Introduction
Sea slugs of the genus Aplysia are commonly known as sea hares because of their characteristic ear-like rhinophores on the top of the head. These euopisthobranch gastropods typically inhabit intertidal and subtidal zones in tropical and temperate regions, where they feed on a variety of macroalgae (Eales, Reference Eales1960; Golestani et al., Reference Golestani, Crocetta, Padula, Camacho-García, Langeneck, Poursanidis, Pola, Yokes, Cervera, Jung, Gosliner, Araya, Hooker, Schroedl and Valdes2019). Their size ranges from the small A. parvula, which rarely exceeds 6 cm in length, to the massive A. gigantea and A. vaccaria, with reported lengths of 60 and 76 cm, respectively (Eales, Reference Eales1960; Winkler and Dawson, Reference Winkler and Dawson1963).
Some sea hares are common on Portuguese shores, including A. depilans, which can grow to a length of 30 cm (Eales, Reference Eales1960). Sea hares are highly studied animals, with thousands of publications focusing on their biology and ecology, mainly because A. californica is an important model organism for research in neurobiology and behaviour (Hawkins et al., Reference Hawkins, Bailey, Kandel, Shepherd and Grillner2017; Kandel, Reference Kandel1979). They are simultaneous hermaphrodites that can function as both sperm donors and receivers (Angeloni et al., Reference Angeloni, Bradbury and Burton2003). Although solitary most of the year, these animals congregate to breed during summer (Pennings SC, Reference Pennings1991), attracted by the water-borne protein pheromones attractin, enticin, temptin, and seductin, which are released during egg laying. Egg masses are also a source of these pheromones that act in concert to attract sea hares and to induce or facilitate reproductive activity (Cummins et al., Reference Cummins, Degnan and Nagle2008, Reference Cummins, Nichols, Warso and Nagle2005; Painter et al., Reference Painter, Cummins, Nichols, Akalal, Schein, Braun, Smith, Susswein, Levy, de Boer, ter Maat, Miller, Scanlan, Milberg, Sweedler and Nagle2004). Mating occurs in pairs or groups of several individuals (Audesirk, Reference Audesirk1979; Carefoot, Reference Carefoot1987; Lee et al., Reference Lee, Kaang and Lee2014).
Euopisthobranchs have a complex reproductive system, with several glands and ducts. Spermatozoa and oocytes are simultaneously produced in the ovotestis of Aplysia (Lobo-da-Cunha et al., Reference Lobo-da-Cunha, Bartolomaeus, Malaquias and Starck2025). Collecting tubes from the ovotestis unite into the upper hermaphroditic duct that conducts the oocytes to the fertilization chamber, where they are mixed with the secretion of the albumen gland and allosperm (sperm received from a donor). From the fertilization chamber, the oocytes go to the membrane gland, also known as the winding gland, and proceed to the coiled duct of the mucous gland. At the distal end of the mucous gland duct, a gelatinous coat covers a string of capsules containing several oocytes. The string passes through the oviductal channel of the lower hermaphroditic duct and receives a final coating before spawning. The lower hermaphroditic duct also contains a vaginal channel for the entrance of the allosperm and a separate groove for the exit of the autosperm (Coggeshall, Reference Coggeshall1972; Klussmann-Kolb, Reference Klussmann-Kolb2001; Lee et al., Reference Lee, Kaang and Lee2015; Painter et al., Reference Painter, Kalman, Nagle, Zuckerman and Blankenship1985; Thompson and Bebbington, Reference Thompson and Bebbington1969). Sea hares spawn egg masses consisting of long, intertwined, cylindrical strings in the intertidal zone. Allosperm was observed swimming in the fluid that filled the capsules containing oocytes of Aplysia. The final stages of oocyte maturation, including the release of polar bodies, occur only after spawning (Thompson and Bebbington, Reference Thompson and Bebbington1969).
The average number of eggs in a capsule was estimated to be approximately 25 in A. depilans strings, 15–25 in A. kurodai, around 40 in A. fasciata, 12‒15 in A. californica, and only four in A. punctata (Barash and Zenziper, Reference Barash and Zenziper1980; Bebbington and Thompson, Reference Bebbington and Thompson1968; Carefoot, Reference Carefoot1987; Chávez-Viteri et al., Reference Chávez-Viteri, Brown and Pérez2017; Lee et al., Reference Lee, Kaang and Lee2014). The total length of a string varies considerably, reaching 40 m in the egg masses of A. fasciata, a species that can measure nearly 40 cm in length (Barash and Zenziper, Reference Barash and Zenziper1980). Considering the number of eggs per capsule, the number of capsules per centimetre, and the total length of the string, a single egg mass of A. depilans can contain approximately 3,300,000 eggs (Bebbington and Thompson, Reference Bebbington and Thompson1968). Production of these large egg masses has a high energetic cost for the animals, and although they can reproduce more than once during their lifespan of about one year, death usually occurs after a breeding season (Audesirk, Reference Audesirk1979; Carefoot, Reference Carefoot1987). Embryos develop within capsules and hatch as planktotrophic veliger larvae. Hatching time varies among species and is approximately 15 days for A. depilans (Carefoot, Reference Carefoot1987). Capsules and gelatinous layers protect gastropod eggs laid in the intertidal zone against predators, desiccation, ultraviolet radiation, temperature changes, and salinity variations (Przeslawski, Reference Przeslawski2004; Przeslawski et al., Reference Przeslawski, Davis and Benkendorff2004; Rawlings, Reference Rawlings1994, Reference Rawlings1996). The scarcity of Aplysia egg predators is an important factor in embryo survival. The distastefulness of egg masses prevents predation. Pieces of sea hare egg masses were consistently rejected by fish and crabs, and extracts from A. juliana egg masses were found to be a feeding deterrent for fish (Pennings SC, Reference Pennings1994). However, starved sea stars (Patiriella regularis) were observed to consume egg masses of A. dactylomela (Johnson and Willows, Reference Johnson and Willows1999; Willan, Reference Willan1979), and the nudibranch Favorinus japonicus feeds on egg masses of A. juliana, A. dactylomela, and other species (Gosliner, Reference Gosliner1979).
The egg masses of 19 heterobranch gastropod species, including the sea hares Aplysia punctata, Bursatella leachii, and Phyllaplysia taylori of the family Aplysiidae, were previously investigated by light and electron microscopy (Klussmann-Kolb and Wägele, Reference Klussmann-Kolb and Wägele2001), but without focus on the associated microorganisms. To protect the eggs and developing embryos from abiotic stressors, harmful microorganisms, and potential predators in the intertidal environment, the gelatinous sheath of A. depilans egg strings must possess a specific structure and composition, which is probably of considerable importance for the breeding success of this species and, therefore, warrants detailed investigation. In the current study, electron microscopy was used to observe the ultrastructure of A. depilans egg strings and their associated microorganisms, which likely have an important ecological interaction with the egg masses. Microscopy observations were complemented by the characterization of the bacterial microbiome through metagenomic analysis of the 16S rRNA gene. Histochemical techniques for the detection of proteins, and acid and neutral polysaccharides were applied to analyse the composition of the different layers that protect the eggs and embryos of A. depilans.
Materials and methods
Collection and sample storage
Egg masses of Aplysia depilans Gmelin, 1791, were collected at low tide in August and early September, on the northern coast of Portugal (Molhe beach, Porto: 41°09′28.9′′N, 8°41′03.7′′W). Egg strings were transported in a container with local seawater, observed fresh, and subsequently processed for microscopy. Four egg strings were rinsed with seawater sterilized by filtration, and an approximately 0.03 g sample from each was used separately for DNA extraction with the E.Z.N.A. DNA Kit (ref. D3373; Omega Bio-tek, Georgia, USA). Two additional egg masses were maintained in a small aquarium to monitor development until veliger hatching. Three 2 L surface seawater samples, collected at the same location in August, were filtered separately through sterile cellulose nitrate membranes (0.2 µm pore size, 47 mm diameter; Whatman), and environmental DNA was extracted using the DNeasy PowerSoil Pro Kit (Qiagen). Extracted DNA samples were stored at −80°C until further processing.
