Implications
Piglets are subject to constant nutritional, psychological and environmental stresses during weaning, which can result in significant swine industry economic losses. Developing strategies to reduce the negative consequences of post-weaning stress is of great concern. Glutamine is an important functional amino acid. It alleviates growth suppression and repairs weaning piglet intestinal epithelium. This article summarizes recent researches which highlight the potential use of glutamine in pig nutrition and underlying mechanisms involved. This review helps to provide references for glutamine use in swine production.
Introduction
The gastrointestinal tract plays crucial roles in digestion and absorption of nutrients, providing barrier functions, as well as amino acid metabolism (Wijtten et al., Reference Wijtten, Van der Meulen and Verstegen2011). Its health is vitally important to host growth and development. With the widespread application of early weaning, piglets are immediately exposed to many environmental and psychosocial stressors that impact on gut structure and function (Spreeuwenberg et al., Reference Spreeuwenberg, Verdonk, Gaskins and Verstegen2001; Yin et al., Reference Yin, Wu, Xiao, Ren, Duan, Yang, Li and Yin2014). These changes cause abnormal digestion and absorption, poor growth performance and increased diarrhea rates (Montagne et al., Reference Montagne, Boudry, Favier, Huërouluron, Lallès and Sève2007). Exploring different nutritional factors, management and strategies that focus on promoting weaning pig growth are critical to optimizing performance. Glutamine has always been classified as a nonessential amino acid when assigning swine nutritional requirement. One of the major reasons for this is that glutamine naturally occurs in conventional feed ingredients such as corn grain, sorghum grain, soybean meal and meat and bone meal. Natural glutamine constitutes about 10% of the amino acid content of total dietary protein (Wu, Reference Wu2014). Also, post-weaning pigs weighing around 5 to 10 kg have high net glutamine synthetic rates (up to 1149 mg/kg BW per day) in their extraintestinal tissues (Wu, Reference Wu2014). However, all tissues utilize glutamine (Wu et al., Reference Wu, Bazer, Burghardt, Johnson, Kim, Knabe, Li, Satterfield, Smith and Spencer2010), and there is growing evidence that pigs have a dietary requirement for glutamine to achieve maximum growth performance and facilitate normal intestinal physiology, particularly in hypercatabolic states (Wang et al., Reference Wang, Chen, Li, Li, Zhou, Wang, Li, Yin and Wu2008; Blachier et al., Reference Blachier, Wu, Yin, Hou, Andriamihaja, Blachier, Wu and Yin2013; Wu, Reference Wu2014). As swine nutrition practices have rapidly changed, the understanding of the underlying mechanisms needs to keep pace. This article provides an overview on the effects of weaning on gastrointestinal structure and function, the effects of glutamine on piglet performance and gut health and information on the underlying mechanisms, and how they are influenced by glutamine.
Intestinal development and health after weaning
A multitude of factors contribute to post-weaning stress, including nutritional (change from sow’s milk to solid feed), psychological (mixing of different litters and removal from the sow), environmental (moving from farrowing crates to nursery pens) and others (Moeser et al., Reference Moeser, Klok, Ryan, Wooten, Little, Cook and Blikslager2007; Yin et al., Reference Yin, Wu, Xiao, Ren, Duan, Yang, Li and Yin2014). Weaning stress is generally accompanied by reduced feed intake, decreased nutrient utilization, poor growth performance and increased disease susceptibility (Montagne et al., Reference Montagne, Boudry, Favier, Huërouluron, Lallès and Sève2007). Several articles demonstrate that weaning stress also negatively impacts intestinal development and function (Montagne et al., Reference Montagne, Boudry, Favier, Huërouluron, Lallès and Sève2007). This review highlights the effects of weaning stress on intestinal epithelial development and its redox status. The common effects of weaning on piglet performance and intestinal development are provided in Table 1.
Table 1 The common effects of weaning on piglet performance and intestinal development and function

PCNA = proliferating cell nuclear antigen; AKP = alkaline phosphatase; CRF = corticotropin-releasing factor; MHC = major histocompatibility complex; 5-HT = 5-hydroxytryptamine; HRP = horseradish peroxidase.
Intestinal epithelium developmental changes after weaning
The gut includes crypts and villi, which are formed by four principal cell types: absorptive enterocytes, mucus-producing goblet cells, hormone-secreting enteroendocrine cells and lysozyme-containing Paneth cells (Chen et al., Reference Chen, Xia, Zhu, Yan, Tan, Deng, Deng, Yin and Ren2018a). The porcine intestinal epithelium structure is shown in Figure 1. The homeostasis and renewal of the intestinal epithelium involve stem cell proliferation at the base of the crypt bottom, cell population expansion in the middle of the villus, terminal differentiation in the upper villus and senescent cell extrusion at the villus tip (Yang et al., Reference Yang, Xiong, Wang, Tan, Li and Yin2016b). This intricate process of epithelial cells along the crypt-villus axis is accompanied by functional specialization in the small intestine (Yang et al., Reference Yang, Wang, Xia and Yin2016a). The proliferation, differentiation and apoptosis of intestinal epithelial cells play important roles in intestinal development, maintenance and recovery from stress-induced tissue damage. In weaning piglets, the altered balance between crypt cell proliferation and epithelial cell apoptosis causes intestinal villus atrophy and crypt hyperplasia resulting in a loss in nutrient absorptive surfaces (Montagne et al., Reference Montagne, Boudry, Favier, Huërouluron, Lallès and Sève2007). There is a decrease in enterocyte differentiation post-weaning accompanied by the loss of digestive enzymes, such as lactase and sucrase, and thus a decrease in intestinal digestive capacity (Pluske et al., Reference Pluske, Hampson and Williams1997).
