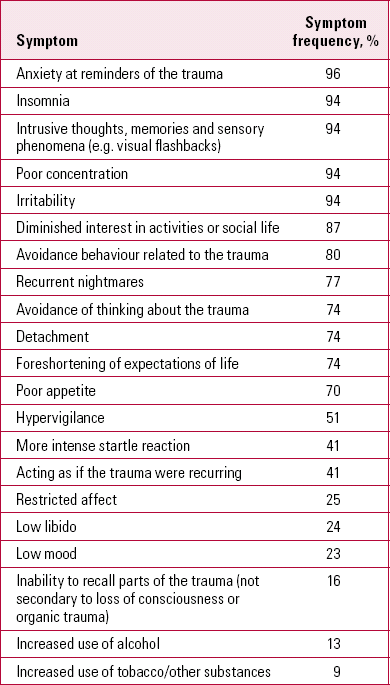
Post-traumatic stress disorder (PTSD) is relatively common, affecting 6–10% of all individuals at some time in their lives (Reference Kessler, Chiu and DemlerKessler 2005). Key symptoms include nightmares, intrusive memories and sensory impressions (flashbacks) and avoidance behaviour. There is a considerable risk of comorbid problems (e.g. depression or alcohol misuse), and PTSD is a source of disability and increased suicide risk. Table 1 shows the relative frequencies of different symptoms in people with PTSD.
TABLE 1 Frequencies of symptoms in 103 trauma survivors with post-traumatic stress disorder
Despite the prevalence of PTSD, traditional treatments are relatively limited in their success. In 1999, the World Health Organization's Management of Mental Disorders concluded that ‘PTSD is a severe disorder that is very difficult to treat’ (Reference Andrews and JenkinsAndrews 1999). Various authors report treatment resistance in about 33% of sufferers. The non-response rate to cognitive–behavioural therapy (CBT) in PTSD is as high as 50% (Reference KarKar 2011) and yet this treatment is purported to have the evidence to justify it as the initial management choice for PTSD by agencies such as the National Institute for Health and Clinical Excellence (NICE; National Collaborating Centre for Mental Health 2005). For comparison, the response rate for selective serotonin reuptake inhibitors (SSRIs) is about 60–80%.
I conducted this review of recent developments in the psychopharmacology of PTSD by searching MEDLINE (from 1946 to 2013) using ‘PTSD’ as a search term and then scanning reviews on its management for treatments other than first-line antidepressants or CBT. I then ran a second search, using suggestions for pharmacological management of severe, chronic or treatment-resistant PTSD as search terms. Treatments backed by research papers are incorporated in this article.
Psychopharmacology for PTSD
Although NICE guidance on PTSD states that ‘the evidence base for drug treatments in PTSD is very limited’ (National Collaborating Centre for Mental Health 2005: p. 19), there are numerous trials indicating that antidepressants are more effective than placebo and at least 18 clinical trials (10 double-blind placebo-controlled, 8 open-label) of atypical antipsychotics for PTSD (Reference Ahearn, Juergens and CordesAhearn 2011). I will return to the antipsychotic trials later.
Antidepressants
Looking at antidepressants, Reference Marshall, Beebe and OldhamMarshall et al (2001) found statistically significant improvements with paroxetine (20–40 mg/day) compared with placebo on all three PTSD symptom clusters (re-experiencing, avoidance/numbing and hyperarousal) in a study involving 551 patients. In a later study involving 70 people with chronic PTSD, Reference Marshall, Lewis-Fernandez and BlancoMarshall et al (2007) found significantly different response rates of 67% for paroxetine v. 27% for placebo after 10 weeks. Exposure therapy (a form of CBT) plus paroxetine has been found superior in producing remission compared with exposure therapy plus placebo (Reference Schneier, Neria and PavlicovaSchneier 2012).
Sertraline has been found to be superior to placebo in studies by Reference Davidson, Rothbaum and van der KolkDavidson et al (2001) and Reference Brady, Pearlstein and AsnisBrady et al (2000), which involved 208 and 187 patients respectively.
Fluoxetine has been found superior to placebo in studies by Reference Connor, Sutherland and TuplerConnor et al (1999) involving 53 patients and Reference Van der Kolk, Dreyfuss and Michaelsvan der Kolk et al (1994) including 64 patients.
