Introduction
The weight of lambs weaned per ewe has a direct influence on the viability of sheep production systems. In order to maintain profitability, it is therefore vital to minimise lamb mortality and maximise lamb growth rate, particularly during the pre-weaning period when lamb growth rate is positively correlated with ewe milk yield and composition. In the UK, approximately four million lambs per year die during the neonatal period on hill and upland farms, representing a loss of £120 million to the sheep industry (Merrell, Reference Merrell1998). A major factor in this high mortality rate is hypothermia due to delayed suckling and exhaustion of brown fat reserves (Slee, Reference Slee1981). Lambs that are vigorous at birth with a short latency of standing and suckling may therefore be more likely to survive during the perinatal period.
The long-chain polyunsaturated fatty acids (PUFA), arachidonic acid (C20:4 (n-6)) and docosahexaenoic acid (C22:6 (n-3)), are essential for the development of neural tissue within the neonatal and sucking animal (Koletzko, Reference Koletzko1992) and may therefore influence neonatal viability. The combination of a low dietary supply of long-chain PUFA and the high level of PUFA biohydrogenation within the rumen (Wachira et al., Reference Wachira, Sinclair, Wilkinson, Enser, Wood and Fisher2000; Chikunya et al., Reference Chikunya, Demirel, Enser, Wood, Wilkinson and Sinclair2004) may limit the quantities of these fatty acids available for absorption at the ovine small intestine. Nevertheless, Cooper et al. (Reference Cooper, Sinclair, Wilkinson, Hallett, Enser and Wood2004) demonstrated that feeding diets high in eicosapentaenoic acid (C20:5 (n-3)) and C22:6 (n-3) to growing lambs substantially increased the concentrations of these fatty acids within muscle phosphatidylglycerols and triacylglycerols. Moreover, Capper et al. (Reference Capper, Wilkinson, Mackenzie and Sinclair2006) and Pickard et al. (2005) reported that the inclusion of PUFA in the diet of pregnant ewes demonstrably improved neonatal lamb vigour, an effect that may be mediated through increased gestation length or enhanced neural tissue development within the foetus. However, there is little information available on the effects of continuing dietary PUFA supplementation into lactation upon ewe milk composition and lamb growth rate. Studies by Chilliard and Doreau (Reference Chilliard and Doreau1997), Keady et al. (Reference Keady, Mayne and Fitzpatrick2000) and Shingfield et al. (Reference Shingfield, Ahvenjärvi, Toivonen, Ärölä, Nurmela, Huhtanen and Griinari2003) have focused on the effects of supplementing lactating dairy cattle with PUFA upon milk yield and composition and have produced conflicting results. Despite physiological similarities in terms of milk synthesis, these results may not be applicable to lactating sheep given the differences in management systems. Although Chikunya et al. (2002), Kitessa et al. (Reference Kitessa, Peake, Bencini and Williams2003) and Cattaneo et al. (Reference Cattaneo, Dell’Orto, Varisco, Agazzi and Savoini2006) have investigated the effects of PUFA supplementation of sheep or goats on milk fatty-acid composition, no data are available relating to the effects of such supplementation upon lamb performance.
Supplementation of pregnant ewes with PUFA would be expected to increase the oxidative challenge both to the dam and sucking lamb (Weber et al., Reference Weber, Bendich and Machlin1997), with concurrent effects upon the antioxidant status. Vitamin E primarily acts as a fat-soluble antioxidant essential for the prevention of lipid peroxidation in cell membranes (Wang and Quinn, Reference Wang and Quinn1999), particularly during periods of rapid cell growth in the foetal and neonatal animal. If the PUFA concentration of milk is increased via dietary supplementation, it is logical to suggest that the dietary vitamin E supply should also be augmented to maintain oxidative stability and antioxidant status of the lamb. Furthermore, supplemental dietary vitamin E has been suggested to improve growth rate in sucking lambs (Gentry et al., Reference Gentry, Ross, Oetting and Birch1992).
Improving neonatal lamb vigour and enhancing the pre-weaning growth rate should improve unit performance with positive effects upon flock profitability. Therefore, the objectives of the current experiment were to evaluate the effects of supplementing pregnant and lactating ewes with fatty acids and vitamin E upon the performance of lactating ewes and sucking lambs.
Materials and methods
Animals
The experiment was carried out at Harper Adams University College in accordance with the Animals (Scientific Procedures) Act 1986. For full details of animal methods, refer to Capper et al. (Reference Capper, Wilkinson, Kasapidou, Pattinson, Mackenzie and Sinclair2005). Briefly, 36 twin-bearing and 12 triplet-bearing ewes were allocated to one of four dietary treatments according to litter size, live weight and condition score (Russel et al., Reference Russel, Doney and Gunn1969).