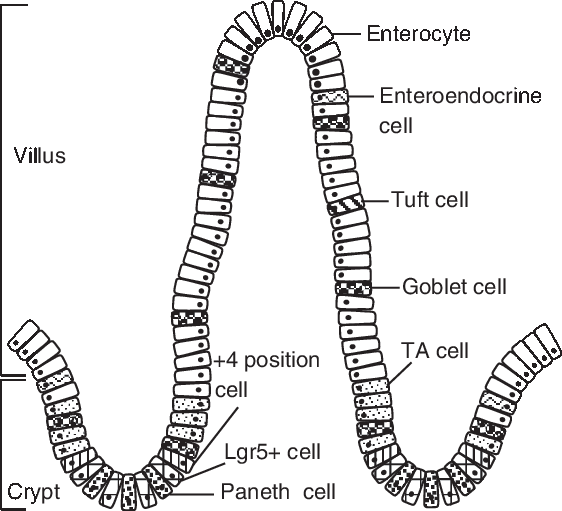
Figure 1 Porcine intestinal epithelium structure. Intestinal stem cells (ISCs) locates at the base of the crypt, which can be identified by marker gene Lgr5 (leucine-rich-repeat-containing G-protein-coupled receptor 5). The +4 position cell resides at the fourth position from the base of the crypt, above the Paneth cell. Paneth cells are located between Lgr5-positive (Lgr5+ ) cells. The daughter cells of ISCs migrate up the crypt-villus axis and pass through a progenitor stage, forming transient amplifying (TA) cells, before becoming differentiated cells (enterocyte, enteroendocrine cell, tuft cell, goblet cell).
Post-weaning distinctive intestinal functional characteristics
An increase in reactive oxygen species (ROS) can induce oxidative stress during animal growth and development, and weaning is often associated with oxidative stress and decreased antioxidant capacity in the intestine and other tissues of pigs (Wang et al., Reference Wang, Chen, Li, Li, Zhou, Wang, Li, Yin and Wu2008; Yin et al., Reference Yin, Wu, Xiao, Ren, Duan, Yang, Li and Yin2014). For example, weaning pigs at 14 days of age resulted in oxidative injury to lipid, protein and DNA and increased the plasma concentrations of damage biomarkers such as malondialdehyde, protein carbonyl and 8-hydroxydeoxyguanosine (Yin et al., Reference Yin, Wu, Xiao, Ren, Duan, Yang, Li and Yin2014). Concurrently, the activity of many antioxidant enzymes, such as superoxide dismutase, catalase and glutathione peroxidase, is also reduced due to weaning (Yin et al., Reference Yin, Wu, Xiao, Ren, Duan, Yang, Li and Yin2014). Glutathione in reduced form (GSH) is an antioxidant that can directly react with reactive-oxygen species, generating oxidized glutathione (GSSG) through glutathione peroxidase. In turn, GGSG can be catalyzed into GSH by glutathione reductase. Since the reduced and oxidized forms of glutathione are simultaneously present in cells, the ratio of GSSG : GSH is an indicator of oxidative stress, that not only determines cellular redox status but also controls intracellular concentrations of both oxygen-reactive and nitrogen-reactive species (Cruzat et al., Reference Cruzat, Macedo Rogero, Noel Keane, Curi and Newsholme2018). In 28-day-old pigs, weaning reduced feed intake (−36%), BW gain (−47%) and small intestine weight (−26%) over the 7-day post-weaning. Meanwhile, weaning increased the expression of genes (+52% to +346%) related to oxidative stress (i.e. glutathione peroxidase-1 and -4) and the GSSG : GSH ratio (+59%) when compared with piglets maintained on the sow (Wang et al., Reference Wang, Chen, Li, Li, Zhou, Wang, Li, Yin and Wu2008). Moreover, pigs weaned at 21 days of age grew more slowly (−89%), had shorter villus height (−42%) than piglets maintained on the sow (Wang et al., Reference Wang, Zhang, Wu, Sun, Wang, He, Dai and Wu2014). In addition, weaning can result in increased intestinal epithelial permeability by decreasing the relative expression of tight junctions including occludin and claudins (Wang et al., Reference Wang, Zhang, Wu, Sun, Wang, He, Dai and Wu2014). These affect the absorption and transportation of nutrients, disrupt barrier functions and may cause additional intestinal inflammatory responses (Wang et al., Reference Wang, Zhang, Wu, Sun, Wang, He, Dai and Wu2014).
