Psychosis is a symptom of severe mental illness characterised by impairment of thought and perception, leading to disconnection from objective reality. Several severe mental illness phenotypes (e.g. bipolar disorder and severe depression) involve psychosis but, contemporary arguments about the classification of mental illness aside, the quintessential psychotic illness is schizophrenia. Schizophrenia is estimated to be the fourth most important cause of life-years lost through disability in the world and it has been described as the ‘leading unsolved disease afflicting humans’ (Reference Carpenter, Murray, Jones and SusserCarpenter, 2003). There is good evidence that both genetic and environmental factors contribute to the aetiology of schizophrenia, but which genes, what aspects of the environment and whether these interact in important ways remains unclear (Reference Mäki, Veijola and JonesMäki et al, 2005). Many environmental exposures have been proposed as causes of schizophrenia, with the initial hope and enthusiasm accompanying the emergence of these hypothesised causes later turning to disappointment and scepticism as subsequent evidence fails to support their candidacy.
An association between cannabis use and psychosis was first observed several decades ago (Reference NegreteNegrete, 1988). More recently, results from several large population-based prospective observational studies have reported associations between a range of cannabis use phenotypes and a variety of measures of psychotic experience (Macleod et al, 2004a). In the largest (and oldest) of these studies, cannabis use in late adolescence was associated with an increased risk of a subsequent diagnosis of schizophrenia (Reference Andreasson, Allebeck and EngstromAndreasson et al, 1987). It is important that clinicians and population health scientists consider the meaning of this evidence and its implications for both policy and clinical practice. This task is made more pressing by the fact that cannabis use is now widespread. Since the late 1960s cannabis use has increased substantially in most high-income countries (Reference Hickman, Vickerman and MacleodHickman et al, 2007). The increase may now be levelling off, but this is hardly a reason for complacency. Cannabis is now well established as the third most widely used psychoactive drug (after alcohol and tobacco) in Europe, the USA and Australasia (Advisory Council on the Misuse of Drugs, 2006). In the UK around half of adolescents will use cannabis at least once and about a fifth of them will use it regularly (monthly or more frequently) in young adulthood. Prior assumptions that most of these will subsequently ‘grow out of’ this pattern of use have no firm evidential basis. Irrespective of any direct effects of cannabis use on physical or mental health, use exposes users to risks of criminalisation, as in most jurisdictions cannabis use is illegal.
Against this background we can now consider the competing, although not necessarily mutually exclusive, reasons why an association between cannabis use and psychosis might be apparent.
Cannabis use might cause psychosis
The first, and perhaps most obvious, possibility is that cannabis may cause psychosis. Plausible neurophysiological mechanisms that might mediate such an effect have been described. Broadly, psychosis may be a disorder of dopamine metabolism and cannabis is one of many substances that appear to influence dopamine metabolism (Reference Hall and SolowijHall & Solowij, 1998). Cannabis use could also cause psychosis through social mechanisms: aspects of the social environment have also been proposed as causes of schizophrenia (Reference Mäki, Veijola and JonesMäki et al, 2005); and cannabis use may give entry to social situations that the user would not be in were it not for their use of the drug. However, as Austin Bradford Hill suggested in his original essay on causal attribution and as has been reiterated more recently, apparent mechanistic plausibility is generally the weakest test of any causal hypothesis (Reference HillHill, 1965; Reference PetittiPetitti, 2004). It is seldom difficult to mobilise a broadly plausible mechanism through which even the most unlikely causal relations might arise. Similarly, the precise mechanism through which demonstrably causal relations do arise is frequently the subject of continuing discussion (Reference Bellosta, Ferri and BerniniBellosta et al, 2000; Reference Beckman and CreagerBeckman & Creager, 2006).
Psychosis might cause cannabis use
It is also possible that psychosis may cause cannabis use, an idea variously referred to as the ‘reverse causality’ or ‘self-medication’ hypothesis. Some aspect of the experience of psychosis may increase the likelihood that a person will use cannabis, through social, neurochemical or other mechanisms. More narrowly, people who are psychotic may use cannabis to in some way ameliorate the unpleasant aspects of their experience (Reference MacleodMacleod, 2007). It seems unlikely that this self-medication would be directed at positive psychotic symptoms in themselves, since acute cannabis intoxication increases rather than decreases unusual thoughts and perceptions. Cannabis use, however, may have other effects valued by users, either effects of the drug itself or those arising out of the social milieu surrounding use (Reference Gregg, Barrowclough and HaddockGregg et al, 2007).
An artefact of study methodology?
An apparent association between cannabis use and psychosis might arise as an artefact of the way the relation is studied. In all but one relevant study, effects of cannabis use on psychosis have been inferred on the basis of associations between self-reports of use and self-reports of unusual thoughts and perceptions. Certain phenomena are likely to have notions of social desirability or undesirability attached to them, particularly among adolescents and in certain subcultures. Reporting bias is a ubiquitous problem in observational epidemiology and a particular issue in the study of illegal, clandestine behaviour (Reference Anthony, Neumark, Van Etten, Stone, Turkkan and BachrachAnthony et al, 2000; Reference Colon, Robles and SahaiColon et al, 2001; Reference Macleod, Hickman and Davey SmithMacleod et al, 2005). It may be assumed that, at worse, such bias simply leads to random misclassification of study measurements, leading to dilution of apparent effects. This assumption, however, is untenable in the situation where bias influences both the exposure of interest (cannabis use) and the outcome it is related to (psychotic symptoms) in the same direction. In other words if adolescents who over- (or under-) report cannabis use also over- (or under-) report unusual thoughts and perceptions then an automatic, yet spurious, association between the two will be apparent. An empirical demonstration of the influence of reporting bias is outlined in Box 1.
