Introduction
Pseudomonas aeruginosa can be responsible of life-threatening diseases, as a consequence of urinary, bone, respiratory, abdominal, and disseminated infections. Reference Pop-Vicas and Opal1 It colonizes human body, being part of the human microbiota (especially in the respiratory tract), and it can also be acquired from exogenous sources. Reference Bertrand, Thouverez and Talon2 Patients hospitalized in intensive care unit (ICU) are at risk of contamination. Potential exogenous sources are tap-water and fomites while patient-to-patient transmission is possible but it can be limited by standard precautions. Reference Rogues, Boulestreau and Lasheras3 Antibiotic pressure is the most relevant factor for P. aeruginosa acquisition in ICU. Reference Boyer, Doussau and Thiébault4
At each antibiotic treatment, germs of the human microbiota are exposed to sub-lethal levels of antibiotics. This event enhances the selection of antibiotic resistance genes, which often are host in transferable plasmids. Reference Gullberg, Albrecht, Karlsson, Sandegren and Andersson5 Otherwise, the antibiotic pressure can trigger chromosomal mutations and transfer of resistance determinants, as is typical for P. aeruginosa. Reference Oliver, Mulet, López-Causapé and Juan6 Because of the risk of antibiotic resistance, the use of broad-spectrum molecules is currently discouraged as antibiotic prophylaxis and treatment. Reference Ioannou, Karakonstantis and Schouten7 The benefits in terms of antimicrobial susceptibility resulting from the reduced consumption of broad-spectrum molecules in ICU was largely demonstrated for gram-negative bacilli. Reference Onorato, Macera and Calò8 However, the results of antimicrobial stewardship programs (ASPs) in ICU concerning P. aeruginosa are often deceiving with no significant reduction of antimicrobial resistance rates. Reference Abbara, Domenech de Cellès and Batista9–Reference Slain, Sarwari and Petros11
At the end of 2010, an ASP started at the ICU of the Melun General Hospital, a 350-bed tertiary care hospital in the Ile-de-France region in France, its ICU accounting for a total of 24 beds. The main objective of the ASP was to restrain the consumption of broad-spectrum molecules (carbapenems, fluoroquinolones (FLQ), and third-generation cephalosporins (3-GC)). This objective was fully achieved. Indeed, a reduction of 50%–85% in the consumption of carbapenems, FLQ, and 3-GC was observed in the following 4-year period (from 2011 to 2014) when compared to the previous one (from 2007 to 2010). Contemporarily, a reduction of AmpC hyperproducing group 3 Enterobacteriaceae, FLQ resistant, and ceftazidime resistant P. aeruginosa was observed. Reference Abbara, Pitsch and Jochmans12
This study pursues the previous one through a P. aeruginosa targeted analysis. It investigates the factors associated with isolation of P. aeruginosa from ICU patients before and after the beginning of the ASP, with a special focus on the risk of antibiotic-resistance and the benefits produced by the ASP in terms of antibiotic susceptibility recovering.
Materials and methods
A monocentric retrospective cohort study was conducted at Melun General Hospital, a 350 tertiary bed hospital in Melun (France). Patients were hospitalized in ICU, accounting for 24 beds. All adult patients admitted to the ICU presenting P. aeruginosa isolates during their hospitalization in ICU from January the 1st, 2007 to December 31st, 2014 were included. Two timeframes were analyzed: (1) before the beginning of the ASP (2007–2010); (2) after the beginning of the ASP (2011–2014).
The previous study by Abbara et al already evaluated the susceptibility of all P. aeruginosa isolated from any site in patients hospitalized in ICU during the same study period. It focused on resistance to single molecules and did not explored the factors associated with P. aeruginosa isolation. Reference Abbara, Pitsch and Jochmans12 For this study, we revised all ICU patient’s files and selected patients with P. aeruginosa isolation from any site during ICU stay and up to 7 days after ICU discharge to explore the possible late impact of ICU stay on P. aeruginosa selection. We included all P. aeruginosa isolates, both infections and colonizations, while in the previous study only isolates of clinical significance were included. We also added analysis about multidrug resistant (MDR) P. aeruginosa. The characteristics of patients with P. aeruginosa isolation were compared to ICU population and an analysis before/after intervention was performed.