Light and electron microscopy
Short portions of freshly collected egg strings were fixed for 2−3 h at 4°C in 2.5% glutaraldehyde and 4% formaldehyde (obtained from hydrolysis of paraformaldehyde), diluted with 0.4 M cacodylate buffer (pH 7.4; final buffer concentration 0.28 M). After washing in buffer, samples were postfixed with 2% osmium tetroxide buffered with cacodylate and dehydrated in increasing concentrations of ethanol. For light and transmission electron microscopy (TEM), the dehydrated samples were embedded in epoxy resin. For light microscopy observations, semithin sections with a thickness of 2 μm were stained with methylene blue and azure II. Additionally, the periodic acid-Schiff (PAS) reaction for polysaccharides, Alcian blue staining for acid polysaccharides, and the tetrazonium coupling reaction for protein detection were applied to semithin sections. For the PAS reaction, the sections were oxidized with 1% periodic acid for 10 min, washed with water, and stained with Schiff reagent for 20 min. For the tetrazonium coupling reaction, sections were treated with a freshly prepared 0.2% solution of fast blue salt B in 0.1 M veronal-acetate buffer (pH 9.2) for 15 min. After washing, sections were treated for 20 min with a saturated solution of β-naphthol in 0.1 M veronal-acetate buffer, pH 9.2 (Nöhammer, Reference Nöhammer1978; Nöhammer and Desoye, Reference Nöhammer and Desoye1981). For Alcian blue staining, the resin was removed using an alcoholic solution of sodium ethoxide, prepared by dissolving sodium hydroxide to saturation in absolute ethanol (Lane and Europa, Reference Lane and Europa1965). After thorough washing in absolute ethanol and water, the sections were stained for 30 min in 1% Alcian blue in acetic acid at pH 2.5 for the detection of carboxylated polysaccharides, or in a pH 1.0 HCl solution for the detection of sulphated polysaccharides. For TEM, ultrathin sections were stained for 20 min with 3% uranyl acetate, washed, and stained for 10 min with lead citrate (Reynolds, Reference Reynolds1963), and then observed with a JEOL 100CXII microscope equipped with a Gatan Orius SC200 digital camera. For scanning electron microscopy (SEM), short segments of egg strings were fixed and dehydrated in ethanol, as described above. Some dehydrated samples were critical point dried in a Bal-Tec CPD 030, while others were air-dried after 5 min in hexamethyldisilazane (Nation, Reference Nation1983). The samples were coated with an Au/Pd thin film by sputtering, using the SPI Module Sputter Coater equipment, before being observed with a FEI Quanta 400 FEG ESEM/EDAX Genesis X4M microscope.
Metagenomic analysis of bacterial microbiomes from A. depilans egg strings and seawater eDNA samples using 16S Ion Torrent sequencing
The DNA extracted from water and from each egg string sample was used for 16S targeted taxonomic profiling of bacteria by Ion Torrent Technology. Ion Torrent sequencing libraries were prepared according to the 16STM Metagenomics Kit protocol following the manufacturer’s instructions. Briefly, the six hypervariable regions of the 16S rRNA gene were amplified using two separate PCR reactions with primer sets V2-4-8 and V3-6, 7-9. Each 30 μl PCR mix contained 15 μl Environmental Master Mix, 3 μl 16S rRNA primer set and 2 μl of DNA sample. The cycling conditions were as follows: denaturation at 95°C for 10 min, 25 cycles of 30 s at 95°C, 30 s at 58°C, and 20 s at 72°C, with a final extension at 72°C for 7 min. Amplicons from each primer set were pooled and end-repaired, and barcoded adapters were ligated into the target amplicons. Libraries were quantified using qPCR with the Ion Universal Library Quantitation Kit, diluted to 40 pM, and pooled equally. Emulsion PCR, templating and 530 chip loading were performed using an Ion Chef Instrument (Thermo-Fisher Scientific). Sequencing was performed on an Ion S5 XLTM sequencer (Thermo-Fisher Scientific). Data were processed using Ion Torrent platform-specific pipeline software Torrent Suite v5.18 to generate sequence reads, trim adapter sequences, filter and remove poor signal reads, and split the reads according to the barcode. Taxonomic assignments were performed using the Ion Reporter 16S Metagenomics workflow (Thermo Fisher Scientific), which employs a two-step BLAST alignment against (1) the MicroSEQ® ID bacterial reference library (Thermo Fisher Scientific, Waltham, MA, USA) and (2) the Greengenes database (DeSantis et al., Reference DeSantis, Hugenholtz, Larsen, Rojas, Brodie, Keller, Huber, Dalevi, Hu and Andersen2006) to achieve comprehensive bacterial identification. According to the Clinical and Laboratory Standards Institute (CLSI) guidelines implemented in the workflow, alignments with <97% identity were assigned at the family level, >97% at the genus level, and >99% at the species level. Sequences not meeting these criteria were retained as classified at lower taxonomic ranks. Bacteria were classified at six levels: phylum, class, order, family, genus, and species. The results are expressed as relative abundance (%).
Statistical analyses of bacterial microbiome composition differences between A. depilans egg string samples and seawater eDNA
Differences in bacterial microbiome composition between A. depilans egg string samples and seawater eDNA samples were analysed separately for two taxonomic resolution datasets: (i) phyla/classes and (ii) families/genera (Supplementary Tables S1, S2). For each dataset, Bray-Curtis dissimilarities were calculated between all pairs of samples. Group-level differences were then tested using permutational multivariate analysis of variance (PERMANOVA) with 999 permutations, yielding a pseudo-F statistic, coefficient of determination (R 2), and permutation-based p-value. Community dissimilarities were visualized by principal coordinates analysis (PCoA) based on Bray–Curtis distances. To identify the taxa contributing most to between-group dissimilarities, a Similarity Percentage (SIMPER) analysis was performed, which decomposes Bray-Curtis dissimilarity into contributions of individual taxa and ranks them by proportional contribution. All analyses were performed in Python (version 3.11.8), using SciPy (1.14.1), NumPy (1.24.0), pandas (1.5.3), and matplotlib (3.6.3).
Results
Fresh egg strings
During the summer season, A. depilans egg masses can be abundant on intertidal rocks at locations on the Portuguese North coast, where individuals of this species congregate to mate and spawn (Figure 1A). Fresh egg strings are approximately 2.5 mm in width and consist of a stratified translucent gelatinous sheath surrounding a long chain of capsules filled with eggs or embryos (Figure 1B, C). Diverse microorganisms were observed on the surface of the egg strings, including numerous bacteria, a few ciliates, some diatoms, and other microalgae. Although quantification was not performed, microalgal fouling was low on strings that were in the initial stages of embryo development (Figure 1C) and increased only modestly with time after spawning. Nematodes and diverse developmental stages of harpacticoid copepods, including adults, copepodites, and nauplii, were also present on the surface of the egg strings and interstitial spaces among the twists of the intertwined egg strings (Supplementary Figure S1). Prior to hatching, the veligers actively swam inside the capsules, suggesting that, at least at this stage, the medium inside the capsules had low viscosity. Veligers hatched in good conditions after approximately two weeks in the aquarium.

Figure 1. Egg masses of A. depilans. (A) An egg mass is attached to rock and algae. (B) Detail of an egg mass showing innumerable capsules (arrows) contained in the long and intertwined string. (C) A stratified gelatinous sheath (gs) envelops the capsules containing several embryos (emb). Microalgae are not visible on the surface of the string. (D) Transverse semithin section of an egg string stained with methylene blue and azure II. The capsule wall (cw) encloses a watery jelly (asterisks) in which the embryos (emb) are immersed.
