Introduction
The Alagoas Antwren Myrmotherula snowi is endemic to the Pernambuco Centre of Endemism in north-east Brazil, and is listed as “Critically Endangered” both nationally and globally (BirdLife International 2023; Ministério do Meio Ambiente e Mundança do Clima 2022; Pereira et al. Reference Pereira, Dantas, Silveira, Roda, Albano and Sonntag2014). The Pernambuco Centre, as with other parts of the Atlantic Rainforest, has suffered extensive deforestation for pasture, sugarcane, bananas, and other agricultural uses, and the small fragments of forest that remain are degraded by decades of tree harvest for timber and charcoal (Mazar Barnett et al. Reference Mazar Barnett, Carlos and Roda2005). At least three endemic bird species have been driven to extinction in the region as a result: the Alagoas Curassow Mitu mitu, Cryptic Treehunter Cichlocolaptes mazarbarnetti, and Alagoas Foliage-gleaner Philydor novaesi (M. mitu is “Extinct in the Wild”, but with a captive population) (Araujo et al. Reference Araujo, Vilela, Phalan, Develey, Pereira Filho, França, Alves and Vasconcellos2023; BirdLife International 2023; Butchart et al. Reference Butchart, Lowe, Martin, Symes, Westrip and Wheatley2018). A further endemic species, the Pernambuco Pygmy-owl Glaucidium mooreorum, has not been recorded for more than 20 years and is listed as “Critically Endangered” (possibly “Extinct”) on the global Red List, and as “Extinct” on the national Red List (Araujo et al. Reference Araujo, Vilela, Phalan, Develey, Pereira Filho, França, Alves and Vasconcellos2023; BirdLife International 2023; Ministério do Meio Ambiente e Mundança do Clima 2022; Roda and Pereira Reference Roda and Pereira2006). While most bird extinctions globally since 1500 have been on islands, these recent losses from the Atlantic Rainforest are suggestive of a new wave of continental bird extinctions (Szabo et al. Reference Szabo, Khwaja, Garnett and Butchart2012).
The known historical distribution of the Alagoas Antwren spans four sites in Alagoas and Pernambuco (Roda et al. Reference Roda, Pereira and Dantas2009; review of all observations on eBird, iNaturalist, WikiAves, and Xeno-Canto up to December 2023). It was described – initially as a subspecies of Unicoloured Antwren M. unicolor, based on three specimens collected at Serra Branca, in the municipality of Murici, in the state of Alagoas in February 1979 (Teixeira and Gonzaga Reference Teixeira and Gonzaga1985). It was elevated to species rank by Collar et al. (Reference Collar, Gonzaga, Krabbe, Madroño Nieto, Naranjo and Parker1992) and Whitney and Pacheco (Reference Whitney and Pacheco1997). There have been many subsequent observations at Murici (note that in some cases, localities are not accurately mapped on citizen science sites, but all are from the same location). Subsequently, it was discovered at three additional sites, all in the neighbouring state of Pernambuco. At Mata de Estado in the municipality of São Vicente Férrer, three specimens were collected in October 1999, with no later records (Roda et al. Reference Roda, Carlos and Rodrigues2003). At Frei Caneca private reserve in the municipality of Jaqueira, several observations were made of individuals and pairs, in February 2003, with later records of single birds in October 2003, December 2007, and November 2009 (Albano Reference Albano2009; Buzzetti Reference Buzzetti2021; Gussoni and Filho Reference Gussoni and Filho2009; Mazar Barnett et al. Reference Mazar Barnett, Carlos and Roda2005; Pereira et al. Reference Pereira, Dantas, Silveira, Roda, Albano and Sonntag2014). Finally, in Mata do Benedito/Engenho Jussará in the municipality of Gravatá, a single male was observed in April 2005, and a pair documented in October 2006 (Albano Reference Albano2014; Albano and Pereira Reference Albano and Pereira2008; Roda et al. Reference Roda, Pereira and Dantas2009).
The type locality is now included within a protected area, Estação Ecológica de Murici, hereafter “Murici”, encompassing 6,116 ha. The protected area has not been fully implemented and the land remains in private ownership. The protected area includes areas known locally as Retiro, Bananeiras, Poço Dantas, and Angelim, and overlaps with the site referred to in the literature as Serra Branca or Pedra Branca. This is the type locality for five species of birds in addition to the Alagoas Antwren, i.e. Alagoas Black-throated Trogon Trogon muriciensis, Orange-bellied Antwren Terenura sicki, Cryptic Treehunter, Alagoas Foliage-gleaner, and Alagoas Tyrannulet Phylloscartes ceciliae (Dickens et al. Reference Dickens, Bitton, Bravo and Silveira2021; Mazar Barnett and Buzzetti Reference Mazar Barnett and Buzzetti2014; Teixeira Reference Teixeira1987; Teixeira and Gonzaga Reference Teixeira and Gonzaga1983a, Reference Teixeira and Gonzaga1983b), and is one of the five localities where the recently described Alagoas Screech-owl Megascops alagoensis occurs (Dantas et al. Reference Dantas, Weckstein, Bates, Oliveira, Catanach and Aleixo2021). The Alagoas Black-throated Trogon and Alagoas Screech-owl are considered sub-populations of T. rufus and M. atricapilla, respectively, by HBW and BirdLife International (2023). Even without these two taxa, Murici is the single site with most “Critically Endangered” birds in Brazil (Develey and Phalan Reference Develey and Phalan2021).
