Article contents
An Analysis of Genetic Discrimination Legislation Proposed by the 105th Congress
Published online by Cambridge University Press: 24 February 2021
Extract
It was the best of times, it was the worst of times, it was the age of wisdom, it was the age of foolishness … it was the spring of hope, it was the winter of despair … we were all going direct to Heaven, we were all going direct the other way.
—Charles DickensThe Human Genome Project (HGP) provides information about the human genome that will forever alter society and the way we view ourselves. The genetic age offers great potential, including a future where gene and germ-cell therapy may virtually eliminate genetic disease. However, genetic information may also result in a world characterized by genetic discrimination and genetic determinism. Although genetic information will be used to develop revolutionary treatments, such as gene therapy and other molecular medicine, it will also bring genetic discrimination and heretofore unrealized invasions into the privacy of our genetic codes.
- Type
- Notes and Comments
- Information
- Copyright
- Copyright © American Society of Law, Medicine and Ethics and Boston University 1998
References
1 CHARLES DICKENS, A TALE OF Two CITIES 1 (Running Press 1986) (1859). This passage provides an apropos introduction into human genetics and the ramifications such knowledge will have. Although it is the genetic “age of wisdom” because of potentially life-saving scientific discoveries, it is also the “age of foolishness” because of our failure to safeguard ourselves from our own designs. Thus, “the double helix reflects the hopes and fears of an age.” Richard Saltus, Sounding the Alarm Like Splitting the Atom, BOSTON GLOBE MAG., May 26, 1996, at 14.
2 See Human Genome Project, About the Human Genome Project (last modified Sept. 2, 1998) <http://www.ornl.gov/TechResources/Human_Genome/home.html> (describing the Human Genome Project (HGP) as “an international 15-year effort formally begun in October 1990 to discover all the 60,000 to 80,000 human genes (the human genome) and make them accessible for further biological study”).
3 See Technological Advances in Genetic Testing: Implications for the Future, 1996: Hearing on H.R. 2748 Before the Subcomm. on Tech. of the House Comm. on Science, 104th Cong. 9 (1996) (statement of Rep. Louise Slaughter) [hereinafter Slaughter Testimony] (stating that “[o]ur genes represent the most personal, private, and fundamental aspects of ourselves. They are inescapable; they cannot be concealed, disguised or avoided. They are the ultimate identification and definition of who we are … . Research efforts like the Human Genome Project are unlocking the secrets of our genes on an almost daily basis.”).
4 See Hudson, Kathy L. et al., Genetic Discrimination and Health Insurance: An Urgent Need to Reform, 270 SCIENCE 391, 391 (1995)CrossRefGoogle Scholar; see also DAVID SUZUKI & PETER KNUDTSON, GENETHICS: THE CLASH BETWEEN THE NEW GENETICS AND HUMAN VALUES 165-66 (rev. ed. 1990) (predicting that gene therapy—the medical repair or replacement of defective genes in human living cells—will become a reality).
5 The motion picture Gattaca provides an excellent glimpse into the future of genetic discrimination and subsequent genetic determinism—where people's lives are preordained by their genome. See Bob Kurson, Dabbling in DNA: ‘Gattaca’ Director Exploring Ethics, CHI. SUN-TIMES, Nov. 16, 1997, at 7, available in 1997 WL 6379554. Gattaca is “a journey into a world where humans are judged not by their characters but by their genes.” Id. This journey describes a world where parents genetically engineer their children, selecting traits such as intelligence, attractiveness and near perfect health. See Kathi Wolfe, Genetic Engineering: Two Sides of the Coin, LAS VEGAS REV.-J., NOV. 28, 1997, at 15B, available in 1997 WL 4558846. Vincent, the genetically inferior protagonist, is doomed to remain a second-class citizen because of poor vision and heart disease resulting from his natural conception. See Roger Ebert, Future Tense: “Gattaca” Sees Flaws in a Perfect World, CHI. SUNTIMES, Oct. 24, 1997, at 35, available in 1997 WL 6375250. The antagonist, Hugo, is Vincent's genetically engineered, and thus superior, brother. See id. In a society predicated on “prejudice with a scientific basis,” genetically inferior Vincent is an “in-valid” destined for menial jobs and low class status. See Rene Downing, ‘Gattaca’ Premise: Future's Not in Stars, but in Our Genes, ARIZ. DAILY STAR, NOV. 20, 1997, at 1C, available in 1997 WL 7934923. Therefore, to avoid genetic stigmatization, Vincent undertakes an elaborate conspiracy to assume the identity of Jerome, a genetically superior “valid” left lame by a car accident. See id. Vincent pays Jerome for his skin cells, blood, urine, hair and other bodily products used for genetic identification through genetic testing. See id. Finally, against all odds, Vincent achieves his life-long dream of becoming an astronaut, a position reserved for the genetically superior because of the substantial investment involved. See id. Gattaca, whose title is comprised of the letters G, T, C, and A, which represent the four nitrogenous bases making up deoxyribonucleic acid (DNA), is a “vision of a possible high-tech-militarized-corporate future” with elements of Aldous Huxley's “social-control dystopia,” Brave New World. See id.
6 See Hudson et al., supra note 4, at 391; see also Privacy, Confidentiality and Discrimination in Genetics, 1997: Hearing on H.R. 272 before the Task Force on Health Records and Genetic Privacy of the House Comm. on Commerce, 105th Cong. 10 (1997) (statement of Francis Collins, Director, National Center for Human Genome Research) [hereinafter Collins 1997 Testimony] (stating that “as knowledge grows about the genetic basis of diseases, so too does the potential for discrimination and stigmatization based on genetic information”). Genetic discrimination has been defined as “the denial of rights, privileges or opportunities on the basis of information obtained from genetically-based diagnostic and prognostic tests.” Gostin, Larry, Genetic Discrimination: The Use of Genetically Based Diagnostic and Prognostic Tests by Employers and Insurers, 17 AM. J.L. & MED. 109, 110 (1991)Google Scholar.
7 See Hudson et al., supra note 4, at 392; see also Collins 1997 Testimony, supra note 6, at 19 (reporting that 19 states enacted laws restricting the use of genetic information in health insurance and that at least 31 states have introduced laws prohibiting genetic discrimination in insurance). For a discussion of state and federal legislation targeting abuse of genetic information, see infra Part VI.
8 Genetic discrimination legislation is being used to refer to legislation protecting genetic privacy and prohibiting discrimination. See Technological Advances in Genetic Testing: Implications for the Future, 1996: Hearing Before the Subcomm. on Tech. of the House Comm. on Science, 104th Cong. 9 (1996) (statement of Alan Goldhammer, Director of Technical Affairs, Biotechnology Industry Organization) (noting that Congress intends to prevent unauthorized use or dissemination of genetic information and use of such information to discriminate against individuals seeking health insurance).
9 See Hudson et al., supra note 4, at 392 (stating that existing state genetic discrimination laws leave some people unprotected because the Employee Retirement Income Security Act of 1974 (ERISA) preempts state regulation of self-funded plans, and because many state laws focus narrowly on mandatory genetic testing rather than more broadly on the use of genetic information); see also infra notes 196-203 and accompanying text (discussing shortcomings of current state genetic discrimination legislation).
10 See, e.g., Holmes, Eric Mills, Solving the Insurance/Genetic Fair/Unfair Discrimination Dilemma in Light of the Human Genome Project, 85 K.Y. L.J. 503, 647 (1996-97).Google Scholar
11 H.R. 341, 105th Cong. (1997).
12 S. 422, 105th Cong. (1997).
13 Thus far, Congress has failed to enact either bill. Search of WESTLAW, Bill Tracking (Nov. 8, 1998).
14 The HGP is a collaboration among many independent international research institutions with the common goal of analyzing the human DNA structure and mapping and sequencing the estimated 100,000 human genes. See Holmes, supra note 10, at 517. The Human Genome Organization (HUGO), a private organization, coordinates genomic research conducted in the United Kingdom, Canada, France and Japan. See id. at 506 n.2. For discussion about the history of the HGP, see id. at 517 n.26. Further information about the HGP is available online at Human Genome Project Information (visited Nov. 18, 1998) <http://www.ornl.gov/TechResources/Human_Genome/home.html>.
15 See Ari Patrinos & Daniel W. Drell, Introducing the Human Genome Project: Its Relevance, Triumphs and Challenges, JUDGES’ J., Summer 1997, at 5, 6. The HGP will cost the federal government an estimated three billion dollars. See Holmes, supra note 10, at 518. This amounts to more than 20% of all biomedical research funding. See Valerio Barrad, Catherine M., Comment, Genetic Information and Property Theory, 87 Nw. U. L. REV. 1037, 1042 n.19 (1993)Google Scholar; see also O'Hara, Susan, The Use of Genetic Testing in the Health Insurance Industry: The Creation of a “Biologic Underclass,” 22 Sw. U. L. REV. 1211, 1215 (1993)Google Scholar (discussing the HGP's $3 billion price tag).
16 See Technological Advances in Genetic Testing: Implications for the Future, 1996: Hearing Before the Subcomm. on Tech. of the House Comm. on Science, 104th Cong. 2 (1996) (statement of Francis Collins, Director, National Center for Human Genome Research) [hereinafter Collins 1996 Testimony]. However, some experts have predicted that the HGP may map 99% of the human genome with 99.9% accuracy by the year 2002. See Holmes, supra note 10, at 516; see also Patrinos & Drell, supra note 15, at 6. The human genome is defined as “[a]ll the information stored in a complete strand of human DNA … [including] the three billion base pairs comprising roughly 100,000 genes.” Holmes, supra note 10, at 521. The HGP's goal is to “develop efficient, cost-effective detailed genetic and physical maps of the human genome, determine the complete nucleotide sequence of human DNA, and create the new technology required to fulfill its purpose.” Id.; see also Barrad, supra note 15, at 1042-43 (describing the HGP as a fifteen-year project with approximately five percent of the total three billion pairs of nucleotides identified in 1993).
17 See Collins 1996 Testimony, supra note 16, at 1.
18 See id. at 3.
19 See Holmes, supra note 10, at 520 (defining genome as “the totality of the genetic information that is stored in cells and passed from one generation to the next … [which] determines how cells behave and how complex organ systems interact… .”); see also Mahlon Hoagland & Bert Dodson, The Molecules of Life, JUDGES’ J., Summer 1997, at 11, 11 (suggesting that if DNA is compared with language, then “there are four … letters (nucleotides, or bases) arranged in paragraphs of 1,000 to 2,000 letters (genes), thence books of a million letters each (chromosomes), thence a library (genome). Our human library contains 46 books.”). See generally A Genetic Glossary for Judges, JUDGES’ J., Summer 1997, at 65, 65 [hereinafter Judges’ Glossary] (defining key terms used in genetics).
20 See Judges’ Glossary, supra note 19, at 65 (defining chromosomes as “[s]tructures found in the nucleus of a cell that contain the genes. Chromosomes come in pairs, and a normal human cell contains forty-six chromosomes, twenty-two pairs of autosomes, and two sex chromosomes.”). Human chromosomes range in size from 50 to 250 million nucleotide base pairs. See Bornstein, Richard A., Genetic Discrimination, Insurability and Legislation: A Closing of the Legal Loopholes, 4 J.L. & POL'Y 551, 556 (1996).Google Scholar
21 See Holmes, supra note 10, at 521.
22 For a good discussion of the difference between somatic and zygotic cells, see Barrad, supra note 15, at 1042 n.20.
23 See id. at 1042.
24 The double helix model of DNA was first proposed in 1953 by James Watson, Rosalind Franklin and Francis Crick. See Holmes, supra note 10, at 519. Watson's 1961 discovery of DNA's genetic code subsequently led to the recognition that “DNA's linear arrangement of paired bases in triplets provide[s] the blueprint for protein synthesis.” See id. at 519-20. The double helix model of DNA “looks much like a ladder that has been twisted.” See id. at 521. The “rungs of the ladder” are the base pairs formed from the “bonding of the four chemical [nucleotide] bases.” See id.
25 See SUZUKI & KNUDTSON, supra note 4, at 31.
26 See id. at 32. Furthermore, “[e]ach nucleotide contains a sugar, a phosphate, and one of four kinds of nitrogen containing bases: adenine (A), guanine (G), cytosine (C), and thymine (T).” Id. The genetic code utilizes these four nucleotides as the chemical instructions for hereditary information. See Holmes, supra note 10, at 521. Nucleotides are complementary, so that pairs are either A-T or C-G base pairs. See id. Moreover, DNA sequences, or ordering of the nucleotides, are composed of three nucleotide units called codons. See id. Sixty-four codons comprise the entire genetic code responsible for making twenty different amino acids, which are the basic building blocks of living organisms. See id. Actually, only 61 codons are responsible for instructing the production of 20 amino acids, and the three remaining codons are “regulatory” or “stop” signals. See Hoagland & Dodson, supra note 19, at 13; see also SUZUKI & KNUDTSON, supra note 4, at 43 tbl. 2.1 (listing the codons in the genetic code and resultant amino acids). Furthermore, “variations in the linear ordering of the [nucleotide] bases make up different genetic sequences.” Bornstein, supra note 20, at 556. Consequently, the “body ‘reads’ these [nucleotide] base pairs in groups of three [i.e. codons], and since every sequential triple identifies a specific amino acid, the body will string together amino acids to form the protein or other bodily chemical dictated by that particular sequence of triple base pairs.” Barrad, supra note 15, at 1042.
27 See SUZUKI & KNUDTSON, supra note 4, at 305; see also Judges’ Glossary, supra note 19, at 65 (defining base pairs as “two complementary, nitrogen rich molecules held together by weak chemical bonds. Two strands of DNA are held together in the shape of a double helix by the bonds between their base pairs.”). Furthermore, each letter in each base pair “is the chemical complement of the letter opposite it… . [and the nucleotide bases], held together by weak bonds, can separate, have new chains laid down along them, and so be perfectly reproduced.” Hoagland & Dodson, supra note 19, at 11.
28 See Cushing, T.H., Should There be Genetic Testing in Insurance Risk Classification?, 60 DEF. COUNS. J. 249, 250 (1993).Google Scholar However, despite the existence of approximately three billion base pairs in the human genome, “most of the DNA in our chromosomes is not genes. We don't know what the function of the excess DNA is.” See Hoagland & Dodson, supra note 19, at 11; see also Denise K. Casey, What Can the New Gene Tests Tell Us?, JUDGES’ J., Summer 1997, at 14, 15 (noting that genes “are interspersed among millions of bases of DNA that do not code for proteins (noncoding DNA) and whose functions are largely unknown. In fact, genes constitute only a tiny fraction of the human genome, a mere 3 percent.”).
29 See Judges’ Glossary, supra note 19, at 65 (defining genes as “unit[s] of inheritance; a working subunit of DNA … [with each gene] containing] the code for a specific product, typically a protein ”).
