Introduction
In the presence of fetal cardiomegaly, when there is no cardiac malformation or dysfunction, systemic or pulmonary arteriovenous malformations that may cause volume loading should be sought. We aimed to present a fetus who had cardiomegaly and left pulmonary artery-left atrial fistula and who underwent transcatheter closure in the early postnatal period
Case presentation
23-week fetus of 28 years age mother referred because of severe cardiomegaly on screening obstetric ultrasonography. Fetal echocardiographic examination revealed marked 4-chamber enlargement of the heart occupying all the left hemithorax and pronounced leftward rotation of cardiac axis. There was no systoli8c dysfunction of the ventricles. Mild pericardial and pleural effusion were noted (Fig 1A). Fetal echocardiography also revealed fistulous connection between dilated left pulmonary artery and left atrium with high velocity continuous flow at the left atrial orifice of fistula (Fig IB-IC, Movie 1-Movie 2 in the Data supplement) and retrograde flow from the ductus arteriosus to the pulmonary artery (Movie 3 in the Data Supplement). Initially, the fetus followed by one-to-two weeks intervals for fetal heart failure and hydrops. Pericardial and pleural effusions disappeared in two weeks, and gradual decrease of heart size (cardiothoracic area ratio was 0.50 initially, then 0.40 at 36 weeks) was observed during pregnancy The fistula size at the site of entry to the left atrium was measured as 2.5 mm and it was not changed during follow-up.
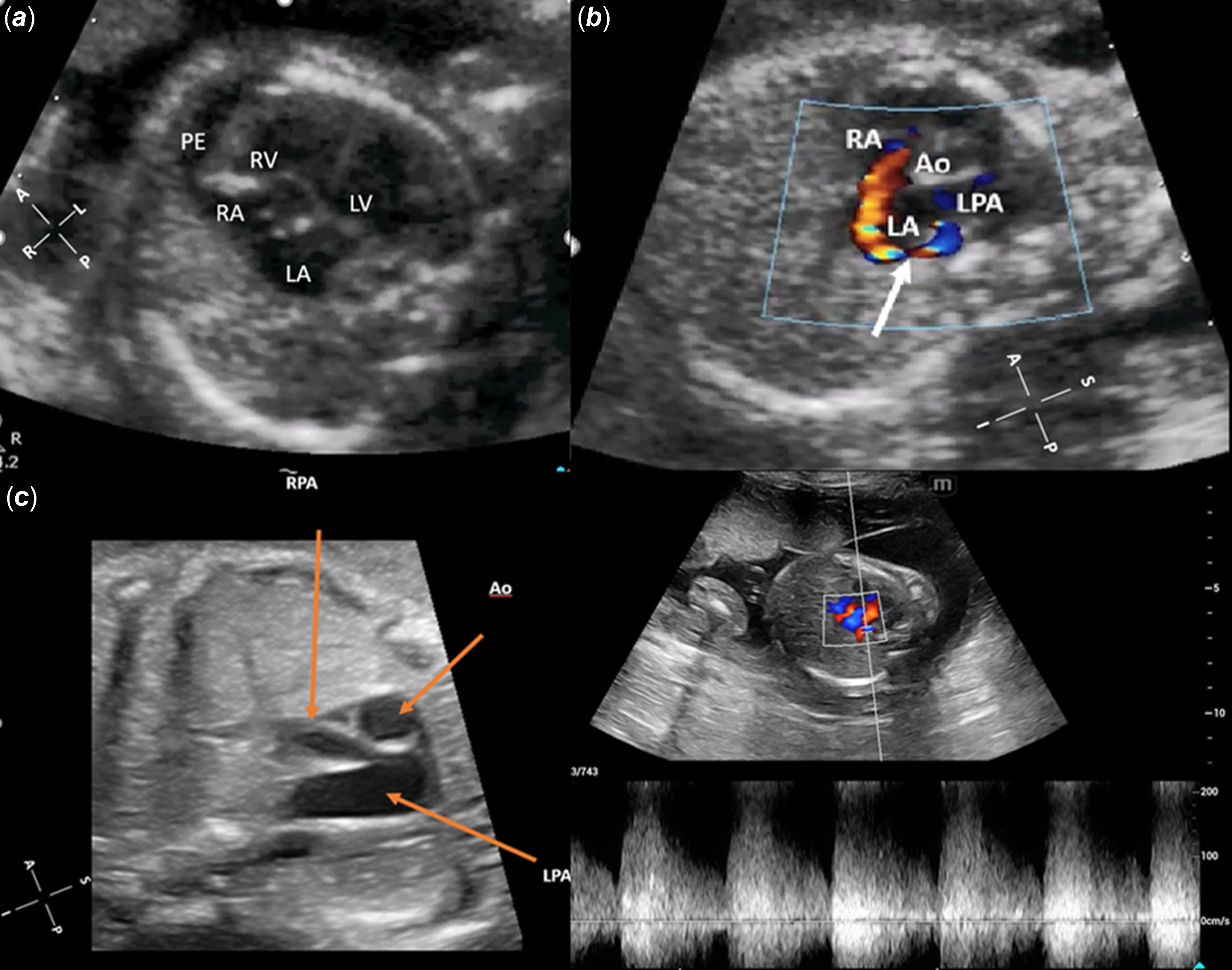
Figure 1. Fetal echocardiographic examination. (a) 4-chamber enlargement of the heart occupying the entire left hemithorax with pronounced leftward rotation of the cardiac axis, mild pericardial, and pleural effusion on echocardiogram. (b) The white arrow indicates the fistulous connection between the dilated LPA and LA, with high-velocity continuous flow at the left atrial orifice of the fistula. (c) Significantly dilated LPA and smaller RPA.