Is trauma-focused psychotherapy the last word in PTSD treatment?
The NICE guidelines gave us a simple message in 2005: ‘All PTSD sufferers should be offered a course of trauma-focused psychological treatment (trauma-focused cognitive behavioural therapy or eye movement desensitisation and reprocessing)’ (National Collaborating Centre for Mental Health 2005: p. 17). The guidelines suggest 8–12 sessions of trauma-focused CBT, but if CBT can begin in the first month after the event, as few as 5 sessions may be needed.
Trauma-focused CBT
Most psychiatrists would be familiar with the therapeutic uses of CBT and with the concepts behind it, derived from the work of the psychiatrist Aaron T. Beck and others (Reference BeckBeck 1963, Reference Beck and Dozois2011). Trauma-focused CBT, however, is qualitatively different. It uses additional components such as stimulus confrontation, in which the patient confronts the trauma by reliving it in the imagination or in real life (Reference Seidler and WagnerSeidler 2006). Daily homework thereafter may consist of repeatedly listening to a recording of a verbal narrative of the trauma, with the aim of producing desensitisation.
Eye movement desensitisation and reprocessing
Another trauma-focused psychotherapy is eye movement desensitisation and reprocessing (EMDR). Based on work by Reference ShapiroShapiro (2001), EMDR is predicated on the theory that traumatic events which overwhelm the cognitive functions of the brain are not processed properly, creating dysfunctional memory systems that reveal themselves as symptoms of PTSD. Therapy involves bilateral brain stimulation using eye movements, sounds or touching while the patient evokes the traumatic visual images or memories and is encouraged to shift between the traumatic past and the safer present. No definitive explanation of how EMDR works is available.
Is EMDR supported by the evidence?
A wealth of controlled trials using EMDR demonstrate a degree of efficacy, but it is probably fair to say that there are no double-blind randomised controlled trials of the intervention, and that EMDR has not shown superior efficacy to other trauma-focused therapies (Reference Lohr, Tolin and LilienfeldLohr 1998). There is also not enough convincing evidence to suggest that the bilateral stimulation techniques are of any use, and some research suggests that EMDR may actually be less effective than CBT (Reference Rothbaum, Astin and MarstellerRothbaum 2005).
In comparative studies, a 50% reduction in PTSD symptoms can be achieved in 32–53% of patients receiving 10 sessions of CBT (Reference Hembree and FoaHembree 2000), but among the rest at least 14% drop out of therapy. For exposure models such as trauma-focused CBT, the drop-out rate may be as high as 50%, as many patients have difficulty re-experiencing the trauma involved in stimulus confrontation.
Nevertheless, many patients prefer a non-drug treatment and find interventions such as CBT and EMDR acceptable because they believe that it is not possible to become ‘hooked’ on psychotherapy. Certainly, there is evidence for the efficacy of trauma-focused CBT and EMDR (Reference Hembree and FoaHembree 2000; Reference Ponniah and HollonPonniah 2009), but can they work for severe, chronic illness and provide a ‘once and for all’ resolution?
