Major depressive disorder (MDD) and insulin resistance-related conditions rank among the leading causes of disability worldwide, and their incidence continues to grow to epidemic proportions.1 Insulin resistance, which is characterised by diminished cellular response to insulin in muscles, fat and liver, is a common feature underlying cardiometabolic conditions such as type 2 diabetes mellitus (T2DM), obesity, dyslipidaemia and cardiovascular diseases (CVDs).Reference James, Stockli and Birnbaum2 These conditions are increasingly recognised as significant risk factors for psychiatric disorders, notably MDD.Reference Possidente, Fanelli, Serretti and Fabbri3
The epidemiological link between MDD and insulin resistance-related conditions is well established.Reference Wimberley, Horsdal, Brikell, Laursen, Astrup and Fanelli4,Reference Rajan, McKee, Rangarajan, Bangdiwala, Rosengren and Gupta5 The risk for insulin resistance-related conditions is higher among patients with MDD and, in turn, people with T2DM and obesity have up to fourfold higher risk for MDD.Reference Possidente, Fanelli, Serretti and Fabbri3 Comorbidity with insulin resistance-related conditions in individuals with MDD adversely affects the clinical trajectory of depression, resulting in increased severity, greater chronicity, and higher mortality rates.Reference Possidente, Fanelli, Serretti and Fabbri3,Reference Fanelli and Serretti6
Recent studies have identified shared genetics and pathophysiological mechanisms between insulin resistance and MDD, including dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, chronic low-grade inflammation and alterations in the gut microbiota and neurotransmitter systems, suggesting a bidirectional relationship where each condition may influence the onset of the other.Reference Possidente, Fanelli, Serretti and Fabbri3,Reference Fanelli and Serretti6,Reference Fanelli, Franke, De Witte, Ruisch, Haavik and van Gils7 In MDD, chronic stress induces HPA axis hyperactivation, resulting in sustained cortisol elevation that promotes gluconeogenesis, impairs insulin-mediated glucose uptake in peripheral tissues and elevates circulating free fatty acids, thereby contributing to insulin resistance.Reference Fanelli, Raschi, Hafez, Matura, Schiweck and Poluzzi8 Concurrently, MDD-associated inflammation can disrupt insulin receptor signalling and contribute to metabolic dysfunction.Reference Possidente, Fanelli, Serretti and Fabbri3,Reference Fanelli, Raschi, Hafez, Matura, Schiweck and Poluzzi8 On the other hand, insulin resistance within the central nervous system impairs synaptic plasticity and affects mood-regulating neurotransmitter systems.Reference Possidente, Fanelli, Serretti and Fabbri3,Reference Fanelli, Raschi, Hafez, Matura, Schiweck and Poluzzi8 These shared pathophysiological mechanisms have also been linked to resistance to treatments.Reference Borgiani, Possidente, Fabbri, Oliva, Bloemendaal and Arias Vasquez9,Reference Murphy, Sarris and Byrne10 The first exploration of insulin’s effects in psychiatric disorders was unfortunately linked to insulin shock therapy, introduced in the mid-20th century as a treatment for severe psychiatric conditions; this approach was abandoned by the 1970s, due to the lack of therapeutic rationale and risks of prolonged hypoglycaemia and other side-effects.Reference Freudenthal and Moncrieff11 As discussed above, in recent years the study of insulin resistance in psychiatric disorders has been based on solid scientific evidence coming from both epidemiological and neurobiological studies.
Traditional antidepressant drugs are a cornerstone of MDD management; they address imbalances of distinct neurotransmitter systems but display inconsistent treatment efficacy. Around 60% of treated individuals, in fact, do not reach complete clinical remission after a full course of treatment.Reference De Carlo, Calati and Serretti12 This variability in response is partly attributed to the high clinical and pathophysiological heterogeneity of MDD, which is not restricted to monoamine system abnormalities;Reference Oliva, Fanelli, Kasper, Zohar, Souery and Montgomery13 one of the most studied MDD subgroups is characterised by metabolic disturbances, and it has been named immune-metabolic depression.Reference Milaneschi, Lamers, Berk and Penninx14 The presence of insulin resistance-related conditions in patients with MDD results in significant clinical challenges. The altered inflammatory and endocrine profile in these patients might reduce the effectiveness of standard antidepressant therapies, contributing to treatment-resistant depression (TRD).Reference Murphy, Sarris and Byrne10 Therefore, understanding the influence of insulin resistance and related conditions on antidepressant response is essential for developing personalised treatment strategies, which is a key goal in precision psychiatry.Reference van Dellen15 Some antidepressants, such as monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs), may exacerbate metabolic disturbances, further complicating treatment.Reference Fanelli and Serretti6 This necessitates a careful balancing, to weight the expected benefits on mental health against the potential metabolic risks.
Genomic studies have highlighted the role of genetic predisposition in the development of both MDD and insulin resistance, hinting at their shared genetic aetiology.Reference Fanelli, Franke, De Witte, Ruisch, Haavik and van Gils7 Polygenic scores (PGSs) quantify the cumulative effect of genetic variants associated with a particular trait or disease; they represent a promising approach for studying the clinical/genetic heterogeneity and treatment response in depression,Reference Oliva, Fanelli, Kasper, Zohar, Souery and Montgomery13 heralding personalised medicine approaches based on individual genetic profiles.
