Implications
The breeding success of a ram, defined as the number of lambs sired, when used in a multi-sire breeding situation was found to be both repeatable and heritable. No negative correlations were observed with other productive traits indicating that selection for ram breeding success could be incorporated into ram selection. This would reduce the number of rams needed for breeding and thus allow for increased selection pressure on the rams and increased genetic gain.
Introduction
Commercial sheep production systems typically use multi-sire breeding groups (i.e. mob mating) for their breeding flocks to produce lambs. In these systems, a group of rams is joined with a large mob of ewes for the breeding season, which typically last 34 to 51 days (i.e. two to three ewe reproductive cycles). In New Zealand, most commercial sheep farms run a mixed livestock system with combined sheep and beef production with some farms also producing deer. The average farm size is ~630 hectares running ~4000 stock units on each farm, with around 2600 sheep (Anonymous, 2018). Mob sizes can range from tens of sheep to thousands of sheep, depending on flock size and breeding requirements. Pasture sizes can range considerably and vary from flat land to hill country with varying stocking rates. Ewe to ram ratios vary with current recommendation in New Zealand suggesting one ram for every 150 mature ewes with guidelines for younger ewes being slightly less, with one ram for every 70 to 100 yearling and 2-year-old ewes (Geenty, Reference Geenty2013). Age of the ram is also an important factor, with a recommendation of rams <1 year old being joined with a reduced number of ewes (Geenty, Reference Geenty2013). In these systems, rams compete for mating of ewes, with many ewes being mated by multiple rams (Allison and Davis, Reference Allison and Davis1976a).
Although overall flock reproductive performance is primarily driven by ewe fecundity, variation in the number of offspring sired by each ram in these group mating systems is observed (Stellflug et al., Reference Stellflug, Cockett and Lewis2006; Alexander et al., Reference Alexander, Cockett, Burton, Hadfield and Moss2012), and this has implication for the genetic potential of the resulting lambs if the rams with the highest genetic potential sire relatively few offspring due to low mating success. In addition, if this trait is repeatable and heritable, selection for ram mating success could allow an increased selection pressure to be applied to rams, as the number of ewes allotted to each ram could be increased, thus increasing genetic gain.
Small focused trials have shown variation in the number of ewes mated and offspring produced in mob mating situations in sheep (Allison and Davis, Reference Allison and Davis1976b; Stellflug et al., Reference Stellflug, Cockett and Lewis2006; Alexander et al., Reference Alexander, Cockett, Burton, Hadfield and Moss2012) and cattle (Holroyd et al., Reference Holroyd, Doogan, De Faveri, Fordyce, McGowan, Bertram, Vankan, Fitzpatrick, Jayawardhana and Miller2002; Van Eenennaam et al., Reference Van Eenennaam, Weber and Drake2014; Abell et al., Reference Abell, Theurer, Larson, White, Hardin, Randle and Cushman2017). However, the repeatability and heritability of this trait has been difficult to measure given limitations in assigning parentage of lambs following mob mating. The validation of parentage tests for use in commercial sheep production (Dodds et al., Reference Dodds, Tate and Sise2005; Clarke et al., Reference Clarke, Henry, Dodds, Jowett, Manley, Anderson and McEwan2014; Heaton et al., Reference Heaton, Leymaster, Kalbfleisch, Kijas, Clarke, McEwan, Maddox, Basnayake, Petrik, Simpson, Smith and Chitko-McKown2014) provides new opportunities to better characterise the repeatability and heritability of ram mating success and its relationship with other productive traits.
