Introduction
In December 2014, researchers from the United Kingdom published a pivotal study forecasting that antimicrobial resistance (AMR) could cause 10 million deaths annually by 2050, surpassing all other global health problems. Reference O’Neil1 This landmark analysis brought AMR to the forefront of public and scientific discourse, highlighting its potential to become the most significant public health crisis of our time. More recently, a 2024 study revealed that over 1 million deaths were attributed to AMR between 1990 and 2021. Reference Naghavi, Vollset and Ikuta2 Using predictive modeling, the study estimated that over 39 million lives could be lost to AMR from 2024 to 2050. These findings underscore the urgent need for coordinated, multidisciplinary efforts to mitigate the growing impact of AMR.
Addressing AMR requires coordinated efforts across clinical and non-clinical sectors, leveraging innovative approaches to infectious diseases (ID) diagnostics, impact assessment, and scientific communication (Figure 1). Superheroes leading the fight are ID clinicians and pharmacists, clinical microbiologists, and infection preventionists (IP). The clinical microbiology laboratory is a core component of the antimicrobial stewardship program (ASP) efforts, helping clinicians answer three critical questions: Is the patient’s condition caused by an infectious pathogen? If so, what pathogen is responsible? Which antimicrobial(s) are optimal for treatment? Advances in diagnostic technologies have enhanced the ability to answer these questions, particularly for bloodstream infections (BSIs). Reference Briggs, Campbell and Gupta3–Reference Peri, Chatfield, Ling, Furuya-Kanamori, Harris and Paterson5
Figure 1. Key elements identified from the antimicrobial resistance (AMR) summit for addressing AMR.
Rapid diagnostic tests (RDTs), when paired with active ASP interventions, have substantially improved patient outcomes and healthcare costs. Reference Bauer, West, Balada-Llasat, Pancholi, Stevenson and Goff6–Reference Timbrook, Morton, McConeghy, Caffrey, Mylonakis and LaPlante8 A recent systematic review and meta-analysis highlighted that RDT plus ASP workflows significantly reduced time to optimal therapy, length of stay (LOS), and mortality rates for BSIs compared to standard blood culture methods. Reference Peri, Chatfield, Ling, Furuya-Kanamori, Harris and Paterson5 While these data are promising, widespread adoption of RDTs and ASPs requires advocacy, strategic business planning, and leadership support. For example, a 2017 study by Goff et al. highlighted how involving hospital administration in ASP mentorship programs led to increased funding for RDTs and protected time for ID pharmacists and clinicians to focus on ASP activities, emphasizing the importance of demonstrating quality, clinical, and economic outcomes to support integration into practice. Reference Goff, Karam and Haines9
Sustained progress in AMR management also requires government and global collaboration. In the U.S., the National Action Plan for Combating AMR prioritizes RDT adoption, while the Centers for Disease Control and Prevention (CDC) provides guidelines for developing and maintaining ASPs. Internationally, organizations like the Foundation for Innovative New Diagnostics and the World Health Organization connect stakeholders, fund diagnostic innovations, enhance AMR surveillance, and advocate for policy changes. Reference Pollack and Srinivasan10–12 Building public support for ASPs and diagnostics requires innovative communication strategies such as patient advocates who can share compelling stories about their impact. Training for future ID and microbiology professionals should emphasize a one-health approach, fostering global awareness of AMR/ASP and skills to effectively communicate with diverse audiences, from the public to policymakers. 13,Reference Jin, Kolis and Parker14
In November 2024, bioMérieux hosted its inaugural AMR Summit in Tampa, Florida, to address the challenges and identify solutions related to AMR. In collaboration with Tampa General Hospital, a bioMérieux Center of Excellence, and the University of South Florida Morsani College of Medicine, the summit convened approximately 90 national experts, including policymakers, ID clinicians and pharmacists, microbiologists, patient advocates, industry and professional society leaders, and health economists. Attendees shared expertise and explored strategies to accelerate progress in addressing AMR and promote coordinated action. Key topics discussed at the summit included:
-
1. The role of multidisciplinary teams in AMR and ASP programs
-
2. Policy, payer, and clinical practice intersections
-
3. Reducing AMR through implementation science
-
4. Advancements in antimicrobial susceptibility testing
-
5. Applications of next-generation sequencing
-
6. Call to action to address research, education, and policy gaps
The role of multidisciplinary teams in AMR
The growing threat of AMR necessitates a coordinated, multidisciplinary approach, with ASPs serving as a critical frontline defense. Antimicrobials stewardship (AMS) includes a variety of targeted strategies aimed at promoting the responsible use of antibiotics for both therapeutic and preventive purposes. Reference Morency-Potvin, Schwartz and Weinstein15 The effective implementation of an ASP depends on a strong collaboration among ID clinicians, ID pharmacists, microbiologists, and IP specialists Reference Hueth, Prinzi and Timbrook16–19 This interdisciplinary teamwork is essential for fostering robust leadership support, which is vital to the success of the ASP. At the AMR Summit, the ASP team from Tampa General Hospital presented on the contributions of various departments in the fight against AMR. Below is a summary of the discussion that involved the microbiology laboratory, ID pharmacy, and IP teams.