The Alagoas Antwren is a discreet species that forages for invertebrates in the understorey and midstorey of closed-canopy humid forest. It is described as an area-sensitive, obligate participant of mixed-species flocks, and is restricted to the interior of tall, upland forest at least 400 m above sea level (Pereira et al. Reference Pereira, Dantas, Silveira, Roda, Albano and Sonntag2014; Roda et al. Reference Roda, Pereira and Dantas2009). A habitat study suggests that the species is restricted to forest away from edges and with larger trees, conditions that are now rare in the Pernambuco Centre of Endemism (Vilela Reference Vilela2020). It lays a clutch of up to two eggs in a deep cup nest of black fungal rhizomorphs, located in the horizontal fork of a small understorey tree (Gonçalves and Efe Reference Gonçalves and Efe2021). Teixeira and Gonzaga (Reference Teixeira and Gonzaga1985) reported that the species occurred “frequently in mixed flocks”, typically in pairs, and, together with Whitney and Pacheco (Reference Whitney and Pacheco1997), they list 17 bird species that occurred in such flocks. More recently, large mixed-species flocks of understory and midstorey insectivores are no longer observed, perhaps as a consequence of the loss and decline of other flocking species (Develey and Phalan Reference Develey and Phalan2021). The factors in the apparent disappearance of the Alagoas Antwren from Pernambuco are likely similar to those that caused the extinction of the Alagoas Foliage-gleaner: extensive habitat loss and degradation, leaving no intact primary forest within its range, and resulting – together with climate change – in the disruption of the supply of moisture to upland forest (Lees et al. Reference Lees, Albano, Kirwan, Pacheco and Whittaker2014; Pereira et al. Reference Pereira, Dantas, Silveira, Roda, Albano and Sonntag2014). The Alagoas Antwren differs from some other endemic birds of the Pernambuco Centre, such as the Long-tailed Woodnymph Thalurania watertonii (Berryman et al. Reference Berryman, Collar, Crozariol, Gussoni, Kirwan and Sharpe2023), in being restricted to a narrower elevation band, avoiding forest edges, and having a lower population density: all characteristics that make it more vulnerable to extinction.
Describing the loss of the Alagoas Foliage-gleaner, Lees et al. (Reference Lees, Albano, Kirwan, Pacheco and Whittaker2014) and Pereira et al. (Reference Pereira, Dantas, Silveira, Roda, Albano and Sonntag2014) identified the Alagoas Antwren as the next species in line for extinction without urgent intervention. In addition to improved forest protection and restoration, they indicate that predator removals and ex situ conservation would likely be needed, including prior work with model species. These suggestions were reinforced and expanded in conservation planning workshops focused on this species held in 2016 and 2019 (Phalan et al. Reference Phalan, Aguiar, Andrade, Barbosa, Bichinski and Bosso2021). To highlight the gravity of the situation, we provide an update on the distribution, population status, and breeding success of the Alagoas Antwren, based on surveys across its historical range as well as a population census and nest monitoring at Murici. We outline conservation efforts implemented from 2019 onwards to help the species, and further interventions underway in 2023–2024.
Methods
We visited all sites with historical records of the Alagoas Antwren, in Alagoas and Pernambuco states, to seek to confirm whether the species is still present at any of them (Table 1). We also visited 15 sites identified with the help of a species distribution model as having potential habitat for the species, focusing on the larger areas of forest with shaded and relatively open understorey (Ferraz et al. Reference K.M.P.M.B, Bovo, Ortiz, Phalan, Aguiar, Andrade, Barbosa, Bichinski and Bosso2021) (Table 1). Three further sites were surveyed prior to 2019; effort and size of all survey sites are summarised in Table 1. At each site, observers with considerable experience with the species (HALSV and ABA) covered as much of the forest area on foot as possible, using playback to increase the chance of detection, as Alagoas Antwrens, especially males, typically respond strongly to playback during the breeding season. These surveys were carried out in December 2019, March 2020, and from September 2020 to January 2021. Alagoas Antwrens are known to breed between October and March (Gonçalves and Efe Reference Gonçalves and Efe2021; Teixeira and Gonzaga Reference Teixeira and Gonzaga1985; personal observations).