Glutamine may affect intestinal function of weaning pigs
Glutamine is considered the most abundant free amino acid in the body, because plasma and tissue glutamine concentrations are 10 to 100 fold higher than any other amino acids (Cruzat et al., Reference Cruzat, Macedo Rogero, Noel Keane, Curi and Newsholme2018). Weaning affects intestinal amino acid metabolism (Sève et al., Reference Sève, Reeds, Fuller, Cadenhead and Hay1986) with many of these changes related to glutamine metabolism. For example, in enterocytes of weaned pigs, l-glutamine is oxidized approximately eight times less slowly than in enterocytes isolated from suckling pigs (Darcy-Vrillon et al., Reference Darcy-Vrillon, Posho, Morel, Bernard, Blachier, Meslin and Duée1994). Weaning also results in reduced glutamine concentrations in the jejunal lumen fluid (−70%), jejunal tissue (−38%), and plasma (−30%) (Wang et al., Reference Wang, Chen, Li, Li, Zhou, Wang, Li, Yin and Wu2008). However, the gut preferentially extracts glutamine as a source of energy within intestinal epithelial cells. For example, approximately 67% of dietary glutamine is catabolized by the small intestine (i.e. enterocytes, lymphocytes and bacteria) in swine, with the remaining glutamine entering the portal circulation (Wu et al., Reference Wu, Bazer, Johnson, Knabe, Burghardt, Spencer, Li and Wang2011). Furthermore, glutamine is the only amino acid in arterial blood that is taken up by the small intestine of swine. Thus, although the small intestine represents only 3% to 4% of BW, it utilizes 30% of total arterial glutamine in pigs (Wu et al., Reference Wu, Bazer, Johnson, Knabe, Burghardt, Spencer, Li and Wang2011). Therefore, glutamine may affect intestinal development, structure and function and growth performance of weaning piglets. Therefore, the potential of feeding higher dietary glutamine levels to weaning pigs, the underlying mechanisms and how they are influenced by glutamine deserves further research.
Glutamine metabolism in the small intestine
The gut derives glutamine from both the diet and blood. The small intestine is the principal organ for glutamine uptake and metabolism into the body. l-glutamate is the principal ingested form that exerts its physiological function in the gut (Blachier et al., Reference Blachier, Wu, Yin, Hou, Andriamihaja, Blachier, Wu and Yin2013). Dietary glutamine is taken up from the intestinal lumen and transported across the apical membranes of the intestinal enterocyte by a variety of amino acid transport systems (i.e. Na+-dependent transport systems) to cytosol and mitochondria. Following that, l-glutamine enters the mitochondria, where it is degraded to l-glutamate and ammonia by the phosphate-dependent glutaminase, which is very abundant in enterocyte mitochondria in both villus and crypt cells (Pinkus and Windmueller, Reference Pinkus and Windmueller1977). Dietary glutamate serves primarily as an important energy source for rapidly dividing porcine mucosal cells (Reeds et al., Reference Reeds, Burrin, Jahoor, Wykes, Henry and Frazer1996 and Reference Reeds, Burrin, Stoll and Jahoor2000; Janeczko et al., Reference Janeczko, Stoll, Chang, Guan and Burrin2007). For example, in pigs weaned at 21 days of age and administered three- to fourfold the normal level of dietary glutamate, the majority (70% to 80%) of absorbed glutamate was utilized by the gut, and a large proportion of this served as an oxidative fuel for the mucosa (Janeczko et al., Reference Janeczko, Stoll, Chang, Guan and Burrin2007).
Second, as a protein synthesis precursor, glutamine has many metabolites, including glutamate, alanine, aspartate, α-ketoglutarate, arginine and proline (Blachier et al., Reference Blachier, Guihot-Joubrel, Vaugelade, Le Boucher, Bernard, Duée and Cynober1999). Transamination (amino acid synthesis and interconversion) appears to be the principal route by which enteral glutamate is converted to α-ketoglutarate in enterocytes in weaning pigs (Madej et al., Reference Madej, Lundh and Lindberg1999). Glutamate is metabolized into alanine and α-ketoglutarate in the presence of pyruvate, and into aspartate and α-ketoglutarate in the presence of oxaloacetate. Then, α-ketoglutarate enters the Krebs cycle to be oxidized to produce energy. Third, less than 10% of total glutamine extracted by the pig gut is used for protein synthesis (Stoll et al., Reference Stoll, Henry, Reeds, Yu, Jahoor and Burrin1998). Lastly, one consequence of glutamine metabolism is to produce glutathione via glutamate. Together with l-cysteine and glycine, enteral l-glutamate is the precursor for the synthesis of glutathione, and among these three amino acids, glutamate probably represents the most important step (Cruzat et al., Reference Cruzat, Macedo Rogero, Noel Keane, Curi and Newsholme2018). Most of the metabolites of glutamine are oxidized with the remaining being exported through the hepatic portal vein to the whole body (Blachier et al., Reference Blachier, Wu, Yin, Hou, Andriamihaja, Blachier, Wu and Yin2013). Note that while cellular glutamine can be metabolized into purines, pyrimidines for the biosynthesis of nucleotides, glutamate cannot replace this function. Conversely, glutamate and ammonia can be synthesized into glutamine by glutamine synthetase in the skeletal muscles or liver, but this reaction consumes energy (Cruzat et al., Reference Cruzat, Macedo Rogero, Noel Keane, Curi and Newsholme2018). A schematic view of catabolic metabolism of glutamine in small intestine is shown in Figure 2.
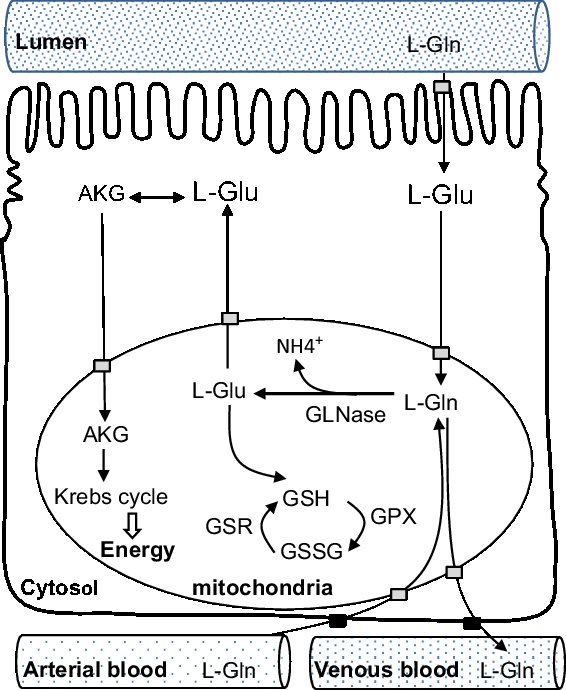
Figure 2 Schematic view of catabolic metabolism of glutamine in the small intestine, adapted from Blachier et al., (Reference Blachier, Wu, Yin, Hou, Andriamihaja, Blachier, Wu and Yin2013). AKG = alpha-ketoglutarate; Gln = glutamine; GLNase = phosphate-dependent glutaminase; Glu = glutamate; GPX = glutathione peroxidase; GSH = reduced form of glutathione; GSR = glutathione reductase; GSSG = oxidized form of glutathione.
