Significant Outcomes
-
No association was found between mental health outcomes and COVID illness severity.
-
Long COVID may be the strongest predictor of neuropsychiatric symptoms amongst people who have been infected with SARS-CoV-2.
-
Changes in immunologic biomarkers were associated with worse outcomes in mental health and quality-of-life measures
Limitations
-
Neuropsychiatric outcomes and quality of life were not measured at admission
-
History of illness prior to COVID infection was not collected
-
No mental health diagnostic interview was administered
-
This study used a small, heterogeneous sample. Studies elsewhere have found an association between psychiatric outcomes and measures of illness severity. It is not possible to determine whether findings of no association was due to the study being underpowered, methodological deficiencies that may have failed to detect an association, or whether there is genuinely no association.
Introduction
Prolonged illness, lasting months after the resolution of acute SARS-CoV-2 infection, continues to gain increased attention. This condition, known as post-COVID condition, post-acute sequelae of SARS-CoV-2 (PASC) or ‘Long COVID’ refers to persistent symptoms usually 3 months from the onset of COVID-19 which generally have an impact on everyday functioning (Soriano et al., Reference Soriano, Murthy, Marshall, Relan and Diaz2021). In addition to the commonly described persistent fatigue and dyspnoea, neuropsychiatric symptoms have also been observed. De novo diagnosis of mood or anxiety, stress, or adjustment disorder in patients without previous history may also occur following SARS-CoV-2 infection, and these symptom clusters may lead to decreased quality of life and increased mortality (Diez-Quevedo et al., Reference Diez-Quevedo, Iglesias-Gonzalez, Giralt-Lopez, Rangil, Sanagustin, Moreira, Lopez-Ramentol, Ibanez-Caparros, Loran and Bustos-Cardona2021). Psychotic symptoms have also been reported from 14 to 90 days following acute infection (Gallo et al., Reference Gallo, Leone, Tarallo, Zoppi and Nicolo2022). Psychiatric symptoms may develop de novo in people with no previous psychiatric history or represent exacerbation of symptoms in people with a history of psychiatric illness, with de novo psychiatric symptoms but not relapse of previous psychiatric illness associated with elevated inflammatory biomarkers interleukin-6 and C-reactive protein (CRP) (Iglesias-Gonzalez et al., Reference Iglesias-Gonzalez, Boigues, Sanagustin, Giralt-Lopez, Cuevas-Esteban, Martinez-Caceres and Diez-Quevedo2022). Reports vary regarding the prevalence of symptoms and associated risk factors, with a 6-month follow-up of 236,379 patients with COVID-19 finding a neurological or psychiatric diagnosis in 33.62% of the cohort with 12.84% as a first diagnosis and a more severe COVID-19 illness found to be a risk factor (Taquet et al., Reference Taquet, Geddes, Husain, Luciano and Harrison2021). Elsewhere, a study of 62,328 COVID-19 patients in China reported rates of stress as 48.1%, depression (26.9%) and anxiety (21.8%) (Bareeqa et al., Reference Bareeqa, Ahmed, Samar, Yasin, Zehra, Monese and Gouthro2021).
Neuropsychiatric symptoms have long been associated with diverse mild to severe infections. They may be caused by the direct effects of the agent on the nervous system, psychological effects of illness, systemic biological effects including immune system activation, adverse effects of medications or combinations of these factors. The real or perceived threat from the COVID-19 pandemic has resulted in increased stress, anxiety and depression in the general community (Salari et al., Reference Salari, Hosseinian-Far, Jalali, Vaisi-Raygani, Rasoulpoor, Mohammadi, Rasoulpoor and Khaledi-Paveh2020), so it is unsurprising that psychological effects of COVID-19 also impact people who are infected. Many viruses like HIV and coronaviruses directly impact the brain (Cheng et al., Reference Cheng, Yang and Gao2020). Activation of immuno-inflammatory systems, especially raised levels of pro-inflammatory cytokines, have been implicated in several psychiatric disorders including schizophrenia, bipolar disorder, major mood disorders, suicidal behaviour, post-traumatic disorder and autism (Leboyer et al., Reference Leboyer, Berk, Yolken, Tamouza, Kupfer and Groc2016). Treatment with antiretroviral agents, including oseltamivir (Tamiflu) (Fuyuno, Reference Fuyuno2007), has been associated with adverse psychiatric effects (Abers et al., Reference Abers, Shandera and Kass2014). A study of people with comorbid COVID-19 and mental illness found low rates of drug–drug interactions between treatments for the two illnesses resulting in mainly drowsiness (4.3% of cases) and borderline QTc prolongation (1.5% of cases) (Arbelo et al., Reference Arbelo, Lopez-Pelayo, Sague, Madero, Pinzon-Espinosa, Gomes-da-Costa, Ilzarbe, Anmella, Llach and Imaz2021).