A basal concentrate was formulated based on barley and sugar-beet pulp, details of which have been described previously (Capper et al., Reference Capper, Wilkinson, Kasapidou, Pattinson, Mackenzie and Sinclair2005). To this diet was added 120 g/kg of either a control fat or fish oil pre-mix and a vitamin/mineral supplement containing either a basal (50 mg/kg) or supranutritional (500 mg/kg) concentration of vitamin E (α-tocopherol acetate; Roche UK Ltd, Heanor, Derbyshire, UK; Table 1). The fish oil pre-mix comprised a mixture of crude unrefined Scandinavian fish oil mixed at a ratio of 0.75:0.25 with Incromega (Trouw Nutrition UK, Northwich, UK), a by-product of omega-3 fatty-acid production for the human market, high in docosahexaenoic acid. Both the fish oil and Incromega were combined with a vermiculite carrier (Trouw Nutrition UK) to protect against PUFA biohydrogenation (Sinclair et al., Reference Sinclair, Cooper, Chikunya, Wilkinson, Hallett, Enser and Wood2005). The control fat source consisted of a calcium soap of palm oil distillate (Megalac; Volac Ltd, Orwell, Royston, Hertfordshire, UK) mixed with straw pellets. The two pre-mixes were formulated to provide equal concentrations of fatty acids (60 g/kg fresh weight) within the concentrate. The treatment diets were therefore: Megalac + basal vitamin E (MB), Megalac + supranutritional vitamin E (MS), fish oil + basal vitamin E (FB) and fish oil + supranutritional vitamin E (FS). The treatment diets were formulated to be isoenergetic (11.4 MJ metabolisable energy (ME) per kg) and isonitrogenous (180 g crude protein (CP) per kg) formulated according to the Agricultural and Food Research Council (1993).
Table 1 Raw material composition of the treatment concentrates differing in vitamin E concentration and fatty acid source

†Abbreviations are: MB = Megalac + 50 mg/kg vitamin E, MS = Megalac +500 mg/kg vitamin E, FB = fish oil + 50 mg/kg vitamin E, FS = fish oil + 500 mg/kg vitamin E.
‡Protected soya-bean supplement; Trouw Nutrition UK, Northwich, Cheshire, UK.
§Ca soap of palm fatty acid distillate; Volac Ltd, Royston, Hertfordshire, UK.
∥By-product of n-3 fatty-acid production for the human market, high in docosahexaenoic acid (Trouw Nutrition UK).
¶Vitamin/mineral supplement (Roche UK Ltd, Heanor, Derbyshire, UK) supplied per kg of diet: Ca, 7.06 g; Na, 2.67 g; P, 1.65 g; Se, 0.36 mg; vitamin A, 4.32 mg; vitamin D, 0.75 mg; vitamin E, 50 mg (basal); or 500 mg (supranutritional).
Experimental procedure
Ewes were housed and individually penned on sawdust from 6 weeks pre-partum to 4 weeks post partum. The concentrate ration was fed in two equal meals during pregnancy at 0800 and 1600 h, stepped from 0.7 kg/day at 103 days of gestation for twin-bearing ewes (0.8 kg/day for triplet-bearing ewes) to 1.2 kg/day at 140 days of gestation (1.3 kg/day for triplet-bearing ewes) and was fed in three equal meals (at 0800, 1200 and 1600 h) at a flat rate of 1.7 kg/day during lactation. Barley straw was offered ad libitum, at a rate of 1.25 × daily intake calculated by weighing back refusals three times per week. Ewe live weight was measured weekly (EziWeigh apparatus, Tru-Test Ltd, Auckland, New Zealand) calibrated using standard weights, and ewes were condition-scored weekly. Ewe blood samples were obtained by jugular venipuncture into evacuated plasma tubes containing potassium oxalate at 1100 h on day 103 of gestation and at 12 h, 14 days and 28 days post partum. Milk samples were taken from ewes using a method detailed by Capper et al. (Reference Capper, Wilkinson, Kasapidou, Pattinson, Mackenzie and Sinclair2005). Milk samples were taken and stored at −20°C before analysis. Lambs were weighed at weekly intervals between birth and 28 days of age (A and D Co., Japan) calibrated using standard weights. Lambs were blood sampled at 1100 h at 14 days of age by jugular venipuncture collected into evacuated tubes containing potassium oxalate.
Chemical analyses
Feed samples were taken weekly and combined to give two samples per diet, which were analysed for dry matter (DM), ash, CP and NDF as detailed in Capper et al. (Reference Capper, Wilkinson, Kasapidou, Pattinson, Mackenzie and Sinclair2005). Plasma was separated by centrifugation at 2290 × g for 5 min before storage at −20°C. Ewe plasma was analysed for β-hydroxy-butyrate (βHB; kit number RB1007, Randox, County Antrim, UK), for urea (kit number TO1-1819-85, Bayer Diagnostics, Newbury, UK) and for non-esterified fatty acids (NEFA; NEFA C Test, Wako Chemicals, USA). Photometric plasma analyses were performed on a Bayer Technicon RA-1000 autoanalyser (Bayer Diagnostics, Berkshire, UK). Fatty acids within plasma and feed samples were saponified in 5 mol/l KOH and aqueous methanol before methylation by diazomethane as detailed in Capper et al. (Reference Capper, Wilkinson, Mackenzie and Sinclair2006). Milk fatty-acid concentration and composition data were provided by extracting fatty acids from a 1 ml sample of milk using methanol and chloroform (2:1 v/v) according to methods modified from Folch et al. (Reference Folch, Lees and Stanley1957) and Christie (Reference Christie1982), and methylated using 100 μl of sodium methoxide. Fatty-acid analysis was achieved using a PerkinElmer 8500 gas chromatograph (PerkinElmer Life and Analytical Sciences Ltd, Boston, MA, USA) comprising a 50 m × 0.22 mm internal diameter fused silica capillary column (model number 50QC2/BPX70 0·25; SGE International Pty Ltd, Ringwood, Victoria, Australia), a flame-ionisation detector and a PerkinElmer AS 8300 autosampler (SGE International Pty Ltd). Fatty-acid separation and identification details have been reported previously (Capper et al., Reference Capper, Wilkinson, Kasapidou, Pattinson, Mackenzie and Sinclair2005). The protein and lactose content of milk samples were determined using a Dairylab 2 IR milk analyser (Foss UK Ltd, Cheshire, UK). The analyser was calibrated for the analysis of ewe milk using standards of known concentrations (Quality Management, Lancashire, UK).