Box 1 Reporting bias: an example
In a study on stress and heart disease among Scottish men (Reference Macleod, Davey Smith and HeslopMacleod et al, 2002) a well-validated measure of baseline stress was associated with a greater than doubling of the risk of angina (diagnosed by a World Health Organization questionnaire) 5 years later. Stress, however, showed no association with any objective index of heart disease. The association with angina arose because men who over- (or under-) reported their experience of stress also tended to over- (or under-) report their experience of angina symptoms.
Whether reporting bias has exerted an important influence on a particular association can be assessed only through a comparison of effects in relation to subjective and objective measures of the factors in question. Both cannabis use and psychosis are difficult to measure objectively.
A result of confounding?
The association may be a product of the fact that both cannabis use and psychosis are independently associated with common antecedents. Confounding is the most serious interpretational issue in observational epidemiology (Reference Davey Smith and EbrahimDavey Smith & Ebrahim, 2002). It arises when the association between two things is real (as opposed to an artefact of bias as discussed above) but has no causal basis. Rather, it has come about because the exposure and outcome under study are both independently related to a third factor, not on any causal pathway between the other two. For example coffee drinking is strongly associated with risk of lung cancer, not because it causes lung cancer, but because both coffee drinking and lung cancer are independently associated with smoking (Reference Ames and GoldAmes & Gold, 1997). This issue of causality is crucial in the context of effective prevention. Preventing coffee drinking would not be an effective public health strategy to reduce rates of lung cancer. Aspects of adversity in early life may underlie both risk of certain patterns of cannabis use (heavier use and earlier use) and risk of psychosis, without the three being linked by a common causal pathway (Reference Maughan and McCarthyMaughan & McCarthy, 1997). In this situation the target for effective prevention of psychosis would be the early-life adversity, not the cannabis use.
Interpretation of observational evidence
Non-causal associations in observational studies can arise in several ways. Other than the play of chance (whose influence can be assessed using statistical tests) the issues of reverse causation, bias and confounding complicate the interpretation of all associations identified in observational studies. This leads to the statistical axiom that association alone should not be taken as evidence of causation. Criteria that can help guide causal inference were proposed around 40 years ago and have been refined on several occasions since (Reference HillHill, 1965; Reference RothmanRothman, 1976; Reference SusserSusser, 1991; Reference Parascandola and WeedParascandola & Weed, 2001). Temporal priority is an obvious prerequisite (a cause must precede an effect) and it can be established only with longitudinal data. Plausibility is discussed above; other considerations include a dose–response association (cause and effect show quantitative covariance); consistency of the association across different places, times and people; and ‘independence’ – that is, persistence of the effect after adjustment for measurable confounding factors.
The last of these criteria, independence, is possibly the most important and certainly the most difficult to establish. There is no ‘test for confounding’ in observational data; rather, there are strategies that provide evidence as to its presence or absence. The most widely used is statistical adjustment. If an unadjusted effect estimate is attenuated towards the null on adjustment for some measure of a potential confounding factor then this is evidence for confounding; conversely, lack of attenuation is evidence against confounding. The problem lies with the assumption, apparently made by some investigators, that a persistent conventionally significant effect apparent after adjustment represents the ‘true effect’ after the influence of confounding has been removed. This is seldom likely to be the case. Imprecision in the measurement of correlated covariates will lead to apparently persistent effects after adjustment, even where an association arises completely through confounding by the adjustment factor (Reference Davey Smith and PhillipsDavey Smith & Phillips, 1990, Reference Davey Smith and Phillips1992; Reference Phillips and Davey SmithPhillips & Davey Smith, 1991). Furthermore, adjustment is possible only for confounding factors that have been anticipated and measured. Residual confounding by unanticipated, unmeasured factors is always possible.
This problem is well recognised in health services research and has led to the dominance of the randomised controlled trial (RCT) as the accepted method of health technology assessment. Random allocation of level of exposure to a putative cause should normally ensure that potential confounding factors, both known and unknown, are evenly distributed across different exposure categories such that their confounding effects are balanced. For this reason the existence of experimental (or ‘counterfactual’) evidence is often seen as an important additional causal criterion (Reference Parascandola and WeedParascandola & Weed, 2001). Epidemiological experience based on instances where apparently robust observational associations were subsequently shown to have no causal basis by experimental studies (for example in relation to intake of various vitamins and risk of chronic disease) have led to suggestions that ‘observational studies propose, RCTs dispose’ with regard to causal hypotheses (Reference Davey Smith and EbrahimDavey Smith & Ebrahim, 2002). Perhaps the best-known recent example is the story of hormone replacement therapy (HRT) and risk of coronary heart disease (Box 2).
Box 2 Hormone replacement therapy (HRT) and coronary heart disease
A large number of observational studies have shown an apparently robust, scientifically plausible, dose–response prospective association between use of HRT and a substantial reduction in risk of heart disease. An authoritative systematic review of 16 prospective studies concluded that ‘overall, the bulk of the evidence strongly supports a protective effect of estrogens that is unlikely to be explained by confounding factors’ (Reference Stampfer and ColditzStampfer & Colditz, 1991). Yet subsequent randomised controlled trials showed no such protective effect: HRT use was in fact associated with a small increased risk of heart disease (Reference Hulley, Grady and BushHulley et al, 1998; Writing Group for the Women's Health Initiative Investigators, 2002).
The anomaly arose because women using HRT were different from those not using it in ways other than the fact of their HRT use. It was these differences, rather than their HRT use, that had led to their decreased risk of heart disease. The differences almost certainly related to socio-economic factors; all the observational studies had recognised the possibility of socioeconomic confounding and had measured and adjusted for social position. These adjustments had simply been unsuccessful in removing this pervasive influence.
