Implications
Dairy cows were offered a blend of essential oils (BEO) and its effect on cation status and performance was investigated. The study provides evidence that recently described effects of essential oils (EO) on calcium transport across the ruminal epithelium in vitro might be applicable in vivo. Supplementing certain EO may improve calcium metabolism, which could impact future hypocalcemia prevention strategies in dairy farming.
Introduction
In recent decades, the use of EO in ruminant nutrition has received increasing attention. So far, the main focus has been on modulating ruminal microbiota. The potential of EO as antimicrobial agents was realized as early as 1965 when Borchers (Reference Borchers1965) demonstrated that thymol arrested deamination of amino acids in vitro in rumen samples. Follow-up studies have since established that, at least in vitro and at appropriately high concentrations, EO modify fermentation patterns with effects on volatile fatty acid production, protein metabolism or both (Calsamiglia et al., Reference Calsamiglia, Busquet, Cardozo, Castillejos and Ferret2007).
At least, as interesting as the antimicrobial potential of EO is their powerful ability to modulate cellular signaling pathways in sensory perception, inflammation and pain through receptors from the transient receptor potential (TRP) family of ion channels (Holzer, Reference Holzer2011; Nilius and Szallasi, Reference Nilius and Szallasi2014). Interestingly, many such EO receptors (e.g. TRPA1 and TRPV3) are found in the epithelia of the intestine (Holzer, Reference Holzer2011), including the rumen of cattle and sheep (Rosendahl et al., Reference Rosendahl, Braun, Schrapers, Martens and Stumpff2016). Recently, the impact of EO or so-called phytonutrients on ruminant immune status, insulin regulation or oxidative stress was reviewed with special notice to the involvement of TRP channels (Oh et al., Reference Oh, Wall, Bravo and Hristov2017).
Essential oils may also influence the expression patterns of other transport proteins expressed by the rumen (Mirzaei-Alamouti et al., Reference Mirzaei-Alamouti, Moradi, Shahalizadeh, Razavian, Amanlou, Harkinezhad, Jafari-Anarkooli, Deiner and Aschenbach2016). The effects of EO may thus well exceed their interaction with microbial populations in the rumen.
In cattle, the absorption of Ca2+ is of particular interest as low plasma Ca2+ levels are the cause of the metabolic disorder milk fever (Martín-Tereso and Martens, Reference Martín-Tereso and Martens2014). In many epithelia, the transport of divalent cations is known to be mediated by certain members of the TRP channel family (Wilkens et al., Reference Wilkens, Kunert-Keil, Brinkmeier and Schröder2009; Martens et al., Reference Martens, Leonhard-Marek, Röntgen and Stumpff2018). Intriguingly, EO have been shown to enhance the transport of Ca2+ and other cations across numerous preparations (Nilius and Szallasi, Reference Nilius and Szallasi2014), with studies of ruminal epithelia in vitro suggesting stimulatory effects on the absorption of Na+, NH4 + and Ca2+, possibly related to the expression of TRPV3 by ruminal tissue (Rosendahl et al., Reference Rosendahl, Braun, Schrapers, Martens and Stumpff2016). In support of this hypothesis, it was recently demonstrated that the bovine TRPV3 mediates the transport of all three ions and that transport is stimulated by the same EO that stimulate transport across the intact ruminal epithelium (Schrapers et al., Reference Schrapers, Sponder, Liebe, Liebe and Stumpff2018). This leads to the hypothesis that offering EO to cows might enhance cation absorption through TRP channels, thus facilitating the absorption of minerals and nutrients with positive effects such as improved production and health status. Accordingly, the present study focuses on the effect of a BEO on feed efficiency, nitrogen metabolism and calcium status in dairy cows.
Material and methods
Experimental procedures, animals and diet
The study was performed using a patented, commercial BEO (BTX12; PerformaNat GmbH, Berlin, Germany, patent US 9693971). A premix of 25 g was added to the concentrate feed, which was added to the total mixed ration (TMR). In total, animals were fed a daily dose corresponding to 1.2 g EO, with menthol as the major active compound (>80%) in addition to smaller amounts of eugenol and anethol.