Material and Methods
Data collection
Information from 15 flocks of varying breeds (Romney, Perendale, Coopworth, Texel, Suffolk (dams only) and composites of these and other breeds) were downloaded from the sheep improvement limited (SIL) database (Young and Newman, Reference Young and Newman2009) for lambs born from 2007 to 2015. These animals were part of commercial ram breeding flocks and were managed to meet the requirements of New Zealand’s Animal Welfare Act 1999 (Codes of Welfare sections 68-79). Typically, ewes are exposed to rams for 2 to 3 reproductive cycles and, when data were recorded (70% of the time) in the database, the average (±SD) length of the mating season was 46±14 days. Data, including parentage information, were collected as part of normal operating procedures for the commercial management of the flock. Information obtained for lambs included year of birth, flock, dam, sire (assigned by DNA parentage assay; Zoetis New Zealand, Dunedin, New Zealand (Dodds et al., Reference Dodds, Tate and Sise2005; Clarke et al., Reference Clarke, Henry, Dodds, Jowett, Manley, Anderson and McEwan2014)), accuracy of sire assignment and ewe mating group. There were a total of 152 156 progeny from 623 mating groups (average size 244±269 ewes (standard deviation)) with 1515 sires represented. Greater than 90% of the lambs produced were from dams and sires of the same breed. Progeny without mating group information, from mating groups with only one sire identified, from mating groups with less than 10 progeny or those born to link sires (i.e. a sire used at two farms in the same year and thus not present during the full mating cycle), were removed. Historical data regarding the pedigree of the animals were obtained either from DNA parentage assays (Dodds et al., Reference Dodds, Tate and Sise2005) or from information recorded at birth from ewes that had been mated to a single, identified sire.
Statistical analyses
The statistical program R (R Core Team (2017)) was used to further analyse the data. Initially, data from sires 7 years and older as well as progeny of dams 8 years and older were removed as were mating groups where the ewe to ram ratio was less than 20:1 due to small group sizes. Ewe to ram ratio ranged from 20:1 to 96.3:1 in the remaining mating groups. Various accuracy combinations for parentage assignments were then examined. The first parameter was the sire assignment accuracy (SAA, (Dodds et al., Reference Dodds, Tate and Sise2005)) where a cut-off of 0.80, 0.90 and 0.95 probability of correct sire assignment was utilised to determine sire as assigned or missing (i.e. for a cut-off of 0.80, any lamb with a probability score for sire assignment of<0.80 was given a missing value for sire). The second criterion removed any mating group with a proportion of >0.05, 0.10 or 0.20 lambs in a mating group whose sire could not be assigned (sire assignment failure (SAF)). From this information, data sets were created to count the number of lambs born to each sire within each mating group. Information from ewes that failed to become pregnant and rams that failed to produce any lambs were not included in the analysis.
Two ram mating traits were assessed: loge transformed number of lambs born per sire (loge(nlamb)) and proportion of lambs sired by each sire in a mating group (proportion). To determine how year of birth, birth flock, sire age, mate group and ram intensity (ewe-to-ram ratio) affected the ram mating success traits, linear models were fitted in R using both forwards and backwards regression. Sire age class was grouped as 1, 2 or 3 years and older (to a maximum of 6 years). For proportion, an additional covariate, 1/number of rams, was fitted to account for the varying number of rams in each mate group. Selection of the most parsimonious model was completed using the least stringent selection criteria for the mate groups. The model terms retained for loge(nlamb) were sire age class, mate group and ram intensity of mate group (as a covariate). For proportion, the model terms retained were sire age class, mate group and 1/number of rams (as a covariate).
Fixed effects were transferred to a full mixed genetic model analysis in ASReml (VSN International (Gilmour et al., Reference Gilmour, Gogel, Cullis, Welham and Thompson2015)) to estimate variance components and to estimate the repeatability and heritability of the traits. Two traits were examined, loge(nlamb) and proportion, using the repeated measures animal model:
where X is an incidence matrix for the fixed effects b (sire age class±mate group) and Z is an incidence matrix relating records of the trait concerned to random animal effects and permanent environmental effects, respectively. The a is the vector of random additive genetic effects with the variance–covariance ![]() ${\rm }A\sigma _{a}^{2} $, where A is the numerator relationship matrix calculated from the pedigree and
${\rm }A\sigma _{a}^{2} $, where A is the numerator relationship matrix calculated from the pedigree and ![]() $\sigma _{a}^{2} $ is the additive genetic variance. The p is a vector of permanent environment (repeatability) effects and e is a vector of residuals. The random effects p and e have variance–covariances
$\sigma _{a}^{2} $ is the additive genetic variance. The p is a vector of permanent environment (repeatability) effects and e is a vector of residuals. The random effects p and e have variance–covariances ![]() $I\sigma _{p}^{2} $ and
$I\sigma _{p}^{2} $ and ![]() $I\sigma _{e}^{2} $, respectively, where
$I\sigma _{e}^{2} $, respectively, where ![]() $\sigma _{p}^{2} $ is the variance due to permanent environmental effects and
$\sigma _{p}^{2} $ is the variance due to permanent environmental effects and ![]() $\sigma _{e}^{2} $ is the residual variance. Initial analysis revealed that ram intensity (or number of sires in the mate group for proportion) was confounded with flock structure as no residual variation was observed. Thus, these factors were removed from the model and mating group was fitted as a fixed effect to account for differences in ram intensity or number of rams in the mating group (for analysis of loge(nlamb) and proportion, respectively). This also removes variation in base fecundity (i.e. the average number of lambs per ewe) between mating groups.
