Introduction
The Clinical and Laboratory Standards Institute (CLSI) recently revised the long-held aminoglycoside breakpoints for Enterobacterales and Pseudomonas aeruginosa in 2023. 1–Reference Humphries3 While aminoglycoside monotherapy is not typical nor recommended for definitive therapy of multidrug-resistant (MDR) gram-negative bacilli (GNB), they have importance as a component of empiric regimens. Reference Humphries3 A recent study from the United States (US) demonstrated that while 94.6% of Enterobacterales remained susceptible to amikacin after the recent breakpoint change, isolates expressing an extended spectrum β-lactamase (ESBL) were significantly impacted, with susceptibility declining from 96.9% to 79.7%. Reference Sader, Mendes, Kimbrough, Kantro and Castanheira4 Comparably large decreases in amikacin susceptibility were seen among carbapenem-resistant Enterobacterales. Reference Sader, Mendes, Kimbrough, Kantro and Castanheira4 Another US institution reported a significant drop in susceptibility to aminoglycosides within GNB, though this was mostly driven by the removal of gentamicin breakpoints and restricting amikacin reporting to urine specimens only for P. aeruginosa. Reference Wangchinda, Aitken, Pierce, Lephart and Pogue5 The impact of the recent breakpoint changes on Canadian institutions remains unexplored.
Aminoglycoside use has decreased in Canada since the early 2000s Reference Liang, Rudnick, Mitchell, Brooks, Bush, Conly, Ellison, Frenette, Johnston, Lavallée and McGeer6 in favor of less nephrotoxic agents including third generation cephalosporins and carbapenems, though the increasing incidence of CPE 7 may lead to increased reliance on aminoglycosides. Despite the growing threat of antibiotic resistance, Canada trails other high-income countries in bringing novel antibiotics to market,8,9 increasing reliance on existing antibiotic options, including many listed in the current IDSA guidance on the treatment of various resistant GNB.10 Here we summarize aminoglycoside susceptibility and the impact of recent breakpoint changes for Enterobacterales and P. aeruginosa from various specimen sources and resistant subsets of isolates identified at a large Canadian academic teaching hospital.
Methods
Retrospective susceptibility testing data from all clinical samples at a large hospital laboratory in Toronto, Ontario, Canada over a one-year period was collected (January 1, 2022 – Dec 31, 2022). The top ten most common Enterobacterales species (ordered by frequency: Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae complex, Proteus mirabilis, Klebsiella oxytoca, Serratia marcescens, Citrobacter koseri, Enterobacter aerogenes, Citrobacter freundii complex, and Morganella morganii) and P. aeruginosa were selected for analysis. Respiratory specimens from cystic fibrosis patients and isolates with incomplete susceptibility data for routinely tested antibiotics were excluded. Interpretations of susceptibility data were defined as follows: MIC values reported by the reference laboratory were viewed as the highest quality of evidence, followed by data from a disk diffusion interpretation, and then by MIC values from the automated susceptibility testing system (Vitek II [Biomerieux]). For this study, the highest quality available evidence was used as the final interpretation based on the CLSI M100E32 1 and for reinterpretation with the CLSI M100E33 breakpoints. 2 Only the first isolate of a given species per patient per year was included for analysis. If more than one isolate of a given species was isolated on the same day for a patient, if the susceptibility pattern was distinct between the isolates, both isolates were included.
Organisms expressing an AmpC or Class A ESBL β-lactamase were identified based on the results of the Kirby–Bauer double-disk diffusion test according to the Institute for Quality Management in Healthcare (IQMH) guidelines. Carbapenemase-producing Enterobacterales (CPE) were identified using either the Rapid Carb screen test (Rosco Diagnostica A/S) with confirmation at the reference Public Health Ontario Laboratory or the NG-Test CARBA 5 (Hardy Diagnostics). The definition of MDR P. aeruginosa was based on the IDSA definitions and the available data in our center. Reference Tamma, Aitken, Bonomo, Mathers, van Duin and Clancy10 MDR was defined as P. aeruginosa not susceptible to at least three of the following: piperacillin-tazobactam, ceftazidime, ciprofloxacin, tobramycin, and meropenem based on the 2023 breakpoints. Data was analyzed using Excel version 2310 and R version 4.1.3. Statistical significance (p-value <0.05) was assessed using the McNemar Test for comparing susceptibility using the 2022 and 2023 interpretations.
Results
Susceptibility to aminoglycosides amikacin, gentamicin, and tobramycin are summarized for Enterobacterales (n = 2713) isolates in Table 1 and P. aeruginosa isolates (n = 442) in Table 2. Enterobacterales organisms remained highly susceptible to aminoglycosides, with small shifts in susceptibility (−0.8%, −0.2%, and −1.6% to amikacin, gentamicin, and tobramycin respectively). Resistance to gentamicin and tobramycin amongst ESBLs and CPEs was significant before and after the breakpoint changes. Amikacin remained the most active aminoglycoside against ESBLs and CPE (93.1% and 50% of isolates susceptible, respectively), though susceptibility of ESBLs to amikacin decreased (−6%, P < 0.001) due to the breakpoint change.
