Introduction
The common liverwort is a thalloid, spore-bearing bryophyte that belongs to the family Marchantiaceae (Durand Reference Durand1908; Budke et al. Reference Budke, Bernard, Gray, Huttunen, Piechulla and Trigiano2018). It grows by developing a flat thalloid structure with the lower surface of the thallus consisting of rhizoids and scales that assist in moisture and nutrient absorption while anchoring it to the growing medium (Budke et al. Reference Budke, Bernard, Gray, Huttunen, Piechulla and Trigiano2018). The liverwort life cycle consists of a sporophytic (sexual) stage and a gametophytic (vegetative) stage (Bold et al. Reference Bold, Alexopoulos and Delevoryas1987; Newby Reference Newby2006). In the sporophytic stage, sperm cells from antheridia (male sexual structure) produced on stalked antheridiophores fertilize the egg cell of archegonia (female sexual structure) borne on a stalked archegoniophore, resulting in the production of spores (Newby Reference Newby2006) (Figure 1). The gametophytic or asexual life cycle begins with the germination of spores formed in the sporophytic stage. Spore germination depends on light availability (Heald Reference Heald1898), and day lengths of 10 h or more are required for germination (Nakazato et al. Reference Nakazato, Kadota and Wada1999). Cup-like structures called gemmae cups are formed on the surface of thalli during the gametophytic life cycle, during which the plant propagates asexually by producing propagules called gemmae (Newby Reference Newby2006; Simpson Reference Simpson2019) (Figure 2). Numerous gemmae are released from each gemmae cup to the immediate surroundings by splashing with irrigation or rainwater (Svenson et al. Reference Svenson, Smith and Briggs1997).

Figure 1. The red arrows are pointed at the male antheridia borne on stalked antheridiophores, and the yellow arrows are pointed at the umbrella-like female archegonia borne on stalked archegoniophores on separate thalli of common liverwort under greenhouse conditions.

Figure 2. The gametophytic life cycle of common liverwort is represented by circular gemma cups (yellow arrows) that contain numerous gemmae.
Liverwort spreads rapidly in nurseries and greenhouses due to its ability to reproduce both sexually via spores and asexually via gemmae (Ross and Puritch Reference Ross and Puritch1981). Liverwort is considered a weed by nursery and greenhouse ornamental container growers because it thrives in environments with low ultraviolet (UV) radiation, high humidity, soil moisture, and fertility (Newby et al. Reference Newby, Altland, Gilliam and Wehtje2006). It competes with ornamental plants for soil/growing medium, water, nutrients, space, and oxygen within the container, and it obstructs the movement of water into the root zone. Ultimately, liverwort reduces the quality and market value of ornamental crops (Svenson et al. Reference Svenson, Smith and Briggs1997). Few herbicides are labeled for use in greenhouses, and even fewer are available for controlling liverwort. Some herbicides such as by quinoclamine and diuron have shown promising results for their ability to control liverwort but they are not registered for greenhouse use thus far in the United States (Altland et al. Reference Altland, Wehtje, Gilliam and Miller2007; Newby et al. Reference Newby, Gillian, Wehtje and Altland2005). Hand-weeding of liverwort is a laborious and time-consuming task. Additionally, in container nursery production, herbicides are applied at higher than recommended rates and multiple times for weed control (Pickens et al. Reference Pickens, Marble and Li2021), which could cause phytotoxicity to sensitive ornamental plants and can have residual effects on the environment.