Despite growing evidence supporting a link between insulin resistance and MDD, there is still a paucity of large-scale studies comprehensively exploring the association between insulin resistance-related conditions and treatment outcomes in MDD.Reference Madsen, Momen, Petersen, Plana-Ripoll, Haarman and Drexhage16,Reference Kraus, Kautzky, Watzal, Gramser, Kadriu and Deng17 In particular, the temporal relationship between the onset of insulin resistance-related conditions and MDD, and how this sequence influences the clinical course of MDD and response to treatment, are not well understood. This gap in knowledge hinders possible considerations for the development of more well -tolerated and effective treatment strategies for patients with MDD and comorbid insulin resistance-related conditions.
The present study investigated whether insulin resistance-related conditions and their PGSs are associated with the clinical course of MDD or response to antidepressant treatment, considering also which condition was diagnosed earlier. This study leveraged data from the UK Biobank (UKB) cohort linked to primary care records, providing the opportunity to examine these relationships in a large population cohort.
Method
UKB cohort and linked primary care data
This study utilised data from the UKB, which is a large-scale, prospective cohort study providing extensive genetic, lifestyle and health data from approximately 500 000 individuals across the UK, aged between 40 and 69 years at recruitment (2006–2010).Reference Bycroft, Freeman, Petkova, Band, Elliott and Sharp18 Primary care data were available for ∼45% of the cohort (230 096 participants), reflecting regional and provider variability.19 Missing or incomplete data were not imputed.
The UKB includes genotypes for 488 377 participants, who were genotyped using the Applied Biosystems UK BiLEVE and UKB Axiom Arrays (Thermo Fisher Scientific, Inc., Waltham, MA, USA).Reference Bycroft, Freeman, Petkova, Band, Elliott and Sharp18 Detailed methodologies for DNA extraction, genotyping, quality control and imputation in UKB are reported elsewhere.19
As part of the UKB’s comprehensive data collection, primary care data were obtained for 230 096 participants, forming the basis of our study.19 This subset includes electronic health records (EHRs) sourced from English, Scottish and Welsh general practitioner practices, using various primary care information systems (EMIS, Vision, TPP). These records include dates and codes for primary care clinical events (e.g. consultations, diagnoses, referrals to specialists or prescription events) coded using Read version 2 (V2) and Clinical Terms Version 3 (CTV3 or V3), the British National Formulary (BNF) and/or the Dictionary of Medicines and Devices (dm+d).19 These codes were used to identify MDD and insulin resistance-related conditions, the time at first diagnosis and antidepressant prescriptions. In cases where prescription or diagnosis dates were missing or implausible (e.g. 01/01/1901, 07/07/2037), diagnostic codes were excluded from temporal analyses but retained for non-temporal analyses to maximise sample size, and prescription records were not considered for derivation of treatment outcome variables. Potential biases arising from missing or incomplete primary care data are addressed in the Discussion.
Ethics statement
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation, and with the Helsinki Declaration of 1975 as revised in 2013. All procedures involving human subjects/patients were approved by the Northwest Multi-centre Research Ethics Committee (MREC), approval no. 11/NW/0382.
Target population: MDD cases with or without insulin resistance-related conditions
We focused on a subset of UKB participants having at least one diagnostic record for a unipolar depressive disorder and at least one prescription code for an antidepressant medication, excluding those with bipolar, psychotic or substance use disorders. These data were extracted according to the steps described in a previous work.Reference Fabbri, Hagenaars, John, Williams, Shrine and Moles20
Similarly, insulin resistance-related conditions were defined based on diagnostic records. We considered the presence of at least one primary care Read code for coronary artery disease (CAD), cerebral ischaemia, CVDs, dyslipidaemia, polycystic ovary syndrome (PCOS), familiar dyslipidaemia, gestational diabetes, hypertension, non-alcoholic fatty liver disease (NAFLD), obesity/overweight, T2DM and Cushing’s disease. Read V2 and CTV3 codes used for the extraction of insulin resistance-related conditions are reported in Supplementary Tables 1 and 2. These IR-related conditions were selected based on their established contribution to, or pathogenic association with, metabolic dysregulations commonly seen in IR.Reference James, Stockli and Birnbaum2,Reference da Silva, do Carmo, Li, Wang, Mouton and Hall21,Reference Hill, Yang, Zhang, Sun, Jia and Parrish22
Outcomes of interest
The outcomes of interest were: (a) antidepressant non-response, defined as ≥1 switch between different antidepressant drugs, with each drug prescribed for at least 6 consecutive weeks, to avoid drug switches due to side-effects; we considered a time interval between consecutive prescriptions of no more than 14 weeks to ensure that treatment had not been suspended, following another recent study;Reference Fabbri, Hagenaars, John, Williams, Shrine and Moles20 (b) TRD, defined as ≥2 switches between different antidepressant drugs, with each drug prescribed for at least 6 consecutive weeks, to ensure an adequate duration of treatment before switching, and a time interval between prescriptions >14 weeks;Reference Fabbri, Hagenaars, John, Williams, Shrine and Moles20 and (c) overall treatment time, used as a proxy for MDD chronicity and calculated as the sum of time windows between 2 consecutive antidepressant prescriptions (if the time interval between 2 consecutive prescriptions was >14 weeks, otherwise it was considered a time window free from antidepressants).
PGS computation
PGSs were estimated in the UKB using PRS-CS-auto, a Bayesian method that applies continuous shrinkage priors on single-nucleotide polymorphism (SNP) effect size, bypassing the need to preselect a Genome-Wide Association Study (GWAS) P-threshold for SNP inclusion.Reference Ge, Chen, Ni, Feng and Smoller23 GWAS summary statistics used for the construction of PGSs were those for body mass index (BMI), CAD, T2DM, fasting plasma glucose (FPG), glucose levels 2 h following oral glucose challenge (2hGlu), glycated haemoglobin (HbA1c), high-density lipoproteins (HDL), homeostatic model assessment of insulin resistance, low-density lipoproteins (LDL) and triglycerides (TGL). GWAS summary statistics were selected based on the largest GWAS sample size available, excluding UKB, to avoid sample overlap between input and target samples (Supplementary Table 3).