$\sigma _{e}^{2} $ is the residual variance. Initial analysis revealed that ram intensity (or number of sires in the mate group for proportion) was confounded with flock structure as no residual variation was observed. Thus, these factors were removed from the model and mating group was fitted as a fixed effect to account for differences in ram intensity or number of rams in the mating group (for analysis of loge(nlamb) and proportion, respectively). This also removes variation in base fecundity (i.e. the average number of lambs per ewe) between mating groups.
Genetic correlations between ram mating success and other productive traits
Available records from progeny of sires represented in the data set restricted to SAA of 0.95 and SAF of 0.05 were obtained from SIL for selected growth and fertility traits, namely weaning weight (wwt), live weight at 8 months of age (lw8), number of lambs born at 1 year of age (NLB1), NLB for ewes 2 to 6 years of age (NLB2-6) and NLB for ewes 1 to 6 years of age (NLB1-6). The effects of variation in birthdate of individual lambs on weaning weight, lw8 and NLB1 measurements were accounted for with inclusion of the deviation of each lamb’s birthdate from the average birthdate in the contemporary group, in the model for these traits (Pickering et al., Reference Pickering, Blair, Hickson, Dodds, Johnson and McEwan2013). Records from a total of 57 284 animals representing progeny from 634 sires were available. Phenotypic and genetic correlations for these traits were calculated from bivariate analyses using ASReml (Gilmour et al., Reference Gilmour, Gogel, Cullis, Welham and Thompson2015). The model described above was used for the ram mating success traits. Given that permanent environmental variance was trending negative when the data set was restricted to SAA of 0.95 and SAF of 0.05, this was set to 0.001 for the analysis. The models used for the growth and female fertility traits varied between traits and are presented in Table 1. For example, the model used for a bivariate analysis of proportion and wt was:
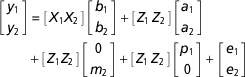 $$\eqalignno{ \left[ {\matrix{ {y_{1} } \cr {y_{2} } \cr } } \right] &{\equals}\left[ {\matrix{ {X_{1} } {X_{2} } \cr } } \right]\left[ {\matrix{ {b_{1} } \cr {b_{2} } \cr } } \right]{\plus}\left[ {\matrix{ {Z_{1} } {\,Z_{2} } \cr } } \right]\left[ {\matrix{ {a_{1} } \cr {a_{2} } \cr } } \right] \cr & {\plus}\left[ {\matrix{ {Z_{1} } {Z_{2} } \cr } } \right]\left[ {\matrix{ 0 \cr {m_{2} } \cr } } \right]{\plus}\left[ {\matrix{ {Z_{1} } {\,Z_{2} } \cr } } \right]\left[ {\matrix{ {p_{1} } \cr 0 \cr } } \right]{\plus}\left[ {\matrix{ {e_{1} } \cr {e_{2} } \cr } } \right]$$
$$\eqalignno{ \left[ {\matrix{ {y_{1} } \cr {y_{2} } \cr } } \right] &{\equals}\left[ {\matrix{ {X_{1} } {X_{2} } \cr } } \right]\left[ {\matrix{ {b_{1} } \cr {b_{2} } \cr } } \right]{\plus}\left[ {\matrix{ {Z_{1} } {\,Z_{2} } \cr } } \right]\left[ {\matrix{ {a_{1} } \cr {a_{2} } \cr } } \right] \cr & {\plus}\left[ {\matrix{ {Z_{1} } {Z_{2} } \cr } } \right]\left[ {\matrix{ 0 \cr {m_{2} } \cr } } \right]{\plus}\left[ {\matrix{ {Z_{1} } {\,Z_{2} } \cr } } \right]\left[ {\matrix{ {p_{1} } \cr 0 \cr } } \right]{\plus}\left[ {\matrix{ {e_{1} } \cr {e_{2} } \cr } } \right]$$where subscript 1 refers to the first trait (proportion), subscript 2 refers to the second trait (wwt), y k refers to the trait values, X k are design matrices of fixed effects, Z k are design matrices relating observations to animals and k refers to the trait. The a are direct breeding values with variance–covariance matrix