Statistical analyses
Data from eight ewes were excluded from the analysis. Two ewes aborted at 131 days of gestation (treatments FB and MS); a further five ewes reared single lambs (one from each of treatments MB, MS and FS, two from treatment FB) and one ewe (treatment MB) suffered from chronic mastitis. Two ewes (treatments MB and MS) bore one live and one stillborn lamb and adopted a lamb of the same breed from six group-housed ewes. The replacement lambs were introduced immediately after expulsion of the dead lamb. Data for these ewes were included; however, records relating to the adopted lambs were not included in the statistical analysis. In total, 12 lambs were excluded from the analysis due to abortion/stillbirth (n = 4), adoption (n = 2), supplemental feeding (n = 2) and being reared singly (n = 4). Consequently, data from 46 ewes was utilised pre partum and from 40 ewes and 84 lambs post partum.
Data were analysed as a 2 × 2 factorial completely randomised-block design with fat source and dietary vitamin E concentration and their interaction as the main effects. The analyses employed the general ANOVA in the statistical package Genstat 6.2 (Lawes Agricultural Trust, 2002). Sex was used as a covariate when analysing lamb birth weight, live weight and growth rate data, and overall lamb growth rate was calculated using linear regression.
Results
Feed analysis
The four concentrates had a similar chemical composition with mean values for DM, organic matter and CP (N × 6.25) of 863 g/kg, 908 g/kg DM and 179 g/kg DM, respectively (Table 2). Concentrations of NDF were lower, and the ash content higher in concentrates of FB and FS as a result of vermiculite inclusion, with mean values of 137 g/kg DM and 111 g/kg DM (fish oil) compared with 211 g/kg DM and 73 g/kg DM (Megalac) for NDF and ash, respectively. Vitamin E concentrations were similar to those predicted, with means of 61 and 522 mg/kg DM for the basal and supranutritional diets, respectively. Total fatty-acid concentrations were higher in concentrates supplemented with Megalac (100 g/kg DM) than those containing fish oil (89.8 g/kg DM). Concentrates supplemented with Megalac had the highest concentrations of C16:0, C18:1cis-9 and C18:2 (n-6), but the long-chain PUFAs C20:5 (n-3) and C22:6 (n-3) were not detectable. By contrast, the inclusion of fish oil plus Incromega in concentrates FB and FS resulted in mean concentrations of C20:4 (n-6), C20:5 (n-3) and C22:6 (n-3) of 0.72 g/kg DM, 3.15 g/kg DM and 3.32 g/kg DM, respectively.
Table 2 Chemical composition of the treatment concentrates differing in vitamin E concentration and fatty-acid source

†Abbreviations are: MB = Megalac + 50 mg/kg vitamin E, MS = Megalac+ 500 mg/kg vitamin E, FB = fish oil + 50 mg/kg vitamin E, FS = fish oil + 500 mg/kg vitamin E.
‡ND = not detected.
Ewe performance
The mean daily straw intake declined between 6 weeks pre partum (0.68 kg DM per day) and 1 week pre-partum (0.55 kg DM per day) across all treatments (Figure 1) before increasing to 1.07 kg DM per day at 3 weeks post partum. There was no effect of dietary treatment on daily straw intake (P > 0.05), although there was an interaction at 6 weeks pre-partum when ewes fed concentrate MB had higher intakes than those offered MS or FB (P < 0.05). There was no effect of dietary treatment upon ewe live weight or condition score change pre-partum (P > 0.05; Table 3). By contrast, post partum live weight loss was lower in ewes supplemented with fish oil, and condition score loss was increased by supranutritional vitamin E supplementation.

Figure 1 Daily straw intakes of ewes fed diets containing Megalac® (M; calcium soap of palm fatty acid, Volac Ltd, Royston, Hertfordshire, UK) or fish oil (F; Trouw UK Ltd, Northwich, Cheshire, UK) supplemented with either 50 (B) or 500 (S) mg/kg α-tocopherol acetate. For details of diets and procedures, see Materials and methods. Values are means with standard errors shown by vertical bars.