Statistical analysis
We compared individuals with MDD having or not having insulin resistance-related comorbidities, while also considering stratification of individuals based on the temporal sequence of MDD-first diagnosis relative to insulin resistance-related condition-first diagnosis, according to primary care records.
Univariable analyses were conducted using two-sample Student’s t-test and Pearson’s χ² test, as appropriate, to examine differences in demographic, socioeconomic, clinical and lifestyle factors among individuals affected by MDD with and without insulin resistance-related conditions. The variables assessed included the age at MDD onset, follow-up duration, mean age during follow-up, patterns of antidepressant prescription, psychological symptoms and treatment outcomes (see Supplementary Table 4 for information on variables and their coding). A subsequent one-way analysis of variance (ANOVA) was used to compare these variables across three defined groups of individuals: MDD without insulin resistance-related conditions (IR–), MDD following diagnosis of an insulin resistance-related condition (MDD-after-IR) and MDD diagnosis preceding insulin resistance-related conditions (MDD-before-IR). Post hoc analyses, employing Tukey’s honestly significant difference (HSD) test, were conducted to identify differences between group pairs.
To examine the association of insulin resistance-related conditions and their PGSs with treatment outcomes, we used either multivariable linear or logistic regression models. These analyses were adjusted for assessment centre, mean age during follow-up, follow-up duration, sex, smoking status, Townsend Deprivation Index and population principal components (the latter only for PGS analyses). PGS analyses were carried out for European individuals only (identified as in Fabbri et alReference Fabbri, Hagenaars, John, Williams, Shrine and Moles20).
We quantified the variance explained in treatment non-response or resistance using Nagelkerke’s pseudo-R 2. For models with overall treatment time as a continuous outcome, variance was quantified using R 2. The Hosmer–Lemeshow χ 2 test was employed to evaluate the goodness of fit of logistic regression models, with P ≥ 0.05 indicating no significant difference between observed and predicted values, suggesting an adequate model fit. While pseudo-R 2 in this study was anticipated to be low due to the multifactorial nature of depression treatment outcomes and insulin resistance-related traits, goodness-of-fit metrics of the Hosmer–Lemeshow test can ensure that the predictions are reliable within the observed data.
This study was hypothesis driven, building on prior evidence and well established biological links between MDD and insulin resistance-related conditions. Although not preregistered, both the analysis plan and selection of variables analysed were informed by previous literature.Reference Possidente, Fanelli, Serretti and Fabbri3,Reference Fabbri, Hagenaars, John, Williams, Shrine and Moles20,Reference Rashidian, Subramaniapillai, Park, Lipsitz, Zuckerman and Teopiz24 To minimise the risk of type I errors due to multiple testing, a stringent Bonferroni correction was applied (α = 0.0006), accounting for 27 predictors and 3 treatment outcomes.
All analyses were performed in R version 4.3.2 (2023-10-31) for Linux (R Foundation for Statistical Computing, Vienna, Austria; see https://cran.r-project.org/), with data cleaning and manipulation streamlined by the R package tidyverse 2.0 for Linux (Posit, PBC, Boston, MA, USA; see https://tidyverse.tidyverse.org/).
Results
Sociodemographic characteristics of the sample
Our study included 30 919 individuals with MDD, of whom 16 063 (51.95%) had a lifetime history of insulin resistance-related conditions (Supplementary Table 5). The mean age during follow-up was 56.12 years (s.d. = 8.35), with males comprising 31.8% of the cohort. A predominant majority (n = 29 581, 95.67%) were of European descent. The most prevalent insulin resistance-related conditions included hypertension (n = 9499, 30.74%), obesity/overweight (n = 5243, 16.97%), CVDs (n = 3650, 11.81%), T2DM (n = 3092, 10.01%) and CAD (n = 2450, 7.92%) (Supplementary Table 5). Of the cohort, 6357 individuals (20.56%) received their first MDD diagnosis following an insulin resistance-related diagnosis; conversely, 9483 (30.67%) had MDD before any insulin resistance-related condition and 14 856 (48.05%) had no history of insulin resistance-related conditions (Supplementary Table 5).
Univariable analyses revealed significant sociodemographic differences among patients with MDD when stratified by the presence or absence of lifetime insulin resistance-related conditions (Table 1). Patients with lifetime insulin resistance-related conditions were older and more frequently male compared with those without (Table 1). The former also reported lower levels of education and lower socioeconomic status, as indicated by the Townsend Deprivation Index and household income (Table 1). Stratification by insulin resistance-related diagnosis timing relative to MDD onset confirmed these findings (Supplementary Tables 7 and 8).
Table 1 Differences in sociodemographic and clinical characteristics between patients with major depressive disorder (MDD) not having a lifetime history of any insulin resistance (IR)-related condition (IR−) and those with MDD having a history of any IR-related condition (IR+). Student’s two-sample t-tests and Pearson’s χ² tests were used for continuous and categorical variables, as appropriate

Only statistically significant differences following Bonferroni correction are reported; non-significant differences are detailed in Supplementary Table 6.