The study was conducted in accordance with Declaration of Helsinki and national and institutional standards. Reference Deplanque, Sénéchal-Cohen and Lemaire13
Data were obtained through the revision of patients’ files which were collected in software used in daily clinical practice (Sillage v17 and CGM Lab channel 1.20.33686). Microbiological identifications and susceptibility tests were performed according to recommendations of the European committee on antimicrobial susceptibility testing. 14 The following outcomes were considered: (1) acquisition MDR bacteria; (2) length of ICU stay; (3) length of hospital stay; (4) in-ICU death; and (5) in-hospital death.
Fisher’s exact test (qualitative variables) and Student’s t-test (quantitative variables) were applied for the univariate analysis. At first, characteristics of P. aeruginosa patients were compared to overall ICU population. Then, clinical and microbiological characteristics of P. aeruginosa patients before (2007–2010) and after (2011–2014) the beginning of the AMP were compared. Analysis according to the origin of the infection (community acquired vs hospital acquired) was also performed. Logistic regression analysis was performed for multivariate analysis. For the multivariate analysis of risk factors of FLQ resistance the parameters included in the analysis were chosen according to univariate analysis results (P ≤ .0001). For the analysis of the risk of MDR P. aeruginosa only the exposition to any class of antibiotics was considered. Statistical significance was set at P < .050.
Results
Overall, 5,263 patients were admitted to the ICU during the study period (2007–2014), 274/5263 (5%) having at least a P. aeruginosa isolate during hospitalization in ICU and up to 7 days after ICU discharge. The percentage of patients with P. aeruginosa isolates reduced significantly before and after the ASP (7% in 2007–2010 vs 4% in 2011–2014, P ≤ .0001). Patients with P. aeruginosa had longer hospital stays and higher rates of in-hospital and in-ICU death (P < .0001) than overall ICU population. They received endotracheal intubation more frequently than patients without P. aeruginosa isolates (P < .0001). Table 1 resumes characteristics of the study population.
Table 1. Characteristics of the population
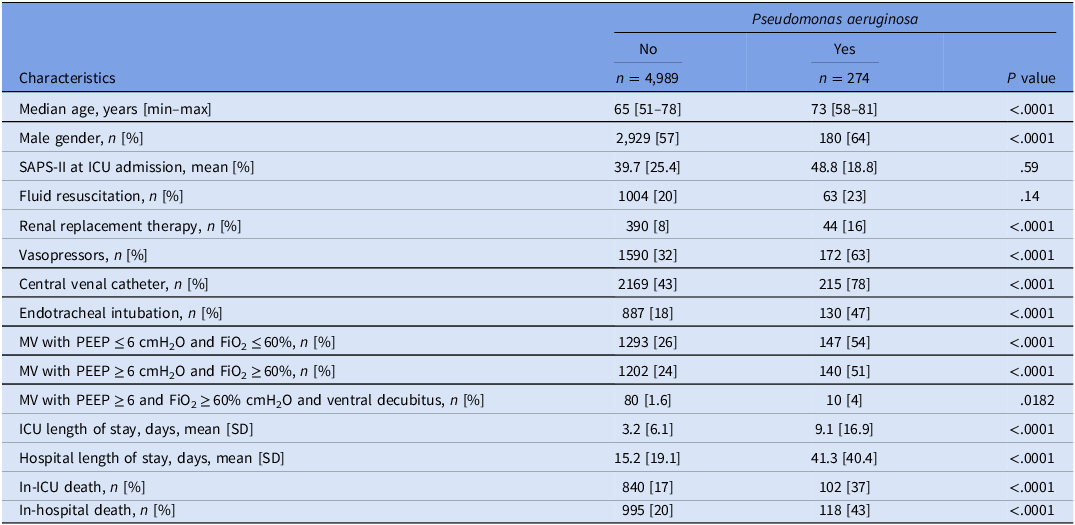
Note. CI, confidence interval; ICU, intensive care unit; FiO2, fraction of inspired oxygen; MV, mechanical ventilation; PEEP, positive end-expiratory pressure; RR, related ratio; SAPS-II, simplified acute physiology score-II; SD, standard deviation.