Table 1. Sites investigated in search of Alagoas Antwren Myrmotherula snowi in Pernambuco (PE) and Alagoas (AL), with fragment size, survey dates, and number of days spent searching at each site
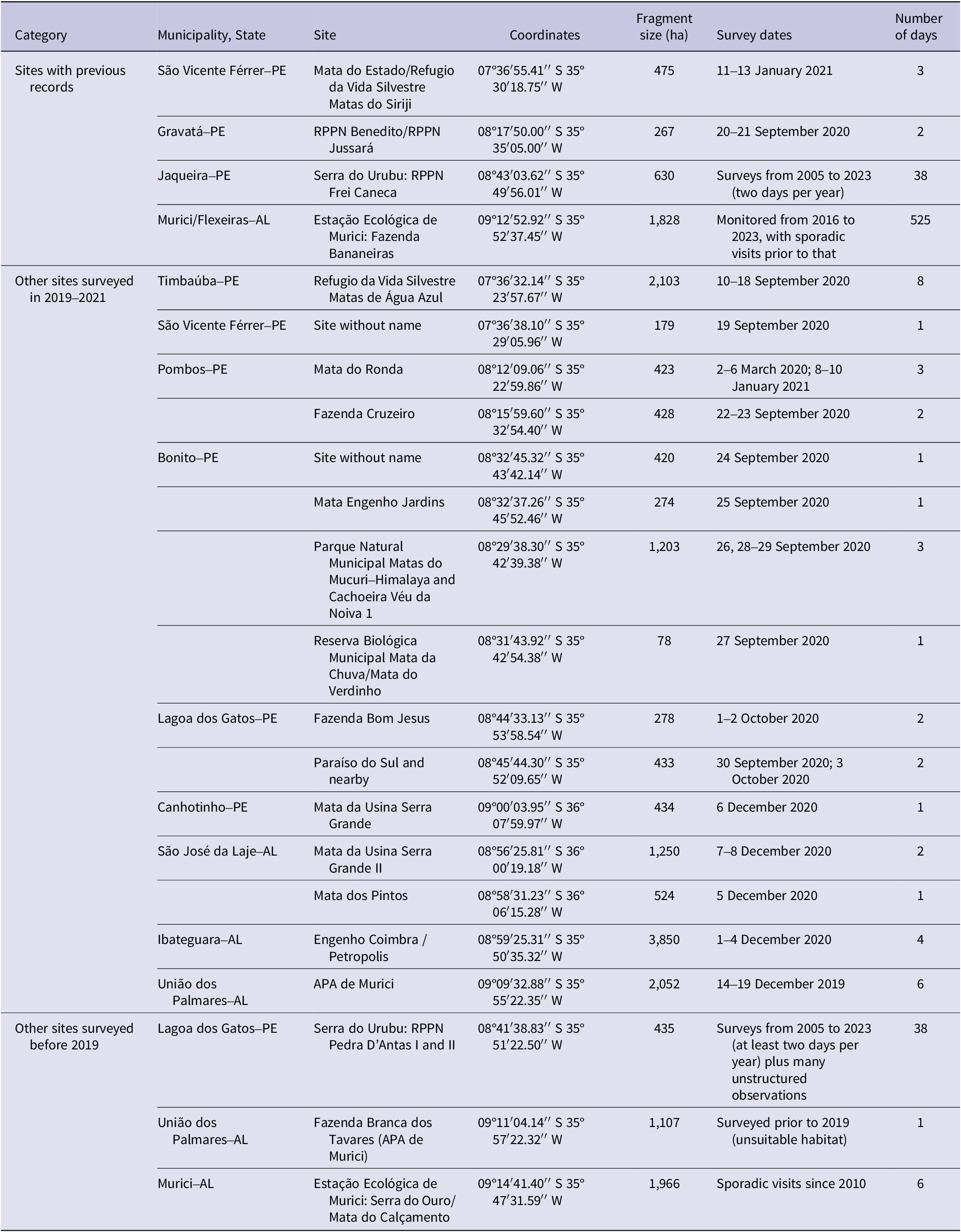
Serra do Urubu, including the Pedra D’Antas and Frei Caneca private reserves (Lagoa dos Gatos/Jaqueira) in Pernambuco, has been thoroughly searched since the 2009 record by the same observers of that last individual to map the territories of all threatened endemic birds, including annual systematic bird surveys over the last 10 years as well as other in-depth studies of some of these species (Gussoni and Pongiluppi Reference Gussoni and Pongiluppi2021; SAVE Brasil unpublished reports). At Murici in Alagoas, more than 150 visits of 2–15 days have been made since 2010 to monitor birds and their nests, including the mist netting of 18 Alagoas Antwrens (Gonçalves and Efe Reference Gonçalves and Efe2021; Vilela Reference Vilela2017, Reference Vilela2020; SAVE Brasil unpublished reports; visits since 2016 shown in Supplementary material Table S1). Some other sites visited prior to 2019 were discarded as having unsuitable habitat (Fazenda Branca dos Tavares) or altitude (Mata do Calçamento).
To complement the survey effort at other locations, and provide information on population trend, we established a grid of sampling plots at Murici, in the largest forest fragment spanning areas known locally as Fazenda Bananeiras, Retiro, and Angelim. Sampling plots deviated from an evenly spaced grid to include all points where the species had been recorded during annual fieldwork since 2010, and those with apparently suitable forest structure, excluding areas with dense understorey (secondary or degraded forest). We established 30 plots, each comprising two non-independent survey points separated by 100 m, and with a minimum distance of 200 m to points in other plots. The locations of these plots were informed by thorough searches carried out throughout the entire fragment between May 2016 and March 2017, using playback at 200-m intervals (Vilela Reference Vilela2017). The number of birds recorded in October–March from that effort is provided for reference, although the methods are not directly comparable with those in later years.