Glutamine regulates intestinal function of weaning pigs
Numerous studies have suggested that glutamine has positive effects on piglet growth performance, intestinal morphology, epithelial cell renewal, antioxidant capacity and barrier functions (Wu et al., Reference Wu, Meier and Knabe1996; Domeneghini et al., Reference Domeneghini, Giancamillo, Bosi and Arrighi2006; Wang et al., Reference Wang, Chen, Li, Li, Zhou, Wang, Li, Yin and Wu2008 and Reference Wang, Zhang, Wu, Sun, Wang, He, Dai and Wu2014; Molino et al., Reference Molino, Donzele, de Oliveira, Saraiva, Haese, Fortes and de Souza2012; Zhu et al., Reference Zhu, Lin, Dai, Zhou, Li, Yuan, Wu, Wu and Wang2015; Jiang et al., Reference Jiang, Chen, Liu, Liu, Yao and Yin2017).
Glutamine improves piglet health, growth and intestinal morphology
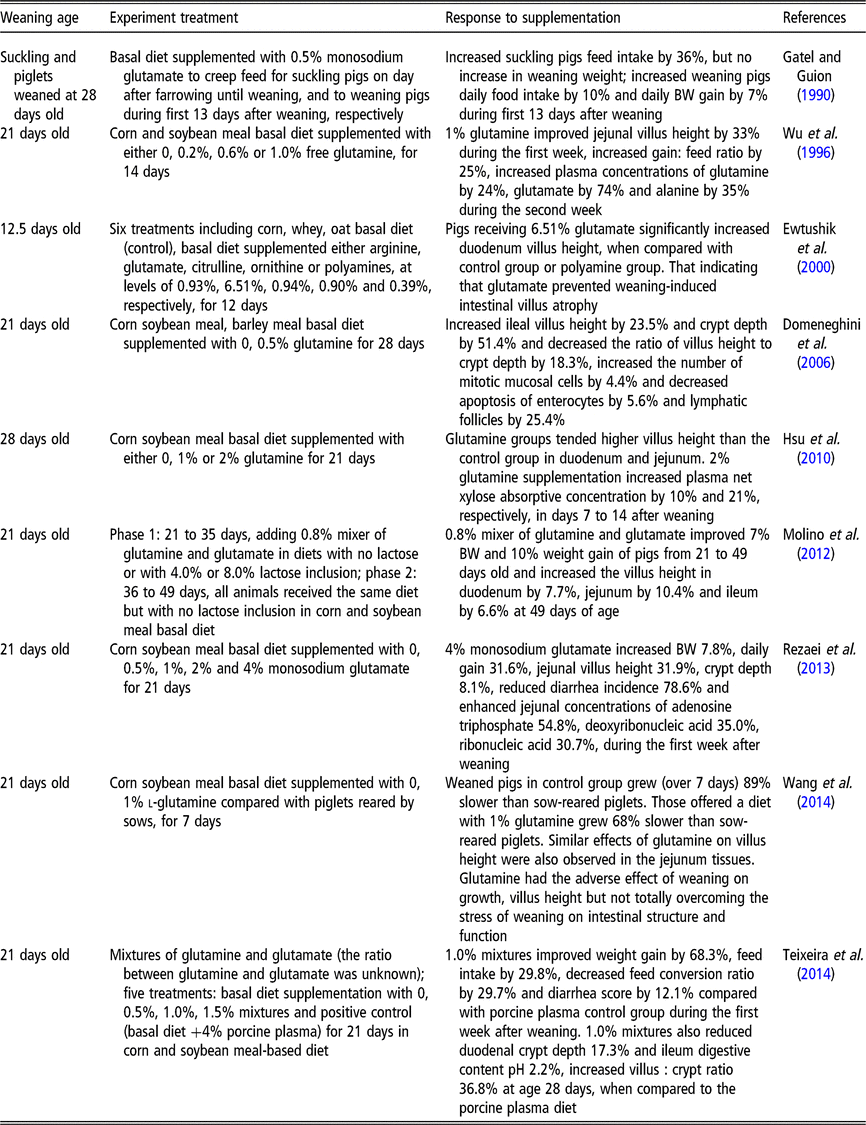
Rhoads et al. (Reference Rhoads, Keku, Quinn, Woosely and Lecce1991) reported that glutamine is an effective oral rehydration solution for rotavirus-induced diarrhea. Compared to normal newborn piglets, the rotavirus-induced diarrhea piglets had decreased villus height (−65%), thinner mucosa and increased thymidine kinase activity (+700%) (Rhoads et al., Reference Rhoads, Keku, Quinn, Woosely and Lecce1991). Meanwhile, the activities of lactase specific (−20%), alkaline phosphatase (−38%) and Na+/K+-ATPase (−50%) were all reduced in response to rotavirus-induced diarrhea (Rhoads et al., Reference Rhoads, Keku, Quinn, Woosely and Lecce1991). Moreover, l-glutamine (30 mmol/l) was found to stimulate in vitro absorption of sodium and chloride in rotavirus-infected pigs, returning the rates those observed in uninfected tissue (Rhoads et al., Reference Rhoads, Keku, Quinn, Woosely and Lecce1991). However, that study did not further investigate whether glutamine affects the villus height and mucosal enzyme activities of infected pigs. In another study, piglets that were weaned at 21 days and supplemented with 0.5%, 1%, 2% and 4% monosodium glutamate for 21 days exhibited a dose-dependent reduction in the incidence of diarrhea in the first week after weaning (Rezaei et al., Reference Rezaei, Knabe, Tekwe, Dahanayaka, Eide, Lovering, Ficken, Fielder and Wu2013). These data indicate that glutamine or glutamate can alleviate diarrhea. Other studies demonstrate that glutamine or glutamate serves as conditionally essential amino acids, and that supplementation with either can improve weaning piglet growth performance (Wu et al., Reference Wu, Meier and Knabe1996; Molino et al., Reference Molino, Donzele, de Oliveira, Saraiva, Haese, Fortes and de Souza2012) and intestinal epithelium structure and function (Rezaei et al., Reference Rezaei, Knabe, Tekwe, Dahanayaka, Eide, Lovering, Ficken, Fielder and Wu2013; Wang et al., Reference