Semithin and ultrathin sections
In semithin sections stained with methylene blue and azure II, four layers could be recognized in the stratified gelatinous sheath that encircles and aggregates the egg capsules (Figures 1D, 2A–C). The thin outermost layer stains light blue and detaches early, thereby exposing the following layer. The second layer is a low-density gelatinous layer formed by 5‒6 strata that stain light purple with clear, round or oval areas. Below the areas where the outermost layer was detached, signs of degradation were observed in the upper strata of the second layer. During embryo development, the low-density gelatinous layer gradually degrades and almost disappears before hatching, thereby reducing the total thickness of the gelatinous sheath. The third layer has a median density and consists of 5‒6 strata that stain dark purple with light purple oval areas. On average, the strata of the third layer are thinner than those of the second layer and seem compressed (Figure 2A, Table 1). The fourth layer is a thinner, highly dense gelatinous layer without visible stratification that stains very dark blue and almost black. This layer functions as a cement that glues the tightly packed capsules to each other and to the outer layers of the gelatinous sheath (Figures 1D, 2A–C). The very thin walls of the capsules stain dark blue and present a rough internal surface (Figure 2C). Inside the capsules, the eggs or embryos were immersed in a fluid aqueous jelly that could be unstained or slightly coloured light purple in semithin sections stained with methylene blue and azure II (Figure 1D).
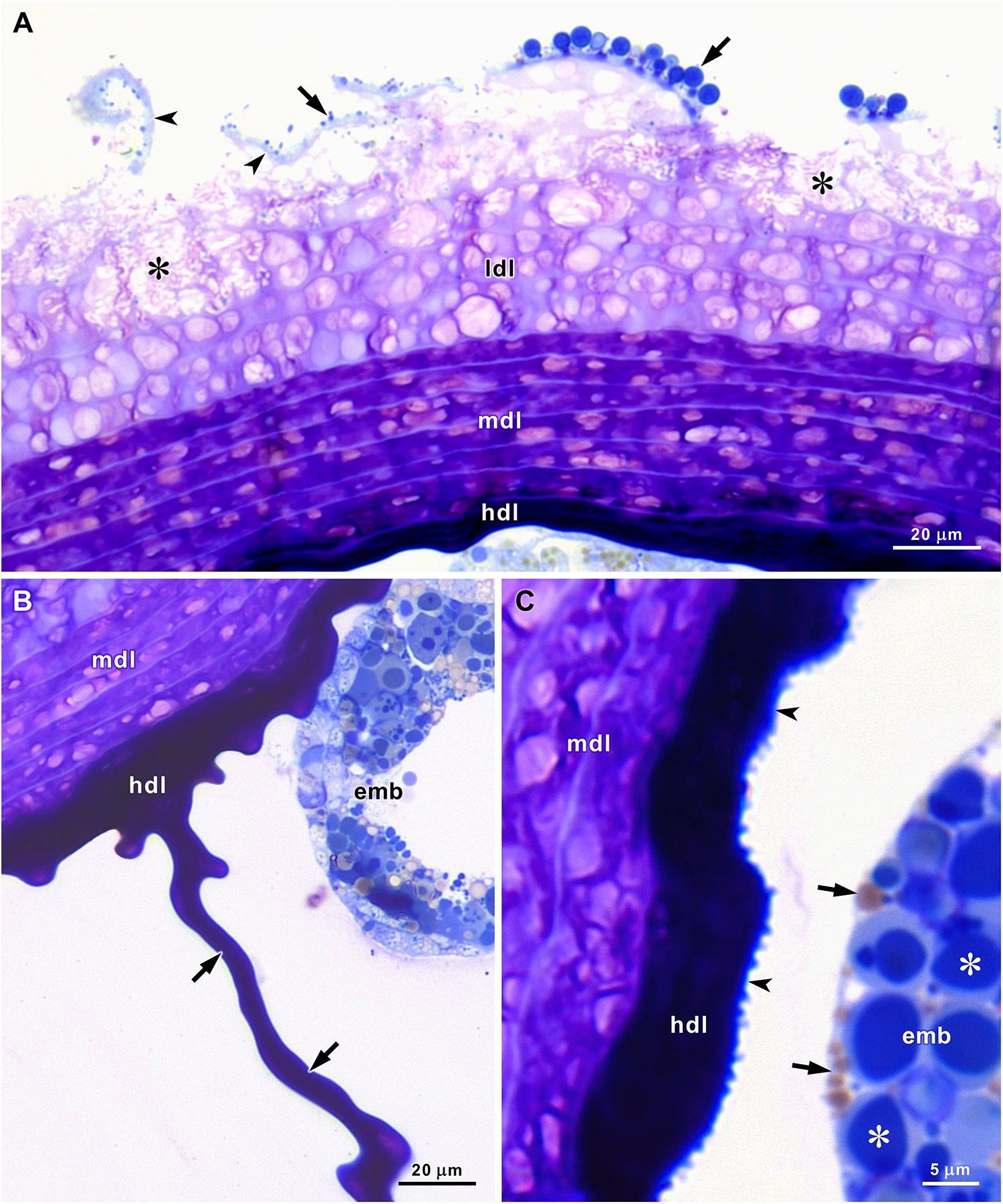
Figure 2. Semithin sections of A. depilans egg strings stained with methylene blue and azure II. (A) Detachment of the thin outermost layer (arrowheads) exposes the upper strata of the low-density layer (ldl) that start to show signs of degradation in some areas (asterisks). Strata of the median-density layer (mdl) stain dark purple with light purple areas, and the high-density layer (hdl) stains very dark blue. Microorganisms (arrows) are attached to the outermost layer. (B) Strands of the high-density layer (hdl) fill the space between the capsules (arrows) that contain the embryos (emb). (C) The thin capsule wall stains dark blue and presents a rough internal surface (arrowheads). Embryos (emb) contain numerous blue-stained yolk vesicles (asterisks) and lipid droplets (arrows).
Table 1. Characterization of A. depilans egg string layers. Measurements were obtained from semithin and ultrathin sections

Staining intensity: −, unstained; +, weak; ++, moderate; +++, strong; ++++, very strong.
Histochemical techniques applied to semithin sections of egg strings revealed differences in the chemical composition of the layers (Figure 3A–D). The outermost layer was not stained by PAS reaction (Figure 3A and inset), but this reaction increased in intensity from the weakly stained low-density gelatinous layer to the strongly stained high-density gelatinous layer that also filled the space between the capsules (Figure 3A, B). The very thin capsule wall was not stained with PAS reaction (Figure 3B). Alcian blue staining was identical at pH 1.0 and pH 2.5. In both cases, staining was strong in the outermost layer and moderate in the low-density gelatinous layer. The strata of the medium-density gelatinous layer were almost unstained, while the high-density gelatinous layer was weakly stained, and the capsule wall was unstained. Thin lines separating each stratum of the second and third layers were evidenced by this stain (Figure 3C and inset). The medium inside the capsules (known as albumen) was weakly stained with Alcian blue, indicating the presence of diluted acid polysaccharides (Figure 3C). The tetrazonium coupling reaction for protein detection revealed the absence of proteins in the outermost layer and an increase in protein concentration from the weakly stained low-density gelatinous layer to the very strongly stained capsule wall (Figure 3D and inset). The features of the layers that shield the eggs of A. depilans are summarized in Table 1.
