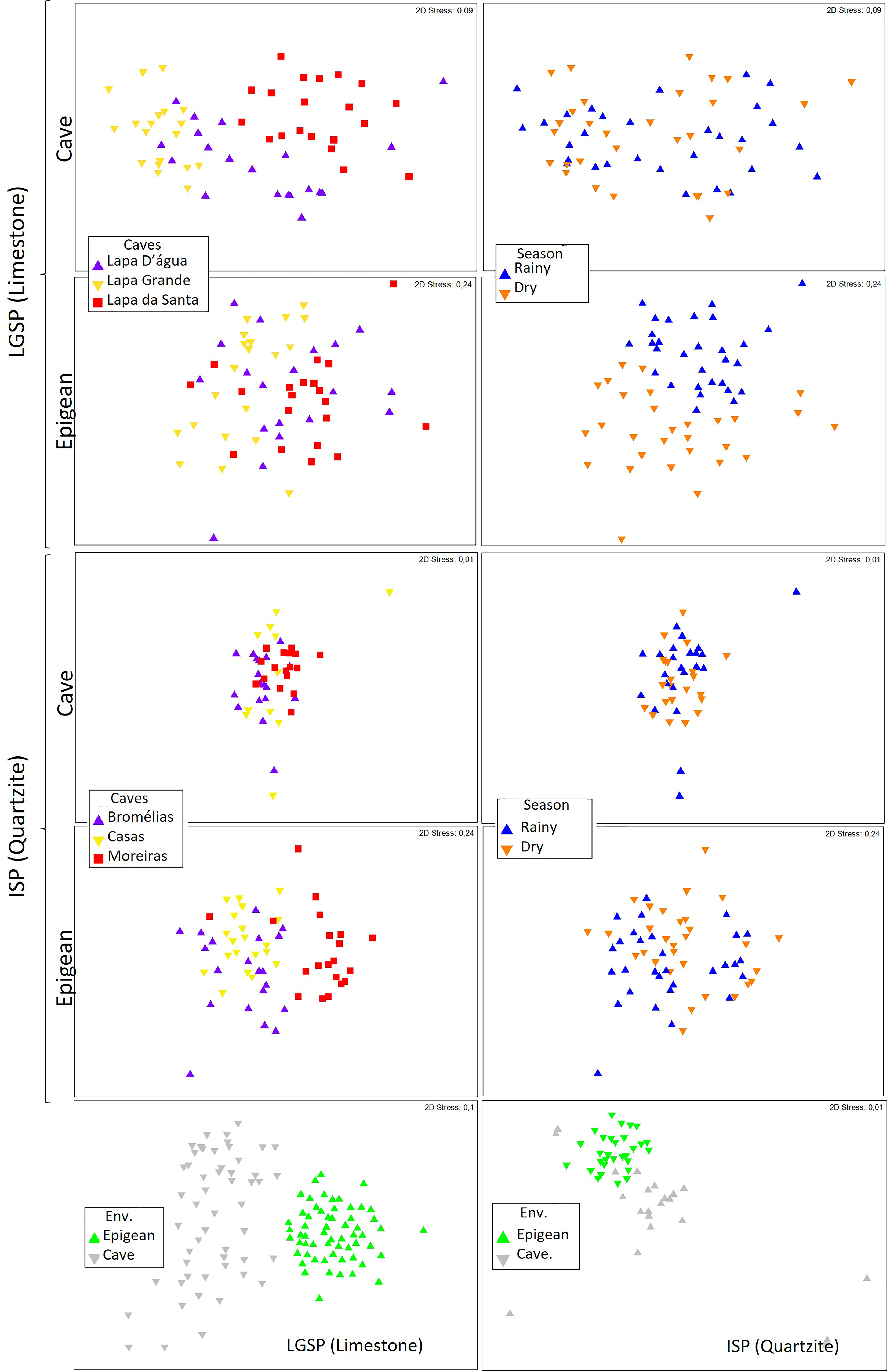
Figure 4. Non-metric Multidimensional Scaling comparisons among caves and epigean habitats of the terrestrial invertebrate Bray-Curtis similarity from Lapa Grande State Park (A, B, C, D, and I) and Ibitipoca State Park (E, F, G, H, and J).