Wang, Zhang, Wu, Sun, Wang, He, Dai and Wu2014). The reported effects of glutamine on the performance, gastrointestinal structure and function of weaned pigs are shown in Table 2. The evidence from these published studies using pure glutamine or a mixture of glutamine and glutamate shows beneficial effects between 0.5% and 2.0% (by weight) throughout the post-weaning period, particularly during the first 2 weeks after weaning (Wu et al., Reference Wu, Meier and Knabe1996; Domeneghini et al., Reference Domeneghini, Giancamillo, Bosi and Arrighi2006; Hsu et al., Reference Hsu, Huang, Wang, Yen and Yu2010; Molino et al., Reference Molino, Donzele, de Oliveira, Saraiva, Haese, Fortes and de Souza2012; Teixeira et al., Reference Teixeira, Nogueira, Kutschenko, Rostagno and Lopes2014; Wang et al., Reference Wang, Zhang, Wu, Sun, Wang, He, Dai and Wu2014). However, some studies also showed that there were no improvements on growth performance of post-weaning piglets supplemented with glutamine or glutamate. For example, a corn soybean meal diet supplemented with 1.0% glutamine did not improve villus height in piglets weaned at 18 days of age, but did improve feed efficiency (Kitt et al., Reference Kitt, Miller, Lewis and Fischer2002). Di et al. (Reference Di Giancamillo, Domeneghini, Paratte, Dell’Orto and Bontempo2003) reported that when pigs weaned at 21 days of age were fed with 0.5% glutamine for 28 days, they showed a significant positive effect on ileal morphology and mucosal cell proliferation (lymphatic follicles and epithelial cells, macrophages and intra-epithelial lymphocytes). However, there were no significant differences among the groups in growth performances (Di et al., Reference Di Giancamillo, Domeneghini, Paratte, Dell’Orto and Bontempo2003). In spite of the positive effects of glutamine on small intestinal villus morphology and plasma net xylose absorptive concentration in 28-day-old weaning pigs, 1% or 2% glutamine supplementation in a corn-soybean meal diet did not significantly affect growth performance during the 21-day treatment period (Hsu et al., Reference Hsu, Huang, Wang, Yen and Yu2010). Similar results were obtained by (Amorim et al., Reference Amorim, Saleh, Miassi and Berto2018) who also did not observe any significant effects of 1% glutamine or 1% glutamate on growth performance variables in pigs fed a rice grit and soybean-based diet. Different diets, weaning age, experimental duration or dosage of glutamine may contribute to differing growth performance responses to glutamine or glutamate supplementation (Kitt et al., Reference Kitt, Miller, Lewis and Fischer2002; Di et al., Reference Di Giancamillo, Domeneghini, Paratte, Dell’Orto and Bontempo2003; Amorim et al., Reference Amorim, Saleh, Miassi and Berto2018).