It is difficult to assess the effect of a pandemic that is in progress; however, there is data from previous pandemics of coronaviruses. A study of the severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) pandemics concluded that, while most patients recover without experiencing mental illness, a significant proportion of patients in the acute stage experienced delirium. There was also a possibility of emergence of depression, anxiety, fatigue, post-traumatic stress disorder and rarer neuropsychiatric syndromes in the longer term (Rogers et al., Reference Rogers, Chesney, Oliver, Pollak, McGuire, Fusar-Poli, Zandi, Lewis and David2020). A similar pattern appears to be emerging for COVID-19.
Neuropsychiatric outcomes and quality of life were investigated in a cross-sectional analysis of 179 people who had been hospitalised for COVID-19 and followed up at 2-month post-discharge. There was moderate impairment of immediate verbal memory and learning (38% of the cohort), delayed verbal memory (11.8%), verbal fluency (34.6%) and working memory (6.1%), as well as neurocognitive impairment in at least one function (58.7%). Rates of anxiety (29.6%), depression (26.8%) and post-traumatic stress disorder (25.1%) were detected using validated screening instruments. Quality of life was assessed using the 12-item Short-Form Health Survey, with low scores for physical and mental components detected in 44.1% and 39.1% of patients, respectively. Delirium and psychiatric morbidity were positively associated with neurocognitive impairment. Female gender was related with increased psychiatric morbidity (Mendez et al., Reference Mendez, Balanza-Martinez, Luperdi, Estrada, Latorre, Gonzalez-Jimenez, Feced, Bouzas, Yepez and Ferrando2021). Elsewhere, 1077 COVID-19 patients were interviewed 2 to 7 months after hospital discharge and administered questionnaires to assess mental health and quality of life. Illness severity was the greatest risk factor for mental health impairments in patients followed up a median of 5.9 months post-discharge (Evans et al., Reference Evans, McAuley, Harrison, Shikotra, Singapuri, Sereno, Elneima, Docherty, Lone and Leavy2021).
More recently, larger studies have confirmed the relationship between SARS-CoV-2 infection and psychiatric symptoms. A study of 236,379 patients found that 33.62% of patients received a neurological or psychiatric diagnosis within 6 months of infection, with 12.84% being a first diagnosis. A more severe COVID-19 illness was the greatest risk factor for a neurological or psychiatric diagnosis (Taquet et al., Reference Taquet, Geddes, Husain, Luciano and Harrison2021).
The ADAPT study is a prospective cohort of adults with confirmed SARS-CoV-2 infection confirmed in Sydney, Australia. Findings from 16- to 32-week follow-ups have been reported elsewhere (Darley et al., Reference Darley, Dore, Byrne, Plit, Brew, Kelleher and Matthews2021; Darley et al., Reference Darley, Dore, Cysique, Wilhelm, Andresen, Tonga, Stone, Byrne, Plit and Masters2021; Phetsouphanh et al., Reference Phetsouphanh, Darley, Wilson, Howe, Munier, Patel, Juno, Burrell, Kent and Dore2022). The aim of the current APADT substudy was to investigate neuropsychiatric outcomes and quality of life in a cohort of patients recovering after SARS-CoV-2 infection including both those managed in hospital and in the community for acute infection. We aim to describe the prevalence, severity and trajectory of persistent psychiatric symptoms. Secondary aims of the study were to identify predictors of mental illness and to investigate the relationship between immunological biomarkers in collected biospecimens and mental illness.