We conducted a population census at Murici in each of three breeding seasons: 2018/19, 2019/20, and 2020/21. Each plot was visited monthly from October to March for a 10-minute point count at each of the two survey points, followed by two minutes of non-continuous playback at one of the points and a five-minute listening period. A total of 946 counts were conducted over these three breeding seasons, with a small number of points not sampled in some months because of poor weather or logistical problems. All points at which antwrens were observed during the period were surveyed in at least four months each year. Additional birds noted outside the systematic point counts were also recorded. Point counts were conducted by HALSV and ABA, with assistance from other observers familiar with the species. As the birds are not individually marked, we summarised observations as apparent individuals, distinguished by sex, age, and location (plot). From our observations, we considered it safe to assume that birds seen more than 200 m apart were different individuals.
Active nest searches were carried out in each of six seasons (2016/17 to 2022/23, with the exception of 2017/18) by walking slowly through areas where Alagoas Antwrens had been observed. Nests were monitored with Bushnell camera traps and in some cases an Intelbras closed-circuit TV camera. From October to March in each of the six seasons, we maintained a team of two to five experienced observers visiting territories of the species each day for at least 10 days each month. Observations of other species, especially those thought to be associated with mixed-species flocks, were made throughout all field expeditions.
Results
We spent a total of 49 days in the field surveying potential sites (13 days in Alagoas and 36 in Pernambuco), not counting the more intensive fieldwork at Murici, which is described in more detail below. We did not locate the Alagoas Antwren at any sites other than Murici (Figure 1, Table 1). Previous survey effort, including by other experienced observers where known, is also summarised in Table 1. The areas assessed as having most potential were not necessarily the largest forest fragments. In addition to Murici, the areas assessed as having most potential by the field team, i.e. extensive areas of tall, closed-canopy forest with relatively open understorey, were in Timbaúba (with good numbers of other endangered species including Orange-bellied Antwren, Ariel Toucan Ramphastos ariel, and Blond Capuchin monkey Sapajus flavius), Serra do Urubu, Gravatá, and the part of Fazenda Usina Serra Grande in São José Lages. There were additional forest fragments in relatively good condition in Gravatá that were not surveyed. While we would not discourage observers from surveying these areas, they are small and subject to extensive edge effects, and if the Alagoas Antwren was not detected in the larger fragments, it is unlikely to be present in the smaller ones.
