Introduction
Unverified penicillin allergy labels are common and associated with harmful consequences to both individuals and health systems. Reference Castells, Khan and Phillips1 The United States Centers for Disease Control and Prevention and various national allergy, infectious disease, and pharmacy specialty societies endorse proactive penicillin allergy testing. A recent American Academy of Allergy, Asthma & Immunology position statement supports such evaluation, even during routine healthcare encounters that do not require antibiotic therapy. 2
Verification of penicillin allergy has historically required an allergist assessment in the outpatient setting using penicillin skin testing (PST), with or without subsequent oral amoxicillin challenge. However, a national shortage of and lack of access to allergy specialists are major obstacles to widespread penicillin allergy evaluation. Reference Mancini, Fu and Zhang3 Though the expansion of penicillin allergy evaluation driven by pharmacists has been successful, Reference Bland and Jones4 there is still a great need for more accessible and equitable approaches to this problem.
A recent international, randomized controlled trial comparing direct oral amoxicillin challenge against PST (with or without subsequent oral amoxicillin challenge) in patients with low-risk penicillin allergies achieved similar success (99.5% vs 97.9%) in removing the allergy label and noninferiority in relation to the incidence of immune-mediated adverse events (0.5% vs 0.5%). Reference Copaescu, Vogrin and James5 Given the reduced time and fewer resources needed to perform oral amoxicillin challenges in relation to PST, this modality of penicillin allergy evaluation could enable widespread delabeling efforts by non-allergy personnel across a variety of healthcare settings.
We initiated a penicillin allergy evaluation program using direct oral amoxicillin challenge in 2022. To inform our implementation strategy, we conducted a retrospective feasibility analysis of potential barriers to removing veterans’ penicillin allergy labels using this approach in the inpatient setting.
Methods
The Dallas VA Medical Center is a 1A critical access facility with 244 acute care inpatient beds. The medical service includes an infectious diseases section with four physician and three pharmacist Full-Time Equivalents but without any on-site allergy/immunology staff. We retrospectively reviewed individual records of veterans with a penicillin allergy label selected from two, three-month convenience samples of acute care admissions within calendar years 2021 and 2022. We included veterans with a documented allergy to any of the following medications: penicillin, amoxicillin, ampicillin, amoxicillin-clavulanate, or nafcillin. For veterans with a penicillin allergy label who were admitted more than once during the analysis period, only the first admission was counted. We collected data on patient demographics, admission diagnoses, penicillin allergy label details, comorbidities, and length of stay. Risk of true penicillin allergy was based on a stratification proposed by Shenoy and colleagues Reference Shenoy, Macy and Rowe6 and modified for our population. No increased risk referred to prior index reaction with proven safe receipt of penicillin after; intolerance history–non-allergy symptoms (e.g., GI upset); low risk–self-limited rash/pruritis (at any point), unknown reaction in childhood or more than 10 years ago, urticaria only more than 10 years ago, family history; moderate-high risk–anaphylaxis or angioedema at any point or the following in the last 10 years: urticaria, bronchospasm, loss of consciousness, severe GI symptoms, or unknown reaction at unknown time documented within last 10 years; very high risk–severe cutaneous adverse reactions, delayed severe reactions (acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, drug reaction with eosinophilia and systemic symptoms, and toxic epidermal necrolysis), serum sickness, acute interstitial nephritis, and drug-induced liver injury. Feasibility of performing an oral amoxicillin challenge to remove a penicillin allergy label was determined based upon a composite criterion of low-risk penicillin allergy, a minimum 2-day admission (a time frame determined by authors that would allow identification of patients, patient interview, and administration of oral amoxicillin challenge and observation), and the absence of the following comorbidities—altered mental status or cognitive impairment, inability to consent, severe cardiac or respiratory failure, suspected drug reaction at time of admission, rash, nausea/vomiting, abdominal pain or inability to take oral medications. Facility leadership reviewed this project and designated it as nonresearch. Thus, Institutional Review Board approval was waived.