Pregnancy was uneventful and the baby was born by caesarean section at 37 weeks, her weight was 2650 g. The heart rate was 140/minute, and oxygen saturation level was 95 %. There were no breathing sounds on the left lung. Transthoracic echocardiography confirmed the prenatal diagnosis of a fistula between the left pulmonary artery and the left atrium (diameter of 3.5–4 mm) from which a high velocity continuous flow sample was taken (Fig 2; Movie 4 in the Data Supplement). The right pulmonary artery measured 4 mm (Z-score: -1.04), which was considerably smaller compared to the left pulmonary artery, which was 9 mm (Z-score: +4.39). Apex of the heart rotated approximately 45 degrees left-up counterclockwise and was located horizontally in the mediastinum left-to-right shunt flow was observed through the ductus arteriosus (4 mm) and the patent foramen ovale. The baby was transferred to the neonatal intensive care unit to monitor for heart failure secondary to volume overload and progression of hypoxia. Computerised tomographic angiography on the 4th postnatal day showed that the left pulmonary artery opened distally into the left atrium at its inferoposterior aspect (Fig 3). Computerised tomographic angiography also showed there was no lung parenchymal tissue and vascular bed in the left lung with rudimentary blind-ended left main bronchus (left lung aplasia). During the follow-up, it was observed that as the patient’s ductus diminished in size, the patient’s oxygen saturation down to (85%) as well. Although the patient did not have heart failure or respiratory distress, transcatheter closure was performed from antegrade route with Amplatzer Piccolo Duct Occluder (5x6 mm) on the 18th postnatal day due to hypoxaemia (Movie 5A–5B in the Data Supplement). Hypoxaemia resolved immediately after transcatheter closure. The baby, who was discharged from the hospital in a short time, showed normal growth and development at 15 months of age without any symptoms. The size discrepancy of branch pulmonary arteries was disappeared during follow-up. Ductus arteriosus (1.3 mm in size) is still open with left-to-right shunt (Movie 6 in the Data Supplement). Since ductus arteriosus does not cause significant volume overload no intervention has been considered yet. There is no sign of pulmonary hypertension during the 15-month follow-up.
