Surveillance for Clostridioides difficile is foundational in the prevention of healthcare-associated transmission. The minimum recommendation for healthcare facilities is to conduct a surveillance program for healthcare-associated C. difficile infection (HA-CDI) to identify increasing rates or outbreaks. Reference McDonald, Gerding and Johnson1 Formally, reporting of C. difficile rates is primarily based on laboratory results, 2 which may contribute to interpretive challenges depending on the method of laboratory detection (ie, molecular, molecular followed by enzyme immunoassay [EIA] (GDH/toxin A/B), or EIA followed by molecular) particularly for indeterminate (PCR+/Tox−) results. Furthermore, using laboratory-based surveillance only can systematically increase the incidence of HA-CDI.
Our laboratory transitioned to a 2-step test reporting algorithm in 2018 (ie, molecular followed by EIA). The change in reporting also prompted an ‘enhanced’ CDI surveillance initiative implemented by the combined infection prevention and control (IPC) and antimicrobial stewardship program (ASP) teams. In addition to the recommended infection control precautions for C. difficile, Reference McDonald, Gerding and Johnson1 the IPC team alerted the ASP team of all inpatient cases with a positive or indeterminate C. difficile result, prompting detailed case review to ascertain colonization or true infection. This process enabled more detailed C. difficile surveillance data to inform IPC practices while enabling the ASP team to optimize treatment for C. difficile infections (and reduce unnecessary concurrent antimicrobial use). We describe the results of our ‘enhanced’ C. difficile surveillance, in which we sought to assess the difference in C. difficile infection rates based on laboratory results compared to the addition of an ASP and clinical review.
Methods
We retrospectively reviewed the IPC CDI surveillance data (April 1, 2018, to March 31, 2022), excluding outpatient clinics, emergency department, and long-term care facilities. Duplicate samples, as well as relapsed cases, were excluded (Fig. 1). The 2-step algorithm included PCR (Xpert C. difficile, Cepheid, Sunnyvale, CA) for all appropriate stool specimens (ie, from patients with 3 or more loose or watery stools—Bristol types 6 and 7–above their baseline within a 24-hour period) followed by enzyme immunoassay (EIA) for toxins A/B and GDH (C. Diff Quik Chek Complete, Techlab, Blacksburg, VA) for all positive samples. The microbiology laboratory reviews all stool submissions and cancels orders for those with formed stool.

Fig. 1. Overview of the Clostridioides difficile results included in the infection prevention and control (IPC) and antimicrobial stewardship (ASP) ‘enhanced’ surveillance.
The process for clinical review has been previously described. For all positive (PCR+/Tox+) and indeterminate (PCR+/Tox−) inpatient cases, the IPC team alerts the ASP team who reviewed with the most responsible physician (MRP) to determine the patient’s clinical status (colonization or infection). Reference Zou, Leung and Champagne3 The case was classified in the IPC surveillance database as colonization or infection when MRP and ASP had concordant assessments. If there was discordance regarding the clinical assessment between ASP and MRP, classification would be based on the final MRP decision. Assessment of infection was based on clinical symptoms (and stool charts), laboratory results (eg, leukocytosis), and an absence of an alternate diagnosis. During the study period, IPC practices and treatment recommendations for C. difficile were unchanged. Statistical analyses were performed using Microsoft Excel (Microsoft, Redmond, WA) and OpenEpi version 3.01 software.
Results
During the 4-year study period, 748 inpatients were reviewed by the IPC and ASP teams, with 247 positive and 501 indeterminate results (Table 1). The proportion of positive and indeterminate cases was generally unchanged over time. On clinical review of all indeterminate cases, the proportion of patients colonized with C. difficile statistically significantly increased from 2018–2019 to 2021–2022 relative to those determined to have acute clinical infection (P < .05). Overall, 288 (57%) of 501 of all indeterminate results were considered colonization. With respect to patients with positive C. difficile results, only 20 (8%) of 247 positive cases were classified as colonization.
Table 1. Clostridioides difficile Cases Identified in the Microbiology Laboratory from 2018–2019 to 2021–2022 and Classification of Cases After Clinical Review (Colonized or Infected)
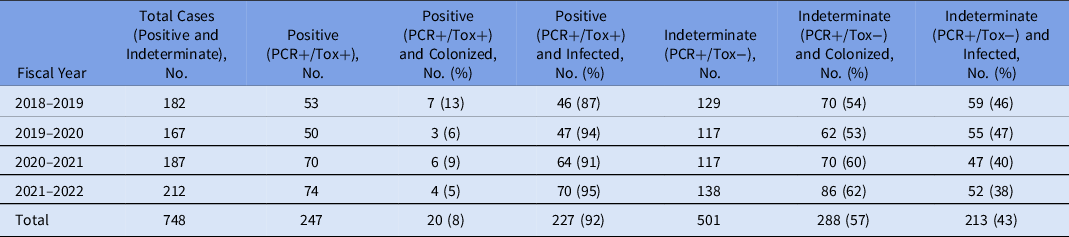
Note. PCR, polymerase chain reaction assay; PCR+/Tox+, positive; PCR+/Tox−, indeterminate.
