Introduction
In general, silvopastoral systems (SPS) such as live fences, dispersed trees in pastures, tree-alley pastures have the potential to mitigate climate change and conserve biodiversity by increasing carbon sequestration, reducing greenhouse gas emissions, enhancing nutrient cycling, preventing soil erosion and compaction, and increasing biodiversity (Montagnini et al., Reference Montagnini, Ibrahim and Murgueitio2013; Villanueva-López et al., Reference Villanueva-López, Lara-Perez, Oros-Ortega, Ramírez-Barajas, Casanova-Lugo, Ramos-Reyes and Aryal2019; Mahmud et al., Reference Mahmud, Raj and Jhariya2021; Aryal et al., Reference Aryal, Morales-Ruiz, López-Cruz, Tondopó-Marroquín, Lara-Nucamendi, Jiménez-Trujillo, Pérez-Sánchez, Betanzos-Simon, Casasola-Coto, Martínez-Salinas, Sepúlveda-López, Ramírez-Díaz, La O Arias, Guevara-Hernández, Pinto-Ruiz and Ibrahim2022). Fodder bank systems (FBS), a type of SPS, consists of establishing shrubs or trees in high densities to feed animals in the dry season and for biomass transfer from green manure to provide an input of nutrients into soils (Nair et al., Reference Nair, Nair, Kumar and Haile2009; George et al., Reference George, Gregory, Robinson, Buresh and Jama2001; Franzel et al., Reference Franzel, Carsan, Lukuyu, Sinja and Wambugu2014).
To maximise economic yields in livestock and crop production, fodder species must have desirable characteristics such as high productivity, in terms of biomass, under repeated cutting or grazing, high crude protein, accumulation of large amounts of nutrients, ands well as high tolerance to adverse biotic or abiotic (George et al., Reference George, Gregory, Robinson, Buresh and Jama2001; Franzel et al., Reference Franzel, Carsan, Lukuyu, Sinja and Wambugu2014). This strategy has proved to increase cattle and crop productivity and has gained considerable importance in Africa and increasing interest in Latin America (Montagnini et al., Reference Montagnini, Ibrahim and Murgueitio2013; Franzel et al., Reference Franzel, Carsan, Lukuyu, Sinja and Wambugu2014; Aryal et al., Reference Aryal, De Jong, Ochoa-Gaona, Mendoza-Vega and Esparza-Olguin2015; Aryal et al., Reference Aryal, Morales-Ruiz, López-Cruz, Tondopó-Marroquín, Lara-Nucamendi, Jiménez-Trujillo, Pérez-Sánchez, Betanzos-Simon, Casasola-Coto, Martínez-Salinas, Sepúlveda-López, Ramírez-Díaz, La O Arias, Guevara-Hernández, Pinto-Ruiz and Ibrahim2022; González-Abraham et al., Reference González-Abraham, Flores-Santana, Rodríguez-Ramírez, Olguin-Alvarez, Flores-Martínez, Torres Rojo, Bocco-Verdinelli, Fernández-Calleros and McCord2023); however, FBS has received little attention from the ecosystem services provided in the production systems and the ecological dynamic of fodder species (Aryal et al., Reference Aryal, Morales-Ruiz, López-Cruz, Tondopó-Marroquín, Lara-Nucamendi, Jiménez-Trujillo, Pérez-Sánchez, Betanzos-Simon, Casasola-Coto, Martínez-Salinas, Sepúlveda-López, Ramírez-Díaz, La O Arias, Guevara-Hernández, Pinto-Ruiz and Ibrahim2022; Casanova-Lugo et al., Reference Casanova-Lugo, Petit-Aldana, Solorio-Sánchez, Ramírez-Avilés, Ward, Villanueva-López and Aryal2018), where the synchronisation of nutrient release and their assimilation by plants are of great importance to net primary production and can contribute to maintain organic matter (Hernández-Ramos et al., Reference Hernández-Ramos, Valdez-Lazalde, Ángeles-Pérez, de los Santos-Posadas, Hernández-Ramos, Peduzzi and Carrero2017; Souza et al., Reference Souza, Veloso, Espírito-Santo, Silva, Sánchez-Azofeifa, Souza e Brito and Fernandes2019; Verma et al., Reference Verma, Kumar, Soni, Pawar, Pradhan, Tanwar and Kumar2021).
Studies of leaf litter production and the dynamic release of nutrients in FBS include a few studies on areas with a specific soil characteristic in monoculture and mix fodder banks (Casanova-Lugo et al., Reference Casanova-Lugo, Petit-Aldana, Solorio-Sánchez, Parsons and Ramírez-Avilés2014, Reference Casanova-Lugo, Petit-Aldana, Solorio-Sánchez, Ramírez-Avilés, Ward, Villanueva-López and Aryal2018; Ramos-Trejo et al., Reference Ramos-Trejo, Canul-Solís, Alvarado-Canché, Castillo-Sánchez, Sandoval-Gío, Campos-Navarrete and Casanova-Lugo2020; Petit-Aldana et al., Reference Petit-Aldana, Casanova-Lugo, Solorio-Sánchez and Ramírez-Avilés2011). In Mexico, litterfall dynamics and biomass production, decomposition, and carbon stocks biomass have been only studied in five woody species in FBS: tree non-legume species (Guazuma ulmifolia Lam., Moringa oleifera Lam., and Morus alba L.) and two legume species (Gliricidia sepium (Jacq.) Kunth ex Walp. and Leucaena leucocephala (Lam.) de Wit) (Petit-Aldana et al., Reference Petit-Aldana, Casanova-Lugo, Solorio-Sánchez and Ramírez-Avilés2011; Casanova-Lugo et al., Reference Casanova-Lugo, Petit-Aldana, Solorio-Sánchez, Parsons and Ramírez-Avilés2014, Reference Casanova-Lugo, Petit-Aldana, Solorio-Sánchez, Ramírez-Avilés, Ward, Villanueva-López and Aryal2018; Ramos-Trejo et al., Reference Ramos-Trejo, Canul-Solís, Alvarado-Canché, Castillo-Sánchez, Sandoval-Gío, Campos-Navarrete and Casanova-Lugo2020). Tithonia diversifolia (Hemsl.) A. Gray have gained considerable interest because it is a growth-fast species with high decomposition rates, with a high concentration of phosphorus, nitrogen, and magnesium (Sánchez et al., Reference Sánchez, Crespo and Hernández2007; Bonilla et al., Reference Bonilla, Belisario-Roncallo and García2008; Partey, Reference Partey2011; Petit-Aldana et al., Reference Petit-Aldana, Casanova-Lugo, Solorio-Sánchez and Ramírez-Avilés2011; Casanova-Lugo et al., Reference Casanova-Lugo, Petit-Aldana, Solorio-Sánchez, Parsons and Ramírez-Avilés2014).