The evidence base for trauma-focused CBT and SSRIs
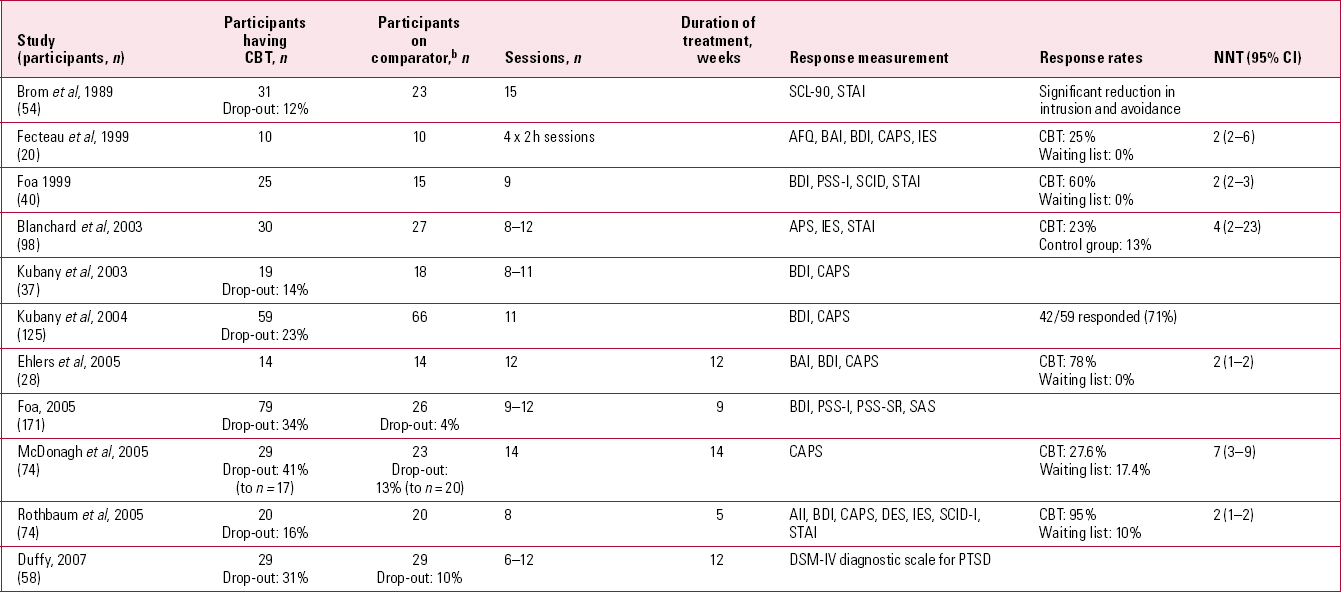
Research on both psychotherapeutic and pharmacological treatments suffers from short-termism. My MEDLINE searches revealed that randomised controlled trials (RCTs) of SSRIs ran for only 5–12 weeks (Table 2), and RCTs of trauma-focused CBT ran for 5–14 weeks (Table 3). Furthermore, such studies often neglect to include patients with chronic, treatment-resistant PTSD, who frequent more naturalistic settings than the research laboratory or university psychology department. Nor can guidelines or research adequately replicate the real world, where supply and demand dictate the non-availability of psychotherapies to the masses of people with PTSD, or provide solutions for patients who have graduated from their second course of CBT and relapsed yet again some months later.
TABLE 2 Randomised controlled trials of selective serotonin reuptake inhibitors (SSRIs) for post-traumatic stress disordera
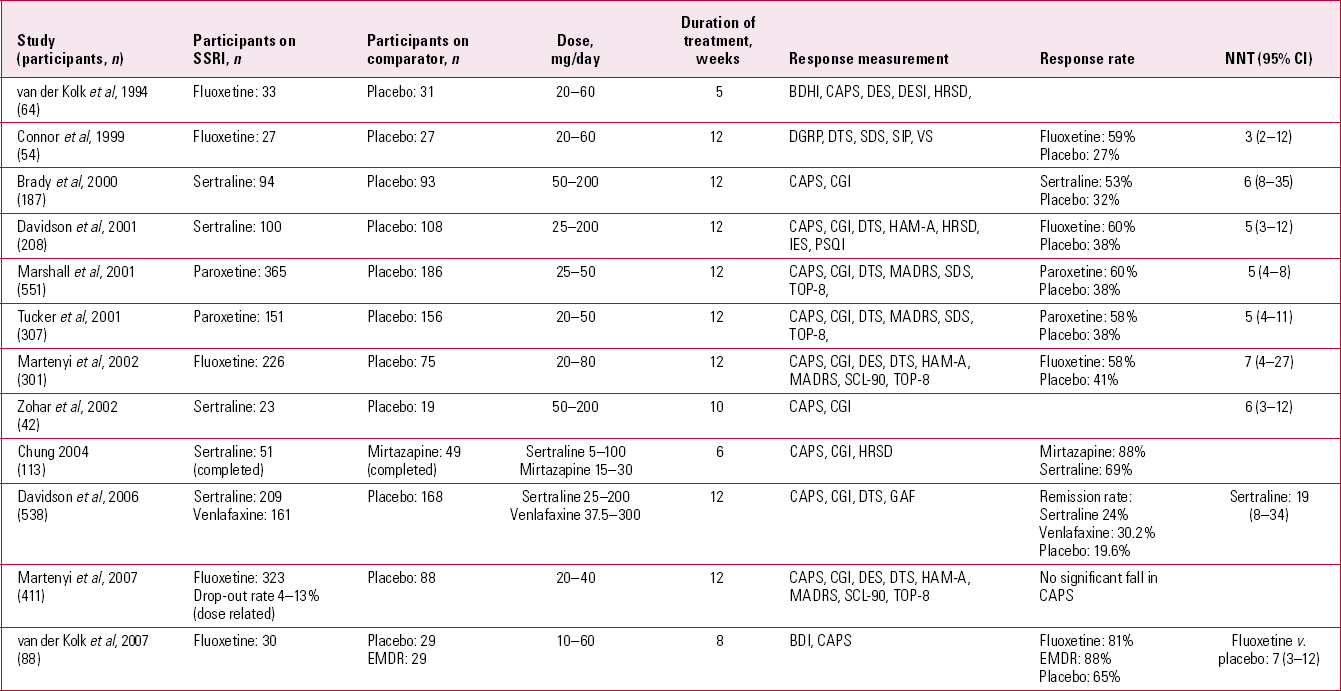
TABLE 3 Randomised controlled trials of trauma-focused cognitive–behavioural therapy (CBT) for post-traumatic stress disordera
NICE recommendations
The NICE guidelines favour the evidence base for CBT rather than antidepressants. Indeed, the numbers needed to treat (NNTs) in individual trials of CBT are generally more favourable (Tables 2 and 3). Certainly CBT seems more efficacious than alternatives such as psychological debriefing. However, the numbers of patients treated with SSRIs in RCTs far exceed the numbers treated with CBT and the numbers of participants in individual trials are low. Over 1600 people with PTSD have been studied in RCTs comparing SSRIs against placebo, roughly 5 times the number studied in trials of trauma-focused CBT. A further point to consider is that, where it is reported, the drop-out rate for trauma-focused CBT is high (12–41%), indicating that it is clearly not tolerated by all. The National Health Service (NHS) Health Technology Assessment Programme (Reference Durham, Chambers and PowerDurham 2005) recommends that those espousing psychotherapies need to develop an evidence base involving ‘longitudinal research designs over extended periods of time (2–5 years), with large numbers of participants (500+)’ before cost-effectiveness could be demonstrated. On the studies that my literature search revealed (Table 3), we are still far from providing such a solid research base for CBT.