While all ASP team members contribute to combating AMR, the microbiology laboratory, despite its critical role, is often underrepresented. Rapid diagnostic results are essential for guiding appropriate antibiotic use and supporting stewardship initiatives. RDTs have significantly reduced turnaround times, enabling earlier intervention and targeted treatment. Reference Morency-Potvin, Schwartz and Weinstein15,Reference Timbrook, Spivak and Hanson20–Reference Palavecino, Williamson and Ohl24 Beyond its diagnostic contributions, the laboratory serves as a surveillance hub, detecting emerging resistance patterns, clusters of infections, and novel multidrug-resistant organisms. The laboratory’s expertise in pathogen detection is indispensable for infection control and outbreak management.
ID pharmacists closely collaborate with the microbiology lab on various facets of antibiotic testing and reporting, playing a pivotal role in treatment protocols and infection prevention strategies. Their oversight in antimicrobial use and susceptibility testing is essential in combating AMR. This collaboration has led to the development of targeted susceptibility comments for specific pathogens, enhancing antibiotic prescribing practices and reducing costs. Collaboration among ID pharmacists and ID physicians is essential particularly when addressing multifaceted patient needs and optimizing therapeutic outcomes. Insights from ID physicians can enhance the pharmacist’s approach to medication management and care strategies in challenging scenarios. This interprofessional dialog is vital for the comprehensive management of patients facing intricate health issues.
The IP team is a key partner in ASP accountability, bringing national best practices and ensuring regulatory compliance. With increased focus on hospital-acquired infections and their implications for Centers for Medicare & Medicaid Services (CMS) funding, the alignment of IP strategies with organizational quality metrics is crucial. Therefore, establishing a strong collaborative relationship between the ASP and IP teams is essential. Reference Knobloch, McKinley, Keating and Safdar25,Reference Tenover and Goff26 The COVID-19 pandemic highlighted the need for adaptive infection control measures and strengthened collaborations between stewardship and IP teams. Reference Assi, Abbas, Nori, Doll, Godbout, Bearman and Stevens27 Furthermore, there is an increasing emphasis on incorporating nursing staff into ASP efforts. This integration presents a valuable opportunity to utilize nurses’ frontline insights and enhance the overall effectiveness of the program. Reference Monsees, Goldman and Popejoy28
Establishing and sustaining a successful interdisciplinary ASP faces challenges including program launch, identifying key priorities, ensuring effective communication, and securing adequate staffing. Realistic objectives build early success and a data-driven business strategy demonstrates cost savings and quality improvements. Stewardship strategies must be tailored to each hospital’s unique environment. One example of this adaptability was highlighted at the summit in which a children’s hospital in Houston, Texas implemented rapid identification systems for gram-positive organism identification from blood cultures. Initially, the microbiology laboratory relayed results directly to bedside nurses; however, this approach often led to delays in physician notification or uncertainty regarding the appropriate course of action, resulting in inconsistent implementation of recommended interventions. To streamline the process, results were instead relayed to ID pharmacists who were equipped with pagers to receive and effectively communicate rapid identification results. This modification significantly reduced time to optimal antibiotic therapy.