In undisturbed ecosystems, leaf litter represents 80% of the total nutrients returned to the soil (Santa-Regina and Tarazona, Reference Santa-Regina and Tarazona2001); this is especially relevant in the Yucatan Peninsula of Mexico, where soils have inherently low fertility (Bautista, Reference Bautista2021; Fragoso-Servón et al., Reference Fragoso-Servón, Pereira Corona, Bautista Zúñiga and Zapata Buenfil2017). Despite the main objective of FBS is significant extracting activities of green biomass, they can contribute to carbon stock biomass, and with adequate management, could increase soil aggregates and organic carbon in the subsoil, wider spacing less intensive pruning, and significant litterfall dynamic that contribute to nutrient cycling and maintain fertility (Petit-Aldana et al., Reference Petit-Aldana, Casanova-Lugo, Solorio-Sánchez and Ramírez-Avilés2011; Casanova-Lugo et al., Reference Casanova-Lugo, Petit-Aldana, Solorio-Sánchez, Ramírez-Avilés, Ward, Villanueva-López and Aryal2018; Fungo et al., Reference Fungo, Buyinza, Sekatuba, Nansereko, Ongodia, Kwaga, Mudondo, Eryau, Akelem, Musinguzi and Agaba2020); which provides a habitat for functional diversity associated with decomposition (Marmolejo et al., Reference Marmolejo, Cantú and Gutiérrez2013; Zhang et al., Reference Zhang, Huang, An, Zeng, Wang, Bai and Huang2023).
The litterfall in FBS soils occurs in a brief lapse of time, between pruning and leaf regrowth, and is the main input of carbon and nutrients that remain after continually moving biomass harvested to feed livestock or as green manure to crop production. Therefore, the aim of this study was to evaluate the potential return of nutrients to the soil of natural litterfall and the litter dynamics in pure fodder banks of T. diversifolia, compared with L. leucocephala and M. oleifera, the most common species used in SPS of south-eastern Mexico. The findings of this study will contribute to understanding valuable insights for sustainable agricultural practices in the sub-humid tropics of Mexico.
Materials and methods
Study site
This study was conducted at the facilities of the Instituto Tecnológico de la Zona Maya, located 21.5 kilometres from the Chetumal-Escárcega highway, in the municipality of Othon P. Blanco in Quintana Roo, Mexico, from March to October 2019. The study site is located at the geographical coordinates 21° 51’ N and 89° 41’ W and presents a warm sub-humid climate (Aw1), with rains in the summer and part of the winter (García, Reference García1988). Its elevation is 15 m.a.s.l., and the topography is flat. The soil of the study site is Gleysol, according to the World Reference Base classification of soil resources (IUSS Working Group, 2022). During the experimental period, the maximum temperature ranged from 32.1 to 37.2 °C, while the minimum temperature ranged from 11.1 to 25.6 °C. The annual precipitation was 998 mm; however, from January to July, the lowest monthly rainfall was reported, ranging from 11.7 to 63.1 mm, and the highest rainfall was during the period from August to December in a range of 64.2 to 279.6 mm, with September being the month with the highest rainfall (Figure 1).
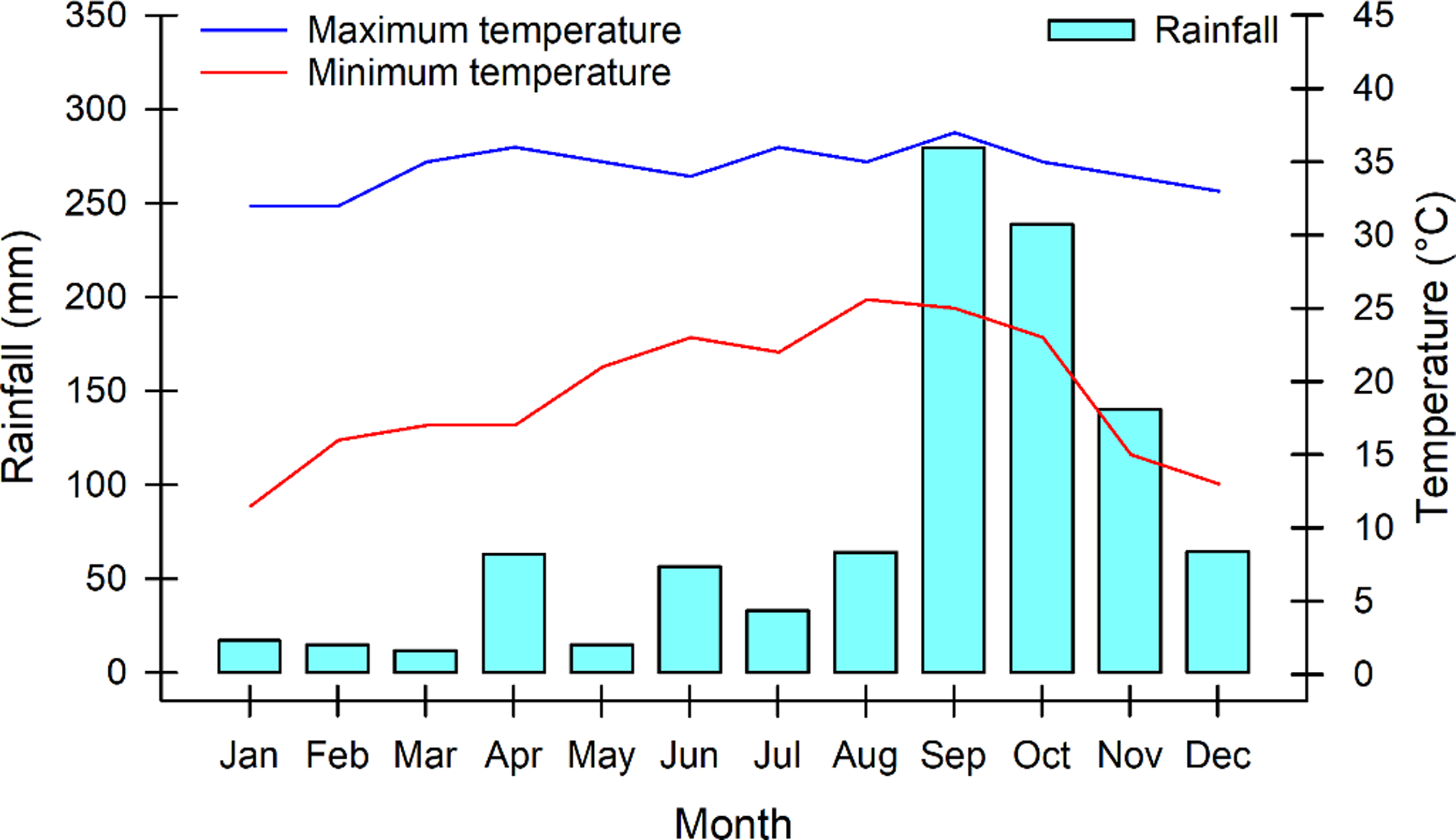
Figure 1. Maximum and minimum air temperatures and rainfall at the study site. Data were taken from the weather station at the Instituto Tecnológico de la Zona Maya in January to December 2019.