In 2011–2014, rates of multidrug-resistant, fluoroquinolone-resistant, and ceftazidime-resistant P. aeruginosa reduced significantly (P = .0020, P < .0001 and P = .0009, respectively). Rates of antibiotic treatments by FLQ, carbapenems, and 3-GC before P. aeruginosa isolation reduced significantly in 2011–2014 (P ≤ .0002). Simultaneously, use of piperacillin (without tazobactam) and trimethoprim-sulfamethoxazole (TMP-SMX) increased (P < .0001 and P = .0023, respectively). P. aeruginosa patients received endotracheal intubation and mechanical ventilation less frequently in 2011–2014 than 2007–2010. Also, the length of hospital and ICU stay decreased during the same period (P < 0.0001 and P = 0.08, respectively). Table 2 shows characteristics of the population with P. aeruginosa isolates.
Table 2. Characteristics of patients with Pseudomonas aeruginosa isolates
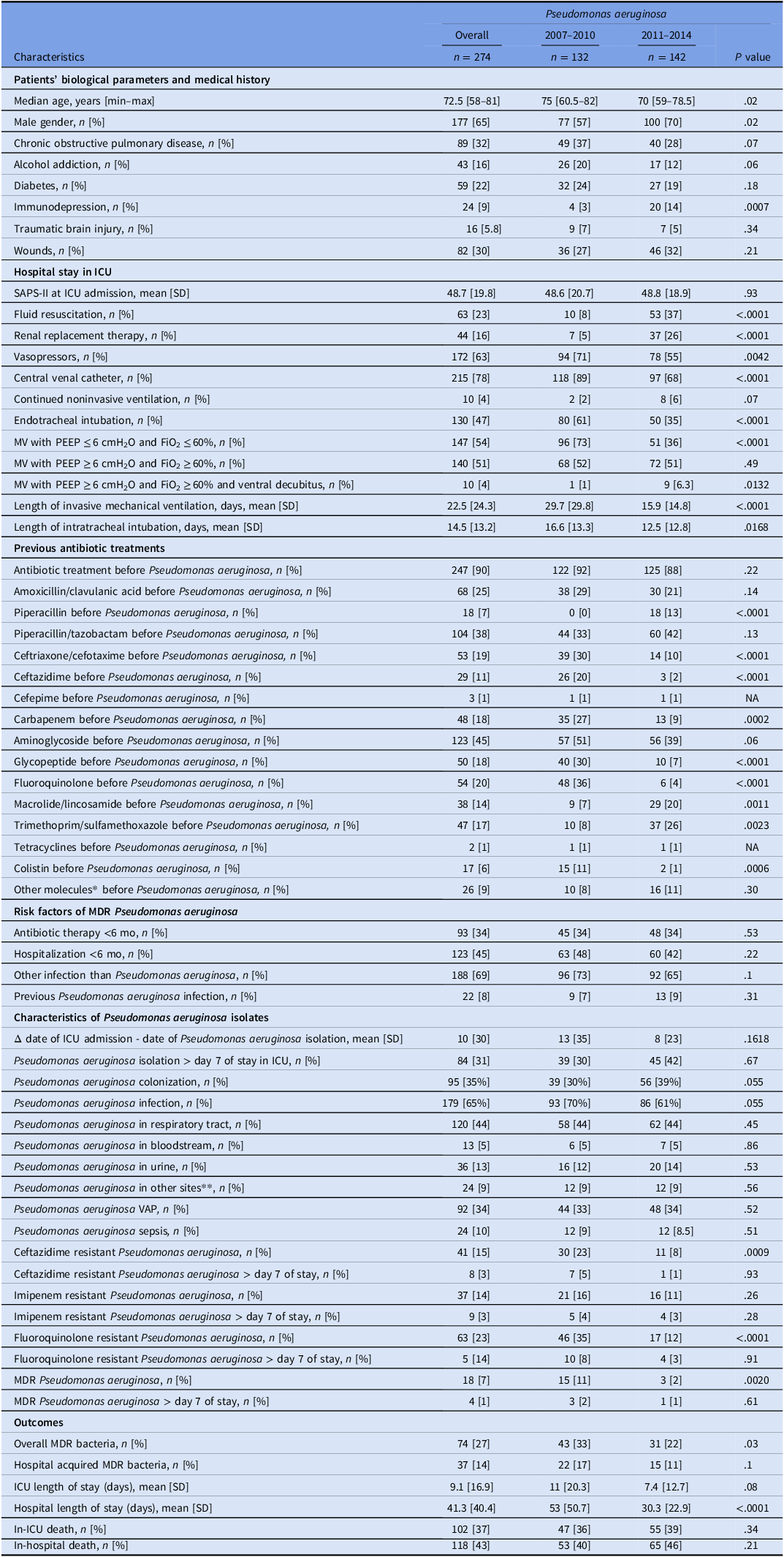
Note. *including: linezolid, fosfomycin, daptomycin, rifampicin; **including: purulent lesions, cutaneous biopsies, vascular catheter, bone biopsies, coprocultures and peritoneal fluids; CI, confidence interval; ICU, intensive