Prop., proportion; BMI, body mass index; SARI, serotonin antagonist and reuptake inhibitor; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant. UK educational qualifications include: College/university degree, which covers undergraduate (Bachelor’s degrees) and postgraduate degrees (Master’s and Doctoral degrees); A/AS levels (Advanced/Advanced Subsidiary levels), qualifications typically pursued by students aged 16 to 18 as a preparatory step for university or vocational training; GCSEs (General Certificate of Secondary Education), the primary set of examinations taken by students at the end of compulsory education around age 16, replacing the historical O-levels (Ordinary levels) and CSEs (Certificate of Secondary Education, a non-academic track phased out in 1988 alongside O-levels); NVQ (National Vocational Qualifications), HND (Higher National Diploma) and HNC (Higher National Certificate), which are vocational qualifications offered post-secondary education to provide practical skills and training in various fields.
MDD clinical profile and insulin resistance
Individuals in the MDD IR+ group had a higher mean age at first diagnosis of depression and longer duration of follow-up (Table 1). This group exhibited more frequently characteristics suggestive of an unhealthy lifestyle, including higher rates of smoking and lower levels of moderate physical activity, but also lower alcohol intake frequency compared with the IR− group (Table 1). The IR+ group also showed higher prevalence of long-term illnesses and disability, as well as higher BMI (Table 1). BMI was highest in the MDD-after-IR group, followed by the MDD-before-IR group (Supplementary Table 9).
Depressive symptoms and traits also varied between groups. The IR+ group reported more feelings of loneliness and being fed-up, but reduced rumination over embarrassing situations, the latter especially in the MDD-after-IR group (Table 1 and Supplementary Tables 7 and 8). Patients in the IR+ MDD-after-IR subgroup were characterised by reduced feelings of nervousness, worry/anxiety, guilt and sensitivity to hurt, but increased feelings of inadequacy and concentration difficulties when compared with IR− individuals (Supplementary Table 8). In contrast, those with pre-existing MDD exhibited higher levels of neuroticism compared with IR− individuals (Supplementary Table 7).
Prescription patterns
IR+ individuals had a higher rate of antidepressant prescriptions per follow-up year, used more drug classes and had more frequent antidepressant switches than those without any lifetime insulin resistance-related condition (Table 1). There were also differences in the prevalence of prescribed antidepressant classes between the groups. Specifically, individuals prescribed serotonin antagonist and reuptake inhibitors (SARIs, nefazodone and trazodone), serotonin-norepinephrine reuptake inhibitors (SNRIs, duloxetine and venlafaxine), tetracyclic antidepressants (mirtazapine) and TCAs were more numerous in the IR+ group, while the opposite was found for selective serotonin reuptake inhibitors (SSRIs); however, the number of prescriptions of individual antidepressants, including SSRIs, was higher in the IR+ group (Table 1). After stratifying the sample based on the timing of the first diagnosis of insulin resistance-related conditions in relation to the first MDD diagnosis, the higher antidepressant prescription level and use of more different antidepressant classes was particularly evident in individuals having MDD onset before insulin resistance-related conditions (Supplementary Tables 7 and 8). A higher number of antidepressant switches and prescriptions of SARIs and SNRIs was found in patients with MDD preceding insulin resistance-related diagnoses versus the IR– group, but not in those with later MDD diagnosis (Supplementary Tables 7 and 8). Among individuals diagnosed with MDD following, but not preceding, an insulin resistance-related condition, a higher proportion was prescribed SSRIs compared with IR– individuals (Supplementary Tables 7 and 8). No differences were observed in antipsychotic use as adjunct treatments or other antidepressant classes among the groups.
Treatment outcomes
IR+ patients had overall longer treatment duration and poorer outcomes, including higher rates of TRD and non-response, than their IR– counterparts (Table 1). After adjusting for confounders, specific insulin resistance-related conditions (i.e. CVDs, CAD, hypertension, NAFLD, obesity/overweight, prediabetes and T2DM) were associated with increased odds of TRD and antidepressant non-response (Fig. 1(a) and (b) and Supplementary Table 10(a) and (b)). This pattern was consistent in both the overall sample and the subgroup of patients who developed MDD prior to each specific insulin resistance-related condition, but not in those who developed MDD after insulin resistance-related conditions (Supplementary Table 11). Regarding the chronicity of MDD, proxied by overall treatment time, a similar result was observed. The presence of insulin resistance-related conditions was associated with longer overall treatment time in the entire sample (Fig. 1(c) and Supplementary Table 10(c)), especially in individuals diagnosed with MDD before the insulin resistance-related condition (Supplementary Table 11). Conversely, in patients who developed MDD following insulin resistance diagnosis, a general association of poorer treatment outcomes and overall treatment time with the presence of any insulin resistance-related condition, rather than with specific insulin resistance-related conditions, was observed (Supplementary Table 12).