The promise of neuroimaging
We now have evidence of consistent structural and functional neuroimaging changes associated with PTSD (Reference Bremner, Elzinga and SchmahlBremner 2008). Structural changes include volume reduction of the hippocampus by 12%. Reduced hippocampal volume is associated with verbal memory deficits and dissociative experiences. These structural changes can reverse with treatment, as demonstrated in separate studies using paroxetine and valproate (Reference Bremner, Elzinga and SchmahlBremner 2008). Functional changes include hyporesponsivity in medial prefrontal cortical areas during reminder cues. Such changes have been demonstrated in studies involving war veterans with PTSD and also in PTSD suffered by survivors of prolonged childhood sexual abuse. Reminder cues included slides of combat or guided memories of childhood abuse. The medial prefrontal cortex is involved in the inhibition of fear responses in the amygdala, and hyporesponsivity therefore leads to greater activation of the amygdala and consequent PTSD symptoms such as an enhanced startle response.
The promise of neuroimaging is to find treatments that can reverse such structural and functional changes.
Novel treatments
Of the recently proposed novel treatments for PTSD, it must be said that none has a body of evidence that approaches the evidence base supporting the use of antidepressants and CBT.
Propranolol
Propranolol is a beta-adrenergic blocker. It blocks the action of adrenaline and noradrenaline on β1 and β2 adrenergic receptors, which also results in indirect α1 agonism. Various studies have implied that effects of propranolol on the central nervous system may be of relevance to models of PTSD. For instance, propranolol has been shown to reduce the reactivity of the amygdala (Reference Hurlemann, Walter and RehmeHurlemann 2010) and it may impair memory formation in animal neocortices by reducing synaptic potentiation (Reference Flores, Núñez and PérezFlores 2010). Propranolol may also impair memory formation by attenuating neuropeptide S-induced memory enhancement (Reference Okamura, Garau and DuangdaoOkamura 2011).