care unit; FiO2, fraction of inspired oxygen; MDR, multidrug resistant; MV, mechanical ventilation; NA, not applicable; PEEP, positive end-expiratory pressure; RR, related ratio; SAPS-II, simplified acute physiology score-II; SD, standard deviation; VAP, ventilator associated pneumonia.
Patients with hospital acquired P. aeruginosa had higher rates of endotracheal intubation (P < .0001), central venous catheter (P = .0316), and previous FLQ treatment (P = .0059) than patients with community acquired P. aeruginosa. Sepsis was more frequent (P = .0063) among patient with community acquired P. aeruginosa than patients with hospital acquired P. aeruginosa (Table 3).
Table 3. Characteristics of patients with Pseudomonas aeruginosa isolates
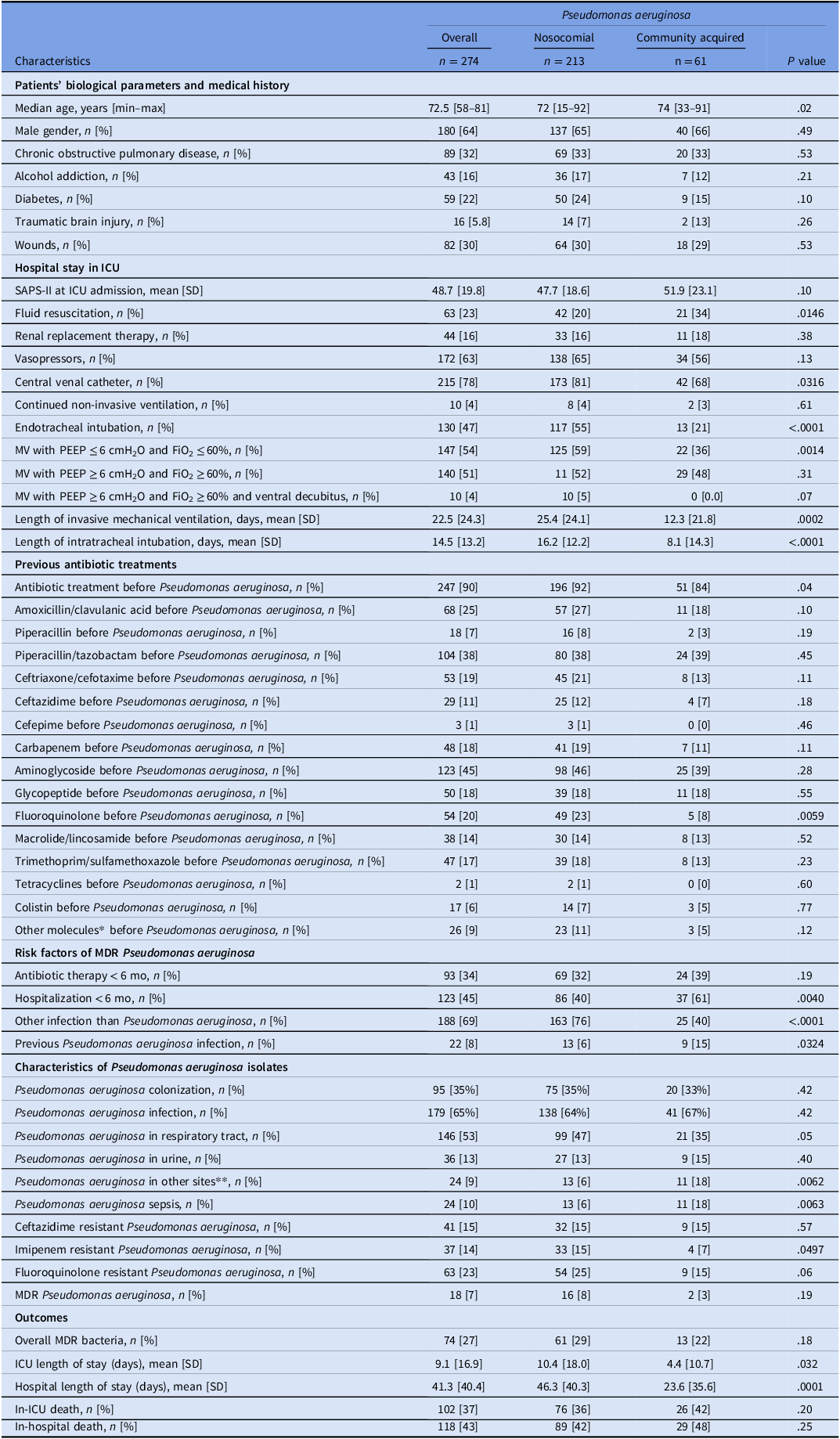
Note. *including linezolid, fosfomycin, daptomycin, rifampicin; **including purulent lesions, cutaneous biopsies, vascular catheter, bone biopsies, coprocultures and peritoneal fluids; CI, confidence interval; ICU, intensive care unit; FiO2, fraction of inspired oxygen; MDR, multidrug resistant; MV, mechanical ventilation; NA, not applicable; PEEP, positive end-expiratory pressure; RR, related ratio; SAPS-II, simplified acute physiology score-II; SD, standard deviation; VAP, ventilator associated pneumonia.