Method
Cohort
In March 2020, a cohort of adults after SARS-CoV-2 infection, confirmed by polymerase chain reaction and who could be contacted, were invited to participate in the ADAPT study. The study was approved by the St Vincent’s Hospital Research Ethics Committee (2020/ETH00964). This cohort includes patients who were diagnosed through both St Vincent’s hospital testing clinics (internal) and patients referred from external testing clinics (external). The cohort was recruited from confirmed COVID-19 cases. Medical history, including psychiatric history, was not a consideration for study inclusion. All patients were followed longitudinally under a defined schedule of assessments at commencing at baseline screening with follow-up (fu_1 to fu_6) visits scheduled at 1, 2, 4, 16, 32 and 48 weeks after the date of positive confirmation of SARS-CoV-2 demonstrated using the polymerase chain reaction (PCR) test, allowing for flexibility between scheduled and actual assessment dates for pragmatic reasons. Baseline demographics and symptoms from the period of acute infection were recalled retrospectively at the enrolment visit. The current analysis uses data up to 48 weeks of follow-up post-baseline. For this substudy, we included patients recruited between March 2020 and March 2021 with a minimum of 5.9 ± 3.5 months follow-up (fu_4 to fu_6).
Measures
Demographic data, comorbidities, symptoms at acute infection and confirmation of COVID-19 diagnosis were collected at baseline for all participants. At each assessment visit from the week 2 follow-up onwards, recovery symptoms were collected and psychiatric outcome scales and screens were performed including: Depression in the Medically Ill 10 item scale where a score of ≥ 9 suggested probable or definite depression (Parker et al., Reference Parker, Hilton, Bains and Hadzi-Pavlovic2002), SPHERE screening tool for mental disorders (Hickie et al., Reference Hickie, Davenport, Hadzi-Pavlovic, Koschera, Naismith, Scott and Wilhelm2001) and the EQ-5D-5L quality-of-life instrument (EuroQol Research Foundation, 2019). The visual analogue scale of fatigue (Lee et al., Reference Lee, Hicks and Nino-Murcia1991) was collected at the 8- and 12-month time points. At each assessment visit, blood for CRP was collected and biobanked for serologic and immunologic research. All data were stored on REDCAP.
Definitions
We defined Long COVID as the presence of persistent fatigue, or shortness of breath, or chest tightness > 4 months after initial infection. Any patients with abnormal mental health measures were offered either ‘This Way Up’ an online mental health tool (Andrews, Reference Andrews2020), or formal review by a hospital psychiatrist.
Statistical analyses
Longitudinal associations between with mental health self-report measures across follow-ups and potential predictive measures, including symptom severity, Long COVID status, pre-existing comorbidities or psychological conditions at baseline and CRP level, were investigated using the generalised estimating equation (GEE) models for continuous outcomes with Gaussian distribution, or dichotomised outcomes with logistic link. Models were adjusted for age, gender and smoking status. We used an unstructured covariance structure in employed GEE models to account for with-subject autocorrelation due to multiple measurements across the follow-ups.