Experimental plots and management
The site has a history of agricultural use. In mid-2016, we established three fodder banks of T. diversifolia, L. leucocephala, and M. oleifera (onwards Tithonia, Leucaena, and Moringa) in an area of 0.6 ha, with a planting arrangement of 0.5 m between plants and 2.0 m between rows, corresponding to a planting density of 10 000 individuals per hectare and with east-to-west orientation. The fodder banks were managed with cut-and-carry practices. That is, the plants were harvested manually every three months at 60 cm from the soil surface and under rain-fed conditions. Weed control was carried out manually and without the application of herbicides. At the end of December 2018, a standardisation cut was made in the three FBS. Subsequently, for every three months, the bushes were pruned during March, June, September, and December.
Litterfall quantification
In each fodder bank, 10 litter traps (replicas) were placed randomly, with 30 traps in total (experimental units). The traps consisted of 1.0 m2 (1.0 × 1.0 m) PVC squares, covered with a 2.0–mm–separated carbon-fibre mesh and were placed on the edge of the rows of woody plants at an approximate height of 0.30 m from the surface to allow water drainage and aeration of the material (Petit-Aldana et al., Reference Petit-Aldana, Casanova-Lugo, Solorio-Sánchez and Ramírez-Avilés2011). Litter production was collected fortnightly for one year (January–December 2019), giving a total of 24 collections per treatment.
The litter samples were transported to the laboratory, where they were separated into leaves and branches grouped by each month of collection. It should be noted that, for the present study, the reproductive structures were discarded since only minute quantities of them were collected, due to the pruning applied to the experimental plots. The samples were dried in a forced air circulation oven at 60 ºC until constant weight. The monthly litter deposition data were extrapolated to units per hectare (t ha–1); additionally, the accumulated litter deposition during the year was calculated (t ha–1 yr–1).
Chemical analysis
All the samples were ground to a particle size of 1.0 mm, and then the carbon (C) and nitrogen (N) contents were determined by the dry combustion method with a PerkinElmer 2400 Series II Elemental Analyzer (PerkinElmer Inc., Massachusetts, USA). Phosphorus (P) content was determined using a PerkinElmer LAMBDA 850 UV/Vis Spectrophotometer at 880 nm (PerkinElmer Inc., Massachusetts, USA). In addition, the C:N ratio was estimated for the litter produced by each of the species studied.
Nutrient return
The nutrient return to the soil (t ha–1) was quantified by multiplying the monthly production of litter and the average fraction of each nutrient of each species: carbon, nitrogen, and phosphorus (% C, N, and P). In addition, the accumulated (annual) deposition of each nutrient (t ha–1 yr–1) for all species was determined.
Data analysis
The data were subjected to a two-way analysis of variance, where the effects of the sampling date, the species, and the interaction between both factors were analysed (Steel and Torrie, Reference Steel and Torrie1980). In addition, Tukey’s multiple comparison test was performed to determine the differences in cumulative deposition and chemical composition among species. Using Pearson’s correlation, the relationship between monthly environmental variables (i.e., temperature and rainfall) and litter deposition and the return of carbon, nitrogen, and phosphorus to the soil was analysed. To test the assumptions of normality and homogeneity of variances of each component of the litter and the chemical composition, the data were subjected to Shapiro–Wilk and Levene´s tests before submitting them to an analysis of variance (Steel and Torrie, Reference Steel and Torrie1980). All statistical analyses were performed with the Statistica© package version 8.0 (StatSoft Inc., 2007).
Results
Litter production (deposition)
There was a significant interaction between the different forage banks and the sampling months on the deposition of leaves, branches, and total litter (Figure 2). Litter production was relatively similar for the three species throughout the months, and they increased the production of leaves and branches in September. However, Tithonia showed the maximum record in that month (0.368 and 0.148 t ha–1, respectively), and September was, in general, the maximum new leaf growth month throughout the year (Figure 2).
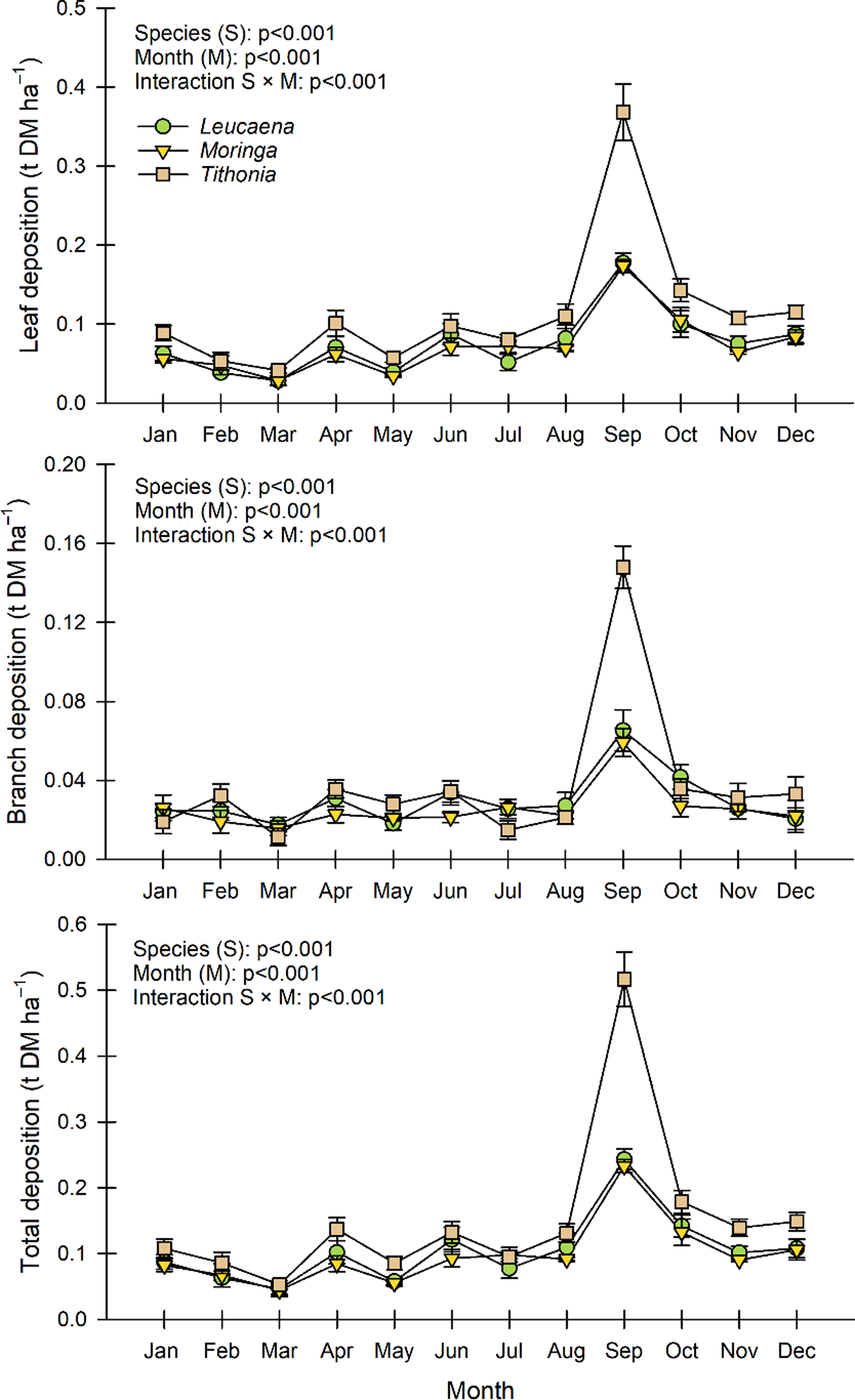
Figure 2. Monthly production of litter and its components: leaves and branches of Tithonia diversifolia, Leucaena leucocephala, and Moringa oleifera in fodder bank systems of southern Quintana Roo, Mexico. Means ± Standard error.