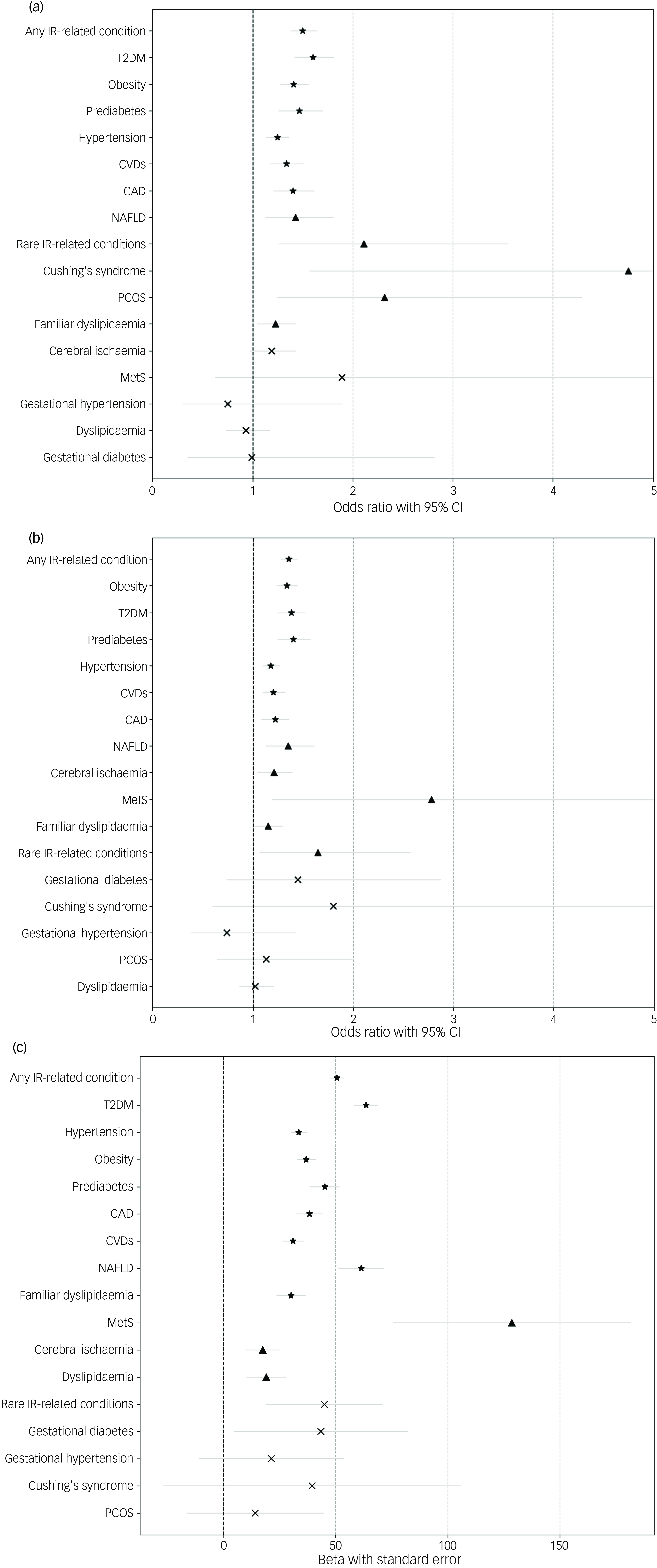
Fig. 1 Associations between insulin resistance (IR)-related conditions and treatment outcomes in major depressive disorder (MDD). These forest plots illustrate the associations of IR-related conditions with (a) treatment-resistant depression, (b) antidepressant non-response and (c) overall treatment time in patients with a lifetime history of MDD. Odds ratios (ORs), along with their 95% CIs, or betas and standard errors, are depicted for each IR-related condition for binary or continuous outcome, respectively. Statistical significance is represented using different symbols: stars (★) for statistically significant results (P < 0.0006), triangles (▴) for nominally significant results (P < 0.05) and crosses (⤬) for non-significant results (P ≥ 0.05). The findings are arranged in a gradient based on significance, with the most statistically significant results at the top and non-significant results at the bottom of the plot. CAD, coronary artery disease; CVDs, cardiovascular diseases; PCOS, polycystic ovary syndrome; MetS, metabolic syndrome; NAFLD, non-alcoholic fatty liver disease; T2DM, type 2 diabetes mellitus.
We did not identify any association between the PGSs of insulin resistance-related diseases/traits and treatment outcomes or overall treatment time; we found nominal associations (P < 0.05) with the PGS of CAD, triglycerides and BMI in certain subgroups defined by diagnosis timing (Supplementary Tables 13–15). The R 2/Nagelkerke’s pseudo-R 2 values for models predicting treatment outcomes ranged from 1.3 to 3.9%, reflecting the multifactorial nature of depression treatment outcomes and insulin resistance-related conditions. Despite this, the Hosmer–Lemeshow χ 2 test indicated an acceptable fit for most models (Supplementary Tables 10–15), supporting the validity of the observed associations.
Discussion
Overview of main findings
This study, leveraging primary care-linked data from UKB, investigated the associations between insulin resistance-related conditions and treatment outcomes, prescription patterns and clinical profiles of patients with MDD. Our analyses revealed a high prevalence of insulin resistance-related conditions among individuals with a history of MDD, emphasising the need to integrate metabolic health into psychiatric care. Those with insulin resistance-related comorbidities showed a later age at first MDD diagnosis, were less often female and exhibited more unhealthy lifestyle factors. Our study is the first to utilise a large, real-world primary care sample with EHRs and genetic information, demonstrating the increased complexity involved in managing depression in this population. This complexity is evidenced by a higher number of antidepressant prescriptions, switches and number of classes ever used among those with insulin resistance-related comorbidities. Most notably, the presence of insulin resistance-related conditions was associated with a higher likelihood of TRD, antidepressant non-response and prolonged treatment duration, particularly when MDD preceded the onset of insulin resistance-related conditions.