Issues relating to effects of drug use
Measuring cannabis use, psychotic illness and the various factors that might confound this association seems no less challenging than measuring use of HRT, heart disease and socio-economic position among middle-aged women. Indeed if the basic issue is that people who use illicit drugs such as cannabis may be different from people who do not, in ways other than the fact of their cannabis use and that measuring and adjusting for all the relevant dimensions of this difference may be difficult, then observational studies on effects of cannabis use may seem even more problematic to interpret.
These problems are illustrated by observational evidence on effects of licit drugs such as tobacco. Several high-quality observational studies show a dose–response association between smoking and increased risk of suicide of a magnitude similar to the association seen between cannabis use and risk of psychosis. This association was first highlighted in a methodological paper discussing the pitfalls of observational epidemiology (Reference Davey Smith and PhillipsDavey Smith et al, 1992). On the basis of similar evidence, some researchers have subsequently argued that smoking may cause suicide, citing plausible mechanisms (Reference Miller, Hemenway and BellMiller et al, 2000). These arguments are undermined by evidence from studies large enough to examine the association that have shown smoking to be associated with an equivalently increased risk of death from homicide (Reference Davey Smith and PhillipsDavey Smith & Phillips, 2001). It is difficult to believe that tobacco use actually causes an increased risk that users will be murdered. Rather, this serves as a reminder that smokers in these populations were different from non-smokers in ways related perhaps to their attitudes to risk, to self-protection and to their social position. These differences had profound implications for their health experience, and expecting that one could anticipate, measure and adjust for all the relevant aspects of difference between smokers and non-smokers in an observational study would be unrealistic.
The evidence
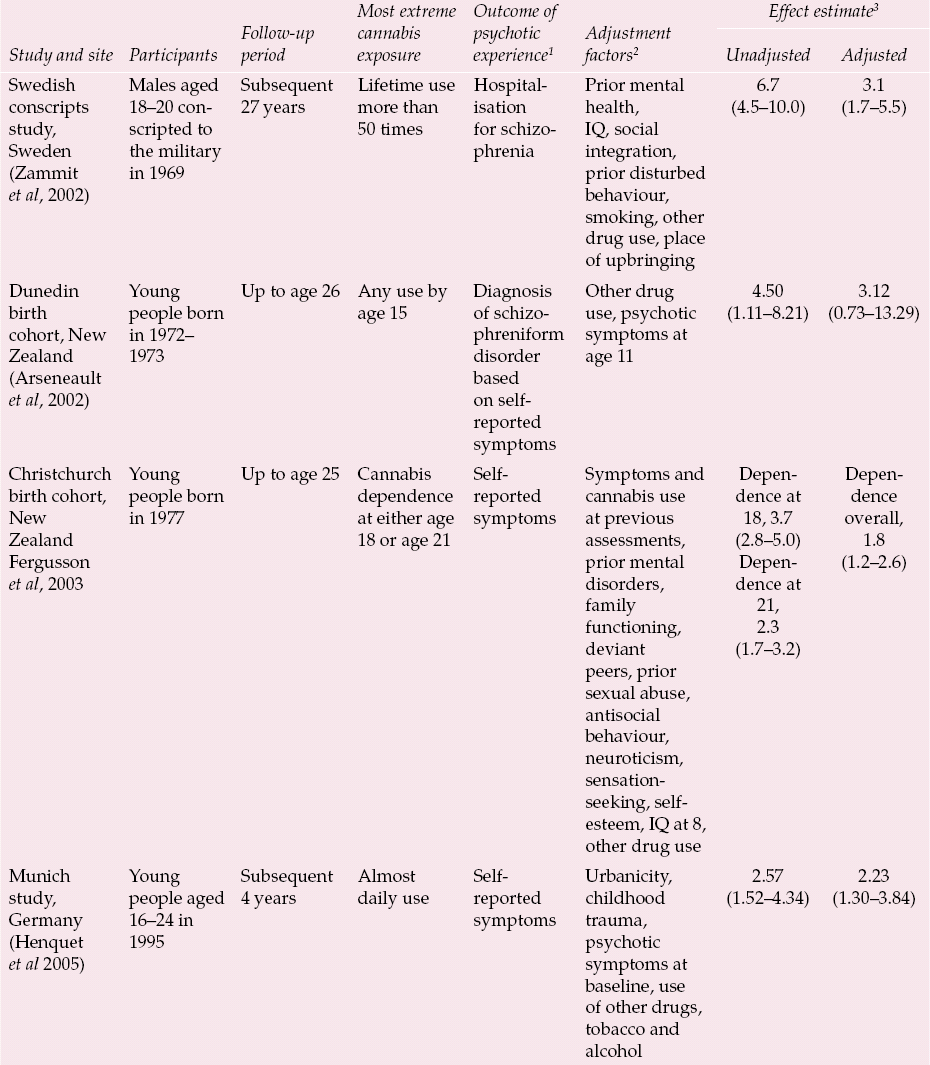
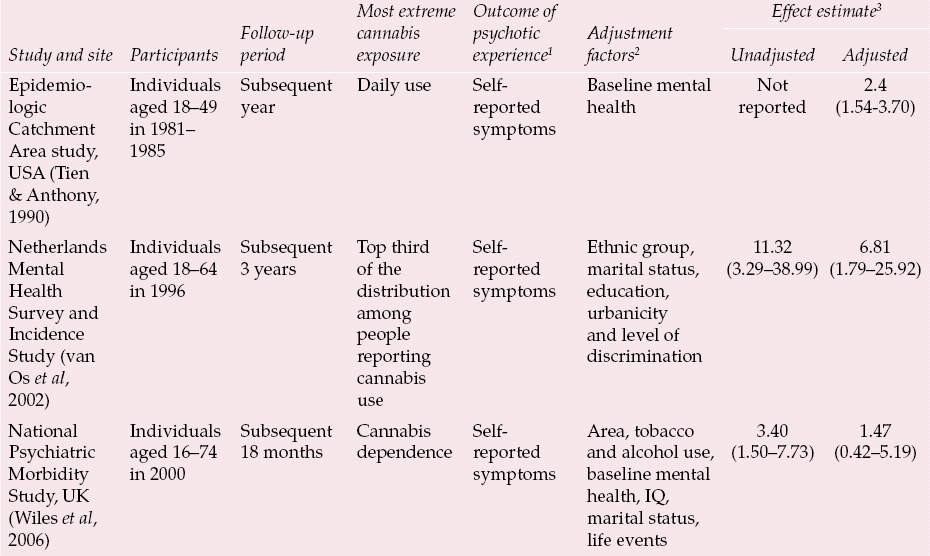
Only one large population-based prospective study has examined the association between adolescent cannabis use and later schizophrenia (Reference Andreasson, Allebeck and EngstromAndreasson et al, 1987; Reference Zammit, Allebeck and AndreassonZammit et al, 2002). Three further studies of adolescent cannabis users and three of adult cannabis users have reported effects on a variety of self-reported psychotic symptom phenotypes (Reference Tien and AnthonyTien & Anthony, 1990; Reference Arseneault, Cannon and PoultonArseneault et al, 2002; Reference van Os, Bak and Hanssenvan Os et al, 2002; Reference Fergusson, Horwood and Swain-CampbellFergusson et al, 2003; Reference Henquet, Krabbendam and SpauwenHenquet et al, 2005; Reference Wiles, Zammit and BebbingtonWiles et al, 2006). These seven studies are summarised in Tables 1 and 2. In general they show varying, but invariably increased, relative risks for whatever psychosis outcome phenotype was measured in relation to whatever their most extreme cannabis exposure category was. These relative risks are (generally substantially) attenuated on adjustment for whatever potential confounding factors were measured. Typically these adjustment factors are broad indices of social position, use of other drugs and history of mental health problems.
