Summations
-
The tripartite system, anandamide, TRPV1, and CB1, may be an important player in regulating fear responses.
-
Anandamide either facilitates or restrains fear, by acting upon TRPV1 or CB1, respectively.
-
The intensity of aversive stimulus and doses of cannabinoids or vanilloids ligands are major determinants in detecting anti-aversive effects.
Considerations
-
This is a narrative review of the interactions among anandamide, TRPV1, and CB1 in the modulation of fear learning. We carefully selected studies contributing to the field.
-
We recognize that some relevant work might not have been cited in this review.
-
Here we suggest potential mechanisms and pathways involved in the regulation of fear by anandamide, TRPV1 and CB1.
Introduction
Fear can be defined as a coordinated reaction, involving autonomic, behavioural and cognitive changes in response to innate or learned threatening stimuli (Fanselow and Pennington, Reference Fanselow and Pennington2018). Fear learning enables an individual to assign motivational content to cues previously paired with threatening stimuli, allowing the prediction of danger and the elaboration of an adaptive response (Krause & Domjan, Reference Krause and Domjan2017). Thus, conditioned fear is crucial for survival and well-being (Krause & Domjan, Reference Krause and Domjan2017). However, beyond certain limits, a mechanism sustaining health may become maladaptive and predispose to psychiatric disorders (Milton, Reference Milton2019). Indeed, alterations in conditioned fear processing have been related to anxiety, depression and post-traumatic stress disorders (Foa et al., Reference Foa, Steketee and Rothbaum1989; Luyten et al., Reference Luyten, Vansteenwegen, van Kuyck, Gabriëls and Nuttin2011; Conoscenti and Fanselow, Reference Conoscenti and Fanselow2019; Bienvenu et al., Reference Bienvenu, Dejean, Jercog, Aouizerate, Lemoine and Herry2021).
In experimental animals, learned fear can be studied using protocols in which a neutral stimulus, such as a context or an auditory cue, is paired with an aversive one (unconditioned, such as a footshock). Thereafter, the formerly neutral stimulus, when subsequently presented, functions as a conditioned stimulus and triggers a conditioned response. Contextual fear conditioning involves the use of a contextual element as a neutral stimulus, while cue fear conditioning often relies on auditore stimuli (a tone). The sound can terminate simultaneously or be delayed in relation to the duration of the aversive stimuli. Alternatively, the aversive stimuli can be delivered after a certain time, characterising the trace-auditory fear conditioning. One advantage of fear conditioning models is the possibility of dissecting the mechanisms underlying specific learning phases. The whole learning and memory process can comprise the following phases: The acquisition phase, when the two stimuli are presented simultaneously (e.g. shock + context, shock + tone) and the processing and initial encoding of memory takes place. This is immediately followed by memory consolidation, a series of complex molecular processes crucial for the duration of the memory (Asok et al., Reference Asok, Leroy, Rayman and Kandel2019; de Oliveira and Do-Monte, Reference de Oliveira and Do-Monte2021). Synaptic consolidation implies the stabilisation of the memory trace (Asok et al., Reference Asok, Leroy, Rayman and Kandel2019; de Oliveira and Do-Monte, Reference de Oliveira and Do-Monte2021), which is encoded by an assembly of neurones called engram (Josselyn et al., Reference Josselyn, Köhler and Frankland2015). Later, when the animals are re-exposed to the previously neutral stimulus, they will display the conditioned response, for which the underlying phenomenon is called memory retrieval, which involves the activation of the engram encoding the memory previously acquired (Josselyn et al., Reference Josselyn, Köhler and Frankland2015; Josselyn and Tonegawa, Reference Josselyn and Tonegawa2020). Likewise, the exposition to the conditioned stimulus can trigger different processes, depending on the duration of this exposition, among other factors (Auber et al., Reference Auber, Tedesco, Jones, Monfils and Chiamulera2013). Long or repeated expositions to the neutral stimuli can induce extinction, in which a new memory trace is formed, decreasing the intensity of the conditioned response. After extinguished, conditioned responses can be restored, by reinstatement, renewal or spontaneous recovery (Asok et al., Reference Asok, Leroy, Rayman and Kandel2019; de Oliveira and Do-Monte, Reference de Oliveira and Do-Monte2021).
In terms of neural circuitry, fear learning requires an extensive network of central structures involved in cognition, emotion, and their corresponding autonomic and behavioural responses. The hippocampus (HPC) is responsible for encoding the contextual information associated with an aversive stimuli (Hennings et al., Reference Hennings, Cooper, Lewis-Peacock and Dunsmoor2022; Marks et al., Reference Marks, Yokose, Kitamura and Ogawa2022; Lee and Kaang, Reference Lee and Kaang2023). Bidirectional projections between the HPC and the amygdala (AMG) are involved in sustaining the emotional valence of the conditioned response (Marks et al., Reference Marks, Yokose, Kitamura and Ogawa2022). The various AMG nuclei process information related to conditioned cues (Li et al., Reference Li, Zhi, Qi, Wang and Hu2023) through neuronal connections with other structures, such as the periaqueductal grey (PAG) and the parabrachial nucleus and the thalamus (Marks et al., Reference Marks, Yokose, Kitamura and Ogawa2022). In addition, cortical structures, especially the prefrontal cortex (PFC), receive substantial connections from subcortical brain regions such as the AMG and the HPC, integrating the fear-learning circuit (Thomas et al., Reference Thomas, Hall and Everitt2002; Alexandra Kredlow et al., Reference Alexandra Kredlow, Fenster, Laurent, Ressler and Phelps2022). Other structures outside the classic circuit have also been investigated in the last years for their contribution to fear conditioning. For instance, the nucleus accumbens (NAC) core is involved in the evaluation of threat degree (Ray et al., Reference Ray, Russ, Walker and McDannald2020). However, its role in fear associated with discrete cues remains uncertain (Thomas et al., Reference Thomas, Hall and Everitt2002).
Thus, different structures and pathways work in concert along the complex process of fear learning, in order to keep the balance between allostasis and allostatic overload (Milton, Reference Milton2019). Various neurochemical mechanisms have been implicated in this process, including the endocannabinoid system (ECS) (Lutz et al., Reference Lutz, Marsicano, Maldonado and Hillard2015), which is briefly described in the following section.