where ![]() $\sigma _{{ak}}^{2} $ is the additive genetic variance for trait k, r g is the genetic correlation and ⊗ is the Kronecker product operator. The m 2 are maternal breeding values with variance
$\sigma _{{ak}}^{2} $ is the additive genetic variance for trait k, r g is the genetic correlation and ⊗ is the Kronecker product operator. The m 2 are maternal breeding values with variance ![]() $A\sigma _{{m2}}^{2} $ where
$A\sigma _{{m2}}^{2} $ where ![]() $\sigma _{{m2}}^{2} $ is the maternal genetic variance for trait 2. The p 1 are permanent environmental effects for trait 1, as previously described. The e are residual effects with variance
$\sigma _{{m2}}^{2} $ is the maternal genetic variance for trait 2. The p 1 are permanent environmental effects for trait 1, as previously described. The e are residual effects with variance
where I k are identity matrices with the same number of rows as y k, I 12 is an indicator matrix with (i,j)th element equal to 1 when the ith observation for trait 1 is on the same animal as the jth observation for trait 2, r e is the residual correlation and ![]() $\sigma _{{ek}}^{2} $ are the residual variances.
$\sigma _{{ek}}^{2} $ are the residual variances.
Table 1 Final models used for calculation of repeatability and heritability of ram mating success traits and the phenotypic and genetic correlations between ram mating success traits and weaning weight (wwt), live weight at 8 months of age (lw8), number of lambs born at 1 year of age (NLB1), NLB for ewes 2 to 6 years of age (NLB2-6) and NLB for ewes 1 to 6 years of age (NLB1-6)
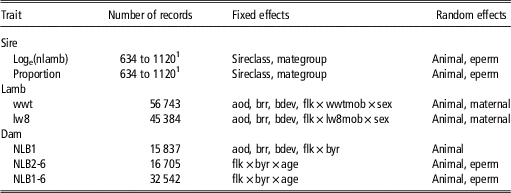
sireclass=age of sire grouped into 1, 2 and 3+ years of age; mategroup=the multi-sire mating group; eperm=permanent environmental effects; aod=age of dam (years); brr=birth-rearing rank (born single, twin or triplet, reared single, twin or triplet); bdev=birth date deviation from the mean of contemporary group; flk=birth flock; wwtmob=wwt grazing mob; sex=sex of the lamb; lw8mob=lw8 grazing mob; byr=birth year; age=ewe age (years).
1 Number of sire records varied according to sire assignment accuracy and sire assignment failure.
Results
Effects of ram age on ram mating success traits
Ram age affected ram mating success traits (P<0.001) with 3-year-old or older rams producing more progeny than either 1- or 2-year-old sires. On average (95% CI) for back-transformed loge(nlamb), 3-year-old or older sires produced 66.3 (53.5 to 82.3, n=147) progeny, whereas 2-year-old rams produced 54.7 (45.0-66.5, n=264) and 1-year-old rams produced 56.9 (42.9 to 75.5, n=379) progeny with SAA set to 0.95 and SAF set to 0.05. Proportion of progeny in a mate group was also affected by ram age (P<0.001). A higher proportion (±SEM) was sired by older rams (3+ years of age) than 1- or 2-year-old rams (0.32±0.02, 0.28±0.02 and 0.27±0.03, respectively).
Effects of differing stringencies for sire assignment accuracy and sire assignment failure on repeatability and heritability of ram mating success traits
Repeatability and heritability of ram mating success trait loge(nlamb) are presented in Table 2 with information on proportion of lambs sired presented in Table 3. In general, as stringency of criteria for including a lamb or mating group in the data set increased, repeatability and heritability for both traits increased. Moderate repeatability is observed at all selection criteria for both traits, with a maximum repeatability of 0.40±0.09 and 0.30±0.09 reached for loge(nlamb) and proportion, respectively. Using the most stringent criteria, namely SAA of 0.95 and SAF of 0.05, heritability of both loge(nlamb) and proportion reaches significance (0.26±0.12 and ±0.30±0.09, respectively; P<0.05).