Regarding the total litter, a similar productive behaviour was observed throughout the year for the three species, and Tithonia reached the highest deposition in September (0.516 t ha–1). On the contrary, Leucaena and Moringa were consistently less productive throughout the observation period, and both species registered their minimum values in March and May (Figure 2).
On the other hand, Tithonia showed a greater accumulated deposition of leaves and total litter, with values of 1.365 and 1.81 t ha–1 yr–1, respectively, compared to the other species (Figure 3a–c). In the case of branch deposition, Tithonia and Leucaena presented the greatest values (0.445 and 0.356 t ha–1 yr–1, respectively), although the latter was like that of Moringa (Figure 3b). In addition to the above, for all the species, the leaves represented between 71.7 and 75.4% of the total litter, while the branches only represented between 24.6 and 28.3% (Figure 3d).
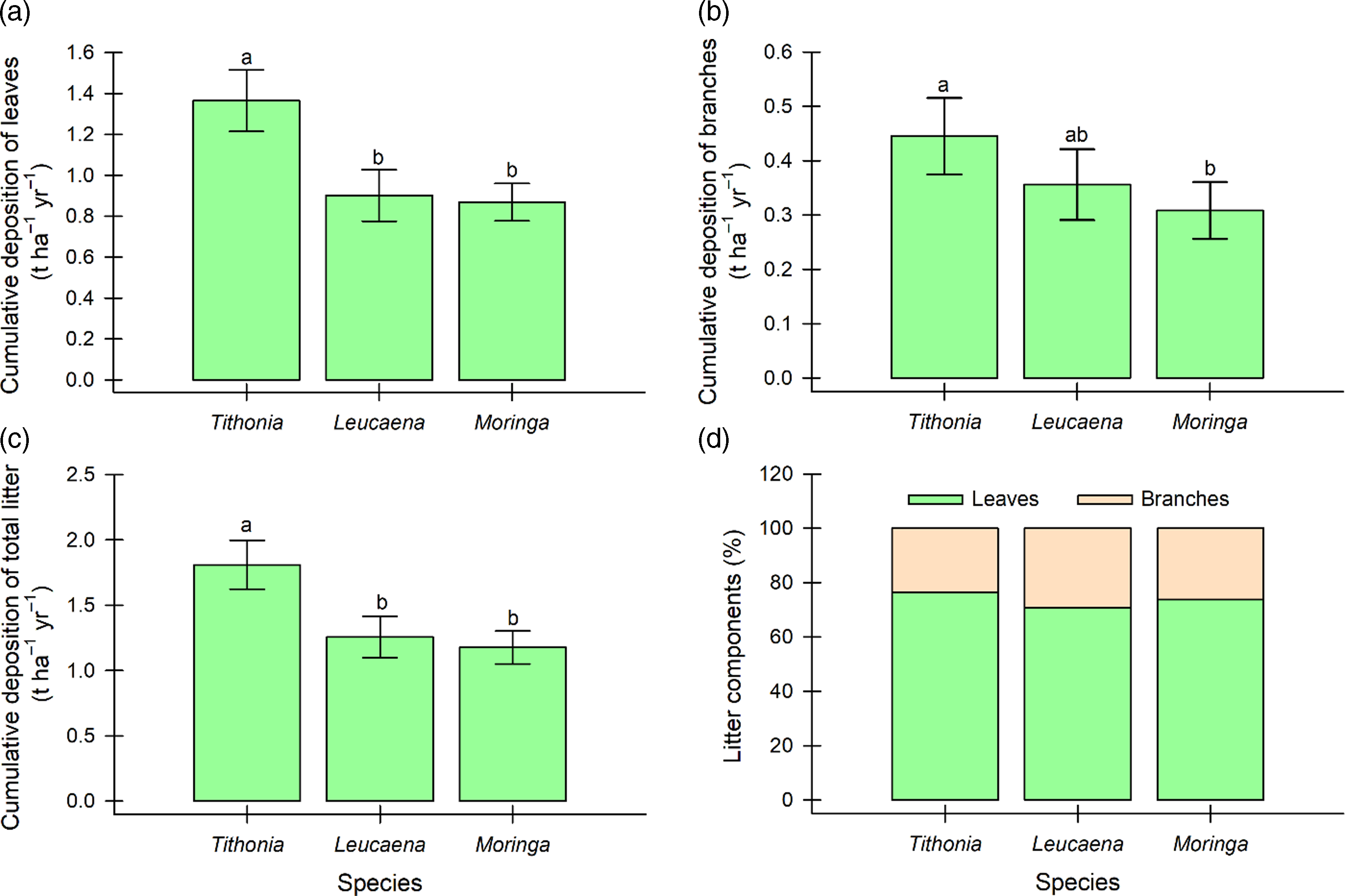
Figure 3. Deposition of leaves (a), branches (b), total (c), and the percentage of litter components (d) of Tithonia diversifolia, Leucaena leucocephala, and Moringa oleifera in fodder bank systems of southern Quintana Roo, Mexico; means ± standard error labelled by different letters are significantly different according to Tukey’s statistic (p < 0.05).
Chemical composition
The chemical composition of the litter showed significant differences between species (Table 1). In this regard, Leucaena had the highest contents of carbon and nitrogen (40.3 and 1.8%), compared to the other species. In contrast, Moringa and Tithonia had the highest phosphorus content (0.43 and 0.35%), compared to Leucaena (0.08%). However, the latter showed the lowest C:N ratio (22.07) compared to Moringa and Tithonia with values of 25.46 and 23.28 (Table 1).
Table 1. Average content of carbon (C), nitrogen (N), and phosphorus (P); the C:N ratio of Tithonia diversifolia, Leucaena leucocephala, and Moringa oleifera litter in fodder bank systems FBS of southern Quintana Roo, Mexico
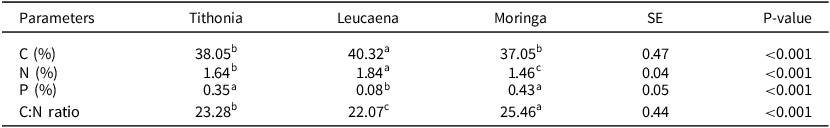
Means followed by different letters indicate significant differences according to Tukey’s statistic (p ≤ 0.05). SE, standard error.