Results
Demographics
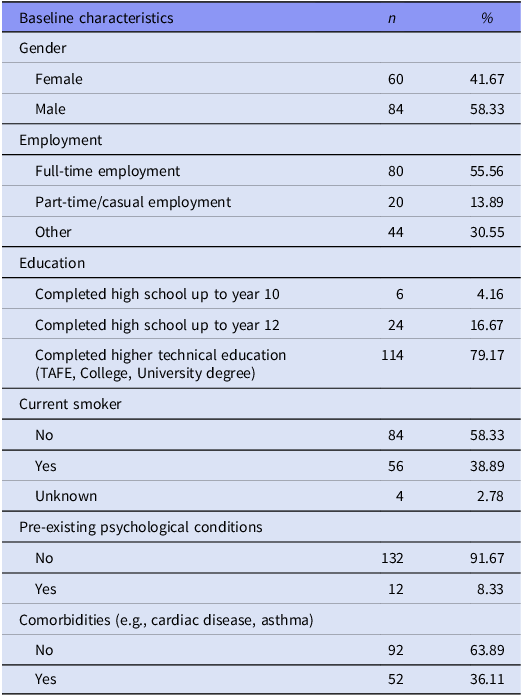
A total of 144 patients completed assessment visits between 6 and 48 weeks and were included for analysis. The demographic and clinical characteristics of participants are described in Table 1. Participants included 84 (58.33%) males and 60 (41.67%) females, with a mean age of 46.9 ± 14.7 years and mean body mass index (BMI) 25.1 ± 4.0. Twelve (8.3%) patients reported a history of psychiatric illness and 95 (66.7%) reported at least one medical comorbidity. Hundred and fourteen (79.2%) patients had achieved higher technical education.
Table 1. Sociodemographic characteristics of participants at baseline
Clinical outcomes and trajectories
Long COVID was present in a similar proportion of patients at follow-up visit 4 (fu_4), 16 weeks post-baseline (n = 38, 26.4%) compared with the follow-up 5 (fu_5), 32 weeks post-baseline (n = 40, 27.8%), reducing to n = 16 (11.1%) at follow-up 6 (fu_6), 48 weeks post-baseline, suggesting that there was little recovery between 16 and 32 weeks, that only reduced as the cohort size decreased with participants lost to follow-up at 48 weeks. The burden of mental illness for the cohort is summarised in Table 2, which shows that by follow-up 3 (fu_3) at 4 weeks post-baseline, measures of quality of life, somatic distress and psychological distress have mean values within the normal range; however, a large subgroup of the cohort has significant impairment. Over 60% of the cohort have significant scores for somatic distress and over 64% for psychological distress at 4, 16 and 32 weeks.
Table 2. Mental health measures at each follow-up
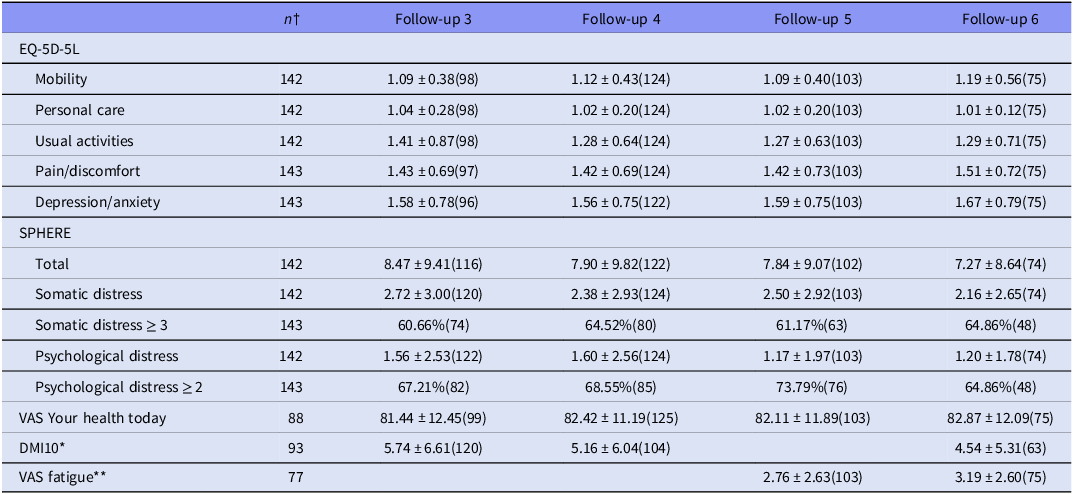
For each measure at each follow-up, n is presented in parentheses.
† Number of participants who completed measure at least once.
* DMI10 was not administered in Wave 5.
** The fatigue VAS was not administered at Wave 3 and Wave 4.
Associations with mental health outcomes: clinical and inflammatory markers
Associations between baseline clinical characteristics at the time of acute infection and the presence of Long COVID were assessed using GEE models. The results are summarised in Table 3. The results for an analysis of predictors of psychological conditions suggest that Long COVID status is the most important predictor of mental impairment.
