Introduction
Garlic mustard, Alliaria petiolata (Marschall von Bieberstein) Cavara and Grande (Brassicaceae), is a weedy biennial plant that was introduced to North America in the 19th century. The plant has few known specialist herbivores in North America and forms dominant patches in forest understoreys across southern Canada and the eastern United States of America (Blossey et al. Reference Blossey, Nuzzo, Hinz and Gerber2001; Yates and Murphy Reference Yates and Murphy2007). Garlic mustard harms forest ecosystems in North America by producing allelopathic chemicals that inhibit the growth of native plant species (Prati and Bossdorf Reference Prati and Bossdorf2004; Stinson et al. Reference Stinson, Kaufman, Durbin and Lowenstein2007; Cipollini Reference Cipollini2016) and modify the growth of arbuscular mycorrhizal fungi that form mutualisms with native plant assemblages (Callaway et al. Reference Callaway, Cipollini, Barto, Thelen, Hallett and Prati2008; Roche et al. Reference Roche, Pearse, Bialic-Murphy, Kivlin, Sofaer and Kalisz2021). The plant also acts as a sink for insect species that oviposit on it but cannot complete their life cycle (Keeler and Chew Reference Keeler and Chew2008; Davis et al. Reference Davis, Frisch, Bjarnholt and Cipollini2015). As a result, its invasion contributes to habitat degradation and loss of biodiversity in this region (Cipollini Reference Cipollini2016). These threats to North American forests have prompted the prioritisation of garlic mustard management for the conservation of forest communities across the range of its invasion.
To supplement existing management options, the development of biological controls for garlic mustard began in 1998 (Blossey et al. Reference Blossey, Nuzzo, Hinz and Gerber2001). After initial screening and extensive host range testing, the first agent to be selected was the specialist root crown–mining weevil, Ceutorhynchus scrobicollis Nerenscheimer and Wagner (Coleoptera: Curculionidae) (Van Riper et al. Reference Van Riper, Gerber, Hinz, Cortat, Katovich, Becker and Marek-Spartz2016). The adults of these weevils consume leaves and oviposit in petioles, leaves, and the apical meristem of first-year rosettes, and their larvae mine leaf stalks, root crowns, and shoot bases from fall to the following spring, reducing plant survival and biomass (Gerber et al. Reference Gerber, Hinz and Blossey2007, Reference Gerber, Cortat, Hinz, Blossey, Katovich and Skinner2009; Evans et al. Reference Evans, Davis, Raghu, Ragavendran, Landis and Schemske2012). In the second year, larvae exit bolted stems in early May and pupate in the soil. In 2018, C. scrobicollis was permitted for release in Canada and, as of 2023, it has been released at field sites in Ontario, British Columbia, and Alberta (McTavish et al. Reference McTavish, Katovich, Becker, Cortat, Smith and Bourchier2024).
A component of post-release monitoring of biological control of invasive plants is the tracking of species interactions following agent release. Other insect taxa may have antagonistic (Ehler and Hall Reference Ehler and Hall1982) or synergistic impacts with biological control agents (Denoth et al. Reference Denoth, Frid and Myers2002; Ong and Vandermeer Reference Ong and Vandermeer2015). Biological control monitoring should also distinguish between activity from agents and other insects. To date, the number of insects found on garlic mustard has been limited (Yates and Murphy Reference Yates and Murphy2007), despite the fact that this widespread invasive weed has been in North America since the 1860s (Nuzzo Reference Nuzzo and McKnight1993). Of the few herbivorous insects found in North America, most are not able to complete their life cycle on the plant or are generalist agricultural pests of Brassicaceae (Blossey et al. Reference Blossey, Nuzzo, Hinz and Gerber2001; Yates and Murphy Reference Yates and Murphy2007; Davis et al. Reference Davis, Frisch, Bjarnholt and Cipollini2015). Here, we describe a novel observation of a widespread insect, reported from across eastern Canada and the United States of America (Global Biodiversity Information Facility 2024), in association with second-year stems of garlic mustard.
In October 2020, as a part of post-release monitoring of C. scrobicollis for the garlic mustard biological control programme, senesced second-year garlic mustard stems were collected from a site in eastern Ontario and dissected to search for C. scrobicollis root crown mining. These dissections revealed several unknown stem-mining larvae and associated frass. The larvae were subsequently identified through DNA barcoding as Mordellina ancilla Leconte (Coleoptera: Mordellidae). Specimen barcoding was performed externally by Dr. Ivo Toševski, at CABI, using the LCO1480hem/HCOhem primer pair. Two specimens were identified through the Barcode of Life Data System as M. ancilla with 99.3% and 100% matches. Mordellina ancilla is a species native to North America (Liljeblad Reference Liljeblad1945; Bousquet et al. Reference Bousquet, Bouchard, Davies and Sikes2017) that had no previously recorded association with invasive garlic mustard.