The endocannabinoid system
The ECS comprises several molecular components, including the cannabinoid type-1 (CB1) (Matsuda et al., Reference Matsuda, Lolait, Brownstein, Young and Bonner1990) and type-2 (CB2) (Munro et al., Reference Munro, Thomas and Abu-Shaar1993) receptors; the endocannabinoids (eCB), N-arachidonoylethanolamide (anandamide) (Devane et al., Reference Devane, Hanuš, Breuer, Pertwee, Stevenson, Griffin, Gibson, Mandelbaum, Etinger and Mechoulam1992) and 2-arachidonoylglycerol (2-AG) (Mechoulam et al., Reference Mechoulam, Ben-Shabat, Hanus, Ligumsky, Kaminski, Schatz, Gopher, Almog, Martin, Compton, Pertwee, Griffin, Bayewitch, Barg and Vogel1995); the enzymes responsible for their synthesis, N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD) and diacylglycerol lipase (DAGL), respectively; and those responsible for their hydrolysis, fatty acid amide hydrolase (FAAH) (Deutsch & Chin, Reference Deutsch and Chin1993) and monoacylglycerol lipase (Dinh et al., Reference Dinh, Carpenter, Leslie, Freund, Katona, Sensi, Kathuria and Piomelli2002), respectively. One particularity of this system is that the eCBs are produced and released on demand; anandamide can be released by either the pre- or the postsynaptic neurones, while 2-AG seems to be released mostly from the postsynaptic neurones (Howlett et al., Reference Howlett, Barth, Bonner, Cabral, Casellas, Devane, Felder, Herkenham, Mackie, Martin, Mechoulam and Pertwee2002; Piomelli and Mabou Tagne, Reference Piomelli and Mabou Tagne2022). Once released, eCBs can act as retrograde messengers or as autocrine modulators (Uchigashima et al., Reference Uchigashima, Narushima, Fukaya, Katona, Kano and Watanabe2007; Busquets-Garcia et al., Reference Busquets-Garcia, Bains and Marsicano2018). CB1 seems to be expressed mostly in presynaptic terminals, although their postsynaptic presence has also been suggested and described as regulating neuronal self-inhibition (Bacci et al., Reference Bacci, Huguenard and Prince2004). Its functions seem to be related to the regulation of dendritic excitability mediating long-term potentiation (LTP), which is necessary for cognition and spatial memory (Maroso et al., Reference Maroso, Szabo, Kim, Alexander, Bui, Lee, Lutz and Soltesz2016; Busquets-Garcia et al., Reference Busquets-Garcia, Bains and Marsicano2018). In addition to these classic members, a more comprehensive description of the ECS may include other targets under the umbrella term of the expanded ECS (Cristino et al., Reference Cristino, Bisogno and Di Marzo2020). The list includes enzymes and receptors modulated directly by phytocannabinoids, eCB or other products of their biosynthetic pathways, such as the transient receptor potential cation channel subfamily V (vanilloid), member 1 (TRPV1) (Cristino et al., Reference Cristino, Bisogno and Di Marzo2020).
TRPV1 and CB1 share some important features. Both were originally discovered as targets for phytochemicals. In the case of CB1, its prototypical agonist is delta-9-tetrahydrocannabinol, the main psychoactive compound of Cannabis sativa (Devane et al., Reference Devane, Dysarz, Johnson, Melvin and Howlett1988); as for TRPV1, its main agonist is capsaicin, a substance present in certain species of chilli peppers and responsible for the burning pain associated with their intake (Caterina et al., Reference Caterina, Schumacher, Tominaga, Rosen, Levine and Julius1997). After their discoveries as orphan receptors, both CB1 and TRPV1 were found to share anandamide as a common endogenous agonist (Devane et al., Reference Devane, Hanuš, Breuer, Pertwee, Stevenson, Griffin, Gibson, Mandelbaum, Etinger and Mechoulam1992; Zygmunt et al., Reference Zygmunt, Petersson, Andersson, Chuang, Sørgård, Di Marzo, Julius and Högestätt1999). TRPV1 is preferentially activated at high temperatures (>42’C) (Caterina et al., Reference Caterina, Schumacher, Tominaga, Rosen, Levine and Julius1997), whereas anandamide binds to this channel with low affinity, as compared to CB1 (Devane et al., Reference Devane, Hanuš, Breuer, Pertwee, Stevenson, Griffin, Gibson, Mandelbaum, Etinger and Mechoulam1992; Ross, Reference Ross2003). These particularities, however, do not refute the possibility that anandamide acts as an endogenous TRPV1 agonist. Indeed, TRPV1 activity is controlled by various other mechanisms in addition to temperature, including calmodulin (Numazaki et al., Reference Numazaki, Tominaga, Takeuchi, Murayama, Toyooka and Tominaga2003), ATP (Lishko et al., Reference Lishko, Procko, Jin, Phelps and Gaudet2007), calcineurin (Docherty et al., Reference Docherty, Yeats, Bevan and Boddeke1996) and several kinases, such as PKA or PKC (Premkumar and Ahern, Reference Premkumar and Ahern2000; De Petrocellis et al., Reference De Petrocellis, Harrison, Bisogno, Tognetto, Brandi, Smith, Creminon, Davis, Geppetti and Di Marzo2001; Numazaki et al., Reference Numazaki, Tominaga, Takeuchi, Murayama, Toyooka and Tominaga2003). The action of these enzymes on TRPV1 may modify its response to its ligands (e.g. anandamide) and enable its activation at physiologic temperatures (Premkumar and Ahern, Reference Premkumar and Ahern2000; De Petrocellis et al., Reference De Petrocellis, Harrison, Bisogno, Tognetto, Brandi, Smith, Creminon, Davis, Geppetti and Di Marzo2001; Numazaki et al., Reference Numazaki, Tominaga, Takeuchi, Murayama, Toyooka and Tominaga2003). Therefore, it is not surprising that anandamide was initially described as a full or partial agonist (Zygmunt et al., Reference Zygmunt, Petersson, Andersson, Chuang, Sørgård, Di Marzo, Julius and Högestätt1999; Ross, Reference Ross2003) depending on the conditions determining TRPV1 conformation.
Notwithstanding these similarities, CB1 and TRPV1 differ in several aspects. Firstly, although both CB1 and TRPV1 are expressed in brain structures related to emotion and cognition, CB1 is usually expressed in presynaptic neurones (Katona et al., Reference Katona, Sperlágh, Sík, Käfalvi, Vizi, Mackie and Freund1999), whereas TRPV1 is thought to predominate in postsynaptic neurones (Tóth et al., Reference Tóth, Boczán, Kedei, Lizanecz, Bagi, Papp, Edes, Csiba and Blumberg2005; Zhao and Tsang, Reference Zhao and Tsang2017). Remarkably, despite different cellular locations, TRPV1 and CB1 are often co-expressed in the same synapsis. Regarding the mechanisms, CB1 is one of the most highly expressed G-protein-coupled receptors in the brain (Tsou et al., Reference Tsou, Brown, Sañudo-Peña, Mackie and Walker1998; Busquets-Garcia, et al., Reference Busquets-Garcia, Bains and Marsicano2018), usually coupled to a Gαi/o protein (Busquets-Garcia et al., Reference Busquets-Garcia, Bains and Marsicano2018). Thus, when activated, CB1 inhibits adenylate cyclase, activates inwardly rectifying K+ channels and decreases neurotransmitters release (Howlett et al., Reference Howlett, Barth, Bonner, Cabral, Casellas, Devane, Felder, Herkenham, Mackie, Martin, Mechoulam and Pertwee2002), regulating depolarisation-induced suppression of both inhibition and excitation (Ohno-Shosaku et al., Reference Ohno-Shosaku, Maejima and Kano2001; Wilson and Nicoll, Reference Wilson and Nicoll2001; Uchigashima et al., Reference Uchigashima, Narushima, Fukaya, Katona, Kano and Watanabe2007). Conversely, TRPV1 is a non-selective cation channel, highly permeable to Ca2+ (Caterina et al., Reference Caterina, Schumacher, Tominaga, Rosen, Levine and Julius1997). Once activated, it promotes an increase in intracellular Na+ and Ca2+, with subsequent increase in neuronal activity (Marinelli et al., Reference Marinelli, Pascucci, Bernardi, Puglisi-Allegra and Mercuri2005; Starowicz et al., Reference Starowicz, Maione, Cristino, Palazzo, Marabese, Rossi, de Novellis and Di Marzo2007). In addition, since eCB synthesis and release can be triggered by Ca2+ (Alger, Reference Alger2002), TRPV1 activation may in turn increase eCB tonus (Maccarrone et al., Reference Maccarrone, Rossi, Bari, De Chiara, Fezza, Musella, Gasperi, Prosperetti, Bernardi, Finazzi-Agrò, Cravatt and Centonze2008). Finally, although both CB1 and TRPV1 are activated by anandamide, this compound has at least twenty times more affinity for the former (Ross, Reference Ross2003; van der Stelt et al., Reference van der Stelt, Trevisani, Vellani, De Petrocellis, Schiano Moriello, Campi, McNaughton, Geppetti and Di Marzo2005).