Table 3. GEE models for investigating associations between pre-existing comorbidities or psychological conditions baseline, C-reactive protein level, symptom severity and Long COVID status, with mental health self-report measures across follow-ups, adjusted for age, gender and smoking status
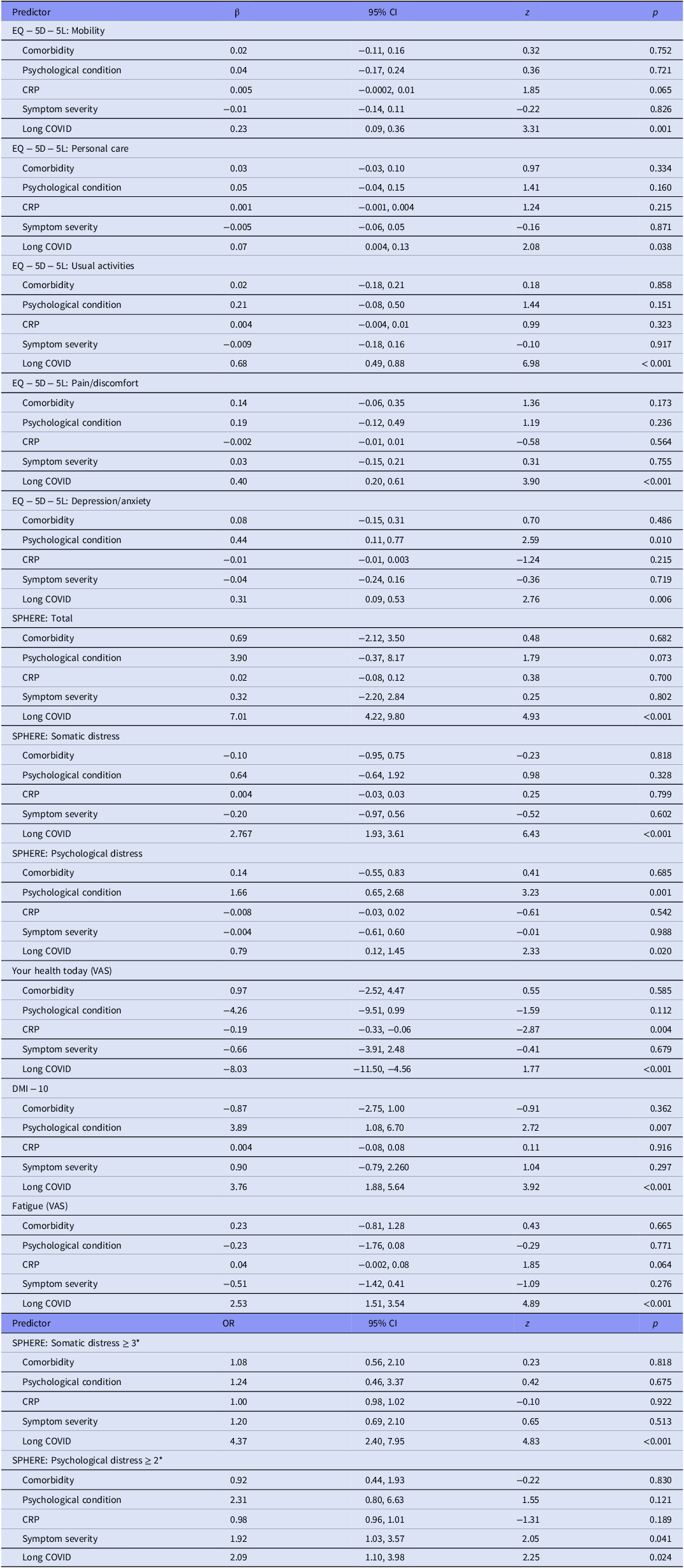
Comorbidity (yes), psych comorbid (yes), Symptom severity (severe), Long COVID (yes).
* Odds ratio and 95% CI are reported.