Nutrient return to the soil
The carbon, nitrogen, and phosphorus return through litter was different depending on the sampling months and the type of fodder banks, with a significant interaction between them (Figure 4). The nutrient deposits followed the same pattern observed in the litter deposition curve of the three FBS. All the species increased their contribution in September, and the fodder bank of Tithonia in that month had the highest observed deposition of carbon (0.197 t ha–1), nitrogen (0.0087 t ha–1), and phosphorus (0.0018 t ha–1). Likewise, the lowest contribution for all species occurred in March, when the Moringa fodder bank recorded the lowest carbon and nitrogen depositions, while the lowest phosphorus contribution was detected in Leucaena (0.0004 t ha–1).
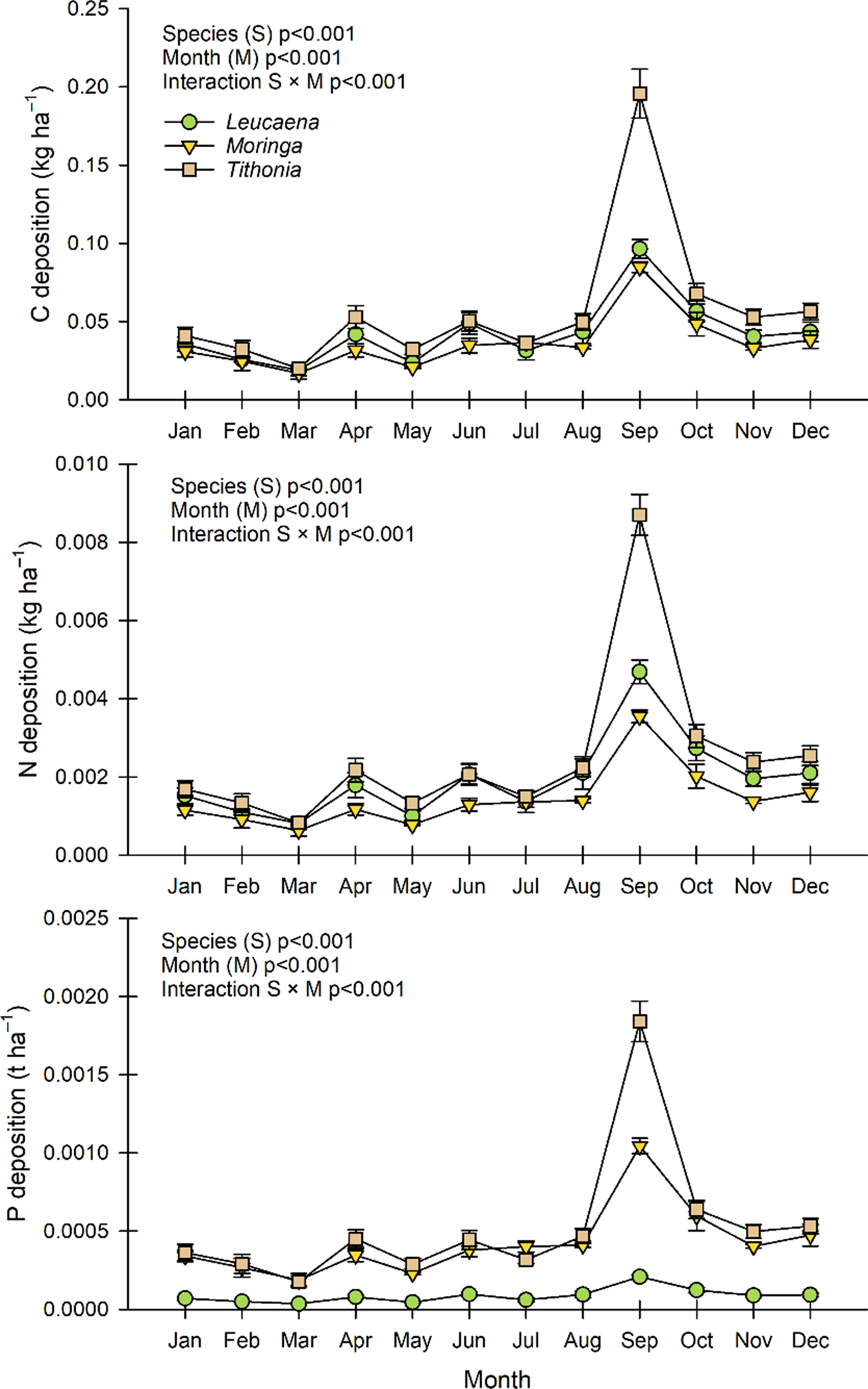
Figure 4. Monthly deposition of carbon (C), nitrogen (N), and phosphorus (P), from Tithonia diversifolia, Leucaena leucocephala, and Moringa oleifera litter in fodder bank systems of southern Quintana Roo, Mexico. Means ± Standard error.
In addition, the fodder bank of Tithonia cumulatively deposited a greater amount (p < 0.05) of carbon (0.687 t ha–1 yr–1), nitrogen (0.30 t ha–1 yr–1), and phosphorus (0.006 t ha–1 yr–1) in the soil, compared to Leucaena and Moringa (Figure 5).
