The first coronavirus disease 2019 (COVID-19) vaccine was authorized for emergency use by the US Food and Drug Administration on December 11, 2020. Reference Polack, Thomas and Kitchin1 Over the past several months, research studies have yielded substantial data on short-term (≤ 3 months) vaccine effectiveness Reference Dagan, Barda and Kepten2–Reference Marra, Kobayashi and Suzuki4 against symptomatic COVID-19. For example, the short-term vaccine effectiveness is known to be very high at 95% for the Pfizer/BioNTech COVID-19 vaccine, 94.1% for the Moderna vaccine, 70.4% for the AstraZeneca vaccine, and 66.3% for the Janssen COVID-19 vaccine. Reference Polack, Thomas and Kitchin1,Reference Voysey, Clemens and Madhi5–Reference Sadoff, Gray and Vandebosch7
In the third year of the pandemic, individuals are still at risk of acquiring COVID-19 even with vaccines available. Reference Brown, Vostok and Johnson8,Reference Glatman-Freedman, Hershkovitz, Kaufman, Dichtiar, Keinan-Boker and Bromberg9 Infection and hospitalization rates among unvaccinated individuals are 5 times and 11–29 times higher than those in vaccinated individuals, respectively. Reference Griffin, Haddix and Danza10,11 Also, the authorized COVID-19 vaccines protect against the δ variant, Reference Lopez Bernal, Andrews and Gower12 even with increased community transmission. Reference Griffin, Haddix and Danza10,Reference Lopez Bernal, Andrews and Gower13
Although these vaccines are effective for a wide range of COVID-19–related outcomes, Reference Polack, Thomas and Kitchin1,Reference Baden, El Sahly and Essink6,Reference Tenforde, Patel and Ginde14,Reference Pilishvili, Gierke and Fleming-Dutra15 the duration of the immune protection following the COVID-19 vaccination is still not well defined, Reference Rossi, Lanuti and Cicalini16–Reference Bayart, Douxfils and Gillot18 and few studies have assessed the long-term vaccine effectiveness of COVID-19 vaccines.
We reviewed the literature on the long-term vaccine effectiveness of COVID-19 vaccines for COVID-19 and COVID-19 hospitalizations. Pooling the results of published studies allows for more precise estimates of the long-term vaccine effectiveness. The information provided from subset analyses during the δ variant period is significantly important given the ongoing pandemic with this variant.
Methods
Systematic literature review and inclusion and exclusion criteria
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement Reference Moher, Liberati, Tetzlaff and Altman19 and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines, Reference Stroup, Berlin and Morton20 and it was registered on Prospero (https://www.crd.york.ac.uk/PROSPERO/) on September 13, 2021 (registration no. CRD42021278162). The approval of our institutional review board was not required.
The inclusion criteria for studies in this systematic review were as follows: original research manuscripts; published in peer-reviewed scientific journals; involved vaccinated and unvaccinated individuals; evaluated the long-term effectiveness of COVID-19 vaccine; and observational study design. Long-term was defined as >5 months after the second dose for mRNA (Pfizer/BioNTech or Moderna) or AstraZeneca COVID-19 vaccine, or 1 dose of Janssen COVID-19 vaccine. The literature search was limited to December 2019 to November 15, 2021. Editorials, commentaries, and published studies from non–peer-reviewed sources (eg, MedRxiv) were excluded. Studies without comparison between vaccinated and unvaccinated individuals (or other vaccinated control group), and studies without vaccine effectiveness data were also excluded.
Search strategy
We performed literature searches in PubMed, Cumulative Index to Nursing and Allied Health (CINAHL), Embase (Elsevier Platform), Cochrane Central Register of Controlled Trials, Scopus (which includes EMBASE abstracts), and Web of Science. The entire search strategy is described in Supplementary Appendix 1. We reviewed the reference lists of retrieved articles to identify studies that were not identified from the preliminary literature searches. After applying exclusion criteria, we reviewed 55 papers, 17 of which met the inclusion criteria and were included in the systematic literature review (Fig. 1).