Table 3 Effect of PUFA and vitamin E supplementation of ewes on performance parameters and metabolic profiles

†Abbreviations are: MB = Megalac + 50 mg/kg vitamin E, MS = Megalac + 500 mg/kg vitamin E, FB = fish oil + 50 mg/kg vitamin E, FS = fish oil + 500 mg/kg vitamin E.
‡Approaching significance (P < 0.1).
Plasma βHB concentrations were reduced by the provision of fish oil at both 12 h (P < 0.001) and 4 weeks (P < 0.05) post partum and were increased by supranutritional vitamin E supplementation (P < 0.01) at 12 h post partum. Plasma NEFA concentrations measured at 12 h post partum were lower in ewes supplemented with fish oil (P < 0.001) and tended to be reduced by basal vitamin E supplementation at 12 h post partum (P = 0.069), although no difference was seen at 4 weeks post partum (P > 0.05). Ewes supplemented with fish oil had higher plasma urea concentrations at 4 weeks post partum (P < 0.01); however, there was no effect of dietary vitamin E supplementation.
Dietary treatment had no effect upon the total concentration of fatty acids in ewe plasma at 2 weeks post partum (P > 0.05; Table 4). Ewes supplemented with fish oil had lower plasma concentrations of C16:0, C18:1cis-9 and C18:2 (n-6) (P < 0.001), and tended to have lower concentrations of C18:0 (P = 0.067) and C20:4 (n-6) (P = 0.051). By contrast, fish oil supplementation increased the concentrations of C18:1trans; cis-9, trans-11 conjugated linoleic acid (CLA) (P < 0.001), C18:3 (n-3) (P < 0.01), C20:5 (n-3) and C22:6 (n-3) (P < 0.001) in plasma. There was no effect of vitamin E supplementation upon ewe plasma fatty acids (P > 0.05).
Table 4 Effect of PUFA and vitamin E supplementation of ewes on ewe plasma fatty acid composition at 2 weeks post partum
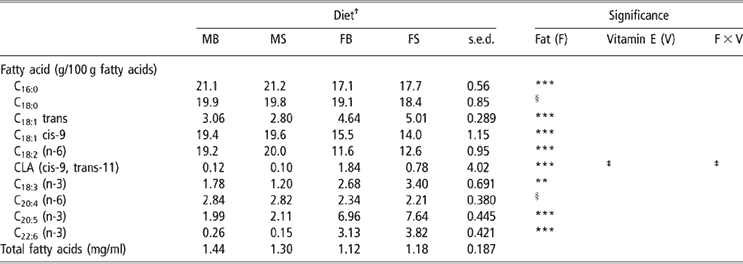
†Abbreviations are: MB = Megalac + 50 mg/kg vitamin E, MS = Megalac + 500 mg/kg vitamin E, FB = fish oil + 50 mg/kg vitamin E, FS = fish oil + 500 mg/kg vitamin E.
‡Approaching significance (P < 0.1).
Ewe milk yield and composition
There was no effect of dietary fat source or vitamin E concentration upon milk secretion rate or yield (P > 0.05; Table 5). However, fish oil supplementation reduced milk fat concentration (P < 0.01) and yield (P < 0.01). There was no main effect of dietary vitamin E concentration upon milk fat concentration or yield (P > 0.05), nonetheless, two interactions between dietary fat source and vitamin E concentration was observed. Ewes fed concentrate MB had lower milk protein concentrations than ewes fed concentrates MS, FB or FS (P < 0.05), and milk protein yield was reduced in ewes fed concentrate MB compared with those fed concentrate FB (P < 0.05). Milk protein yield (g/h) was increased by the provision of concentrates containing fish oil (P < 0.05); however, no effects of dietary vitamin E concentration were observed upon milk protein concentration or yield (P > 0.05). There was no effect of dietary treatment upon milk lactose concentration or yield (P > 0.05).
Table 5 Effect of PUFA and vitamin E supplementation of ewes on milk yield, milk composition and lamb growth rate

†Abbreviations are: MB = Megalac + 50 mg/kg vitamin E, MS = Megalac + 500 mg/kg vitamin E, FB = fish oil + 50 mg/kg vitamin E, FS = fish oil + 500 mg/kg vitamin E.
‡Approaching significance (P < 0.1).
§Growth rates calculated by linear regression of live weight data from birth to 28 days of age.
Fish oil supplementation of ewes reduced the concentrations of C16:0, C18:0 and C18:1cis-9 within milk fat (P < 0.001; Table 6). By contrast, concentrations of C18:1trans (P < 0.01), C18:3 (n-3) (P < 0.05), C20:5 (n-3) (P < 0.01) and C22:6 (n-3) (P < 0.001) in milk fat were increased by the provision of fish oil in the diet and cis-9, trans-11 CLA tended to be increased (P = 0.098). There was no effect of dietary fat source upon the concentrations of milk C18:2 (n-6) or C20:4 (n-6) (P > 0.05). Vitamin E supplementation level had no effect upon milk fatty-acid composition save for a decrease in C20:5 (n-3) conferred by supranutritional supplementation (P < 0.05).