30 Holmes, supra note 10, at 519. For a good description of the interaction between genes and proteins, see Hoagland & Dodson, supra note 19, at 11-13 (noting that “[p]roteins are workers, machinery; they do life's jobs. Genes are information molecules; they carry life's ideas. This relationship between genes and proteins is recursive: the two are locked together in a single, interdependent .system.”)(emphasis in the original).
31 See Judges’ Glossary, supra note 19, at 66; see also SUZUKI & KNUDTSON, supra note 4, at 344-46 (defining genotype and phenotype, respectively).
32 O'Hara, supra note 15, at 1213.
33 Id. A genetic locus is an identifiable area or “marker” on a chromosome indicating that a specific trait will be expressed by the gene. See id.
34 See Holmes, supra note 10, at 522-23. Detailed genetic maps of the 46 human chromosomes can be found at A Gene Map of the Human Genome (visited Nov. 18, 1998) <http://www.ncbi.nlm.nih.gov/genemap98>.
35 Holmes, supra note 10, at 523.
36 O'Hara, supra note 15, at 1213. Genomic research “suggests that specific genes or groups of genes predispose individuals to some forms of cancer, emphysema, juvenile diabetes, Alzheimer's disease, … heart disease and mental illness.” Id. Moreover, “[g]enes have been identified that are associated with breast cancer, cystic fibrosis, [and] skin cancer … .” Slaughter Testimony, supra note 3, at 7. Daily updates regarding genetic discoveries may be found on the Internet at The Institute for Genomic Research (visited Nov. 18, 1998) <http://www.tigr.org>.
37 See Holmes, supra note 10, at 522. More than 5,000 diseases currently affect over half of the population of the United States. See Bornstein, supra note 20, at 557.
38 See Judges’ Glossary, supra note 19, at 66 (defining mutation as “[a] change in the number, arrangement, or molecular sequence of a gene”). Most genetic mutations are in-consequential. For example, “changes in an amino acid here and there in proteins whose key function is unaffected.” Hoagland & Dodson, supra note 19, at 13. Yet, some genetic mutations “are detrimental [because] they alter an amino acid at a vulnerable location in a protein.” Id.; see also Casey, supra note 28, at 16 (discussing the role genetic mutation plays in disease pathology).
39 Monogenic disorders are relatively rare, comprising only three percent of all diseases. See Casey, supra note 28, at 16 (discussing monogenic disorders, including sickle cell anemia, cystic fibrosis (CF), Huntingtons disease (HD) and Tay-Sachs disease).
40 Holmes, supra note 10, at 522. See Bornstein, supra note 20, at 557-59; see also O'Hara, supra note 15, at 1219 (stating that the “manifestation of genetic disease also depends upon specific genes acting in concert with other genes [because] neighboring genes have various effects upon one another … creating] yet another variable in the manifestation of disease”).
41 See Collins 1996 Testimony, supra note 16, at 28 (stating that “[A]s a result of the Human Genome Project, new disease genes are discovered almost weekly. Once a disease gene is identified, it is often only a matter of months before a diagnostic test can be made available.”).
42 Casey, supra note 28, at 17.
43 See Berry, Roberta M., The Human Genome Project and the End of Insurance, 7 U. FLA. J.L. & PUB. POL'Y 205, 206-07 (1996)Google Scholar.
44 Patrinos & Drell, supra note 15, at 6.
45 Id. In other words, medical diagnostics will vastly improve “by monitoring human DNA variability and correlating it with patients’ therapeutic responsiveness, [creating] new methods for treating or curing diseases.” Jim Graves, Should Evolution be Guided by Genetic Control?: University Lecture Wrestles with Ethics of Tomorrow's Biomedicine, B.U. BRIDGE, Oct. 24, 1997, at 3.
46 The HGP's penultimate goal, by definition, must be realized before the ultimate goal. In other words, “[o]nce the human genome has been deciphered, medical researchers intend to use the information to cure genetically-based diseases by designing interventions to prevent the manifestation of these diseases.” Holmes, supra note 10, at 518.
47 The ultimate goal of the HGP has been described as “an attempt to erase the uncertainty of humankind regarding genetic illness.” O'Hara, supra note 15, at 1214; see also Casey, supra note 28, at 19 (stating that gene therapy “holds great potential for treating or even curing genetic and acquired diseases, using normal genes to replace or supplement a defective gene or bolster immunity to disease”). Over 150 clinical gene therapy trials were in progress as of summer 1997, mostly involving different kinds of cancers. See id.; see also Patrinos & Drell, supra note 15, at 6-7 (discussing the future of medicinal applications of genomic research).
48 Casey, supra note 28, at 82; see also Leslie Sowers, The Basics on Genes, HOUST. CHRON., Aug. 17, 1997, at 1, available in 1997 WL 13056793 (stating that a “genetic cure for a disease will take much more knowledge and experimentation” because scientists need to learn “how to send a correct version of the instructions into the body on a cellular level and get the corrected version to keep reproducing itself”).
49 SUZUKI & KNUDTSON, supra note 4, at 166 (emphasis in the original); see also Barrad, supra note 15, at 1043 (noting that “[i]dentifying the genetic cause of disease could suggest new methods of treatment, aid early identification, or lead to eradication of the syndrome through gene therapy”).
50 See Casey, supra note 28, at 17-19; see also Berry, supra note 43, at 206-07 (stating that with the “knowledge gained from the HGP, diagnostic tests for genetic defects will soon be widely available, and cures for diseases caused by these genetic defects will follow”).
51 Casey, supra note 28, at 19.
52 See Holmes, supra note 10, at 524; Cushing, supra note 28, at 250. Gene testing is defined as an examination of “a sample of blood or other body fluid or tissue for biochemical, chromosomal, or genetic markers that indicate the presence or absence of genetic disease.” Judges’ Glossary, supra note 19, at 66; see also Casey, supra note 28, at 18-19 (discussing gene tests for HD, CF, Alzheimer's disease and breast cancer). For a table listing diseases for which gene tests were available as of 1996 in New York State approved clinical laboratories, see Casey, supra note 28, at 15.
53 See Cushing, supra note 28, at 250-51. Genetic technicians accomplish genetic screening by testing the DNA “in blood cells to determine the presence of certain genetic markers associated with various genetic diseases.” Obinata, Naomi, Comment, Genetic Screening and Insurance: Too Valuable an Underwriting Tool to be Banned from the System, 8 SANTA CLARA COMPUTER & HIGH TECH. L.J. 145, 148 (1992)Google Scholar.
54 See SUZUKI & KNUDTSON, supra note 4, at 145-47.
55 A gene probe is defined as a “specific sequence of single-stranded DNA, typically labeled with a radioactive atom, which is designed to bind to, and thereby single out, a particular segment of DNA.” Judges’ Glossary, supra note 19, at 67. Gene probes involve researchers designing “short pieces of DNA, called probes, whose sequences are complementary to the mutated sequences. These probes will seek their complement among the three billion base pairs of an individual's genome. If the mutated sequence is present in the patient's genome, the probe will bind to it and flag the mutation.” Casey, supra note 28, at 17.
56 A linkage test is defined as a “gene-hunting technique that traces patterns of heredity in large, high risk families, in an attempt to locate a disease-causing gene mutation by identifying traits that are co-inherited with it.” Judges’ Glossary, supra note 19, at 66.
57 See Cushing, supra note 28, at 251.
58 See id.
59 See id. at 251-52.
60 See id. at 252. Because of the complexity in determining which sequence of base pairs causes a particular disease, diseases must be studied extensively in order to develop gene probes. See id. at 251. Consequently, gene probes are fewer in number than less research intensive linkage tests. See id.
61 See Casey, supra note 28, at 17.
62 See Gostin, supra note 6, at 116.
63 See id. at 116-17.
64 See O'Hara, supra note 15, at 1215; Holmes, supra note 10, at 518-19.
65 See Patrinos & Drell, supra note 15, at 8 (discussing issues including: (1) fair use of genetic information; (2) impact on genetic counseling and medical practice; (3) effects on personal reproductive decisions; (4) past uses and abuses of genetic information; (5) privacy implications of personal genetic information; and (6) commercialization and intellectual property protection of genome results); see also Holmes, supra note 10, at 519-20 (discussing the patentability of DNA sequences identified by the HGP); Bowman, James E., The Road to Eugenics, 3 U. CHI. L. SCH. ROUNDTABLE 491, 491 (1996)Google Scholar (discussing the possibility of a new eugenics movement); Wendy McGoodwin, A Look at … DNA Dilemmas, WASH. POST, May 5, 1996, at C3 (describing the situation where an health maintenance organization (HMO) told a pregnant woman whose fetus tested positive for CF that the HMO would not cover the infant under the family's medical policy if the expectant mother chose to carry the pregnancy to term).
66 See Patrinos & Drell, supra note 15, at 8.
67 See O'Hara, supra note 15, at 1215; Holmes, supra note 10, at 518-19.
68 See id. (noting that “the ELSI program can contribute to the integration of HGP results in ways that are less disruptive, painful, or destructive than [the manner in which genetic information has been used] in the past”).
69 See O'Hara, supra note 15, at 1215.
70 See Paul Recer, Group Warns Against Genetic Bias, DENV. POST, Mar. 21, 1997, at A14, available in 1996 WL 6067905 (noting concerns expressed by researchers); Slaughter Testimony, supra note 3, at 10 (discussing several cases of genetic discrimination by health insurers); Nancy E. Kass, The Implications of Genetic Testing for Health and Life Insurance, in GENETIC SECRETS: PROTECTING PRIVACY AND CONFIDENTIALITY IN THE GENETIC ERA 299, 299 (Mark A. Rothstein ed., 1997) (noting concerns expressed by academics).
71 See Barrad, supra note 15, at 1047; Lombardo, Paul A., Genetic Confidentiality: What's the Big Secret?, 3 U. CHI. L. SCH. ROUNDTABLE 589, 589 (1996)Google Scholar; see also Patrinos & Drell, supra note 15, at 7-8 (stating that electronic archives facilitate genetic data storage, provide increased access to research results and foster dissemination of information, leading to substantial increases in the number of individuals having access to medical information in electronic form).
72 Lombardo, supra note 71, at 590; Siegler, Mark, Confidentiality in Medicine—A Decrepit Concept, 307 NEW. ENG. J. MED. 1518, 1518-19 (1982).CrossRefGoogle Scholar
73 See Patrinos & Drell, supra note 15, at 7. Genomic databases are “informantics tools” developed to manipulate genetic information concerning the human genome, which if printed out in its complete letter sequence would fill 200 major city phone books. See id. at 7-8; see also The Consumer Protection and Medical Record Confidentiality Act of 1998: Hearing before the Subcomm. on Gov't Management, Info., and Tech. of the House Comm. on Gov't Reform and Oversight, 105th Cong. (1998) (testimony of Janlori Goldman, Director, Health Privacy Project Institute) (discussing concerns regarding the integrity of genetic information); David Brown, Individual ‘Genetic Privacy’ Seen as Threatened: Officials Say Explosion of Scientific Knowledge Could Lead to Misuse of Information, WASH. POST, Oct. 20, 1991, at A6 (discussing privacy concerns with respect to use of genetic information); Sally Lehrman, Genetic Privacy Debated Rapidly Developing New Technology Raises Questions, S.F. EXAMINER, Nov. 21, 1990, at B1, available in 1990 WL 7773740 (discussing concerns over the accuracy of genetic information).
74 See Annas, George J., Privacy Rules for DNA Databanks: Protecting Coded “Future Diaries ”, 270 JAMA 2346, 2346 (1993)CrossRefGoogle ScholarPubMed. But cf. Dahm, Lisa L., Using DNA Profile as the Unique Patient Identifier in the Community Health Information Network: Legal Implications, 15 J. MARSHALL J. COMPUTER & INFO. L. 227, 254-55 (1997)Google Scholar (arguing that the effect of electronic medical records and DNA fingerprints on individual privacy would be minimal).
75 See Rothstein, Mark A., Discrimination Based on Genetic Information, 33 JURIMETRICS J. 13, 13-14 (1992)Google Scholar [hereinafter JURIMETRICS] (listing entities interested in receiving genetic information, including insurers, employers, mortgage lenders, educational institutions, adoption agencies, potential business partners and courts involved in custodial disputes).
76 Genetic discrimination has been defined as “discrimination against an individual or against members of that individual's family solely because of real or perceived differences from the ‘normal’ genome in the genetic constitution of the individual.” Smith, George P. II, Accessing Genomic Information or Safeguarding Genetic Privacy, 9 J.L. & HEALTH 121, 124 (1994-95)Google ScholarPubMed (quoting Natowicz, Marvin R. et al., Genetic Discrimination and the Law, 50 AM. J. HUM. GENETICS 465, 466 (1992)Google Scholar). Genetic discrimination is not based on any notion of the present function of the individual, because the discriminating party relies on that individual's genotype to assess the probability of future risks. See id. at 124 n. 15. For examples of various forms of genetic discrimination, see Berry, supra note 43, at 209; Technological Advances in Genetic Testing: Implications for the Future, 1996: Hearing Before the Subcomm. on Tech. of the House Comm. on Science, 104th Cong. 11-12 (1996) (statement of Rep. Jim McDermott) [hereinafter McDermott Testimony].
77 Smith, supra note 76, at 124. Employers and insurers normally have access to detailed medical records of employees and customers. See id. at 124-25. Consequently, discrimination cases based on “the dissemination of genetic information to official or private entities” will shortly be heard in courts across the country. See Patrinos & Drell, supra note 15, at 5.
78 See Casey, supra note 28, at 14; see also infra text accompanying notes 82-85 (noting how genetic discrimination may arise).
79 See Casey, supra note 28, at 14; see also Technological Advances in Genetic Testing: Implications for the Future, 1996: Hearing Before the Subcomm. on Tech. of the House Comm. on Science, 104th Cong. 79 (1996) (statement of Karen Rothenberg, Director, Law & Healthcare Program, University of Maryland School of Law) [hereinafter Rothenberg Testimony] (stating that the risk of misusing genetic information is significant and unique because of the information's intergenerational impact); Hudson et al., supra note 4, at 393 (discussing third parties’ ability to “obtain sensitive genetic information about individuals, families and even populations”).
80 See Hudson et al., supra note 4, at 391 (describing the plight of a father unable to obtain insurance for his four-year-old son who was fatally diagnosed with a genetic predisposition for long QT syndrome); Rothstein, Mark A., Genetic Discrimination in Employment and the Americans with Disabilities Act, 29 Hous. L. REV. 23, 27 (1992)Google Scholar (reporting on “considerable anecdotal evidence of genetic discrimination in employment”); see also Bornstein, supra note 20, at 564-68 (reporting various studies detailing genetic discrimination by health insurers, concluding that millions of Americans are at risk of losing health coverage as a result of carrying genes that make them vulnerable to diseases); Joyce Lain Kennedy, Genetic Testing in the Workplace Holds Potential for Discrimination, BUFF. NEWS, Aug. 23, 1997, at A13 (expressing concerns about the potential impact of employer-mandated testing).