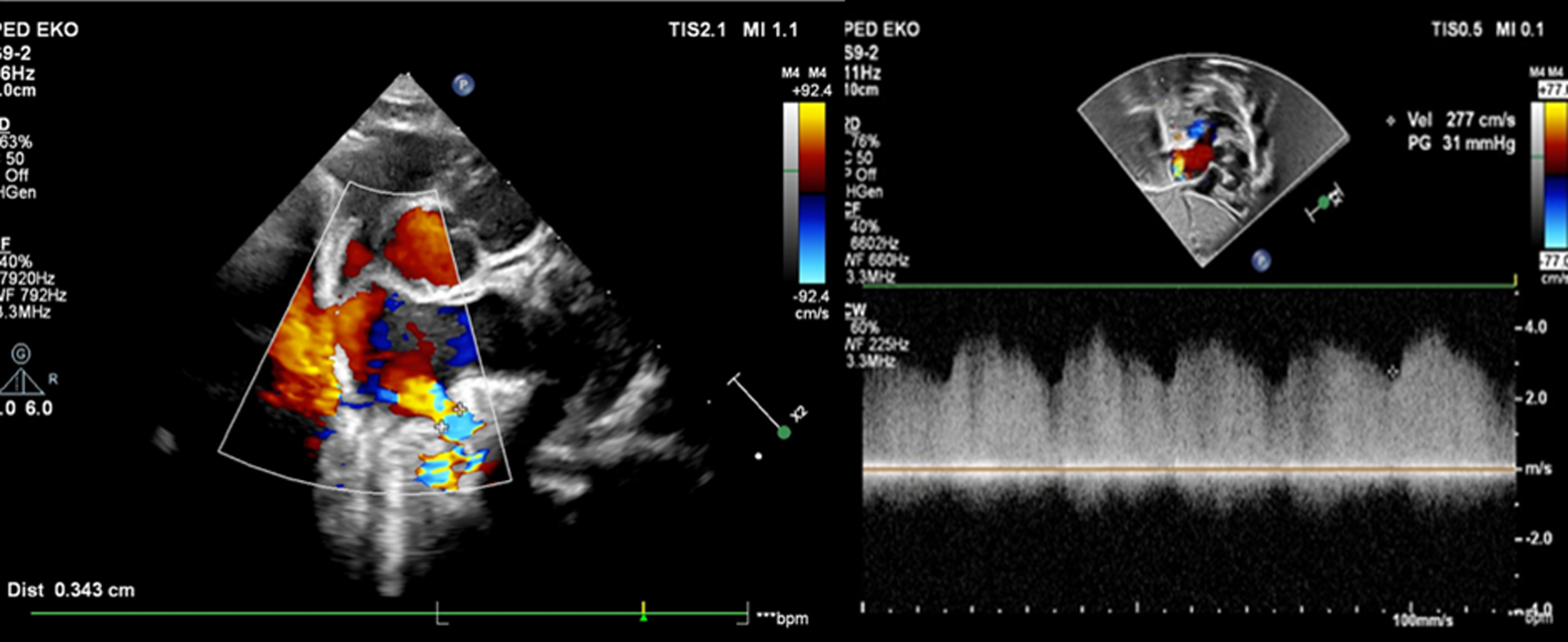
Figure 2. Transthoracic echocardiogram. Transthoracic echocardiogram showing the LPA-LA fistula and high-velocity continuous flow on Doppler examination.