Table 6 Effect of PUFA and vitamin E supplementation of ewes on milk fatty-acid composition
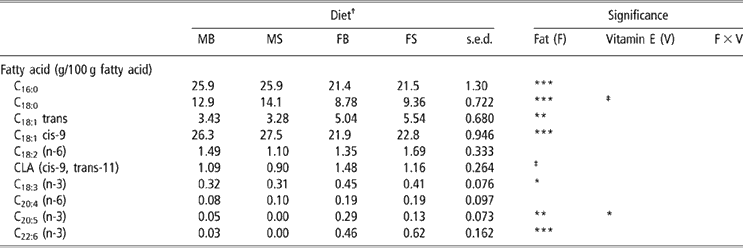
†Abbreviations are: MB = Megalac + 50 mg/kg vitamin E, MS = Megalac + 500 mg/kg vitamin E, FB = fish oil + 50 mg/kg vitamin E, FS = fish oil + 500 mg/kg vitamin E.
‡Approaching significance (P < 0.1).
Lamb performance
Individual lamb growth rate tended to be reduced by fish oil supplementation of the ewe (P = 0.054), an effect that was manifested as a decrease in litter growth rates from birth until 4 weeks of age (P < 0.05; Table 5). Supranutritional vitamin E supplementation of the ewe also tended (P = 0.074) to reduce litter growth rates when fed in combination with fish oil (concentrate FS) but not with Megalac (concentrate MS).
The total fatty-acid concentration in sucking lamb plasma was reduced by fish oil supplementation of the ewe (P < 0.01; Table 7). Two interactions between maternal dietary fat source and vitamin E concentration were observed: lambs sucking ewes fed concentrate FB had greater plasma C20:5 (n-3) concentrations than those from treatment FS (P < 0.05); and lambs on the MS treatment had increased plasma C22:6 (n-3) concentrations compared with those on the MB treatment (P < 0.05). Maternal fish oil supplementation reduced the plasma concentrations of C16:0, C18:0, C18:1cis-9, C18:2 (n-6) and C20:4 (n-6) in sucking lambs. By contrast, increases in C18:1trans; cis-9, trans-11 CLA, C18:3 (n-3), C20:5 (n-3) and C22:6 (n-3) (P < 0.001) were conferred by supplementing the ewe with fish oil. There was no effect of vitamin E supplementation upon plasma fatty-acid composition, save for a reduction in C20:5 (n-3) conferred by supranutritional supplementation (P < 0.05).
Table 7 Effect of PUFA and vitamin E supplementation of ewes on sucking lamb plasma fatty-acid composition at 2 weeks post partum
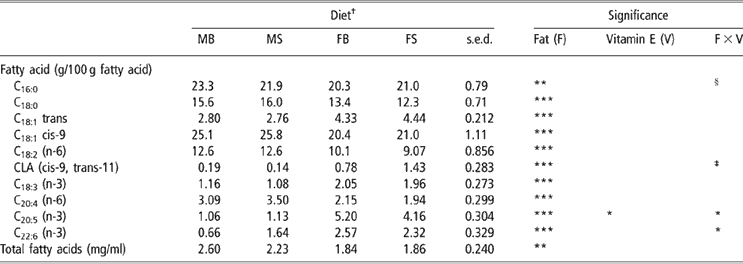
†Abbreviations are: MB = Megalac + 50 mg/kg vitamin E, MS = Megalac + 500 mg/kg vitamin E, FB = fish oil + 50 mg/kg vitamin E, FS = fish oil + 500 mg/kg vitamin E.
‡Approaching significance (P < 0.1).
Discussion
The aim of the current study was to determine the effects of fish oil and vitamin E supplementation of the pregnant and lactating ewe on ewe milk yield and composition and lamb growth rate. Previously published data (Capper et al., Reference Capper, Wilkinson, Kasapidou, Pattinson, Mackenzie and Sinclair2005 and Reference Capper, Wilkinson, Mackenzie and Sinclair2006) suggested that neonatal lamb vigour may be improved by fish oil and vitamin E supplementation of the pregnant ewe. However, fish oil supplementation of lactating cattle has been reported to reduce both milk yield and milk fat concentration (Shingfield et al., Reference Shingfield, Ahvenjärvi, Toivonen, Ärölä, Nurmela, Huhtanen and Griinari2003); effects that may potentially reduce lamb growth rate.