The univariate analysis showed that patients who received endotracheal intubation had higher rates of fluoroquinolone resistant P. aeruginosa isolation (P = .0197; Table 4). At the multivariate analysis, the factors associated with fluoroquinolone-resistance were study period (2007–2010) and previous treatment by fluoroquinolones (P = 0.0020 and P = 0.0462, respectively), as shown in Table 5. No previous use of any class of antibiotics was associated with the risk of MDR P. aeruginosa (Table 6).
Table 4. Resistance to fluoroquinolones among Pseudomonas aeruginosa isolates from patients receiving endotracheal intubation

Table 5. Multivariate analysis of factors associated with fluoroquinolone resistance in Pseudomonas aeruginosa isolates
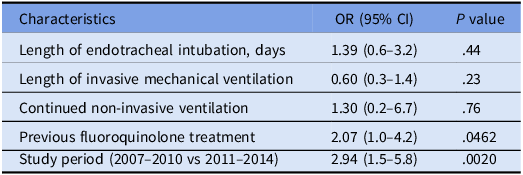
Table 6. Multivariate analysis of factors associated with presence of multi drug resistant Pseudomonas aeruginosa isolates
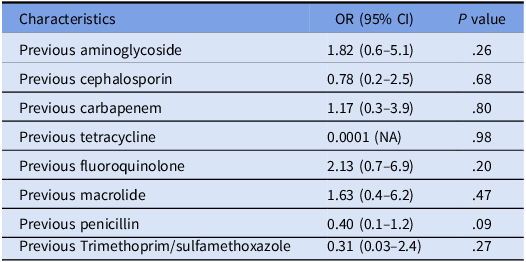
Note. NA, not applicable.