Therefore, it is important to study alternative methods of liverwort management. Strategic placement of controlled release fertilizers (CRFs) is a nonchemical, cultural method of weed control that refers to adding CRFs to containerized plants in a manner that is different from the traditional practice of mixing fertilizer into the substrate, or simply top-dressing the medium with the fertilizer (Di Tomaso Reference Di Tomaso1995). Alternative methods of fertilizer placement that can influence weed management include dibbling (placing fertilizer in a pocket directly below the root zone at a depth of a few centimeters) or subdressing the CRF (placing the CRF in a uniform layer a few centimeters below the root zone) (Stewart et al. Reference Stewart, Marble, Jackson, Pearson and Wilson2018). The strategic placement of fertilizer near the root zone has been shown to provide a competitive advantage over traditional methods of fertilizer application by reducing weed growth in agronomic (Chauhan and Ahugho Reference Chauhan and Ahugho2013; Mashingaidze et al. Reference Mashingaidze, Lotz, Van der Werf, Chipomho, Kropff and Nabwami2012) and ornamental crops (Fain et al. Reference Fain, Knight, Gilliam and Olive2003; Marble et al. Reference Marble, Koeser and Hasing2015). Strategic placement of CRFs can reduce liverwort growth by limiting their access to nutrients because their rhizoids are relatively short, while increasing the crop’s ability to access nutrients, which ultimately improves its competitive ability (Nkebiwe et al. Reference Nkebiwe, Weinmann, Bar-Tal and Muller2016; Di Tomaso Reference Di Tomaso1995). In a study conducted by Khamare et al. (Reference Khamare, Marble, Pearson, Chen and Devkota2023), the growth of bittercress (Cardamine hirsuta L.) and liverwort increased in top-dressed (fertilizer applied in a layer on the top of the substrate) containers versus containers in which CRFs were mixed with the substrate. Saha et al. (Reference Saha, Marble, Torres and Chandler2019) reported that CRF dibbling and subdressing resulted in reduced growth and seed production of eclipta [Eclipta prostrata (L.) L.], large crabgrass [Digitaria sanguinalis (L.) Scop.], and spotted spurge (Euphorbia maculata L.) compared to the industry standard practice of CRF incorporation or top-dressing. Incorporating CRFs in the medium resulted in increased germination of spotted spurge (from 77% to 183%) versus subdressing and dibbling. In similar studies conducted by Altland et al. (Reference Altland, Fain and Von Arx2004) and Broschat and Moore (Reference Broschat and Moore2003), weed germination and growth were both reduced with CRF dibbling. Another study demonstrated that dibble CRF placement also resulted in faster plant establishment and superior plant quality (Meadows and Fuller Reference Meadows and Fuller1983).
Liverwort growth is directly correlated with increasing nitrogen levels (Svenson Reference Svenson1998). Liverwort establishment slows down at nitrogen application rates <75 mg L–1 (Svenson et al. Reference Svenson, Smith and Briggs1997), but that amount of nitrogen is usually not sufficient for growth of ornamental crops. For example, poinsettia (Euphorbia pulcherrima Willd. Ex Klotzsch), zonal geranium [Pelargonium zonale (L.) L’Her.ex Aiton], and ivy geranium [Pelargonium peltatum (L.) L’Her.ex Aiton] require 250, 250, and 250 mg L–1 of nitrogen, respectively, for optimal growth (Cox Reference Cox1997). So, altering the placement of CRFs within containers can aid in reducing the growth of traditional weeds. However, additional research is required to determine whether varying the location and depth of CRFs can help to control liverwort in container production and not negatively influence crop growth. Therefore, this study was undertaken to evaluate the effectiveness of various methods of CRF placement including incorporation, subdressing, and dibbling on liverwort control in containerized greenhouse production.
Materials and Methods
Experiments were conducted in a greenhouse with polycarbonate sidewalls and a double-layer polyethylene roof at the Michigan State University Horticulture Teaching and Research Center in 2021 and 2022. The minimum, maximum, and average daily temperatures throughout the study were 21.0, 26.6, and 23.8 C, respectively. The study was conducted in a greenhouse because it allowed temperature and irrigation management and reduced pest pressure and weed competition compared to outdoor studies (Gallina et al. Reference Gallina, Cregg, Patterson, Hill and Saha2023).
Experiment 1: CRF Placement
Containers were filled with a commercial medium consisting of 70% peat moss, 21% perlite, and 9% vermiculite (Suremix; Michigan Grower Products Inc., Galesburg, MI). It was amended with a CRF of N:P:K 17-5-11 (Osmocote ICL Specialty Fertilizers, Dublin, OH) with an 8- to 9-mo release period and applied at the manufacturer’s recommended highest labeled rate (35 g per 3.8 L). Each 5.7-L container (East Jordan Plastics Inc., East Jordan, MI) received 52.5 g of CRF. Strategic CRF placement in the containers included top dressing, subdressing, medium incorporation, and dibbling. For top-dressing CRFs were added to the top layer of the medium. Incorporation consisted of thoroughly mixing the CRF with the medium and then filling the container. For subdressing, the CRF was added at depths of 2.5, 5.1, and 7.6 cm from the top. For the dibble method, the CRF was placed into small pockets at depths of 2.5, 5.1, and 7.6 cm from the top. Finally, a nontreated control did not receive any fertilizer.
After 1 or 2 d, gemmae of common liverwort were applied every other week throughout the study, to the top of the medium in each container. For gemmae collection, gemmae cups were scraped off vigorous liverwort stock plants and put into a bowl of tap water, thus releasing gemmae upon separation from their clumps. A spoon was used to apply approximately 5 mL of water from the bowl, which contained gemmae, across the surface of each container. Overhead sprinklers inside the greenhouse provided all containers with approximately 0.51 cm of irrigation twice daily. The percentage of container surface covered by liverwort thalli was visually estimated at 2, 4, 6, 8, 10, and 12 wk after treatment (WAT). At 12 wk, the number of gemma cups (asexual reproductive structures) produced on the liverwort thallus were counted in each container. The liverwort thalli were allowed to continue to grow and monitored on a regular basis to identify the development of sexual reproductive structures. After approximately 28 wk, the number of sexual reproductive structures (male, antheridiophores; female. archegoniophores) were recorded in each container to determine any differential responses. At the end of the experiment, the total liverwort fresh biomass was recorded.