Prevalence of insulin resistance conditions in depression: mechanisms and unhealthy lifestyle
The high prevalence of hypertension, obesity/overweight, CVDs and T2DM within our MDD sample aligns with existing research, underscoring the influence of these comorbidities on mental health.Reference Possidente, Fanelli, Serretti and Fabbri3,Reference Wimberley, Horsdal, Brikell, Laursen, Astrup and Fanelli4,Reference Kraus, Kautzky, Watzal, Gramser, Kadriu and Deng17,Reference Kangethe, Lawrence, Touya, Chrones, Polson and Evangelatos25 Metabolic dysregulation and MDD share overlapping pathophysiological mechanisms, including chronic inflammation, impaired insulin signalling, neuroendocrine dysfunction and oxidative stress (e.g. Milaneschi et alReference Milaneschi, Lamers, Berk and Penninx14). These disturbances contribute to depressive symptomatology by disrupting neural circuits related to reward, verbal/numerical reasoning and processing speed,Reference Fanelli, Mota, Salas-Salvado, Bullo, Fernandez-Aranda and Camacho-Barcia26,Reference Milaneschi, Simmons, van Rossum and Penninx27 thereby exacerbating core depressive symptoms such as anhedonia and cognitive dysfunction and hindering treatment response.Reference Martone, Possidente, Fanelli, Fabbri and Serretti28 Furthermore, behavioural and affective symptoms of depression may foster an unhealthy lifestyle, predisposing individuals to insulin resistance-related conditions. This underscores the necessity of integrated treatment approaches.
The higher prevalence of unhealthy behaviours, such as smoking and reduced physical activity, in the IR+ group resonates with existing evidence linking lifestyle factors to both depression and metabolic disturbances.Reference Kandola, Ashdown-Franks, Hendrikse, Sabiston and Stubbs29 This observation, coupled with the evidence indicating poorer treatment outcomes in the same group, highlights the potential benefits of incorporating lifestyle interventions in MDD management.Reference Kandola, Ashdown-Franks, Hendrikse, Sabiston and Stubbs29 However, lower alcohol intake frequency was noted, which aligns with certain clinical characteristics of the IR+ group. Given the comorbidities and more complex medication regimens in this group, it is likely that they had prudently reduced alcohol intake for medical reasons (as a form of tertiary prevention and to avoid possible pharmacokinetic interactions with their medicationsReference Chan and Anderson30). The link between chronic health conditions, such as MDD and insulin resistance and lower socioeconomic status (SES), is probably bidirectional. The risk of chronic diseases is increased in groups with lower SESReference Sommer, Griebler, Mahlknecht, Thaler, Bouskill and Gartlehner31 but, at the same time, these conditions negatively impact well-being and social/work functioning, escalating medical expenses.Reference Cabral, Dantas de Souza, Barbosa, Jerez-Roig and Souza32
Distinct clinical and emotional profiles
Our study also suggests that individuals with a lifetime history of both MDD and insulin resistance-related conditions exhibit a distinct clinical profile of depression. The higher mean age at MDD first diagnosis in the IR+ group could possibly have resulted from an intersection of age-related reduction in insulin sensitivity, lifestyle and psychosocial stressors inherent to ageing, such as social isolation. Age-related factors, including chronic health challenges, retirement and shifts in social roles, may contribute to the simultaneous emergence of depression and insulin resistance-related conditions.Reference Stenholm, Westerlund, Salo, Hyde, Pentti and Head33 The prevalent feelings of loneliness and being fed-up in the MDD IR+ group resonate with the heightened susceptibility to perceived social isolation associated with atypical depression.Reference Lojko and Rybakowski34 This subtype of depression, frequently connected with inflammatory and metabolic disturbances, may also be reflected in the elevated BMI observed in the same group, consistent with the weight gain characteristic of atypical depression.Reference Lojko and Rybakowski34 The distinctive emotional profiles observed in relation to the timing of MDD onset versus insulin resistance-related diagnoses provide potential hints for targeted preventive interventions. Higher neuroticism in individuals with pre-existing MDD suggests that these patients might have personality characteristics that could predispose not only to depression but also to metabolic changes. Neuroticism, characterised by a tendency towards anxiety, depression and emotional instability, is a well established risk factor for developing both mood disorders and cardiometabolic conditions.Reference Lee, Grimm, Spiro and Kubzansky35 Conversely, the reduced presence of classical anxiety-related symptoms, coupled with increased feelings of inadequacy and difficulty concentrating in the MDD-after-IR subgroup, could reflect the negative psychological impact of experiencing a chronic cardiometabolic condition prior to depression. Living with a chronic insulin resistance-related condition may lead to adaptation to some emotional responses, shifting from anxiety and worry to feelings of inadequacy and difficulty concentrating. This could be attributed to the constant coping and management demands of a chronic physical illness, which may lead to a sense of cognitive overload and being overwhelmed.
Prescription patterns and insulin resistance-related comorbidities
An increased frequency of prescriptions of SSRIs, SARIs, SNRIs and tetracyclic and tricyclic antidepressants in the IR+ group was found, suggesting a more challenging treatment course. This is confirmed by the higher frequency of antidepressant switches and the use of a wider array of antidepressant classes, particularly in patients with MDD preceding insulin resistance-related diagnoses. The metabolic side-effect profiles of these antidepressant classes warrant careful consideration. SSRIs are typically preferred for their relatively favourable side-effect profile, especially in patients with comorbid medical conditions.Reference Gold, Kohler-Forsberg, Moss-Morris, Mehnert, Miranda and Bullinger36 SSRIs have been shown to improve glycaemic control in adults with comorbid MDD and T2DM in short-term studies, and have no long-term deleterious effects on glycaemic homeostasis.Reference Possidente, Fanelli, Serretti and Fabbri3 Conversely, SNRIs and tetracyclic and tricyclic antidepressants, despite their efficacy, are associated with significant cardiometabolic side-effects, including hypertension, weight gain and dyslipidaemia,Reference Gold, Kohler-Forsberg, Moss-Morris, Mehnert, Miranda and Bullinger36,Reference Serretti and Mandelli37 posing potential exacerbation risks in the presence of underlying insulin resistance predisposition. The use of TCAs in these patients, often a choice of last resort due to their lower tolerability, suggests a clinical pivot towards more pharmacodynamically complex treatment options when first-line treatments fail. Conversely, a less frequent use of some antidepressant classes in the MDD-after-IR group probably reflects clinicians’ attention to the metabolic side-effects of certain antidepressants and, consequently, a more conservative approach. Overall, these findings emphasise the importance of a personalised treatment strategy for MDD, especially for individuals with a personal or familiar history of insulin resistance-related conditions. Antidepressant selection must carefully weigh the risk:benefit ratio, prioritising patient safety and overall health in the context of pre-existing or heightened risk of insulin resistance.