Reductions in feed intake have been reported as a result of fish oil supplementation of ewes (Annett et al., 2004) or lactating cattle (Donovan et al., Reference Donovan, Schingoethe, Baer, Ryali, Hippen and Franklin2000) but dietary fat source had no significant effect upon the daily forage intake of pregnant and lactating ewes within the current study. It should be noted, however, that the aforementioned studies did not include a control fat source in the non-fish oil-supplemented diets, therefore it is possible that the observed results may have resulted from similar effects of Megalac and fish oil upon DM intake. The depressive effect of long-chain PUFAs upon DM intake has been attributed to toxic effects upon rumen microflora, although the use of protected fish oils appears to negate these effects (Kitessa et al., Reference Kitessa, Gulati, Ashes, Fleck, Scott and Nichols2001a). It is therefore postulated that the adsorbent nature of the vermiculite carrier included in the current study may have rendered the long-chain PUFAs less available to ruminal bacteria, thereby negating potential negative effects. Alternatively, PUFA supplementation may have metabolic effects upon DM intake via shifts in ruminal biohydrogenation leading to an increased supply of unsaturated fatty acids at the duodenum as suggested by Shingfield et al. (Reference Shingfield, Reynolds, Lupoli, Toivonen, Yurawecz, Delmonte, Griinari, Grandison and Beever2006). Measures of ruminal biohydrogenation were not made within the current study; however, the increases in C18:1trans and cis-9, trans-11 CLA within plasma of ewes fed fish oil indicate that biohydrogenation of C18:2 (n-6) and C18:3 (n-3) to C18:0 may have been inhibited in favour of intermediaries such as trans isomers of C18:1.
Although fish oil supplementation reduced live-weight loss, dietary supranutritional vitamin E was associated with the mobilisation of body fat reserves, with increases in condition score loss and plasma βHB and NEFA concentrations. As vitamin E plays an essential role in preventing oxidation of unsaturated fatty acids, increasing the dietary supply might augment the release of these fatty acids from tissues to compensate for a reduced energy balance during lactation. However, this does not concur with the results of either Kott et al. (Reference Kott, Thomas, Hatfield, Evans and Davis1998) or Merrell (Reference Merrell1998) who reported no effect of vitamin E supplementation on ewe body condition score.
Changes in ewe plasma fatty acids largely reflected the fatty acid composition of the dietary concentrates. The replacement of Megalac by fish oil in the diets of pregnant and lactating ewes conferred a significant reduction in the amount of C16:0 within plasma, proportional to differences in dietary supply. The increased amounts of C16:0 observed in plasma of ewes offered Megalac concur with Petit (Reference Petit2002) who reported higher amounts of C16:0 in plasma of dairy cows fed Megalac compared with linseed or soya beans. Addition of fish oil to the ruminant diet has been shown to increase the plasma concentrations of both C18:1trans isomers and CLA produced by incomplete ruminal biohydrogenation of PUFA (Baumgard et al., Reference Baumgard, Corl, Dwyer, Saebo and Bauman2000; Wachira et al., Reference Wachira, Sinclair, Wilkinson, Enser, Wood and Fisher2000; Chikunya et al., Reference Chikunya, Demirel, Enser, Wood, Wilkinson and Sinclair2004), results that concur with the increase in C18:1trans fatty acids conferred by fish oil supplementation within the current study. Although concentrations of C18:2 (n-6) were lower in the diets containing fish oil compared with Megalac, proportionally, the reduction in plasma concentrations was higher, suggesting significant ruminal biohydrogenation of C18:2 (n-6). This concurs with data presented by Chikunya et al. (Reference Chikunya, Demirel, Enser, Wood, Wilkinson and Sinclair2004) and Sinclair et al. (Reference Sinclair, Cooper, Chikunya, Wilkinson, Hallett, Enser and Wood2005) in fish oil-supplemented sheep.
Increases in the n-3 PUFA status conferred by fish oil supplementation are often accompanied by a corresponding increase in plasma C20:4 (n-6) concentration resulting from increased dietary supply (Kitessa et al., Reference Kitessa, Gulati, Ashes, Fleck, Scott and Nichols2001a). However, despite increased dietary intakes of C20:4 (n-6), and in agreement with data presented by Sinclair et al. (Reference Sinclair, Cooper, Chikunya, Wilkinson, Hallett, Enser and Wood2005), fat source had no effect upon plasma C20:4 (n-6) concentration within the current study. The significant increase in plasma C20:5 (n-3) and C22:6 (n-3) in ewes offered diets containing fish oil were a direct result of the increased dietary supply of preformed long-chain PUFAs. However, Wachira et al. (Reference Wachira, Sinclair, Wilkinson, Hallett, Enser and Wood2002) and Chikunya et al. (Reference Chikunya, Demirel, Enser, Wood, Wilkinson and Sinclair2004) reported the presence of C20:5 (n-3) and C22:6 (n-3) within muscle and plasma fatty acids of sheep fed diets in which these fatty acids were not detected. The endogenous synthesis of C20:5 (n-3) and C22:6 (n-3) from C18:3 (n-3) may therefore provide an explanation for the presence of long-chain n-3 PUFA within plasma of Megalac-supplemented ewes. Rates of biohydrogenation (Gulati et al., Reference Gulati, Ashes and Scott1999; Sinclair et al., Reference Sinclair, Cooper, Chikunya, Wilkinson, Hallett, Enser and Wood2005) and transfer into milk (Shingfield et al., Reference Shingfield, Ahvenjärvi, Toivonen, Ärölä, Nurmela, Huhtanen and Griinari2003) are comparable between C20:5 (n-3) and C22:6 (n-3), yet despite significant increases in the amounts of both C20:5 (n-3) and C22:6 (n-3) in plasma as a response to fish oil supplementation, the increase was proportionally higher for C 20:5 (n-3).