Table 1 Prospective observational studies examining the association between cannabis use in adolescence and subsequent experience of psychotic symptoms
| Effect estimate 3 | |||||||
|---|---|---|---|---|---|---|---|
| Study and site | Participants | Follow-up period | Most extreme cannabis exposure | Outcome of psychotic experience 1 | Adjustment actors 2 | Unadjusted | Adjusted |
| Swedish conscripts study, Sweden (Reference Zammit, Allebeck and AndreassonZammit et al, 2002) | Males aged 18–20 con- scripted to the military in 1969 | Subsequent 27 years | Lifetime use more than 50 times | Hospitalisation for schizo-phrenia | Prior mental health, IQ, social integration, prior disturbed behaviour, smoking, other drug use, place of upbringing | 6.7 (4.5–10.0) | 3.1 (1.7–5.5) |
| Dunedin birth cohort, New Zealand (Reference Arseneault, Cannon and PoultonArseneault et al, 2002) | Young people born in 1972– 1973 | Up to age 26 | Any use by age 15 | Diagnosis of schizo- phreniform disorder based on self- reported symptoms | Other drug use, psychotic symptoms at age 11 | 4.50 (1.11–8.21) | 3.12 (0.73–13.29) |
| Christchurch birth cohort, New Zealand Reference Fergusson, Horwood and Swain-CampbellFergusson et al, 2003 | Young people born in 1977 | Up to age 25 | Cannabis dependence at either age 18 or age 21 | Self- reported symptoms | Symptoms and cannabis use at previous assessments, prior mental disorders, family functioning, deviant peers, prior sexual abuse, antisocial behaviour, neuroticism, sensation- seeking, self- esteem, IQ at 8, other drug use | Dependence at 18, 3.7 (2.8–5.0) Dependence at 21, 2.3 (1.7–3.2) |
Dependence overall, 1.8 (1.2–2.6) |
| Munich study, Germany (Reference Henquet, Krabbendam and SpauwenHenquet et al 2005) | Young people aged 16–24 in 1995 | Subsequent 4 years | Almost daily use | Self- reported symptoms | Urbanicity, childhood trauma, psychotic symptoms at baseline, use of other drugs, tobacco and alcohol | 2.57 (1.52–4.34) | 2.23 (1.30–3.84) |
Table 2 Prospective observational studies examining the association between cannabis use in adulthood and subsequent experience of psychotic symptoms
| Effect estimate 3 | |||||||
|---|---|---|---|---|---|---|---|
| Study and site | Participants | Follow-up period | Most extreme cannabis exposure | Outcome of psychotic experience 1 | Adjustment factors 2 | Unadjusted | Adjusted |
| Epidemiologic Catchment Area study, USA(Reference Tien and AnthonyTien & Anthony, 1990) | Individuals aged 18–49 in 1981– 1985 | Subsequent year | Daily use | Self- reported symptoms | Baseline mental health | Not reported | 2.4 (1.54–3.70) |
| Netherlands Mental Health Survey and Incidence Study (Reference van Os, Bak and Hanssenvan Os et al, 2002) | Individuals aged 18–64 in 1996 | Subsequent 3 years | Top third of the distribution among people reporting cannabis use | Self- reported symptoms | Ethnic group, marital status, education, urbanicity and level of discrimination | 11.32 (3.29–38.99) | 6.81 (1.79–25.92) |