Table 2 Repeatability and heritability (h 2) for the sire mating success trait loge(number(n)lambs)

NS, not significant.
1 untransformed, NS (P>0.05).
Table 3 Repeatability and heritability (h 2) for the sire mating success trait, proportion of lambs sired
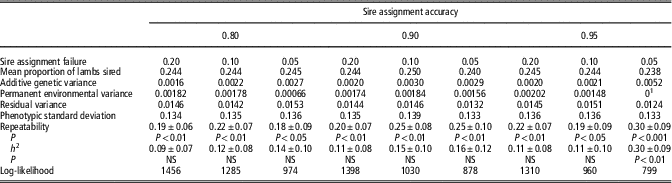
NS=not significant.
Note that information regarding the number of sires and mate groups is the same as provided in Table 2.
1 Value fixed at 0 as trending negative; NS (P>0.05).
Correlations between traits
Using the most stringent criteria, namely SAA of 0.95 and SAF of 0.05, phenotypic (r p) and genetic correlations (r g) between loge(nlamb) and proportion were 0.88±0.01 and 0.94±0.06, respectively. The estimates of repeatability and heritability from the bivariate model were similar to those obtained from the univariate model. For loge(nlamb), the repeatability and heritability of the bivariate model were 0.40±0.09 and 0.27±0.12, respectively. For proportion, the estimates for repeatability and heritability in the bivariate model were 0.31±0.10 and 0.25±0.13, respectively. Phenotypic and genetic correlations between the ram mating success traits and growth and female fertility traits are presented in Table 4. The phenotypic correlations were small and non-significant. The genetic correlations observed were all low and positive but none reached significance.
Table 4 Genetic and phenotypic correlations (±SEM) of sire mating success traits, loge transformed number of lambs born per sire (loge(nlamb)) and proportion of lambs sired by each sire in a mating group (proportion) with weaning weight (wwt), live weight at 8 months of age (lw8), number of lambs born at 1 year of age (NLB1), NLB for ewes 2 to 6 years of age (NLB2-6) and NLB for ewes 1 to 6 years of age (NLB1-6)

Discussion
Using a large data set collected from commercial flocks in New Zealand, we have shown that ram mating success, as defined by the number of offspring sired or proportion of progeny sired, is moderately repeatable for an individual when assessed over multiple observations. Heritability of ram mating success is also moderate, suggesting that selection for the trait could be undertaken to improve the number of ewes each ram was capable of mating, thus improving genetic gain through increased selection pressure on rams. No significant genetic correlations between the ram mating success traits and the growth and female fertility traits were observed with all having low positive estimates. Antagonistic relationships between these traits, which would complicate selection to improve ram mating success, appear unlikely. Conversely, selection for female fertility or growth traits as defined in this study is also unlikely to provide much benefit for improving ram mating success. Thus, improved direct measurement of ram mating success, or identification of a stronger predictor trait, would be beneficial.
The ram mating success trait is potentially influenced by multiple factors, including the number of ewes a ram mates and whether or not he sires one (or more) lambs from each ewe mated. Both of these factors can be influenced by the mating group the ram is included in through variations in the number of ewes the ram is exposed to and the average number of lambs each ewe would produce. A ram in a mating group with more lambs sired would have a greater opportunity to sire increased numbers of lambs v. those in a group with fewer lambs sired. Similarly, the proportion of lambs sired in each mating group is highly dependent on the number of rams included in the mate group. Fitting of the mate group as a fixed effect in the model allowed removal of the effects of variations in number of lambs sired per mating group (for loge(nlamb)) or number of rams in the mate group (for proportion). Given the high phenotypic and genetic correlation between the two traits, it is likely that the models used were effective in removing the variation caused by differences in mate group composition.