Building trust and collaboration with key stakeholders requires sustained effort and a long-term commitment. Effectively showcasing the clinical impact of RDTs requires strategic data utilization and consistent communication of key findings and updates to testing algorithms. This endeavor is often hindered by delays in data collection and interpretation, staffing limitations, fluctuations in patient demographics, and institutional changes. Regular interdisciplinary meetings are essential for maintaining transparency, providing updates, and evaluating new test methods or processes.
Despite the progress in fostering multidisciplinary collaboration, it is important to continuously explore innovative strategies to optimize the communication processes. Ongoing commitment to advancing patient care and streamlining laboratory operations is reflected in these improvement initiatives. Addressing challenges and fostering trust among team members are vital to ensuring success and sustainability of ASP initiatives. Prioritizing open communication and collaboration not only enhances the team’s effectiveness but also lays the foundation for long-term impact of these antimicrobial stewardship efforts.
Intersection of policy, payers, clinical practice
RDTs are integral to improving clinical outcomes and combatting AMR, but their effectiveness hinges largely on clinician adoption and payor reimbursement. Increasing RDT use requires a collaborative approach involving healthcare providers, payors, and policymakers. This process often involves generating robust evidence that elucidates the clinical utility Reference Ramsey, Veenstra, Garrison, Carlson, Billings, Carlson and Sullivan29 and economic value of RDTs, developing and disseminating clinical guidelines, Reference Kredo, Bernhardsson, Machingaidze, Young, Louw, Ochodo and Grimmer30 and active advocacy to influence payor policies while securing the necessary funding. 31–34 Clinicians are ideally suited to conduct research validating the efficacy of RDTs and, where possible, collaborate with manufacturers on test development and integration.
Clinicians contribute significantly to guideline development committees, which evaluate evidence and recommend test strategies. Establishing high-quality evidence and comprehensive guidelines is critical for shaping payor policy decisions. Chief medical officers (CMOs) within payor organizations may be receptive to conversations with representatives from diagnostic companies and clinical advocates, presenting an avenue to influence payor perspectives based on current data.
Legislative action is equally important in the fight against AMR. Clinicians, scientific organizations, and patient advocates can collaborate to influence policymakers and support initiatives like ASPs, public health surveillance, antibiotic prescribing regulations, and research funding. A policy publication from the Infectious Diseases Society of America (IDSA) outlines several recommendations for combating AMR, which include implementing economic incentives for antibiotic research and development, streamlined antibiotic approvals, enhanced federal coordination, strengthened surveillance, improved infection control, expanded support for rapid diagnostics, workforce training, and eliminating inappropriate antibiotic use in agriculture. Reference Spellberg, Blaser and Guidos33
A panel discussion at the AMR summit involving clinicians, payers, and government officials explored critical issues related to test reimbursement, policy alignment, and clinical needs. Table 1 summarizes key insights from this discussion, highlighting opportunities for collaboration, barriers to adoption, and strategies to enhance market access for rapid diagnostic tests. Stronger stakeholder partnerships are critical to overcoming current obstacles and maximizing the impact of RDTs.