| National Psychiatric Morbidity Study, UK (Reference Wiles, Zammit and BebbingtonWiles et al, 2006) | Individuals aged 16–74 in 2000 | Subsequent 18 months | Cannabis dependence | Self- reported symptoms | Area, tobacco and alcohol use, baseline mental health, IQ, marital status, life events | 3.40 (1.50–7.73) | 1.47 (0.42–5.19) |
It is also possible to summarise the results of these studies through meta-analysis, a statistical technique that weights study results according to study size (or variance, which is generally a function of study size) and allows the results of several studies to be averaged. Some reviewers of the observational evidence on cannabis use and psychosis have adopted this approach (Reference Semple, McIntosh and LawrieSemple et al, 2005). Results of meta-analysis are typically presented in ‘Forest plots’ – in some ways these presentations (of a series of ‘box and whisker’ plots from individual studies, along with a combined estimate derived by meta-analysis of these) have become the visual embodiment of evidence-based medicine, as reflected in the well-known logo of the Cochrane Collaboration (http://www.cochrane.org). Forest plots provide a useful means of visually comparing the results of several studies investigating a similar question. However, when the studies summarised are observational and have considered different measures in different populations observed over different periods, presentation of a combined effect estimate derived through meta-analysis may not be a reliable guide to causal inference (Reference Egger, Schneider and Davey SmithEgger et al, 1998). Figure 1 illustrates this problem using the evidence on smoking and suicide discussed above. Ostensibly, this Forest plot provides convincing evidence, according to some conventional criteria, that smoking causes suicide. A consistent association is seen across several independent studies; the estimates from these studies are adjusted for potential confounding factors, yet they are still apparent and are generally statistically ‘significant’ at conventional levels; further, each study appears to show a convincing dose–response association between quantity of exposure and magnitude of outcome, and the combined estimate at the bottom of the plot is reassuringly distinct from the line of no effect (the vertical broken line). The reason for this apparent paradox is probably straightforward. Confounded associations are likely to be apparent in any population with a similar structure of potential confounding factors (i.e. in this case where the psychosocial characteristics of smokers are similar). Where confounding factors covary with the exposure of interest in a quantitative fashion (as they often will) then spurious dose–response associations will be apparent. As discussed above, a sceptical interpretation of the evidence on smoking and suicide seems appropriate, irrespective of the appearance of the plot in Fig. 1.
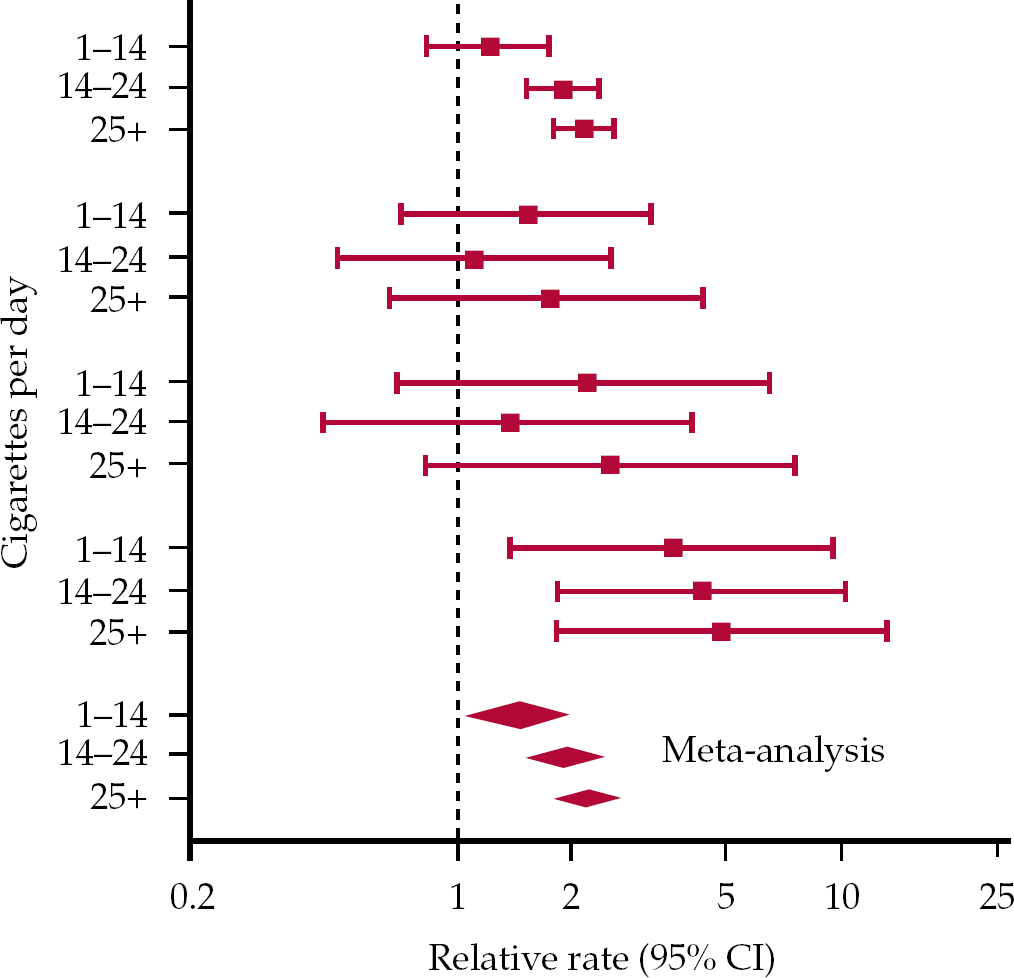
Fig. 1 Forest plot of estimates of the association between tobacco smoking and mortality from suicide. The four sets of three box and whisker plots show the results for the four different samples. (Reference Davey Smith and PhillipsDavey Smithet al, 1992. With permission.)