This body of evidence endorses the hypothesis that anandamide, CB1 and TRPV1 configure a tripartite system regulating neuronal activity in the brain (Fig. 1). In synapsis expressing both receptors, low levels of anandamide activate presynaptic CB1 receptors, decreasing neurotransmitter release, whereas higher levels of anandamide may also recruit TRPV1 receptors, increasing neuronal activity and counterbalancing CB1-mediated effects. Here, we make the case for the possibility that the dual action of anandamide upon CB1 and TRPV1 may also participate in the modulation of learned fear. Our hypothesis will be built upon pharmacological and genetic studies to investigate the role of each receptor in fear-conditioned paradigms, considering the various phases of fear-learning processing.
Role of CB1 in fear conditioning
Studies using either genetic or pharmacological approaches support the involvement of CB1 in the regulation of fear conditioning. Indeed, initial studies by Marsicano and colleagues revealed that CB1-deficient mice presented impaired fear extinction when subjected to a tone previously paired with footshock (Marsicano et al., Reference Marsicano, Wotjak, Azad, Bisogno, Rammes, Cascio, Hermann, Tang, Hofmann, Zieglgänsberger, Di Marzo and Lutz2002). Regarding the effects of pharmacological interventions, the modulation of fear by drugs targeting CB1 and other molecular components of the ECS may differ depending on the specificity of the compound, dosage and intensity of conditioning. As elaborated below, it also varies according to the memory phase in which the intervention occurred (acquisition, consolidation, expression and extinction).
Acquisition of fear memory
Several studies, using pharmacological approaches, have implicated CB1 in the acquisition of fear memory. The administration of CB1 antagonists and inverse agonists, such as AM4113 (6mg/kg) and AM251 (4 and 8 mg/kg), respectively, impaired fear acquisition in an auditory fear conditioning task (Sink et al., Reference Sink, Segovia, Collins, Markus, Vemuri, Makriyannis and Salamone2010). However, in another study, AM251 (5mg/kg) was found to enhance the subsequent fear responses in both trace (HPC-dependent) and delayed (amygdala-dependent) fear conditioning; these effects were further increased when the drugs were administered before both conditioning and expression (Reich et al., Reference Reich, Mohammadi and Alger2008). Similarly, Sink and colleagues observed that AM251 enhanced the acquisition of fear conditioned to a context, while no effect was noticed after AM4113 treatment (Sink et al., Reference Sink, Segovia, Collins, Markus, Vemuri, Makriyannis and Salamone2010). Regarding cannabinoid agonists, the non-selective CB1, agonist WIN55,212-2 (2.5 and 5mg/kg), impaired contextual but not auditory fear conditioning (Pamplona and Takahashi, Reference Pamplona and Takahashi2006). These effects were prevented by pre-treatment with selective CB1 antagonists, such as SR141716A or SR147778 (Pamplona and Takahashi, Reference Pamplona and Takahashi2006).
Consolidation
Studies focusing on the role of the ECS on fear memory consolidation observed that anandamide release restrained this process, an effect prevented by a subeffective concentration of AM251 (Scienza-Martin et al., Reference Scienza-Martin, Lotz, Zanona, Santana-Kragelund, Crestani, Boos, Calcagnotto and Quillfeldt2022). Also, the CB1 agonists, HU-210 (Maćkowiak et al., Reference Maćkowiak, Chocyk, Dudys and Wedzony2009) and ACPA (Nasehi et al., Reference Nasehi, Davoudi, Ebrahimi-Ghiri and Zarrindast2016), impaired fear consolidation in both contextual and auditory fear conditioning. As expected, HU-210 effect was blocked when co-administered with AM251 (Maćkowiak et al., Reference Maćkowiak, Chocyk, Dudys and Wedzony2009). Similarly, the phytocannabinoid cannabidiol impaired memory consolidation via CB1, since its effect was prevented by AM251, although also by the CB2 antagonist, AM630 (Stern et al., Reference Stern, da Silva, Raymundi, de Souza, Hiroaki-Sato, Kato, Guimarães, Andreatini, Takahashi and Bertoglio2017). This effect was observed either with systemic or local (dorsal HPC) administration (Stern et al., Reference Stern, da Silva, Raymundi, de Souza, Hiroaki-Sato, Kato, Guimarães, Andreatini, Takahashi and Bertoglio2017).
Retrieval
In studies investigating the role of CB1 in fear memory retrieval, the CB1 antagonist, SR141716 (1, 5 mg/kg), was ineffective (Mizuno et al., Reference Mizuno, Matsuda, Tohyama and Mizutani2022). However, the non-selective agonist WIN55,212-2 (0.25 mg/kg) decreased contextual fear responses when animals were subjected to a more intense, but shorter protocol (1 × of 1.5mA) (Pamplona et al., Reference Pamplona, Bitencourt and Takahashi2008). When the intensity of the aversive stimulus was lower, but its duration was longer (3 × of 0.75 mA), WIN55,212-2 enhanced fear memory retrieval at both doses tested (0.075mg/kg and 0.75 mg/kg) in males, but just at the highest one in females (Mizuno et al., Reference Mizuno, Matsuda, Tohyama and Mizutani2022).
Regarding compounds that indirectly facilitated the ECS, JZL184, an inhibitor of 2-AG hydrolysis, increased freezing in females (8 mg/kg), but not in male mice, an effect mediated by CB1, but not CB2 receptors. The FAAH inhibitor URB597 (0.3, 1, 3 mg/kg) was ineffective (Mizuno et al., Reference Mizuno, Matsuda, Tohyama and Mizutani2022).