Table 1. Discussion panel perspectives
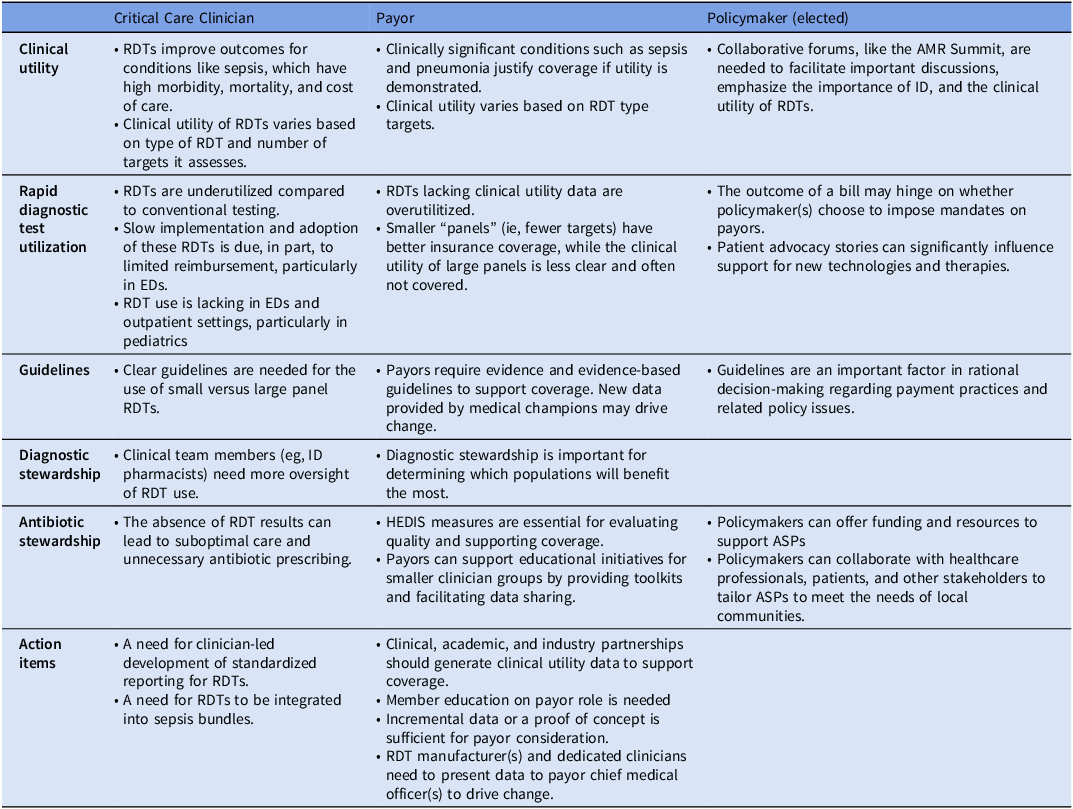
ASP, antimicrobial stewardship program; ID, infectious diseases. HEDIS; Healthcare Effectiveness Data and Information Set; RDT, Rapid diagnostic test; EDs, Emergency Departments.
Reducing AMR through implementation science
Leonardo da Vinci’s observation that “everything connects to everything else” highlights the complex nature of human behavior, a critical factor in addressing AMR. A major contributor to AMR is the inappropriate use of antimicrobials. Antibiotic prescribing is a social act, Reference Svd, Whyte and Hardon35 serving as a tool clinicians use to navigate patient demands, manage diagnostic uncertainty, and conclude clinical encounters. Reference Szymczak, Barlam, Neuhauser, Tamma and Trivedi36 Social determinants, including clinician-patient dynamics and factors like risk, anxiety, fear, emotional responses, and misperceptions, influence prescribing behaviors. Understanding these complexities is crucial for tackling antibiotic overuse and AMR.
While valuable, ASP interventions such as direct education, preauthorization, and audit and feedback are insufficient. Research demonstrates that direct educational initiatives often fail to result in sustained improvement, and policies can be circumvented. Reference LaRosa, Fishman, Lautenbach, Koppel, Morales and Linkin37–Reference van der Velden, Pijpers, Kuyvenhoven, Tonkin-Crine, Little and Verheij41 Developing interventions and technologies without considering socio-behavioral context reduces their impact, delays implementation, and compromises sustainability. Integrating research into practice is a slow process, with only 14% of research translated into clinical practice or evidence-based interventions (EBIs) within 17 years. Reference Lenfant42–Reference Brownson, Colditz and Proctor44 Recognizing these timelines and barriers is essential for achieving meaningful change in the fight against AMR.