Interpreting the evidence
So how should we interpret the evidence on the association of psychosis with cannabis use in light of the above competing explanations? Systematic reviews are essentially a form of observational study in which the units of observation are other studies rather than individuals. As such they are subject to most of the potential biases that afflict any observational study and their interpretation is substantially subjective. It is therefore not surprising that different groups of reviewers can draw different conclusions based on review of the same evidence. What is perhaps more surprising is that the numerous recent reviews of the evidence on cannabis and psychosis have come to very similar conclusions (Reference Arseneault, Cannon and WittonArseneault et al, 2004; Macleod et al, 2004a; Reference Smit, Bolier and CuijpersSmit et al, 2004; Reference Semple, McIntosh and LawrieSemple et al, 2005; Reference Degenhardt and HallDegenhardt & Hall, 2006). These conclusions have, broadly, been as follows.
Evidence of the association between cannabis use and increased risk of psychosis is reasonably consistent and is compatible with the possibility of a causal relationship. This evidence, however, is not currently conclusive. Non-causal mechanisms may have generated the association. Probably the most difficult of these to discount is the possibility of residual confounding by factors that independently increase risk of both certain patterns of cannabis use – especially earlier and heavier use – and psychosis. This possibility is signalled by the fact that unadjusted effect estimates in all studies in which these were available for comparison were attenuated towards the null value on adjustment for the sometimes limited range of potential confounders measured. The study with the most sophisticated consideration of confounding, the Christchurch birth cohort, reported some of the smallest effects of cannabis in relation to one of the largest apparent exposure doses (cannabis dependence) (Reference Fergusson, Horwood and Swain-CampbellFergusson et al, 2003). Different reviewers have varied only in how emphatically they have acknowledged this finding. Some of the studies summarised in Tables 1 and 2 have also provided evidence suggestive of the other non-causal mechanisms discussed above. For example in the Dunedin birth cohort, children reporting psychotic symptoms at age 11 were more likely to report subsequent cannabis use (Reference Arseneault, Cannon and PoultonArseneault et al, 2002). When this association was considered in the analysis, the association between cannabis use and subsequent psychotic symptoms was attenuated and lost conventional statistical significance. However, this suggestion of reverse causality was not consistently seen across all seven studies. Similarly, most studies related uncorroborated self-reports of cannabis use to uncorroborated reports of psychotic symptoms, leaving them prone to an influence of reporting bias. Nevertheless, in the Swedish conscript study, psychotic symptoms were, in effect, corroborated by a health professional as they were indexed by hospital diagnoses of schizophrenia (Reference Andreasson, Allebeck and EngstromAndreasson et al, 1987; Reference Zammit, Allebeck and AndreassonZammit et al, 2002). Cannabis effects in this cohort were very similar to those seen in others, making an important role for reporting bias seem less likely.
The ecological perspective
Ecological studies consider events in populations, rather than in individuals. Interpretation of them is potentially prone to ‘ecological fallacy’ if their results are extrapolated to individuals (Reference LastLast, 1988: p. 40). Two populations with different rates of cannabis use may also have different rates of psychosis, but it does not necessarily follow that it was individual differences in cannabis use that caused these. Rather than compare populations with different rates of cannabis use at a particular point in time, ecological perspectives on the relation between cannabis use and psychosis have compared population changes in cannabis use over time with rates of psychosis. If, as some studies have suggested, cannabis use genuinely doubles or even trebles the risk of psychosis over a relatively short period from exposure to effect then populations in which cannabis use has increased should see increases in psychosis, all other influences on psychosis being equal. The ability to test this hypothesis depends on the availability and quality of data on rates of cannabis use and psychosis in the population. Often these data are rudimentary, and of dubious quality. Using Australian data, Degenhardt and colleagues found little evidence to suggest that apparently substantial changes in cannabis use had been followed by the increases in psychosis one might expect if cannabis use causes psychosis (Reference Degenhardt, Hall and LynskeyDegenhardt et al, 2003). More recently, Hickman and colleagues looked at the same question using UK data (Reference Hickman, Vickerman and MacleodHickman et al, 2007). Their results were more equivocal. No reliable prospective data on changing rates of cannabis use are available in the UK. The authors instead relied on a single national survey in which people of different ages reported when they had first and most recently used cannabis and their approximate frequency of use between these points; from these data time trends in use by age could be constructed. Large increases in cannabis use between the early 1970s and the late 1990s were apparent, but the biggest increases among young people were relatively recent. High-quality data on psychosis incidence were also relatively recent, and therefore a possible prior influence of cannabis on this incidence could only be modelled. Model projections suggested that increases in psychosis might have been less substantial, and consequently less noticeable, than some had assumed. They also suggested, however, that a truly causal relationship would lead to larger increases, unlikely to be missed by reliable surveillance, by around 2010.
Obtaining better evidence
The evidence summarised above indicates that cannabis use may be clinically relevant as an important cause of psychosis and may provide a rational target for primary prevention. Clarifying this question is crucial; psychosis is estimated to be the fourth largest contributor to disability in the world and the effectiveness of secondary prevention through treatment is often disappointing (Reference Carpenter, Murray, Jones and SusserCarpenter, 2003).
Better observational studies
Compared with epidemiological evidence on more established behavioural and physiological influences on chronic disease, longitudinal evidence on the relationship between cannabis use and psychosis is limited both in quantity and quality. The evidence on which effective primary prevention might be based must come from longitudinal research that commences in early life, ideally before either cannabis use or psychosis is present, in order to address the question of direction of causality. These studies must include repeated measures of all the important socio-environmental and temperamental factors that might confound an association between cannabis use and psychosis, if they are to consider the influence of such confounding. If they are to detect subtle effects that might be obscured by exposure misclassification they need to include repeat assessments of both frequency and quantity of cannabis used. To address the question of possible reporting bias these qualitative and quantitative self-reports should be corroborated with toxicological assessments (Reference Wolff, Farrell and MarsdenWolff et al, 1999). Psychosis outcomes should be assessed with standard psychometric instruments and these assessments should be augmented with data on actual clinical events, probably obtained through linkage to existing clinical databases. This may seem like a counsel of perfection. Fortunately, however, it is precisely the approach that studies with a serious intent to answer these important questions are already taking (see, for example, the Avon Longitudinal Study of Parents and Children at http://www.alspac.bris.ac.uk).