Propranolol is licensed in the UK for various conditions, including hypertension and migraine. It is less often prescribed for anxiety disorders in settings such as primary care, the literature on its efficacy as an anxiolytic is very limited. Furthermore, the drug has very real side-effects, which include bradycardia, hypotension and falls, and so certain baseline investigations (electrocardiogram and blood pressure, among others) must be performed before, and monitored during, prescription.
The propranolol protection theory
There has been media interest over the past decade in a potential protective effect conferred by propranolol against the development of PTSD. The level of media interest could be argued to have been disproportionate, as it was triggered by relatively small-scale studies (e.g. Reference Pitman, Sanders and ZusmanPitman 2002; Reference Vaiva, Ducrocq and JezequelVaiva 2003) that showed a lower incidence of PTSD in people who had been treated with propranolol in the aftermath of trauma. The general concept was that propranolol, as a beta-adrenergic blocker, might prevent the excess activation of the central nucleus of the amygdala and the locus ceruleus after trauma, thought at the time to provide the neurological basis for PTSD.
This promising theory has not been confirmed by larger studies. For instance, research involving 29 children randomised to receive propranolol or placebo within 12 hours of physical trauma showed no significant between-group difference in scores on the Clinician-Administered PTSD Scale for Children and Adolescents (CAPS-CA) at 6 weeks (Reference Nugent, Christopher and CrowNugent 2010).
In another trial, physically injured patients were randomised to receive 14 days of propranolol (n = 17), gabapentin (n = 14) or placebo (n = 17) within 48 hours of injury. None of the interventions showed any effect at follow-up (Reference Stein, Kerridge and DimsdaleStein 2007).
Similarly, Reference McGhee, Maani and GarzaMcGhee et al (2009) found no reduction in PTSD in US soldiers injured in Iraq who had been given propranolol. They reviewed 603 patients at a military burns centre, 226 of whom completed a PTSD checklist. Thirty-one soldiers had received propranolol and 34 matched soldiers had not. The prevalence of PTSD in the propranolol group was 32.3%, against 26.5% in the comparison group (P = 0.785).
The most recent study, a randomised placebo-controlled trial by Reference Hoge, Worthington and NagurneyHoge et al (2012), evaluated the proactive use of propranolol in a clinical sample of 41 emergency department patients who had just experienced psychological trauma. Participants received either up to 240 mg/day of propranolol or placebo for 19 days. The drug did not significantly affect rates of PTSD diagnosis or severity of symptoms between the groups.
Animal research reports that propranolol given 1 hour after trauma was ineffective in preventing PTSD-like behavioural responses (e.g the startle response or freezing response on reminder cues) (Reference Cohen, Kaplan and KoreshCohen 2011).
Ketamine
Ketamine is a glutamatergic N -methyl-d-aspartate receptor antagonist which can, very rarely nowadays, be used as an anaesthetic agent. There has been recent interest in the use of intravenous ketamine for depression. A small-scale study of intravenous ketamine in 17 patients with treatment-resistant unipolar depression showed positive effects on mood lasting for a week after a dose of the drug (Reference Zarate, Singh and CarlsonZarate 2006). Suicidal ideation was found to reduce in the hours following intravenous administration of ketamine in a study of people with treatment-resistant major depressive disorder (Reference Diazgranados, Ibrahim and BrutscheDiazgranados 2010a). There is also research on the use of ketamine in depression associated with chronic regional pain syndrome (Reference Romero-SandovalRomero-Sandoval 2011) and treatment-resistant bipolar depression (Reference Diazgranados, Ibrahim and BrutscheDiazgranados 2010b).