Implementation science (IS) offers a solution by focusing on methods to enhance the adoption and fidelity of EBIs in practice, bridging the gap between evidence and application. While not new to medicine, IS is a relatively emerging field within ASP and diagnostic stewardship. It begins by identifying the EBI—such as novel diagnostic tool, an electronic medical record alert, or a training program designed to mitigate inappropriate prescribing or optimize patient outcomes. Reference Curran45 Implementation strategies involve deliberate actions to promote EBI adoption, execution, and sustainability. Several theories, models, and frameworks guide this process. Progress models describe the translational research journey, while determinant frameworks, classic theories, and implementation theories provide insights into factors influencing implementation outcomes. Evaluation frameworks assess the effectiveness of the implementation process. Reference Nilsen46 These validated tools standardize concepts and definitions, enhancing generalizability and applicability for others aiming to refine their practice. Reference Brown, Curran and Palinkas47–Reference Waltz, Powell and Matthieu49
This use of models and frameworks distinguishes IS from quality improvement (QI) efforts. IS aims to generate generalizable insights applicable in various settings, while QI efforts typically focus on a single healthcare center and are highly contextualized. Reference Tyler and Glasgow50 However, elements of both can be combined in ASP projects, facilitating the adaptation of a larger-scale EBI to the local context of a single institution or disseminating QI findings to broader settings. Reference Tyler and Glasgow50 For example, Claeys et al., used a determinant framework with qualitative methods to understand local practices and perceptions of potential diagnostic stewardship interventions for urinary tract infections (UTIs) within US Veterans Affairs medical centers. Reference Claeys, Weston, Pineles, Morgan and Krein51 Their findings revealed barriers and facilitators that can inform tailored ASP interventions for increased adoption and sustainability.
Effective implementation is essential not only for designing interventions but also for optimizing clinical outcomes in research, particularly in the realm of in-vitro diagnostics. Outcomes such as prescribing rates are influenced by the diagnostic test itself and by human behaviors involving patients, providers, and healthcare teams. A test’s efficacy depends on its adoption; otherwise, its impact on outcomes remains unclear. Optimizing behaviors related to test collection, reporting, interpretation, and prescribing is crucial for achieving significant results with advanced ID diagnostics. Similar to standalone ASP interventions neglecting socio-behavioral dynamics, diagnostic tests alone are insufficient in combating AMR. Reference Thampi, Szymczak and Leis39
The most vulnerable are often the most at risk for infectious diseases and AMR. Utilizing IS to comprehend local contexts may help mitigate these inequities. Integrating bioethical inquiry into ASP and IS ensures that populations are appropriately served. Reference Yen and Cutrell52–Reference Tompson, Manderson and Chandler55 The risks and benefits of any AMR-focused intervention should be evaluated, considering alignment with local needs and priorities, stakeholder identification, community impact, and the initiative’s effect on the community and healthcare system. Reference Luyckx, Reis, Maher and Vahedi56,Reference Gopichandran, Luyckx and Biller-Andorno57 Given that prescribing and diagnostic practices are shaped by local context, social influences, and inherent biases, IS provides a more equitable framework for addressing the complex challenges of AMR.
Advancing antimicrobial susceptibility testing
The availability of rapid antimicrobial susceptibility testing (rAST) is crucial for optimizing management and improving patient outcomes, particularly in sepsis. While traditional phenotypic AST methods require 48 to 96 hours, rAST can deliver results within 6 to 24 hours, enabling more targeted treatment and improved outcomes. Reference Babowicz, LaPlante, Mitchell, O’Donnell, Tobin, George and Carreno58,Reference MacVane and Dwivedi59 Studies on rAST have yielded mixed results. Reference Banerjee and Humphries60 Although most studies demonstrate faster reporting times and some treatment adjustments, they often fail to show significant improvements in mortality or length of stay (LOS), likely due to small sample sizes and limited statistical power. Reference MacVane and Dwivedi59 Studies integrating rAST with an ASP have demonstrated significantly improved outcomes, Reference Banerjee and Humphries60 a finding corroborated by a meta-analysis that showed a substantial reduction in LOS and mortality when rapid diagnostics were coupled with ASP. Reference Peri, Chatfield, Ling, Furuya-Kanamori, Harris and Paterson61 The AMR summit discussions highlighted key implementation challenges, focusing on laboratory workflow logistics (including cost, testing scope, and reporting) and the crucial role of collaborating with ID pharmacists and other stakeholders for improved AMS.