The life history and plant associations of M. ancilla are poorly understood, likely due to the numerous taxonomic changes the species has undergone and the difficulties in observing endophagous insects that do not produce externally visible signs of damage (Jackman and Lu Reference Jackman and Lu2001; Lisberg Reference Lisberg2003). Endophagous feeding by larvae in this family (Mordellidae) is common (Traylor et al. Reference Traylor, Ulyshen and McHugh2023), and M. ancilla larvae, specifically, have been previously found feeding inside the thorns of honey locust, Gleditsia triacanthos Linnaeus (Fabaceae) (Ford and Jackman Reference Ford and Jackman1996; Jackman and Lu Reference Jackman and Lu2001). However, the species likely occupies other host plant species: for example, it was found recently in New Brunswick, Canada, where the thorn-bearing form of honey locust has not been naturalised (Webster et al. Reference Webster, Sweeney and DeMerchant2012). To our knowledge, M. ancilla had not been found in an herbaceous plant before our collection in 2020, and it was unclear at that time whether the species could interact with C. scrobicollis.
This study investigates the basic life history of M. ancilla and its association with garlic mustard. We sought to identify how common this association was, what life stages of garlic mustard M. ancilla uses, and how it uses the plant. We conducted a geographic survey for M. ancilla at eight sites across southern Ontario to determine the extent of the insect’s association with garlic mustard. We also performed a 12-month longitudinal sampling at a single site near King City, Ontario to determine the phenology of M. ancilla on garlic mustard. A more complete understanding of the life history of M. ancilla will fill a key gap in our knowledge about insect communities associated with garlic mustard and will help inform the developing biological control programme for this weed.
Methods
Geographic survey
We sampled second-year bolted garlic mustard stems across southern Ontario during 2021 and 2022 to determine the geographic extent of the association between garlic mustard and M. ancilla. We selected eight forested sites with known populations of garlic mustard across a range of 1.87 degrees latitude (Table 1). The sites were sampled in early November 2021 to match the timing of the initial detection of M. ancilla in eastern Ontario in the fall of 2020.
Table 1. Coordinates and description of garlic mustard sampling sites in southern Ontario. Sampling occurred in a 20-m × 20-m sampling grid at each site in the first week of November 2021, except for at the Koffler Scientific Reserve (KSR), which was sampled periodically from August 2021 to August 2022
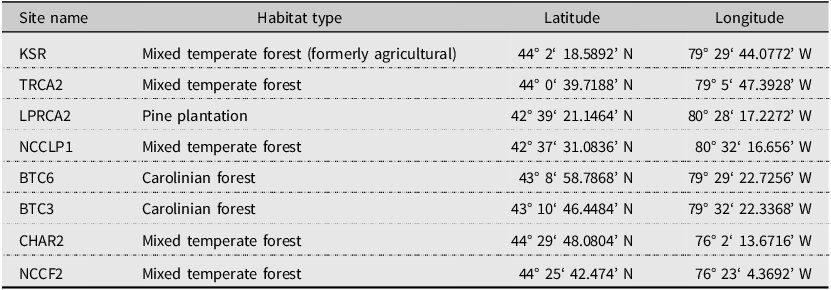
Site names: KSR, Koffler Scientific Reserve; TRCA2, Toronto and Region Conservation Authority Site 2; LPRCA2, Long Point Region Conservation Authority Site 2; NCCLP1, Nature Conservancy of Canada Long Point Site 1; BTC6, Bruce Trail Site 6; BTC3, Bruce Trail Site 3; CHAR2, Charleston Lake Site 2; NCCF, Nature Conservancy of Canada Battersea Site 2.
At each site, we set up a 20-m × 20-m sampling grid in the middle of a dense patch of garlic mustard. We walked transects within the entire sampling grid, randomly collecting bolted garlic mustard stems while leaving a minimum of one metre between collections. At least 100 whole stems with attached roots were collected per site, wrapped in plastic, and transferred to the lab for dissection.
Longitudinal survey
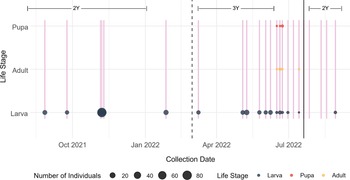
The longitudinal survey was conducted at a single site selected from the geographic survey (Koffler Scientific Reserve, King City, Ontario, Canada) over a period of one year to determine the seasonal life history of M. ancilla on garlic mustard. Using the same sampling protocol described for the geographic survey, we collected 100 stems once every 1–2 months during fall 2021 and winter and early spring of 2022. Sampling frequency was intensified to weekly and bi-weekly sampling during late spring and summer 2022 to capture expected changes in M. ancilla life stages at that time. A minimum of 100 stems were collected during each collection event, although six collections failed to reach this threshold due to depletion of stems in the chosen sampling grid. A total of 2031 stems were collected over 20 collection events carried out over the course of 13 months (Fig. 1).