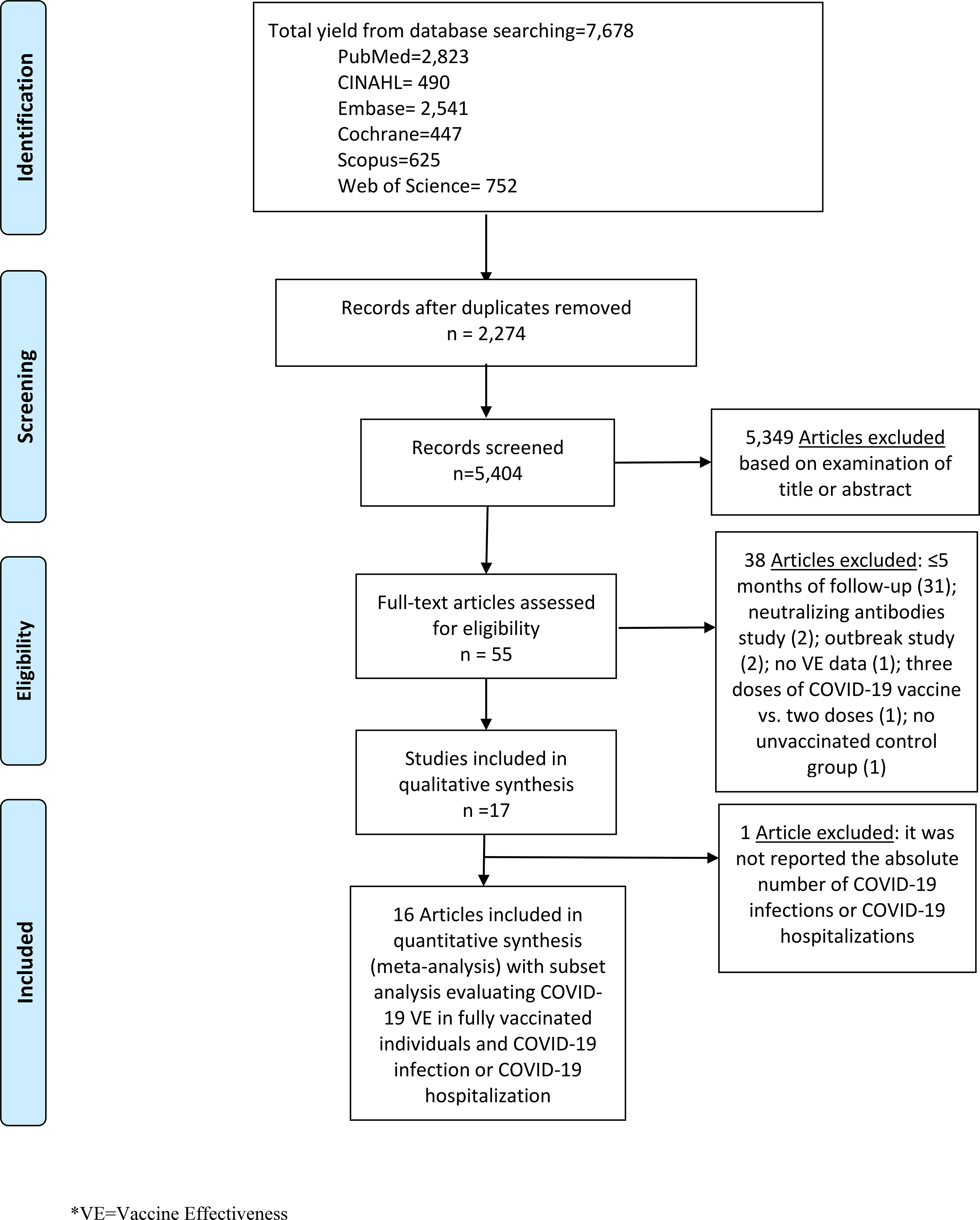
Fig. 1. Literature search for articles on the long-term COVID-19 vaccine effectiveness among general population.
Data abstraction and quality assessment
Titles and abstracts of all articles were screened to assess whether they met inclusion criteria. The reviewers (A.R.M., B.M.T., H.S., L.M.B., M.A., M.A.A., and T.K.) abstracted data for each article. Reviewers resolved disagreements by consensus.