Concerning the brain regions involved, no effect was verified by administering AM251 into the ventromedial PFC (Lisboa et al., Reference Lisboa, Reis, da Silva, Corrêa, Guimarães and Resstel2010; Simone et al., Reference Simone, Green, Hodges and McCormick2015), although this CB1 antagonist did increase freezing in animals exposed to a less aversive conditioning protocol (Lisboa et al., Reference Lisboa, Reis, da Silva, Corrêa, Guimarães and Resstel2010). Local injection of anandamide (5 pmol/200 nl) or the anandamide transport inhibitor, AM404 (50 pmol/200 nl), into this region, attenuated the fear-conditioned responses, a result prevented by local pre-treatment with AM251 (100 pmol/200 nl). No effect was also reported in auditory fear conditioning after ACEA, another CB1 agonist (Simone et al., Reference Simone, Green, Hodges and McCormick2015). Finally, when administered locally into the PAG, 2-AG decreased freezing response, an effect prevented by AM251 (Brianis et al., Reference Brianis, Lima, Moreira and Aguiar2022).
Extinction
The importance of CB1 in the extinction of aversive memories was observed through several strategies. For instance, CB1-deficient mice showed impaired short- and long-term extinction, and this was mimicked by the administration of the CB1 antagonist, SR141716A, to wild-type animals (Marsicano et al., Reference Marsicano, Wotjak, Azad, Bisogno, Rammes, Cascio, Hermann, Tang, Hofmann, Zieglgänsberger, Di Marzo and Lutz2002). The extinction of the auditory fear conditioning was impaired by the administration of another CB1 antagonist/inverse agonist, AM251 (3 mg/kg), and facilitated by the selective agonist, ACEA (0,1 and 0,5 mg/kg) (Simone et al., Reference Simone, Green, Hodges and McCormick2015).
Accordingly, a selective CB1 antagonist disrupted extinction, while administration of WIN55,212–2 resulted in the opposite effect (Pamplona et al., Reference Pamplona, Prediger, Pandolfo and Takahashi2006). The facilitation of fear extinction was also observed in the extinction of remote memories, an effect prevented by a CB1 antagonist (Pamplona et al., Reference Pamplona, Prediger, Pandolfo and Takahashi2006). In another study, however, the administration of WIN55,212-2 (0.075, 0.75 mg/kg) inhibited fear extinction in both sexes (Mizuno et al., Reference Mizuno, Matsuda, Tohyama and Mizutani2022). Regarding the direct modulation of endocannabinoid hydrolysis, extinction impairments were reported in animals treated with a DAGL inhibitor as well as in animals lacking DAGL (DAGLα−/− mice) (Cavener et al., Reference Cavener, Gaulden, Pennipede, Jagasia, Uddin, Marnett and Patel2018). In addition, 2-AG or anandamide facilitation, with JZL184 (4 or 8 mg/kg) and URB597 (0.3, 1, 3 mg/kg), respectively, inhibited fear extinction on day 5; however, such differences disappeared on the last day of extinction (day 28) for both sexes (Mizuno et al., Reference Mizuno, Matsuda, Tohyama and Mizutani2022).
Therefore, the predominant effect of facilitating endocannabinoid signalling is the inhibition of fear responses, whereas its blockade tends to induce the opposite response. These effects tend to occur particularly in protocols in which the animals are exposed to aversive stimuli of moderate intensity.
Role of TRPV1 in fear conditioning
The literature on TRPV1 and fear is scant, in comparison to the more robust evidence discussed for CB1. One of the earliest studies implicating TRPV1 in fear memory reported phenotypic differences between TRPV1 KO and wild-type animals in the auditory fear conditioning (Marsch et al., Reference Marsch, Foeller, Rammes, Bunck, Kössl, Holsboer, Zieglgänsberger, Landgraf, Lutz and Wotjak2007); knock out animals displayed lower levels of freezing when evaluated shortly or remotely after conditioning, while unconditioned fear responses and pain threshold remained unchanged as compared to controls (Marsch et al., Reference Marsch, Foeller, Rammes, Bunck, Kössl, Holsboer, Zieglgänsberger, Landgraf, Lutz and Wotjak2007). The responses seemed to be dependent on the intensity of the conditioning (Marsch et al., Reference Marsch, Foeller, Rammes, Bunck, Kössl, Holsboer, Zieglgänsberger, Landgraf, Lutz and Wotjak2007). This early study suggested that TRPV1 may be involved in fear memory, but whether its role was relevant for acquisition, consolidation or retrieval was still unknown.
Acquisition
Pharmacological studies revealed no effect after the administration of a TRPV1 blocker, capsazepine, before conditioning, although administration of capsaicin, a TRPV1 agonist, had biphasic effects – low doses enhanced acquisition and high doses impaired it (Almeida et al., Reference Almeida, Levin, Peres, Suiama, Vendramini, Santos, Silva, Zuardi, Hallak, Crippa and Abílio2019). A potential explanation for this biphasic profile is the fast desensitisation of TRPV1 induced by even single doses of capsaicin (Szallasi and Di Marzo, Reference Szallasi and Di Marzo2000; Almeida et al., Reference Almeida, Levin, Peres, Suiama, Vendramini, Santos, Silva, Zuardi, Hallak, Crippa and Abílio2019).
Consolidation
In agreement with the results obtained from experiments in knockout mice, intra-hippocampal administration of capsazepine impaired memory consolidation when the animals were exposed to high intensities of the aversive stimulus, although no effect was observed after capsaicin administration (Genro et al., Reference Genro, de Oliveira and Quillfeldt2012). Interestingly, this effect was replicated in a more recent study (Scienza-Martin et al., Reference Scienza-Martin, Lotz, Zanona, Santana-Kragelund, Crestani, Boos, Calcagnotto and Quillfeldt2022).
Retrieval
Local administration of TRPV1 blockers (capsazepine or 6-iodonordihydrocapsaicin) into the ventromedial PFC decreased behavioural and autonomic responses to contextual fear learning (Terzian et al., Reference Terzian, dos Reis, Guimarães, Corrêa and Resstel2014). However, the effect of 6-iodonordihydrocapsaicin was not observed when the animals were exposed to aversive stimuli of low intensity (Terzian et al., Reference Terzian, dos Reis, Guimarães, Corrêa and Resstel2014). In spite of this, at this intensity, this compound was able to prevent the enhancement of conditioned responses induced by central administration of capsaicin (Terzian et al., Reference Terzian, dos Reis, Guimarães, Corrêa and Resstel2014). The augmentation of behavioural and autonomic responses induced by capsaicin administration into the ventromedial PFC was replicated in another study (Uliana et al., Reference Uliana, Antero, Borges-Assis, Rosa, Vila-Verde, Lisboa and Resstel2020). Finally, TRPV1 blockers directly administered into the dorsal HPC impaired memory retrieval when the mice were exposed to footshocks of moderate-to-high, but not low, intensities (Iglesias et al., Reference Iglesias, Fernandes, de Miranda, Perez, Faccioli, Sorgi, Bertoglio, Aguiar, Wotjak and Moreira2023).