Table 2 The reported effects of glutamine on the performance, gastrointestinal structure and function of weaned pigs
Glutamine and cellular redox state
Glutamine metabolism can provide glutamate required for cellular glutathione synthesis. Glutathione can directly react with ROS and thereby protect cellular macromolecules such as DNA, proteins and lipids against oxidative stress (Cruzat et al., Reference Cruzat, Macedo Rogero, Noel Keane, Curi and Newsholme2018). Glutamine has been shown to preserve total glutathione levels after injury/ischemia to gut (Harward et al., Reference Harward, Coe, Souba, Klingman and Seeger1994). In response to cobalt chloride (375 µmol/kg BW)-generated oxidative stress, rats showed increased liver thiobarbituric acid reactive substances content (+50%) and decreased GSH (−40%) activity compared to control values (Gonzales et al., Reference Gonzales, Polizio, Erario and Tomaro2005). The level of antioxidant enzymes catalase, glutathione peroxidase and superoxide dismutase in liver also was decreased by about 30% after cobalt injection, while that of hepatic heme oxygenase-1 induction was increased over 14-fold after cobalt treatment. However, these dramatic effects of cobalt on enzyme activities, including thiobarbituric acid reactive substances and GSH, were not observed in rats that had been pretreated with dietary glutamine (300 mg/kg BW). Also, the marked increase in the liver heme oxygenase-1 (protein expression and activity) due to cobalt challenge was partially prevented by glutamine administration (Gonzales et al., Reference Gonzales, Polizio, Erario and Tomaro2005). These data indicate that glutamine supplementation prevents glutathione depletion in the liver of rats, thus exerting protective effects during oxidant stress. Similarly, in pigs weaned at 21 days age and supplemented with 4% monosodium glutamate, there was an increase in jejunal GSH (+56%) and a decrease in GSSG (−24%) and the ratio of GSH : GSSG (−54%) during the first week after weaning (Rezaei et al., Reference Rezaei, Knabe, Tekwe, Dahanayaka, Eide, Lovering, Ficken, Fielder and Wu2013). Dietary glutamate also alleviated intestinal oxidative stress in weaning pigs, when animals were challenged by the potent oxidants diquat and hydrogen peroxide (Yin et al., Reference Yin, Liu, Ren, Duan, Yang, Zhao, Fang, Chen, Li and Yin2015b; Duan et al., Reference Duan, Yin, Ren, Liu, Cui, Huang, Wu, Kim, Liu and Wu2016). Diquat is an herbicide that generates superoxide anions through redox cycling and has been used to induce oxidative stress in vivo (Lv et al., Reference Lv, Yu, Mao, Zheng, He and Chen2012). A diquat injection to piglets significantly decreased final BW (−22%), average daily weight gain (−125%) and feed intake (−57%) (Yin et al., Reference Yin, Liu, Ren, Duan, Yang, Zhao, Fang, Chen, Li and Yin2015b). Meanwhile, supplementation with 2% glutamate after diquat treatment partially restored final BW to 91.2%, average daily feed intake to about 50%, and average daily weight gain to 19% of control levels (Yin et al., Reference Yin, Liu, Ren, Duan, Yang, Zhao, Fang, Chen, Li and Yin2015b). Diquat disrupted antioxidant enzymes balances, such as decreased serum nitric oxide about onefold, elevated serum malondialdehyde by about 80%. Diquat also decreased serum concentrations of threonine (−42%), serine (29%), glycine (26%) and glutamate (−5.5%) (Yin et al., Reference Yin, Liu, Ren, Duan, Yang, Zhao, Fang, Chen, Li and Yin2015b). Pigs treated with 2% glutamate increased nitric oxide and reduced malondialdehyde. Meanwhile, 2% supplemental glutamate significantly enhanced serum glutamate level by 21% and associated amino acid transporters expression. Glutamate supplementation increased serum threonine, serine and glycine concentrations, although they did not return completely to control values (Yin et al., Reference Yin, Liu, Ren, Duan, Yang, Zhao, Fang, Chen, Li and Yin2015b).
Glutamine and intestinal epithelial cells renewal
Glutamine and intestinal epithelial cell proliferation and differentiation
Glutamine has long been recognized as having an important role in cell survival and proliferation (Ehrensvard et al., Reference Ehrensvard, Fischer and Stjernholm1949). Glutamine promotes intestinal cell proliferation in rats (Swaid et al., Reference Swaid, Sukhotnik, Matter, Berkowitz, Hadjittofi, Pollak and Lavy2013), piglets (Wang et al., Reference Wang, Chen, Li, Li, Zhou, Wang, Li, Yin and Wu2008) and various cell lines (Chen et al., Reference Chen, Tseng, Yao, Li and Tsai2018b). A recent study with weaning mice by Chen et al. (Reference Chen, Xia, Zhu, Yan, Tan, Deng, Deng, Yin and Ren2018a) found that 10 mg/ml glutamine promotes Lgr5 (leucine-rich-repeat-containing G-protein-coupled receptor 5, also known as Gpr49), a stem cell marker, expression in intestinal cells. Another study found that Lgr5 stem cell growth is closely associated with epidermal growth factor (EGF), Wnt3 and the Notch-ligand. These are all essential signals required for stem cell proliferation and differentiation (Sato et al., Reference Sato, van Es, Snippert, Stange, Vries, van den Born, Barker, Shroyer, van de Wetering and Clevers2011). Further work is necessary to determine if and how glutamine changes these signal pathways to promote Lgr5 stem cell development. Some studies indicate that supplemental glutamine had no effect on lysozyme and angiogenin expressions that are Paneth cell markers (Liu et al., Reference Liu, Ren, Fang, Hu, Guan, Al-Dhabi, Yin, Duraipandiyan, Chen and Peng2017; Chen et al., Reference Chen, Xia, Zhu, Yan, Tan, Deng, Deng, Yin and Ren2018a). For example, glutamine at a dosage of 2% did not promote mRNA expression in other Paneth cell-associated factors such as α-defensins (cryptdin-1, -4 and -5), lysozyme C and angiogenin 4 in the ileum of mouse models (Liu et al., Reference Liu, Ren, Fang, Hu, Guan, Al-Dhabi, Yin, Duraipandiyan, Chen and Peng2017). Glutamine at a dosage of 10 mg/ml fed to weaning mice did not affect the number of absorptive enterocytes, Paneth cells, goblet cells, enteroendocrine cells or the gene expression of these cell markers (Chen et al., Reference Chen, Xia, Zhu, Yan, Tan, Deng, Deng, Yin and Ren2018a). However, the in vitro administration of 10 mmol glutamine increased chromogranin A and mucin 2 expression. These are differentiation markers for enteroendocrine and goblet cells, respectively (Chen et al., Reference Chen, Tseng, Yao, Li and Tsai2018b). This apparent discrepancy may result from differences between in vivo and in vitro studies. Whether glutamine affects weaning piglet intestinal cell differentiation warrants further investigation.