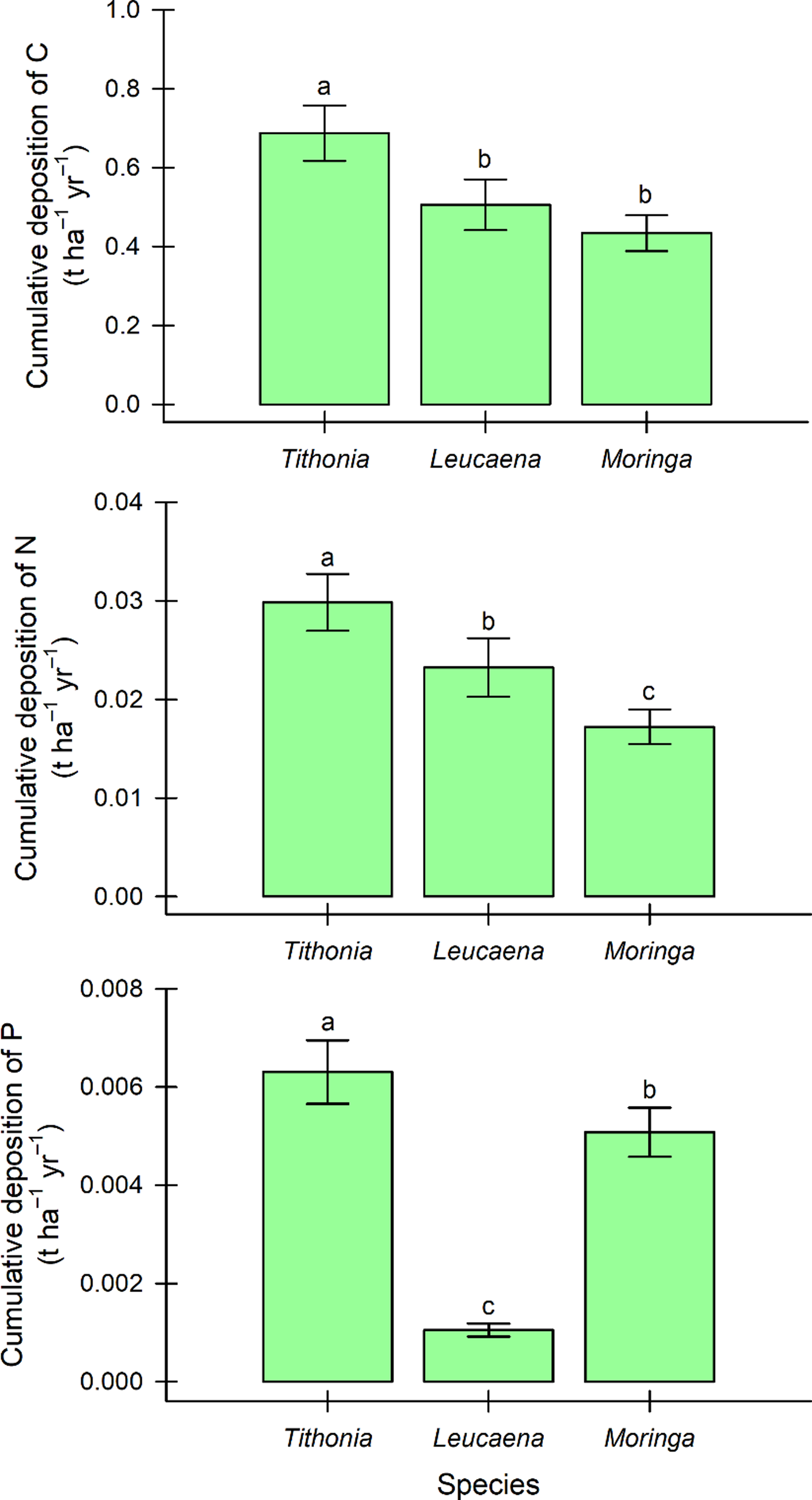
Figure 5. Accumulated deposition of carbon (C), nitrogen (N), and phosphorus (P), from Tithonia diversifolia, Leucaena leucocephala, and Moringa oleifera litter in fodder bank systems of southern Quintana Roo, Mexico; means ± standard error labelled by different letters are significantly different according to Tukey’s statistic (p < 0.05).
Relationship between environmental variables and litter deposition
On the other hand, the Pearson correlation analysis showed that the monthly rainfall during the experimental period was positively related to the litter deposition of Leucaena (r = 0.88, p < 0.001), Moringa (r = 0.87, p < 0.001), and Tithonia (r = 0.83, p < 0.001). However, the maximum and minimum temperatures did not show any correlation (p > 0.05) with the monthly litter deposition (Table 2).
Table 2. Pearson correlation analysis between environmental variables and monthly litter deposition (kg ha–1), of Tithonia diversifolia, Leucaena leucocephala, and Moringa oleifera in fodder bank systems of southern Quintana Roo, Mexico

***, p < 0.001; NS, not-significant (p > 0.05).
Discussion
Litter production
The greatest litter deposition in the months of maximum rainfall (September and October) in the fodder bank of Leucaena, Moringa, and Tithonia, coincided with the monthly dynamics of litter deposition of perennial and deciduous species in plantations and tropical agroforestry systems (Pennington and Sarukhán, Reference Pennington and Sarukhán2005; Kumar, Reference Kumar, Batish, Kohli, Jose and Singh2008; Rivera-Vázquez et al., Reference Rivera-Vázquez, Soto-Pinto, Núñez-Colín, De Jong, Hernández-Rivera and Ordóñez-Díaz2013). However, it is contrary to the behaviour of the predominant vegetation in the region (medium and low sub-evergreen and deciduous forest), where the greatest deposition of leaf litter occurs in March and April, the driest months of the year (Miranda and Hernández, Reference Miranda and Hernández1963; Aryal et al., Reference Aryal, De Jong, Ochoa-Gaona, Mendoza-Vega and Esparza-Olguin2015; García-Domínguez et al., Reference García-Domínguez, Cámara-Cabrales, Van Der Wal and Martínez-Sánchez2020), due to the deciduous tree species that make up the natural vegetation (Miranda and Hernández, Reference Miranda and Hernández1963; Pennington and Sarukhán, Reference Pennington and Sarukhán2005; García-Domínguez et al., Reference García-Domínguez, Cámara-Cabrales, Van Der Wal and Martínez-Sánchez2020). The greater litter production in September could also be directly related to its tolerance to pruning, domestication status, and regrowth capacity during rainy months (Lugo et al., Reference Lugo, Molina, Gonzáles, Gonzáles and Sánchez2012). In addition, the plants of the three species respond favourability to humidity and management practices (prunes), indicating that the optimal conditions for the growth of these species are met in this period (Castillo-Mestre et al., Reference Castillo-Mestre, Betancourt-Bagué, Toral-Pérez and Iglesias-Gómez2016; Vargas-Velázquez et al., Reference Vargas-Velázquez, Pérez-Hernández, López-Ortiz, Castillo, Cruz and Jarillo2022).
Other studies have also reported that factors such as harvest age and height influence biomass production and the quality of forage plants (Gallego-Castro et al., Reference Gallego-Castro, Mahecha-Ledesma and Ángulo-Arizala2017; Guatusmal-Gelpud et al., Reference Guatusmal-Gelpud, Escobar-Pachajoa, Meneses-Buitrago, Cardona-Iglesias and Castro-Rincón2020). For example, Partey (Reference Partey2011), Guatusmal-Gelpud et al. (Reference Guatusmal-Gelpud, Escobar-Pachajoa, Meneses-Buitrago, Cardona-Iglesias and Castro-Rincón2020), and Gallego-Castro et al. (Reference Gallego-Castro, Mahecha-Ledesma and Ángulo-Arizala2017) found higher biomass production by Tithonia at 80 days after cutting and at 50–60 cm of height; they also showed a trend of higher production at older plant ages.