Table 4. Analysis of Similarities differences in terrestrial invertebrate dissimilarity fauna from Lapa Grande State Park (LGSP) and Ibitipoca State Park (ISP) under different approaches (R = Global R statistics). Outside the caves (epigean adjacent surrounding areas)
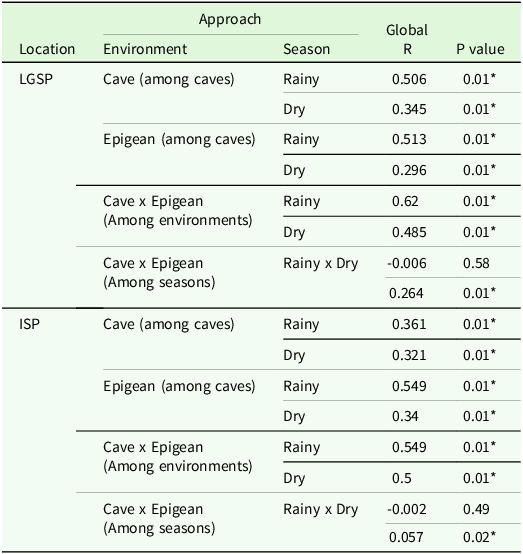
The ANOSIM revealed significant differences in fauna composition driven by environmental variations and seasonal fluctuations. Cave habitats and epigean environments showed considerable dissimilarities, particularly during the RS (Table 4). Furthermore, the epigean fauna exhibited differences in species compositions between rainy and DSs, whereas the cave environment did not (Table 4).
Quartzitic caves (ISP)
In the nMDS plots, we observed a more pronounced distinction in fauna composition between different caves when comparing epigean and cave environments (Figure 4 E to H and J). Although these differences were not as conspicuous for the cave environment in the nMDS plots, they were significant, as evidenced by ANOSIM (Table 4). Additionally, while the epigean fauna displayed distinct patterns between seasons, no such differentiation was observed in the cave habitats (Table 4).
The ANOSIM revealed significant differences in fauna composition based on environmental factors and seasons. Notably, the cave and epigean environments exhibited significant dissimilarities among caves, though these differences were more balanced across seasons. Furthermore, the epigean fauna showed similarities between RSs and DSs (Table 4).
Influence of environmental variables on the invertebrate community composition
Limestone caves (LGSP)
The DistLM model analysis revealed that the selected predictor variables significantly impact species composition in all studied scenarios. However, the variables with the highest explanatory power and the strength of these relationships varied significantly between environments and among limestone caves.
In LGSP, models considering cave and epigean environments during the RS explained 49.83% and 29.04% of the variations in species composition, respectively. However, for the DS, models accounted for 29.26% and 11.76% of the variations in species composition in cave and epigean cave environments, respectively (Table 5).