Mating performance of rams has previously been subjectively measured using a libido test in sheep (Snowder et al., Reference Snowder, Stellflug and Van Vleck2002). The score is based on the number of mounts and ejaculations recorded for each ram when exposed to three ewes in oestrus for 30 min. This trait is also highly repeatable and moderately heritable (0.22±0.04) (Snowder et al., Reference Snowder, Stellflug and Van Vleck2002). The correlation between libido test and number of lambs sired in a mob mating situation have not been thoroughly investigated. In a smaller pen test, when rams of high and low mating performance (as measured by a libido test) were included together in a mob mating situation (approximately one ram per 67 ewes), rams with high mating performance sired more than double the number of lambs than those with a low mating score (Stellflug et al., Reference Stellflug, Cockett and Lewis2006). Thus, ram libido likely accounts for at least some of the variation observed in mating performance and variability in number of lambs sired by each ram. Furthermore, this likely contributes to the heritability observed in number of lambs sired per ram. It is also important to note that associations between pen libido test and number of ewes mated in a group mating situation were not well correlated in some experiments (Kelly et al., Reference Kelly, Allison and Shackell1975; Moore and Whyman, Reference Moore and Whyman1977). These differences may be related to differing ram to ewe ratios, with effects potentially only observed when the ram is required to mate a large number of ewes. Alternatively, differing variations in libido in the rams tested may also affect outcome with the potential for libido needing to be very poor to suppress number of ewes mated.
Ram mating success was influenced by ram age, with young rams having lower success than older rams. Previous studies in sheep have shown an effect of ram age on libido test (Snowder et al., Reference Snowder, Stellflug and Van Vleck2002) and number of ewes mated (Kelly et al., Reference Kelly, Allison and Shackell1975; Allison, Reference Allison1978; Ch’ang and Evans, Reference Ch’ang and Evans1979), although this was not observed with all breeds (Ch’ang and Evans, Reference Ch’ang and Evans1979). Variability in the number of offspring produced in multi-sire breeding groups on commercial farms was also observed in Bos taurus bulls in California (Van Eenennaam et al., Reference Van Eenennaam, Weber and Drake2014) and in Bos indicus or Bos indicus cross bulls in Australia (Holroyd et al., Reference Holroyd, Doogan, De Faveri, Fordyce, McGowan, Bertram, Vankan, Fitzpatrick, Jayawardhana and Miller2002). In these analyses, which each included around 230 to 260 sires, sire age did not consistently affect the number of offspring produced although calf number increased with age for Brahman bulls.
Although overall flock reproductive performance is primarily driven by ewe fecundity, there is also some evidence that rams with low mating performance can lead to reduced flock fertility. In single sire mating situations, oestrus synchronised ewes (30 per ram) exposed to rams that had low mating scores (less than two ejaculations during a 30-min test with ewes in oestrus) were less apt to become pregnant than those exposed to rams that had high mating scores (at least six ejaculations). This was linked to the number of ewes mated by each ram (Perkins et al., Reference Perkins, Fitzgerald and Price1992). As highlighted by the authors, the reduced pregnancy rate was likely linked to the high number of ewes in oestrus at the same time as oestrus was synchronised in the ewes. Other studies have failed to link ram mating libido to reduced flock fertility when oestrus had not been synchronised and relatively low ewe to ram ratios were used (Kelly et al., Reference Kelly, Allison and Shackell1975; Mickelsen et al., Reference Mickelsen, Paisley and Dahmen1982). Thus, use of rams with low libido may not affect overall pregnancy rate or number of lambs born per ewe. However, if ewe to ram ratio is high, it may result in some ewes not being mated during the first 17 days of the breeding season resulting in more ewes lambing later during the lambing period (Perkins et al., Reference Perkins, Fitzgerald and Price1992). How the inclusion of a ram with low mating success in a multi-sire mating situation might affect overall flock fecundity is not well understood. It seems likely that if other rams with high libido were present in the sire group, all ewes would likely be covered as most ewes are mated by multiple rams even at relatively high ewe to ram ratios (equivalent to 180 ewes per ram) (Allison, Reference Allison1978) although paddock size can affect this with fewer rams breeding each ewe in larger paddocks (Allison and Davis, Reference Allison and Davis1976b).
However, if ram mating success is linked to increased embryo loss in ewes, fertility of ewes bred by rams with poor ram mating success could affect overall flock fertility. Although the effects of poor libido that have been observed on overall flock performance have been linked to failure of the ewe to be mated, whether the ram mating success traits examined in the current study could also contain an element of increased embryo loss in the ewes, which would suppress flock performance, is not well understood. Additional work is needed to understand how including one or more sires with poor mating success in multi-sire mating schemes affects the overall flock fertility. Understanding if there is a link between ram mating success and other female reproductive traits not examined in this study, such as ovulation rate and particularly embryo survival, could provide further information. If ram mating success was phenotypically and genetically linked to embryo survival, which has low heritability (Shorten et al., Reference Shorten, O’Connell, Demmers, Edwards, Cullen and Juengel2013), selection for ram mating success could potentially provide a means to improve flock embryo survival.