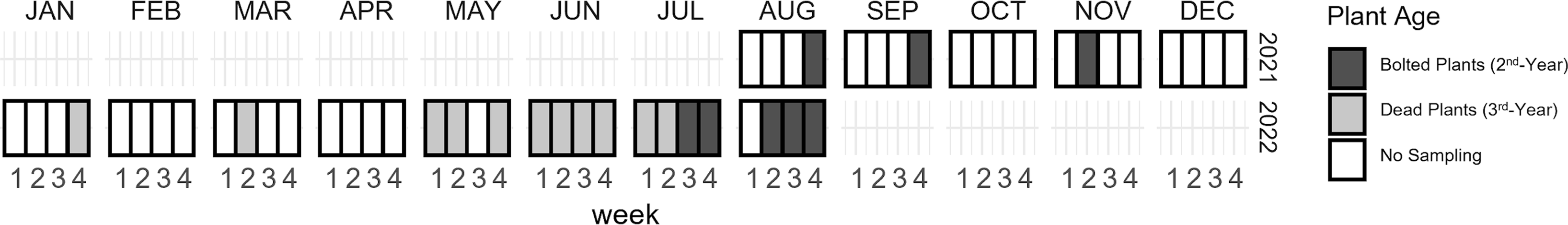
Figure 1. Timing of Mordellina ancilla collection from garlic mustard stems from August 2021 to August 2022 at Koffler Scientific Reserve, King City, Ontario, Canada. Dead third-year plants were collected in 2022 to document the development of M. ancilla post-diapause. Collection of second-year stems resumed in July 2022 to target eggs and early-instar larvae of M. ancilla. Note: two collection events were made during the last week of June 2022.
Stem measurements and insect collection
Before dissection, stems were stored at 4–6 °C to limit insect development. Collected plants were measured to determine whether stem characteristics (stem mass, stem length, and stem basal diameter) influenced the likelihood of occupancy by M. ancilla. Single stems were isolated by discarding secondary stems branching off from the main stem above the root–shoot junction. The root–shoot junction was determined by cutting a longitudinal slit at the base of each plant and marking the transition point between the spongy stem pith and the woody root pith. Stem mass was taken after removing siliques, leaves, and any debris remaining attached to the plants. Stem length and root length were measured separately to account for plants with missing or broken roots. Stem basal diameter was measured using calipers placed at each plant’s root–shoot junction.
Following measurements, we dissected each stem longitudinally to search for M. ancilla and any other stem-dwelling arthropods. Although the main focus of this study was M. ancilla, other arthropods were also collected to find other species that may interact with this system. We recorded the position of each specimen within a stem (stem diameter, distance from root–shoot junction), as well as life stage and any potential evidence of feeding. Arthropods were categorised by general morphology and either were placed back in stems for rearing or were stored in 95% EtOH for morphological identification and barcoding. A total of 1–2 specimens of each presumed new species were allocated to barcoding, and all other uninjured specimens were reared.
For reared specimens, individuals found during dissections were placed into 10-cm fragments of their original stems. The two halves of each stem segment were tied together using metal wire and were placed in individual corked glass containers. The cork allowed for external humidity to enter vials while avoiding the buildup of liquid that could drown growing larvae. Vials were placed outside in a clear waterproof container to trigger diapause and overwintering. Vials were examined once a month between March and August to collect any emerging adults (Fig. 2). At the end of this emergence period, stems were inspected to find adults that had failed to exit stems.

Figure 2. Photos of A, the dorsal habitus and B, the lateral habitus of Mordellina ancilla. Photos were taken on a Leica M205 dissecting microscope (Leica Microsystems, Wetzlar, Germany), and images were compiled using Zerene Stacker (Zerene Systems, Richland, Washington, United States of America).
Due to the limited availability of morphological keys for the identification of larvae, pupae, and eggs (Liu et al. Reference Liu, Erwin and Yang2018), the specimens were identified through a combination of DNA barcoding and examination of adult forms following rearing. The DNA barcoding was performed by Tyler Nelson at Agriculture and Agri-food Canada (Summerland, British Columbia, Canada), using the universal cytochrome c oxydase 1 primers from Folmer et al. (Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994). Adult M. ancilla specimens were verified by Serge Laplante (Liljeblad Reference Liljeblad1945) at the Canadian National Collection of Insects, Arachnids, and Nematodes (Ottawa, Ontario, Canada), where the voucher specimens produced from this study have been deposited.