Table 5. Distance-based linear models (DistLMs) best models after the sequential tests between predictor variables and species composition from Lapa Grande State Park (LGSP) and Ibitipoca State Park (ISP) during rainy seasons and dry seasons. (Adj. R² = Adjusted R-squared; Prop = Proportion of variable explanation within the model; Cumul. Prop. = Cumulative proportion of model explanation; * = statistically significant variables)
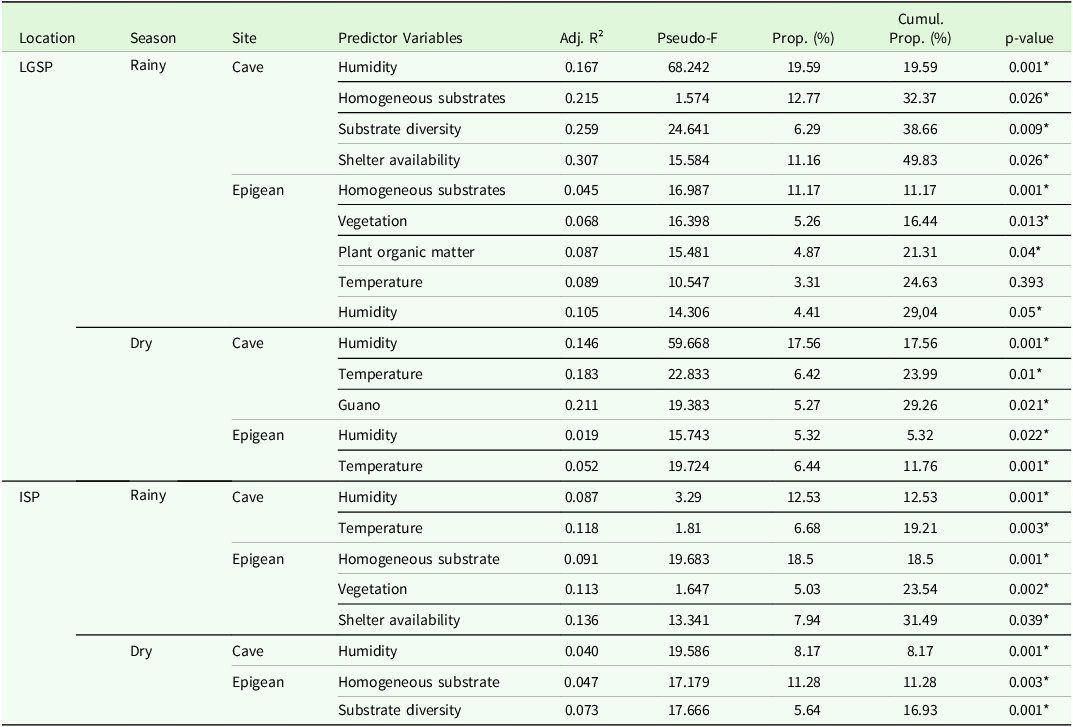
The model focused on the cave environment during the RS demonstrated the highest capacity for explaining variations in species composition. This highlights the significant influence of the cave’s specific conditions on its fauna. During the RS, humidity, homogeneous substrates, substrate diversity, and shelter availability were the primary environmental factors influencing cave species composition. In contrast, during the DS, the key variables were humidity, temperature, and guano.
Finally, in the epigean environment, the dominant factors influencing variations in species composition during the RS were homogeneous substrates, vegetation, plant organic matter, temperature, and humidity. In the DS, species composition was primarily influenced by moisture and temperature (Table 5).
Overall, the models for epigean fauna showed lower explanatory power than those for cave fauna. However, it is important to note that many influential variables were shared across models, albeit with varying degrees of significance.
Humidity emerged as the most significant variable explaining variations in species composition within LGSP, appearing in all models and serving as the primary explanatory variable in three. Temperature was the second most influential factor, crucial in three scenarios (Table 5).
Quartzitic caves (ISP)
Within ISP, the DistLM analyses revealed that the predictor variables significantly affected species composition in the cave environments. Like LGSP, the strength of this relationship varied across environments. However, a notable difference emerged: the epigean environments showed more model explanations than the cave environments in both seasons.
In the cave environments of ISP caves, the models exhibited lower explanatory power, accounting for 19.21% and 8.17% of the variation in species composition during the rainy and DSs, respectively. In contrast, the epigean environment models explained 31.49% and 16.93% of the variations in species composition during the rainy and DSs, respectively (Table 4).
In the cave environment, the most influential variables were humidity and temperature during the RS, with humidity alone being the key factor during the DS. In contrast, the most significant variables for the epigean environment were the presence of homogeneous substrates, vegetation, and shelter availability during the RS, as well as homogeneous substrate and substrate diversity during the DS (Table 5).
Humidity was the most critical variable in cave environments, accounting for 12.53% of the variation in species composition during the RS and 8.17% during the DS. It was the only variable selected by the model (Table 5).
In the epigean cave environments, homogeneous substrates were the most influential factor, explaining 18.5% of the variation in species composition during the RS and 11.28% during the DS (Table 5).