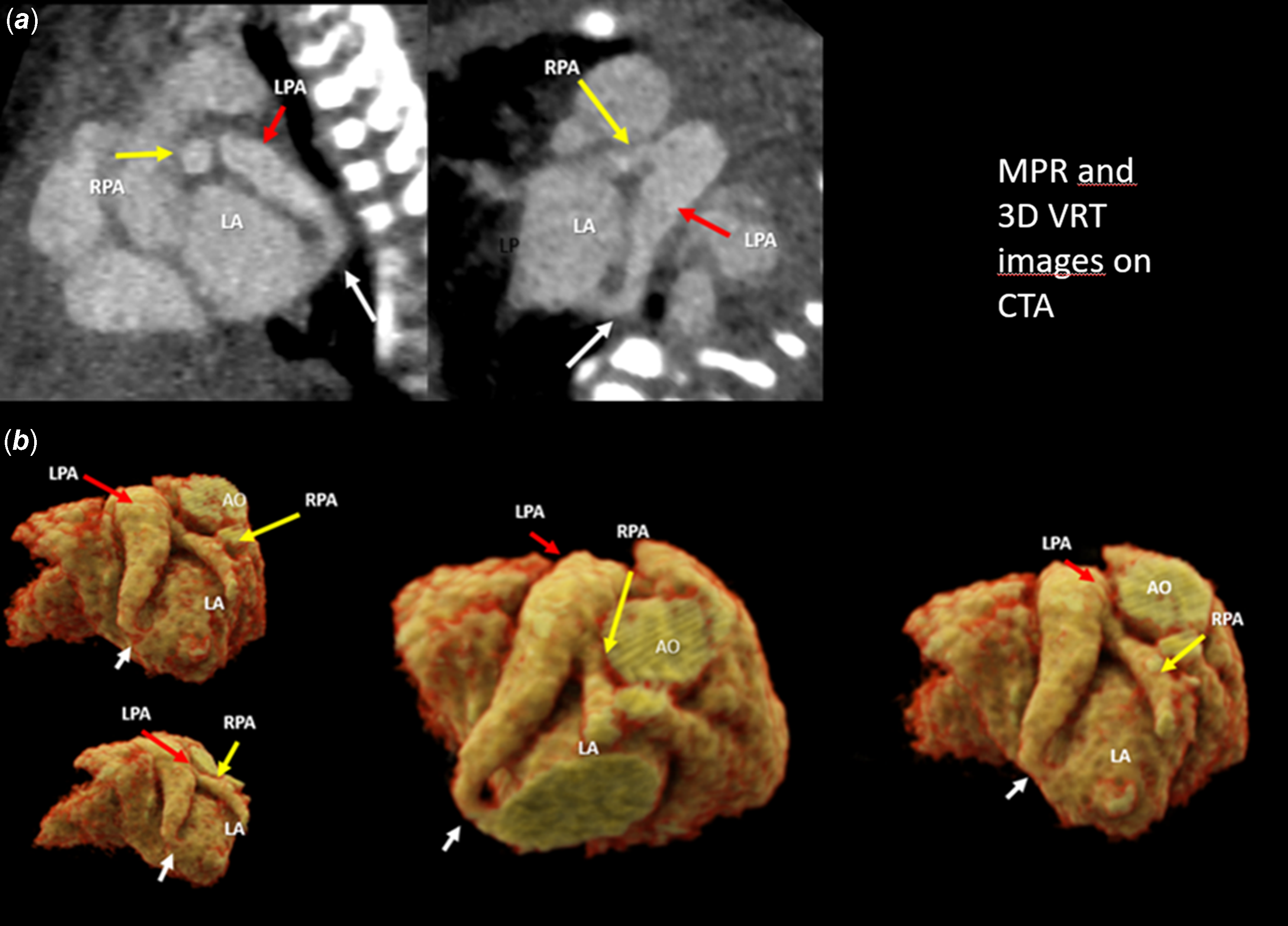
Figure 3. CTA. (a) Multiplanar reconstruction (MPR) images: the white arrow indicates the LPA-LA fistula, the red arrow indicates the dilated LPA, and the yellow arrow indicates the smaller RPA. (b) 3D volume rendering technique (VRT) images: these images show the LPA-LA fistula, a significantly dilated LPA, and a smaller RPA.