Statistical analyses
Analysis of all data was conducted using R, version 4.3.2 (https://www.r-project.org/foundation/). We generated basic descriptive statistics to characterise detections of M. ancilla within and across sample sites. In order to determine which variables influenced the presence of M. ancilla, we performed a set of logistic regressions using a generalised linear model in the lme4 package. Presence and absence of M. ancilla were used as the response variable with site and stem diameter at base as predictors. A first logistic regression was performed with these parameters on the geographic sampling data because this dataset includes the largest sample of stem diameters from sites across the range of garlic mustard in southern Ontario. However, because stem diameter and site were associated, a second logistic regression was performed with these same parameters on the longitudinal sampling data to remove the effect of site on the presence of M. ancilla. For this second logistic regression, we used samples only up to the first detection of adult M. ancilla because, after this time, the beetles would begin exiting stems (increasing false negatives). To characterise the occupancy of stems by M. ancilla, chi-squared tests were used to identify associations between potential evidence of feeding and the presence of the beetle in roots and stems. Data from both the longitudinal and the geographic surveys were included for these chi-squared tests, although the longitudinal survey data were similarly bounded by the first observation of an adult mordellid, as above.
Results
Summary of collected specimens
A total of 2826 garlic mustard stems were collected – 845 from geographic sampling and 2090 from the longitudinal sampling (109 stems belonged to both sampling efforts). Mordellina ancilla was the most common insect found during stem dissections (n = 336, 11.9% of stems). Other insects found in garlic mustard stems were a wasp in the genus Heterospilus (Hymenoptera: Braconidae) (n = 73, 2.6% of stems) and a midge in the genus Dasineura (Diptera: Cecidomyiidae) (n = 35, 1.2% of stems). The Heterospilus sp. was reared from white pupal casings to adulthood and identified through DNA barcoding. This Heterospilus sp. is suspected to be a parasitoid of Mordellina ancilla because members of this genus are known to parasitise mordellids (Viereck Reference Viereck1911). The Heterospilus sp. was not the only parasitoid found during this study. Reared mordellid larvae occasionally yielded a second braconid wasp, Aliolus marginatus (Hymenoptera: Braconidae). Specimens were identified by Dr. José Fernández-Triana (Agriculture and Agri-Food Canada, Ottawa, Ontario), with voucher specimens deposited at the Canadian National Collection of Insects, Arachnids, and Nematodes. Other arthropods found in the stem material were not associated specifically with garlic mustard: Formicidae (n = 336, 11.9% of stems), Chilopoda (n = 15, 0.5% of stems), and Myriapoda (n = 15, 0.5%). These generalist arthropods were found incidentally in stems due to their prevalence in the broader sampled habitats.
Life history of Mordellina ancilla in garlic mustard
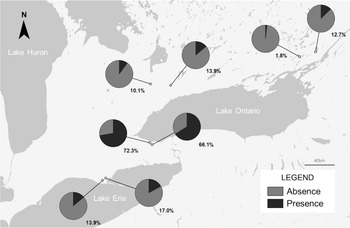
Mordellid larvae were found at all sites sampled in the geographic survey, with an occupation rate ranging from 1.8% to 72.3% of collected stems (Fig. 3). Occupation rate varied across southern Ontario, with the two sites near Grimsby, Ontario containing the highest densities of individuals (72.3 and 66.1%). All individuals collected during the sampling period in November were larvae.
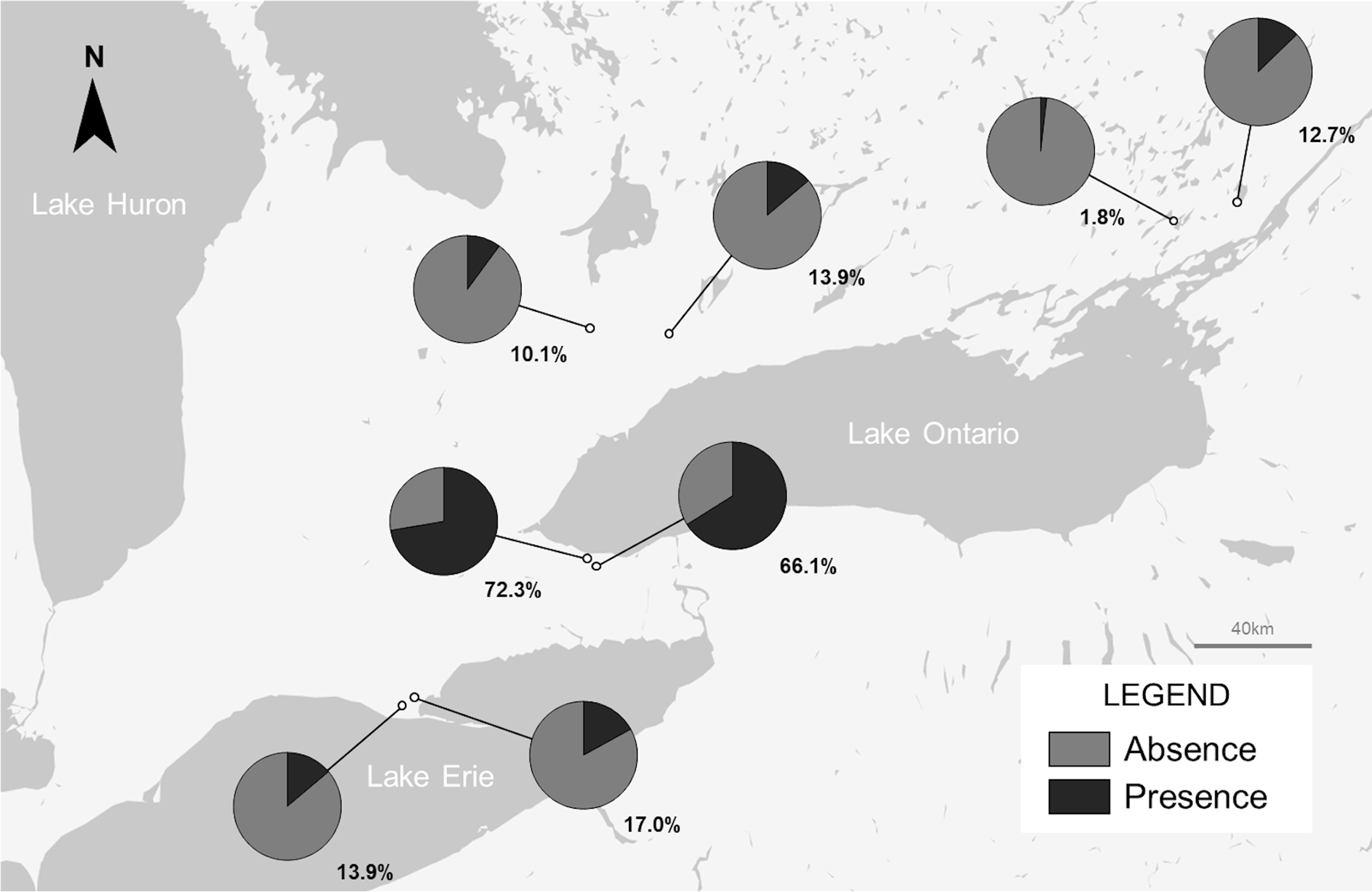
Figure 3. Map of southern Ontario, Canada, showing presence and absence of Mordellina ancilla in garlic mustard stems in material collected during 2021–2022. Percentage presence (% of sampled garlic mustard stems containing M. ancilla) is noted next to each corresponding pie chart.
From the longitudinal sampling, we collected larvae, pupae, and adults of M. ancilla. The species was found overwintering as larvae within dead second-year garlic mustard stems and began pupation and adult emergence from stems from around mid-June to mid-July (Fig. 4).