The feeding trial followed a 2×2 cross-over design and was carried out at the agricultural teaching and testing institute of the state of Schleswig Holstein at Futterkamp approved by the responsible authority, the Ministry for Energy Transition, Agriculture, Environment and Rural Areas, State of Schleswig Holstein (V242-7224.121-25). A herd of 72 primi- and multiparous Holstein–Friesian cows in mid-lactation were randomly assigned to two groups which were fed either BEO or the control diet. The first BEO feeding period lasted 20 days followed by a period of 40 days in which cows of both groups were fed a standard diet without BEO to prevent carry-over effects. The second BEO feeding period of 20 days followed in which the cows that had previously been fed the control diet were fed BEO and vice versa.
Each group consisted of nine or 11 heifers and 25 or 27 multiparous cows, with the total mean for both groups at 2.3±0.1 lactations, 170±8 days in milk and milk yield at 37±1 kg/day at the start of the trial. Cows were milked twice daily in a milking parlour at 0600 and 1500 h. A TMR was presented directly after milking. Water and feed were available ad libitum. TMR consisted of grass–maize silage and concentrate feed. Samples of TMR were taken for analysis of dry matter and chemical composition. Ingredients and chemical analyses are summarized in Table 1.
Table 1 Diet ingredients and chemical composition of the total mixed ration for dairy cows
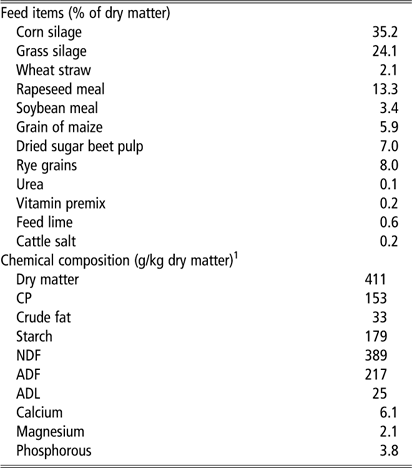
1 Chemical composition was analysed in three samples of the total mixed ration.
Sample collection and analysis
All data were collected both before the start and at the end of the feeding period. Individual feed intake was measured by feed weighing troughs (Insentec, Marknesse, the Netherlands) as previously described (Pahl et al., Reference Pahl, Hartung, Mahlkow-Nerge and Haeussermann2015). In brief, troughs opened for cows identified by a transponder and closed after the individual animals left the trough. Feed intake per cow was summed up for each day. Individual milk yield was recorded at morning and afternoon milking using automatic milk counters and was summed up for daily milk yield. The last 3 days before the start and at the end of each experimental period were used to calculate individual milk yield and feed intake.
Milk fat, protein and urea were analysed twice weekly and the corresponding values for fat corrected and energy corrected milk yield (FCM and ECM) were calculated. Feed efficiency was calculated by dividing milk yield by the dry matter intake (DMI). To investigate the effect of BEO on metabolites and minerals, blood samples were collected both on the day before and on the last day of each feeding period directly after morning milking. Blood samples were taken from the coccygeal vessel using vacutainers (Greiner Bio-One International GmbH, Kremsmünster, Austria). After centrifugation, plasma was separated and stored at −20°C until analysis of metabolites and minerals. In serum samples, calcium, magnesium, phosphorus, total protein, creatinine, urea, β-hydroxybutyrate (BHB) and non-esterified fatty acids (NEFA) were analysed using an automatic biochemistry analyser (COBAS C-311; Roche, Mannheim, Germany). Unfortunately, due to technical problems, NEFA values were unreliable and had to be discarded.
Spontaneous urine was collected on 2 consecutive days directly after morning milking before the start and at the end of feeding period. The results of both samples were averaged to minimize daily fluctuations. In urine samples, calcium, magnesium, urea and creatinine were analysed. Urinary pH was measured immediately after collection (pH-Meter 1140; Mettler Toledo, Gießen, Germany), after which urine samples were stored at −20°C for further analysis. Urinary net acid excretion was determined in duplicate after a modified method described in Chan (Reference Chan1972). In brief, 10 ml urine was constantly stirred and titrated to pH of 3.5 using 1M HCl to measure titratable base. Then, samples were boiled for 2 min to remove CO2. After cooling down, samples were titrated to pH of 7.4 using 1 M NaOH for determination of titratable acid. To determine total ammonia concentration, acidified urine (1 ml of 4M HCl/40 ml of urine) was frozen and later analysed using an ammonia electrode via the addition method (Orion; Thermo Scientific, Bremen, Germany). Net acid excretion was calculated from titratable base minus the sum of titratable acid and ammonia. Base:acid ratio was determined by dividing titratable base by the sum of titratable acid and ammonia.