The notion that ketamine might have an off-label part to play in the treatment of PTSD is perhaps less easy to understand, as the drug is associated with side-effects such as tachycardia, nightmares and hallucinations. Nevertheless, interest in ketamine arose from the observation that glutamate, which is released in stress, is important in the generation of dissociative symptoms (Reference Chambers, Bremner and MoghaddamChambers 1999). The Mount Sinai School of Medicine has been conducting a trial (NCT00749203) to see whether ketamine infusions may be a treatment for PTSD. It should be noted that the role ketamine might play in long-term treatment of PTSD would be limited by the need for intravenous administration.
Of three studies of accident and burns victims given ketamine during emergency treatment or surgery, two reported negative effects. In 2005, Schönenberg et al studied injured accident victims who had received either (S)-ketamine or opioids as analgesia in hospital a year previously. They found that retrospectively assessed dissociation, re-experiencing and avoidance behaviour and current PTSD were higher in the (S)-ketamine group.
Further work by Reference Schönenberg, Reichwald and DomesSchönenberg et al (2008) found that ketamine aggravated PTSD in burns victims, producing early acute stress disorder, dissociative states, re-experiencing, hyperarousal and avoidance behaviours. The study looked at patients who had received a dose of either ketamine (n = 13), opioids (n = 24) or non-opioid analgesics (n = 13) during initial emergency treatment.
Reference McGhee, Maani and GarzaMcGhee et al (2008), however, reported reduced levels of PTSD among 119 soldiers with burns who had received ketamine during surgery compared with 28 patients who had not. The PTSD prevalence in the ketamine group was 27%, compared with 46% in the non-ketamine group (P = 0.044).
If ketamine's future in PTSD is not as a treatment, its potential role in stimulating PTSD symptoms may guide further research. One area of interest might be N -methyl-d-aspartate receptor agonists (rather than antagonists) such as d-cycloserine, which facilitates learning and hippocampal activity.
MDMA (3,4-methylenedioxymethamphetamine)
Before its use as a recreational drug (‘ecstasy’) in the 1990s, MDMA was used as an adjunctive agent to psychotherapy in the 1970s and 1980s. The use of stimulants in psychotherapy for PTSD has a long history. William Sargant used nikethamide and amphetamines in a therapy akin to flooding (exposure therapy) in cases of battle shock during the Second World War (Reference SargantSargant 1957). Sargant called his technique abreaction, but rather than using sedation, he used stimulants to provoke a sudden and profound emotional crisis or collapse to produce a cure.
The hypothesis behind the use of MDMA in the psychotherapy of PTSD is that MDMA ameliorates fear activation during therapy and increases trust in the therapist, making exposure therapy easier. The drug may also increase blood flow in ventromedial prefrontal and occipital cortex and reduce it in the left amygdala, reversing known PTSD abnormalities (Reference Mithoefer, Wagner and MithoeferMithoefer et al, 2011).
The MDMA dose used in therapy is typically between 50 and 125 mg. The drug is given when the patient is relaxed on a couch, possibly listening to music through headphones. Patients can alternate between the inner experience and talking to the therapist. Sessions are non-directive and patients are encouraged to fully experience and express whatever arises, rather than avoiding or suppressing thoughts. The format for the psychotherapy mimics that used in past decades with lysergic acid diethylamide (LSD) (Reference Pahnke, Kurland and UngerPahnke 1971).
Precautions are usually taken to monitor for tachycardia and raised blood pressure. Small-scale studies have also monitored anxiety, depression and neurocognitive abilities. Participants often report tightness of the jaw, nausea, headache, dizziness and anxiety.
Reference Bouso, Doblin and FarréBouso et al (2008) studied six individuals with PTSD who had been treated with 50–75 mg MDMA. The small number reflects the fact that the study was closed down through what the authors termed ‘political pressure’. The authors concluded that, as far as their study was concerned, MDMA was ‘safe’ with ‘promising signs of efficacy’ and suggested studies with larger samples and higher doses.
Reference Mithoefer, Wagner and MithoeferMithoefer et al (2011) studied 20 individuals with PTSD, 12 of whom were allocated to psychotherapy with MDMA and 8 to psychotherapy with placebo. The outcomes measured through the Clinician-Administered PTSD Scale (CAPS) and Impact of Events Scale (IES) were significantly improved in the MDMA group (P <0.015 and P <0.027). At 1 year, 82% (14/17) of the MDMA group still did not meet criteria for PTSD.