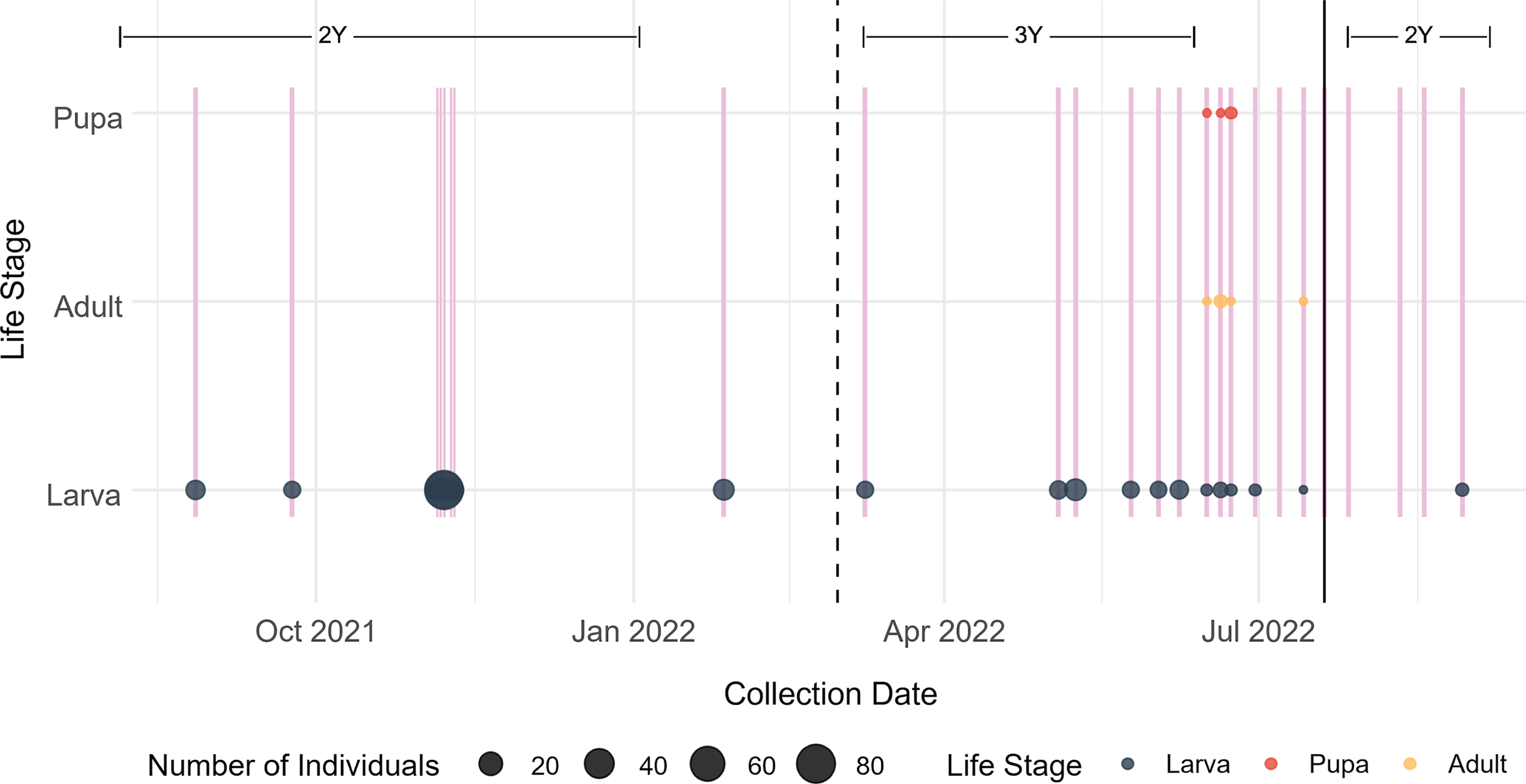
Figure 4. Life stages of Mordellina ancilla collected over the 13 months of sampling garlic mustard stems from southern Ontario, Canada, in 2021 and 2022. Vertical pink lines denote every sampling event. Vertical black lines separate life stage of plants: 2Y = second-year plants in 2021 and 2022, 3Y = third-year plants at the beginning of 2022. The third-year plants (3Y) consist of dead biomass of bolted second-year stems in which M. ancilla overwinters. Collection of second-year bolted plants resumed in July 2022.
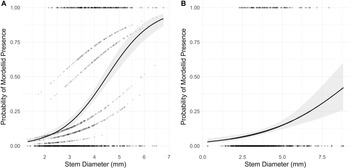
Mordellina ancilla occurred more often in stems with larger diameters in both the geographic sampling data (LR X 2 = 162.07, df = 1, P < 0.001) and the longitudinal sampling data (LR X 2 = 23.023, df = 1, P < 0.001; Fig. 5).
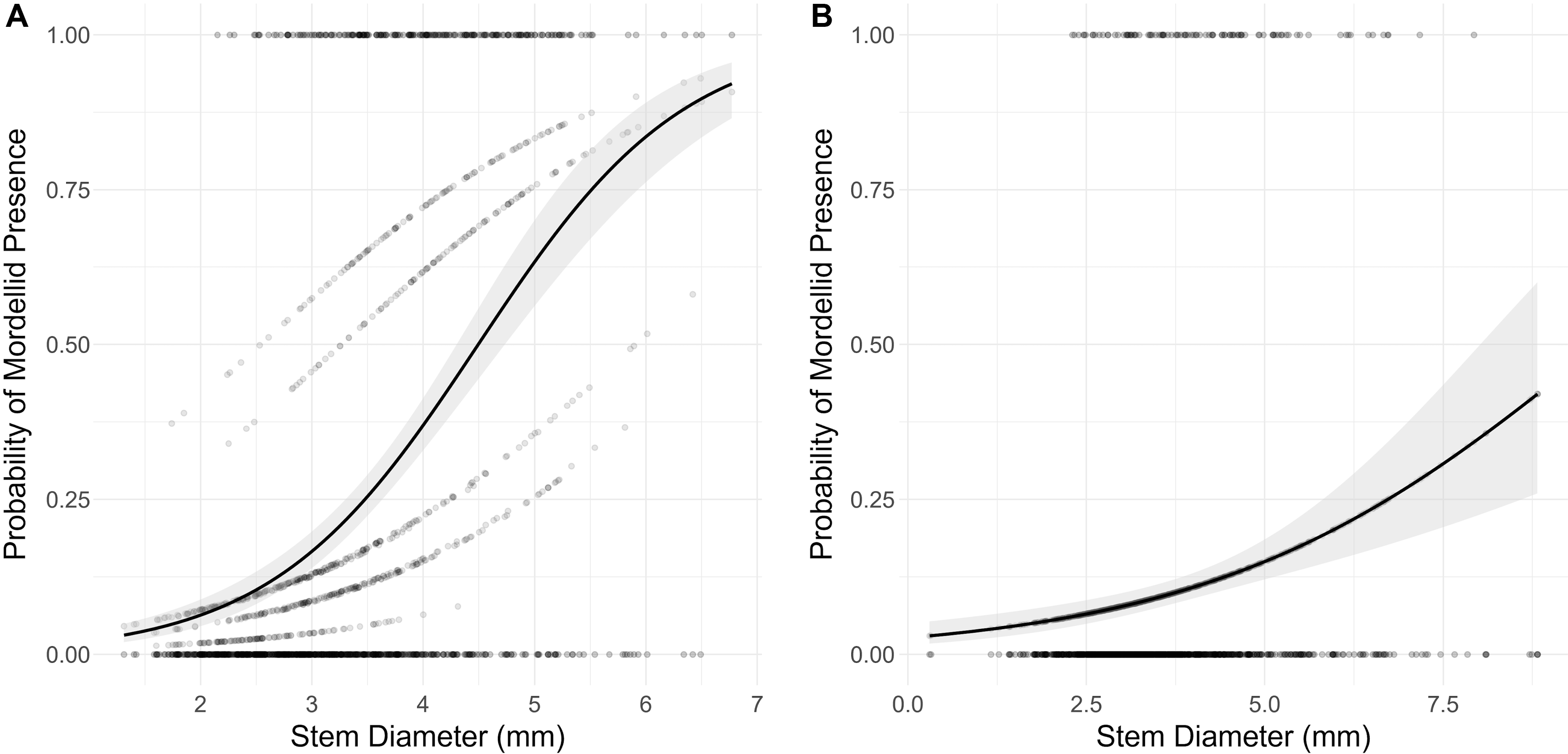
Figure 5. Logistic regression of the probability of Mordellina ancilla relative to garlic mustard stem diameter for the A, geographic sampling data with site as a random effect (data from southern Ontario, fall 2021), and B, longitudinal sampling data at Koffler Scientific Reserve, King City, Ontario, Canada (2021–2022). Samples were excluded if they occurred after the date of the first emergence of M. ancilla adults.
Specimens kept in stem fragments for rearing produced fine granular light-beige frass, leaving this frass behind in mined tunnels. This frass is closely associated with the presence of larvae in both the stem (X 2 = 625.25, df = 1, P < 0.001) and the root (X 2 = 62.851, df = 1, P < 0.001; Table 2), and no other species has been observed producing similar frass (Fig. 6). Along with the frass, M. ancilla is also associated with hollow stem sections where the pith has been entirely consumed (X 2 = 14.32, df = 1, P < 0.001; Tab. 2). Mordellina ancilla was also found in association with dark root pith (X 2 = 7.8954, df = 1, P = 0.005).
Table 2. Chi-square test results for association between the presence of Mordellina ancilla in stems and modified appearances of the pith (“pith state”) in garlic mustard collected in southern Ontario, Canada, from 2021 to 2022