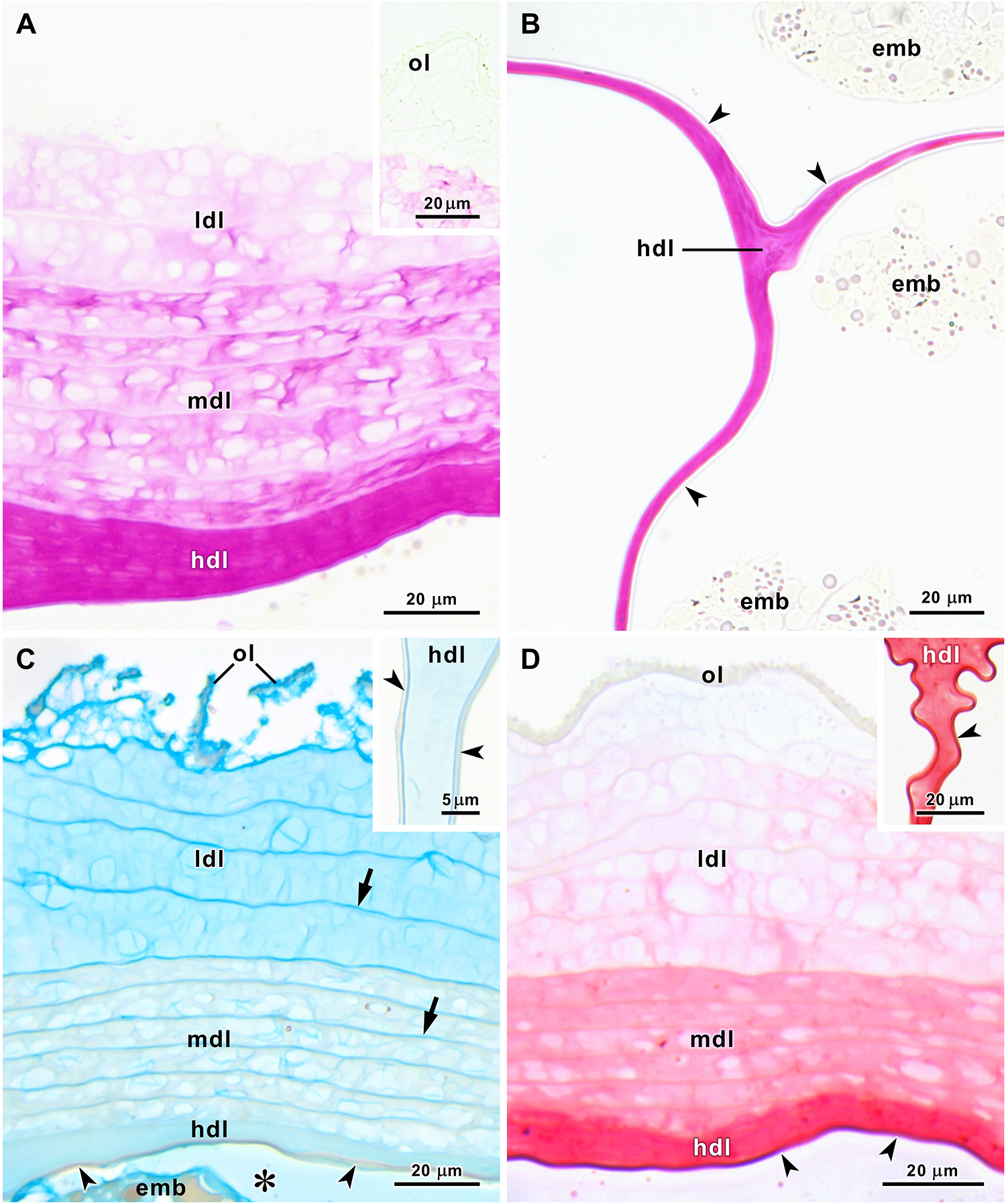
Figure 3. Histochemical stains applied to semithin sections of A. depilans egg strings. (A and inset) PAS reaction is absent in the outermost layer (ol), weak in the low-density layer (ldl), moderate in the median-density layer (mdl) and strong in the high-density layer (hdl). (B) PAS reaction is strong in the high-density layer (hdl) between capsules, but negative in the capsule wall (arrowheads). (C and inset) Alcian blue staining at pH 2.5. Staining is strong in the outermost layer (ol), moderate in the strata of the low-density layer (ldl), almost absent in the strata of the median-density layer (mdl) and weak in the high-density layer (hdl). The capsule wall (arrowheads) is not stained, but the lines between strata (arrows) are well stained. The medium inside the capsule (asterisk) is weakly stained. (D and inset) Tetrazonium coupling reaction for protein detection. Reaction is absent in the outermost layer (ol), weak in the low-density layer (ldl), moderate in the median-density layer (mdl), strong in the high-density layer (hdl) and very strong in the capsule wall (arrowheads). emb, embryos.
TEM revealed a fluffy structure in the outermost layer of the egg strings and an electron-dense thin line separating this layer from the underlying stratum (Figure 4A). The strata of the low-density gelatinous layer are formed by a reticulated matrix that is more open in some zones, creating round and oval areas with lower electron densities in ultrathin sections. In the strata of the medium-density gelatinous layer, the reticulated matrix is more compacted, increasing the electron density of this layer, but in less electron-dense oval zones, the matrix is more open. Thin electron-dense lines formed by a denser matrix separated the strata in both these layers (Figure 4B, C). Moreover, TEM revealed that the high-density gelatinous layer is formed by a similar reticulated matrix that, although much more compacted than in the previous layers, also included zones with less electron density. The capsule wall consisted of a homogeneous, highly electron-dense material with a rough surface in contact with the electron-lucent watery jelly that filled the capsules (Figure 4D).
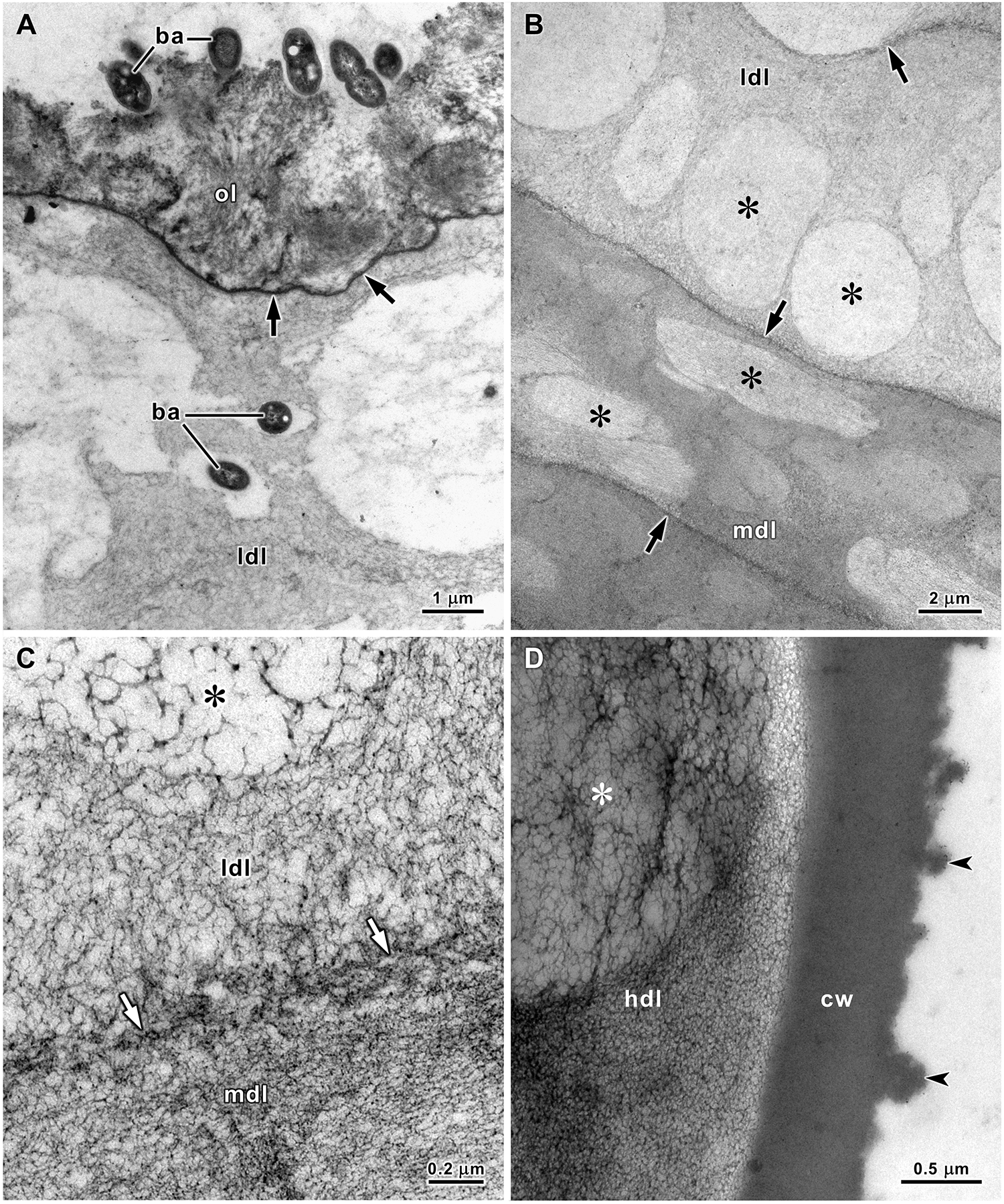
Figure 4. Transmission electron micrographs of the stratified gelatinous sheath of A. depilans egg string. (A) A thin electron-dense line (arrows) separates the fluffy outermost layer (ol) from the upper stratum of the low-density layer (ldl). Several bacteria (ba) are attached to the surface of the egg string, and some have already penetrated the low-density layer. (B) Inferior strata of the low-density layer (ldl) and upper strata of the middle-density layer (mdl). Regions with lower electron-density (asterisks) are visible in these strata that are separated by thin electron-dense lines (arrows). (C) At high magnification it is possible to see a reticulated matrix with different densities: greater at the line separating strata (arrows) and lesser in areas of lower density (asterisk) in the low-density layer (ldl). (D) The high-density layer (hdl) is also formed by a reticulated matrix and includes regions with less electron-density (asterisk). The capsule wall (cw) is a homogeneous, highly electron-dense layer with a rough internal surface (arrowheads).