Statistics
The effects of BEO were analysed using the univariate ANOVA procedure of SPSS 22 (SPSS Inc., Chicago, IL, USA). The samples collected before the treatment period were used as covariate. Treatment was chosen as the fixed effect and the animal was set as the random effect. Results are presented as means±SEM. Statistical differences were considered to be significant when P<0.05 and trends are discussed when P<0.10.
Results
Feed intake and milk parameters
Supplemental BEO did not affect feed intake (Table 2). Milk yield (milk, FCM and ECM) was significantly increased by BEO feeding. Feed efficiency (ECM/DMI and FCM/DMI) significantly increased for cows receiving BEO supplementation, milk yield per DMI tended to increase (P=0.066, Table 2). Fat and protein content of the milk was not influenced by BEO. Fat and protein yield were significantly higher for the BEO group (P=0.007 and P=0.003, respectively). Urea in milk significantly decreased in the BEO group (P=0.006, Table 2).
Table 2 Response of feed intake, milk parameters and feed efficiency of dairy cows following dietary supplementation with a blend of essential oils (BEO) for 20 days

DMI=dry matter intake; ECM=energy corrected milk; FCM=fat corrected milk.
Blood parameters
Feeding BEO was associated with a significant rise in plasma calcium levels (control: 2.46±0.015 mmol/l, BEO: 2.53±0.02 mmol/l, P<0.001) (Table 3). No significant changes were observed in serum levels of magnesium, phosphorus, total protein or creatinine. β-Hydroxybutyrate significantly decreased in the BEO group (control: 0.78±0.02 mmol/l, BEO: 0.65±0.02 mmol/l, P=0.001), even though cows in mid-to-late lactation state were not prone to ketosis. Plasma urea levels dropped significantly when BEO was fed (P<0.001, Table 3).
Table 3 Response of the plasma parameters of dairy cows following dietary supplementation with a blend of essential oils (BEO) for 20 days
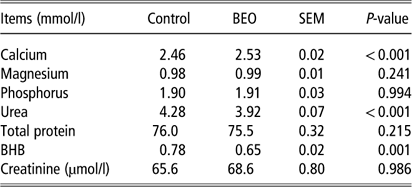
BHB=β-hydroxybutyrate.
Urine parameters
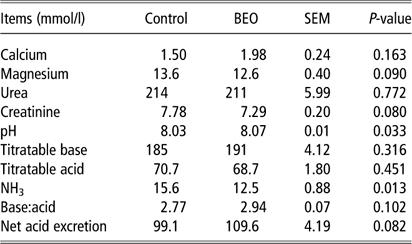
In urine, concentrations of calcium and urea were not affected by BEO feeding (Table 4). Urinary excretion of magnesium and creatinine tended to decrease in the BEO feeding group (P=0.09 and 0.08, respectively). Urinary pH significantly increased (control: 8.03±0.014 mmol/l, BEO: 8.07±0.014 mmol/l, P=0.033). Changes in titratable acid and base were not significant, whereas net acid excretion and acid:base ratio tended to increase (P=0.082 and P=0.102, respectively). Ammonia excretion was significantly lower in the BEO group (P=0.013).
Table 4 Response of urine parameters of dairy cows following dietary supplementation with a blend of essential oils (BEO) for 20 days
Discussion
In the current study, cows were fed 1.2 g of a commercial BEO containing menthol as the major active compound (>80%) and smaller amounts of eugenol and anethol. In response to this feed additive, an increase of milk yield, milk fat and protein yield at constant DMI was observed, resulting in a significantly elevated feed efficiency. Plasma calcium concentration increased significantly, whereas urea in plasma and milk decreased. Although this particular BEO has not been studied before, a number of other studies have addressed the effects of isolated EO or mix of these agents in feeding trials, with results highly variable and depending on the preparation studied and the study design (Khiaosa-ard and Zebeli, Reference Khiaosa-ard and Zebeli2013).