The current analysis highlighted that stringent selection criteria for both assignment of a lamb to a sire and inclusion of the mate group as a fixed effect within the analysis allowed for a more accurate estimation of heritability. Thus, one key method to improve direct measurement of ram mating success is to use stringent criteria for SAA and SAF when assessing the trait. Historically, parentage testing all progeny from a mob mating situation was expensive given average number of progeny per ram was over 60. Alexander et al. (Reference Alexander, Cockett, Burton, Hadfield and Moss2012) used proportional testing within the progeny groups, but its effect on accuracy of the trait was not analysed. A more fruitful approach might be to find indirect traits that were well correlated with ram mating success and can be measured at a young age. Given the similarities between the heritability of libido and the ram mating success traits examined in this study, these traits may be highly correlated. Recently, Alhamada et al. (Reference Alhamada, Debus and Bocquier2017) described an automated method for evaluation of ram libido and this may provide another approach to evaluate ram mating success.
Whether there were permanent environmental effects that affect ram mating success was unclear as, when examined as a proportion with the most stringent data selection conditions (i.e. SAA at 0.95 and SAF at 0.05), the permanent environmental variance trended negative, indicating that there was little or no permanent environmental effect. However, this was not observed for the trait expressed as loge(nlamb). Potential factors that could affect a ram mating success could include differences in gestational or early life environment such as nutrition or environmental toxins, that affected development of the male reproductive tract (Kotsampasi et al., Reference Kotsampasi, Balaskas, Papadomichelakis and Chadio2009; Pang et al., Reference Pang, Li, Feng, Yang, Han, Fan, Nie, Wang, Wang and Zhang2018; Scully et al., Reference Scully, Estill, Amodei, McKune, Gribbin, Meaker, Stormshak and Roselli2018).
Somewhat surprisingly, we also observed that when calculating heritability of ram mating success, inclusion of mating group in the model as a random effect with 1/number of rams in the mating group as a covariate resulted in no residual variation when examining proportion of lambs sired, indicating that the original model was not adequate. Factors such as mating group, where there are a large number of levels, and specific levels are not of interest, are often fitted as random effects. Fitting mating group as a fixed effect which will account for variation of number of rams in the mating group (or ram intensity for loge(nlambs)) allowed estimation of heritability for both ram mating success traits. Further examination of the data suggested that the number of rams included in a mating group was confounded with the data structure as estimation of heritability of 1/number of rams was highly significant for this data set. Thus it was critical that the model used for analysis was able to address and adjust for these confounding issues in the data set.
In conclusion, ram mating success in multi-sire mob mating situations was variable, thus the genetic merit of the offspring produced from multi-sire mob mating situations will not necessarily reflect the average genetic merit of the team of rams used for mating. Ram mating success was moderately repeatable and heritable and this information can be used to select for rams capable of breeding an increased number of ewes. Given that negative genetic correlations were not observed with the growth and female fertility traits measured in this study, selection for increased mating ability of rams can be utilised to reduce the number of rams required for breeding, thereby allowing for increased selection pressure on the rams and increased genetic gain.
Acknowledgements
The authors would like to thank Silong Liao and Matt Bixley for preliminary analysis and discussions on the project, Neville Amyes for downloading of data from the SIL database, Natalie Pickering for helpful advice on interpretation of data traits and Mary Wheeler for helpful discussion during the project. The authors would also like to thank Beef + Lamb New Zealand Genetics and FarmIQ Systems Limited for providing access to data required for the experiment. In addition, the authors would like to thank the farmers who allowed us to use information from their flocks. Funding was provided from AgResearch’s Strategic Investment Fund from the New Zealand Government.
Declaration of interest
The authors declare no conflict of interest.
Ethics statement
The animals used in the study were part of commercial flocks and were managed to meet the requirements of New Zealand’s Animal Welfare Act 1999 (Codes of Welfare sections 68-79).
Software and data repository resources
The data used in this study are in the Sheep Improvement Limited (https://www.sil.co.nz/) database. These data were collected from commercial sheep and thus the information is accessible only with permission from the flock owners.