The experiment was conducted in a completely randomized design and there were six single-container replications per treatment. The experiment was repeated twice over time and the data from both replications were pooled for statistical analysis, since there were no significant differences among the results of two runs. All data were analyzed using the GLIMMIX procedure with SAS software (v.9.4; SAS Institute, Cary, NC) to conduct an analysis of variance. The replications were considered as random effects and CRF placement methods were considered fixed effects. When ANOVA results revealed significant effects, mean comparisons for fixed factors were performed using Tukey’s HSD test to separate the means. All the effects were considered significant at α = 0.05. Furthermore, pair-wise contrasts were conducted between different fertilizer placement methods at 12 wk using the contrast statement with the MIXED procedure using SAS software, and results were expressed as P-values.
Experiment 2: Liverwort Competitiveness
In this experiment, 3.8-L containers (East Jordan Plastics) were filled with Suremix media. The Osmocote CRF (ICL Specialty Fertilizers) was applied at the highest labeled rate according to the manufacturer’s recommendation. CRF placement in the containers included the four previously mentioned methods. For subdressing and dibbling, we placed the CRF at only the 7.6-cm depth because results from Experiment 1 indicated that it was the most effective depth. Wax begonia, a dicot, and dracaena spike, a monocot, were planted after the fertilizer was added to the medium. Containers were irrigated daily with approximately 1.02 cm of water via overhead sprinkles inside the greenhouse.
After 1 or 2 d, gemmae of common liverwort were applied on top of the medium in each container. One week after planting, gemmalings were thinned to contain 0, 3, or 9 per container. Different densities of gemmalings per container were considered a treatment factor. Another treatment factor was the presence or absence of ornamental plants. Containers were hand-weeded throughout the experiment to ensure that no other weeds were growing in the nontreated control or treatment containers.
Growth indices of the ornamental plants were recorded at the initiation and conclusion of the study (2 and 12 wk after planting) by averaging the height of the plant and two crosswise widths from top of each plant. Percent increase in growth index of plants was measured using Equation 1:
At 12 wk after planting, liverwort thalli and ornamental plant shoot fresh biomass were recorded.
The experiment was conducted in a 4 × 3 × 2 factorial arrangement with four fertilizer placement methods, three gemmae densities, and the presence or absence of an ornamental crop in a completely randomized design. There were four single-container replications per treatment, and the experiment was repeated in time. All data were analyzed using the GLIMMIX procedure with SAS software (v.9.4) to conduct an analysis of variance. The replications were considered as random effects and fertilizer placement methods, gemmae densities, and ornamental presence or absence were considered fixed effects. When ANOVA results revealed significant effects, mean comparisons for fixed factors were performed using Tukey’s HSD test. All the effects were considered significant at α = 0.05, to separate out the means.
Results and Discussion
Experiment 1: CRF Placement
The effect of fertilizer placement method significantly influenced (P < 0.001) liverwort coverage on the top of the medium surface from 2 to 12 WAT (Table 1). Subdressing and dibbling CRFs at greater depths (5.1 and 7.6 cm) had the most effect on reducing liverwort coverage, while the most liverwort coverage occurred in containers in which the CRF was incorporated into the media. At 12 WAT, liverwort coverage was 39% in containers that had CRFs subdressed at 7.6 cm, 34% in containers in which CRFs were dibbled at 7.6 cm, and 22% in the nontreated control containers. The most liverwort coverage (99%) occurred when CRF was incorporated, followed by 91% coverage when CRF was subdressed at 2.5 cm, 70% when CRF was dibbled at 2.5 cm, and 65% with top-dressing. Notably, all the fertilizer placement methods with the exception of incorporation demonstrated up to 30% liverwort coverage until 6 WAT (Table 1). A further analysis of pair-wise contrasts of various fertilizer placement methods at 12 WAT revealed that the effects of dibbling were significantly different in contrast to incorporation, top dressing, and no treatment. Similarly, the effects of subdressing were also significantly different from incorporation and the nontreated controls but not the top-dress treatment (Table 2). Given that liverwort thallus anchors to the top layer of the growing medium with rhizoids and scales (Budke et al. Reference Budke, Bernard, Gray, Huttunen, Piechulla and Trigiano2018) that mostly obtain moisture and nutrients from the top 1 to 2 cm of the medium, CRF subdressing and dibbling provided significant control of liverwort. A previous study (Hoskins et al. Reference Hoskins, Owen and Niemiera2014) reported that when CRF top-dressing and incorporation were applied at the same rate to containers, CRF incorporation rapidly released nutrients compared to top-dressing. Similar results were observed by Khamare et al. (Reference Khamare, Marble and Chandler2020), who indicated that subdressing at a depth of 7.5 cm effectively reduced the growth of eclipta by 50% in comparison to top-dressing. However, subdressing at 7.5 cm reduced the growth of little leaf boxwood (Buxus microphylla Siebold & Zucc.) and glossy privet (Ligustrum lucidum W.T. Aiton), when growing in competition with eclipta. Other studies have also found improved weed control from dibbling compared to top dressing (Altland and Fain Reference Altland and Fain2003; Altland et al. Reference Altland, Fain and Von Arx2004; Fain et al. Reference Fain, Knight, Gilliam and Olive2003).