Microorganisms associated with the egg masses
The microorganisms on the surface of A. depilans egg strings were observed by light microscopy and SEM. In this case, the two drying methods (critical-point drying and hexamethyldisilazane air drying) applied to the samples destined for SEM observations yielded indistinguishable results. Bacteria covered the surface of the egg strings, including very long filamentous bacteria (Figure 5A, B). After bacteria, diatoms were the second most common microorganisms observed on the surface of egg strings (Figure 5C). Detachment of the outer layers removed the attached microorganisms (Figure 2A), which were less abundant on surfaces recently exposed after the removal of the upper layers. In addition to those on the surface, several other bacteria penetrated the low-density gelatinous layer. Around them, the gelatinous matrix was usually less dense, suggesting digestion by bacteria (Figure 4A, 5D–F). No microorganisms were found inside the egg capsules.

Figure 5. Scanning electron micrographs (A–C) and transmission electron micrographs (D–F) of microorganisms associated with the gelatinous sheath of A. depilans egg strings. (A–C) Long filamentous bacteria (arrows), other bacteria (arrowheads in B) and diatoms (asterisks in C) on the surface of egg strings. (D–F) Bacteria (ba) are inside the gelatinous sheath of the egg strings.
Metagenomic analysis revealed a large taxonomic diversity of bacteria associated with the egg masses of A. depilans and in seawater eDNA samples (Supplementary Dataset S1). The 16S amplicon sequencing analysis on the Ion Torrent platform generated an average of 257,424.5 ± 44,736.73 valid reads per egg string sample (sample 1: 193,903; sample 2: 297,717; sample 3: 262,880; sample 4: 275,198), of which an average of 173,138.25 ± 26,334.81 (sample 1: 140,264; sample 2: 203,624; sample 3: 180,147; sample 4: 168,518) could be successfully mapped. These reads were classified into 15 phyla, 25 classes, 55 orders, 101 families, 133 genera, and 153 species (Table 2). In turn, seawater eDNA samples yielded an average of 319,299.3 ± 12,854.6 valid reads (sample 1: 325,097; sample 2: 328,234; sample 3: 304,567), of which 131,737.7 ± 2,278.4 reads (sample 1: 133,731; sample 2: 132,228; sample 3: 129,254) were mapped. These were classified into 19 phyla, 34 classes, 68 orders, 121 families, 156 genera, and 162 species (Table 2).
Table 2. Bacterial taxonomic diversity in the samples of A. depilans egg strings and seawater eDNA. Identification was made almost always down to the level of families, but the identification of genera and species was not possible in several cases. The two most relevant phyla and the total numbers are highligth in bold
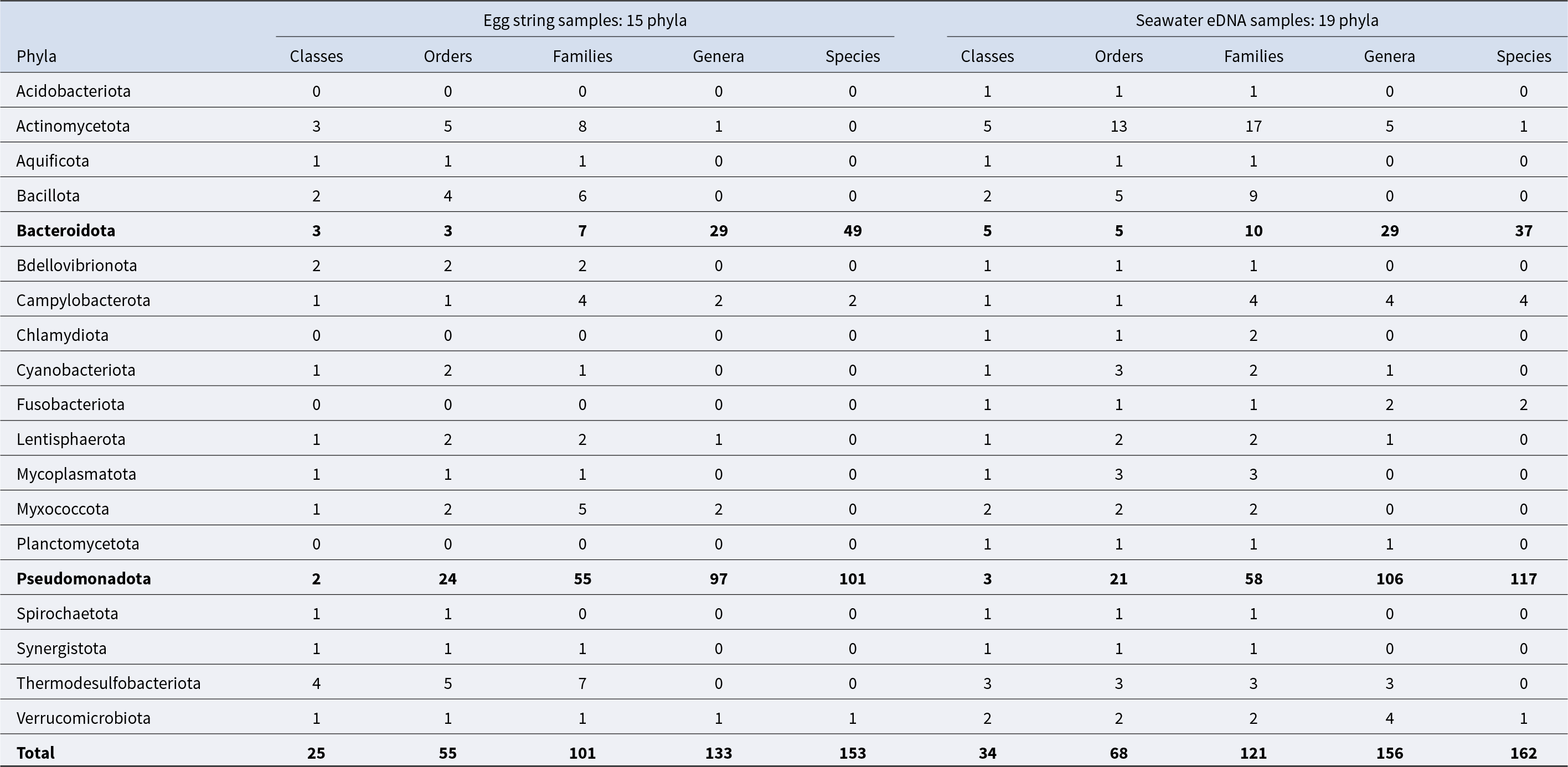
In general, variability in bacterial microbiome composition was low between seawater eDNA samples, but higher between egg string samples, revealing heterogeneity in bacterial distribution along the egg strings. Although bacteria belonging to 15 phyla were detected in the egg string samples (11 present in all samples and 4 present only in some), 95.6% ± 2.1% of the relative abundance corresponded to the phyla Bacteroidota (25.7% ± 18.0%), Lentisphaerota (30.0% ± 10.8%) and Pseudomonadota (39.9% ± 9.9%). The higher variability observed in the relative abundance of Bacteroidota was mainly caused by the uneven distribution of the genus Tenacibaculum, which reached very high relative abundance in one sample (35.2%). In seawater eDNA samples, Lentisphaerota was detected at very low relative abundance (0.5% ± 0.2%), while Pseudomonadota (70.1% ± 0.1%) largely predominated. Although the average relative abundance of Bacteroidota was similar between egg string samples and seawater eDNA (23.7% ± 0.6%), differences were observed in the relative abundance of families and genera within this phylum. The class Betaproteobacteria of the phylum Pseudomonadota was detected in seawater eDNA samples (0.5% ± 0.1%) but not in the egg strings (Figure 6, Supplementary Table S1).