The reviewers abstracted data on study design, population and location, study period (months) and the calendar time, demographic and characteristics of participants, types of COVID-19 vaccine, and the date of whole-genome sequencing if available. Laboratory-confirmed COVID-19 was considered the primary outcome to calculate vaccine effectiveness after 2 doses of a COVID-19 vaccine. COVID-19 hospitalization was considered as a secondary outcome. We collected the hazard ratio (HR), the relative risk (RR), the odds ratio (OR), and vaccine effectiveness with 95% confidence intervals (CIs). We have also described the statistical analysis performed per each study to describe the estimated COVID-19 vaccine effectiveness. Risk of bias was assessed using the Downs and Black scale. Reference Downs and Black21 Reviewers followed all questions from this scale as written except for question 27 (a single item on the power subscale scored 0–5), which was changed to a yes or no. Two authors performed component quality analyses independently, reviewed all inconsistent assessments, and resolved disagreements by consensus. Reference Alderson22
Statistical analysis
To meta-analyze the extracted data, we calculated the pooled diagnostic odds ratio (DOR) for COVID-19 or COVID-19 hospitalization between vaccinated and unvaccinated individuals. Vaccine effectiveness was estimated as 100% × (1 − DOR). We performed stratified analyses by vaccine type (eg, PfizerBioNTech COVID-19 vaccine [2 doses], Janssen COVID-19 vaccine [1 dose]), by COVID-19 status (ie, COVID-19 or COVID-19 hospitalization), and by the δ variant period. Reference Bajema, Dahl and Prill23–Reference Tartof, Slezak and Fischer33 We performed statistical analysis using R version 4.1.0 with mada package version 0.5.4. Reference Doebler and Holling34 Analogous to the meta-analysis of the odds ratio methods for the DOR, an estimator of random-effects model following the approach of DerSimonian and Laird is provided by the mada package. Reference Doebler and Holling34 For our meta-analysis of estimates of COVID-19 vaccine effectiveness, we used a bivariate random effects model, adopting a similar concept of performing the diagnostic accuracy, which enables simultaneous pooling of sensitivity and specificity with mixed-effect linear modeling while allowing for the trade-off between them. Reference Reitsma, Glas, Rutjes, Scholten, Bossuyt and Zwinderman35,Reference Goto, Ohl, Schweizer and Perencevich36 Heterogeneity between studies was evaluated with I2 estimation and the Cochran Q statistic test.
Results
Characteristics of included studies