Table 1. Liverwort coverage on top of the container surface from 2 to 12 WAT as affected by different fertilizer placement methods. a, b
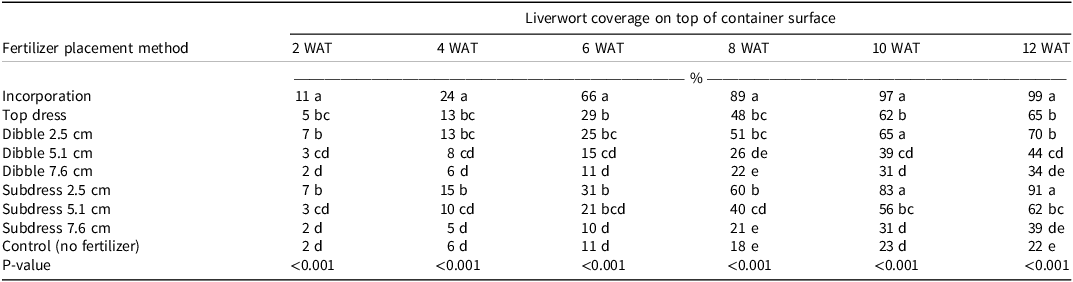
a Abbreviation: WAT, weeks after treatment.
b Means followed by the same letter are not significantly different according to Tukey’s HSD within a column (α = 0.05).
Table 2. Contrasts between different fertilizer placement methods at 12 wk after treatment for the effects of fertilizer placements on liverwort coverage

In the current study, the number of gemmae cups and antheridiophores was significantly influenced by the CRF placement method (Table 3). At 12 WAT, the greatest number of gemmae cups were recorded with CRF incorporation (284) and top-dressing (231). The number of gemmae cups was lowest when CRFs were dibbled at depths of 7.6 cm and 2.5 cm, when 90 and 124 cups, respectively, were counted, which was similar to the number of gemmae cups (55) counted in the nontreated control. In contrast, the number of antheridiophores was significantly higher when CRFs were dibbled at 5.1 cm and subdressed at 7.6 cm (259 and 228, respectively), compared to top-dressing (63). The fresh biomass of liverwort thallus in containers was also significantly greater under either of the CRF placement methods compared with the nontreated control. The fresh biomass of liverwort from the nontreated control was 11 g, followed by 61g from dibbling at 5.1 cm, 32 g from dibbling at 7.6 cm, and 38 g from subdressing at 7.6 cm (Table 3). The greatest liverwort biomass was recorded in containers that were top-dressed, incorporated, subdressed at 2.5 gm, and dibbled at 2.5 cm. A similar study conducted by Altland and Fain (Reference Altland and Fain2003) reported that weed shoot dry weight was 60% lower in containers in which CRFs were dibbled as opposed to being incorporated or top-dressed.
Table 3. Number of gemmae cups, male structures, female structures, and fresh mass of liverwort under different fertilizer placement methods at 12 WAT. a, b
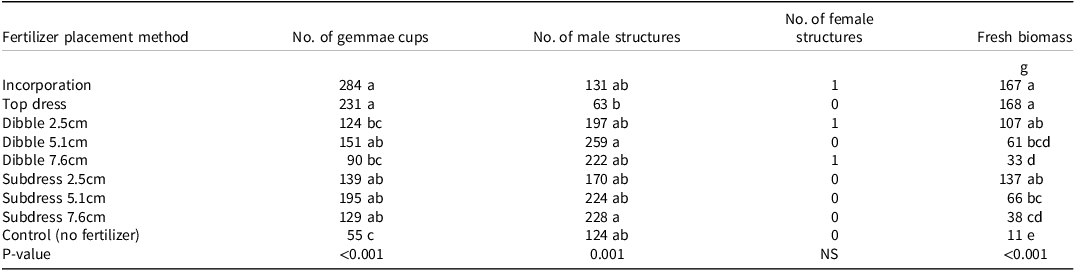
a Abbreviations: WAT, weeks after treatment; NS, nonsignificant,
b Means followed by the same letter are not significantly different according to Tukey’s HSD within a column (α = 0.05).