Extinction
The potent TRPV1 inhibitor iodoresiniferatoxin administered before conditioning, retrieval and extinction enhanced fear extinction without affecting other phases (Laricchiuta et al., Reference Laricchiuta, Centonze and Petrosini2013). However, in the auditory fear conditioning, an acute administration of SB366791 had no effect (Llorente-Berzal et al., Reference Llorente-Berzal, Terzian, di Marzo, Micale, Viveros and Wotjak2015).
In summary, the scarce literature suggests that activation of TRPV1 channels exacerbates fear memory with a biphasic profile, possibly associated with fast desensitisation of TRPV1 channels at certain doses of agonists (Szallasi and Di Marzo, Reference Szallasi and Di Marzo2000; Almeida et al., Reference Almeida, Levin, Peres, Suiama, Vendramini, Santos, Silva, Zuardi, Hallak, Crippa and Abílio2019). On the contrary, genetic deletion as well as pharmacological blockade of TRPV1 promotes anti-aversive responses. This effect depends on the intensity of the aversive experience, probably due to the tonus of the ECS, as will be discussed below.
TRPV1 and (endo)-cannabinoid interactions
Although the aforementioned studies focused on CB1 and TRPV1 separately, we argue that these receptors function in concert to mediate opposite functions of anandamide. In this section, we built on the hypothesis that anandamide, CB1 and TRPV1 form a tripartite system modulating fear memory based on three sets of evidence: First, CB1 and TRPV1 are co-localized in fear-related brain regions; second, they interfere with common downstream pathways involved in fear memory. Finally, they depend on each other to mediate the effects of selective pharmacological interventions.
CB1 and TRPV1 are co-localized in fear-related structures
The presence of CB1 in brain circuits modulating fear learning is supported by an enormous and consistent body of evidence (Tsou et al., Reference Tsou, Brown, Sañudo-Peña, Mackie and Walker1998; Wilson-Poe et al., Reference Wilson-Poe, Morgan, Aicher and Hegarty2012; Lazenka et al., Reference Lazenka, Selley and Sim-Selley2013; Gomes-de-Souza et al., Reference Gomes-de-Souza, Bianchi, Costa-Ferreira, Tomeo, Cruz and Crestani2021). Moreover, histological studies with specific antibodies found CB1 to be co-expressed with TRPV1 in several synapses of fear-related regions (Cristino et al., Reference Cristino, de Petrocellis, Pryce, Baker, Guglielmotti and Di Marzo2006). Additional studies in specific brain regions observed TRPV1 and CB1 colocalization in the PFC (Fogaça et al., Reference Fogaça, Aguiar, Moreira and Guimarães2012; Diniz et al., Reference Diniz, Biojone, Joca, Rantamäki, Castrén, Guimarães and Casarotto2019), the PAG (Casarotto et al., Reference Casarotto, Terzian, Aguiar, Zangrossi, Guimarães, Wotjak and Moreira2012) and the dorsomedial hypothalamus (Dos Anjos-Garcia & Coimbra, Reference Dos Anjos-Garcia and Coimbra2019). In the dorsal HPC, the use of high-resolution confocal microscopy and z-stack three-dimensional analysis also revealed co-expression of CB1 and TRPV1 (Iglesias et al., Reference Iglesias, Fernandes, de Miranda, Perez, Faccioli, Sorgi, Bertoglio, Aguiar, Wotjak and Moreira2023). Therefore, studies applying histological immuno-histochemical techniques, along with microscopy analysis, support the possibility that CB1 and TRPV1 can be simultaneously activated by anandamide in fear-related brain regions, provided the local synaptic concentration of these endocannabinoids reaches levels high enough to bind both receptors.
CB1 and TRPV1 functions modulate common fear memory-related pathways
Neurotrophic signalling exerts several functions in the brain, including modulation of fear memory (Notaras & van den Buuse, Reference Notaras and van den Buuse2020). Activation of tyrosine receptor kinase B (TrkB) by brain-derived neurotrophic factor (BDNF) leads to the regulation of several downstream pathways involved in plasticity, such mTOR, Akt, CamKII or ERK, all of them crucial for the consolidation of fear memory (Minichiello, Reference Minichiello2009). The link between neurotrophic and anandamide-CB1 signalling has been extensively explored in recent years. On the one hand, BDNF and TrkB activation enhances endocannabinoids release (Yeh et al., Reference Yeh, Selvam and Levine2017; Wu et al., Reference Wu, Liu, Guo, Ye, Ge and Xue2020); on the other hand, certain effects derived from CB1 activation are mediated by BDNF (Blázquez et al., Reference Blázquez, Chiarlone, Bellocchio, Resel, Pruunsild, García-Rincón, Sendtner, Timmusk, Lutz, Galve-Roperh and Guzmán2015; Navabpour et al., Reference Navabpour, Rezayof and Ghasemzadeh2021). As for TRPV1, its relation with BDNF remains unknown. However, one study showed that TRPV1-mediated synaptogenesis in the HPC seems to require BDNF (Hurtado-Zavala et al., Reference Hurtado-Zavala, Ramachandran, Ahmed, Halder, Bolleyer, Awasthi, Stahlberg, Wagener, Anderson, Drenan, Lester, Miwa, Staiger, Fischer and Dean2017). Despite the scarcity of data, one could hypothesise that, if neurotrophic signalling increases endocannabinoid tonus, this may facilitate the recruitment of TRPV1 and its involvement in some of the BDNF-related effects. Indeed, an in vitro study showed that anandamide enhances TrkB phosphorylation (Diniz et al., Reference Diniz, Biojone, Joca, Rantamäki, Castrén, Guimarães and Casarotto2019). Interestingly, the mechanism underlying this effect is dose-dependent, with low doses of anandamide acting through CB1, whereas at higher doses, anandamide action occurs through TRPV1 activation (Diniz et al., Reference Diniz, Biojone, Joca, Rantamäki, Castrén, Guimarães and Casarotto2019).
Another important player in fear and memory is the nitric oxide (NO) pathway (Susswein et al., Reference Susswein, Katzoff, Miller and Hurwitz2004; Medeiros et al., Reference Medeiros, Almeida-Souza, Silva, Santos, Santos, Gois, Leal and Santos2022). In postsynaptic neurones, calcium influx and calmodulin activation promote neuronal nitric oxide synthase (nNOS) activity, with the subsequent retrograde effects of NO and enhancement in neurotransmitter release (Huang, Reference Huang1997). Several pieces of evidence point to the involvement of nNOS/NO in plasticity and specifically in the modulation of certain fear memory phases (Sadeghi et al., Reference Sadeghi, Hemmati, Nassireslami, Yousefi, Hosseini, Abbasian and Chamanara2022). More important for the scope of this review, the ECS and the nitric oxide pathway seem to interact to modulate learned fear. For instance, the enhancement of the endocannabinoid tonus, by means of FAAH inhibition, prevented the extinction deficits in mice with genetic deletion of inducible NOS, iNOS (Lisboa et al., Reference Lisboa, Gomes, Silva, Uliana, Camargo, Guimarães, Cunha, Joca and Resstel2015), suggesting that the ECS may be a downstream effector of NO or at least able to compensate for some of its effects. Moreover, facilitation of fear retrieval induced by TRPV1 agonism or CB1 antagonism was prevented by a subeffective dose of a NO scavenger, NO inhibitors and a soluble guanylate cyclase inhibitor (Uliana et al., Reference Uliana, Hott, Lisboa and Resstel2016). Similar results were observed in the ventromedial PFC (Uliana et al., Reference Uliana, Antero, Borges-Assis, Rosa, Vila-Verde, Lisboa and Resstel2020). In addition, NOS inhibition blocked the LTP induced by a TRPV1 agonist in the AMG, the same effect being elicited by a CB1 antagonist (Zschenderlein et al., Reference Zschenderlein, Gebhardt, von Bohlen und Halbach, Kulisch, Albrecht and Baccei2011). These data suggest that TRPV1 and CB1 act in opposite directions upon the NO pathway, which may partially explain their contrasting role in modulating fear memory.