In total, 17 studies met the inclusion criteria Reference Bajema, Dahl and Prill23–Reference Tartof, Slezak and Fischer33,Reference Braeye, Cornelissen and Catteau37–Reference Thompson, Stenehjem and Grannis42 and were included in the final review (Table 1). Almost all of these studies were nonrandomized (16 studies), and of these, 12 were retrospective cohort studies. Reference Bajema, Dahl and Prill23,Reference Bozio, Grannis and Naleway24,Reference Cohn, Cirillo, Murphy, Krigbaum and Wallace26–Reference Embi, Levy and Naleway28,Reference Nanduri, Pilishvili and Derado30–Reference Tartof, Slezak and Fischer33,Reference Braeye, Cornelissen and Catteau37,Reference Self, Tenforde and Rhoads39,Reference Tande, Pollock, Shah, Binnicker and Berbari40 Also, 1 study was a prospective cohort study Reference Fowlkes, Gaglani, Groover, Thiese, Tyner and Ellingson29 and 3 studies were case–control studies. Reference Chemaitelly, Tang and Hasan25,Reference Kissling, Hooiveld and Sandonis Martín38,Reference Thompson, Stenehjem and Grannis42 Only 1 study was a randomized clinical trial. Reference Thomas, Moreira and Kitchin41 All but 1 of these studies evaluated the Pfizer/BioNTech vaccine (16 studies). Reference Bajema, Dahl and Prill23–Reference Cohn, Cirillo, Murphy, Krigbaum and Wallace26,Reference Embi, Levy and Naleway28–Reference Tartof, Slezak and Fischer33,Reference Braeye, Cornelissen and Catteau37,Reference Self, Tenforde and Rhoads39–Reference Thompson, Stenehjem and Grannis42 Of these studies, 13 analyzed the Moderna vaccine Reference Bajema, Dahl and Prill23,Reference Bozio, Grannis and Naleway24,Reference Cohn, Cirillo, Murphy, Krigbaum and Wallace26,Reference Embi, Levy and Naleway28–Reference Nunes, Rodrigues and Kislaya32,Reference Braeye, Cornelissen and Catteau37,Reference Self, Tenforde and Rhoads39,Reference Tande, Pollock, Shah, Binnicker and Berbari40,Reference Thompson, Stenehjem and Grannis42 ; 7 studies analyzed the Janssen vaccine, Reference Cohn, Cirillo, Murphy, Krigbaum and Wallace26,Reference Corchado-Garcia, Zemmour and Hughes27,Reference Fowlkes, Gaglani, Groover, Thiese, Tyner and Ellingson29,Reference Braeye, Cornelissen and Catteau37–Reference Self, Tenforde and Rhoads39,Reference Thompson, Stenehjem and Grannis42 1 of which evaluated only the Janssen vaccine Reference Corchado-Garcia, Zemmour and Hughes27 ; and 3 studies analyzed the AstraZeneca vaccine, Reference Nordström, Ballin and Nordström31,Reference Braeye, Cornelissen and Catteau37,Reference Kissling, Hooiveld and Sandonis Martín38 1 of which evaluated mixing COVID-19 vaccines. Reference Nordström, Ballin and Nordström31
Table 1. Summary of Characteristics of Studies Included in the Systematic Literature Review
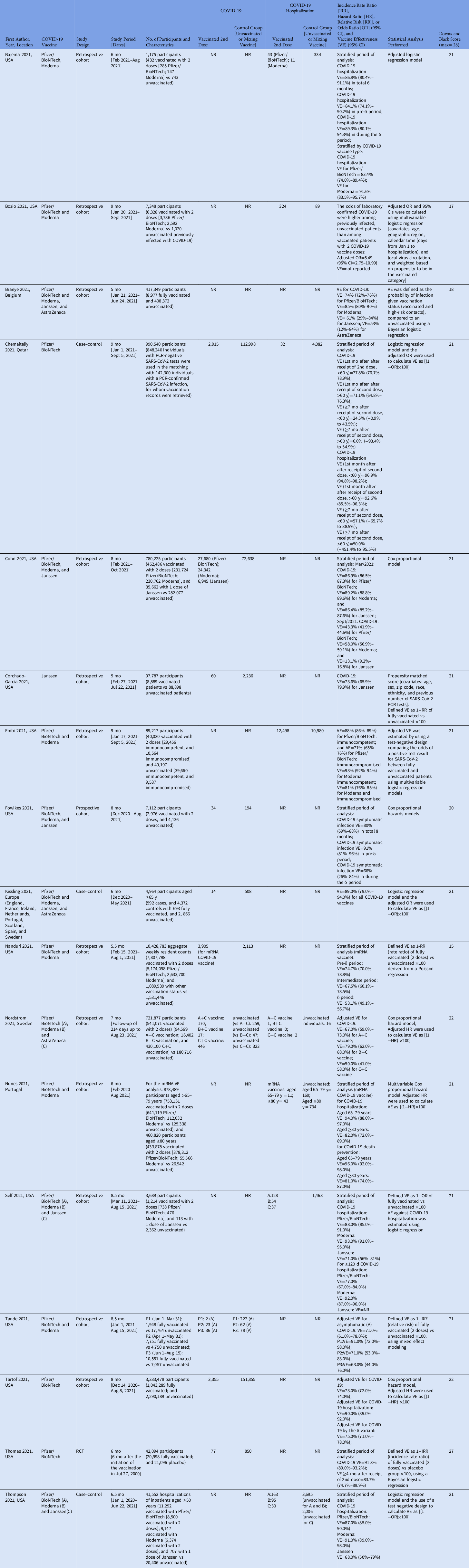
Note. A, asymptomatic; S, symptomatic; SD, standard deviation; IQR, interquartile range; IRR, incidence rate ratio; HR, hazard ratio [HR]; RR’, relative risk; OR, odds ratio; 95% CI, 95% confidence interval; VE, vaccine effectiveness; NR, not reported; N, number reported; RCT, randomized controlled trial.
Table 2. Subset Analyses Evaluating Long-Term COVID-19 Vaccine Effectiveness Among Fully Vaccinated Individuals
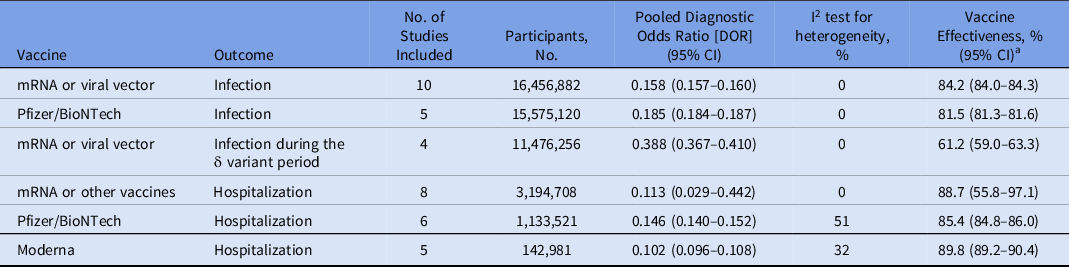
Note. CI, confidence interval; mRNA, Pfizer/BioNTech and Moderna; viral vector, AstraZeneca, and Janssen.
a Vaccine effectiveness was estimated as 100% × (1 − DOR).
Fully vaccinated is defined as receiving 2 doses of Pfizer/BioNTech, Moderna, or AstraZeneca vaccine, or 1 dose of Janssen vaccine.