A possible explanation for the greater number of male reproductive structures recorded from dibbling and subdressing could be that under nutrient stress conditions, liverwort may produce more reproductive structures to ensure its multiplication for subsequent generations. CRF placement method did not influence the number of archegoniophores, which was likely due to the fact that archegoniophores usually appear later than the antheridiophores in the liverwort sexual reproduction cycle (Newby Reference Newby2006), and the present study was terminated before all the archegoniophores may have appeared. The age of liverwort, photoperiod, and light intensity inside the greenhouse could also have influenced the number of female reproductive structures. These factors were not controlled in the study because they were out of the scope of our research objectives. Mache and Loiseaux (Reference Mache and Loiseaux1973) reported that light intensities ranging from 370 to 555 µmol·m–2·s–1 promoted vegetative growth of liverwort, whereas higher light intensities impeded it. In a separate study, asexual reproduction (gemmae production) occurred more rapidly at shorter daylengths (∼8 h) than under longer daylengths (17 to 18 h) (Voth and Hamner Reference Voth and Hamner1940). Long days, high light intensities, and natural diffused daylight promote formation of antheridiophores (Terui Reference Terui1981). Future experiments of longer duration and under more controlled environmental conditions may help to elucidate the response of liverwort to different fertilizer placement methods in terms of number of archegoniophores.
Experiment 2: Liverwort Competitiveness
The interaction of fertilizer placement method and liverwort gemmae density influenced the growth index and fresh biomass of the begonia (Table 4). At a liverwort gemmae density of 3 and 9 per container, the percentage increase in growth index among containers was significantly different from each other under different fertilizer placement methods. In CRF-incorporated containers, the growth index was significantly greater at higher liverwort gemmae pressure (density of 9) and in the absence of gemmae as compared to a gemmae density of 3. However, for top-dressed treatments the growth index was greatest when weed pressure was low (liverwort gemmae density 3). Broschat and Morre (Reference Broschat and Moore2003) reported that dibbling or subdressing had either a greater effect or no effect on the growth of Chinese hibiscus (Hibiscus rosa-sinensis), plumbago (Plumbago auriculata Lam.), and downy jasmine [Jasminum multiflorum (Burm.f.) Andrews]. Altland and Fain (Reference Altland and Fain2003) reported a reduction in the growth index of azalea (Rhododendron spp. L.) and holly (Ilex aquifolium L.) with CRF incorporation and top-dressing versus dibbling. Meadows and Fuller (Reference Meadows and Fuller1983) reported that higher-quality azalea (Rhododendron spp.) resulted when CRFs were dibbled compared to incorporated.
Table 4. Growth index and fresh mass of begonia as influenced by interaction between fertilizer placement methods and liverwort gemmae density. a, b
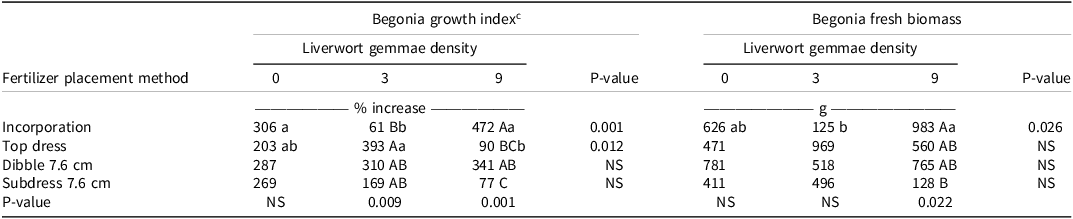
a Abbreviation: NS: nonsignificant.
b Means followed by different capital letters in column for gemmae density for each fertilizer placement methods or lower case letters in the row which compare a specific fertilizer placement method across gemmae densities are significantly different according to Tukey’s HSD (α = 0.05).
c The percent increase in begonia growth index was obtained using Equation 1.