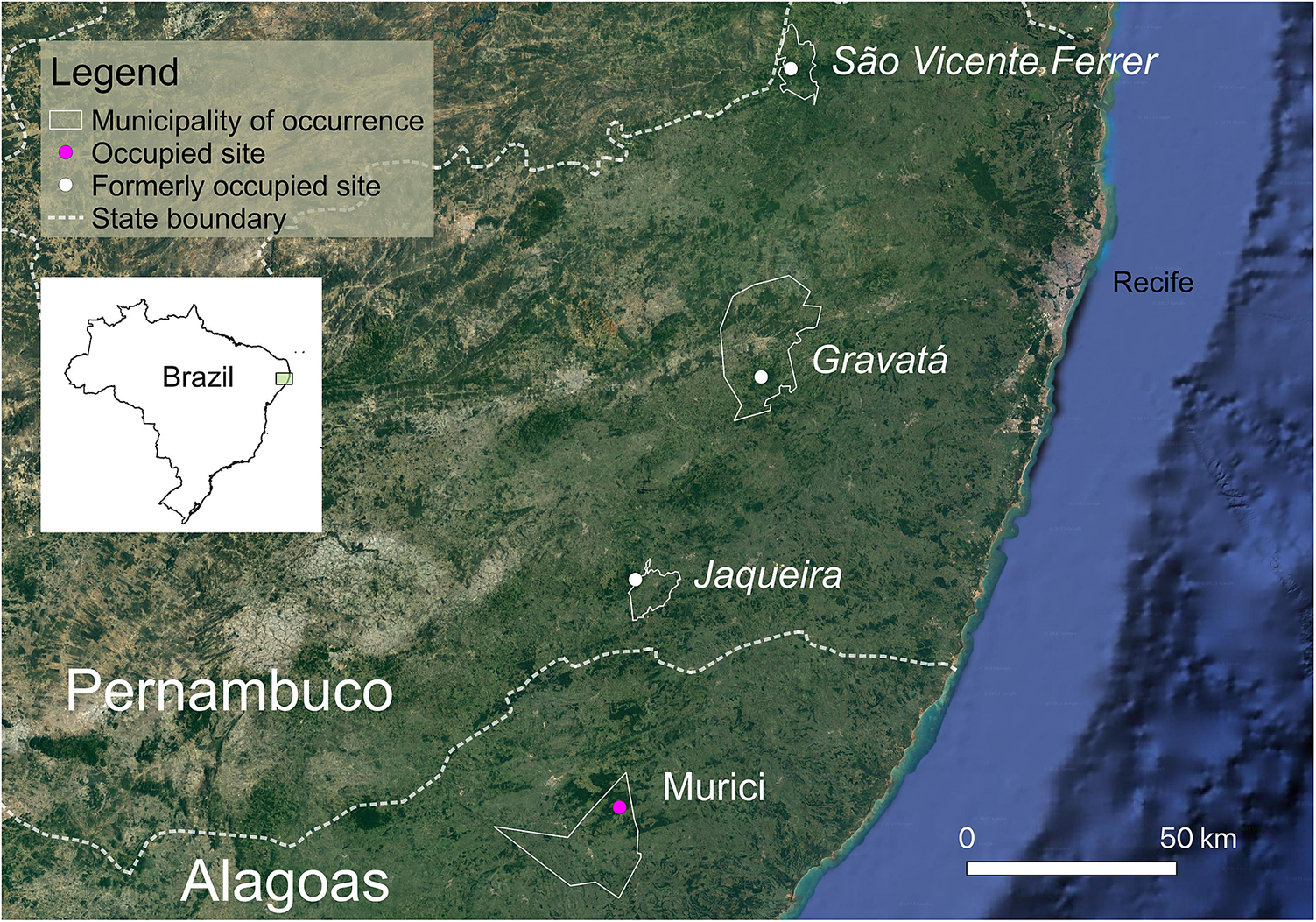
Figure 1. Map showing the four sites at which the Alagoas Antwren Myrmotherula snowi has been recorded. The relevant municipalities are outlined in white, with sites where the species could not recently be found indicated by white circles and italic text, and the one site where the species is still known to persist (Murici) indicated by a pink circle.
At Murici, we spent a total of 525 days in the field from 2016 to 2023, with an estimated total of 1,758 person-hours spent monitoring the population (average of 290 person-hours per season) and 7,875 person-hours searching for nests of understorey birds (average of 1,310 person-hours per season) (see Table S1). During this period, the total number of apparent individuals of Alagoas Antwren, including those outside the systematic point counts, declined from 18 to 6, a decline of 67% over the period of seven years (Table 2). The number of apparent individuals recorded during the systematic counts declined by 50% over a period of three years, from 12 to 6, including both adults and juveniles. If only adults were considered, the rate of decline was even more severe (64%) over the three years. None of these counts was adjusted for detectability.
Table 2. Total apparent number of individuals of Alagoas Antwren Myrmotherula snowi from six breeding seasons (birds were not surveyed in 2017/18), and results of systematic censuses at Estação Ecológica de Murici in three breeding seasons, based on monthly visits from October to March each year. The systematic census is in principle the most comparable from season to season, while the records from all observations provide a minimum population estimate for the species
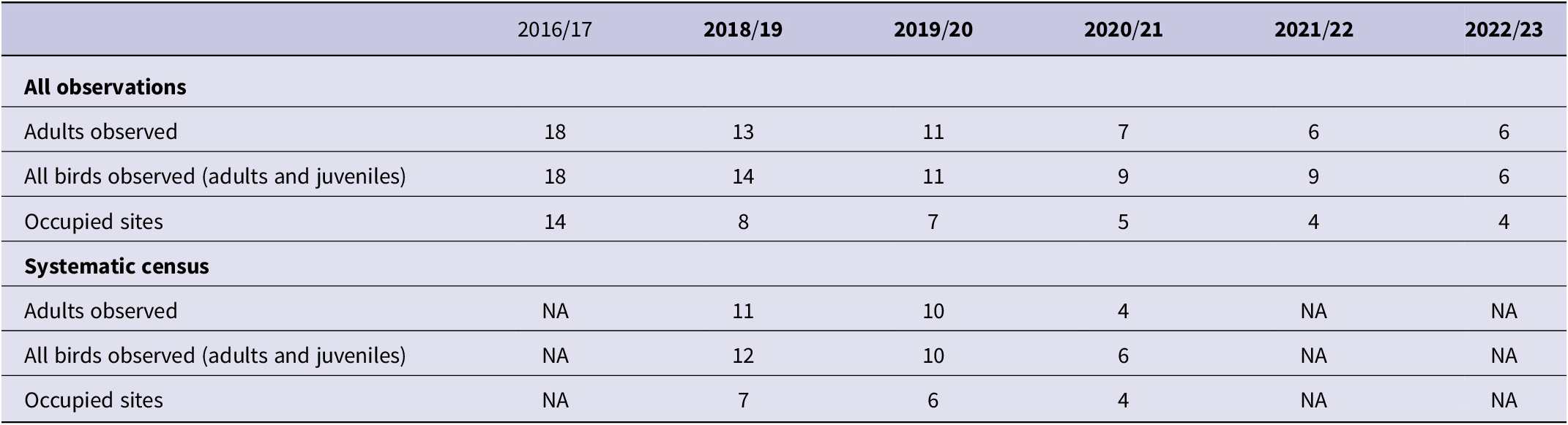
NA = not applicable, because systematic censuses did not take place in those years. Systematic censuses were started in 2018/19, and interrupted after the 2020/21 breeding season to focus on nest finding and protection. In 2016/17 we were in the process of identifying areas of apparently suitable habitat and using playback to find individuals. The totals reported in that year are from October to March, a subset of the year of observations reported by Vilela (Reference Vilela2017).