Most of the studies included in our review were conducted in the United States (12 studies) Reference Bajema, Dahl and Prill23,Reference Bozio, Grannis and Naleway24,Reference Cohn, Cirillo, Murphy, Krigbaum and Wallace26–Reference Nanduri, Pilishvili and Derado30,Reference Tartof, Slezak and Fischer33,Reference Self, Tenforde and Rhoads39–Reference Thompson, Stenehjem and Grannis42 ; 1 study was a multicenter study performed in Europe (assembling data from England, France, Ireland, Netherlands, Portugal, Scotland, Spain, and Sweden) Reference Kissling, Hooiveld and Sandonis Martín38 ; and 1 study was performed in each of these countries: Belgium, Reference Braeye, Cornelissen and Catteau37 Qatar, Reference Chemaitelly, Tang and Hasan25 Sweden, Reference Nordström, Ballin and Nordström31 and Portugal. Reference Nunes, Rodrigues and Kislaya32 All studies were performed between December 2020 and October 2021. Reference Bajema, Dahl and Prill23–Reference Tartof, Slezak and Fischer33,Reference Braeye, Cornelissen and Catteau37–Reference Thompson, Stenehjem and Grannis42
Moreover, 10 studies evaluated long-term vaccine effectiveness for COVID-19, Reference Chemaitelly, Tang and Hasan25–Reference Corchado-Garcia, Zemmour and Hughes27,Reference Fowlkes, Gaglani, Groover, Thiese, Tyner and Ellingson29–Reference Nordström, Ballin and Nordström31,Reference Tartof, Slezak and Fischer33,Reference Kissling, Hooiveld and Sandonis Martín38,Reference Tande, Pollock, Shah, Binnicker and Berbari40,Reference Thomas, Moreira and Kitchin41 8 studies evaluated long-term vaccine effectiveness for COVID-19 hospitalizations, Reference Bajema, Dahl and Prill23–Reference Chemaitelly, Tang and Hasan25,Reference Embi, Levy and Naleway28,Reference Nordström, Ballin and Nordström31,Reference Nunes, Rodrigues and Kislaya32,Reference Self, Tenforde and Rhoads39,Reference Thompson, Stenehjem and Grannis42 with 2 studies overlapping. Reference Chemaitelly, Tang and Hasan25,Reference Nordström, Ballin and Nordström31 The study duration varied from 5 to 14 months. Reference Bajema, Dahl and Prill23–Reference Tartof, Slezak and Fischer33,Reference Braeye, Cornelissen and Catteau37–Reference Thompson, Stenehjem and Grannis42
Furthermore, 13 studies reported genomic surveillance data. Reference Bajema, Dahl and Prill23–Reference Cohn, Cirillo, Murphy, Krigbaum and Wallace26,Reference Embi, Levy and Naleway28–Reference Tartof, Slezak and Fischer33,Reference Braeye, Cornelissen and Catteau37,Reference Kissling, Hooiveld and Sandonis Martín38 Also, 11 studies reported detecting the new SARS-CoV-2 B.1.617.2 δ (delta) variant Reference Bajema, Dahl and Prill23–Reference Tartof, Slezak and Fischer33 ; 7 studies reported only δ variant during the long-term vaccine effectiveness evaluation Reference Bajema, Dahl and Prill23,Reference Bozio, Grannis and Naleway24,Reference Cohn, Cirillo, Murphy, Krigbaum and Wallace26,Reference Embi, Levy and Naleway28–Reference Nordström, Ballin and Nordström31 ; 2 studies reported the B.1.1.7 α (alpha) variant and δ variant Reference Corchado-Garcia, Zemmour and Hughes27,Reference Nunes, Rodrigues and Kislaya32 ; 1 study reported the B.1.351 β (beta) variant and δ variant, Reference Chemaitelly, Tang and Hasan25 and 1 study reported α, β, γ (gamma or P.1), and δ variants. Reference Chemaitelly, Tang and Hasan25