Glutamine and intestinal cell apoptosis
Various factors, such as glutamine deprivation (Papaconstantinou et al., Reference Papaconstantinou, Hwang, Rajaraman, Hellmich, Townsend and Ko1998), toxins (Wu et al., Reference Wu, Xiao, Ren, Yin, Hu, Duan, Liu, Tan, Xiong and Oso2014) and inflammation (Crespo et al., Reference Crespo, San-Miguel, Prause, Marroni, Cuevas, González-Gallego and Tuñón2012), have been reported to induce intestinal cell apoptosis. Glutamine administered rectally to rat with colitis reduced activating transcription factor 6 (ATF6), activating transcription factor 4 (ATF4) and spliced X-box-binding protein-1 (XBP-1) mRNA levels. These are transmembrane proteins in the endoplasmic reticulum that have pro-endoplasmic reticulum stress effects. Glutamine (25 mg/dl) administration inhibited the pro-apoptotic protein expression of phospho-p53 and cytochrome c, and decreased activities of apoptotic markers including caspase-9, caspase-8 and caspase-3 (Crespo et al., Reference Crespo, San-Miguel, Prause, Marroni, Cuevas, González-Gallego and Tuñón2012). Double immunofluorescence staining showed co-localization of C/enhancer binding protein homologous protein (CHOP) and cleaved caspase-3 in colon sections (Crespo et al., Reference Crespo, San-Miguel, Prause, Marroni, Cuevas, González-Gallego and Tuñón2012). When 2% glutamate was given to piglets weaned at 28 days of age, plasma superoxide dismutase and glutathione peroxidase activities were increased after being challenged with deoxynivalenol for 30 days (Wu et al., Reference Wu, Xiao, Ren, Yin, Hu, Duan, Liu, Tan, Xiong and Oso2014). Significantly increasing proliferating indexes and markedly decreasing caspase-3 activities in the jejunum and the ileum were observed in toxin-challenged piglets treated with 2% glutamate (Wu et al., Reference Wu, Xiao, Ren, Yin, Hu, Duan, Liu, Tan, Xiong and Oso2014). These results indicate that glutamine is beneficial to alleviate intestinal cell apoptosis.
Glutamine and intestinal cell endoplasmic reticulum stress
Glutamine (0.45 g/l) lessened endoplasmic reticulum stress in intestinal porcine epithelial cell line J2 (IPEC-J2) treated with tunicamycin, an endoplasmic reticulum stress inducer (Jiang et al., Reference Jiang, Chen, Liu, Liu, Yao and Yin2017). First, IPEC-J2 was exposed to tunicamycin and spontaneous endoplasmic reticulum stress was observed. It was exhibited by upregulation of the glucose-regulated protein 78 (GRP78). Second, prolonged tunicamycin treatment induced apoptosis mediated by CHOP, and this was accompanied by GRP78 downregulation (Jiang et al., Reference Jiang, Chen, Liu, Liu, Yao and Yin2017). Third, stressed cells that were treated with 0.45 g/l l-glutamine maintained a high level of GRP78 while CHOP-mediated apoptosis was alleviated. The inositol requiring enzyme1α (IRE1α)-XBP1 axis was also activated and cell proliferation promoted. Finally, a specific inhibitor of the IRE1α-XBP1 axis reversed the protective effect of l-glutamine by blocking the expression of IRE1α/XBP1s (Jiang et al., Reference Jiang, Chen, Liu, Liu, Yao and Yin2017). These findings indicate that l-glutamine facilitates intestinal health partly through modulating endoplasmic reticulum stress and CHOP-mediated apoptosis. These data give some insight into the mechanism by which glutamine repairs intestinal damage in weaned piglets.
Glutamine and intestinal cell autophagy
Glutamine affects autophagy and coordinates cell growth and proliferation in various cells, including cancer cells (Nicklin et al., Reference Nicklin, Bergman, Zhang, Triantafellow, Wang, Nyfeler, Yang, Hild, Kung and Wilson2009) and porcine intestinal cells (Zhu et al., Reference Zhu, Lin, Dai, Zhou, Li, Yuan, Wu, Wu and Wang2015). Glutamine deprivation in porcine intestinal epithelial cells (IPEC-1) increased the autophagy marker light chain 3B-II in a time-dependent manner and decreased cell growth number in a time-dependent manner (Zhu et al., Reference Zhu, Lin, Dai, Zhou, Li, Yuan, Wu, Wu and Wang2015). Glutamine supplementation at 5 mmol/l increased cell growth and cellular protein content and repressed IPEC-1 cell light chain 3B-II protein abundance (Zhu et al., Reference Zhu, Lin, Dai, Zhou, Li, Yuan, Wu, Wu and Wang2015). Glutamine deprivation reduced mammalian target of rapamycin (mTOR) activation and its downstream protein p70S6 kinase. It reduced mitogen-activated protein kinase (MAPK) phosphorylation and its downstream extracellular signal-related kinase (ERK) 1/2 protein (Zhu et al., Reference Zhu, Lin, Dai, Zhou, Li, Yuan, Wu, Wu and Wang2015) indicating that glutamine may suppresses cell autophagy by activating mTOR and MAPK signaling pathways. Nuclear factor kappa B (NF-κB) and nuclear factor erythroid 2-related factor 2 (Nrf2) are both known to act as transcription factors in stress adaptation and tissue protection (Ringseis et al., Reference Ringseis, Kynast, Couturier, Most and Eder2016). Weaning piglets exposed to hydrogen peroxide were found to form cell autophagosome in the jejunum (Yin et al., Reference Yin, Duan, Cui, Ren, Li and Yin2015a). Nuclear factor kappa B was triggered along with Nrf2, and Keap1, a Kelch-like ECH-associated 23 protein 1, signaling pathway (Yin et al., Reference Yin, Duan, Cui, Ren, Li and Yin2015a). When 6-week-old pigs were fed a diet with oxidized fat (rapeseed oil that had been heated to 175°C for 72 h) for 29 days, mRNA concentrations in Nrf2 target genes, including NAD(P)H dehydrogenase, quinone 1, peroxiredoxin 6, glutathione peroxidase 2 and thioredoxin reductase 1 in the duodenal mucosa, were upregulated (Ringseis et al., Reference Ringseis, Kynast, Couturier, Most and Eder2016). Protein concentrations of active or cleaved caspase-3 in the duodenal mucosa increased approximately 30% (Ringseis et al., Reference Ringseis, Kynast, Couturier, Most and Eder2016). The association between glutamine regulation of piglet small intestinal autophagy and Nrf2/Keap1 signal pathway remains unknown.