81 Technological Advances in Genetic Testing: Implications for the Future, 1996: Hearing on H.R. 2690 Before the Subcomm. on Tech. of the House Comm. on Science, 104th Cong. 4 (1996) (statement of Rep. Cliff Stearns) [hereinafter Stearns Testimony].
82 For example, a young attorney from Ohio, Theresa Morelli, was denied disability insurance because her father suffers from HD. See Myk Cherskov, Fighting Genetic Discrimination, A.B.A. J., June 1992, at 38, 38. Although Morelli has not received genetic test results indicating that she has the Huntington's genotype, she has a 50% chance of inheriting the debilitating disorder. See id.; see also Theresa E. Morelli, Genetic Discrimination by Insurers: Legal Protections Needed from Abuse, HEALTHSPAN, Sept. 1992, at 8 (describing Morelli's experience with genetic discrimination); Bornstein, supra note 20, at 565-66 (describing example of an eight-year-old girl who tested positive for phenylketonuria at birth who was denied insurance, despite the fact that she is asymptomatic).
83 See Cherskov, supra note 82, at 38; see also O'Hara, supra note 15, at 1220 (noting that genetic discrimination by insurers will likely increase because experts predict genetic screening will seep into insurance underwriting); Morelli, supra note 82, at 10 (stating that unless state and federal laws ban genetic discrimination, insurance companies will continue to insure only the genetically healthy in order to maximize profits).
84 See Morelli, supra note 82, at 10.
85 See Berry, supra note 43, at 209. Inevitable genetic discrimination litigation will likely affect insurance industry practices. See George J. Annas, Genetic Prophecy and Genetic Privacy, TRIAL, Jan. 1, 1996, at 19, 20. Furthermore, the genomic information age may bring other profound changes to the insurance industry. See Berry, supra note 43, at 209. For example, if insurance companies are not given access to genetic information, the consequence may be the end of insurance because insureds predisposed to cost-intensive afflictions will not be assessed a proportional premium and “an adverse selection spiral will result… [that] may well conclude with the insurance company's insolvency.” Id. at 218. However, even if insurance companies have access to the genetic information, the consequence may still be the end of insurance, because the insurance mechanism is premised on the insureds’ guarding against financial loss associated with vulnerability and ignorance about diseases, which will inevitably be reduced with increased knowledge through genetic testing. See id. at 209.
86 See Rothstein, supra note 80, at 66-67. Alternatively, some people pay cash for their genetic testing so that no insurance claim is submitted, which keeps insurers and employers ignorant of the existence of any genetic test results that may be used for discriminatory purposes. See id.; see also O'Hara, supra note 15, at 1222 (stating that some people who believe that they may be carriers of disease-causing genes have declined new genetic testing for fear of putting “black marks” on their medical records and losing insurance coverage).
87 Barrad, supra note 15, at 1046. For a discussion of cost-containment pressures on insurance providers, see JURIMETRICS, supra note 75, at 15-16 (reporting the findings of a study indicating that “about 5% of health insurance claimants represent about 50% of healthcare expenditures, while the top 10% of health insurance claimants represent 75% of expenditures. Excluding even a few of these potential claimants could save substantial sums of money [for insurers and self-insured employers].”). See also infra notes 90-92 (discussing cost-containment pressures on employers).
88 See Stearns Testimony, supra note 81, at 4.
89 See Barrad, supra note 15, at 1046 (discussing the problem of genetic discrimination by insurers and employers).
90 Rothstein, supra note 80, at 28. For example, from 1985 to 1991, employer health insurance costs per employee increased from $1,724 to $3,605 per year. See id. Moreover, insurance costs continue to increase at least 10% to 20% a year. See id. To streamline costs, “[b]oth large, self-insured companies and small-to-medium sized, experience rated companies are acting much like commercial health insurers who have financial incentives to screen out health risks.” Id. at 29-30; see also JURIMETRICS, supra note 75, at 15 (remarking that “[i]f employers have been placed in the role of health insurance underwriters, then it is hardly surprising that some of them are behaving as an insurance company behaves—limiting costs by excluding those at high risk. Furthermore, if some employers are willing to screen out people based on [risk] factors … then scientific genetic screening would have great appeal.”).
91 See Rothstein, supra note 80, at 26-28 (discussing the 1989 survey Office of Technology Assessment (OTA) conducted of Fortune 500 companies, large utilities and major unions). This study found that of the 330 companies that responded, only 12 reported using biochemical genetic screening and none anticipated using direct DNA screening over the next five years. See id. at 26. However, OTA's separately published survey data indicated that 42% of employers considered applicants’ health risks in hiring decisions. See id. at 27. Additionally, 36% of employers took a proactive role in assessing applicants’ health insurance risks. See id. These numbers are evidence of “[t]he growing concern among employers over the rising costs of employee health insurance, and the increased efforts to reduce these costs to the employer, [which is] likely to increase the scope of health insurance screening in the workplace.” Id. at 28. Thus, OTA concluded that employers may increase genetic testing to identify applicants with “atypical subsequent health care demands” as a means of health insurance cost-containment. See id. at 27-28.
92 See Smith, supra note 76, at 125-26. Reasons for genetic discrimination by employers include “increased medical and insurance premiums, absenteeism, lowered productivity, increased risk in the line of duty and increased liability for workers compensation.” See id.; see also Melanie Payne, Genetic Fears, CHI. TRIB., Apr. 29, 1998, at 7 (quoting Mark Rothstein as stating that “[e]mployers have a tremendous economic incentive to discriminate based on perceived future health status … [because the] key motivation for employers is to save money on health costs”).
93 See JURIMETRICS, supra note 75, at 15. If the employment system is unable to create adequate levels of health care coverage, more people will become uninsured, underinsured, or placed on public health finance, leading to more cost-shifting to the remaining private payers underwriting uncompensated care. See id. Furthermore, genetic discrimination “seems to fly in the face of the letter and spirit of our antidiscrimination laws.” Id.
94 See O'Hara, supra note 15, at 1224. See infra Part VI for discussion of various legislative proposals targeting genetic discrimination.
95 See Holmes, supra note 10, at 558-63 (discussing abuses by insurers); Brokaw, Katherine, Genetic Screening in the Workplace and Employer's Liability, 23 COLUM. J.L. & SOC. PROBS. 317, 317-18 (1990)Google Scholar (discussing abuses by employers).
96 See McDermott Testimony, supra note 76, 11 (stating that “[s]ince we all carry some genetic mutations, [the scenario of genetic discrimination by health insurers] carried to its conclusion would leave us all uninsurable”).
97 See Holmes, supra note 10, at 558. Individuals with the least need for health insurance will be able to obtain comprehensive coverage at reasonable rates, whereas those with genetic predispositions for illness may be unable to afford their insurance premiums or may be denied insurance altogether. See id.; see also Berry, supra note 43, at 254 (predicting that as “the HGP progresses and genetic testing advances in its wake, the class of those excluded from the benefits of insurance will grow”).
98 See Barrad, supra note 15, at 1046-47. Employers may attempt to use genetic information to ascertain whether a prospective employee is “predisposed to job-related disabilities, low productivity, or absenteeism.” See id. Employers will be increasingly tempted to use genetic screening tests “in the face of pressure from insurers and top management to cut medical costs.” See Brokaw, supra note 95, at 318.
99 See, e.g., Dana Hawkins, Dangerous Legacies: New Gene Tests Provide Fresh Grounds for Discrimination, U.S. NEWS & WORLD REP., Nov. 10, 1997, at 7; David Stipp, Health: Genetic Testing May Mark Some People as Undesirable to Employers, Insurers, WALL ST. J., July 9, 1990, at B1; David Smith, Rite and Reason, IRISH TIMES, June 4, 1996, at 12, available in WL 10575420.
100 See O'Hara, supra note 15, at 1212. In essence, genetic discrimination by insurers and employers “penalizes those who do not conform to the institutional norm of ‘healthy’ individuals.” See id.; see also Brokaw, supra note 95, at 319 (stating that “[g]enetic screening in the workplace has undeniable potential for … the ‘leperizing’ of hundreds of thousands of American workers … [and that genetic information could] become a substantial barrier to employment with little basis other than the fears, prejudices and misunderstandings of people who know little about genetics”).
101 See Brokaw, supra note 95, at 320; see also Morelli, supra note 82, at 8 (stating that insurers “shift the risk to individuals and ultimately to the taxpayers when uninsurable and impoverished people are forced onto Medicaid”).
102 See Morelli, supra note 82, at 8.
103 See id. Because private health insurance is the means by which many Americans pay for health care, genetic discrimination resulting in uninsurability leads to a denial of health care for a large number of people. See Bornstein, supra note 20, at 577; Kass, supra note 70, at 299, 305.
104 See Widiss, Alan I. & Gostin, Larry, What's Wrong with the ERISA “Vacuum“?: The Case Against Unrestricted Freedom for Employers to Terminate Employee Health Care Plans and to Decide What Coverage is to be Provided When Risk Retention Plans are Established for Health Care, 41 DRAKE L. REV. 635, 636 (1992).Google Scholar
105 See Lombardo, supra note 71, at 613. Genetic discrimination is one part of a much larger problem of competitive risk-rating, which puts many potential policyholders beyond the ambit of insurance coverage. See id. Until this problem is fixed, “the attempt to carve out some portion of the public for unfavorable treatment because of medical condition—genetic or not—will remain endemic.” See id.
106 See Holmes, supra note 10, at 565. If insurers are not permitted to calculate known genetic risks of individuals seeking health insurance coverage in rate setting, then the insurers will be forced to distribute the costs for present and future diseases more evenly among their insureds. See id. at 567.
107 See SUZUKI & KNUDTSON, supra note 4, at 347. Polygenic means “controlled by or associated with more than one gene.” See id.
108 See Holmes, supra note 10, at 527. There are basically four types of genetic anomalies. See id. at 527-29. First, monogenic disorders involve “one improperly functioning gene.” See id. at 527-28. Although there are 3,600 monogenic disorders, only “three percent of the population will develop a single gene disease.” See id. at 528. Second, chromosomal disorders result from an abnormal chromosomal number or structure (i.e., Down's Syndrome). See id. Third, there are noninherited disorders with “genetic changes occurring during one's [lifetime],” such as cell mutation leading to cancer. See id. at 528-29. Finally, multi-factorial disorders comprise the “largest genetic-disorder classification” and are diseases that “result from the interaction of environmental factors and many abnormal genes presumably on different chromosomes.” See id. at 528. Heart disease is an example of a multi-factorial genetic disorder. See id.
109 See id. at 529. In the majority of cases, the results of genetic tests simply reflect an increased or decreased susceptibility to a particular disease or to diseases associated with environmental influences. See id. Furthermore, despite links made between diseases and genes, “the degree of severity, timing of onset, or whether the disease will ever manifest itself at all, remain a matter of statistical probability.” See id. Therefore, most genetic test results reflect probabilities and are less conclusive than one may assume. See id.
110 See Bornstein, supra note 20, at 569-71.
111 See O'Hara, supra note 15, at 1218-19.
112 See Holmes, supra note 10, at 524; Miller, Frances H. & Huvos, Philip A., Genetic Blueprints, Employer Cost-Cutting, and the Americans with Disabilities Act, 46 ADMIN. L. REV. 369, 372 (1994).Google Scholar
113 See Graves, supra note 45, at 3. For example, gene tests “rarely provide definitive information about the function of particular genes” because they only indicate an abnormality in one gene, and are thus unable to predict the onset of a disease caused by the interaction of several genes. See id.
114 See Holmes, supra note 10, at 530. Historically, misinterpretation and subsequent misuse of genetic information has caused unreasonable discrimination, most notably in the 1970s among the population carrying the sickle cell trait. See Cushing, supra note 28, at 258.
115 See Holmes, supra note 10, at 530.
116 Cushing, supra note 28, at 258. Despite their imperfections, genetic tests are accorded an illusion of precision. See O'Hara, supra note 15, at 1215-16. Thus, the danger in genetic testing lies not in the test's imperfect powers of prediction, but rather in the hasty adoption of unreliable test results by “institutions eager to minimize costs [in a health care system where] costs are rising at the rate of twenty percent per annum.” See id. at 1216.
117 See Bornstein, supra note 20, at 559; see also Casey, supra note 28, at 17 (explaining that genetic test results are difficult to interpret because they only provide a predictive guess).
118 See Holmes, supra note 10, at 530-31. The report further warned against two major misinterpretations of genetic information: reductionism and determinism. See id. at 531. Reductionism occurs when genetic test results reduce particular traits to the “expression of particular genes” and neglect to consider fully the importance of external factors. See id. Determinism occurs when an individual is “inappropriately” labeled as “sick or abnormal.” See id.
119 See O'Hara, supra note 15, at 1224. It is estimated that every human being has between four to eight genetic defects. See id.
120 See Berry, supra note 43, at 232.
121 See id.
122 See id. at 236.
123 See id. at 235-36. For example, insurers may agree to insure an individual genetically predisposed to heart disease, but contractually exclude coverage for claims involving heart disease. See id. The strategy reflects the fact that insurers lack the economic motivation to eliminate every insured with genetic defects because that would completely eliminate the market for insurance, and that insurers have an economic incentive to write as many policies as possible. See id. at 232.
124 See infra notes 157-62 and accompanying text (discussing the use of adverse selection by insurers).
125 See Underwood, Richard H. & Cadle, Ronald G., Genetics, Genetic Testing, and the Specter of Discrimination: A Discussion Using Hypothetical Cases, 85 KY. L.J. 665, 686 (1996-97).Google Scholar
126 See id.
127 Bornstein, supra note 20, at 609.
128 See id.
129 Universal health care may alleviate the problem of adverse selection caused by genetic discrimination legislation. See Bornstein, supra note 20, at 609 n.299. Since medical underwriting is unnecessary in universal health care, it would eliminate the need for the disclosure of genetic information. See id. Insurers could also adopt “genetic community rating,” a variant of “community rating.” Cf. Jacobi, John V., The Ends of Health Insurance, 30 U.C. DAVIS L. REV. 311, 316 (1997)Google Scholar (discussing use of community rating by Blue Cross in the 1930s). Community rating involves charging all insureds a premium based on the average risk experienced by people living in a particular community. See id. Genetic community rating, however, would only be successful in a competitive insurance market if legislation prevents insurers from using genetic information to offer cheaper insurance to insureds. Cf. Jacobi, supra, at 316 (discussing commercial insurers’ attempt to undermine Blue Cross's market by charging low-risk groups based on experience rating rather than community rating). Moreover, insurers could adopt insurance pooling arrangements to cover high-risk insureds. Cf. Lowe, Robert, Genetic Testing and Insurance: Apocalypse Now?, 40 DRAKE L. REV. 507, 515 (1991)Google Scholar (discussing state plans pooling those who have been rejected by insurers and whose premiums are exorbitantly high, require insurers to pay into the pool and then charge the insurers a separate fee to make up for any shortfalls in the pool). State legislatures could require insurers to cover a percentage of high-risk insureds in proportion to each insurer's market share thereby eliminating adverse selection. See id. Pooling insurance for genetic risk assumes that genetic information would be available to insurers and would thus be a compromise between absolute genetic privacy and unrestricted access to genetic information. Cf. Holmes, supra note 10, at 543 (suggesting that insurers and insureds should have equal access to relevant genetic data to avoid unfairness to insurers and adverse selection by insureds). Alternatively, insurers could determine the average or median levels of insurance and charge a premium reflecting the increased costs of health insurance associated with adverse selection to all insureds purchasing insurance coverage who fall too far outside of a predetermined range. Cf. id. at 540 (suggesting that rates should discriminate fairly among insureds to reflect their health status).