Studies varied with regards to the type of statistical analysis performed. Nine studies used logistic regression Reference Bajema, Dahl and Prill23–Reference Chemaitelly, Tang and Hasan25,Reference Embi, Levy and Naleway28,Reference Braeye, Cornelissen and Catteau37–Reference Self, Tenforde and Rhoads39,Reference Thomas, Moreira and Kitchin41,Reference Thompson, Stenehjem and Grannis42 ; 5 studies used Cox proportional hazard analysis Reference Cohn, Cirillo, Murphy, Krigbaum and Wallace26,Reference Fowlkes, Gaglani, Groover, Thiese, Tyner and Ellingson29,Reference Nordström, Ballin and Nordström31–Reference Tartof, Slezak and Fischer33 ; 1 study used propensity matched scoring Reference Corchado-Garcia, Zemmour and Hughes27 ; 1 study used Poisson distribution for adjusted logistic regression Reference Nanduri, Pilishvili and Derado30 ; and 1 study used mixed-effects modeling. Reference Tande, Pollock, Shah, Binnicker and Berbari40
Regarding the quality assessment scores of the 17 included studies, >75% of the studies (13 studies) were considered good quality (ie, 19–23 of 28 possible points) per the Downs and Black quality tool. Reference Bajema, Dahl and Prill23,Reference Chemaitelly, Tang and Hasan25–Reference Fowlkes, Gaglani, Groover, Thiese, Tyner and Ellingson29,Reference Nordström, Ballin and Nordström31–Reference Tartof, Slezak and Fischer33,Reference Kissling, Hooiveld and Sandonis Martín38–Reference Tande, Pollock, Shah, Binnicker and Berbari40,Reference Thompson, Stenehjem and Grannis42 Also, 3 studies were considered fair quality (ie, 14–18 points) Reference Bozio, Grannis and Naleway24,Reference Nanduri, Pilishvili and Derado30,Reference Braeye, Cornelissen and Catteau37 and 1 study was considered high quality (ie, >24 points). Reference Thomas, Moreira and Kitchin41
Results pooled by COVID-19 vaccine type and COVID-19 outcome
Overall, we included 17,939,172 individuals from 16 studies in the meta-analysis. Reference Bajema, Dahl and Prill23–Reference Tartof, Slezak and Fischer33,Reference Kissling, Hooiveld and Sandonis Martín38–Reference Thompson, Stenehjem and Grannis42 Among them, 10 studies evaluated the long-term vaccine effectiveness of mRNA or viral vector vaccines (ie, AstraZeneca or Janssen). Reference Chemaitelly, Tang and Hasan25–Reference Corchado-Garcia, Zemmour and Hughes27,Reference Fowlkes, Gaglani, Groover, Thiese, Tyner and Ellingson29–Reference Nordström, Ballin and Nordström31,Reference Tartof, Slezak and Fischer33,Reference Kissling, Hooiveld and Sandonis Martín38,Reference Tande, Pollock, Shah, Binnicker and Berbari40,Reference Thomas, Moreira and Kitchin41 The estimated long-term vaccine effectiveness for COVID-19 was 84.2% (95% CI, 84.0%–84.3%). Also, 5 studies evaluated the long-term vaccine effectiveness of the Pfizer/BioNTech vaccine, Reference Chemaitelly, Tang and Hasan25,Reference Cohn, Cirillo, Murphy, Krigbaum and Wallace26,Reference Nanduri, Pilishvili and Derado30,Reference Tartof, Slezak and Fischer33,Reference Thomas, Moreira and Kitchin41 and 2 studies evaluated the Moderna vaccine. Reference Cohn, Cirillo, Murphy, Krigbaum and Wallace26,Reference Nanduri, Pilishvili and Derado30 The estimated long-term vaccine effectiveness against COVID-19 of the Pfizer/BioNTech COVID-19 vaccine was 81.5% (95% CI, 81.3%–81.6%). Furthermore, 4 studies evaluated vaccine effectiveness of the mRNA or viral vector vaccines during the δ variant period Reference Chemaitelly, Tang and Hasan25,Reference Fowlkes, Gaglani, Groover, Thiese, Tyner and Ellingson29,Reference Nanduri, Pilishvili and Derado30,Reference Tande, Pollock, Shah, Binnicker and Berbari40 ; 2 studies evaluated vaccine effectiveness of the Pfizer/BioNTech COVID-19 vaccine only Reference Chemaitelly, Tang and Hasan25,Reference Nanduri, Pilishvili and Derado30 , and 2 studies reported vaccine effectiveness of the Moderna COVID-19 vaccine only. Reference Chemaitelly, Tang and Hasan25,Reference Nanduri, Pilishvili and Derado30 The estimated long-term vaccine effectiveness for COVID-19 with mRNA or viral vector vaccines during the δ variant–dominant period was 61.2% (95% CI, 59.0%–63.3%).