Discussion
In the presence of fetal cardiomegaly, when there is no cardiac malformation or dysfunction, systemic (mainly hepatic and intracranial arteriovenous malformations) or pulmonary arteriovenous malformations that may cause volume loading should be sought. Pulmonary artery-to-left atrial fistula is a rare anomaly and is frequently described between the right pulmonary artery and the left atrium. Reference Kehr, Stirling, Bagnall, Long, Artrip and Gentles1 There are very few case reports of left pulmonary artery to left atrial fistula and has never been reported in fetal age before. Presentation of age depends on the size of the fistulous connection. Patients with large connections are presented in fetal age with cardiomegaly and heart failure or presented in early infancy with profound cyanosis. Small connections, however, may not be present until adolescence or early adulthood. All the reported patients with pulmonary artery-to-left atrial fistula in fetal period have cardiomegaly. The volume overload due to fistula if it is large enough may lead to heart failure and/or hydrops fetalis. Reference Kehr, Stirling, Bagnall, Long, Artrip and Gentles1 Our patient also had cardiomegaly and pericardial effusion initially. We assume that the regression of our patient’s cardiomegaly and pericardial effusion during follow-up may be related to the fistula size being relatively smaller as the fetus grew. In our patient, the right pulmonary artery was found to be significantly smaller compared to the left pulmonary artery, which was notably dilated. The fact that the right pulmonary artery is smaller compared to the left pulmonary artery can be easily explained by the flow “stolen” from the right pulmonary artery through the fistula, along with the significant dilation of the left pulmonary artery.
Although lung hypoplasia has been reported in patients with pulmonary artery-to-left atrial fistula/connection lung aplasia has never been reported in these patients. It is known that lung parenchyma and pulmonary vascular bed development occur simultaneously and depend on each other (3). It may be expected left lung hypoplasia in our case because of two reasons: 1) occupying of all left thorax by enlarged heart and 2) impairment of vascular and consequently parenchymal development secondary to arterial steal through the fistula. It was quite surprising that our patient had lung aplasia. By definition, there should be no pulmonary artery belonging to the aplastic lung, the presence of the left pulmonary artery in our case is a unique finding that has never been reported and therefore needs to be explained from a developmental standpoint. Due to severe cardiomegaly occupying the entire left hemithorax, left lung aplasia was not clearly visualised on fetal echocardiography in our case. However, postnatal imaging clearly revealed the condition. As a result, fetal MRI was not performed, but in cases like ours, where lung pathology is suspected during the fetal period, fetal MRI should be considered as a valuable diagnostic tool for further evaluation. Reference Kasprian, Balassy, Brugger and Prayer4
Surgical or transcatheter closure can be achieved successfully in these patients at neonatal period or early infancy like in our case. Reference Kehr, Stirling, Bagnall, Long, Artrip and Gentles1 , Reference Mongé, Russell, Popescu and Robinson2 In our patient, we opted for an appropriately sized Piccolo duct occluder for transcatheter closure. However, alternative devices such as vascular plugs have also been reported in the literature for transcatheter closure Reference Kumar, Varghese and George5
It is known that patients with lung aplasia/agenesis should be monitored for pulmonary hypertension. It has been stated that the risk of developing pulmonary hypertension in the absence of the right lung is higher than in the absence of the left lung (6). In short-term follow-up, our patient did not show any sign of pulmonary hypertension.
Summary
Pulmonary arteriovenous malformations are rare but important causes of fetal cardiomegaly and heart failure. When it was detected, close follow-up for haemodynamic consequences before and after closure is mandatory.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1047951125001714.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Author contribution
Bilgehan Betül Biçer, Follow-up of the patient and writing the manuscript.
Hayrettin Hakan Aykan, performing the intervention and follow-up of the patient.
Tevfik Karagöz, performing the intervention and follow-up of the patient.
Ercan Tutar, fetal diagnosis, follow-up of the patient, and editing the manuscript.
Financial support
This study was not supported by any sponsor or funder.
Competing interests
There is no conflict of interest.
Ethical statement
An informed consent form containing information to be used in scientific publications was obtained from the patient’s parents.