Furthermore, CB1 and TRPV1 interact in the modulation of electrophysiological processes underlying memory and neuronal plasticity. For instance, in the neocortex and striatum, spike time-dependent LTP was blocked by both TRPV1 and CB1 antagonism (Cui et al., Reference Cui, Paillé, Xu, Genet, Delord, Fino, Berry and Venance2015, Reference Cui, Perez and Venance2018). Similarly, anandamide induced long-term depression (LTD) through both CB1 and TRPV1 in the Nac (Grueter et al., Reference Grueter, Brasnjo and Malenka2010). In the dentate gyrus, CB1 and TRPV1 agonists seem to modulate excitatory postsynaptic field potentials and LTP in opposite directions (Tahmasebi et al., Reference Tahmasebi, Komaki, Karamian, Shahidi, Sarihi, Salehi and Nikkhah2015). However, this intersection between CB1 and TRPV1 remains controversial. For example, anandamide facilitated LTD through TRPV1 but not CB1 (Yang et al., Reference Yang, Lei, Xie, MacDonald and Jackson2014), while other reports indicated that anandamide rescued impaired hippocampal LTP through CB1 activation (Basavarajappa et al., Reference Basavarajappa, Nagre, Xie and Subbanna2014).
Some of these processes may be explained by the capacity of TRPV1 and CB1 to modulate glutamatergic neurotransmission. For example, CB1 activation in the hypothalamus decreases glutamate release, while the opposite goes for TRPV1 (Jamieson et al., Reference Jamieson, Kim and Iremonger2022). Similarly, CB1 and TRPV1 presented opposite effects on NMDA-induced autonomic responses (Lagatta et al., Reference Lagatta, Kuntze, Ferreira-Junior and Resstel2018) and plasticity (Back & Carobrez, Reference Back and Carobrez2018). These examples suggest that TRPV1 and CB1 may act upon common mechanism in the regulation of fear memory, usually leading to opposite outcomes.
Anandamide, CB1 and TRPV1 interact to modulate fear responses
Complementing the histological and neurochemical evidence, the last section will focus on studies using pharmacological interventions in animals exposed to fear conditioning. Administration of anandamide itself, compounds that inhibit its hydrolysis (by inhibiting FAAH blockers, such as URB597) or compounds that exert dual TRPV1 and FAAH blockade (e.g., AA-5-HT) provide evidence of opposite functions for CB1 and TRPV1 in mediating the actions of anandamide in different phases of fear responses.
Acquisition
The systemic administration of the FAAH inhibitor URB597 had no effect on the acquisition of fear memory (Laricchiuta et al., Reference Laricchiuta, Centonze and Petrosini2013; Balogh et al., Reference Balogh, Szente, Biro, Varga, Haller and Aliczki2019). Similarly, local administration of this drug into the ventral HPC, prelimbic PFC or AMG had no effects on memory retrieval (Balogh et al., Reference Balogh, Szente, Biro, Varga, Haller and Aliczki2019). However, direct administration of anandamide into the NAC core impaired the acquisition of contextual, but not auditory, fear (Pedroza-Llinás et al., Reference Pedroza-Llinás, Méndez-Díaz, Ruiz-Contreras and Prospéro-García2013). Likewise, after systemic FAAH inhibition by the compound OL-135, an impairment in the acquisition of the contextual fear conditioning was observed (Burman et al., Reference Burman, Szolusha, Bind, Kerney, Boger and Bilsky2016), but no effect was detected in the auditory fear conditioning (Burman et al., Reference Burman, Szolusha, Bind, Kerney, Boger and Bilsky2016). Contextual and auditory fear conditioning rely on different brain structures, specifically, the dorsal portion of the HPC seems involved in contextual but not in auditory fear conditioning (Phillips and LeDoux, Reference Phillips and LeDoux1992). Even though FAAH is expressed in the amygdala, which is involved in both tasks (Gulyas et al., Reference Gulyas, Cravatt, Bracey, Dinh, Piomelli, Boscia and Freund2004), its inhibition might not be relevant to the acquisition of the auditory fear conditioning (Burman et al., Reference Burman, Szolusha, Bind, Kerney, Boger and Bilsky2016). Instead, the disruption observed in the contextual task after increasing endocannabinoid tonus (Burman et al., Reference Burman, Szolusha, Bind, Kerney, Boger and Bilsky2016) may be related to the action of this drug in structures such as the dorsal HPC, which is not involved in auditory fear conditioning. However, this effect seems to depend on the type of modulation, since the impairment in fear acquisition was observed with OL-135 (Burman et al., Reference Burman, Szolusha, Bind, Kerney, Boger and Bilsky2016), but not with URB597 (Laricchiuta et al., Reference Laricchiuta, Centonze and Petrosini2013; Balogh et al., Reference Balogh, Szente, Biro, Varga, Haller and Aliczki2019). This may be related to differences in the way how these compounds inhibit FAAH (Naidu et al., Reference Naidu, Varvel, Ahn, Cravatt, Martin and Lichtman2007) or off target enzymes (Zhang et al., Reference Zhang, Saraf, Kolasa, Bhatia, Zheng, Patel, Lannoye, Richardson, Stewart, Rogers, Brioni and Surowy2007).
Resembling URB597 effects, the dual FAAH/TRPV1 blocker, AA-5-HT, had no effect on the acquisition of contextual fear conditioning (Gobira et al., Reference Gobira, Lima, Batista, de Oliveira, Resstel, Wotjak, Aguiar and Moreira2017). However, the non-selective anandamide reuptake blocker and TRPV1 agonist, AM404, impaired fear acquisition, an effect dependent on both TRPV1 and CB1, since it was prevented by capsazepine and by rimonabant (Almeida et al., Reference Almeida, Levin, Peres, Suiama, Vendramini, Santos, Silva, Zuardi, Hallak, Crippa and Abílio2019). Similarly, intra-HPC administration of AM404 prevented memory acquisition via CB1 activation (Lin et al., Reference Lin, Yang, Liu, Sun, Dang, Liang, Wang, Chen and Li2011).