Among the 16 studies, 8 studies evaluated the long-term vaccine effectiveness of mRNA or viral vector vaccines for COVID-19 hospitalization. Reference Bajema, Dahl and Prill23–Reference Chemaitelly, Tang and Hasan25,Reference Embi, Levy and Naleway28,Reference Nordström, Ballin and Nordström31,Reference Nunes, Rodrigues and Kislaya32,Reference Self, Tenforde and Rhoads39,Reference Thompson, Stenehjem and Grannis42 The estimated long-term vaccine effectiveness against COVID-19 was 88.7% (95% CI, 55.8%–97.1%). In stratified analyses, 6 studies evaluated long-term vaccine effectiveness for COVID-19 hospitalization with the Pfizer/BioNTech vaccine, Reference Bajema, Dahl and Prill23–Reference Chemaitelly, Tang and Hasan25,Reference Embi, Levy and Naleway28,Reference Self, Tenforde and Rhoads39,Reference Thompson, Stenehjem and Grannis42 and 5 studies with the Moderna vaccine. Reference Bajema, Dahl and Prill23,Reference Bozio, Grannis and Naleway24,Reference Embi, Levy and Naleway28,Reference Self, Tenforde and Rhoads39,Reference Thompson, Stenehjem and Grannis42 The estimated long-term vaccine effectiveness for COVID-19 hospitalization with the Pfizer/BioNTech vaccine was 85.4% (95% CI, 84.8%–86.0%). The estimated long-term vaccine effectiveness for COVID-19 hospitalization with the Moderna vaccine was 89.8% (95% CI, 89.2%–90.4%). Only 1 study evaluated COVID-19 hospitalization during the δ variant period with mRNA vaccines. Reference Bozio, Grannis and Naleway24 This study did not report the COVID-19 vaccine effectiveness but reported that the adjusted odds of COVID-19 was higher among unvaccinated and previously infected patients compared with fully vaccinated individuals (adjusted odds ratio, 5.49; 95% CI, 2.75–10.99). Reference Bozio, Grannis and Naleway24
The results of meta-analyses were homogeneous for COVID-19 with mRNA or viral vector vaccines (heterogeneity P = .76; I2 = 0%); studies evaluating individuals vaccinated with the Pfizer/BioNTech vaccine alone (heterogeneity P = .55; I2 = 0%); and studies evaluating individuals vaccinated with mRNA or viral vector vaccines during the δ variant period (heterogeneity P = .50; I2 = 0%).
Meta-analysis results were also homogeneous for COVID-19 hospitalization (studies evaluating individuals vaccinated with mRNA or viral vector vaccines (heterogeneity P = .67; I2 = 0%); and studies evaluating individuals vaccinated with the Moderna vaccine alone (heterogeneity P = .28; I2 = 20%). However, results were not homogenous for studies of COVID-19 hospitalization only evaluating individuals vaccinated with the Pfizer/BioNTech vaccine alone (heterogeneity P = .07; I2 = 51%) or for studies of COVID-19 hospitalization only evaluating individuals vaccinated with the Moderna vaccine alone (heterogeneity P = .21; I2 = 32%).
Discussion
This systematic literature review and meta-analysis showed that the long-term of vaccine effectiveness with COVID-19 vaccines (primarily the mRNA vaccines) for COVID-19 and COVID-19 hospitalization were high at 84.2% and 88.7%, respectively. However, the long-term vaccine effectiveness against COVID-19 during the δ-variant–dominant period was lower at 61.2%. These results suggest that 2 doses of the COVID-19 vaccine may lose effectiveness after a few months, and more prospective studies are needed to investigate the short- and long-term vaccine effectiveness after the third dose of the COVID-19 vaccines.
A growing body of early global research shows that the authorized COVID-19 vaccines remain highly protective against the disease’s worst outcomes over time with some exceptions among older and immunocompromised people. Reference Chodick, Tene and Rotem43,Reference Del Rio, Malani and Omer44 In our systematic literature review, we analyzed only the estimated pooled vaccine effectiveness for the mRNA COVID-19 vaccines and the viral vector COVID-19 vaccines. These are the first COVID-19 vaccines authorized by the FDA and around the world, 45–48 and they prevent COVID-19 and COVID-19 hospitalization. Reference Dagan, Barda and Kepten2,Reference Marra, Kobayashi and Suzuki4,Reference Griffin, Haddix and Danza10,Reference Lopez Bernal, Andrews and Gower12,Reference Pilishvili, Gierke and Fleming-Dutra15,Reference Tenforde, Patel and Ginde49 The long duration of the studies (from 5 to 14 months, as shown in Table 1) included in our systematic literature review helps to better elucidate the long-term vaccine effectiveness in the context of a global pandemic with new SARS-CoV-2 variants Reference Lopez Bernal, Andrews and Gower12,Reference Lopez Bernal, Andrews and Gower13 and to better understand that the decrease of vaccine effectiveness is associated with a waning of humoral immune response after a few months. Reference Lopez Bernal, Andrews and Gower13,Reference Levin, Lustig and Cohen17 Although the overall long-term vaccine effectiveness against COVID-19 and COVID-19 hospitalization were moderately high (∼80%), a number of published studies demonstrated significantly lower vaccine effectiveness (∼60%) during the δ-variant period. Reference Chemaitelly, Tang and Hasan25,Reference Cohn, Cirillo, Murphy, Krigbaum and Wallace26,Reference Fowlkes, Gaglani, Groover, Thiese, Tyner and Ellingson29,Reference Nanduri, Pilishvili and Derado30,Reference Self, Tenforde and Rhoads39,Reference Thomas, Moreira and Kitchin41