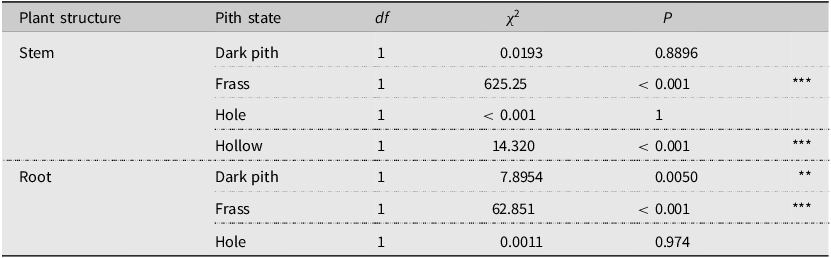
The last column denotes significance of P-values (* for P < 0.05, ** for P < 0.01, and *** for P < 0.001). No test was conducted for the hollow root state as this was never observed in any dissections.

Figure 6. Photos showing various conditions of second-year bolted garlic mustard stems and roots collected from 2021 to 2022 in southern Ontario, Canada: A, larva of Mordellina ancilla within a mined stem gallery; B, normal white pith of garlic mustard with no stem occupation by M. ancilla; C, frass left behind by stem-mining M. ancilla; D, dark root pith found near root–shoot junction of sampled plants; and E, large gallery mined by M. ancilla larva. Galleries were often filled with frass such as is shown in C.
Discussion
Our results confirm that M. ancilla uses garlic mustard as a host to complete its development. Larvae feed on the pith of second-year bolted stems, undergo winter diapause, continue to feed during the following spring, and then emerge as adults in early summer. This overwintering strategy is consistent with observations of other species in the family that undergo winter diapause within plant tissue (Baust et al. Reference Baust, Grandee, Condon and Morrissey1979). Despite extensive dissections, we were not able to find eggs of M. ancilla during any of the sampling periods. Although no eggs were discovered, larvae were often found at the base of the stems, along with a central gallery originating from the top of the stem. This suggests that eggs may be oviposited near the top of the plant on the stem or on other undissected plant structures, such as leaf petioles, similar to what is seen in other species of this family (Schwitzgebel and Wilbur Reference Schwitzgebel and Wilbur1942) and in stem-mining taxa more broadly (Stiling and Strong Reference Stiling and Strong1983). Future efforts should include dissections of various upper plant structures of second-year garlic mustard plants in order to locate the exact location of M. ancilla oviposition.