Implementing rAST in the laboratory presents several complexities. Reference Gonzalez and Yarbrough62 Decisions regarding making testing universally available versus targeted application of testing, types of specimens to test, replacement of existing methods, and 24/7 availability versus centralized or batched testing must be addressed. Most hospitals prioritize rAST directly from positive blood cultures due to the critical nature of bloodstream infections, where timely antimicrobial optimization significantly impacts patient outcomes. Reference Dickinson and Kollef63,Reference Weinberger, Rhee and Klompas64 This focus ensures rapid identification of effective therapy, helping to reduce mortality and improve ASP efforts. Laboratories must choose between phenotypic and genotypic AST, each with advantages and limitations. For example, while some resistance genes (eg mecA, vanA/B) reliably predict phenotypic outcomes and inform treatment, others (eg AmpC, OXA-48) pose challenges due to inducibility and variable AST profiles. Reference MacVane and Dwivedi59
The financial burden of rAST implementation remains one of the most significant obstacles. Beyond the initial cost of new testing platforms, laboratories must allocate considerable resources and time to validate assays per the Clinical Laboratory Standards Institute guidelines. 65,66 Given these constraints, it is crucial for microbiology laboratories to collaborate with ID and ASP teams to optimize implementation strategies and maximize clinical impact.
A well-functioning ASP is integral to the success of rAST. A significant barrier to ASP interventions is the lack of ASP pharmacists to act on results in real-time. Reference Timbrook, Morton, McConeghy, Caffrey, Mylonakis and LaPlante8 The impact of rAST is limited without timely reporting, communication, and action. Reference MacVane and Dwivedi59 A quality improvement project conducted by Texas Children’s Hospital, one of the participating sites at the Summit, reinforced this idea, demonstrating that direct communication of rAST results to an ASP pharmacist reduced time to appropriate vancomycin de-escalation from 44 to 4 hours.
AMR is a critical global health problem that can be addressed through faster and more accurate AST, enabling timely adjustments to antimicrobial therapy. While the benefits of rAST coupled with ASP are well supported, implementation requires careful planning to overcome logistical, financial, and communication barriers. Successful implementation depends on interdisciplinary collaboration among ASP/ID pharmacists, physicians, nurses, IP specialists, and microbiologists. The 2024 US Antibiotic Awareness Week theme “Fighting Antimicrobial Resistance Takes All of Us,” aptly captured this shared responsibility. 67
Future directions in NGS
Next-generation sequencing (NGS) technology has transformed healthcare, significantly impacting cancer research and care, Reference Kamps, Brandão, Bosch, Paulussen, Xanthoulea, Blok and Romano68 rare disease identification, and personalized medicine. While its applications in clinical microbiology and ID continue to expand, the full potential of NGS in these fields is yet to be realized. ID diagnostics still primarily rely on conventional microbiology methods, despite the advances in molecular technologies. NGS holds considerable promise in four key areas of ID medicine: organism identification, bacterial and viral genotyping, Reference Lima, Bruzek, Lasher, Vestal and Silbert69 AMR detection, and epidemiological surveillance. Reference Mitchell and Simner70,Reference Dunne, Westblade and Ford71 Traditionally, organism identification has relied on time-consuming methods, such as biochemical tests. The introduction of mass spectrometry has notably improved turnaround times for these tests. Additionally, molecular diagnostics, including laboratory-developed tests, have revolutionized microbial identification. Nonetheless, challenges persist, particularly with slow-growing organisms, such as Mycobacterium and other fastidious bacteria, which often lead to delayed identification. NGS has the potential to dramatically reduce the turnaround time for identifying these challenging pathogens. Reference Wang and Xing72,Reference Cabibbe, Moghaddasi, Batignani, Morgan, Marco and Cirillo73 When implemented effectively for direct-from-specimen use in early patient care, NGS could also lower overall healthcare costs. Reference Rodino and Simner74