Lactose serves as the primary determinant of milk osmotic potential and hence of milk yield (Mansbridge and Blake, Reference Mansbridge and Blake1997). Given the lack of significant treatment effects upon milk lactose production between treatments, it is therefore not surprising that milk yields were similarly unaffected by diet within the current study. Within the literature, effects of fish oil on milk yield are equivocal. Cattaneo et al. (Reference Cattaneo, Dell’Orto, Varisco, Agazzi and Savoini2006) reported a decrease in milk yield induced by fish oil supplementation of lactating goats, but Shingfield et al. (Reference Shingfield, Reynolds, Hervas, Griinari, Grandison and Beever2005) did not observe any significant effect of fish oil supplementation upon bovine milk yield. By contrast, Chilliard and Doreau (Reference Chilliard and Doreau1997) and Keady et al. (Reference Keady, Mayne and Fitzpatrick2000) reported increases in milk yield when fish oils were fed to lactating dairy cows.
The phenomenon of milk fat depression (MFD) induced by PUFA supplementation of lactating ruminants has been reviewed in detail (Peterson et al., Reference Peterson, Matitashvili and Bauman2003; Bauman et al., Reference Bauman, Mather, Wall and Lock2006) and it is clear that specific fatty acids produced as intermediates during ruminal biohydrogenation of C18:2 (n-6) play a significant role in reducing milk fat synthesis through inhibitory effects on mammary gene expression (Peterson et al., Reference Peterson, Matitashvili and Bauman2003). This mechanism would explain the 27% decrease in milk fat concentration conferred by fish oil supplementation, and, in combination with effects of diet upon milk yield, the 29% decrease in milk fat yield. Although plasma and milk concentrations of C18:1trans- and the cis-9, trans-11 isomer of CLA were increased by fish oil supplementation, it was not possible accurately to detect trans-10, cis-12 CLA or individual C18:1trans isomers within the current study, therefore this hypothesis cannot be verified. However, similar MFD responses to those observed in the current study were reported by Lock et al. (Reference Lock, Teles, Perfield, Bauman and Sinclair2006). CLA supplementation of lactating dairy ewes repartitioned nutrients away from milk fat synthesis towards increased milk yield and milk protein synthesis. Additionally, Keady et al. (Reference Keady, Mayne and Fitzpatrick2000) also reported increases in milk protein yield as a consequence of fish oil supplementation of lactating dairy cows. Diet had no significant effect upon milk yield within the current study; however, milk protein yields were higher in ewes supplemented with fish oil indicating that nutrient partitioning may have been affected by dietary fat source. This effect may also have led to reduced body fat mobilisation, a finding that corresponds with the lower live weight losses observed in ewes supplemented with fish oil. Vitamin E supplementation had no main effect upon milk yield or composition and the metabolic significance of the interaction between fat source and vitamin E concentration resulting in lower milk protein production in ewes fed concentrate MB is not clear, particularly as vitamin E supplementation of lactating cattle did not affect milk protein yield in the studies of Politis et al. (Reference Politis, Bizeli, Tsiaras and Baldi2004), Bell et al. (Reference Bell, Griinari and Kennelly2006) or Pottier et al. (Reference Pottier, Focant, Debier, De Buysser, Goffe, Mignolet, Froidmont and Larondelle2006).
Endogenous milk fat synthesis principally results in the secretion of short- and medium-chain fatty acids, with a significant contribution made by C16:0 (Bauman and Griinari, Reference Bauman and Griinari2001). In contrast to results observed by Kitessa et al. (Reference Kitessa, Peake, Bencini and Williams2003) and Shingfield et al. (Reference Shingfield, Ahvenjärvi, Toivonen, Ärölä, Nurmela, Huhtanen and Griinari2003), the concentration of C16:0 within milk fat appeared to be depressed in ewes offered fish oil within the current study. However, rather than a depressive effect of PUFA supplementation per se, this may be attributed to the high dietary concentration of this fatty acid in the control (Megalac) diets. By contrast, the lower concentration of C18:0 within milk fat conferred by fish oil supplementation concurs with results reported by Donovan et al. Reference Donovan, Schingoethe, Baer, Ryali, Hippen and Franklin(2000), Kitessa et al. (Reference Kitessa, Gulati, Ashes, Fleck, Scott and Nichols2001b) and Gulati et al. (Reference Gulati, McGrath, Wynn and Scott2003). The methods used to analyse milk fatty-acid composition within the current study did not differentiate between lipid classes, thus both phosphatidylglycerols and triacylglycerols are presented in the results. The major differences observed between plasma and milk concentrations of C18:2 (n-6) and C18:3 (n-3) may be partly attributable to their location within the phosphatidylglycerols, which contribute a relatively small proportion of milk lipids (Chilliard et al., Reference Chilliard, Ferlay, Mansbridge and Doreau2000). Moreover, the requirement for both fatty acids as precursors for endogenous synthesis of long-chain fatty acids and for C18:2 (n-6) as a precursor for prostaglandin synthesis may have reduced their deposition into milk fat. Considerable research has been devoted to increasing the concentration of C20:5 (n-3) and C22:6 (n-3) within milk products. Gulati et al. (Reference Gulati, McGrath, Wynn and Scott2003) reported that C20:5 (n-3) and C22:6 (n-3) were transferred into milk at rates between 6.8% and 8.1% of dietary intake; values lower than those observed within the current study at approximately 10% for C20:5 (n-3) and 17% for C22:6 (n-3) in ewes fed fish oil. However, these transfer rates do not take into account the possible confounding effects of the incorporation of both fatty acids into adipose tissue prior to parturition, and their subsequent mobilisation. Despite the presence of C20:5 (n-3) and C22:6 (n-3) within plasma of all ewes, albeit in low concentrations for the control group, the concentrations of C20:5 (n-3) and C22:6 (n-3) were significantly higher in milk fat from ewes fed fish oil compared with Megalac where they were almost undetectable. These results are in agreement with those observed by Kitessa et al. (Reference Kitessa, Peake, Bencini and Williams2003) as a result of fish oil supplementation of lactating ewes. It is interesting to note that although Pottier et al. (Reference Pottier, Focant, Debier, De Buysser, Goffe, Mignolet, Froidmont and Larondelle2006) reported changes in biohydrogenation patterns induced by supernutritional vitamin E supplementation of dairy cows, no such effect was observed in plasma or milk fatty acids within the current study.