130 Otherwise, the people most in need of health insurance will be unable to acquire it. See Cushing, supra note 28, at 257.
131 See Holmes, supra note 10, at 563-64. However, gene therapies may soon change this immutable nature. See supra notes 48-49 and accompanying text (noting the promise of gene therapy).
132 See Holmes, supra note 10, at 516. Moreover, because “genetic differences are morally arbitrary, the notion of good or bad genetic luck ought not be the reason that one person receives better or worse insurance” coverage. Id. at 564.
133 See Hudson et al., supra note 4, at 391. The high costs of health care in the United States make insurance coverage necessary in order to access health care. See id.
134 See Cushing, supra note 28, at 258. Essentially, “'genetically healthy’ people who do not need health insurance will be able to get it at a reduced premium rate, [while] the vast majority who do not need health insurance will not be offered the chance or will not be able to afford what's offered.” Id. at 257.
135 See Holmes, supra note 10, at 563.
136 See Warren, Samuel D. & Brandeis, Louis D., The Right to Privacy, 4 HARV. L. REV. 193, 193 (1890).CrossRefGoogle Scholar
137 See Bornstein, supra note 20, at 572; see also Cushing, supra note 28, at 258-59 (noting that “genetic information is fundamentally different from other types of medical information readily accessible to insurers [because] the immutable quality of genetic information sets it apart from other sorts of medical information, such as a person's blood pressure or cholesterol level”).
138 See Annas, supra note 74, at 2346; Holmes, supra note 10, at 570-71. Modern technology provides insurers with practical and possibly clandestine means of obtaining, storing, organizing, retrieving and disseminating genetic and other actuarial data about applicants and policyholders. See Holmes, supra note 10, at 570-71. A “justifiable fear arises that widespread delineation of genotypes and genetic profiles may, like credit information, culminate in centralized genetic information databases.” Id.
139 Holmes, supra note 10, at 565 (quoting NIH-DOE WORKING GROUP ON ETHICAL, LEGAL, AND SOCIAL IMPLICATIONS OF HUMAN GENOME RESEARCH, NATIONAL CENTER FOR HUMAN GENOME RESEARCH, GENETIC INFORMATION AND HEALTH INSURANCE, REPORT OF THE TASK FORCE ON GENETIC INFORMATION AND INSURANCE 12 (1993)).
140 See id. at 574. Genetic autonomy is defined as “the right of persons to make an informed, independent judgment about whether they wish to be tested,” including whether they wish to know the results of the genetic test. See Bornstein, supra note 20, at 572.
141 See Bornstein, supra note 20, at 573.
142 See id.
143 See id. at 575.
144 See Annas, supra note 85, at 19.
145 See Marilyn Chase, Genetic Testing Needs Clear Plans for How to Handle Treatment, WALL ST. J., Feb. 26, 1996, at B1.
146 See Holmes, supra note 10, at 571-72.
147 See id. at 571-73. Although genomic information is not dispositive of a person's future health, people place the same over-reliance on the value of genetic testing results that insurers and employers do, and may thus cause themselves unnecessary angst. See id. at 572-73. Having knowledge about one's own genetic make-up may have a devastating psychological impact when the individual is told that he or she will develop a fatal, incurable disease. See id. Historical experience with sickle cell anemia in the 1970s exemplifies the psychological damage that injudicious use of genetic information can cause. See id. at 572.
148 See id. at 573. The genetic information may adversely affect an individual's decisions regarding education, work, marriage and reproduction. See id.
149 Although arguments for the right to discriminate genetically against certain individuals are primarily made by insurers, they apply with equal force to employers inasmuch as employers provide health care to employees and are thus similarly interested in containing the cost of health care. See id. at 557. Furthermore, employers are also interested in genetically discriminating against individuals to increase job-related productivity. See Nobles, Kimberley, Note, Birthright or Life Sentence: Controlling the Threat of Genetic Testing, 65 S. CAL. L. REV. 2081, 2089 (1992).Google ScholarPubMed
150 See Holmes, supra note 10, at 557.
151 For a general discussion of the mechanics of underwriting in the insurance industry, see Berry, supra note 43, at 216-18, and Meyer, Roberta B., Justification for Permitting Life Insurers to Continue to Underwrite on the Basis of Genetic Information and Genetic Test Results, 27 SUFFOLK U. L. REV. 1271, 1279 (1993).Google Scholar Underwriting is done in the individual insurance market when the “private health insurance industry offers two types of insurance: individual health policies and large employer group health policies.” See Holmes, supra note 10, at 533-34. Approximately 90% of private health insurance policies are group policies, with the remaining 10% comprised of individual policies or policies for small groups of fewer than 10 to 15 people. See id. Medical underwriting generally only occurs for individual and small group policies and not for large group policies. See id. at 534. “Because of their ability to average utilization over a large number of employees, large employer groups customarily are not medically underwritten.” Id. Moreover, “[n]ew employees [working for large employers] are eligible for coverage without medical underwriting by the insurer.” Id. The group's insurance premiums will vary based on the group's size because large groups are experience rated, whereby the insureds’ premiums change based on their total claims. See id. As such, these large groups are typically not denied health insurance due to the poor health of some employees or their dependents. See id. Hence, “concern over [genetic] discrimination is greatly diminished when medical premiums are based on experience rather than calculated through underwriting.” Id.
152 See Holmes, supra note 10, at 531-34. “Fair discrimination” requires efficient, actuarial analysis establishing risk transference and risk distribution, which are the keys to understanding insurance underwriting. See id. at 531. Insurance, by its nature, is discriminatory because individuals with a higher risk are routinely charged a higher premium. See id. at 533. Consequently, “the goal of insurance underwriting is equity; that is, equitable, but not equal, treatment of applicants and policyholders. To achieve that goal, insurers must differentiate among policyholders by risk classifications and discriminate fairly so that each insured will pay a premium at a level consistent with the risk represented by each individual insured.” Id.; see also Obinata, supra note 53, at 146—47 (discussing the necessity of genetic discrimination in the underwriting process to achieve fairness).
153 See Holmes, supra note 10, at 532.
154 See id. at 538. However, inasmuch as everyone has a few bad genes, no insured poses an absolutely lower risk, because all insureds pose a greater risk for some disease. See O'Hara, supra note 15, at 1224. Therefore, insurers would refine the risk classification to such a degree either that no insured would be able to afford health insurance or, alternatively, that no insured would be able to get insurance that covers whatever condition an individual insured would be predisposed to suffer. See Holmes, supra note 10, at 558.
155 See Holmes, supra note 10, at 534-35. Prohibiting insurers’ use of genetic information yields two alternative scenarios:
(1) If an insurer under-assesses risks, it will have insufficient funds to pay claims submitted unless it overcharges people who represent low risk. If insurers have inadequate funds and are unable to meet their contractual obligations to pay claims, the insurers will go bankrupt, leaving people uninsured.
(2) If an insurer over-assesses risk and overcharges, the free market and competitive nature of business logically dictate that people will purchase insurance elsewhere. Thus, accurate risk assessment is essential to the business of insurance.
Id. at 538; see also Meyer, supra note 151, at 1279 (discussing the possibility that deficient risk classification systems will make insurers insolvent); Obinata, supra note 53, at 149 (stating that “[p]remium rates must be high enough to assure permanent financial security for the company and, at the same time, low enough to enable the company to be competitive”).
156 See Holmes, supra note 10, at 539 (discussing insurers’ arguments supporting the view that genetic tests are efficient and equitably fair). However, the argument for equitable treatment of insureds fails for two reasons. First, risk underwriting is only used for the individual insurance market, which comprises just 10% to 15% of the total insurance market. See id. at 534. This relatively small size undermines the argument that under-assessing insureds will result in financial insolvency for the insurance industry. See id. Rather, insurers may be attempting to hide a naked desire to eliminate expensive policyholders from coverage or at least to limit coverage by excepting an insured's expensive condition. See Gaulding, Jill, Race, Sex and Genetic Discrimination in Insurance: What's Fair?, 80 CORNELL L. REV. 1646, 1651-52 (1995).Google Scholar Second, if all insurers were denied the ability to use genetic information in their risk classifications, then over-assessing premium rates would not result in a loss of business among insureds because all insurers would be similarly disadvantaged with respect to incorporating genetic information into actuarial analysis. See Jacobi, supra note 129, at 403. Preventing insurers from using genetic information will merely maintain the status quo because insurers have operated profitably for many years without the benefit of genetic information. See, e.g., Philip R. Reilly, Laws to Regulate the Use of Genetic Information, in GENETIC SECRETS: PROTECTING PRIVACY AND CONFIDENTIALITY IN THE GENETIC ERA 369, 374-79 (Mark A. Rothstein ed., 1997).
157 Adverse selection is the tendency of insureds to apply for insurance based on the knowledge that the insured is in poor health, while concealing this fact from the insurer. See Cushing, supra note 28, at 254; Berry, supra note 43, at 217; see also Meyer, supra note 151, at 1289 (defining adverse selection as the “tendency of persons who are poorer risks to seek insurance to a greater extent than do persons who are better risks”).
158 See Cushing, supra note 28, at 254-55.
159 Holmes, supra note 10, at 544.
160 See Meyer, supra note 151, at 1290. Typically, applicants at a higher risk for a specific disease would be more likely to seek insurance covering that disease than the average person. See Holmes, supra note 10, at 544. “If insurers charge an equitably rated premium without knowledge of the high risk or charge an equal premium for all applicants, the high risk person will select to apply and obtain insurance in greater proportion than low risk people … [leading to a cycle of] adverse consequences.” Id. This cycle starts with increased premiums resulting from greater claims made by high-risk individuals, leading to a discontinuance of insurance by low-risk insureds, which in turn leads to a residual of extremely high-risk insureds, which finally leads to another premium increase that renews the cycle. See id. However, this argument assumes that there are low-risk insureds to drive out of the health insurance market. See O'Hara, supra note 15, at 1224.
161 Meyer, supra note 151, at 1290.
162 See Holmes, supra note 10, at 545; see also Berry, supra note 43, at 218 (stating that the genetically spurred “adverse selection price spiral may well conclude with … [an] insurance company's insolvency”).
163 Cf. Jacobi, supra note 129, at 389 (suggesting that “even risk-averse, low-risk people will select the lower price offered by a risk-rated [health insurance policy]” rather than opting for no coverage at all).
164 See Privacy of Individual Genetic Information, 1998: Hearings on S. 422 Before the Senate Comm. on Labor, 105th Cong. (1998) (statement of Senator Jim Jeffords ) [hereinafter Jeffords 1998 Testimony].
165 See Holmes, supra note 10, at 555; Kass, supra note 70, at 300. Moreover, only a fraction of this 10% slice of the market involves monogenic diseases, see Holmes, supra note 10, at 536, which in turn represent only 3% of diseases. See Casey, supra note 28, at 16. Accordingly, insurers will probably only face increased costs from adverse selection by an extremely small fraction of the insurance market, which would not not be enough to make the industry insolvent.
166 Berry, supra note 43, at 221.
167 See Glazier, Alexandra K., Genetic Predispositions, Prophylactic Treatments and Private Health Insurance: Nothing is Better Than a Good Pair of Genes, 23 AM. J.L. & MED. 45, 63-64 (1997)Google Scholar; cf. Weaver, Kirke D., Genetic Screening and the Right to Know, 13 ISSUES IN L. & MED. 243, 249 (1997)Google Scholar (discussing the impact of adverse selection on the insurance industry and the industry's response to the problem).
168 See Cushing, supra note 28, at 254.
169 See Hudson et al., supra note 4, at 391.
170 See Cushing, supra note 28, at 254.
171 Holmes, supra note 10, at 557.
172 See Glazier, supra note 167, at 63.
173 See Holmes, supra note 10, at 556.
174 See Brokaw, supra note 95, at 326.
175 See id.
176 See Holmes, supra note 10, at 555. However, the marginal effect genetic information will have on insurance availability also undermines the insurers’ argument that such information is needed to maintain solvency.
177 See Cushing, supra note 28, at 252. Furthermore, insurers contend that the average price for genetic tests, between $450 and $1,000 per test, would be prohibitively expensive if performed on insureds in the aggregate. See id.
178 See generally Malinowski, Michael J. & Blatt, Robin J.R., Commercialization of Genetic Testing Services: The FDA, Market Forces, and Biological Tarot Cards, 71 TUL. L. REV. 1211 (1997)Google Scholar (discussing the growing market in commercial genetic tests and the need for regulating such tests); Casey, supra note 28, at 15 (listing available genetic tests).
179 See Rothstein, supra note 80, at 28.
180 See Jeffords Continues Push to Markup on Health Bills, HEALTH LEGIS. & REG. WKLY., May 27, 1998, available in 1998 WL 10395863 (stating that “[i]nsurers argue that, if a bill is passed at all, it must define gene information narrowly to include only knowledge gleaned from directly testing an individual's DNA and ribonucleic acid (RNA), and exclude information about inheritable traits gleaned from family histories or other medical tests.”).
181 See id.
182 See Jeffords 1998 Testimony, supra note 164.
183 See Holmes, supra note 10, at 578.
184 See Smith, supra note 76, at 121. However, balancing these divergent interests is easier said than done.
185 Ch. 20, 59 Stat. 33 (1945) (codified as amended at 15 U.S.C. §§1011-1015 (1998)).
186 See Cushing, supra note 28, at 260. For a general background of the McCarran-Ferguson Insurance Regulation Act of 1945, see Holmes, supra note 10, at 582-84.
187 Holmes, supra note 10, at 584.
188 Pub. L. No. 93-406, 88 Stat. 829 (1974).
189 Telephone Interview with Brian O'Connor, Communications Director, Office of U.S. Representative Joseph P. Kennedy II (Feb. 18, 1998) (stating that the insurance industry was able to secure freedom from state regulations by lobbying for the McCarran Act); see also Karen Rothenberg, Miracles of Genetics Can Bear Heavy Cost, BALT. SUN, July 20, 1997, at 6F (stating that “state insurance laws cannot apply to self-funded employer plans because of [ERISA]”).