Our systematic review included 11 studies evaluating the widespread circulation of the δ variant contributing to the majority of recent COVID-19 and COVID-19 hospitalizations. Reference Bajema, Dahl and Prill23–Reference Tartof, Slezak and Fischer33 The studies in this systematic review antedate the emergence of the B.1.1.529 (omicron) variant announced by the World Health Organization (WHO) on November 26, 2021. 50 We need more studies on the SARS-CoV-2 variants of concerns (VOC) that have multiple spike-protein changes and that may be more infectious or cause more severe disease than other circulating variants. Reference Challen, Brooks-Pollock, Read, Dyson, Tsaneva-Atanasova and Danon51 Some deletions in the spike-protein gene can alter the shape of the spike and may help it evade antibodies. Reference Wang, Nair and Liu52 No COVID-19 vaccine is 100% effective against SARS-CoV-2 infection, as demonstrated by breakthrough infections, Reference Brown, Vostok and Johnson8,Reference Hacisuleyman, Hale and Saito53 but they are highly effective at preventing severe disease and death. Reference Chemaitelly, Tang and Hasan25 Although the long-term vaccine effectiveness was not as high as the short-term vaccine effectiveness, it is not clear whether the waning of immunity is due to the passage of time or the coincident spread of the δ variant (from June to September 2021). Reference Bajema, Dahl and Prill23–Reference Tartof, Slezak and Fischer33
Our study had several limitations. Most of the studies included in the meta-analysis were observational studies, which are subject to multiple biases. Reference Harris, Lautenbach and Perencevich54 However, this is the most common study design in the infection prevention literature. Reference Harris, Lautenbach and Perencevich54 None of the included studies reported possible adverse events after vaccine administration. We could not perform further analyses stratified by immunocompromised status due to the limited number of studies. Only 1 study compared immunocompromised individuals to immunocompetent individuals and reported that the effectiveness of mRNA vaccination against COVID-19 hospitalization was lower (77%) among immunocompromised individuals than among immunocompetent individuals (90%). Reference Embi, Levy and Naleway28 Because our study focused on the long-term vaccine effectiveness after the second dose, we could not evaluate the impact of a third dose. Because of the low number of included studies of viral vector vaccines, it was not possible to perform a stratified analysis for these. It was not possible to evaluate the long-term vaccine effectiveness of the Moderna vaccine against COVID-19 because there were not enough studies. Reference Cohn, Cirillo, Murphy, Krigbaum and Wallace26,Reference Nanduri, Pilishvili and Derado30 There are not enough studies comparing each 1 of the 2 mRNA vaccines to draw conclusions about the vaccine effectiveness for COVID-19 during the δ variant dominant period. Reference Chemaitelly, Tang and Hasan25,Reference Nanduri, Pilishvili and Derado30 Also, it was not possible to evaluate the COVID-19 hospitalization vaccine effectiveness during the δ-variant–dominant period. It was not possible to make any conclusions about the long-term vaccine effectiveness of mixing vaccines because just 1 study assessed this. Reference Nordström, Ballin and Nordström31 From that study, mixing COVID-19 vaccines (first dose with the AstraZeneca vaccine adding a mRNA prime-boost showed a higher vaccine effectiveness (68%) than that of 2 doses of AstraZeneca vaccine (50%). Reference Nordström, Ballin and Nordström31 Lastly, each study used a different approach to report the incidence of COVID-19 (eg, incidence rate per person years). Therefore, we decided to perform our meta-analysis and stratified analysis with a bivariate approach to preserve the 2-dimensional nature of the original data from the selected studies. Reference Bajema, Dahl and Prill23–Reference Tartof, Slezak and Fischer33,Reference Kissling, Hooiveld and Sandonis Martín38–Reference Thompson, Stenehjem and Grannis42
In conclusion, COVID-19 vaccines can effectively prevent COVID-19 and COVID-19 hospitalization for a relatively long period. These vaccines are also effective in preventing COVID-19 during the δ-variant period, though vaccines were less effective. These data are very important to help motivate individuals to seek vaccination. More observational studies are needed to evaluate other types of COVID-19 vaccine (eg, viral vector or inactivated virus) effectiveness, vaccine effectiveness of a third dose, vaccine effectiveness of mixing COVID-19 vaccines, COVID-19 breakthrough infection after vaccination, and genomic surveillance for better understanding vaccine effectiveness against the new viral variants.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ash.2021.261
Acknowledgments
We thank Jennifer Deberg, MLS, from the Hardin Library for the Health Sciences, University of Iowa Libraries, for assistance with the search methods.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.