Results
Baseline demographic characteristics of 136 veterans with a penicillin allergy label are shown in Table 1. Most patients were male (89.7%) and white (61.0%), with the median age of 71 years (IQR, 63–76). The median Charlson Comorbidity Index was 5 (IQR, 3–7), driven primarily by high prevalence of diabetes mellitus (51.5%), solid tumors (29.4%), and congestive heart failure (27.9%). Nearly two-thirds of admissions were for noninfectious reasons.
Table 1. Baseline characteristics of veterans admitted with penicillin allergy label
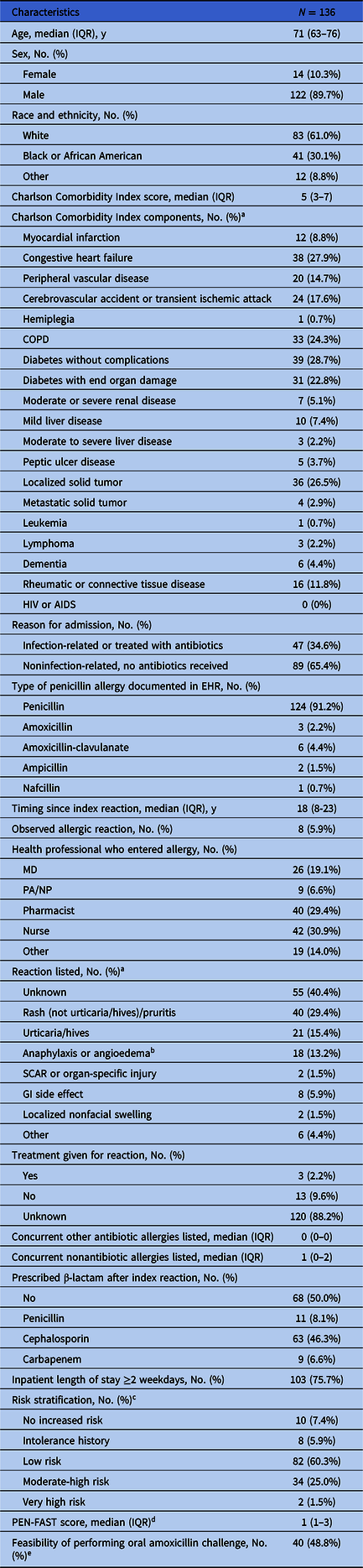
Note. EHR, electronic health record; GI, gastrointestinal; PEN-FAST, Penicillin Allergy Decision Rule; SCAR, severe cutaneous adverse reaction.
a Categories are not mutually exclusive as patients may have had more than one comorbidity or symptom listed; percentages may add to more than 100%.
b Includes reactions documented as “swelling” and for which angioedema could not be excluded.
c Patients with confirmed safe receipt of any penicillin-class antibiotic other than piperacillin-tazobactam after the index date were classified as “No increased risk.” Those who received piperacillin-tazobactam were reclassified as “No increased risk” only if the original history was consistent with a “low-risk” allergy.
d PEN-FAST: PEN, penicillin allergy reported by patient; F, five years or less since reaction (two points); A, anaphylaxis or angioedema (two points); S, severe cutaneous adverse reaction (two points); T, treatment required for reaction (one point); 0 points: very low risk of positive penicillin allergy test <1%, 1–2 points: low risk 5%, three points: moderate risk 20%, 4–5 points: high risk 50%. Reference Trubiano, Vogrin and Chua11
e Percentage was calculated based on a denominator of n = 82 low-risk patients.
Of the penicillin-class of antibiotics listed as an allergy, penicillin was the most frequently listed (91.2%), followed by amoxicillin-clavulanate (4.4%), amoxicillin (2.2%), ampicillin (1.5%), and nafcillin (0.7%). The most frequently documented reaction was non-urticarial rash (29.4%), followed by urticaria/hives (15.4%), though a plurality (40.4%) of reactions were unknown. Based on careful review of the electronic health record (EHR), we determined 18 (13.2%) patients had allergy histories consistent with anaphylaxis or angioedema, and two patients (1.5%) had histories of severe cutaneous adverse reactions, organ-specific injury (interstitial nephritis), or serum sickness syndrome. Notably, the median time from the index reaction to admission (as determined from the EHR) was 18 years. Most of the allergy documentation in this cohort was historical (94%) and not directly observed.