190 See Rothenberg, supra note 189, at 6F.
191 See Bornstein, supra note 20, at 589; Holmes, supra note 10, at 644. One source noted that there are genetic discrimination laws in 24 states that restrict insurers’ use of genetic information, and that as many as 250 genetic discrimination bills are currently pending in 44 state legislatures. See Laws to Forbid Gene-Test Penalties by Insurers Languish in Congress; Thousands Wanting to Know of Risk Fear Being Denied Coverage, BALT. SUN, Apr. 12, 1998, at 4A. Some states have begun to require insurers to use “community rates” whereby premiums for individual and group policies are based solely on the basis of age, geography and family composition, thus preventing “any health insurer from seeking genetic testing of applicants and from using genetic information in fixing rates and premiums.” See Holmes, supra 10, at 648-69.
192 See ALA. CODE §§ 27-53-1 to -4 (1998); ALASKA STAT. §§ 21.54.100-.54.110 (Michie Supp. 1998); ARIZ. REV. STAT. ANN. §§ 20-448 to -448.02, 20-1379 (West 1997); ARK. CODE ANN. §§ 23-86-304 to -306 (Michie Supp. 1997); CAL. CIV. CODE §§ 56.17-.18 (West 1998); CAL. HEALTH & SAFETY CODE § 1374.7 (West 1990); CAL. INS. CODE §§ 742.405-.407, 10123.3-.35, 10123.9, 10140, 10140.1, 10143, 10146, 10148-10149.1, 10198.9, 10705, 11512.7-11517 (West 1993 & Supp. 1998); COLO. REV. STAT. ANN. §§ 10-3-1104.7, 25-1-122.5 (1997); CONN. GEN. STAT. § 38a-816(19) (1998); FLA. STAT. ANN. §§ 627.4301, 627.65625, 636.0201, 641.31073, 641.438, 760.40 (West 1997 & Supp. 1998); GA. CODE ANN. §§ 33-54-1 to -8 (1996); HAW. REV. STAT. §§ 431:10A-118, 432:1607, 432D-26 (1998); IDAHO CODE §§ 41-2221, -3940, -4708 (Michie 1997); 215 ILL. COMP. STAT. 5/356t, 97/20-97/25, 513/45 (West 1998); IND. CODE ANN. §§ 16-39-5-2, 27-8-26-1 to -12 (West Supp. 1998); IOWA CODE ANN. § 729.6 (West 1993); KAN. STAT. ANN. § 40-2259 (1998); LA. REV. STAT. ANN. §§ 22:213.7, :250.3, :1214, 40:1299.6, :2210 (West 1998); ME. REV. STAT. ANN. tit. 24-A, § 2850 (West Supp. 1997); MD. CODE ANN., INS. §§ 27-208, -909 (1997); MINN. STAT. ANN. § 72A.139 (West Supp. 1998); MONT. CODE ANN. §§ 33-18-206, -22-514, -22-526 (1997); NEB. REV. STAT. §§ 44-5246.02, -6916 (1997); NEV. REV. STAT. §§ 629.101-.201, 689A.417, .545, .585, 689B.069, .420, .550, 689C.193, .198, .207 (1997); N.H. REV. STAT. ANN. §§ 141-H:1 to -H:5, 420-G:6 to -G:7 (1996); N.J. STAT. ANN. §§ 10:5-5, :5-12, :5-43 to-49, 17:48-6.18, 17:48A-6.11, 17.48E-15.2, 17B:26-3.2, :27-36.2, :27-57, :30-12, 26:2M5.1 (West 1993 & Supp. 1998); N.M. STAT. ANN. §§ 24-21-1 to -7, 59A-23E-11 (Michie Supp. 1998); N.Y. Civ. RIGHTS LAW §§ 48-a, 48-b, 79-1 (McKinney 1992 & Supp. 1998); N.Y. EXEC. LAW § 296 (McKinney 1993 & Supp. 1997); N.Y. INS. LAW §§ 2612, 3221, 3232, 4305, 4318 (McKinney 1985 & Supp. 1998); N.C. GEN. STAT. §§ 58-68-30, -35, 95-28.1A (Supp. 1997); N.D. CENT. CODE §§ 26.1-08-12, -36.3-01, -36.3-06, -36.4-03.1 (1995 & Supp. 1997); OHIO REV. CODE ANN. §§ 1751.18, 1751.65, 3901.21, 3901.49, 3901.491, 3901.50, 3901.501, 3924.031, 3924.27 (Anderson 1997 & Supp. 1997); OR. REV. STAT. §§ 659.036, 659.227, 659.324, 659.340, 659.700-.720, 746.135 (1997); R.I. GEN. LAWS §§ 28-6.7-1 to -4 (1995); S.C. CODE ANN. §§ 38-41-45, 38-71-670, -840, -860 (Law Co-op. Supp. 1997); S.D. CODIFIED LAWS §§ 58-17-84, 58-18-45, 58-18B-27 (Michie Supp. 1998); TENN. CODE ANN. §§ 56-7-2701-7-2708, 56-7-2802 (Supp. 1997); TEX. LAB. CODE ANN. §§ 21.401-405 (West Supp. 1998); TEX. REV. Civ. STAT. ANN. art. 9031 (West Supp. 1998); VA. CODE ANN. §§ 32.1-67.1 to -69.1, 38.2-508.4, 38.2-613 (Michie 1997 & Supp. 1998); W. VA. CODE §§ 33-15-2a, -16-la, -16-3k (Supp. 1998); Wis. STAT. §§ 111.372, 631.89, 632.746, 632.748 (1998); WYO. STAT. ANN. §§ 26-19-107, -306 (Michie 1998).
193 See H.B. 10, 1st Spec. Sess. (Ala. 1997); H.B. 218, Reg. Sess. (Ala. 1997); S.B. 113, Reg. Sess. (Ala. 1997) (enacted); H.B. 415, 20th Leg., 2d Reg. Sess. (Alaska 1998); S.B. 104, 20th Leg., 1st Reg. Sess. (Alaska 1997) (enacted); H.B. 2600, 43d Leg., 2d Reg. Sess. (Ariz. 1998); H.B. 2144, 43rd Leg., 1st Reg. Sess. (Ariz. 1997) (enacted); H.B. 2011, 81st Leg., Reg. Sess. (Ark. 1997); A.B. 2688, 97-98 Reg. Sess. (Cal. 1997); A.B. 310, 97-98 Reg. Sess. (Cal. 1997); A.B. 34, 97-98 Reg. Sess. (Cal. 1997); S.B. 1654, 97-98 Reg. Sess. (Cal. 1997); H.B. 5471, 139th Leg., 2d Reg. Sess. (Conn. 1998); H.B. 6527, 139th Leg., 1st Reg. Sess. (Conn. 1997) (enacted); H.B. 5349, 139th Leg., 1st Reg. Sess. (Conn. 1997); H.B. 5134, 139th Leg., 1st Reg. Sess. (Conn. 1997); H.B. 5053, 139th Leg., 1st Reg. Sess. (Conn. 1997); S.B. 85th, 139th Leg., 2d Reg. Sess. (Conn. 1998); S.B. 80, 139th Leg., 2d Reg. Sess. (Conn. 1998) (enacted); S.B. 89, 139th Leg., 1st Reg. Sess. (Conn. 1997); S.B. 166, 139th Leg., 1st Reg. Sess. (Del. 1997) (enacted); S.B. 153, 139th Leg., 1st Reg. Sess. (Del. 1997) (enacted); S.B. 152, 139th Leg., 1st Reg. Sess. (Del. 1997); H.B. 127, 100th Leg., 1st Reg. Sess. (Fla. 1997); S.B. 1850, 100th Leg., 1st Reg. Sess. (Fla. 1997); S.B. 138, 100th Leg., 1st Reg. Sess. (Fla. 1997); H.B. 386, 144th Leg., Reg. Sess. (Ga. 1997); H.B. 1822, 19th Leg., Reg. Sess. (Haw. 1997); H.B. 1566, 19th Leg., Reg. Sess. (Haw. 1997); H.B. 476, 19th Leg., Reg. Sess. (Haw. 1997); S.B. 2160, 19th Leg., Reg. Sess. (Haw. 1997); S.B. 1565, 19th Leg., Reg. Sess. (Haw. 1997) (enacted); S.B. 112, 19th Leg., Reg. Sess. (Haw. 1997); H.B. 388, 90th Leg., 1st Reg. Sess. (Ill. 1997); H.B. 8, 90th Leg., 1st Reg. Sess. (Ill. 1997) (enacted); S.B. 802, 90th Leg., 1st Reg. Sess. (Ill. 1997) (enacted); S.B. 672, 90th Leg., 1st Reg. Sess. (Ill. 1997); S.B. 165, 110th Leg., 1st Reg. Sess. (Ind. 1997); H.F. 701, 77th Leg., 1st Reg. Sess. (Iowa 1997) (enacted); H.F. 171, 77th Leg., 1st Reg. Sess. (Iowa 1997); S.F. 100, 77th Leg., 1st Reg. Sess. (Iowa 1997); H.B. 2734, 77th Leg., 1st Reg. Sess. (Kan. 1997); H.B. 2417, 77th Leg., 1st Reg. Sess. (Kan. 1997); S.B. 463, 77th Leg., 1st Reg. Sess. (Kan. 1997); S.B. 204, 77th Leg., 1st Reg. Sess. (Kan. 1997) (enacted); S.B. 334, 97-98 Reg. Sess. (Ky. 1998); H.B. 2071, 97-98 Reg. Sess. (La. 1997); S.B. 771, 97-98 Reg. Sess. (La. 1997); S.B. 526, 97-98 Reg. Sess. (La. 1997); H.B. 893, 118th Leg., 1st Reg. Sess. (Me. 1997); H.B. 845, 118th Leg., 1st Reg. Sess. (Me. 1997); S.B. 384, 118th Leg., 1st Reg. Sess. (Me. 1997) (enacted); H.B. 297, 412th Leg., 1998 Reg. Sess. (Md. 1998); H.B. 920, 411th Leg., 1997 Reg. Sess. (Md. 1997); H.B. 776, 411th Leg., 1997 Reg. Sess. (Md. 1997); S.B. 324, 412th Leg., 1998 Reg. Sess. (Md. 1998); H.B. 4485, 180th Gen. Ct., Reg. Sess. (Mass. 1997); H.B. 4419, 180th Gen. Ct., Reg. Sess. (Mass. 1997); H.B. 4162, 180th Gen. Ct., Reg. Seos. (Mass. 1997); H.B. 2669, 180th Gen. Ct., Reg. Sess. (Mass. 1997); H.B. 2668, 180th Gen. Ct., Reg. Sess. (Mass. 1997); S.B. 2018, 180th Gen. Ct., Reg. Sess. (Mass. 1997); S.B. 1933, 180th Gen. Ct., Reg. Sess. (Mass. 1997); S.B. 659, 180th Gen. Ct., Reg. Sess. (Mass. 1997); H.B. 5963, 89th Leg., Reg. Sess. (Mich. 1997); H.B. 5960, 89th Leg., Reg. Sess. (Mich. 1997); H.B. 5462, 89th Leg., Reg. Sess. (Mich. 1997); H.B. 5461, 89th Leg., Reg. Sess. (Mich. 1997); H.B. 5460, 89th Leg., Reg. Sess. (Mich. 1997); H.B. 4991, 89th Leg., Reg. Sess. (Mich. 1997); H.B. 4990, 89th Leg., Reg. Sess. (Mich. 1997); H.B. 4882, 89th Leg., Reg. Sess. (Mich. 1997); H.B. 4881, 89th Leg., Reg. Sess. (Mich. 1997); H.B. 4880, 89th Leg., Reg. Sess. (Mich. 1997); H.B. 4836, 89th Leg., Reg. Sess. (Mich. 1997); H.B. 4118, 89th Leg., Reg. Sess. (Mich. 1997); S.B. 134, 89th Leg., Reg. Sess. (Mich. 1997); S.B. 110, 89th Leg., Reg. Sess. (Mich. 1997); S.B. 109, 89th Leg., Reg., Sess. (Mich. 1997); S.B. 108, 89th Leg., Reg. Sess. (Mich. 1997); S.B. 107, 89th Leg., Reg. Sess. (Mich. 1997); S.B. 70, 89th Leg., Reg. Sess. (Mich. 1997); S.B. 69, 89th Leg., Reg. Sess. (Mich. 1997); S.B. 68, 89th Leg., Reg. Sess. (Mich. 1997); S.B. 67, 89th Leg., Reg. Sess. (Mich. 1997); S.B. 66, 89th Leg., Reg. Sess. (Mich. 1997); H.B. 1316, 89th Gen. Assembly, 2d Reg. Sess. (Mo. 1998); A.B. 549, 69th Leg., Reg. Sess. (Nev. 1997) (enacted); H.B. 241, 115th Leg., 1997 Reg. Sess. (N.H. 1997); A.B. 1716, 208th Leg., Reg. Sess. (N.J. 1998); A.B. 1641, 208th Leg., Reg. Sess. (N.J. 1998); A.B. 331, 208th Leg., Reg. Sess. (N.J. 1998); S.B. 565, 208th Leg., Reg. Sess. (N.J. 1998); H.B. 331, 43d Leg., 2d Reg. Sess. (N.M. 1998) (enacted); H.B. 395, 43d Leg., 1st Reg. Sess. (N.M. 1997); S.B. 176, 43d Leg., 2d Reg. Sess. (N.M. 1998) (enacted); A.B. 8412, 220th Leg., Reg. Sess. (N.Y. 1997); A.B. 5553, 220th Leg., Reg. Sess. (N.Y. 1997); A.B. 4512, 220th Leg., Reg. Sess. (N.Y. 1997); A.B. 4510, 220th Leg., Reg. Sess. (N.Y. 1997); S.B. 4009, 220th Leg., Reg. Sess. (N.Y. 1997); S.B. 3284, 220th Leg., Reg. Sess. (N.Y. 1997); S.B. 3283, 220th Leg., Reg. Sess. (N.Y. 1997); S.B. 3198, 220th Leg., Reg. Sess. (N.Y. 1997); S.B. 1497, 220th Leg., Reg. Sess. (N.Y. 1997); H.B. 1495, 141st Leg., 2d Reg. Sess. (N.C. 1998); H.B. 350, 141st Leg., 1st Reg. Sess. (N.C. 1997); S.B. 254, 141st Leg., 1st Reg. Sess. (N.C. 1997) (enacted); H.B. 698, 122d Leg., 2d Reg. Sess. (Ohio 1998); H.B. 3169, 46th Leg., 2d Sess. (Okla. 1998) (enacted); H.C.R. 2021, 46th Leg., 1st Reg. Sess. (Okla. 1997); H.B. 1012, 46th Leg., 1st Reg. Sess. (Okla. 1997); H.B. 2779, 182d Leg., 1997 Reg. Sess. (Pa. 1997); S.B. 100, 182d Leg., 1997 Reg. Sess. (Pa. 1997); H.B. 7590, 97-98 Reg. Sess. (R.I. 1997); H.B. 6349, 97-98 Reg. Sess. (R.I. 1997); H.B. 5157, 97-98 Reg. Sess. (R.I. 1997); S.B. 2805, 97-98 Reg. Sess. (R.I. 1997); S.B. 2202, 97-98 Reg. Sess. (R.I. 1997); S.B. 181, 112th Leg., Reg. Sess. (S.C. 1997); S.B. 56, 73d Leg., 1998 Reg. Sess. (S.D. 1998); S.B. 989, 100th Leg., 1st Reg. Sess. (Tenn. 1997); H.B. 413, 75th Leg., 1997 Reg Sess. (Tex. 1997); H.B. 263, 75th Leg., 1997 Reg. Sess. (Tex. 1997); H.B. 39, 75th Leg., 1997 Reg. Sess. (Tex. 1997) (enacted); S.B. 98, 75th Leg., 1997 Reg. Sess. (Tex. 1997); H.B. 271, 52d Leg., 1998 Gen. Sess. (Utah 1998); S.B. 78, 65th Leg., 97-98 Reg. Sess. (Vt. 1997); H.B. 1384, 1998 Reg. Sess. (Va. 1998); H.B. 854, 1998 Reg. Sess. (Va. 1998); H.B. 781, 1998 Reg. Sess. (Va. 1998); S.B. 652, 1998 Reg. Sess. (Va. 1998); S.B. 6663, 55th Leg., 1997 Reg. Sess. (Wash. 1997); S.B. 5298, 55th Leg., 1997 Reg. Sess. (Wash. 1997); A.B. 69, 93d Leg., Reg. Sess. (Wis. 1997); A.B. 157, 93d Leg., Reg. Sess. (Wis. 1997) (enacted). See generally Lisa Seachrist, A Plethora of Genetic Privacy Bills Floods State Legislatures, BlOWORLD TODAY, Apr. 10, 1997, available in 1997 WL 7473568 (discussing common provisions found in various state genetic privacy bills).