In a recent study of cows fed a ketogenic diet, a commercial BEO significantly increased milk fat content, energy-corrected milk and feed efficiency (ECM/DMI) (Drong et al., Reference Drong, Meyer, von Soosten, Frahm, Rehage, Breves and Dänicke2016). However, this occurred with a concomitant rise in BHB and NEFA, both of which rose more strongly when EO were added to the ketogenic diet, suggesting a negative impact of the preparation on energy balance in these challenged animals. In a study of cows fed a more balanced diet, BHB and NEFA were not affected by this blend (Tassoul and Shaver, Reference Tassoul and Shaver2009), which is in contrast to the results of this study showing decreased BHB values in cows offered BEO feeding. However, more work is clearly needed to assess the impact of EO on fat mobilization and energy balance of lactating cows, in particular in the transition period.
An interesting observation in this study was the significant drop in plasma urea levels, which most likely explains the concomitant lower milk urea levels during BEO supplementation. As milk urea nitrogen (MUN) can be used to predict total urinary nitrogen excretion (Nousiainen et al., Reference Nousiainen, Shingfield and Huhtanen2004; Spek et al., Reference Spek, Dijkstra, Van Duinkerken, Hendriks and Bannink2013), a reduction in total nitrogen excretion can be inferred. A drop in MUN was also observed in a study of ewes fed another commercial BEO (Giannenas et al., Reference Giannenas, Skoufos, Giannakopoulos, Wiemann, Gortzi, Lalas and Kyriazakis2011), whereas in lactating cows, MUN values that were elevated by monensin supplementation were normalized (Benchaar et al., Reference Benchaar, Petit, Berthiaume, Whyte and Chouinard2006). As daily milk protein yield increased, nitrogen might have been incorporated more efficiently into protein and amino acids such as glutamine rather than non-protein nitrogen like urea.
To the extent that they have been validated, the mechanisms behind the in vivo effects of EO are thought to reflect the well-documented antimicrobial effects of EO (Borchers, Reference Borchers1965; Calsamiglia et al., Reference Calsamiglia, Busquet, Cardozo, Castillejos and Ferret2007). Various mechanisms for the bactericidal effects of these compounds are discussed, such as an interaction with the lipid membrane itself or proteins embedded therein, ultimately leading to a breakdown in membrane barrier (Jouany and Morgavi, Reference Jouany and Morgavi2007) with a differential impact on Gram-negative and Gram-positive bacteria (Chao et al., Reference Chao, Young and Oberg2000).
However, in particular, at the concentrations fed in vivo, the mode of action of EO fed to dairy cows may exceed the antimicrobial effects. We found higher plasma calcium levels – a fact that might relate to the well-documented stimulatory interaction of EO with non-selective cation channels from the TRP channel family (Holzer, Reference Holzer2011; Nilius and Szallasi, Reference Nilius and Szallasi2014; Rosendahl et al., Reference Rosendahl, Braun, Schrapers, Martens and Stumpff2016; Schrapers et al. Reference Schrapers, Sponder, Liebe, Liebe and Stumpff2018). Effects of EO on the transport of various ions across epithelia of the gastrointestinal tract have been reported not only in human and rat colon (Kaji et al., Reference Kaji, Karaki and Kuwahara2011) but also notably in the ruminal epithelium of cattle and sheep (Rosendahl et al., Reference Rosendahl, Braun, Schrapers, Martens and Stumpff2016). Furthermore, we were able to show that menthol and thymol stimulated the transport not just of Na+, but also of NH4 + and Ca2+ across the isolated ruminal epithelia of sheep and cattle in an ex vivo approach (Rosendahl et al., Reference Rosendahl, Braun, Schrapers, Martens and Stumpff2016). In addition to these acute effects, a change in the expression pattern of acid base transporting proteins was recently shown in the ruminal epithelium of sheep fed a blend of extracts from a variety of plants that included peppermint (Mirzaei-Alamouti et al., Reference Mirzaei-Alamouti, Moradi, Shahalizadeh, Razavian, Amanlou, Harkinezhad, Jafari-Anarkooli, Deiner and Aschenbach2016).
Both menthol and thymol stimulate certain non-selective cation channels of the TRP family that are expressed by the tissue (Nilius and Szallasi, Reference Nilius and Szallasi2014; Rosendahl et al., Reference Rosendahl, Braun, Schrapers, Martens and Stumpff2016). With due caution, the low-dose BEO fed in this study may have stimulated the transport of calcium from rumen into blood, which might explain the increase of plasma Ca2+ observed. An increased ruminal uptake of calcium should enhance calcium homeostasis, with particular relevance for the reduction of hypocalcemia around calving (Goff, Reference Goff2008).