Eighty-two patients (60.3%) were considered to have histories suggestive of low-risk penicillin allergy and so were eligible candidates for allergy label removal following direct oral amoxicillin challenge (Table 1). After accounting for insufficient duration of inpatient stay (n = 16) and precluding comorbidities (n = 26), approximately half (40/82) of these low-risk patients would have met our composite feasibility criterion for an amoxicillin challenge.
Discussion
We conducted a retrospective feasibility analysis in our inpatient veteran population to determine the potential for penicillin allergy removal using direct oral amoxicillin challenge. We identified ample opportunities to remove penicillin allergy labels either via oral amoxicillin challenge or directly, via history and careful review of the EHR, lending support to the initiation of a pilot penicillin allergy removal program at our facility. Based on our results (a total of 100 inpatients over 6 months total with low-risk penicillin allergy, intolerance, or no increased risk history), we estimated approximately 10–15 patients per month could have their penicillin allergy label removed, requiring about 20–25 hours per month of combined physician/pharmacist/nursing time.
A short duration of inpatient stay or certain comorbidities would have reduced the feasibility of an amoxicillin challenge among our low-risk patients by approximately 50%. These findings highlight the need for thoughtful, context-specific implementation strategies to remove penicillin allergies in an elderly veteran population. Examples may include third-party consent to oral amoxicillin challenge for patients with cognitive impairment or systematized, close patient follow-up to reassess the feasibility of performing challenges should their acute admission-related diagnosis resolve.
Because the prevalence of penicillin allergy increases with age, Reference Macy7 it is important to increase delabeling efforts in older persons such as our hospitalized veteran cohort. In the US Drug Allergy Registry, it was recently reported that 286 out of a potential 296 adults aged 65 years or older were able to have their penicillin allergy removed. Reference Accarino, Ramsey and Samarakoon8 A review of published literature on penicillin allergy within the Veterans Health Administration revealed that allergist-provided or pharmacy-driven PST remained the most common modality of penicillin allergy evaluation. Reference Accarino, Ramsey and Samarakoon8 Although a voluntary pharmacy-driven penicillin allergy label removal program (including the use of oral amoxicillin challenges) has begun across the Veterans Health Administration, Reference Kouma, Guastadisegni, Yang, Maxwell, Storey and Arasaratnam9 our findings suggest more detailed study is needed with regard to potential implementation barriers.
This study has some limitations. These data were collected to inform a quality improvement initiative specific to our facility and thus cannot be generalized across other facilities and non-VA populations. EHR review is unlikely to confer the same accuracy with respect to penicillin allergy history as in-person interviews and thus may have led to the misclassification of penicillin allergy risk. Furthermore, the retrospective nature of this report could not identify point-of-care barriers to delabeling such as provider and patient hesitancy.
In summary, we found ample opportunity to remove penicillin allergy labels with an oral amoxicillin challenge among inpatients at our facility. Careful consideration of other patient-specific and/or health systems variables may improve the feasibility of this approach among an elderly population of hospitalized veterans.
Acknowledgements
We thank the Quality, Safety and Value Section at the VA North Texas Health Care System for their support in this quality improvement initiative. We thank Allison Herrera for her thoughtful input and comments regarding this manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the US Department of Veterans Affairs or the US government. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Academy of Medicine or the National Academy of Sciences.
Author contribution
The authors confirm their contribution to the paper as follows: study conception and design: R.J.A. and J.M.G.; data collection: M.K.K., J.M.C., and J.M.G.; draft manuscript preparation: R.J.A., J.M.G., M.A.K., and D.F.S. All authors reviewed the results and approved the final version of the manuscript. Overall work was supervised by R.J.A.
Financial support
This publication is supported by the National Academy of Medicine of the National Academy of Sciences under award number 2000001346 and the Dallas VA Research Corporation.
Competing interests
All authors report no conflicts of interest relevant to this article.