Table 1. Percent of amikacin, gentamicin, and tobramycin susceptible Enterobacterales isolates based on the 2022 and 2023 breakpoints, as well as the absolute change in susceptibility and the significance of that change (P ≤ 0.05, McNemar Test). Organisms listed as AmpC, ESBL, AmpC and ESBL, and CPE are classed based on their phenotypic expression or genotypic evidence of a β-lactamase. ESBL; Extended spectrum beta-lactamase Class A. CPE; Carbapenemase-producing Enterobacterales
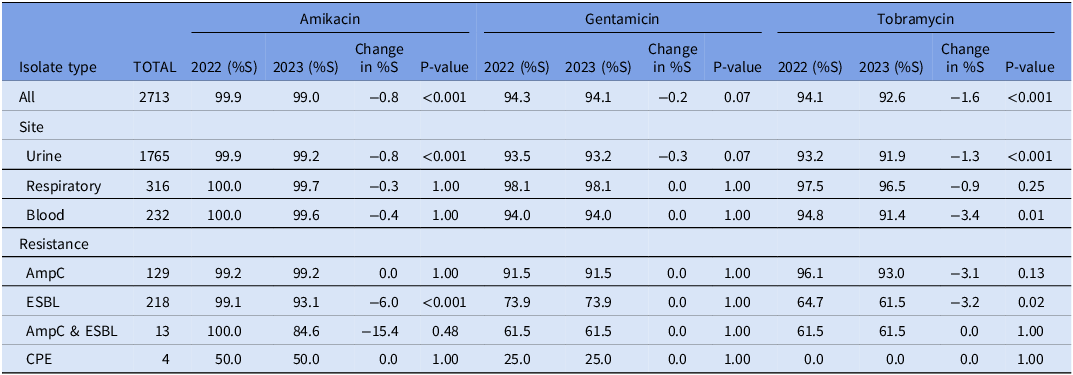
Table 2. Percent of amikacin, gentamicin, and tobramycin susceptible P. aeruginosa isolates based on the 2022 and 2023 breakpoints, as well as the absolute change in susceptibility and the significance of that change (P ≤ 0.05, McNemar Test). MDR; Multidrug-resistant

*This result reflects the removal of the breakpoint for amikacin for non-urine isolates or the removal of the breakpoint for gentamicin altogether for P. aeruginosa in 2023.
Non-susceptible organisms included those that were intermediate, resistant, or non-susceptible based on the breakpoint changes, hence the susceptibility of P. aeruginosa to gentamicin based on the 2023 breakpoints is 0% for all isolates (Table 2). Tobramycin remained highly active against P. aeruginosa isolates in aggregate, though the percentage of susceptible isolates identified in respiratory specimens decreased by 5% after the breakpoint change. MDR P. aeruginosa susceptibility to aminoglycosides shifted with the breakpoint change, though this was statistically insignificant.
Discussion
In contrast to recent data from the US, Reference Sader, Mendes, Kimbrough, Kantro and Castanheira4 our institution did not observe large decreases in the proportion of Enterobacterales susceptible to aminoglycosides when applying the recent CLSI breakpoint changes. Our data suggests comparatively fewer isolates were close to the breakpoint values. One reason for this observation may be that Canadian hospitals use aminoglycosides infrequently compared to other centers, though we could not identify directly comparable use data between the US and Canada. Although we had low numbers of CPE, our findings corroborate the significant aminoglycoside resistance within CPEs documented by the Canadian Nosocomial Infection Surveillance Program (CNISP). 7 This is concerning in the context of a threefold rise in healthcare-associated CPE colonization in Canada between 2015 and 2019. 7 Of concern, Canada trails in bringing novel antibiotics to market (many which are now preferentially recommended by IDSA guidelines for treating MDR GNB), Reference Tamma, Aitken, Bonomo, Mathers, van Duin and Clancy10 due to relatively low market-share compared to other countries, and limited incentives to entice companies to undertake the Canadian regulatory approval process.8,9
Aminoglycoside susceptibility among P. aeruginosa was altered when applying the 2023 breakpoints, though tobramycin remained active against 94.8% of P. aeruginosa isolates. Encouragingly, the number of MDR P. aeruginosa we identified was small, though susceptibility to aminoglycosides within this subgroup was low. While tobramycin is often used as dual coverage in empiric regimens for patients likely to be harboring resistant strains, our data, though underpowered, suggests resistant subgroups of P. aeruginosa isolates are not well covered by this agent. Additional research with a greater sample size of MDR isolates is needed to make definitive conclusions. Our definition of MDR was modified slightly from the IDSA guidelines based on the agents that are routinely tested and reported at Canadian institutions. Reference Tamma, Aitken, Bonomo, Mathers, van Duin and Clancy10 Of note, aminoglycoside breakpoints are not aligned across breakpoint setting organizations which surely impacts our results and should be considered when comparing data from other regions not using the CLSI breakpoints. USCAST breakpoints are set at lower MICs than CLSI, while EUCAST is similar to CLSI, though divergences in these documents exist.
Conclusion
Recent aminoglycoside breakpoint changes impacted ESBL-producing Enterobacterales organisms, though these agents remained active against most isolates. Our data, although not robust in CPE or MDR P. aeruginosa representation, demonstrates that these subgroups are not well covered by aminoglycosides, supporting the ongoing concern that patients may be at risk of harm in the future if development or commercialization of new antimicrobials stalls. Clinical availability of novel antimicrobials remains problematic in countries where access limitations are observed, 9 which likely influences the high mortality rate associated with CPE infections in Canada. 7 Future research should address susceptibility to novel antimicrobials within resistant GNBs to ensure that the introduction of novel antimicrobials in Canada addresses current gaps in available treatment options.
Acknowledgements
None.
Author contribution
EL and LM conceived the study.
EA, EL, and TL collected and cleaned data.
EA, KB, LM, and EL performed data analysis.
EA, EL, and LM drafted the manuscript.
EA, LM, KB, TL, and EL have read, contributed to, and approved of the final draft.
Financial support
None.
Competing interests
None.