Combining data from all methods, the number of survey plots in Murici at which the species was detected declined from 14 to 4 over seven years. Looking only at the more standardised systematic census, the number (percentage) of occupied plots – naïve occupancy – declined from seven (23%) to four (13%) over three years (Figure 2, Table 2). The monthly detection probability of Alagoas Antwren was low, ranging between 0.1 and 0.5 depending on the month of survey, with highest detection probabilities from October to December (Vilela Reference Vilela2020). All plots with detection of the species were at least 400 m from the forest edge.
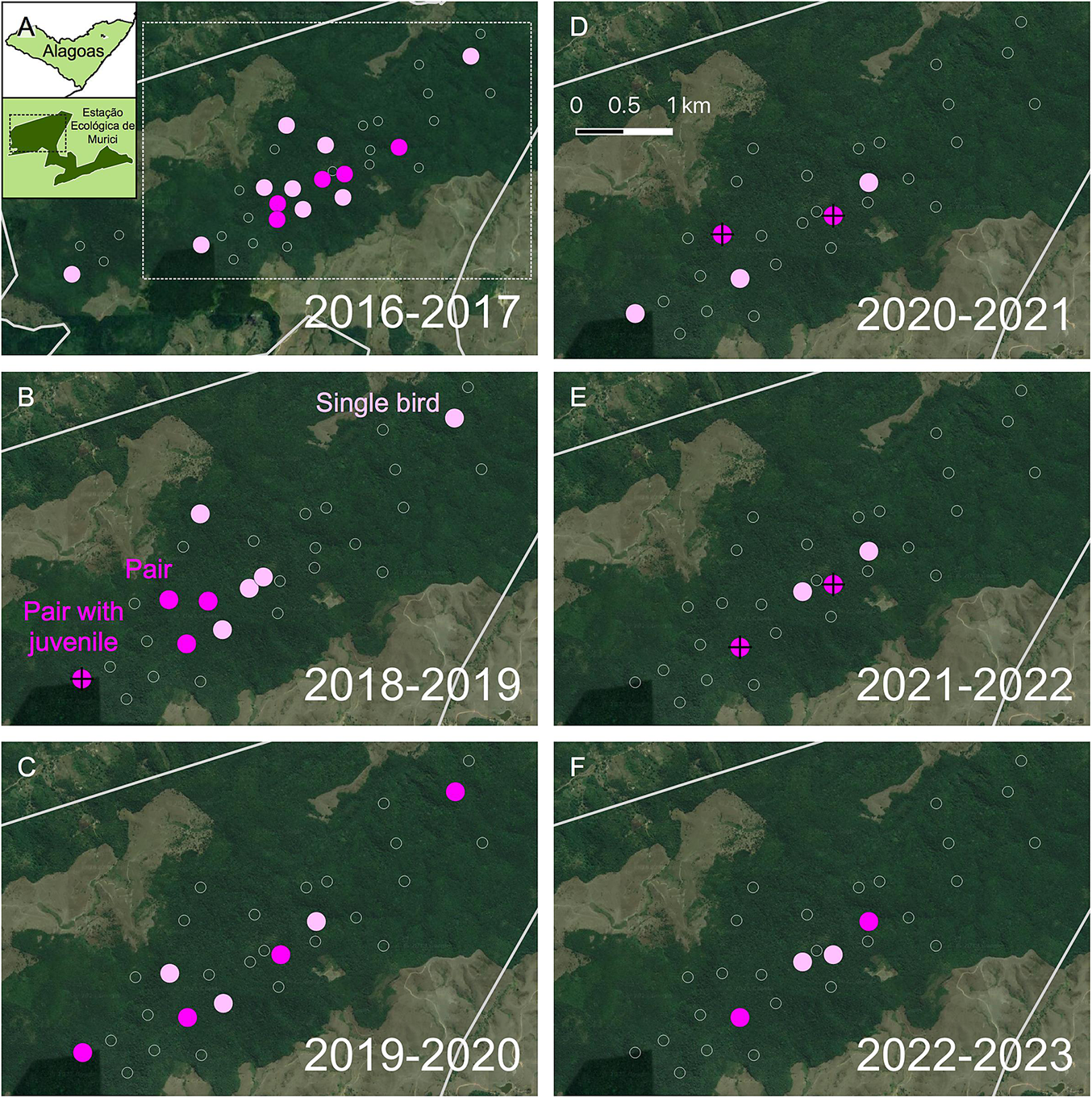
Figure 2. Plots with the occurrence of Alagoas Antwren Myrmotherula snowi from 2016 to 2023. (A–F) Plots with detections of the species in each breeding season from 2016/17 to 2022/23 (surveys were not carried out in 2017/18). The number of plots with detected presence declined from 14 to 4 over the period. Surveyed plots without detections are shown as white outlines.