The growth index of begonia was significantly different among various fertilizer placements at gemmae densities of 3 and 9, with incorporation and dibbling at 7.6 cm producing the highest percentage increase in growth index at a gemmae density of 9 (472% for incorporation, 341% for dibbling at 7.6 cm) (Table 4). The fresh biomass of begonia under different fertilizer placements was not significantly different from each other at gemmae densities of 0 and 3, but there was a significant difference at gemmae density 9 (Table 4). In the fertilizer-incorporated treatment, and at gemmae densities of 9 and 0, begonia plants exhibited a higher percent increase in growth index (472% and 306%, respectively) and higher plant biomass (983 g and 626 g, respectively). The overall higher fresh biomass recorded at the higher gemmae density of 9 compared with the lower densities of 0 and 3 may indicate that there was competition between liverwort and the ornamental plants in obtaining nutrients. Also, considering the size of the container, the fibrous roots of begonia were able to reach a depth below the top of the container, which enabled them to utilize nutrients from deeper placement zones (dibble and subdress) compared to liverwort, which has a shallow rhizoid system. Khamare et al. (Reference Khamare, Marble, Pearson, Chen and Devkota2023) investigated the effect of top-dressing versus incorporation with mulched or stratified medium on growth of hibiscus cultivar Snow Queen, and bittercress (Cardamine flexuosa With.) and liverwort. Both weeds exhibited greater growth with top-dressing compared to incorporation. Surface CRF application and/or dibbling provided better shoot growth compared to incorporation for sweet viburnum (Viburnum odoratissimum Ker Gawl.), azalea [Rhododendron obtusum (Lindl.) Planch ‘Hinodegiri’], and golden privet (Ligustrum × vicaryi) (Blessington et al. Reference Blessington, Garvey and Howell1981; Cobb Reference Cobb1985; Conover and Poole Reference Conover and Poole1985), whereas incorporation produced superior quality gardenia (Gardenia jasminoides J. Ellis ‘Radicans’) (Cobb Reference Cobb1985).
For dracaena, the percent increase in growth index was nonsignificant for effects of fertilizer placement method and liverwort gemmae density (Table 5). However, significant differences were observed in fresh biomass at the end of the experiment with different CRF placement methods and gemmae densities. When there was no weed pressure (gemmae density 0), the maximum fresh biomass was recorded when CRFs were subdressed at 7.6 cm (962 g), which was significantly different from all other treatments. Out of all the fertilizer placement methods investigated, the fresh mass of dracaena was significantly influenced by top-dressing and subdressing at 7.6 cm, and by varying liverwort gemmae densities (Table 5). Similar to begonia, dracaena have a fibrous root system that allows them to grow well below the container surface. Broschat and Morre (Reference Broschat and Moore2003) reported that subdressing (layering beneath the medium surface) or dibbling had an increased or no effect on the growth of bamboo palm (Chamaedorea seifrizii Burret), fishtail palm (Caryota mitis Lour.), Areca palm [Dypsis lutescens (H. Wendl.) Beentje & J. Dransf.], Alexandra palm [Archontophoenix alexandrae (F. Muell.) H. Wendl. & Drude], foxtail palm (Wodyetia bifurcata A.K. Irvine), and Macarthur palm (Ptychosperma macarthurii H. Wendl. Ex H.J. Veitch). Layering resulted in greater shoot dry mass than incorporation in Alexandra palm and foxtail palm. Out of all the species studied, only Areca palm performed best with incorporated fertilizer.
Table 5. Growth index and fresh mass of dracaena as influenced by interaction between fertilizer placement methods and liverwort gemmae density. a, b
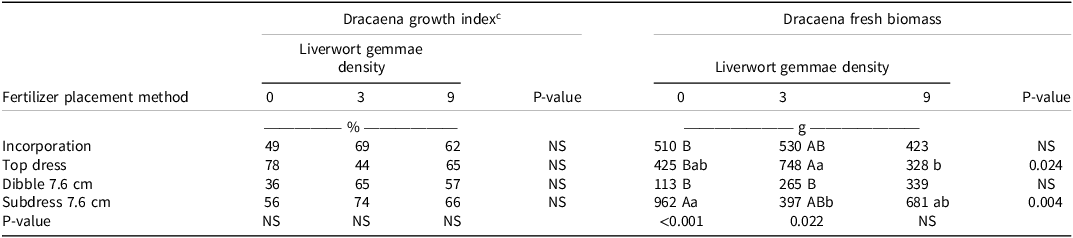
a Abbreviation: NS, nonsignificant.
b Means followed by different capital letters in column for gemmae density for each fertilizer placement methods or lower case letters in the row that compares a specific fertilizer placement method across gemmae densities are significantly different according to Tukey’s HSD (α = 0.05).
c The percent increase in dracaena growth index was obtained using Equation 1.
An analysis of variance of the effects of fertilizer placement method (F), gemmae density (D) and presence or absence of an ornamental (O) plant on the fresh biomass of liverwort (measured in grams) at 12 WAT, for both ornamental crops is presented in Table 6. For begonia, there were significant two-way interactions between F and O, and between D and O (Table 6). For dracaena, there was significant three-way interaction between F, D, and O for their effect on the fresh biomass of liverwort (P < 0.05; Table 6).