Discussion
The study highlights a remarkable diversity of terrestrial invertebrate species within the caves and surrounding areas across the rainy and DSs. Although there were no significant differences in species richness between the two sampled areas (limestone and quartzite), substantial variations were observed in the species richness and composition of invertebrates between cave environments and their adjacent epigean areas in both lithologies. These differences reflect the effect of environmental filters that restrict the colonisation of cave environments. Specifically, species richness was higher during the RS in the limestone areas, while no similar trend was observed in the quartzite region. These findings emphasise the role of various environmental variables in shaping invertebrate fauna in both areas, with climatic factors emerging as one of the most significant drivers of species composition.
We observed significant species richness and composition differences across lithologies and seasons when investigating the relationship between epigean and cave environments. In general, epigean environments tend to have a greater diversity of invertebrate species compared to cave environments due to the availability of food resources (Souza-Silva et al. Reference Souza-Silva, Martins and Ferreira2011a, Cardoso Reference Cardoso2012, Mendes-Rabelo et al. Reference Mendes-Rabelo, Souza-Silva and Ferreira2020). Additionally, cave habitats showed a tighter variation in the number of species among seasons, while epigean environments displayed considerable variability. This consistent pattern in cave environments underscores the sensitivity of these habitats to environmental changes. The lower turnover in species richness may result from the relatively stable conditions within caves, which can act as a selective filter, favouring species pre-adapted to these specific conditions (Poulson & White Reference Poulson and White1969, Prous et al. Reference Prous, Ferreira and Martin2004, Reference Prous, Ferreira and Jacobi2015).
The seasonal variations in species richness enhance our understanding of the influence of seasonality on terrestrial invertebrate fauna. During the RS, epigean environments exhibited higher species richness compared to the DS, likely due to increased organic resource availability and productivity during the rainy period (Silva et al. Reference Silva, Frizzas and Oliveira2011). Like epigean environments, cave habitats also tend to experience an increase in organic matter input during the RS (Souza-Silva et al. Reference Souza-Silva, Ferreira, Bernardi and Martins2007, Reference Souza-Silva, Martins and Ferreira2011a, Reference Souza-Silva, Bernardi, Martins and Ferreira2012), as a significant portion of this input occurs through bats and current water (Simon et al. Reference Simon, Pipan and Culver2007, Souza-Silva et al. Reference Souza-Silva, Martins and Ferreira2011a).
Although seasonal differences in cave species richness have been reported in the literature (Bento et al. Reference Bento, Ferreira, Prous, Souza-Silva, Bellini and Vasconcellos2016, Lunghi et al. Reference Lunghi, Manenti and Ficetola2017), our study did not find differences across seasons. The lack of seasonal variation in species richness in our study areas underscores the importance of environmental stability for cave ecosystems and their invertebrate fauna.
Our investigation into the invertebrate community composition revealed that both lithologies exhibited similar trends, with the cave environment displaying stability in species composition across seasons, in contrast to the epigean environment. This stability in species composition between seasons highlights the importance of caves’ stable environmental conditions. Such stability provides a refuge for species adapted to cave conditions, shielding them from the pronounced seasonal variations experienced by epigean fauna (Mammola Reference Mammola2019).
Moreover, the observed dissimilarities in epigean fauna composition between rainy and DSs align with studies highlighting the impact of seasonal fluctuations on epigean environments (Tonkin et al. Reference Tonkin, Bogan, Bonada, Rios-Touma and Lytle2017) and their invertebrate fauna (Frith & Frith Reference Frith and Frith1990, Kai & Corlett Reference Kai and Corlett2002). Seasonal variations influence temperature, humidity, precipitation, and organic resource availability in epigean ecosystems, contributing to their dynamic nature. In contrast, cave ecosystems are more stable across seasons, with these factors fluctuating less around the annual average observed for the epigean environment (Badino Reference Badino2004, Reference Badino2010, Mejía-Ortíz et al. Reference Mejía-Ortíz, Christman, Pipan and Culver2020, Reference Mejía-Ortíz, Christman, Pipan and Culver2021).