Milk was the sole nutrient source available to sucking lambs within the current study and milk component yield was therefore a significant regulator of lamb growth rate. The observed depression in lamb growth rate conferred by fish oil supplementation may be attributed to reductions in energy intake of approximately 21% as a consequence of decreased milk fat supply, despite the increase in protein yield. Nevertheless, it is interesting to note that the decreased growth rate conferred by fish oil may have been confounded by high growth rates in lambs from treatment MS; particularly as previously published data from this study (Capper et al., Reference Capper, Wilkinson, Kasapidou, Pattinson, Mackenzie and Sinclair2005) indicated that supranutritional vitamin E supplementation improved lamb birth weight. The growth rate effect concurs with the results of Maiorano et al. (2007) although the mechanism by which vitamin E may increase birth weight and growth rate is not known. It may be related to an improvement in immune function as reported in other ruminant species (for review see McDowell et al. (Reference McDowell, Williams, Hidiroglou, Njeru, Hill, Ochoa and Wilkinson1996)), although this was not demonstrated in sheep by Daniels et al. (Reference Daniels, Hatfield, Burgess, Kott and Bowman2000) or Hatfield et al. (Reference Hatfield, Robinson, Minikhiem, Kott, Roth, Daniels and Swenson2002) as a result of vitamin E supplementation. The sucking lamb, with no access to long fibre, effectively functions as a monogastric (Valvo et al., Reference Valvo, Lanza, Bella, Fascone, Scerra, Biondi and Priolo2005); therefore, fatty acids absorbed at the small intestine are not subject to modification by ruminal bacteria. In consequence, the presence of C18:1trans-11 and cis-9, trans-11 CLA within sucking lamb plasma must have resulted either from the secretion of these fatty acids into maternal milk, or the action of Δ9-desaturase on trans-vaccenic acid as suggested by Valvo et al. (Reference Valvo, Lanza, Bella, Fascone, Scerra, Biondi and Priolo2005). Indeed, Knight et al. (Reference Knight, Tavendale, Death and Agnew2004) proposed that the increased concentration of cis-9, trans-11 CLA in muscle of lambs borne by ewes selected for high cis-9, trans-11 CLA in milk fat was due to desaturation of trans-vaccenic acid rather than due to up-regulation of Δ9-desaturase in mammary or muscle tissue. By contrast, although a positive relationship existed between ewe and lamb plasma concentrations of C18:2 (n-6), this relationship was not reflected by the proportions of C18:2 (n-6) within milk fat. Furthermore, the reduction in plasma C20:4 (n-6) exhibited by lambs sucking ewes fed fish oil does not appear to be related to ewe plasma and milk concentrations, but may result from reduced endogenous synthesis within the lamb. Comparison of the concentrations of C20:5 (n-3) and C22:6 (n-3) in sucking lamb plasma with the amounts supplied by maternal milk suggests that a system for the preferential conservation and deposition of n-3 PUFA exists in animals with a habitually low preformed dietary supply. However, the precise biological mechanisms by which n-3 PUFA status may be maintained in the ruminant is yet to be elucidated.
Conclusion
The results of the current study demonstrate that the previous reported benefits of long-chain n-3 PUFA and vitamin E supplementation upon neonatal lamb vigour and performance are not maintained by extending the supplementation into lactation, whereby milk fat concentration and lamb growth rate are reduced. The mechanisms by which dietary fish oil depresses milk fat synthesis and increases milk protein synthesis warrant further investigation. To exploit fully the potentially beneficial effect of maternal diet upon lamb performance, a strategy that combines PUFA supplementation during pregnancy with a saturated fatty-acid source fed during lactation may improve both neonatal vigour and sucking lamb performance.
Acknowledgements
The authors wish to thank the Oldacre Foundation for financial assistance, Trouw Nutrition UK for the provision of feedstuffs and Roche UK Ltd for the provision of vitamin supplements and vitamin analysis within feed.