Figure 6. Relative abundance (RA) of bacterial phyla and classes associated with each egg string sample of A. depilans (ES1–ES4) and with seawater eDNA samples (SW, average values), based on 16S rRNA gene metagenomic analysis. Relative abundance values represent the percentage of mapped reads.
Most of the families, genera and species that could be identified belonged to the phyla Bacteroidota and Pseudomonadota (Table 2). Based on the percentage of 16S rRNA gene mapped reads, most identified taxa had a relative abundance below 0.5%. In the egg string samples of A. depilans, only 18 families out of 101 had a relative abundance above 0.5%, with Flavobacteriaceae, Lentisphaeraceae and Rhodobacteraceae being the most relevant (Supplementary Table S2). In several cases, genus and species could not be identified. Within Lentisphaeraceae, the family with the highest average relative abundance in egg string samples (27.1% ± 11.7%), only a minor percentage of 16S rRNA gene sequences could be attributed to a genus, Lentisphaera, and no species were identified. This family had a much lower relative abundance in seawater eDNA samples (0.4% ± 0.2%). Among the genera identified in the egg string samples, Tenacibaculum had the highest average relative abundance (14.2%), with values ranging from 4.5% to 35.2% (Supplementary Table S2). Nine species of this genus were identified (T. aestuarii, T. aiptasiae, T. crassostreae, T. dicentrarchi, T. gallaicum, T. lutimaris, T. ovolyticum, T. skagerrakense, T. soleae), but a large part of the 16S rRNA gene sequences belonging to this genus could not be attributed to a known species (Supplementary Dataset S1). The most abundant was T. lutimaris (2.3%–11.1%), followed by T. ovolyticum (0.1%–1.5%). This genus was also present in seawater eDNA samples, though at much lower relative abundances (2.0%–2.4%), where only T. ovolyticum and T. soleae were identified (0.02%–0.08%).
The family Thiotrichaceae had a relative abundance of 1.3% ± 0.7% in egg string samples (Supplementary Table S2) and included the genera Leucotrix and Thiothrix, which encompass long filamentous marine bacteria that may correspond to the filamentous bacteria observed by light microscopy and SEM (Figure 5A–C). In seawater eDNA samples, Thiotrichaceae occurred at a lower relative abundance (0.6% ± 0.1%). The genera Cellulophaga, Loktanella and Rubritalea, among others, were more abundant in egg string samples than in seawater eDNA (Supplementary Table S2), with Cellulophaga geojensis (1.5%), Loktanella rosea (0.8%) and Rubritalea marina (2.4%) being the most abundant species of their respective genera. In contrast, the families Alteromonadaceae (7.6% ± 0.9%), Cryomorphaceae (4.3% ± 0.5%), Pseudoalteromonadaceae (3.5% ± 1.3%), and Vibrionaceae (5.6% ± 0.2%) were more abundant in seawater eDNA samples (Supplementary Table S2). The family Legionellacea, which also showed a higher relative adundance in seawater eDNA samples (4.7% ± 0.4%), was detected in only one egg string sample at a very low abundance (0.07%). The species Photobacterium aplysiae (Vibrionaceae) was detected at very low abundance in seawater eDNA samples (0.01%) but was absent from the egg string samples (Supplementary Dataset S1).
Statistical analysis using PERMANOVA revealed significant differences in the overall bacterial microbiome composition between A. depilans egg string samples and seawater eDNA from the sampling location (phyla/classes dataset: pseudo-F = 7.63, R 2 = 0.60, p = 0.018; families/genera dataset: pseudo-F = 9.13, R 2 = 0.65, p = 0.031). Visualization by PCoA was consistent with the PERMANOVA results and supported the presence of distinct microbial assemblages in A. depilans egg string samples compared to the surrounding water eDNA (Supplementary Figure S2). SIMPER analysis of the phyla/classes dataset (taxa listed in the Supplementary Table S1) identified the classes Flavobacteriia, Lentisphaeria, Alphaproteobacteria, Gammaproteobacteria, and Verrucomicrobiia as the major contributors to the dissimilarity between the bacterial microbiomes of egg string and seawater eDNA samples at this taxonomical level. This result is consistent with the SIMPER analysis of the families/genera dataset (taxa listed in the Supplementary Table S2), in which several families and genera belonging to these classes contributed to the bacterial community dissimilarity. Together, these analyses indicate that the divergence between the bacterial microbiomes of egg string samples and seawater eDNA samples resulted from coordinated shifts across multiple taxa (Supplementary Table 3).
Discussion
Marine gastropods living in shallow or deep seas enclose fertilized eggs in capsules and gelatinous matrices for protection (Pechenik, Reference Pechenik1986; Gustafson et al., Reference Gustafson, Littlewood and Lutz1991; Wägele, Reference Wägele1996; Rawlings, Reference Rawlings1994, Reference Rawlings1996; Averbuj et al., Reference Averbuj, Penchaszadeh and Pastorino2018). The external morphology of gastropod egg masses differs considerably among species (Soliman, Reference Soliman1987; Wilson, Reference Wilson2002), and their internal structures are diverse (Klussmann-Kolb and Wägele, Reference Klussmann-Kolb and Wägele2001; Pal and Hodgson, Reference Pal and Hodgson2003). In the egg strings of A. depilans, five layers of protection with sharp differences between them were identified based on light microscopy stains and TEM. The thin outermost layer had a similar ultrastructural appearance in the egg strings of both A. depilans and A. punctata. In A. depilans, this layer was strongly stained by Alcian blue but not by PAS and the tetrazonium coupling reaction, indicating that acid polysaccharides are the essential components of this layer, in which neutral polysaccharides and proteins were not detected. In the egg strings of both species, an electron-dense line separates the outermost fluffy layer from the underlying layer (Klussmann-Kolb and Wägele, Reference Klussmann-Kolb and Wägele2001). The most superficial layer must be the last one to be added during the passage of the egg string through the reproductive system before spawning (Klussmann-Kolb, Reference Klussmann-Kolb2001; Thompson and Bebbington, Reference Thompson and Bebbington1969). This outermost layer is naturally responsible for the attachment of Aplysia egg masses to algae and rocks, to avoid being swept away by tidal flows and waves.