Significant dissimilarities among the epigean environments, although these differences were less pronounced than in the cave environment, can result from local habitat heterogeneity. It is important to note that although caves have more stable microclimates, trophic characteristics and microhabitat structure can vary between caves even in a short distance. Some caves may have guano, while others may contain roots, plant debris, mud, sand, gravel, etc. (Souza-Silva et al. Reference Souza-Silva, Iniesta and Ferreira2020, Reference Souza-Silva, Cerqueira, Pellegrini and Ferreira2021). This variation in organic resources and microhabitats leads to more differentiated faunal compositions among caves in the same region, in contrast to the epigean invertebrate communities primarily associated with the surrounding vegetation and having high turnover (Kitching et al. Reference Kitching, Dahlsjö and Eggleton2020). Likewise, Simões et al. (Reference Simões, Souza-Silva and Ferreira2015) found dissimilar invertebrate fauna among caves, albeit in a broader sampling scale.
Research in tropical regions has shown that environmental factors like rock type, water presence, cave size, number and size of entrances, distance between caves, and habitat availability can influence the diversity and composition of invertebrate communities in caves (Souza-Silva et al. Reference Souza-Silva, Iniesta and Ferreira2020, Simões et al. Reference Simões, Souza-Silva and Ferreira2015, Bento et al. Reference Bento, Ferreira, Prous, Souza-Silva, Bellini and Vasconcellos2016). Therefore, this intricate interplay between community similarities underscores the need for larger-scale studies, as some invertebrate community properties only emerge at larger scales and are often masked at restricted analysis scales, as observed in this study (Moseley Reference Moseley2009, Mammola Reference Mammola2019, Reis-Venâncio et al. Reference Reis-Venâncio, Rabelo, Pellegrini and Ferreira2022).
The relationship between environmental factors and species composition varied across the studied environments and seasons. The variables measured in limestone environments provided a more robust explanation for species composition variation than those in quartzite environments. For limestone, the influence of environmental factors was more pronounced inside the caves during the RS. Cave environments are often referred to as ‘natural laboratories’ due to their simplified and stable biotic and abiotic conditions (Poulson & White Reference Poulson and White1969, Mammola et al. Reference Mammola, Paschetta and Isaia2014, Mammola Reference Mammola2019, Sánchez-Fernández ). Our model for the cave environment explained a much more significant percentage of the variation in fauna composition compared to the epigean region, aligning with these assumptions. The inherent stability of cave environments, with relatively constant temperatures and high humidity levels, can create a more predictable and less dynamic setting for invertebrate communities. This stability likely accounts for the increased explanatory power of the models, as the limited external influences in cave environments enhance the models’ effectiveness in capturing the intricate relationships between environmental factors and invertebrate communities.
Regarding quartzite, the predictor variables had a more modest influence on species composition variations than limestone. The strength of this relationship varied between environments, with a more significant influence on the epigean environment than the cave one. This unexpected result may stem from the fact that the quartzite caves in this study have more entrances than the limestone caves, making them more susceptible to external environmental factors.
Multiple entrances in quartzite caves have been shown to influence species diversity. This indicates that entrances could function as shelter locations and pathways for colonising various invertebrate species (Souza-Silva et al., Reference Souza-Silva, Iniesta and Ferreira2020). Quartzite caves with more entrances might provide more opportunities for colonisation, potentially leading to a greater variety of invertebrate species, especially those associated with the transitional areas near cave entrances (Prous et al. Reference Prous, Ferreira and Jacobi2015).
The importance of climatic factors, such as humidity and temperature, in shaping cave environments across both lithologies aligns with existing literature, highlighting these variables’ role in structuring cave ecosystems (Simões et al. Reference Simões, Souza-Silva and Ferreira2015, Lunghi et al. Reference Lunghi, Manenti and Ficetola2017). This consistency underscores the fundamental role of these climatic variables in shaping cave community structure by providing a stable habitat less affected by the significant environmental fluctuations typical of epigean ecosystems.