In particular, the biomass deposition results obtained for Tithonia in this research were lower than those reported by Gallego-Castro et al. (Reference Gallego-Castro, Mahecha-Ledesma and Ángulo-Arizala2017) of 19 t ha–1 yr–1, Guatusmal-Gelpud et al. (Reference Guatusmal-Gelpud, Escobar-Pachajoa, Meneses-Buitrago, Cardona-Iglesias and Castro-Rincón2020) of 30.6 t ha–1 yr–1, and Vargas-Velázquez et al. (Reference Vargas-Velázquez, Pérez-Hernández, López-Ortiz, Castillo, Cruz and Jarillo2022) who reported 9 t ha–1 yr–1. Regarding Leucaena, Sánchez et al. (Reference Sánchez, Crespo and Hernández2007) in tropical SPS, in Matanzas, Cuba, found that the litter production of this species reached 9.1 t ha–1 yr–1, which widely exceeded the production obtained in the present study. However, it does coincide with the 0.978 t ha–1 yr–1 produced in another SPS of Leucaena in the same country (Alonso et al., Reference Alonso, Ruiz, Febles and Achan2003). Such differences could be related to the age of the plants in the FBS, which were six years old for this study. According to Gallego-Castro et al. (Reference Gallego-Castro, Mahecha-Ledesma and Ángulo-Arizala2017), Guatusmal-Gelpud et al. (Reference Guatusmal-Gelpud, Escobar-Pachajoa, Meneses-Buitrago, Cardona-Iglesias and Castro-Rincón2020), and Vargas-Velázquez et al. (Reference Vargas-Velázquez, Pérez-Hernández, López-Ortiz, Castillo, Cruz and Jarillo2022), the leaf–stem ratio decreases as the plants grow because plants accumulate more stems or branches as a support structure, although this may vary depending on the climatic conditions. Petit-Aldana et al. (Reference Petit-Aldana, Casanova-Lugo, Solorio-Sánchez and Ramírez-Avilés2011) affirmed that the annual production of litter in FBS of Moringa and Leucaena was variable and reached up to 1.0 t ha–1 yr–1, which was like what we found in the present study. Despite the above, these findings showed that these species could become an optimal option to have forage for animal feeding throughout the year, particularly during the dry season when the availability of additional food is very limited.
Regarding the proportion of leaves (71.7–75.4%) and branches (24.6–28.3%) in the litter, the values found in this study were like those reported by Santa-Regina and Tarazona (Reference Santa-Regina and Tarazona2001) in various agroecosystems, which comprised between 50 and 80% of the total litter components. Although other studies (González-Rodríguez et al., Reference González-Rodríguez, Domínguez-Gómez, Cantú-Silva, Gómez-Meza, Ramírez-Lozano, Pando-Moreno and Fernández2011; Domínguez, Reference Domínguez2009; López et al., Reference López, González, Ramírez, Cantó, Gómez, Pando and Estrada2013; García-Domínguez et al., Reference García-Domínguez, Cámara-Cabrales, Van Der Wal and Martínez-Sánchez2020) have reported proportions of 40–86% in natural ecosystems, showing that FBS produce important amounts of organic matter to the soil, in a way like natural forests. These findings may be related to the harvesting height of the plant for this study of 60 cm, which allowed a greater number of stems to remain and consequently, a greater number of buds that become stems to give way to the production of leaves (Guatusmal-Gelpud et al., Reference Guatusmal-Gelpud, Escobar-Pachajoa, Meneses-Buitrago, Cardona-Iglesias and Castro-Rincón2020). Other studies (Ella et al., Reference Ella, Blair and Stür1991; Stür et al., Reference Stür, Shelton, Gutteridge, Gutteridgeand and Shelton1994) have also reported that older plants have a greater number of ramifications due to a greater number of buds and reserves, allowing more regrowth, regardless of the remaining leaves on the plants after cutting.
Concerning the effect of pruning on litter production, no significant decrease was observed, except in March, which could be related to the strongest period of drought in the study region. However, the phenological patterns of the plants were modified; so, the number and weight of reproductive structures (flowers, fruits, and seeds) were too small to be considered in the composition of the litter. Likewise, Harmand et al. (Reference Harmand, Forkong, Bernhard–Reversat and Puig2004) and Petit-Aldana et al. (Reference Petit-Aldana, Casanova-Lugo, Solorio-Sánchez and Ramírez-Avilés2011) found that the management of the system influences litter production, where periodic pruning decreases the deposition of litter dumped on the ground by up to 40% compared to trees without pruning. This is because the carbohydrate reserves are reallocated after pruning, and the plant invests more resources in producing new branches and leaves, altering the growth patterns of the plant (Casanova-Lugo et al., Reference Casanova-Lugo, Caamal, Petit, Solorio and Castillo2010). In addition to the above, our findings could indicate that the three evaluated species have a high range of adaptation to the edaphoclimatic conditions of the study region, great capacity to recover nutrients from the soil and low demand for inputs for their cultivation that potentiate their forage yields during the year.
Chemical composition
The differences in the chemical composition of the evaluated species are attributable to their intrinsic characteristics and their environmental interaction (Petit-Aldana et al., Reference Petit-Aldana, Casanova-Lugo, Solorio-Sánchez and Ramírez-Avilés2011). For example, Leucaena, as a legume, can associate symbiotically with N-fixing bacteria of the genus Rhizobium. In addition to increasing this nutrient, it reduces the contribution of phosphorus, which is used in nodulation and consumed by the bacterial symbiont. In addition, this species has a pivoting and dense root system, which facilitates its regrowth, a greater resprouting of aboveground forage biomass, and higher carbon and nitrogen contents (Casanova-Lugo et al., Reference Casanova-Lugo, Caamal, Petit, Solorio and Castillo2010).
In the case of Tithonia, it is part of the early pioneer flora of the study area, presents great adaptation as a fodder bank and is a great litter contributor, which corresponds to its Mesoamerican origin and its productivity in different soil and climate conditions (González-Castillo et al., Reference González-Castillo, Hahn von-Hessberg and Narváez-Solarte2014). This species has been described as having a low carbon content but a high phosphorus content (Lezcano et al., Reference Lezcano, Soca, Ojeda, Roque, Fontes, Montejo, Santana, Martínez and Cubillas2012; Guatusmal-Gelpud et al., Reference Guatusmal-Gelpud, Escobar-Pachajoa, Meneses-Buitrago, Cardona-Iglesias and Castro-Rincón2020) as found in this study. This is due to its dense and superficial root system, with the ability to explore and capture the greatest amount of nutrients that are translocated in the humus accumulation zone to the formation of deciduous aerial structures. These, in turn, can easily be humified by the sequential action of the macro-, meso-, and micro-fauna of the soil, compared to other woody species. Due to these qualities, the species has been pointed out as a soil improver due to its excellent biomass yields, its high nitrogen, phosphorus, and potassium content and its rapid decomposition capacity in the soil (Crespo et al., Reference Crespo, Ruiz and Álvarez2011). Other studies (Gallego-Castro et al., Reference Gallego-Castro, Mahecha-Ledesma and Ángulo-Arizala2017; Cardona-Iglesias et al., Reference Cardona-Iglesias, Mahecha-Ledesma and Angulo-Arizala2017) in SPS have also reported values of 0.27% and 0.35% phosphorus, respectively, like what was found in this study. These values can be considered high compared to other species used in animal feed (Mahecha and Rosales, Reference Mahecha and Rosales2005).
Moringa oleifera showed slightly lower nitrogen contents than the other two species. These results coincide with reports that it is surpassed by legumes such as Gliricidia sepium and Piscidia piscipula (L.) Sarg.; however, it stands out as an extractor of deep nutrients from the soil and as a reserve of water and nutrients (Casanova-Lugo et al., Reference Casanova-Lugo, Cetzal-Ix, Díaz-Echeverría, Chay-Canul, Oros-Ortega, Piñeiro-Vázquez and González-Valdivia2019). In dry areas, Moringa promotes interactions with mycorrhizal fungi, such as Rhizoglomus (Glomus) intraradices (N.C. Schenck & G.S. Sm.) Sieverd., G.A. Silva & Oehl, and Sclerocystis (Glomus) sinuosum Gerd. & B.K. Bakshi, which favour the acquisition of water and phosphorus from the soil (Oros-Ortega et al., Reference Oros-Ortega, Lara-Pérez, Casanova-Lugo, Díaz-Echeverría, Villanueva-López, Ramírez-Barajas, Cetzal-Ix, Varma, Tripathi and Prasad2020); therefore, being exotic, inoculation should be considered to improve production efficiency in tropical SPS.