194 See Bornstein, supra note 20, at 589-608; see also Holmes, supra note 10, at 629-47 (providing an extensive discussion and examination of state genetic discrimination laws).
195 See O'Hara, supra note 15, at 1224; Sally Lehrman, Clinton Backs Laws Against Genetic Discrimination, BIOTECHNOLOGY NEWSWATCH, July 21, 1997, at 1, available in 1996 WL 8791009.
196 See Collins 1997 Testimony, supra note 6, at 19. Almost two-thirds of U.S. employers are self-insured. See Morelli, supra note 82 at 9. Therefore, even if every state passed comprehensive genetic discrimination laws, millions of American employees with employer self-funded plans would not be protected. See Holmes, supra note 10, at 648.
197 O'Hara, supra note 15, at 1225.
198 See Holmes, supra note 10, at 586.
199 See O'Hara, supra note 15, at 1225.
200 Collins 1997 Testimony, supra note 6, at 14. Thus, insurers may still be permitted to use phenotype indicators, pattern of inheriting genetic characteristics or a request for genetic testing as the basis for genetic discrimination. See id. Consequently, “meaningful protection against genetic discrimination requires that insurers be prohibited from using all information about genes, gene products, or inherited characteristics to deny or limit health insurance coverage.” Id.
201 See Holmes, supra note 10, at 645. When compared with a comprehensive approach to protecting genetic privacy, “piecemeal approach in enacting hundreds of statutes seems politically impractical and ineffective.” See id.
202 Id. at 647.
203 See id. at 663.
204 Pub. L. No. 101-336, 104 Stat. 327 (1990). See also Gurd, Charles B., Whether a Genetic Defect is a Disability Under the Americans with Disabilities Act: Preventing Genetic Discrimination by Employers, 1 ANNALS HEALTH L. 107, 111 (1992)Google Scholar (stating that the ADA “prevents discrimination by private employers who hire more than 15 employees … [and] prohibits discrimination based on a disability”).
205 pub. L. No. 104-191, 110 Stat. 1936(1996).
206 See Bornstein, supra note 20, at 581-82. However, the ADA may not prohibit the underwriting and classification of risks by employers or insurers, thus permitting employers and insurers to treat employees differently for purposes of insurance coverage based on their disabilities. See Rothstein, supra note 80, at 79.
207 See Dee Lord, Something in the Genes, A.B.A. J., Apr. 1996, at 86. The ADA provides a three-prong definition of “disability” as follows: (1) a physical or mental impairment that substantially limits one or more of a person's major life activities; (2) a record of such impairment; or (3) being regarded as having such an impairment. See id. For a discussion of how the ADA extends protection for people with the gene for HD, see generally Gin, Brian R., Note, Genetic Discrimination: Huntington's Disease and the Americans with Disabilities Act, 97 COLUM. L. REV. 1406 (1997)CrossRefGoogle Scholar.
208 See Bornstein, supra note 20, at 581; see also Lord, supra note 207, at 86 (explaining that the Equal Employment Opportunity Commission (EEOC) compliance manual “categorizes a genetic susceptibility to a disease … as a ‘disability'”). But see Rothstein, supra note 80, at 39-43 (arguing that although the ADA protects “qualified individuals who have an already-expressed genetic disease” that substantially limits their major life activities, the ADA does not protect those with minor genetic conditions without such limitations).
209 Bornstein, supra note 20, at 581-82. Nonetheless, despite the inadequacies of ADA protections from genetic discrimination, the EEOC compliance manual “codifies a principle that you are not to be held responsible for what's in our [sic] genes … .” See Richard Saltus, US Ruling Bars Discrimination Based on Genes, BOSTON GLOBE, Apr. 11, 1995, at A4 (quoting Francis Collins, Director, National Center for Human Genome Research).
210 See Rothenberg, Karen et al., Genetic Information and the Workplace: Legislative Approaches and Policy Changes, 275 SCIENCE 1755, 1756 (1997)CrossRefGoogle Scholar.
211 See Rothstein, supra note 80, at 47.
212 See Bornstein, supra note 20, at 582.
213 See Rothenberg et al., supra note 210, at 1756.
214 See Norman-Bloodshaw v. Lawrence Berkeley Lab., 135 F.3d 1260, 1273 (9th Cir. 1998). The court in Norman-Bloodshaw explained that the ADA does not impose restrictions on the scope of medical entrance exams of employees. See id. Thus, genetic make-up information may be disclosed in such examinations. See id.
215 See Rothenberg et al., supra note 210, at 1756.
216 See Patrinos & Drell, supra note 15, at 9.
217 Holmes, supra note 10, at 658-59. The Act prohibits genetic discrimination against asymptomatic people, pre-symptomatic people with a genetic predisposition and unaffected genetic carriers. See id. at 659.
218 See id.
219 See id. at 658; see also Barbara A. Ryan, Health Law Promises Access, Portability Obstacles to Insurance Coverage Addressed, N.Y.L.J., Feb. 18, 1997, at S5 (stating that the Health Insurance Portability and Accountability Act of 1996 (HIPAA) may prevent insurers from denying health insurance coverage based on risk and claims history for a group of employees).
220 See Holmes, supra note 10, at 659; see also Slaughter Testimony, supra note 3, at 8-10 (stating that HIPAA represents an important step toward ending genetic discrimination in health insurance, but fails to close loopholes that leave many people vulnerable to genetic discrimination).
221 See Collins 1997 Testimony, supra note 6, at 19; see also Lisa Seachrist, Clinton Endorses Legislation to Protect Genetic Privacy, BIOWORLD TODAY, July 15, 1997, available in 1997 WL 11130570 (noting that HIPAA left loopholes that H.R. 306 would close).
222 See Holmes, supra note 10, at 659.
223 See id.; see also Rothenberg Testimony, supra note 79, at 85 (stating that HIPAA “does not require insurers to obtain authorization before disposing genetic information”); Collins 1997 Testimony, supra note 6, at 20 (stating that HIPAA does not protect “against unauthorized people or institutions having access to the genetic information contained in their medical records”).
224 See Rothenberg Testimony, supra note 79, at 83; Collins 1997 Testimony, supra note 6, at 20.
225 See Slaughter Testimony, supra note 3, at 10; Collins 1997 Testimony, supra note 6, at 19; see also Rothenberg Testimony, supra note 79, at 84 (stating that HIPAA “provides even less protection for employees not in group plans and provides no coverage for the uninsured”).
226 See Slaughter Testimony, supra note 3, at 11; see also Rothenberg, supra note 189, at 6F (stating that HIPAA does not prohibit insurers from raising rates, excluding coverage for certain conditions, imposing lifetime caps on benefits, requesting or requiring genetic testing and protecting the privacy of genetic test results); Holmes, supra note 10, at 659-60 (stating that the HIPAA “is only a patchwork solution to the insurance/genetic dilemma”).
227 See Rothenberg Testimony, supra note 79, at 84; see also Collins 1997 Testimony, supra note 6, at 19 (stating that HIPAA “leaves open the possibility that all individuals within a group could be charged a higher premium based on the genetic information of one or more members of the group”).
228 Federal genetic discrimination bills introduced in past sessions of Congress include: The Genetic Confidentiality and Nondiscrimination Act of 1996, S. 1898, 104th Cong.; Genetic Information Nondiscrimination in Health Act of 1996, S. 1694, 104th Cong.; Genetic Fairness Act of 1996, S. 1600, 104th Cong.; Health Insurance Reform Act of 1996, H.R. 3185, 104th Cong.; Health Coverage and Availability and Affordability Act of 1996, H.R. 3160, 104th Cong.; Health Insurance Reform Act of 1996, H.R. 3103, 104th Cong.; Working Families Health Access Act of 1996, H.R. 3043, 104th Cong.; Genetic Information Nondiscrimination in Health Insurance Act of 1995, H.R. 2748, 104th Cong.; The Genetic Privacy and Nondiscrimination Act of 1995, S. 1416, 104th Cong., also introduced in the House as H.R. 2690, 104th Cong. (1995); The Genetic Information Privacy Act of 1992, H.R. 5838, 102d Cong.; The Human Genome Privacy Act of 1990, H.R. 5612, 101st Cong. It should be noted that H.R. Res. 392, 104th Cong. (1996) amended H.R. 3103 by substituting and combining the bill with H.R. 3160, which did not contain any genetic information provisions. See Bornstein, supra note 20, at 585 n.149. For a general discussion of federal genetic discrimination legislation introduced between 1995 and 1996, see Reilly, supra note 156, at 379-86.
229 Genetic discrimination legislation introduced prior to the 105th Congress failed to pass because of staunch opposition from a “very powerful insurance industry lobby.” See Interview with O'Connor, supra note 189. Insurance companies have a strong economic incentive to use genetic discrimination to “cherry pick” the healthiest insureds, thereby eliminating health insurance for those people most in need of it. See id. Thus, insurers’ opposition to the “fair and equal access to health care” offered by genetic discrimination legislation makes it necessary to include genetic discrimination protection provisions into unrelated bills that are more likely to pass of their own accord. See id. However, the strong insurance lobby and the “ponderous pace of politics [are] not the only reason[s] for the lag” in enacting genetic discrimination legislation. See Cindy Schreuder, Can Laws Protect Us From Our Genes?, CHI. TRIB., July 20, 1997, at 1. Rather, the “complexity, uncertainty and the fast pace of genetic research” have hampered efforts to enact federal genetic discrimination legislation. See id.
230 See Bornstein, supra note 20, at 579. This bill was introduced by Representative John Conyers (D-MI). See H.R. 5612, 101st Cong. (1990).
231 See Bornstein, supra note 20, at 579-80.
232 See id. at 579. For a list of genetic discrimination bills introduced in the 104th Congress, see supra note 228.
233 Several legislative findings made by the 104th Congress were expressly made by the 105th Congress. For example, in H.R. 341, the 105th Congress adopted the same legislative findings as those adopted by the 104th Congress in H.R. 2690 and S. 1416. Both Congresses found that:
(1) The DNA molecule contains information about an individual's probable medical future.
(2) Genetic information is uniquely private and personal information that should not be disclosed without the authorization of the individual.
(3) The improper disclosure of genetic information can lead to significant harm to the individual, including stigmatization and discrimination in areas such as employment, education, health care, and insurance.
(4) An analysis of an individual's DNA provides information not only about an individual, but also about the individual's parents, siblings and children.
(5) Current legal protections for genetic information, tissue samples and DNA samples are inadequate to protect genetic privacy, and require further attention.
(6) Laws for the collection, storage and use of identifiable DNA samples and private genetic information obtained from those samples are needed both to protect individual privacy and to permit legitimate genetic research.
H.R. 341, 105th Cong. § 2 (1997); H.R. 2690, § 2; S. 1416, § 2.
234 Genetic information is defined as “the information about genes, gene products or inherited characteristics that may derive from an individual or a family member.” H.R. 341, § 3; S. 1600, 104th Cong. § 2(2) (1996); H.R. 2690, § 3; S. 1416, § 3.
235 Genetic test is defined as “a test for determining the presence or absence of genetic characteristics in an individual, including tests of nucleic acids such as DNA, RNA and mitochondrial DNA, chromosomes or proteins in order to diagnose a genetic characteristic.” H.R. 341, § 3; H.R. 2690, § 3; S. 1416, § 3.
236 An insurer is defined as “an insurance company, health care service contractor, fraternal benefit organization, insurance agent, third party administrator, insurance support organization or other person subject to regulation under State insurance laws. Such term includes self-funded health plans and health plans regulated under the Employee Retirement Income Security Act of 1974.” H.R. 341, § 3; H.R. 2690, § 3; S. 1416, § 3.
237 For example, genetic services are defined as “health services to obtain, assess, and interpret genetic information for diagnostic and therapeutic purposes, and for genetic education and counseling.” H.R. 306, 105th Cong. § 2(a) (1997); H.R. 2748, 104th Cong. § 2(e) (1995). Employee health benefit plans are defined as “any employee welfare benefit plan, governmental plan, or church plan” by ERISA. See H.R. 2748, § 2(e).
238 H.R. 2690; S. 1416. Representative Clifford Stearns (R-FL) and Senator Mark Hatfield (ROR) introduced the bill in their respective Houses during the 104th Congress. Representative Stearns re-introduced this bill during the 105th Congress as the Genetic Privacy and Nondiscrimination Act of 1997. See infra text accompanying notes 257-77 (discussing the scope of the proposed Genetic Privacy and Nondiscrimination Act of 1997). Whenever possible, discussion of the Genetic Privacy and Nondiscrimination Act of 1995 hereinafter will reference provisions in both H.R. 2690 and S. 1416.
239 See H.R. 2690, § 4(a)(1); S. 1416, § 4(a)(1)-(2); see also S. 1898, 104th Cong. §§ 201-205 (1996) (stating that entities may not disclose or be compelled to disclose an individual's genetic information unless disclosure is provided for by one of the enumerated exceptions).
240 See H.R. 2690, § 4(a)(2); S. 1416, § 4(a)(2). Both bills specify that an individual's genetic information may be disclosed if authorized under federal or state criminal laws, required under a specific order of a federal or state court, authorized to establish paternity, used for medical diagnosis of a decedent's blood relatives or used to identify a body. See H.R. 2690, § 4(a)(2); S. 1416, § 4(a)(2). Furthermore, the Genetic Privacy and Nondiscrimination Act of 1995 would have allowed disclosure of genetic information if the individual specifically authorized such disclosure in a writing, indicating the information to be disclosed, the entity authorized to receive the information and the purpose of the disclosure. See H.R. 2690, § 4(a)(1); S. 1416, § 4(a)(1).