The increased influence of vegetation, shelter availability, and substrate diversity on epigean invertebrate communities suggests that these factors may serve as refuges from the significant fluctuations typically observed in epigean environments. Although our study measured temperature and humidity directly at sampling sites, the complexity of variables affecting epigean communities means that the direct impact of these climatic factors might not be apparent. Instead, vegetation and shelter availability indirectly reflect the animals’ preference for more stable climatic conditions (Kolasa & Pickett Reference Kolasa and Pickett1991, Ficetola et al. Reference Ficetola, Lunghi and Manenti2020).
Research shows a compelling connection between epigean-dwelling and cave invertebrate diversity (Mendes-Rabelo et al. Reference Mendes-Rabelo, Souza-Silva and Ferreira2020, Oliveira & Ferreira Reference Oliveira and Ferreira2024). However, the pronounced differentiation observed between epigean and cave environments in our study underscores the significant selective pressures imposed by cave environments. Fauna must overcome substantial environmental filters to successfully colonise and persist in these habitats over the long term (Chapman, Reference Chapman1982, Sket Reference Sket1999, Romero Reference Romero2009, Prous et al. Reference Prous, Ferreira and Jacobi2015).
Final considerations
This study offers valuable insights into the ecological relationships of invertebrate communities in cave environments, highlighting the importance of viewing both caves and their surrounding areas as an interconnected ecosystem rich in biodiversity. The differences observed in species richness and composition between the study areas highlight the complexity of these ecological interactions. Furthermore, investigations regarding other compartments of the subterranean ecosystem are encouraged (e.g. the Mesovid Shallow Substratum - MSS). The MSS is known to harbour specialised and diverse invertebrate communities, which can provide a more comprehensive understanding of subterranean biodiversity and connectivity. Future research integrating MSS sampling would offer deeper insights into the ecological dynamics between surface and subterranean habitats and further clarify the processes shaping invertebrate community structure.
The significant influence of climatic variables on fauna composition in cave and epigean environments suggests that conservation strategies focusing on preserving microclimatic conditions could enhance the connectivity between these environments. These findings deepen our understanding of cave systems and their surroundings, underscoring the need to consider regional and cave-specific factors in conservation strategies. This comprehensive approach is critical in global warming, where understanding and preserving these unique ecosystems is paramount.
Finally, understanding the ecological patterns in both epigean and cave environments is essential for grasping the connections between these ecosystems. The stability observed in cave environments and their potential role as refuges amidst global environmental fluctuations like climate change highlight the need to incorporate cave ecology into broader ecological frameworks.
Data availability statement
The data supporting this study’s findings are available on request from the corresponding author.
Acknowledgements
The authors thank the team at the Center for Studies in Subterranean Biology (CEBS/UFLA) for their support during field trips; additionally, they extend acknowledgement to CNPq (National Council for Scientific and Technological Development) for the productivity scholarship awarded to RLF (CNPq n. 302925/2022-8). Thanks to the teams at Ibitipoca and Lapa Grande state parks.
Author contributions
Rodrigo Lopes Ferreira and Gabrielle Soares Muniz Pacheco were responsible for the conceptualisation and methodology. Gabrielle Soares Muniz Pacheco, Rodrigo Lopes Ferreira, and Marconi Souza Silva participated in field activities. Gabrielle Soares Muniz Pacheco prepared the original draft. Gabrielle Soares Muniz Pacheco, Rodrigo Lopes Ferreira, and Marconi Souza Silva were responsible for statistical analysis, artwork, reviewing, and editing. All authors have read and agreed to the published version of the manuscript.
Financial support
This research received financial support from Fundação de Amparo à Pesquisa do Estado de Minas Gerais and Vale company S/A, which sponsored the field and laboratory activities of the study (CRA-RDP-00070-18), and from the scholarship granted to Gabrielle Soares Muniz Pacheco. The data used in this article were part of her thesis in Applied Ecology at the Federal University of Lavras.
Competing interests
The corresponding author confirms on behalf of all authors that no involvement might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated.