In general, organic materials with nitrogen concentrations greater than 20 mg·g–1 are of high quality, although lignin and polyphenol contents must also be considered. Phosphorus contents higher than 2.5 mg·g–1 can also be considered of high quality (Petit-Aldana et al., Reference Petit-Aldana, Casanova-Lugo, Solorio-Sánchez and Ramírez-Avilés2011). The results of this study show the importance of combining the three species in FBS, to achieve the contribution of carbon, nitrogen, and phosphorus through the incorporation of litter to the soil and thus contribute to its fertility and the ecological dynamics of the SPS.
The physical and structural characteristics of the plant species (i.e., leaf area, the moisture content in the leaves), as well as the phenology and even the taxonomic affinity of the species (family to which it belongs), could explain the monthly variations and leaf composition chemistry (Sánchez-Silva et al., Reference Sánchez-Silva, De Jong, Aryal, Huerta-Lwanga and Mendoza-Vega2018). Given that the forage banks were subjected to frequent pruning for their use (i.e., every three months), removing 70–80% of the foliage, management could influence the productive attributes of litter depending on the characteristics inherent to the crop species, but they were not analysed in this study, which could limit its scope.
Nutrient return to the soil
The values obtained coincide with what was reported by Jha and Prasad-Mohapatra (Reference Jha and Prasad-Mohapatra2010), who point out that in Leucaena plantations they obtained values ranging from 0.0023 to 0.0762 t ha–1 yr–1 nitrogen and from 0.0018 to 0.0514 t ha–1 yr–1 phosphorus. In tropical forage banks, Petit-Aldana et al. (Reference Petit-Aldana, Casanova-Lugo, Solorio-Sánchez and Ramírez-Avilés2011) reported contributions of carbon, nitrogen, and phosphorus of 0.0124, 0.0010, and 0.00004 t ha–1·yr–1 for Moringa and 0.4344, 0.0210, and 0.0010 t ha–1·yr–1 for Leucaena, respectively. The above shows the important contribution of these production systems, considering that the soils of the region are poor in these nutrients, due to their karstic origin, with calcium carbonate contents (CaCO3) greater than 70% (Cabadas et al., Reference Cabadas, Solleiro, Sedov, Pi and Alcalá2010). Furthermore, in the case of phosphorus, it is known that the main sources of this element in terrestrial ecosystems come from the transport of dust from Africa, marine sediments, and volcanic remains, among other sources (Cabadas et al., Reference Cabadas, Solleiro, Sedov, Pi and Alcalá2010; Estrada-Medina et al., Reference Estrada-Medina, Jiménez-Osornio, Álvarez-Rivera and Barrientos-Medina2019). Therefore, the use of species such as Tithonia and Moringa can contribute to the deposition of phosphorus, which is essential for soil fertility. However, the scientific literature regarding the nutrient contribution of Tithonia in the modality of FBS is still scarce. Despite the above, these findings show potential application; even in moderate contributions of nutrients, from the integration of species such as Tithonia, Moringa, and Leucaena, in FBS, compared to natural ecosystems (Aryal et al., Reference Aryal, De Jong, Ochoa-Gaona, Mendoza-Vega and Esparza-Olguin2015). Likewise, they showed the potential to promote more efficient nutrient recycling due to the high quality of the litter and thus promote the strategic recovery of degraded areas, as mentioned by Sileshi et al. (Reference Sileshi, Mafongoya and Nath2020) and Williams-Linera et al. (Reference Williams-Linera, Bonilla-Moheno, López-Barrera and Tolome2021).
Relationship between environmental variables and litter deposition
In the present study, a positive and statistically significant association was observed between the monthly litter production of the species and the precipitation, probably due to a foliage renewal process preceded by the rains or maximum precipitation, as pointed out by Bonilla et al. (Reference Bonilla, Belisario-Roncallo and García2008). Indeed, it agrees with what was reported by Petit-Aldana et al. (Reference Petit-Aldana, Casanova-Lugo, Solorio-Sánchez and Ramírez-Avilés2011), who pointed out that the highest litter production in FBS in Yucatan, Mexico, occurred in the periods of highest rainfall, just when the greatest decomposition occurs due to relative humidity and the greatest presence of arthropods and earthworms (Bonilla et al., Reference Bonilla, Belisario-Roncallo and García2008).
Despite the above, our results are inconsistent with what was reported by Sánchez et al. (Reference Sánchez, Crespo and Hernández2007, Reference Sánchez, Lama and Suantunce2008), who did not find a significant correlation between litter production and precipitation, which depends on the caducifolious character of species studied, where the highest litter production values occurred during the natural fall of the leaves that occurs at the end of the year, where temperatures and humidity are low. Likewise, our findings are also distinct from what was reported by Sánchez et al. (Reference Sánchez, Lama and Suantunce2008), who pointed out that precipitation and temperature appear as the most decisive climatic variables in the production and decomposition of litter. This is because they influence both the development of vegetation and the activities of microorganisms, which are fundamental factors in soil formation. Furthermore, these differences could be directly related to the management, prevailing climatic conditions at the site, and the specific response of the three species.
Conclusion
Litter production showed a similar trend in the three species evaluated during the study period, with an increase in September. Tithonia diversifolia was the most productive species and showed the highest contributions of carbon, nitrogen, and phosphorus under the fodder bank silvopastoral design. Regarding the return of carbon, nitrogen, and phosphorus, all species followed the same trend observed in the litter deposition curve which showed that both measures can work like a correlated variable.
Finally, it can be considered that the potential biomass yield and the contribution of nutrients to the soil shown by the three species gave them a high productive potential in tropical regions. Therefore, in the future, studying the combination of Tithonia, Leucaena, and Moringa for the complementary effects, could compensate for or reinforce the reintegration of nutrients to the soil in agroecosystems that include trees and/or shrubs as fodder banks. Moreover, our results suggest that leaving the remains of the forage bank on the ground like cycling nutrients can be an alternative for managing soil fertility instead of hauling forage for livestock. This can be a viable alternative to produce food and ecosystem services because these species can sustainably enhance soil fertility and animal production without agrochemical adds and subsequent contamination.
Acknowledgements
The authors thank Tecnológico Nacional de México for financial support throughout this project (No. 14328.22-P and 1129.21-P). We are also grateful to the Consejo Nacional de Humanidades, Ciencias y Tecnologías for financing infrastructures project (No. 316492) to carry out field and laboratory works and obtain a M. Sc. degree (of the first author) in Sustainable Agroecosystems.
Competing interests
The authors state that they have no conflicts of interest.