Insulin resistance-related conditions and treatment outcomes in depression
The association between insulin resistance-related conditions and higher odds of poorer treatment outcomes and overall treatment duration supports the hypothesis that metabolic dysregulation may be linked with difficult-to-treat depression. The association with poorer treatment outcomes was particularly evident when MDD diagnosis preceded insulin resistance-related conditions. This trajectory may suggest that the neurobiological and behavioural effects of depression, including stress-related hormonal imbalances and reduced physical activity, may predispose individuals to metabolic disturbances that probably worsen treatment response.Reference Milaneschi, Lamers, Berk and Penninx14,Reference Milaneschi, Simmons, van Rossum and Penninx27,Reference Horstmann and Binder38 Chronic inflammation and oxidative stress, which are associated with insulin resistance, can impair serotonin signalling and synaptic plasticity, processes involved in antidepressant response.Reference Milaneschi, Lamers, Berk and Penninx14,Reference Pilar-Cuellar, Vidal, Diaz, Castro, dos Anjos and Pascual-Brazo39,Reference Mehdi, Wani, Krishna, Kinattingal and Roohi40 Notably, the observed higher prescription of antidepressants and diverse pharmacological classes in IR+ individuals raises questions about the potential of pharmacotherapy in triggering or worsening insulin resistance-related conditions; indeed, patients with difficult-to-treat MDD may be more frequently exposed to medications with metabolic side-effects.Reference Serretti and Mandelli37 On the other hand, the observation in our sample that a broad phenotype of insulin resistance pathology – defined by the presence of any insulin resistance-related condition rather than specific ones – is linked to worse treatment outcomes when insulin resistance precedes MDD and may support a direct influence of metabolic health on psychiatric treatment effectiveness. Of note, the larger sample size of the cumulative insulin resistance phenotype probably increased the statistical power of this analysis, thus revealing associations not apparent in more narrowly defined groups. However, future research is needed to clarify whether insulin resistance-related conditions primarily aggravate depressive symptoms through metabolic dysregulation or directly impair antidepressant efficacy, because the current study design does not establish causality.
PGSs and future directions
Our study did not identify significant associations between PGSs for insulin resistance-related conditions and treatment outcomes, although nominal associations were observed with PGS for CAD, triglycerides and BMI in certain subgroups. The multifactorial nature of treatment outcomes, with a relatively modest contribution of common genetic variants,Reference Pain, Hodgson, Trubetskoy, Ripke, Marshe and Adams41 and methodological limitations, may have impacted on the possibility of reaching statistical significance for these results. For example, the PGS approach used was not biologically informed, i.e. it did not prioritise SNPs based on their known or predicted functional impact, which may have improved PGS prediction accuracy.Reference Sharew, Clark, Schubert and Amare42 Consistent with our findings, previous studies reported limited explanatory power of PGSs for insulin resistance-related conditions in antidepressant treatment outcomes. For instance, PGSs for CAD and BMI explained only 1.3 and 0.8% of SSRI treatment response variance, respectively, with notable cohort-specific and quartile-dependent differences in effect size.Reference Amare, Schubert, Tekola-Ayele, Hsu, Sangkuhl and Jenkins43 In one cohort, associations were evident only among individuals in the highest PGS quartile, while intermediate quartiles showed stronger effects in another.Reference Amare, Schubert, Tekola-Ayele, Hsu, Sangkuhl and Jenkins43 Similarly, research on PGS for T2DM and depression has shown that significant associations were particularly evident in early-onset cases, or only nominally significant across ancestrally diverse cohorts.Reference Fanelli, Raschi, Hafez, Matura, Schiweck and Poluzzi8
In addition to lifestyle modifications, addressing inflammatory-metabolic dysfunctions of MDD with new pharmacological interventions offers promising opportunities. Anti-inflammatory agents, such as anti-interleukin-6 antibodies and tumor necrosis factor-α inhibitors, have shown potential in alleviating depressive symptoms, particularly in individuals with elevated inflammatory biomarkers.Reference Fanelli, Raschi, Hafez, Matura, Schiweck and Poluzzi8,Reference Wittenberg, Stylianou, Zhang, Sun, Gupta and Jagannatha44 Similarly, glucagon-like peptide-1 receptor agonists (GLP-1 RAs), such as liraglutide, offer dual benefits by improving glycaemic control and reducing systemic inflammation, with preliminary evidence of antidepressant effects.Reference Possidente, Fanelli, Serretti and Fabbri3,Reference Fanelli, Raschi, Hafez, Matura, Schiweck and Poluzzi8 Integrating these pharmacological interventions with precision psychiatry tools, such as multivariable models incorporating more advanced PGS approaches, could optimise treatment personalisation.