In the egg strings of the sea hares A. punctata and Bursatella leachii, a distinction was made between an outer mucous cover and a more compact mucous matrix around the egg capsules (Klussmann-Kolb and Wägele, Reference Klussmann-Kolb and Wägele2001). The outer mucous cover of these egg strings, with a thickness of up to 80 μm in A. punctata and 60 μm in B. leachii, is composed of several strata and seems to correspond to the assemblage of the low- and median-density gelatinous layers of A. depilans egg strings. In the egg strings of these three sea hares, these layers contained clear regions in which the reticulated matrix was less dense. The mucous matrix surrounding the egg capsules of A. punctata and B. leachii correspond to the high-density gelatinous layer of A. depilans egg strings. In the egg strings of A. punctata and B. leachii, acid and neutral polysaccharides were detected in the outer mucous cover and mucous matrix, but proteins were not detected in these layers using bromophenol blue (Klussmann-Kolb and Wägele, Reference Klussmann-Kolb and Wägele2001). In contrast, with the tetrazonium coupling reaction, proteins were detected in the corresponding layers of A. depilans egg strings, in addition to polysaccharides detected by PAS reaction and Alcian blue. The capsules that contain the eggs are limited by a wall that presents a homogeneous electron-dense structure in A. depilans and A. punctata. The capsule wall is thinner in A. punctata (0.25 μm) than in A. depilans (0.7 μm), but thicker in B. leachii (1 μm). The capsule wall of A. punctata contains proteins, and acid and neutral polysaccharides, but in the capsule walls of A. depilans and B. leachii, only proteins were detected by histochemical stains (Klussmann-Kolb and Wägele, Reference Klussmann-Kolb and Wägele2001).
In general, in addition to eggs, capsules of heterobranch gastropods contain a substance known as albumen that, in some cases, can be consumed by the larvae before hatching (Bayne, Reference Bayne1968; Clark and Jensen, Reference Clark and Jensen1981). Histochemical staining revealed some variability in the albumen composition. In A. depilans egg capsules, albumen was weakly stained by Alcian blue, whereas in B. leachii this substance was strongly stained by PAS reaction and weakly stained by Alcian blue and bromophenol blue, and in A. punctata it was only weakly stained by PAS reaction (Klussmann-Kolb and Wägele, Reference Klussmann-Kolb and Wägele2001). In the egg capsules of A. punctata, albumen appears in TEM as flocculent material filling the space around the embryos (Klussmann-Kolb and Wägele, Reference Klussmann-Kolb and Wägele2001). This was not observed in A. depilans egg capsules, in which the embryos were surrounded by electron-lucent fluid. The egg masses of the small eelgrass sea hare Phyllaplysia taylori were considerably different from those of other sea hares. They are almost rectangular flat packets attached to a Zostera leaf with layers of capsules, each containing a single egg (Bridges, Reference Bridges1975). The gelatinous matrix of these egg masses presents an arrangement of fibres that are more compact and longitudinally oriented along the upper surface. In the lower side that is in contact with the leaf, longitudinal fibres are absent. The walls of P. taylori egg capsules, with a thickness of up to 2 μm, are thicker than those of other sea hares (Klussmann-Kolb and Wägele, Reference Klussmann-Kolb and Wägele2001).
The effects of microorganisms on the development and survival of encapsulated embryos have been investigated in several gastropods. In certain species, high levels of surface fouling by microalgae and protozoans were associated with higher mortality rates of encapsulated embryos (Biermann et al., Reference Biermann, Schinner and Strathmann1992; Przeslawski and Benkendorff, Reference Przeslawski and Benkendorff2005). However, according to other studies, microalgae associated with egg masses can be beneficial by improving the oxygen supply to embryos, at least in some cases (Cohen and Strathmann, Reference Cohen and Strathmann1996; Peyton et al., Reference Peyton, Hanisak and Lin2004). Our observations suggest that the presence of diatoms and other microalgae on the surface of A. depilans egg strings should not have a significant influence on embryo development and survival, because the level of microalgae fouling was low to moderate. The association between invertebrates and gastropod egg masses has also been investigated (Fox, Reference Fox1980). Aplysia egg masses with narrow interstitial spaces among the twists of the intertwined egg string can provide occasional refuge and foraging sites for small invertebrates, such as amphipods, which were the most abundant invertebrates associated with egg masses of Aplysia brasiliana in Florida (Rey and Stoner, Reference Rey and Stoner1984). In the present study, some copepods and nematodes were associated with the egg masses of A. depilans, and grazing by these micro-invertebrates may have a controlling effect on the proliferation of microalgae and other microorganisms on the surface of the egg strings, thereby reducing biofouling.
Bacteria have also been observed in the egg masses of gastropods (Klussmann-Kolb and Wägele, Reference Klussmann-Kolb and Wägele2001; Pal and Hodgson, Reference Pal and Hodgson2003; Wägele, Reference Wägele1989), despite the presence of antimicrobial activity having been detected in the egg mass extracts of several species (Benkendorff et al., Reference Benkendorff, Davis and Bremner2001; Peters et al., Reference Peters, Collins and Benkendorff2012; Ramasamy and Murugan, Reference Ramasamy and Murugan2007; Smoot et al., Reference Smoot, Plante and Podolsky2015). In egg mass extracts of Aplysia extraordinaria and Dolabella auricularia (Aplysiidae), this activity was substantially lower than the antimicrobial activity detected in egg mass extracts of muricids and other gastropods (Ramasamy and Murugan, Reference Ramasamy and Murugan2005). However, because extracts from whole egg masses (including protective layers and eggs) were used in the antimicrobial assays, it is not possible to determine exactly where the compounds with antimicrobial activity are located in the egg masses (Benkendorff et al., Reference Benkendorff, Davis, Rogers and Bremner2005). Glycoproteins isolated from egg masses and albumen glands of Aplysia kurodai and D. auricularia exhibited antimicrobial activity that was considered bacteriostatic rather than bactericidal, stopping bacterial growth without causing lysis (Yamazaki, Reference Yamazaki1993). Although the egg masses of these sea hares contained a large quantity of glycoproteins with antibacterial activity, this activity was no longer detected approximately one week after egg laying. Therefore, the physiological functions of these glycoproteins as antibacterial agents in egg masses are not clear (Yamazaki, Reference Yamazaki1993). According to Kamiya et al., (Reference Kamiya, Muramoto and Ogata1984), the weak antibacterial activity detected in some extracts of the mucous gland of A. kurodai was probably due to contamination with the albumen gland. Thus, the detection of glycoproteins with antibacterial activity in the albumen gland and the likely absence of antimicrobial activity in the secretion released by the mucous gland, indicate that these glycoproteins are present in the albumen that fills the egg capsules and not in the gelatinous layers of the egg string produced by the mucous gland.