Quetiapine
Antipsychotics are often prescribed off-label for treatment-resistant PTSD (Reference Leslie, Mohamed and RosenheckLeslie 2009). A review by Reference Ahearn, Juergens and CordesAhearn et al (2011) identified 18 clinical trials (10 double-blind placebo-controlled, 8 open-label) of atypical antipsychotics for PTSD. The authors reported that the double-blind placebo-controlled trials showed only modest effect sizes, but these were positive for risperidone and quetiapine. The greatest improvement was seen on intrusive thoughts and hypervigilance symptom subscales. A 6-month randomised double-blind placebo-controlled multicentre study of adjunctive risperidone published after Ahearn et al 's review (Reference KrystalKrystal 2011) did not find it to be of significant help in reducing PTSD symptoms.
The evidence indicates a potential role for quetiapine, which is an antagonist at the D1, D3 and D4 dopamine receptors and the 5-HT serotonin receptor. An 8-week open-label trial of adjunctive quetiapine (at a mean dose of 216 mg/day) in 15 adults with severe PTSD reported a 42% improvement in CAPS score, a 45% improvement on the Davidson Trauma Scale and a 44% improvement on the Sheehan Disability Scale (Reference Ahearn, Mussey and JohnsonAhearn 2006).
Another open-label trial, this time involving 6 adolescents, saw improvement in symptom checklist scores with flexibly dosed quetiapine (50–200 mg/day) over 6 weeks (Reference Stathis, Martin and McKennaStathis 2005).
Reference Kozaric-Kovacic and PivacKozaric-Kovacic & Pivac (2007) conducted an open-label study of quetiapine (25–400 mg/day) for 53 war veterans with PTSD and psychotic symptoms. Quetiapine treatment led to significant reductions in scores on the CAPS, the Positive and Negative Syndrome Scale (PANSS) and Clinical Global Impression – Severity (CGI-S) scale at 2, 6 and 8 weeks.
Two short-term open-label trials (Reference Hamner, Deitsch and BrodrickHamner 2003; Reference Sokolski, Denson and LeeSokolski 2003) showed improvement with low-dose quetiapine (25–300 mg/day) as an adjunct to SSRIs for chronic PTSD. Sokolski et al reported improvements on the DSM-IV PTSD criterion of re-experiencing in 35% of participants, on avoidance/numbing in 28% and on arousal in 65%. Hamner et al reported significant improvement in CAPS and improvement in depression scores.
It is worth noting that several US soldiers and veterans have died while taking high-dose quetiapine (>1600 mg) for PTSD. It is not known whether the drug contributed to the deaths, but the situation has resulted in some controversy in the USA regarding off-label prescribing for the disorder (Reference PerronePerrone 2010).
Quetiapine has various side-effects, including somnolence and postural hypotension (Reference GreenGreen 1999).
Prazosin
Prazosin is an alpha-adrenergic blocker currently licensed for hypertension, cardiac failure, Raynaud's phenomenon and benign prostatic hyperplasia. The drug is also used in the management of scorpion stings. Any potential use for PTSD would be off-label, but there is now a research base to justify its further study for the disorder (Table 4) – arguably a much more substantial base than that for some other treatments considered above. There are important side-effects to be aware of, such as hypotension (especially with the first dose, which should be given in surroundings where monitoring can be done). Other adverse effects include gastrointestinal disturbance, oedema, palpitations, dyspnoea, depression, anxiety and nasal stuffiness.