Based on our in vitro study (Rosendahl et al., Reference Rosendahl, Braun, Schrapers, Martens and Stumpff2016), we initially expected to see higher absorption of ammonia as NH4 + from the rumen, and thus higher plasma and milk urea levels in the feeding group receiving EO. However, two factors may have intervened. First, degradation of protein may have been reduced by inhibition of proteolytic bacteria (McIntosh et al., Reference McIntosh, Williams, Losa, Wallace, Beever and Newbold2003). Second, given the very high pK value of the strong base ammonia (NH3) of 9.2, a shift away from the absorption of NH3 and towards the absorption of the protonated cationic form (NH4 +) through a TRP channel can be expected to induce a shift in the acid–base equilibrium of the blood towards less alkaline values. A decrease in pH will cause an activation of glutamine synthetase in the liver (Taylor and Curthoys, Reference Taylor and Curthoys2004; Weiner and Verlander, Reference Weiner and Verlander2017). This normalizes the blood pH as the protons, which would be released in the course of urea synthesis are incorporated into the glutamine molecule. In the form of the non-toxic glutamine, protons and ammonia can then be transferred to the kidney and excreted, which may explain the higher net acid excretion observed under the BEO in this study.
As the most abundant amino acid in plasma, glutamine plays a central role not only as a shuttle for ammonia and protons but also as a key player in protein metabolism (Taylor and Curthoys, Reference Taylor and Curthoys2004), with the supply limited in stress situations such as calving (Meijer et al., Reference Meijer, van der Meulen and van Vuuren1993). An increase in glutamine synthesis from ammonium leaving the rumen would certainly appear to facilitate protein metabolism in animals challenged by lactation, which might be supported by the reduced NH3 excretion in cows fed BEO. The possibility that certain BEO have the capacity not only to enhance proton absorption from the rumen but also to shift ammonia detoxification towards glutamine production is speculative. This hypothesis clearly merits further investigation.
Currently, the assumption that beneficial effects of feeding EO are caused by influencing the gastrointestinal flora represents the most established hypothesis. However, the ability of certain EO to affect epithelial ion transport in vitro might also contribute to their in vivo effects. This approach might provide an explanation for the high variability in the outcome of studies, as the effects of EO on epithelial ion transport are highly dependent on the dose and the substance. Further research is needed to elucidate the complex interaction of EO, gastrointestinal bacteria and the ruminal epithelium, contributing to the multiple observed positive effects on milk yield, feed efficiency and possible impacts on calcium homeostasis.
In conclusion, the tested BEO increased feed efficiency and plasma calcium levels. Possibly, this reflects stimulatory effects of EO on ruminal calcium absorption, which might help to cover the increased calcium demand during lactation.
Conclusion
The blend of plant bioactive EO tested in this study had effects that suggest improved feed efficiency and calcium homeostasis. We propose that in addition to their effects on fermentational patterns; EO activate specific cation-transporting proteins expressed by the rumen, resulting in an increased uptake of cations like calcium and ammonium. Further studies are required to elucidate the fate of the absorbed nutrients with special regard to calcium and nitrogen.
Acknowledgements
The authors gratefully acknowledge the help of M. Grunau, Prof. J. R. Aschenbach (Freie Universität Berlin, Germany) and the staff of the Chamber of Agriculture Schleswig-Holstein in Futterkamp. The project was funded by the European Social Fund (ESF) and the German Ministry of Economics and Technology based on a decision of the German Parliament (03EUABE057).
Declaration of interest
This study was performed primarily for scientific reasons within a conventional academic framework. However, the results of previous studies have led to a patent (US 9693971, involving the authors Friederike Stumpff and Julia Rosendahl, both as employees of the Freie Universität Berlin). At present, Julia Rosendahl, Hannah-Sophie Braun and Katharina T. Schrapers work for PerformaNat, an animal nutrition company with commercial interest in feed additives.
Ethics statement
The feeding trial was carried out at the agricultural teaching and testing institute of the state of Schleswig-Holstein at Futterkamp approved by the responsible authority, the Ministry for Energy Transition, Agriculture, Environment and Rural Areas, State of Schleswig-Holstein (V242-7224.121-25).
Software and data repository resources
All data are available upon request.