Table 6. Analysis of variance of the effects of fertilizer placement method, gemmae density, and the presence or absence of an ornamental plant on the fresh mass of liverwort in a container at 12 WAT. a
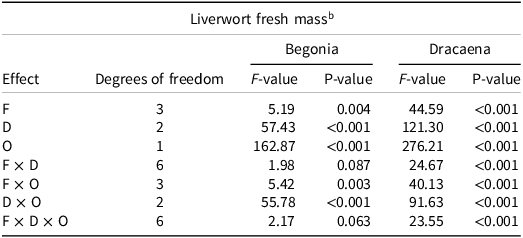
a Abbreviations: F, fertilizer placement method; D, gemmae density; O, presence/absence of ornamental; WAT, weeks after treatment.
b Liverwort fresh mass was measured in grams.
For begonia, there was no significant difference in liverwort fresh mass under different fertilizer placements or in the presence of the ornamental plant, but it was significantly different in the absence of the ornamental plant (Table 7). When no ornamental was present, there was a significantly lower liverwort fresh mass when CRFs were subdressed at 7.6 cm and dibbled at 7.6 cm (6.5 g and 9.1 g, respectively) compared to incorporation and top-dressing. Each of the fertilizer placement methods also had a significant influence on liverwort fresh biomass between the presence or absence of the ornamental plant. Liverwort fresh biomass was significantly higher under all the fertilizer placement methods when the ornamental plants were not present, and there was no competition for the resources within the container. There were no differences in liverwort fresh biomass under different fertilizer placements in the presence of begonia, which indicates that in plant-plant competition, neither of the fertilizer placements was promoting liverwort growth and the ornamental plant was able to use the fertilizer, whereas liverwort could not. However, the fresh mass of liverwort was greatest when no ornamental plant was present, and with surface CRF application methods (top-dress and incorporation). In a previous study, Altland and Fain (Reference Altland and Fain2003) reported that weed shoot dry mass was 60% lower in containers in which CRFs were dibbled compared to CRFs being incorporated and top-dressed.
Table 7. Liverwort fresh mass (g) at 12 WAT as influenced by interaction between fertilizer placement methods and presence/absence of the ornamental plant begonia. a, b
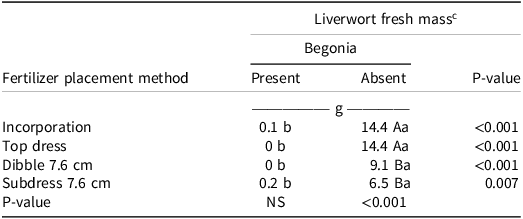
a Abbreviations: NS, nonsignificant; WAT, weeks after treatment.
b Means followed by different capital letters in column for ornamental plant presence or absence for each fertilizer placement methods or small letters in the row that compares a specific fertilizer placement method across presence or absence of plant are significantly different according to Tukey’s HSD (α = 0.05).
c Liverwort fresh mass was measured in grams.
Similarly, for the interaction of gemmae density and the presence or absence of an ornamental plant, there was no significant difference in liverwort fresh mass under liverwort gemmae densities in the presence of begonia, but it was significantly different in its absence (Table 8). When only the liverwort was growing in the container, the highest liverwort fresh mass was recorded when the gemmae density was highest (22.5 g). Liverwort gemmae density (3 and 9 gemmae per container) also had a significant influence on liverwort fresh mass between the presence or absence of an ornamental plant. Liverwort fresh mass was greater under all the liverwort gemmae densities when there was no competition with begonia.
Table 8. Liverwort fresh mass (g) at 12 WAT as influenced by interaction between gemmae density (0, 3, 9) and presence/absence of the dicot ornamental plant begonia. a b,
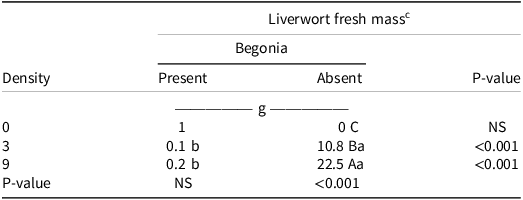
a Abbreviations: NS, nonsignificant; WAT, weeks after treatment.
b Means followed by different capital letters in column for ornamental plant presence or absence for each gemmae density or lower case letters in the row that compares a gemmae density across presence or absence of plant are significantly different according to Tukey’s HSD (α = 0.05).
c Liverwort fresh mass was measured in grams.