TABLE 4 Studies involving prazosin as a treatment for post-traumatic stress disorder
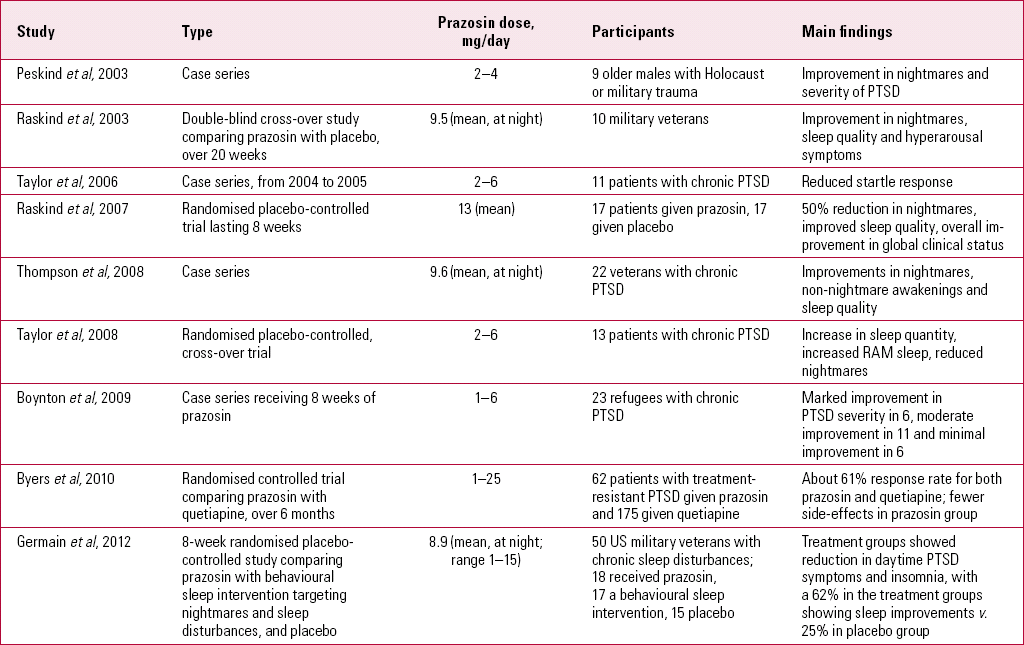
At least eight studies indicate a role for prazosin in treating PTSD and, given the nature of chronic PTSD and its notable treatment resistance, these are of considerable interest and larger-scale RCTs would be welcome.
Conclusions
Traditional PTSD treatments recommended by agencies such as NICE (e.g. CBT, EMDR and SSRIs) do have an evidence base, but they are of limited efficacy and trauma-focused CBT in particular has a high drop-out rate. Developments in neuroimaging could lead to new pharmacological treatments. Although current research has not demonstrated a significant role for either ketamine or propranolol, studies of prazosin for the treatment of severe, chronic PTSD suggest anti-nightmare efficacy and long-term tolerability. Larger-scale RCTs on prazosin are now justified.
MCQs
Select the single best option for each question stem
-
1 Prazosin is:
-
a an alpha-adrenergic blocker
-
b a beta-adrenergic antagonist
-
c an antagonist at D1, D3 and D4 receptors
-
d a glutamatergic drug
-
e a selective serotonin reuptake inhibitor.
-
-
2 Trauma-focused CBT:
-
a has efficacy rates of 80% within 10 weeks of starting therapy
-
b should be offered to all sufferers of PTSD, according to the 2005 NICE guidelines
-
c is demonstrably superior to EMDR for PTSD
-
d has a negligible drop-out rate of less than 5%
-
e has less efficacy than psychological debriefing in the treatment of PTSD.
-
-
3 Propranolol:
-
a is licensed in the UK for the treatment of anxiety
-
b prevents PTSD if given immediately after trauma, according to substantial, replicated double-blind randomised trials
-
c can be prescribed for anxiety without first performing any physical examination or investigations
-
d can cause bradycardia
-
e is a specific alpha-adrenergic blocker.
-
-
4 Quetiapine:
-
a is superior to prazosin in treating chronic PTSD
-
b is licensed in the UK for the treatment of PTSD
-
c is an agonist at D1, D3 and D4 receptors
-
d has been associated with fatalities at low doses in US military personnel
-
e can cause postural hypotension.
-
-
5 PTSD:
-
a has a lifetime prevalence of 4%
-
b responds to SSRIs within 2 months in 90% of cases
-
c occurs in less than 10% of people involved in road traffic accidents
-
d is associated with increased alcohol consumption
-
e is associated with irritability in less than 50% of cases.
-
MCQ answers

| 1 | a | 2 | b | 3 | d | 4 | e | 5 | d |
Acknowledgements
I am indebted to Dr Chris Cates for his advice on the calculation and presentation of NNTs.







eLetters
No eLetters have been published for this article.