The presence of M. ancilla on garlic mustard is a new host record: the species has been previously sampled only from thorns of honey locust, Gleditsia triacanthos (Ford and Jackman Reference Ford and Jackman1996). Because G. triacanthos is not present over the entire recorded range of M. ancilla (Gold and Hanover Reference Gold and Hanover1993; Ford and Jackman Reference Ford and Jackman1996; Welk et al. Reference Welk, Schubert and Hoffmann2002; Webster et al. Reference Webster, Sweeney and DeMerchant2012), it may have other native host plants, or the introduction of garlic mustard may have allowed M. ancilla to expand its range. Further sampling from other plant species from across the insect’s known range will contribute to a better understanding of the relationship between M. ancilla and its host plants.
In terms of larval behaviour within stems, we found more M. ancilla larvae in stems with larger stem diameters, suggesting that survival may be greater in these stems or that the species may favour larger stems. The relationship between stem size, larval fitness, and female oviposition preference should be examined further to understand host plant selection in this system. This trend is not uncommon in stem-miners and has been documented in a variety of other species (Holmes and Peterson Reference Holmes and Peterson1960; Rathcke Reference Rathcke1976; Niveyro and Salvo Reference Niveyro and Salvo2017). It is possible that either a threshold in stem size affects oviposition or larval survival, where larvae do not occur in stems smaller than a specified diameter, or stem occupancy may decrease gradually as stem diameter decreases. Sampling stems of various diameters during the oviposition window of M. ancilla would help identify the mechanism behind this observed pattern.
Our improved understanding of the life history of M. ancilla on garlic mustard suggests that this species is unlikely to have any direct effect on the use of C. scrobicollis as a biological control agent for garlic mustard. The insects use different life stages of the garlic mustard: C. scrobicollis exits second-year garlic mustard plants in the spring (∼March–April; Gerber et al. Reference Gerber, Hinz and Blossey2007), well before M. ancilla larvae are first found in garlic mustard stems in late summer. The two species also feed on different types of plant material: C. scrobicollis feeds on live garlic mustard tissue earlier in the season, whereas M. ancilla larvae begin consuming dried stem pith later in the summer. The difference in diet may also explain why the frass of C. scrobicollis is considerably darker than M. ancilla’s light-beige frass (Fig. 6). Similarly, M. ancilla is also unlikely to directly interact with a second species, Ceutorhynchus constrictus Marsham (Coleoptera: Curculionidae), another species being considered as a biocontrol agent for garlic mustard in North America. Larvae of C. constrictus feed on garlic mustard seeds on second-year plants in June and July before leaving the siliques to pupate in the soil (Davis et al. Reference Davis, Landis, Nuzzo, Blossey, Gerber and Hinz2006). As with C. scrobicollis, C. constrictus feeds on living tissue earlier in the season than M. ancilla does, and the species are thus unlikely to interact directly.
These findings suggest that M. ancilla does not affect the growth and reproduction of garlic mustard during its development because much of the tissue being consumed by the insect is no longer contributing to the plant’s growth and reproductive output. Conversely, root crown feeding by C. scrobicollis could affect the availability of suitable or high-quality host plants for M. ancilla. Stems with lower biomass and smaller stem diameters would be less suitable for M. ancilla larvae, as was found in the present study. However, garlic mustard control alone is not likely to affect M. ancilla populations because the beetle can still rely on other host plant(s) to sustain its populations.
In conclusion, this study confirms the presence of M. ancilla as a stem-miner capable of completing its life cycle in the end-stages of second-year bolted and dead garlic mustard stems. By characterising the species’ life cycle, we determined that M. ancilla is unlikely to affect the growth or reproduction of garlic mustard or its proposed biocontrol agent C. scrobicollis. As a species native to North America, M. ancilla appears to be taking advantage of the introduction of garlic mustard and likely has other, as-of-yet-unidentified host plants. This new record expands our existing knowledge of M. ancilla, finding it to be at least oligophagous, a potential explanation for its geographic range beyond that of its previous known host plant, the honey locust. Future sampling of other plant species may identify additional hosts for M. ancilla and may help to contribute to our knowledge of the Mordellidae, a relatively common group of endophagous beetles.
Acknowledgements
The authors thank Carla Timm, Jolie Nguyen, Ayumi Akimoto, Matt Wells, H.W. Colin Chiu, and Adonis Doherty for assistance in data collection and field work. The authors also thank José L. Fernández-Triana at the Canadian National Collection of Insects, Arachnids, and Nematodes and Tyler Nelson at Agriculture and Agri-Food Canada for identifying unknown specimens found during stem dissections, as well as Serge Laplante at the Canadian National Collection of Insects, Arachnids, and Nematodes for confirming the identification of M. ancilla. They are grateful to the Invasive Species Centre (Sault Ste. Marie, Ontario, Canada) and the Ontario Ministry of Natural Resources for providing funding for this project.
Competing interests
The authors declare that they have no competing interests.