Strengths and limitations
This study should be viewed in the context of its strengths and limitations. Its strengths lie in its large sample size and the use of a comprehensive data-set from UKB. The inclusion of primary care data enriched the findings, providing a real-world perspective on the management of MDD in relation to insulin resistance-related conditions. However, its observational nature precludes causal inferences, and generalisability of the findings may be limited to similar healthcare settings. While the results demonstrate a strong association between insulin resistance-related conditions and poorer treatment outcomes in MDD, they cannot determine whether insulin resistance-related conditions primarily aggravate MDD, directly contribute to resistance or result from prolonged treatment resistance and pharmacological burden. Future longitudinal and experimental studies are required to disentangle the temporal and causal dynamics between insulin-resistance conditions, MDD severity and treatment outcomes. The demographic composition of UKB, predominantly consisting of females, older individuals and those of higher SES, does not mirror the general UK population.Reference Fry, Littlejohns, Sudlow, Doherty, Adamska and Sprosen45 Additionally, our analysis relied on proxy measures such as antidepressant switches for treatment non-response/resistance. While these proxies are well established in the literature,Reference Lage, McCoy, Perlis and Doshi-Velez46,Reference Wigmore, Hafferty, Hall, Howard, Clarke and Fabbri47 they depend on the completeness of EHRs and are not direct measures of treatment response. The interpretation of our results should consider potential biases introduced by missing data, such as gaps in prescription or diagnosis dates. Furthermore, our analysis did not consider prescription dosages, nor did it differentiate based on symptom severity or MDD phase (acute versus non-acute). Regarding PGS calculation, to prevent results inflation we could not use some of the larger GWAS sample, which was overlapping with our target UKB sample. While our findings demonstrate statistically significant associations between various insulin resistance-related conditions and treatment outcomes in depression, the predictive impact of these associations, as indicated by Nagelkerke’s pseudo-R 2 and R 2 values, was limited. This aligns with expectations for complex, multifactorial conditions such as MDD and insulin resistance-related traits, where a substantial portion of variance arises from unmeasured genetic, environmental and clinical factors. Nonetheless, the Hosmer–Lemeshow test results (P > 0.05 in most models) indicated acceptable model fit, supporting the validity of the observed associations. Future research should incorporate other variables and advanced modelling approaches to better capture the full complexity of biopsychosocial factors that contribute to depression treatment outcomes.
Discussion
In conclusion, this study highlights a substantial prevalence of insulin resistance-related conditions among individuals with a history of MDD, highlighting a demographic profile characterised by later age of MDD onset, a propensity towards unhealthy lifestyle and a distinct clinical profile. Notably, the presence of insulin resistance-related conditions was found to be associated with heightened complexity in managing depression, as evidenced by an increase in antidepressant prescriptions, treatment non-response/resistance and prolonged treatment duration, particularly when MDD diagnosis preceded that of insulin resistance. These results advocate for careful antidepressant selection, mindful of potential metabolic adverse effects. Overall, these insights endorse the implementation of a holistic care model that surpasses traditional psychiatric management, incorporating metabolic assessments and lifestyle interventions to improve outcomes in patients with MDD. It is important for healthcare providers to regularly monitor metabolic health in patients with MDD, because early detection/treatment of insulin resistance-related conditions holds the potential to enhance psychiatric and physical outcomes, particularly in patients with persistent or treatment-resistant MDD.
Supplementary material
The supplementary material can be found online at https://doi.org/10.1192/bjp.2025.82
Data availability
The data that support the findings of this study are available from the UKB project site, subject to registration and application process. Further details can be found at https://www.ukbiobank.ac.uk. Returned data and code from single publications are also available through the UKB resource.
Acknowledgement
This research has been conducted using the UKB Resource under application no. 56514, ’Stratification of health outcomes in mood disorders’.
Author contributions
G.F.: writing – original draft preparation, conceptualisation, methodology, formal analysis, software, validation, investigation, data curation and visualisation; J.B.: writing – review and editing; B.F.: writing – review and editing; N.R.M.: writing – review and editing; A.R.A.: writing – review and editing; D.D.R.: writing – review and editing; A.M.M.: funding acquisition and writing – review and editing; L.G.: funding acquisition and writing – review and editing; MNESYS – Mood and Psychosis Sub-Project (Spoke 5): writing – review and editing and funding acquisition; A.S.: supervision, conceptualisation, writing – review and editing, funding acquisition and resources; C.F.: supervision, conceptualisation, writing – review and editing, funding acquisition and resources.
Funding
This work was supported by NEXTGENERATIONEU (NGEU) and funded by the Italian Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project no. MNESYS (PE0000006) – A multiscale integrated approach to the study of the nervous system in health and disease (no. DN. 1553 11.10.2022). BF’s contribution was supported by funding from the European Community’s Horizon 2020 Programme (no. H2020/2014 – 2020) under grant agreement no. 847879 (PRIME), and from the PRISM2 project (www.prism-project.eu; grant agreement no. 101034377) through the Innovative Medicines Initiative 2 Joint Undertaking. She also received relevant funding from the National Institute of Mental Health of the National Institutes of Health, under award no. R01MH124851.
The content of the publication has been solely the responsibility of the authors and does not necessarily represent the official views of the funding organisations.
Declaration of interest
A.S. is, or has been, a consultant/speaker for Abbott, Abbvie, Angelini, AstraZeneca, Clinical Data, Boehringer, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Innovapharma, Italfarmaco, Janssen, Lundbeck, Naurex, Pfizer, Polifarma, Sanofi, Servier and Taliaz. B.F. has received educational speaking fees and travel support from Medice. The other authors declare no conflicts of interest.





eLetters
No eLetters have been published for this article.