Genetically informed studies
There are other approaches that could help: genetics is one example (Reference Nestler and LandsmanNestler & Landsman, 2001). Twin studies suggest a substantial genetic influence on both initiation and level of drug use in populations in which a particular drug is available (Reference Kendler, Jacobson and PrescottKendler et al, 2003; Reference Rhee, Hewitt and YoungRhee et al, 2003; Reference Maes, Sullivan and BulikMaes et al, 2004). These influences may be general, suggesting mediation through genes that influence temperament or common physiological pathways, or they may be specific to particular drugs. The precise genes involved remain to be securely identified, but once found genes exerting an influence on level of use (contingent on initiation) of specific drugs potentially provide useful instrumental variables allowing clarification of true causal influences of the drug in question. This is because genotype is effectively randomly assigned at meiosis (Reference Davey Smith and EbrahimDavey Smith & Ebrahim, 2003; Reference Little and KhouryLittle & Khoury, 2003). There is preliminary evidence that some polymorphisms may have a specific influence on level of cannabis use and may therefore prove useful in this regard (Reference Comings, Muhleman and GadeComings et al, 1997; Reference Gadzicki, Muller-Vahl and StuhrmannGadzicki et al, 1999; Reference Sipe, Chiang and GerberSipe et al, 2002).
Taking action
Valuable though better evidence from the above sources is likely to be, it is not necessary to wait for it before taking public health action. Most cannabis users seem to smoke cannabis with tobacco, indeed use of the two drugs appears intimately linked (Reference Amos, Wiltshire and BostockAmos et al, 2004). Cannabis use may provide a route into tobacco use and a block on routes out of it (Reference Patton, Coffey and CarlinPatton et al, 2005). This relationship with tobacco use may explain apparent adverse effects of cannabis use on adolescent respiratory health (Reference Taylor, Fergusson and MilneTaylor et al, 2002). Moreover, cannabis use in most places is illegal and criminalisation is unlikely to exert a healthy influence on the life trajectory of most young people.
The above evidence alone provides public health justification enough to develop and evaluate interventions to prevent cannabis use. These interventions should fulfil the normal criteria that most prevention or screening interventions are required to satisfy (Reference GrayGray, 2001). In particular, the intervention should be cost-effective, acceptable to the people receiving it and any benefits should demonstrably exceed any collateral harm caused. Criminal justice-based interventions appear politically popular and should not be dismissed out of hand. However, they seem likely to fail in relation to all these requirements. School-based educational interventions are also popular, and may be more acceptable and humane. They can, however, still have unexpected adverse effects (Reference Aveyard, Cheng and AlmondAveyard et al, 1999). They are also expensive and, currently, appear to have limited long-term positive effects (Reference White and PittsWhite & Pitts, 1998; Advisory Council on the Misuse of Drugs, 2006). This notwithstanding, school-based interventions will probably improve, and other individually focused approaches, motivational interviewing for example, show promise. In other words there are a range of candidate interventions to reduce or prevent cannabis use whose effectiveness should be established in randomised controlled trials. If these interventions do reduce cannabis use then their influence on later psychosis will provide some of the most robust evidence on the true basis of the cannabis–psychosis association.
Clinical implications
The clinical implications of the debate on whether cannabis use causes psychosis have probably been overstated. As discussed above, irrespective of the answer to this question there are sound reasons to try to help young people avoid initiation into cannabis use and to stop or reduce use already initiated. Health workers outside of specialist substance use or mental health services may seldom find themselves in situations where prevention of cannabis use is high on the agenda, although cannabis smoking has arguably the same relevance to management of common cardio-respiratory illnesses as tobacco smoking. Young people seem unlikely to seek medical advice as to whether they should initiate cannabis use. This notwithstanding, clinical situations where a person, or perhaps more often people around them, seeks help in relation to problems in which cannabis use may play a part are likely to become more common – since cannabis use has become more widespread and its status as a medical (rather than social) issue has gained greater currency. In these situations the advice that can be provided is unequivocal – there are many good reasons that a person should stop or, if this is not possible, reduce use of cannabis. The risk that it may cause psychosis is not at the top of the list of these but is an additional consideration. If simple evidence-based advice is not sufficient, community drugs agencies increasingly see cannabis-related problems as potentially falling within their remit.
Clinicians caring for people with established psychosis face the issue that a large proportion of their patients use drugs and that alongside tobacco, cannabis is one of the more common of these substances. This may partly reflect the fact that patients with psychosis perceive some benefit from their cannabis use (Reference Gregg, Barrowclough and HaddockGregg et al, 2007; Reference MacleodMacleod, 2007). This may seem paradoxical to health workers caring for them, since one of the most reliable short-term effects of cannabis use (i.e. as distinct from any enduring effect on psychosis) is the precipitation of unusual thoughts and perceptions – or ‘psychosis’. Because of this, and because of the general risks associated with cannabis use discussed above, most psychiatrists are likely to conclude that they should try to prevent cannabis use by patients who experience psychosis. The effectiveness of interventions to modify cannabis use has recently been discussed in APT by Reference Maddock and BabbsMaddock & Babbs (2006).