The discrepancies between the administration of FAAH dual blockers and AM404 may rely on two different but complementary hypotheses. First, the levels of anandamide depend on intensity/aversiveness of the experience (Morena et al., Reference Morena, Roozendaal, Trezza, Ratano, Peloso, Hauer, Atsak, Trabace, Cuomo, McGaugh, Schelling and Campolongo2014; Iglesias et al., Reference Iglesias, Fernandes, de Miranda, Perez, Faccioli, Sorgi, Bertoglio, Aguiar, Wotjak and Moreira2023), thus low intensities may not promote enough release of anandamide and its hydrolysis inhibition will not reach a substantial effect. Indeed, Gobira (2017) showed no effects of AA-5-HT, while Almeida and colleagues (2019) did observe fear inhibition after AM404 administration. However, the intensity of the conditioning was much higher in the latter study. Alternatively, AM404 may act as a partial agonist at TRPV1 (Ross, Reference Ross2003).
Consolidation
During the consolidation of fear memory, increased levels of eCB were observed in the basolateral AMG and the HPC, but not in the PFC (Marsicano et al., Reference Marsicano, Wotjak, Azad, Bisogno, Rammes, Cascio, Hermann, Tang, Hofmann, Zieglgänsberger, Di Marzo and Lutz2002; Morena et al., Reference Morena, Roozendaal, Trezza, Ratano, Peloso, Hauer, Atsak, Trabace, Cuomo, McGaugh, Schelling and Campolongo2014). In the HPC, anandamide levels seem to depend on intensity of aversive stimuli (Morena et al., Reference Morena, Roozendaal, Trezza, Ratano, Peloso, Hauer, Atsak, Trabace, Cuomo, McGaugh, Schelling and Campolongo2014). However, post-training administration of OL-135 (Burman et al., Reference Burman, Szolusha, Bind, Kerney, Boger and Bilsky2016) or anandamide (intra-NAc) (Pedroza-Llinás et al., Reference Pedroza-Llinás, Méndez-Díaz, Ruiz-Contreras and Prospéro-García2013) did not impair contextual or auditory fear conditioning. On the other hand, similarly to the acquisition, the administration of AM404, an inhibitor of anandamide reuptake, disrupted contextual fear consolidation when administered into the CA1 area of the dorsal HPC (Scienza-Martin et al., Reference Scienza-Martin, Lotz, Zanona, Santana-Kragelund, Crestani, Boos, Calcagnotto and Quillfeldt2022).
Retrieval
Anandamide levels increase in the basolateral AMG after retrieval of fear memory (Gaspar et al., Reference Gaspar, Okine, Dinneen, Roche and Finn2022). The same was observed in the HPC, where anandamide levels increase as a function of fear intensity (Iglesias et al., Reference Iglesias, Fernandes, de Miranda, Perez, Faccioli, Sorgi, Bertoglio, Aguiar, Wotjak and Moreira2023). Moreover, the direct administration of anandamide into the medial PFC (Lisboa et al., Reference Lisboa, Reis, da Silva, Corrêa, Guimarães and Resstel2010) or the PAG (Resstel et al., Reference Resstel, Lisboa, Aguiar, Corrêa and Guimarães2008) reduced freezing. Furthermore, administration of AM404 systemically (Pamplona et al., Reference Pamplona, Bitencourt and Takahashi2008), into the PFC (Lisboa et al., Reference Lisboa, Reis, da Silva, Corrêa, Guimarães and Resstel2010), PAG (Resstel et al., Reference Resstel, Lisboa, Aguiar, Corrêa and Guimarães2008) or into the HPC (Scienza-Martin et al., Reference Scienza-Martin, Lotz, Zanona, Santana-Kragelund, Crestani, Boos, Calcagnotto and Quillfeldt2022), mimicked anandamide effects. AM404 effects on retrieval were prevented by a CB1 antagonists (Lisboa et al., Reference Lisboa, Reis, da Silva, Corrêa, Guimarães and Resstel2010; Llorente-Berzal et al., Reference Llorente-Berzal, Terzian, di Marzo, Micale, Viveros and Wotjak2015) and by a TRPV1 blocker (Llorente-Berzal et al., Reference Llorente-Berzal, Terzian, di Marzo, Micale, Viveros and Wotjak2015). In addition, AA-5-HT (a dual FAAH/TRPV1 blocker) administered systemically or into the HPC impaired fear memory retrieval, an effect prevented by pre-treatment with AM251 (Gobira et al., Reference Gobira, Lima, Batista, de Oliveira, Resstel, Wotjak, Aguiar and Moreira2017). Remarkably, AA-5-HT effects were mimicked by co-administration of subeffective doses of a FAAH inhibitor with a TRPV1 blocker (Gobira et al., Reference Gobira, Lima, Batista, de Oliveira, Resstel, Wotjak, Aguiar and Moreira2017), supporting the hypothesis of opposite roles for CB1 and TRPV1. In agreement with this possibility 1) the administration of a CB1 antagonist or a TRPV1 agonist into the dorsolateral PAG induced the same effect on fear expression (Uliana et al., Reference Uliana, Hott, Lisboa and Resstel2016); 2) a CB1 antagonist prevented the retrieval deficits induced by TRPV1 blockers in the HPC (Iglesias et al., Reference Iglesias, Fernandes, de Miranda, Perez, Faccioli, Sorgi, Bertoglio, Aguiar, Wotjak and Moreira2023) and 3) a TRPV1 blocker prevented the enhancement of memory retrieval induced by CB1 antagonists in the PAG (Uliana et al., Reference Uliana, Hott, Lisboa and Resstel2016). Altogether, these data support our proposal that CB1 and TRPV1 act in concert to mediate opposite functions of anandamide in the control of fear responses.
Extinction
Anandamide infusion into the dorsal HPC facilitates extinction, an effect prevented by pre-treatment with AM251 (de Oliveira et al., Reference de Oliveira, Pasqualini, Diehl, Molina and Quillfeldt2008). In addition, administration of AM404 systemically (Pamplona et al., Reference Pamplona, Bitencourt and Takahashi2008), via intracerebroventricular (Bitencourt et al., Reference Bitencourt, Pamplona and Takahashi2008) or directly into the dorsal HPC (Abush and Akirav, Reference Abush and Akirav2010), facilitates fear extinction. These effects seem to depend on the activation of CB1, but not TRPV1 (Bitencourt et al., Reference Bitencourt, Pamplona and Takahashi2008). However, TRPV1 involvement in AM404 effects on extinction was observed in auditory fear conditioning (Llorente-Berzal et al., Reference Llorente-Berzal, Terzian, di Marzo, Micale, Viveros and Wotjak2015).
Conclusion and future directions
The evidence reviewed here supports our hypothesis that anandamide, CB1 and TRPV1 act in concert as a neurochemical system regulating fear memory. Low-intensity aversive stimuli could promote moderate anandamide release, which activates CB1 receptors and decreases the release of glutamatergic neurotransmission, with subsequent inhibition of fear. However, in response to highly aversive stimuli, anandamide levels would further increase; as a result, TRPV1 channels would be activated to promote Ca+2 influx, increase neuronal firing and, finally, activate the neuronal mechanisms promoting fear.