On average, across the three seasons of systematic counts, twice as many males as females were recorded (average encounter of 5.3 males:2.7 females per year (Table 3, Table S2), which is likely a consequence of their responses to playback and higher detectability when vocalising. While no females were recorded in systematic counts in the 2020/21 breeding season, three were observed in 2021/22.
Table 3. Age and sex of Alagoas Antwren Myrmotherula snowi apparent individuals recorded in six breeding seasons (birds were not surveyed in 2017/18), and during systematic censuses in three breeding seasons at Estação Ecológica de Murici. The total of apparent individuals detected during systematic counts is given in parentheses. The apparent sex ratio of males to females, based on the average of the three years of systematic censuses, was 2:1, but may not reflect the true sex ratio, as males are believed to be more detectable than females. The “unknown” individuals were heard vocalising, but not seen. Systematic censuses were started in 2018/19 and interrupted after the 2020/21 breeding season to focus on nest finding and protection
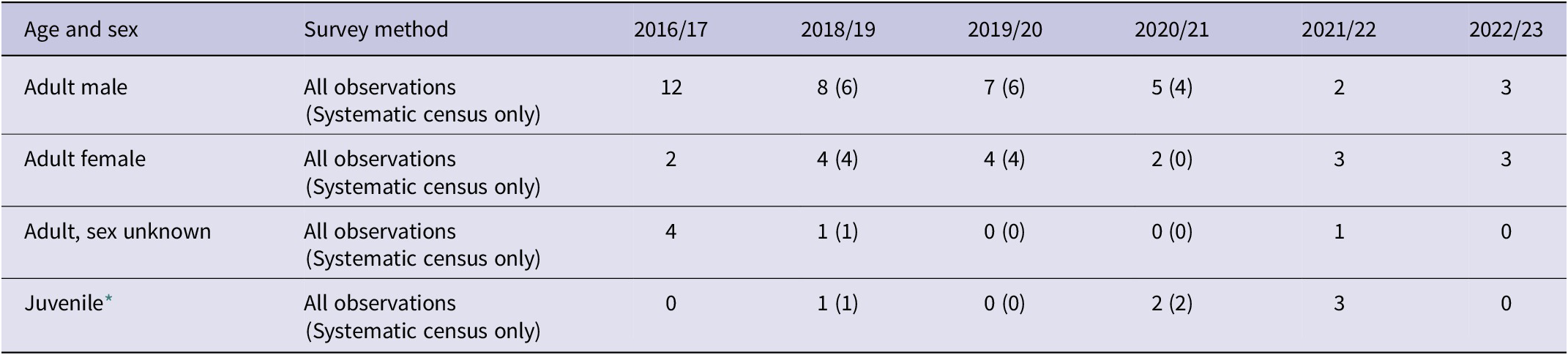
* In the first three years of this series, juveniles were not specifically searched for, and the small number of juveniles recorded in those years is likely a sampling artefact until 2020/21.
Five active nests (with eggs or nestlings) were found: in January and October 2021, and in January, October, and November 2022. Only one previous active nest of the species had ever been found – it was later predated (Gonçalves and Efe Reference Gonçalves and Efe2021). Of the five nests, the contents of three were predated at the nestling stage, one was abandoned early in incubation, and in one, the single nestling fledged successfully. The successful nest was one at which we installed smooth plastic barriers and tubes to exclude climbing predators (snakes or mammals). The nest abandonment may have been related to our activity near the nest when it was found, or to an especially wary female, as every effort was made to minimise time spent at the nest. The male returned to incubate for several days, and the female returned but did not sit on the eggs. Without both members of the pair incubating, the eggs did not survive. The same female started a new clutch within one month of abandoning the nest, re-utilising the nest from which a chick fledged in the previous breeding season. We installed protective barriers at this nest, but a nest camera recorded the nestlings being predated by a snake, the Yellow-bellied Hissing Snake Spilotes sulphureus. Several inactive nests in different states of degradation were also found, and Gonçalves and Efe (Reference Gonçalves and Efe2021) reported finding a nest with damaged eggs in January 2019.
During nest searches in 2019–2023, nests of 33 other bird species were found and monitored, providing information on nest predators in the wider community of understorey birds at Murici. Of 120 active nests for which the outcome could be determined, only 30% produced at least one fledgling. More than half (53%) were predated, with losses from a range of predators including mammals, birds, and reptiles. A smaller number (18%) were abandoned before or during incubation (personal observations, in prep.).