241 See H.R. 2748, § 2(b); S. 1694, 104th Cong. § 2(b) (1996). Both bills prohibit disclosure of an individual's genetic information without the written authorization of the individual or the individual's legal representative, and require the authorization to identify to whom the disclosure is made. See H.R. 2748, § 2(b); S. 1694, § 2(b).
242 See H.R. 2690, § 5(a); S. 1416, § 5(a); see also S. 1898, 104th Cong. § 301 (1996) (prohibiting employers or potential employers from using genetic information to discriminate against employees or applicants).
243 H.R. 2690, § 5(a); S. 1416, § 5(a).
244 See H.R. 3160, 104th Cong. § 101(b) (1996) (prohibiting insurers from classifying genetic predispositions as preexisting conditions that are excluded from coverage); H.R. 3103, 104th Cong. § 101 (1996) (prohibiting insurers from using genetic information as a preexisting condition so long as the patient has not sought treatment for the condition to which the genetic information is related during the six-month period before the effective date of the insurance coverage); H.R. 2748, § 2(a) (prohibiting insurers from altering any terms of the insurance contract based on genetic information). The Working Families Health Access Act of 1996 would have imposed a tax on health plan providers who use genetic predisposition to establish or impose eligibility, continuation or contribution requirements. See H.R. 3043, 104th Cong. § 4986(a) (1996). That bill regulated health insurers, self-insured employers and ERISA plans by imposing a tax of 25% on collected premiums, or, in the case of self-insured employers, a tax of 25% on health coverage expenditures. See id. § 4986(a)-(b). In addition, the bill protected people in both individual and group health insurance markets. See H.R. 3043, § 4987(a)-(b).
245 See Bornstein, supra note 20, at 582-88.
246 H.R. 2690, § 6(a); S. 1416, § 6(a). However, insurers other than those providing health care coverage would be able to require applicants to undergo genetic testing. See H.R. 2690, § 6(c); S. 1416, § 6(c).
247 See S. 1694, 104th Cong. § 2(c)(1)-(2) (1996); see also S. 1600, 104th Cong. § 2(6)(a)-(c) (1996) (defining “insurer” to include insurance companies, managed care organizations and ERISA plans).
248 See H.R. 3185, 104th Cong. §§ 101, 110 (1996); see also supra note 151 (discussing differences between group and individual insurance policies).
249 See H.R. 2690, § 7; S. 1416, § 7. The National Bioethics Advisory Commission (NBAC) report would include recommendations on the development and implementation of standards “to provide increased protection for the collection, storage, and use of identifiable DNA samples and genetic information obtained from those samples” and “for the acquisition and retention of genetic information in all settings, including appropriate exceptions.” H.R. 2690, § 7; S. 1416, § 7.
250 The Working Families Health Access Act of 1996, for example, varied the provision prohibiting health insurers from genetically discriminating by imposing a substantial tax for noncompliance rather than by commanding an outright ban against genetic discrimination. See H.R. 3043, 104th Cong. § 4986(a) (1996). This approach to addressing genetic discrimination by health insurers appears more effective because insurers may be more averse to confronting the Internal Revenue Service than they would individual insureds. However, the approach may also offer less protection from genetic discrimination if the tax imposed was less than the amount saved by genetically discriminating.
251 See H.R. 2748, 104th Cong. § 2(c)(3) (1995).
252 See Bornstein, supra note 20, at 587.
253 Several other bills introduced during the 105th Congress indirectly address the issue of genetic discrimination, but are beyond the scope of this Note. For example, Congress introduced several bills prohibiting genetic discrimination in the issuance of Medicare supplemental policies. See H.R. 1738, 105th Cong. § 1(a)(2) (1997); S. 386, 105th Cong. § 103(a)(3) (1997); S. 302, 105th Cong. § 2(a)(3) (1997); H.R. 625, 105th Cong. § 2(a)(3) (1997); H.R. 598, 105th Cong. § 1(b)(2) (1997); H.R. 451, 105th Cong. § 1(b)(2) (1997). The 105th Congress also introduced two bills prohibiting genetic discrimination in state-based health care coverage programs for children and uninsured pregnant women. See H.R. 1229, 105th Cong. § 2852(a)(5) (1997); S. 13, 105th Cong. § 104(b)(1) (1997).
254 President Bill Clinton “urged Congress to pass laws that would prevent health care companies from discriminating against consumers on the basis of genetic information.” Lehrman, supra note 195, at 1. Furthermore, Senator Pete Domenici (R-NM) “hopes that the 105th Congress will reach conclusions about ‘what we are to set as national standards regarding gene confidentiality and discrimination.’” M.B., Genetic Privacy, Discrimination Issues Could Cripple Biotech Industry, BIOTECHNOLOGY NEWSWATCH, Oct. 7, 1996, at 13.
255 See Patients’ Bill of Rights Act, S. 2330, 105th Cong. (1998); Family Genetic Privacy and Protection Act, H.R. 3299, 105th Cong. (1998); Medical Information Privacy and Security Act, S. 1368, 105th Cong. (1997); Genetic Employment Protection Act of 1997, H.R. 2275, 105th Cong.; Genetic Justice Act, S. 1045, 105th Cong. (1997); Genetic Nondiscrimination in the Workplace Act, H.R. 2215, 105th Cong. (1997); Genetic Protection in Insurance Coverage Act, H.R. 2216, 105th Cong. (1997); Genetic Privacy and Nondiscrimination Act of 1997, H.R. 2198, 105th Cong.; Medical Privacy in the Age of New Technologies Act of 1997, H.R. 1815, 105th Cong.; Children's National Security Act, H.R. 1726, 105th Cong. (1997); Genetic Confidentiality and Nondiscrimination Act of 1997, S. 422, 105th Cong.; Genetic Information Nondiscrimination in Health Insurance Act of 1997, S. 89, 105th Cong, (companion bill to H.R. 306, 105th Cong. (1997)); Genetic Information Health Insurance Nondiscrimination Act of 1997, H.R. 328, 105th Cong.; Genetic Information Nondiscrimination in Health Insurance Act of 1997, H.R. 306, 105th Cong.; Genetic Privacy and Nondiscrimination Act of 1997, H.R. 341, 105th Cong.
256 For example, the Genetic Privacy and Nondiscrimination Act of 1995 was reintroduced in 1997 without change. Compare H.R. 2690, 104th Cong. (1995) (The Genetic Privacy and Nondiscrimination Act of 1995) with H.R. 341 (Genetic Privacy and Nondiscrimination Act of 1997). See also supra note 233 (discussing similar congressional findings motivating H.R. 2690 and H.R. 341).
257 H.R. 341. Representative Cliff Stearns (R-FL) introduced H.R. 341. See id. Representative Stearns, however, subsequently introduced H.R. 2198 on July 17, 1997, which is also entitled the Genetic Privacy and Nondiscrimination Act of 1997. See H.R. 2198. Unless otherwise stated, the discussion herein involving the Genetic Privacy and Nondiscrimination Act of 1997 will be based on H.R. 341.
258 H.R. 341, § 2(a).
259 See, e.g., The Medical Privacy in the Age of New Technologies Act of 1997, H.R. 1815, § 2(a). This Act includes the following legislative findings:
(1) Health information plays a vital role in every aspect of an individual's life [and]… includes some of the most sensitive information available about an individual.
(2) An individual's health information is currently accessible to many people who do not need the information to provide health care to the individual, often without the individual's knowledge or consent.
(3) Individuals will be deterred from using the health care system unless they are assured that the confidentiality of their health information will be respected.
(4) There exists little Federal protection of the confidentiality of an individual's health information.
(5) While health information often is transferred across State lines, protection of the confidentiality of health information varies greatly from State to State, with little protection in some States.
(6) New technologies increase the importance of addressing new threats to the confidentiality of health information. For example, technologies that permit an individual's health information to be computerized increase the possibility of unauthorized electronic access to the information. Technologies that provide genetic information provide information not just about an individual's current health but also about the individual's potential future health and the health of the individual's relatives. This creates potential new uses and abuses of genetic health information that need to be addressed by legislation.
(7) The potential benefits from new genetic technologies will not be realized if individuals cannot trust that their health information is safe from unauthorized uses.
Id.
260 See H.R. 341, § 4(a)(1); see also S. 422, § 3(3) (defining disclose as “to convey, or provide access to, the genetic information” of an individual to someone other than that individual); H.R. 1815, § 3(3) (defining disclose as “to release, transfer, provide access to, or otherwise divulge the [genetic] information to any person other than an individual who is the subject of the information”). As defined under H.R. 1815, “disclose” includes “the placement of protected health information into a computerized data base, networked computer system, or any other electronic or magnetic data system, that more than one person may access by any means.” H.R. 1815, § 3(3). The term, however, “does not include oral communication between an individual who is the subject of protected health information and a health care provider delivering health care to such individual.” Id.
261 See H.R. 341, § 3(4) (defining genetic information as “information about genes, gene products or inherited characteristics that may derive from an individual or a family member”). Several other bills defined genetic information with the same or similar language. See S. 2330, 105th Cong. §§ 302(b), 303(c), 304(c) (1998); H.R. 3299, 105th Cong. §§ 2(a)(1)(C), 2(a)(2)(C), 3(d)(3) (1998); H.R. 2216, 105th Cong. § 2(2) (1997); H.R. 2198, § 2(a)(1)(C); H.R. 1726, 105th Cong. § 105(a)(1) (1997); H.R. 328, 105th Cong. § 2(a)(1)(C) (1997); H.R. 306, 105th Cong. § 3(a)(1) (1997); see also S. 422, § 3(10) (defining genetic information as “information from a human DNA sample about molecular genotype, information from mutation analysis, or information about nucleotide sequence of a gene”). However, the Genetic Employment Protection Act of 1997 and its companion bill, the Genetic Justice Act, defined genetic information more broadly to include “a physical medical examination, [and/or] a family history [in addition to] a direct analysis of genes or chromosomes.” H.R. 2275, 105th Cong. § 2(2) (1997) (Genetic Employment Protection Act of 1997); S. 1045, 105th Cong. § 2(2) (1997) (Genetic Justice Act).
262 H.R. 341, 105th Cong. § 4(a)(1). This prohibition also applies to the redisclosure of genetic information by any entity properly possessing such information. See id.; S. 422, § 201(b); see also H.R. 3299, § 2(a)(1)(B) (detailing prohibitions against the use or disclosure of genetic information that might adversely affect health benefits). The prohibition on disclosure or redisclosure presumably refers to entities with authorized access to genetic information, such as health care professionals requiring genetic information for medical diagnoses and therapy. Several other bills also use the same or similar language as H.R. 341 in prohibiting unauthorized disclosure of genetic information. See S. 2330, §§ 302(b), 303(c), 304(b); H.R. 3299 §§ 2(a)(1)(B), 2(a)(2)(B), 3(b); S. 1368, 105th Cong. § 202(b) (1997); H.R. 2198, § 2(a)(1)(B); H.R. 1726, § 105; S. 422, § 302; H.R. 328, § 2(a)(1)(B); H.R. 306, § 3(a)(1). H.R. 341, however, includes several exceptions where competing policy reasons outweigh a blanket prohibition against disclosure. See H.R. 341, § 4(a)(2). This section permits disclosure of genetic information under five circumstances: (1) when disclosure is authorized under federal or state criminal laws; (2) when disclosure is required under the specific order of a federal or state court; (3) when disclosure is authorized to establish paternity; (4) when disclosure is needed for the medical diagnosis of a decedent's blood relatives; or (5) when disclosure is needed to identify bodies. See id. Other bills also include the same or similar exceptions. See H.R. 3299, §§ 2(a)(1)(B), 2(a)(2)(B), 3(a)(2); H.R. 2198, § 2; S. 1368, §§ 211-215; H.R. 328, § 2.
263 H.R. 341, § 4(a)(1). For other bills that use the same or similar language in discussing disclosure authorization requirements, see H.R. 3299, §§ 2(a)(1)(B), 2(a)(2)(B); S. 1368, § 202; H.R. 2215, § 2; H.R. 2198, § 2; H.R. 1726, § 105; H.R. 306, § 3.
264 See H.R. 1815, § 2(b). The Act had seven defined purposes. First, the Act intended to “recognize that there is a right to privacy with respect to health information, including genetic information, and that this right must be protected accordingly.” Id. Second, the Act was designed to “ensure that an individual's interest in the privacy of [his] health information cannot be overridden without meaningful notice and informed consent, except in limited circumstances where there is a compelling public interest.” Id. Third, the Act aimed to “provide individuals [with] access to health information of which they are the subject [and the] power to challenge the accuracy and completeness of, and amend or correct, records containing such information.” Id. Fourth, the Act intended to “establish a minimum Federal standard for the protection of health information which will promote confidentiality while allowing efficient transfer of health information between States.” Id. Fifth, the Act aimed to “help ensure the confidentiality of computerized or electronically transferred health information.” Id. Sixth, the Act was designed to “restrict the gathering of aggregate health information for financial gain or other purposes without each subject's knowledge or consent.” Id. Finally, the Act intended to “establish strong and effective remedies for [violators].” Id.
265 See H.R. 328, § 2 (stating that “health insurance issuer[s] may not request or require an individual to whom the issuer provides health insurance coverage in connection with a group health plan … to disclose any genetic information or to obtain any genetic test”); see also H.R. 2275, § 3(2) (preventing employers from using genetic information in any way that would deprive an individual employment opportunities or that would affect that status of the individual as an employee); H.R. 1726, §105 (amending ERISA to include exceptions provided in H.R. 306); H.R. 306, § 3 (stating that group health plans cannot request or require participants, beneficiaries, or potential participants or beneficiaries to disclose genetic information); S. 422, §§ 101, 202, 203 (requiring written authorization and informed consent to collect an individual's DNA sample and giving individuals the right to inspect and copy the records containing their genetic information, in addition to the right to amend inaccurate records). Moreover, the Genetic Confidentiality and Nondiscrimination Act of 1997 would promulgate standards for scientists engaged in the collection of DNA samples for research purposes. See S. 422, § 501.
266 See H.R. 341, § 5(a); see also Geri Aston, Preventing Genetic Discrimination, AM. MED. NEWS, Aug. 4, 1997, at 3.
267 H.R. 341, § 5(a). Several bills also use similar language to prohibit genetic discrimination by employers. See H.R. 3299, § 3; H.R. 2275, § 3; S. 1045, 105th Cong. § 3 (1997); H.R. 2215, § 2; H.R. 2198, §3; S. 422, §401.