Glutamine and intestinal paracellular permeability
The intestinal epithelium provides several extrinsic and intrinsic defense mechanisms. One is the permeability barrier that is regulated predominantly by tight junctions (Turner, Reference Turner2009). The beneficial effect of glutamine on intestinal paracellular permeability has been well described in humans, animals (Wang et al., Reference Wang, Zhang, Wu, Sun, Wang, He, Dai and Wu2014) and various cell lines (Li and Neu, Reference Li and Neu2009; Wang et al., Reference Wang, Wu, Ji, Sun, Dai and Wu2016a). For example, glutamine depletion results in porcine villus atrophy (Wu et al., Reference Wu, Meier and Knabe1996). Intestinal permeability was increased by 40% by weaning (Wang et al., Reference Wang, Zhang, Wu, Sun, Wang, He, Dai and Wu2014), and this was accompanied by villus atrophy and reduced jejunal protein expression, including occludin, claudin-1, zonula occludens-2 (ZO-2) and ZO-3. Dietary 1% l-glutamine supplementation attenuated the weaning stress–induced mucosal barrier dysfunctions although it did not return growth performance, villus morphology, tight junction protein expression (occludin, claudin-1, zonula occludens-2) to levels exhibited by sow-reared piglets (Wang et al., Reference Wang, Zhang, Wu, Sun, Wang, He, Dai and Wu2014). The underlying mechanism for glutamine’s regulatory effect on paracellular permeability may involve multiple signal pathways. Glutamine at 4.0 mmol/l may regulate claudin-expression via the PI3K/AKT pathway in Caco-2 cells (Li and Neu, Reference Li and Neu2009). Glutamine at 2.0 mmol/l was found to regulate claudin-1, claudin-4 and ZO-1 distributions through activated phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) but via CaMKK2-AMPK, calmodulin-dependent kinase 2 to AMP-activated protein kinase, in IPEC-1 cells (Wang et al., Reference Wang, Wu, Ji, Sun, Dai and Wu2016a). One recent study observed that 2.0 mmol/l glutamine prevented EGF receptor degradation and activated EGF receptor tyrosine kinase in intestinal epithelial-6 cells. Glutamine-mediated EGF receptor signaling activated ERK1/2 and inactivated p38MAPK in the heat-stressed cells (Niederlechner et al., Reference Niederlechner, Baird, Petrie, Wischmeyer and Wischmeyer2013). These results suggest that glutamine is an important metabolic checkpoint and may have distinct regulating pathways in different cells. The type of glutamine being added may influence outcomes. When Caco-2 cells are treated with acetaldehyde (600 µmol), there is a time-dependent decrease in transepithelial electrical resistance and an increase in inulin permeability. However, supplementation with l-glutamine (2 mmol) effectively prevented the acetaldehyde-induced changes in transepithelial electrical resistance and inulin permeability. Interestingly, d-Glutamine (2 mmol) or a glutaminase inhibitor by themselves did not influence transepithelial electrical resistance or inulin flux in control or acetaldehyde-treated Caco-2 cell monolayers (Seth et al., Reference Seth, Basuroy, Sheth and Rao2004). Glutaminase is responsible for catalysing glutamine into glutamate and ammonia suggesting that l-glutamine rather than d-glutamine or glutamate protects the barrier function in Caco-2 cell monolayers from acetaldehyde-induced injury.
Conclusions
Despite glutamine not being considered essential in the traditional sense, there is growing evidence that glutamine supplementation alleviates the negative effects induced by weaning. A healthy gut is extremely important for animal growth and development. Glutamine is a functional amino acid involved in many metabolic processes that are especially important for small intestinal health and development. Our review addressed the mechanisms involved and the physiological significance for dietary glutamine in small intestine of weaning pigs. This nutrient is necessary to support maximum growth by reducing weaning piglet intestinal oxidative stress, preventing intestinal epithelium damage and improving normal barrier function.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31330075, No. 31402089, No. 31640078, No. 31872991), the Key Programs of Frontier Scientific Research of the Chinese Academy of Sciences (QYZDY-SSW-SMC008), the Key Project of Hunan Provincial Education Department (16A096) and the Innovation Platform and Talents Program of Hunan Provincial Science and Technology Department (2018RS3105) and Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences (No. 1630032019022).
H. S. Yang 0000-0003-1164-5771
Declaration of interest
None.
Ethics statement
All ethical standards have been met.
Software and data repository resources
None.