Discussion
Our institution’s ‘enhanced’ surveillance for C. difficile called for a detailed review of the clinical presentation for patients tested with a 2-step algorithm (ie, PCR and toxin EIA). There continues to be uncertainty regarding the clinical relevance of PCR+/Tox−, though C. difficile testing with molecular methods alone have been associated with overdiagnosis of infection. Reference Polage, Gyorke and Kennedy4 In our experience, interpretation of indeterminate (PCR+/Tox−) results requires a clinical assessment. We previously identified that 54% of PCR+/Tox− cases were considered to be true infections. Reference Zou, Leung and Champagne3 Longitudinal follow-up in this study revealed that trends in infection versus colonization shifted over time, with more indeterminate cases representing colonization. Although the underlying reason for this shift is not clear yet and requires further investigation, familiarity over time with the 2-step algorithm and ASP review may have contributed to increasing comfort level among MRPs to classify cases as colonization. It further underscores the importance of diagnostic stewardship for C. difficile testing, and for the IPC and ASP teams to encourage clinicians to order testing only when there is clinical suspicion, rather than for any patient with a reported case of loose stool.
For patients with PCR+/Tox− results who were considered colonized, our earlier study suggested that there were no differences in adverse outcomes on follow-up 8 weeks after assessment, despite those patients not prescribed treatment for CDI. This finding is similar to a recent study assessing outcomes for untreated patients with PCR+/Tox−, which found noninferiority for unresolved diarrhea at 7 days and 30-day all-cause mortality. Reference Hogan, Hitchcock and Frost5 For facilities where the microbiology laboratory tests by a molecular method and for the presence of toxin production, indeterminate (PCR+/Tox−) results represent a significant potential for ASP to reduce inappropriate antibiotic use given that C. difficile therapy (eg, oral vancomycin) may have unintended sequelae due to its impact on the human intestinal microbiota. Reference Isaac, Scher and Djukovic6 However, clinical review is essential to ensure that appropriate patient populations are targeted to avoid missing treatment given that 43% of indeterminate cases in this study were considered true infections.
Surveillance for HA-CDI is inherently impacted by laboratory methodology. Molecular methods alone may overestimate rates, while toxin EIA as a primary test may underestimate rates. Reference Fang, Polage and Wilcox7 Based on the CDC NHSN definitions, for laboratories that utilize a 2-step testing algorithm with a molecular assay first, PCR+/Tox− would not be a reportable case (though the exact same results, except with an EIA for GDH/toxin A/B as the primary test would be considered a reportable case). 2 Our study has highlighted that although most PCR+/Tox− cases represent colonization, 43% of cases represent infection. Relying solely on the laboratory definition may lead to delayed treatment, underreporting of CDI, and potential for C. difficile transmission in the absence of precautions in this subset of PCR+/Tox− patients.
This study had several limitations. These results may not be generalizable to other settings; our results were based on 2 hospitals in our institution. In addition, the rates of colonization versus infection may be underestimated because final classification was adjudicated by the MRP, which has not yet been evaluated to determine the reliability or validity in comparison to established definitions for C. difficile. If the MRP decides to treat for CDI despite ASP indicating a likely case of colonization, the case would be classified as an infection, deferring to the clinical judgement of the physician caring for the patient.
Both IPC and ASP teams contribute to the prevention of C. difficile transmission in healthcare facilities. Reference McDonald, Gerding and Johnson1 However, active antimicrobial stewardship interventions have been reported to be infrequent among the SHEA Research Network hospitals, which is a missed stewardship opportunity to reduce C. difficile–specific antibiotics as well as education regarding the need (or lack thereof) for concurrent antimicrobials. Reference Sullivan, Gallagher and Leekha10 The collaboration enabled an ASP intervention for C. difficile treatment and allowed for more detailed surveillance data for HA-CDI—specifically, further clarification of CDI on a clinical review rather than solely on a laboratory result.
Acknowledgments
We thank the St. Paul’s Hospital microbiology laboratory staff for their commitment to quality testing. We are also grateful to the ICP staff at Providence Health Care for supporting this project and their dedication to the prevention of C. difficile transmission.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.