268 See H.R. 341, § 5(b) (incorporating private remedies provided under §§ 705-709 of the Civil Rights Act of 1964). Several other genetic discrimination bills provide a private right of action. See H.R. 3299, § 3(c) (incorporating private remedies provided under §§ 705-709 of the Civil Rights Act of 1964); S. 1368, § 323 (permitting an individual to recover compensatory, punitive and consequential damages); H.R. 2275, § 8 (providing private right of action with equitable and legal relief); S. 1045, § 8 (same); H.R. 2215, § 2(d) (providing private right of action with remedies including actual damages and equitable relief); H.R. 1815, § 302 (providing a private right of action with remedies including equitable relief, actual or liquidated damages, punitive damages and attorney's fees); H.R. 1726, § 105 (permitting recovery for compensatory) consequential and punitive damages); S. 422, § 701(d) (permitting recovery for: actual damages or a specified amount of money, whichever is greater; treble damages in cases of violations made for pecuniary gain; and/or reasonable attorney's fees and civil penalties).
269 See H.R. 2198, § 3(a)(2)(A). Three other bills also contain similar exceptions that permit genetic discrimination by employers in cases of “business necessity.” See H.R. 3299, § 3(a)(2)(A); H.R. 2275, § 3(3)(B); S. 422, § 401(a)(2); see also S. 1045, § 3(3) (permitting employers to disclose employees’ genetic information if the employer requests the information after an offer of employment, the information requested is a business necessity and the employee voluntarily consented in writing). However, the Genetic Employment Protection Act of 1997 and the Genetic Justice Act would expand protection by prohibiting employment agencies and labor organizations from genetically discriminating. H.R. 2275, §§ 4-5; S. 1045, §§ 4-5.
270 H.R. 2215.
271 See id. § 2 (1997). An employer who wants genetic information about an employee or prospective employee “shall provide the employee or prospective employee with a written statement of the uses which the employer intends for such genetic information.” Id. The bill also would allow individuals to sue employers who fail to use genetic information in accordance with their stated purpose. See id.
272 See id. § 2. The section states that “[n]othing in this section authorizes an employer to obtain, disclose, or use genetic information about an employee or prospective employee in violation of the Americans with Disabilities Act of 1990 or any other Federal or State law that restricts access to, disclosure of, or use of genetic information.” Id.
273 See H.R. 2275, § 3(2); see also S. 1045, § 3(2) (making it unlawful for employers to “limit, segregate, or classify the employees of the employer in any way that would deprive or tend to deprive any individual of employment opportunities or otherwise adversely affect the status of the individual as an employee, because of genetic information with respect to the individual, including an inquiry by the individual regarding genetic services”).
274 H.R. 341, 105th Cong. § 6(a) (1997). Similarly, other bills would prohibit health insurers and self-insured employers from “deny[ing], cancel[ling], or refus[ing] to renew such benefits or such coverage, or vary[ing] the premiums, terms, or conditions for such benefits or such coverage, for any participant or beneficiary … on the basis of genetic information … or on the basis that the participant or beneficiary has requested or received genetic services.” H.R. 1726, 105th Cong. § 105 (1997); see also S. 2330, 105th Cong. §§ 302(a), 303(a)(1)(A), 304(a) (1998) (providing similar prohibitions as H.R. 1726); H.R. 3299, § 2(a)(1)(A), (B) (same); S. 422, § 402(a) (same); H.R. 306, 105th Cong. § 3 (1997) (same). Genetic services is defined as “health services provided to obtain, assess, and interpret genetic information for diagnostic and therapeutic purposes, and for genetic education and counseling.” H.R. 1726, § 105; H.R. 306, § 3. However, the Genetic Privacy and Nondiscrimination Act of 1997 would permit life insurers to require applicants to undergo genetic testing. See H.R. 341, § 6(c). But see H.R. 2216, 105th Cong. § 3 (1997) (proposing to prohibit life and disability insurers from genetically discriminating). Genetic test is defined as “a test for determining the presence or absence of genetic characteristics in an individual, including tests of nucleic acids such as DNA, RNA and mitochondrial DNA, chromosomes or proteins in order to diagnose a genetic characteristic.” H.R. 2216, § 2(3); H.R. 2198, § 2; H.R. 341, § 3(5); see also H.R. 328, 105th Cong. § 2(a)(1)(B) (1997) (defining genetic test with similar language).
275 See H.R. 341, § 3(6). The term insurer is defined as “insurance company, health care service contractor, fraternal benefit organization, insurance agent, third party administrator … or other such person subject to regulation under State insurance laws [as well as] self-funded health plans and health plans regulated under [ERISA].” Id. Other bills impose similar proscriptions on health insurers and ERISA plans. See S. 2330, §§ 302(a), 303(a)(1)(A), 304(a); H.R. 3299, § 2(a)(1)-(2); H.R. 1726, § 105(a)-(b); S. 422, § 402(b).
276 See S. 2330, § 303(a)-(b); H.R. 3299, § 2(b); H.R. 2198, § 2; H.R. 1726, § 105; H.R. 328, § 2; H.R. 306, § 3; see also Collins 1997 Testimony, supra note 6, at 15 (stating that H.R. 328 closed some of the loopholes left by HIPAA by “addressing discrimination in the individual health insurance market”).
277 See H.R. 341, § 7(1)-(2). Under a similar bill, H.R. 2198, the NBAC would be required to make recommendations on
(1) the development and implantation of standards to provide increased protection for the collection, storage, and use of identifiable DNA samples and genetic information obtained from those samples; and
(2) the development and implementation of appropriate standards for the acquisition and retention of genetic information in all settings, including appropriate exceptions.
H.R. 2198, § 4(1)-(2). This provision is also contained in H.R. 2690, 104th Cong. § 7 (1995). In addition, H.R. 3299 proposed to establish elaborate procedures for the creation of the National Bipartisan Commission on the Use of Genetic Information. See H.R. 3299, § 4.
278 See H.R. 341.
279 See S. 1368, 105th Cong. §§ 101-103 (1997); H.R. 1815, 105th Cong. §§ 101-103 (1997); S. 422,§§ 202-203.
280 The HGP and the genomic information that it reveals implicate various ethical, legal, social, political and economic issues. See Patrinos & Drell, supra note 15, at 8.
281 See Elizabeth Shogren, White House to Seek Genetic Test Safeguards, L.A. TIMES, Jan. 20, 1998, at A1, available in 1998 WL 2390404 (quoting Chris Jennings, President Clinton's senior health care advisor, as stating that genomic research “has extraordinary potential for diagnoses and treatment”); see also Reilly, Philip R., Public Policy and Legal Research Issues Raised by Advances in Genetic Screening and Testing, 27 SUFFOLK U. L. REV. 1327, 1333 (1993)Google Scholar (suggesting that genetic testing will revolutionize medicine).
282 See Shogren, supra note 281 (quoting from a preliminary draft of a study, entitled Genetic Information and the Work Force stating that “[w]hile genetic technology increases the ability to detect and prevent health disorders, it can also be misused to discriminate against or stigmatize individuals”); .see also Steve Ginsberg, The New Resume: Jobs, Schools, Genetic Makeup?, S.F. BUS. TIMES, Nov. 21, 1997, at 8A, available in 1997 WL 15945933 (discussing workers’ fears of genetic discrimination in the workplace).
283 See Annas, supra note 74, at 2346. The collection, storage and use of genetic information have already created litigation. See Mayfield v. Dalton, 109 F.3d 1423, 1424 (9th Cir. 1997). The plaintiff's, two U.S. Marines, challenged the constitutionality of the Department of Defense's DNA registry, fearing that “information obtained from the repository samples, regarding the donors’ propensities for hereditary diseases and genetic disorders, might be used to discriminate against applicants for jobs, insurance or benefit programs.” Id.
284 See Rothenberg Testimony, supra note 79, at 85-86 (stating that “[a]s the flow of genetic information increases, so too will the risk of its misuse”); see also Norman-Bloodshaw v. Lawrence Berkeley Lab., 135 F.3d 1260, 1268-70 (9th Cir. 1998) (holding unanimously that the “constitutionally protected privacy interest in avoiding disclosure of personal matters” and the Fourth Amendment's search and seizure clause prohibit employers from secretly collecting and using employees’ genetic information).
285 See, e.g., G. J. V. NOSSAL & Ross L. COPPEL, RESHAPING LIFE: KEY ISSUES IN GENETIC ENGINEERING 139 (2d ed. 1989).
286 See Collins 1997 Testimony, supra note 6, at 12; see also Stearns Testimony, supra note 81, at 3 (stating that “each and every one of us could be affected once the mapping and sequencing phase of [HGP] research has been completed”).
287 See H.R. 341, 105th Cong. § 3(6) (1997) (defining insurer to include HMOs and self-insured employers); see also Hudson et al., supra note 4, at 393 (defining “insurance provider” broadly to include “an insurance company, employer, or any other entity providing a plan of health insurance or health benefits”).
288 See Hudson et al., supra note 4, at 393.
289 See H.R. 341, §§ 5-6; see also Hudson et al., supra note 4, at 393 (listing several legislative recommendations of the Working Group on Ethical, Legal, and Social Implications that would prohibit insurers from “using genetic information, or an individual's request for genetic services, to deny or limit any coverage or establish eligibility, continuation, enrollment, or contribution requirements … [or from] establishing differential rates or premium payments based on genetic information or an individual's request for genetic services”).
290 See H.R. 341, § 5(a); see also Hudson et al., supra note 4, at 393 (recommending that “[i]nsurance providers should be prohibited from requesting or requiring collection or disclosure of genetic information”). But see H.R. 341, § 6(c) (permitting life insurers to request genetic tests). For some compelling reasons that would permit mandatory genetic testing, see Raffone, Kristin M., Note, The Human Genome Project: Genetic Screening and the Fundamental Right of Privacy, 26 HOFSTRA L. REV. 503, 534-44 (1997)Google Scholar (citing preventative medicine, inheritable diseases, genetic diversity and societal interest in potential life as compelling reasons given by states for instituting genetic screening programs).
291 See Lauran Neergaard, Doctor Lobbies for Genetic Privacy Legislation, BUFF. NEWS, Sept. 30, 1995, at A4; see also Hudson et al., supra note 4, at 393 (recommending that “[i]nsurance providers and other holders of genetic information should be prohibited from releasing genetic information without prior written authorization of the individual [and that written] authorization should be required for each disclosure and include to whom the disclosure would be made”); H.R. 341, § 4(a)(1)-(2) (prohibiting disclosure or redisclosure of genetic information, subject to several narrowly tailored exceptions).
292 See Andrews, Lori B. & Jaeger, Ami S., Confidentiality of Genetic Information in the Workplace, 17 AM. J.L. & MED. 75, 108 (1991).Google Scholar
293 See bills discussed in supra note 268.
294 See H.R. 341, § 7 (requiring the NBAC to make recommendations to Congress in order to keep genetic legislation adaptable to subsequent scientific developments).
295 See id. §§ 4-7.
296 Several genetic discrimination bills introduced in the 105th Congress offer adequate protections. Table 1 indicates the protections offered by each of the 105th Congress's genetic discrimination bills. Although different bills may include provisions of the same general type (for example, provisions protecting genetic privacy), not all of the provisions were equally comprehensive.
Table 1 does not reflect the superiority of some provisions relative to those in other bills.
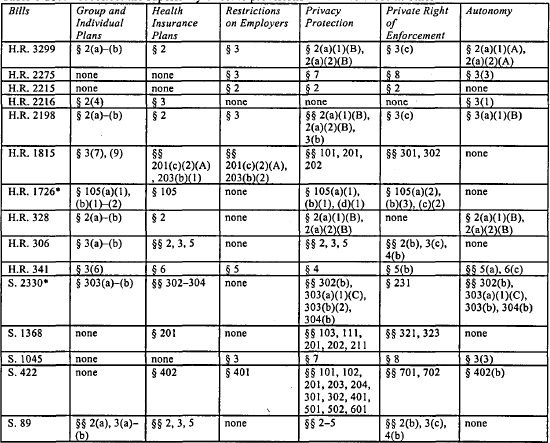
Table 1. 105th Congress's Genetic Discrimination Provisions.
* S. 2330. entitled the Patients’ Bill of Rights Act, is not strictly a genetic discrimination bill. Title III of S. 2330, cited as Genetic Information Nondiscrimination in Health Insurance Act of 1998, provides the relevant provisions relating to genetic discrimination. Similarly, H.R. 1726, entitled Children's National Security Act, is also not strictly a genetic discrimination bill.
297 The odds that any of the 105th Congress's genetic discrimination bills would be enacted were low. See Search of WESTLAW, Billcast (Oct. 6, 1998). Table 2 represents the odds of passing for each of the 105th Congress's genetic discrimination bills.
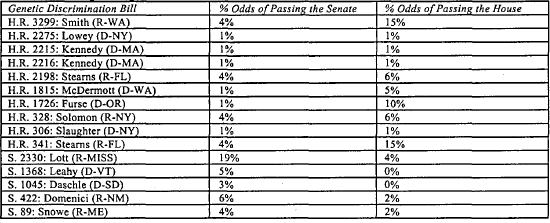
Table 2. Odds of Success for 105th Congress's Genetic Discrimination Bills.
298 A staff member from Representative Kennedy's office indicated that, in light of heavy opposition from insurance lobbyists, genetic discrimination legislation might have to be inserted as a rider into bills supported by the Republican majority. See Interview with O'Connor, supra note 189.
299 See Bornstein, supra note 20, at 609; O'Hara, supra note 15, at 1227.
300 See O'Hara, supra note 15, at 1212.
301 See Holmes, supra note 10, at 663.
302 See Gostin, supra note 6, at 113.
303 See supra note 5 (describing a fictional, though plausible, account of a society incorporating genetic discrimination).
304 See, e.g., H.R. 2198, 105th Cong. (1997) (limiting “the disclosure and use of genetic information in connection with group health plans and health insurance coverage,” and prohibiting “employment discrimination on the basis of genetic information and genetic testing, and for other purposes”); S. 422, 105th Cong. (1997) (defining “the circumstances under which DNA samples may be collected, stored, and analyzed”). Restrictions imposed by S. 422 would include genetic discrimination by employers and health insurers, as well as unauthorized disclosure of genetic information by any entity. See S. 422, § 201. Moreover, comprehensive genetic discrimination protections would be enacted under two bills introduced by Representative Kennedy. See H.R. 2215, 105th Cong. (1997) (restricting “employers in obtaining, disclosing, and using genetic information”); H.R. 2216, 105th Cong. (1997) (limiting the “disclosure and use of genetic information by life and disability insurers”).
305 See H.R. 341, 105th Cong. (1997). The Genetic Privacy and Nondiscrimination Act of 1997 would prohibit genetic discrimination by employers and health insurers. See id. §§ 5-6. The bill would also prohibit unauthorized disclosure of genetic information. See id. § 4. Furthermore, genetic discrimination prohibitions would apply to HMOs and other ERISA-regulated entities. See id. § 3(6). Finally, the bill would require the NBAC to prepare subsequent reports for Congress regarding new legislative needs in light of scientific advances. See id. § 7.
- 16
- Cited by