Our 16S rRNA gene metagenomic analysis showed significant differences between the bacterial microbiome associated with the egg strings and that of the surrounding environment. Generally, the bacteria identified in the egg strings were also present in the local water, but with different relative abundances. The families Flavobacteriaceae (Bacteroidota), Lentisphaeraceae (Lentisphaerota) and Rhodobacteraceae (Pseudomonadota) constituted 67.9% ± 11.6% of the microbiome of A. depilans egg string samples. Members of the phylum Lentisphaerota can be found in seawater, sediments, and animal gut, but have been difficult to isolate and characterize in pure culture. Nevertheless, the limited available data indicate that these bacteria have potential as degraders of polysaccharides (Zhang et al., Reference Zhang, Zheng, Liu, Li and Sun2022). The genus Tenacibaculum (Flavobacteriaceae), with an average relative abundance of 14.2%, was the most represented among the identified genera. Several species of Tenacibaculum were identified in our samples, among which T. lutimaris and T. ovolyticum were the most abundant; however, more than half of the sequences belonging to this genus could not be attributed to a particular species. This genus comprises several species of rod-shaped Gram-negative marine bacteria that can adhere to the surface of marine organisms, in some cases causing serious infections in fish and shellfish, especially in aquaculture facilities (Avendaño-Herrera et al., Reference Avendaño-Herrera, Toranzo and Magariños2006; Nowlan et al., Reference Nowlan, Lumsden and Russell2020; Suzuki et al., Reference Suzuki, Nakagawa, Harayama and Yamamoto2001; Tsertou et al., Reference Tsertou, Triga, Droubogiannis, Kokkari, Anasi and Katharios2023). Tenacibaculum ovolyticum (as Flexibacter ovolyticus) was originally isolated from the adherent bacterial epiflora of Atlantic halibut (Hippoglossus hippoglossus) eggs and was considered an opportunistic pathogen for halibut eggs and larvae (Hansen et al., Reference Hansen, Bergh, Michaelsen and Knappskog1992). However, the presence of this and other bacterial species did not affect the viability of sardine eggs on the Northwest Spanish coast (Míguez et al., Reference Míguez, Combarro, Guisande, Vergara and Riveiro2004). Tenacibaculum lutimaris had a higher average relative abundance (4.8%) than any other species identified in A. depilans egg string samples. This species, which was first isolated from tidal mud sediments, is capable of degrading gelatine, but not starch (Yoon et al., Reference Yoon, Kang and Oh2005), and was not previously reported in association with marine organisms. The ability of environmental Tenacibaculum species to proliferate when polysaccharides are available, either by taking advantage of the enzymatic activity of other microorganisms or because of their diverse and extensive set of enzymes that break down complex polysaccharides, can explain their abundance in the gelatinous layers of A. depilans egg strings. The Gram-negative rod-shaped bacteria of the species C. geojensis (Flavobacteriaceae), which can decompose agar, carrageenan, gelatine, and starch (Park et al., Reference Park, Oh, Lee, Oh and Yoon2012), despite being detected with less relative abundance (1.5%), also seem capable of participating in the degradation of the gelatinous layers of A. depilans egg strings.
Other bacteria also possessing enzymes that can decompose polysaccharides and glycoproteins contribute significantly to carbon cycling in marine environments and can degrade the gelatinous layers of egg masses during embryo development and after hatching. For example, bacteria of the family Colwelliaceae (Gammaproteobacteria), represented in the microbiome of A. depilans egg strings by the genera Colwellia and Thalassomonas, play a role in the decomposition of polysaccharides, proteins, and lipids in marine environments (Bunse et al., Reference Bunse, Koch, Breider, Simon and Wietz2021; Lee et al., Reference Lee, Yang, Kim, Park, Park and Kwon2025). Bacteria of the family Saprospiraceae (Bacteroidota) are also likely important in the breakdown of complex organic compounds in the marine environment (McIlroy and Nielsen, Reference McIlroy, Nielsen, Rosenberg, DeLong, Lory, Stackebrandt and Thompson2014). Other representative genera and species in the microbiome of A. depilans egg masses are known to be associated with marine organisms. Rubritalea marina (Verrucomicrobiota) was originally isolated from a Mediterranean sponge (Scheuermayer et al., Reference Scheuermayer, Gulder, Bringmann and Hentschel2006), but has not been previously reported to be associated with egg masses. Most of the long filamentous bacteria that were prominent on microscopic observations probably corresponded to the genus Leucotrix, which was the most abundant genus of the family Thiotrichaceae in our samples. This is a widespread genus of epiphytic bacteria present on marine algae, benthic crustaceans, and fish eggs (Brock, Reference Brock, Dworkin, Falkow, Rosenberg, Schleifer and Stackebrandt2006; Johnson et al., Reference Johnson, Sieburth, Sastry, Arnold and Doty1971). Contrary to other bacteria identified in the egg masses of A. depilans, Loktanella rosea and other species of Loktanella (Alphaproteobacteria, Rhodobacteraceae) do not degrade gelatine, chitin, agar, or starch and have limited ability to utilize carbon sources (Ivanova et al., Reference Ivanova, Zhukova, Lysenko, Gorshkova, Sergeev, Mikhailov and Bowman2005). Therefore, despite having a relative abundance of approximately 5%, the genus Loktanella does not seem to be involved in the degradation and recycling of gelatinous layers of Aplysia egg strings. Photobacterium aplysiae is a lipolytic bacterial species able to hydrolyse gelatine that was isolated from egg masses of Aplysia kurodai (Seo et al., Reference Seo, Bae, Yang, Lee and Kim2005). This species was found in our seawater eDNA samples collected during the breeding season at a spot of high spawning activity for A. depilans, but was not detected in the egg string samples used in this study.
In conclusion, it seems that under natural conditions, embryo development is not affected by the presence of microorganisms on the surface or within the outer layers of the gelatinous sheath of A. depilans egg strings. Despite the presence of bacteria and other microorganisms, the veligers hatched after approximately two weeks in an aquarium, apparently in good conditions. The detachment of the outermost layer removes all microorganisms that attach to the surface of the egg strings during the early stages of embryonic development, thereby reducing biofouling. Partial degradation and dissolution of the gelatinous sheath caused by bacteria and abiotic factors, such as UV radiation or sea water, may even contribute to reducing the thickness and strength of the gelatinous layers, thereby facilitating hatching. The gelatinous sheath of A. depilans egg strings, with its structural complexity, is well adapted to provide adequate protection from adverse biotic and abiotic factors during intracapsular development stages. Despite the penetration of bacteria in the upper layers of the gelatinous sheath, the thickness and toughness of the inner layers and the possible presence of some antimicrobial compounds prevent the entrance of microorganisms into the capsules that contain the developing embryos until hatching of the veligers, approximately 2 weeks after spawning. Likewise, bacteria, protozoa, and diatoms were found in the gelatinous matrix of Siphonaria diemeninsis egg masses, but never inside the egg capsules (Peters et al., Reference Peters, Collins and Benkendorff2012). The diversity and abundance of bacteria, several of which lack known gene sequences, show that egg masses of Aplysia and other marine gastropods are promising sources for the discovery of new bacterial species, especially those involved in polysaccharide degradation.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315425100908.
Acknowledgements
The authors thank Ana Machado of ICBAS for the extraction of DNA from the environmental samples, Cátia Bartilotti of IPMA for copepod identification, Daniela Silva of CEMUP for operating the SEM, Eduardo Rocha of ICBAS for carrying out the statistical analyses, and Gilberto Mendes Silva of ICBAS for performing critical point drying. The authors further acknowledge Ana Mafalda Rocha of the i3S Genomics Scientific Platform (Porto, Portugal) for her help with the Ion Torrent analysis.
Author contributions
A.L.C.: research conception, specimen collection, microscopy studies and writing; A.A.: microscopy studies; S.R.: molecular studies and writing. All authors reviewed and approved the manuscript.
Funding
This study was supported by funds from Instituto de Ciências Biomédicas Abel Salazar (ICBAS), Universidade do Porto, Portugal.
Competing interests
None.
Ethical standards
This study was not covered by any regulations, and formal ethical approval was not required.
Availability of data and materials
Raw sequence data have been deposited in the NCBI Sequence Read Archive (SRA) under BioProject accession number PRJNA1356730. Processed taxonomic assignments and relative abundance data are available in the Supplementary Information.