Genotyping bacterial and viral pathogens provides valuable insights into treatment strategies, vaccine development, and infection severity, particularly through the detection of virulence factors like toxin production. Reference Miyoshi-Akiyama, Watanabe and Kirikae75 One emerging area of interest is using NGS to identify drug-resistant tuberculosis, a disease that poses significant treatment challenges due to its complex regimens, drug interactions, side effects, and the need for prolonged therapy. Reference Schwab, Perrig and Göller76 Accurately identifying resistance mutations can lead to personalized treatment plans, ultimately enhancing patient outcomes. Furthermore, NGS plays a crucial role in detecting and preventing outbreaks, especially for communicable diseases like tuberculosis. Reference Sundermann Alexander, Rosa and Harris Patrick77 Recent research has shown that combining NGS methodologies with machine learning applied to electronic health records can enhance outbreak detection methods in hospital settings, supporting infection prevention efforts and significantly reducing costs associated with outbreaks. Reference Sundermann, Chen and Kumar78 On a global level, NGS offers the opportunity to improve AMR surveillance comprehensively, but this requires collaboration between high-income and low-income countries. 79 Additionally, accessible reference databases that include genetic data and metadata, formed through inter-regional and international collaborations, are essential to fully realize the potential of NGS for surveillance. 79
While AMR detection with NGS in routine clinical microbiology practice is feasible, it requires comprehensive databases or the simultaneous use of multiple databases. Targeted or enrichment approaches via direct-from-specimen NGS, such as in blood cultures, will also be required for effective AMR detection. Reference Rodino and Simner74 Despite its potential, several challenges have prevented NGS from becoming a routine tool in clinical microbiology. Cost remains a major limitation, making NGS inaccessible to many laboratories. The technology requires significant preparatory work, and interpreting results requires a high level of expertise to translate data into actionable clinical decisions. Reference Lesho, Clifford and Onmus-Leone80 For NGS to be adopted for routine use, quality control, guidelines, user-friendly bioinformatics pipelines, validation, and data storage infrastructure are necessary. Reference Miller, Chiu, Rodino and Miller81 Even with user-friendly informatics software, clinical and microbiological expertise is required to determine the significance of detected organisms. Despite these obstacles, the future of NGS in ID diagnostics appears promising. Realizing its full potential will require collaboration between laboratory professionals and clinicians to overcome challenges and integrate NGS into routine clinical practice.
Call to action
The escalating threat of AMR demands decisive, coordinated action. Failure to act with urgency and collectively will have severe repercussions for healthcare systems and communities globally. The AMR Summit confirmed that we have the knowledge and the tools to make significant progress against this global threat. However, sustained success hinges on interdisciplinary collaboration, advocacy for supportive policies, and adequate resources. We urge healthcare providers, policymakers, researchers, and the public to unite to advance RDTs, optimize ASPs, and promote responsible antibiotic use. Each of us has a role to play in this fight. We call upon every individual and organization invested in the fight against AMR to consider the initiatives outlined in Table 2. The time to act is now. By uniting across disciplines and committing to collaboration, we can mitigate the threat of AMR and protect the health of current and future generations.
Table 2. AMR initiatives

ASPs, antimicrobial stewardship programs; AMR, antimicrobial resistance; RDTs, rapid diagnostic tests; ID, infectious diseases.
Acknowledgments
We acknowledge all of the speakers at the 2024 AMR summit, including Jose Alexander, MD, Kimberly Atrubin MPH, CIC, CPHQ, FAPIC, Esther Babady, PhD, D(ABMM), FIDSA, FAAM, Virginia Calega, MD, MBA, FACP, Kimberly Claeys, PharmD, PhD, Daniel Feinstein, MD, FACP, Debra Goff, PharmD, FCCP, Karen Gonzalez Pittman, David McAdams, PhD, Jessina McGregor, PhD, Jose Montero, MD, FACP, FIDSA, Nicholas Moore, Ph.D., D(ABMM), MLS(ASCP)CM, Melissa O’Neal, PharmD, Denver Niles, MD, D(ABMM), Asa Oxner, MD, Nick Piccicacco, PharmD, BCIDP, AAHIVP, Brittany A Rodriguez, PharmD, BCIDP, Heinz Salazar, MBA-HCM, MLS, Suzane Silbert, PhD, Diane Shader Smith, Julia Szymczak, PhD, Robert Tibbetts, PhD D(ABMM), F(CCM), F(IDSA), Christina Yen, MD.
Author contribution
BAR, AMP, BKH, RT, HS ARA, and SS were responsible for content ideation, all authors contributed to manuscript drafting and editing.
Financial support
None.
Competing interests
AMP, MW, and BKH are paid employees of bioMérieux. DTN is a consultant for bioMérieux. BAR, RT, HS, ARA, and SS work at medical facilities that have been designated as Centers of Excellence (COE) by bioMérieux but receive no financial compensation for COE-related projects; they have no relevant COI to disclose. DM has no relevant COI to disclose.