A stronger association was found between Long COVID and all mental health measures, where Long COVID was designated to participants who still had respiratory symptoms, malaise or fatigue at fu_4. No statistically significant association was found between severity of medical symptom of COVID and any of the psychiatric measures.
Associations with mental health outcomes: immunologic biomarkers
A battery of 27 immunologic biomarkers was measured from blood specimens collected at 62 participants at fu_4 (16 weeks) and from 20 participants at fu_5 (32 weeks) and tested for associations with mental health and quality-of-life measures (DMI/SPHERE/EQ-5D-5L/VAS) were investigated using GEE models. This demonstrated several significant associations of various effect sizes (Table 4), where a small effect is < 0.01, medium is 0.06 and > 0.14 is a large effect. A significant association was found between raised levels of CRP and VAS your health today score (p = 0.004). A trend towards significance was found for the VAS fatigue score (p = 0.064).
Table 4. GEE models for investigating associations between biomarkers and mental health self-report measures across follow-ups, adjusted for age, gender and smoking status

Comorbidity (yes), psych comorbid (yes), Symptom severity (severe), Long COVID (yes).
* Odds ratio and 95% CI are reported.
Discussion
Our findings suggests that a designation of Long COVID is the study variable of greatest concern for an increased risk of psychiatric symptoms following SARS-CoV-2 infection. Long COVID appears to be associated with diverse symptoms, including depression, fatigue and reduced quality of life and may be consistent with an emerging chronic fatigue like syndrome.
No association was detected between severity of medical symptom of SARS-CoV-2 infection, such as ventilator use or intensive care unit (ICU) admission and psychiatric outcomes.
A weak association was demonstrated between increased CRP and mental health measures. CRP was only measured at follow-up (fu3 onwards), and measures were generally low. At fu3, there was one person with CRP 58.9, one who scored 15.7 and a third who scored 10.8. All others scored<10. CRP scores at other follow-ups were even lower. This leaves open the possibility that a stronger association between CRP and mental health measures was not detected because of the small sample size and missing the peak for CRP that may have occurred earlier in the illness.
Conclusion
SARS-CoV-2 infection is associated with some psychiatric comorbidity, significantly in people with Long COVID. Further prospective follow-ups are required to determine the duration and characteristics of psychiatric sequaelae of Long COVID. Studies of larger populations are required to fully characterise the association between SARS-CoV-2 infection and psychiatric comorbidity. This study suggests that Long COVID may be the strongest predictor of neuropsychiatric symptoms amongst people who have been infected with SARS-CoV-2.
Acknowledgments
The ADAPT study was supported by the St Vincent’s Clinic Foundation. MB is supported by a National Health and Medical Research Council (NHMRC) Senior Principal Research Fellowship (APP1059660 and APP1156072)
Author contribution
SD, JO’D and MB are mental health researchers who prepared the first draft of the manuscript. MM conducted statistical analyses. GM and DRD are involved in COVID treatment and research and collected the data for this study. All authors approved the final manuscript.
Competing interests
SD has received grant support from the Stanley Medical Research Institute, NHMRC, Beyond Blue, ARHRF, Simons Foundation, Geelong Medical Research Foundation, Harry Windsor Foundation, Fondation FondaMental, Eli Lilly, Glaxo SmithKline, Organon, Mayne Pharma and Servier, speaker’s fees from Eli Lilly, advisory board fees from Eli Lilly and Novartis, and conference travel support from Servier. MB has received Grant/Research Support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, Medical Benefits Fund, National Health and Medical Research Council, Medical Research Futures Fund, Beyond Blue, Rotary Health, A2 milk company, Meat and Livestock Board, Woolworths, Avant and the Harry Windsor Foundation, has been a speaker for Abbot, Astra Zeneca, Janssen and Janssen, Lundbeck and Merck and served as a consultant to Allergan, Astra Zeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Janssen and Janssen, Lundbeck Merck, Pfizer and Servier – all unrelated to this work. MM, JO’D, GM and DRD have no conflicts of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The study was approved by the St Vincent’s Hospital Research Ethics Committee (2020/ETH00964).