For dracaena, there was a significant interaction between fertilizer placement method, gemmae density, and the presence or absence of an ornamental plant for their effect on the fresh biomass of liverwort at the end of the experiment (Table 9). In the presence of an ornamental plant, significant differences were observed in liverwort fresh mass under different fertilizer treatments when the gemmae density was high (3 and 9). The maximum recorded liverwort fresh mass (3.3 g) was observed with CRF incorporation at gemmae density 9. Similarly, different fertilizer placement strategies influenced liverwort fresh mass recorded at the end of the experiment, under the liverwort gemmae densities of 3 and 9, when no ornamental plant was present. The greatest liverwort fresh mass was recorded at gemmae density of 9 for CRF top-dressing (40.9 g), followed by incorporation (16.6 g). Liverwort fresh mass was not different between dibbling (5.1 g) and subdressing (9 g) at 7.6 cm; however, a lower fresh biomass was recorded at gemmae density 9 compared to other treatments. There were also significant differences within the top-dressing and subdressing at 7.6 cm treatments, for all three gemmae densities, when ornamental plants were not present (Table 9). For liverwort that grew alongside dracaena, liverwort was competitive for nutrient uptake among different fertilizer placement methods. Based on the higher liverwort fresh biomass recorded from the CRF incorporation treatment at higher gemmae densities, it seemed to be competitive and used a significant amount of nutrients in the presence of dracaena. As expected, when the ornamental plant was absent, liverwort fresh biomass was higher than the situation described above. Additionally, liverwort fresh biomass was higher from the surface fertilizer application method (top-dressing), followed by incorporation, which were the application methods that provided maximum availability of nutrients for liverwort growth. Berchielli-Robertson et al. (Reference Berchielli-Robertson, Gilliam and Fare1990) reported that competition from weeds growing in containers significantly reduces crop growth. Out of different fertilizer placements, dibbling and top-dressing resulted in the highest quality plants compared to incorporation. Stewart et al. (Reference Stewart, Marble, Jackson, Pearson and Wilson2018) also mentioned that plant response to fertilizer placement methods is species-specific, thereby underlining the need of conducting these studies for various ornamental plants for developing specific recommendations.
Table 9. Liverwort fresh mass (g) at 12 WAT as influenced by interaction between fertilizer placement methods, liverwort gemmae density and presence/absence of the monocot ornamental plant dracaena. a, b

a Abbreviations: NS, nonsignificant; WAT, weeks after treatment.
b Means followed by different capital letters in column for gemmae density for each fertilizer placement methods or lower-case letters in the row which compare a specific fertilizer placement method across gemmae densities are significantly different according to Tukey’s HSD (α = 0.05).
c Liverwort fresh mass was measured in grams.
Overall, the results indicate that subdressing or dibbling at 7.6 cm are effective fertilizer placements for controlling liverwort growth in ornamental container production considering both the quality of ornamentals and their competitiveness with liverwort. These treatments are also promising for improving the overall growth of the begonia and dracaena considered in this study. The surface fertilizer application methods may have had comparatively greater availability of fertilizer for liverwort rhizoids, thus promoting its growth, compared to subsurface methods of placement. Future studies need to focus on any possible toxic effects of dibbling because placing a significant amount of fertilizer right below the roots may have a detrimental effect on the ornamental plant. More study is needed to determine how different water regimes and media would affect liverwort growth under different fertilizer placement methods. Additionally, these methods of fertilizer placement will have varying results for different ornamental plants, especially those with deeper root systems. In integrated weed management programs, these fertilizer placements in combination with other control methods, such as hand-weeding or application of preemergence herbicides, may also provide beneficial results for effective liverwort control.
Practical Implications
Liverwort is a major weed problem in nursery container production systems, and a limitation to its control in greenhouses is the lack of herbicide options, because most of the herbicides are not labeled for greenhouse use. Hand-removal of liverwort is difficult because it forms a mat-like structure on top of the container medium. Therefore, the cultural approach of strategically placing fertilizers in containers can be directly used to successfully control liverwort both in greenhouses and outdoor container nurseries. By altering the placement of fertilizer, the availability of nutrients will increase for ornamental plants and decrease for liverwort. In the current study, CRF subdressing and dibbling at 7.6 cm were highly effective in reducing liverwort coverage and biomass and helped in improving the growth of both begonia and dracaena. The traditional approach of fertilizer incorporation resulted in higher liverwort coverage in general. As a result of strategic fertilizer placement, the ornamentals will potentially out-compete liverwort thallus growth, reducing the need for hand-weeding and labor costs. Nursery growers and greenhouse operators can thus opt for this simple approach and improve their overall profitability through effective liverwort control.
Acknowledgments
We thank research aide Carolyn Fitzgibbon and undergraduate research assistants McKinleigh Cordahl and Shriya Kethireddy for their help in setting up the greenhouse experiments and with data collection. We also thank the Michigan State University College of Agriculture and Natural Resource Statistical Consulting Unit for consultation with data analysis.
Funding
Funding for this project was provided by the Western Michigan Greenhouse Association and by US. Department of Agriculture–National Institute of Food and Agriculture, Hatch project MICL02670.
Competing interests
The authors declare they have no competing interests.