Discussion
This study showed that the frequency of P. aeruginosa infection in ICU reduced after the beginning of an ASP, principally based on saving of broad-spectrum antibiotics. The antimicrobial susceptibility of P. aeruginosa recovered after the reduction of 3-GC and fluoroquinolone consumption and the increased prescription of alternative “old” molecules, such as piperacillin and trimethoprim-sulfamethoxazole. Endotracheal intubation was associated with FLQ resistance.
Although ASP are strongly recommended, reducing antibiotic consumption in ICU is extremely difficult because patients’ uncertain diagnosis and compromised hemodynamic state push prescribers keeping long-course broad-spectrum antibiotic treatments. A consequence of this attitude is an increased risk of P. aeruginosa infection which occurs almost exclusively in patients who received a previous antibiotic treatment. Reference Pickens and Wunderink15 In fact, broad-spectrum antibiotics interact with the environment and facilitate P. aeruginosa acquisition. Reference Boyer, Doussau and Thiébault4 Our ASP succeeded in reducing both P. aeruginosa antibiotic resistance and infection incidence. One of the key of this success was the increased use of molecules with low ecological footprint to treat infections others than P. aeruginosa.
The use of FLQ as empirical treatment for P. aeruginosa is inadvisable because of the risk of treatment failure and the increased mortality due to the development of antibiotic resistance. Reference Nguyen, Hsu, Ganapathy, Shriner and Wong-Beringer16 Indeed, P. aeruginosa has many mechanisms of resistance, frequently based on GyrA, ParC, and MexR enzymes. Reference Nguyen, Nguyen, Nguyen and Le17 In ICU setting, an important virulence and resistance factor is biofilm which contributes reducing susceptibility to antimicrobial molecules and host-immune factors. Reference Das, Sehar and Manefield18 This effect disappears when bacteria are deprived of the capacity of producing the extracellular matrix made by polysaccharides, proteins, and metabolites. Reference Walters, Frank, Amandine, Franklin and Stewart19 As a consequence, biofilm reduces antibiotic efficacy by several mechanisms: reduction of antibiotic penetration, microenvironment modifications, and increased inflammatory response. Reference Pang, Raudonis, Glick, Lin and Cheng20 FLQ activity is largely limited by biofilm production. Reference Cepas, López and Muñoz21 In ICU, the main sources of biofilm producing P. aeruginosa are invasive devices. Reference Boyer, Doussau and Thiébault4 Moreover, endotracheal intubation is the most relevant determinant of P. aeruginosa acquisition and ventilator-associated pneumonia (VAP). Reference Coppadoro, Bellani and Foti22 The restriction of endotracheal intubation was adopted in our ICU to reduce respiratory infection rate. It was obtained through new standard of care, such as protocol-based sedation, favoring noninvasive ventilation over invasive ventilation whenever possible, and improved ventilation weaning process in mechanical ventilation. No change in devices and patient admission policy was adopted during the study period. Results of this study are in line with other studies which showed that restriction of endotracheal intubation was associated with reduction of mortality and MDR bacterial infection. Reference Villemure-Poliquin, Costerousse and Lessard Bonaventure23,Reference Wang, Li and Dai24 This study showed that previous FLQ treatment was associated with FLQ resistance in P. aeruginosa strains afterward isolated. It also showed that patients who received endotracheal intubation had higher rates of FLQ resistance. We can hypothesize that the reduction of endotracheal intubation observed from 2011 to 2014 could have contributed in reducing rates of FLQ resistant P. aeruginosa. This study advocates against the use of FLQ in intubated patients because of the increased risk of FLQ resistance in P. aeruginosa strains.
FLQ are frequently prescribed for atypical pneumonia and intracellular bacterial infections but their collateral damages in term of selection of MDR bacterial impose their limitation as empirical antibiotic treatment. For this reason, our ASP suggested macrolides as alternative molecules. Reference Abbara, Pitsch and Jochmans12 Indeed, macrolides can be preferred to FLQ in many situations. At first, macrolides are not inferior to FLQ for the treatment of Legionella pneumonia. Reference Jasper, Musuuza and Tischendorf25 Second, the treatment of severe community acquired pneumonia with beta-lactam plus macrolides resulted more effective than treatment with FLQ alone in reducing mortality and length of hospitalization in ICU. Reference Ito, Ishida and Tachibana26 Third, because of their immunomodulatory effects, macrolides are an interesting alternative for the treatment of low respiratory tract infections in patients affected by chronic respiratory diseases. Reference Pollock and Chalmers27 In this study, macrolides contributed to reduce FLQ prescriptions.