However, some limitations should be considered. For instance, most studies have focused on male rodents as experimental subjects. Since only recently has sex been included as an experimental variable, little is known regarding differences in anandamide/CB1/TRPV1 interactions between males and females. A recent study showed that eCB signalling facilitation had no effect on fear extinction in males, while extinction was impaired in females, probably via TRPV1 activation (Morena et al., Reference Morena, Nastase, Santori, Cravatt, Shansky and Hill2021). Another biological variable to be taken into consideration is development, since TRPV1 (Huang et al., Reference Huang, Min, Liu, He and Peng2014) and CB1 (Liu et al., Reference Liu, Bilkey, Darlington and Smith2003) expression change along the lifespan. Future research should address the impact of these variables in anandamide/CB1/TRPV1 interactions in specific brain regions. Finally, a remaining question is how this tripartite system could be targeted for developing new drugs for the treatment of certain psychiatric disorders, particularly those resulting from exacerbated responses to aversive stimuli.
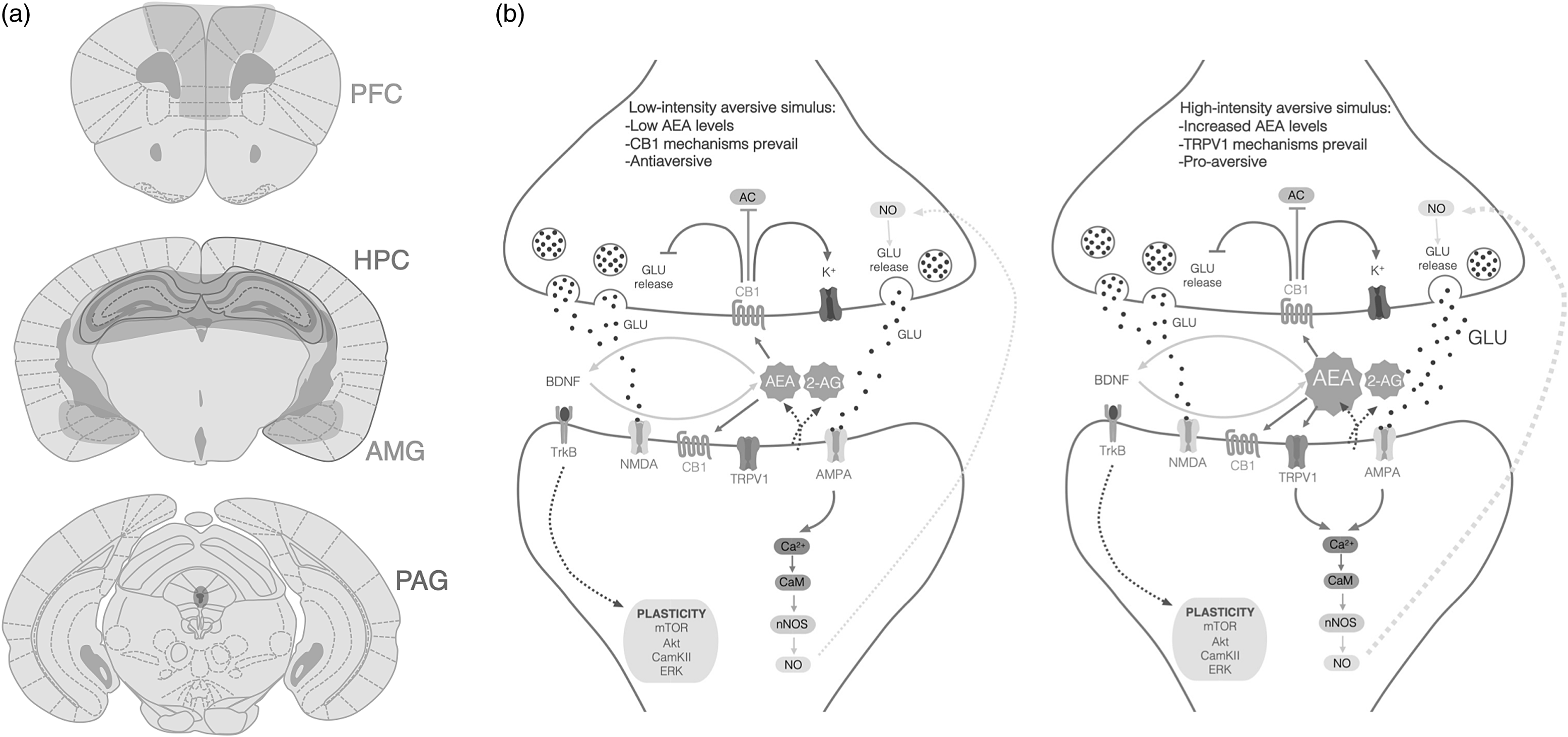
Figure 1. a ) CB1 and TRPV1 receptors are co-localized in brain regions that modulate fear responses, such as the prefrontal cortex (PFC), hippocampus (HPC), amygdala (AMG) and periaqueductal grey (PAG). b ) Molecular pathways involved in fear memory modulated by CB1. receptors and TRPV1 channels in response to low or high aversive stimuli. Under low-intensitiy, aversive stimuli (left panel) CB1 activation by anandamide inhibits adenylate cyclase. (AC), reduces glutamate (GLU) release and activates rectifying potassium (K+) channels. However, as the intensity of the aversive stimulus increases (right panel), anandamide binds to TRPV1 receptors and causes calcium (Ca+2) influx. This leads to calmodulin (CaM) activation and neuronal nitric oxide synthase (nNOS) activity, resulting in nitric oxide (NO) production and its retrograde activity, increasing GLU release (2-AG, 2-arachidonoylglicerol; AC, adenylate cyclase; anandamide, N-arachidonoylehtanolamide or anandamide; Akt, protein kinase B; AMPA, α-amino-3-hydroxy-5-methyl- 4-isoxazolepropionic acid channel; BDNF, brain-derived neurotrophic factor; Ca+2, calcium; CaM, calmodulin; CamKII, calcium–calmodulin (CaM)-dependent protein kinase II; CB1, cannabinoid type 1 receptor; ERK, extracellular signal-regulated kinase; GLU, glutamate; K+, potassium; mTOR, mammalian target of rapamycin; NMDA, N-mehtyl-d- aspartate channel; nNOS, neuronal nitric oxide synthase; NO, nitric oxide; TrkB, receptor tyrosine kinase B; TRPV1, transient receptor vanilloid type-1 channel).
Acknowledgements
FAM thanks Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG; project APQ-00741-21); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; research productivity fellowship, level 2); and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES): ‘O presente trabalho foi realizado com o apoio da Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Código de Financiamento 001’ – ‘ This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001’.
Author contribution
LPA was the main responsible for designing the review. All the authors have contributed to this study by participating in the literature search and writing the article. All authors have agreed on the final version of the manuscript.
Competing interests
None.