Policy implications
Scientific evidence on whether cannabis use does or does not cause psychosis seems unlikely to exert an important influence on policy concerning the regulation and control of the cannabis market (Reference Macleod, Oakes and OppenkowskiMacleod et al, 2004b ). Arguments on the pros and cons of different policy approaches to drug use have been extensively rehearsed (Reference SingleSingle, 1989; Reference Strang, Witton and HallStrang et al, 2000; Reference Wodak, Reinerman and CohenWodak et al, 2002). However, it seems difficult to remove from the equation the short-term political concerns of any administration that might implement change. As long as it is perceived that a particular policy change, however scientifically rational, may be politically unpopular either domestically or internationally it seems unlikely that this change will be pursued.
For example, during his time as UK Home Secretary David Blunkett, perhaps mindful of the need to make rational use of police resources in light of changing criminal justice and international security priorities in the early 21st century, recently introduced minor reductions to criminal penalties related to cannabis use in the UK (Reference May, Duffy and WarburtonMay et al, 2007). Presumably, Mr Blunkett hoped that in making these changes he would reduce the apparently disproportionate amount of criminal justice resources consumed by activity related to the personal possession of cannabis. In practice the reclassification of cannabis within the UK Misuse of Drugs Act 1971 seems to have had less of an impact on police activity than proponents had hoped and opponents had feared (Reference May, Duffy and WarburtonMay et al, 2007). This, along with the resulting political furore, makes further liberalisation of the regulatory framework appear unlikely in the foreseeable future.
Summary and conclusions
Psychotic illness, in particular schizophrenia, has a huge impact on individuals, families and the wider community. Because of limited understanding of the causes of psychosis, attempts to ameliorate this impact have met with limited success. Against this background, evidence of an association between cannabis use and psychosis has recently emerged. By normal epidemiological conventions, evidence that this association has a causal basis is currently not strong. However, cannabis may cause psychosis and this possibility presents a tantalising glimpse of a means to effectively reduce the population burden of illnesses such as schizophrenia (Reference McGrath and SahaMcGrath & Saha, 2007). Irrespective of this possibility there are good reasons to develop effective interventions for the primary and secondary prevention of cannabis use now. Rigorous evaluation of these interventions, along with better basic epidemiological research, should clarify whether cannabis use causes psychosis and whether psychosis can be prevented by preventing cannabis use.
Declaration of interest
J. M. is supported by a Career Scientist Fellowship from the Department of Health. The views expressed are those of the author and not necessarily of the Department of Health.
MCQs
-
1 With regard to cannabis use and psychotic illness in the UK population over the past 30 years:
-
a most evidence suggests that cannabis use by young people has increased
-
b rates of psychosis have clearly fallen
-
c rates of psychosis have clearly increased
-
d increasing rates of cannabis use should inevitably have led to increasing rates of psychosis, if cannabis use causes psychosis
-
e only a small minority of young people today are likely to have used cannabis.
-
-
2 In relation to the evidence that cannabis use may cause psychotic illness:
-
a several large prospective studies among young people in the general population have shown cannabis use to be associated with increased diagnosis of schizophrenia
-
b the fact that there are plausible neurophysiological mechanisms through which cannabis use might cause psychosis is probably the strongest evidence that it does
-
c confounding occurs when people exaggerate both their use of cannabis and their experience of psychotic symptoms
-
d in several studies, increased frequency of reported cannabis use has been associated with increased reporting of unusual thoughts and perceptions
-
e it is likely that the association between cannabis use and psychotic symptoms seen in several studies has arisen by chance.
-
-
3 In relation to cannabis use and public health:
-
a if cannabis use does not cause psychosis then there is no public health justification for preventing cannabis use
-
b recent evidence suggests that cannabis use probably causes more harm to public health than tobacco or alcohol use
-
c there is good evidence that the prohibition of cannabis use is an effective strategy to reduce use among young people
-
d cannabis use appears to have dramatically increased since the recent reclassification of cannabis under the UK Misuse of Drugs Act
-
e preventing cannabis use may lead to reductions in population rates of psychosis.
-
-
4 In relation to interventions to prevent drug use among young people:
-
a school-based educational interventions appear very effective in reducing young people's drug use
-
b school-based interventions may occasionally have unexpected adverse effects
-
c there is no evidence that motivational interviewing is effective as an intervention to reduce drug use
-
d one of the most effective prevention strategies seems to be the prohibition of drugs such as cannabis
-
e reductions in drug use seen with school-based educational campaigns may be small but tend to be sustained over several years.
-
-
5 In relation to different kinds of scientific evidence and their interpretation:
-
a randomised controlled trials have become the gold standard because of their power to remove the role of chance
-
b bias in the measurement of risk factors and the outcomes that they may be causally related to will only ever lead to effect dilution and can therefore be overcome by increasing sample size
-
c random allocation of level of exposure to a possible cause addresses the problem of confounding
-
d confounding is an important theoretical issue in epidemiology but it has few practical implications
-
e confounded associations between a possible cause and its effect frequently lead to effective interventions.
-
MCQ answers
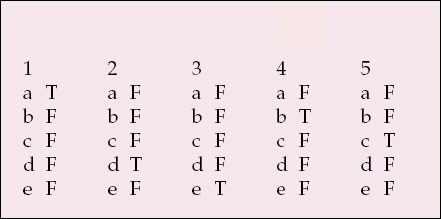
| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | T | a | F | a | F | a | F | a | F |
| b | F | b | F | b | F | b | T | b | F |
| c | F | c | F | c | F | c | F | c | T |
| d | F | d | T | d | F | d | F | d | F |
| e | F | e | F | e | T | e | F | e | F |






eLetters
No eLetters have been published for this article.