Piperacillin is a broad-spectrum beta-lactam. It is active against gram-positive bacteria and it shows high activity against gram-negative bacilli, both aerobic and anaerobic (Klebsiella pneumoniae, Serratia marcescens, and P. aeruginosa). Reference Drusano, Schimpff and Hewitt28 It is hydrolyzed by beta-lactamases (as TEM-1) and, therefore, it is almost always administrated in association with tazobactam, a beta-lactamase inhibitor which successfully restores the activity of piperacillin against many beta-lactamases. Reference Bryson and Brogden29 However, P. aeruginosa may rapidly develop resistance to tazobactam by the production of extended spectrum beta-lactamases and AmpC beta-lactamases. Reference Karaiskos and Giamarellou30 The use of piperacillin “alone” without the adding of tazobactam for documented infection caused by gram-negative bacteria was adopted in our ICU with the rationale of sparing tazobactam and, therefore, reducing the antimicrobial selective pressure on targeted pathogens, bacteria of the human microbiota and invasive device’s contaminants. Results of this study suggest that this strategy could have contributed in reducing rates of MDR and ceftazidime resistant P. aeruginosa strains. Further studies are needed to confirm this hypothesis.
TMP-SMX represents an alternative to FLQ and beta-lactams for the treatment of infection by gram-negative (Enterobacteriaceae) and gram-positive (Staphylococcus aureus) bacteria, although it is not active against P. aeruginosa. In France, it is currently the first choice for treatment of documented urinary infection according to French national recommendations. 31 In our establishment, TMP-SFX is successfully used for the treatment of VAP by bacteria other than P. aeruginosa. Reference Strazzulla, Postorino and Youbong32 According to our ASP, TMP-SMX was preferred to FLQ and beta-lactams whenever the antimicrobial susceptibility test confirmed the sensibility to TMP-SMX. Aim of this choice was to reduce antibiotic “collateral damages” and in particular the selection of MDR bacteria. The reduction of P. aeruginosa’s resistance rates to FLQ and beta-lactams was likely influenced by the reduced consumption of broad-spectrum molecules (FLQ and 3-GC) and their replacement by molecules with a lower ecological footprint, such as TMP-SMX and piperacillin.
Results of this study were limited by its retrospective design. Indeed, a loss of data was expected and direct comparison between molecules were not possible. Also, a longer period analysis was necessary to confirm the positive results of the ASP. Because of the study design, the number of variables was limited and many factors potentially associated with P. aeruginosa isolation were not investigated. Notwithstanding, results of this study are encouraging and justify the pursue of exploration by further studies. In particular, factors associated with P. aeruginosa isolates occurring in patients hospitalized in non-ICU units needs to be explored. Also, risk factors of P. aeruginosa VAP need to be investigated. A direct comparison between different molecules necessitates to be performed.
Conclusions
The antibiotic stewardship program implemented in our institution achieved in reducing rates of antibiotic resistance in P. aeruginosa isolates obtained from ICU patients. Among the factors investigated by this study, the decreasing consumption of 3-GC and FLQ and the increased use of TMP-SMX and piperacillin contributed in achieving this result. Also, the decreasing use of endotracheal intubation was observed and likely participate in reducing rates of P. aeruginosa isolation. Further studies are needed to verify the effectiveness of this strategy in other settings.
Acknowledgments
None.
Author contribution
AS drafted the work; VA gave substantial contribution in data acquisition, RH revised the draft, SD contributed in interpretation of data; OB contributed in interpretation of data; SA contributed in interpretation of data; SH contributed in interpretation of data; MM gave substantial contributions to conception or design of the work; SD gave.
Financial support
This study did not receive any funding from public or private entities.
Competing interests
All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.