Large mixed-species flocks of understorey or midstorey insectivores (involving more than five species) were not observed at Murici. White-backed Fire-eye Pyriglena leuconota and one or two species of woodcreepers would sometimes assemble at swarms of army ants Eciton sp. The Alagoas Antwren was observed to forage at times with White-flanked Antwren Myrmotherula axillaris or Plain Antvireo Dysithamnus mentalis, and occasionally in loose association with other species such as Plain Xenops Xenops minutus. However, these associations were often weak and short-lived, and Alagoas Antwrens often continued foraging alone. Bird species believed to be mixed-flock leaders are now absent or rare at Murici. The Alagoas Foliage-gleaner is “Extinct”. A single Cinereous Antshrike Thamnomanes caesius was observed in December 2020, 16 years after the last documented record (Xeno-Canto recording XC237593 by J. Minns), but not thereafter. Red-crowned Ant-tanagers Habia rubica have been recorded as mixed-flock leaders elsewhere, but not at Murici. They have been observed frequently from 2018 onwards in parts of the protected area, after 15 years without records, suggesting a population recovery.
Discussion
Our results show that the Alagoas Antwren is perilously close to extinction. Despite surveying all historical sites and other suitable areas within its historical distribution, we found this species only at a single site, Murici, to which it is now likely restricted. The Alagoas Antwren can be difficult to detect: it is small, unobtrusive, difficult to observe, and in our experience, rarely vocalises outside a brief period between 05h00 and 07h00. Our surveys were of short duration at many sites, leaving open the possibility of false negatives. However, we have several reasons to think it unlikely that the species still exists at sites other than Murici. First, the species responds readily to playback during the breeding season. We avoided using playback in confirmed territories to avoid causing disturbance, but used it at potential sites where the species had not yet been detected – this was especially the case during the prospective surveys in 2016/17. Second, there are few sites with such a large area of good-quality forest as Murici. The sites with equivalent forest quality were smaller than Fazenda Bananeiras, and larger sites were either fragmented, degraded, at unsuitable altitudes, or a combination of these. If the species is disappearing even at Murici, then it seems highly unlikely that other populations could have persisted for decades, stable but undetected, in smaller or more degraded fragments.
At Murici, the population is very small and rapidly declining. It declined by around 50% over a period of three years, and we recorded just six adult individuals in the most recent breeding season (2022/23). We recorded more males than females. We caution against assuming that this indicates a biased sex ratio in the population as our reliance on vocalisations and playback means that males are more likely than females to be detected. This is shown clearly by the apparent 6:1 sex ratio in 2016/17 fieldwork, which relied most heavily on playback. The fact that we detected no females during systematic counts in the 2020/21 breeding season, but three in the subsequent breeding season, indicates that some individuals, perhaps especially females, can go undetected through a breeding season. We did, however, detect females in all years when combining all observations. It is therefore probable that the true population size is somewhat larger than the number of birds we recorded. Our subjective impression is that it is unlikely to be very much larger, and is probably now in single figures. Points at which birds were detected only once or twice may have been occupied by non-breeding individuals who did not stay in place for the full breeding season, or even by birds who had already been recorded at other points. Individual marking would be needed to resolve this question.
Like the other species who have disappeared from the Pernambuco Centre of Endemism in recent decades, the Alagoas Antwren has undoubtedly been strongly affected by deforestation and forest degradation. The decline and disappearance of forest birds in this region is an example of extinction debt, or the time lag between habitat loss and extinction (Brooks et al. Reference Brooks, Tobias and Balmford1999; Develey and Phalan Reference Develey and Phalan2021). Habitat availability alone seems unlikely to explain recent declines. At Serra do Urubu, for example, a fragment with more than 1,000 ha protected in private reserves, the species disappeared despite no apparent change to vegetation structure. However, reduced to small, fragmented populations by habitat loss, the Alagoas Antwren and other species become vulnerable to a range of other potential problems, incuding inbreeding and demographic stochasticity. The proximate factors in the species’ recent decline, reducing survival or reproductive success, are impossible to identify with any certainty (Robinson and Sherry Reference Robinson and Sherry2012). Our main hypotheses for this decline include the following.
-
1) Collapse of mixed-species flocks, resulting in reduced foraging success and increased predation risk for antwrens (Greenberg Reference Greenberg, Boinski and Garber2001; Mangini et al. Reference Mangini, Gandoy, Areta and Blendinger2023). Alagoas Antwrens are still often seen in the company of White-flanked Antwrens and occasionally one or two other species, but larger mixed-species flocks are no longer observed in the understorey and midstorey (Develey and Phalan Reference Develey and Phalan2021).
-
2) Low breeding success caused by increases in population density of mesopredators, resulting from alterations to vegetation structure, exposure to edge effects in a fragmented landscape, and the indirect effects of hunting (Gonçalves and Efe Reference Gonçalves and Efe2021). Our observations suggest low breeding success for Alagoas Antwren and other understorey birds at Murici, with the most important predators of open-cup nests being mouse opossums Marmosa sp. and Yellow-bellied Hissing Snakes.
-
3) Reduced foraging success caused by declines in insect prey availability related to changes in forest structure, edge effects, climatic changes, and pesticide drift. Since 2000, Alagoas has experienced drier conditions in many more years than occurred in the 1970s, 1980s or 1990s (Oliveira-Júnior et al. Reference Oliveira-Júnior, de Gois, Silva, de Oliveira Souza, Jardim and Silva2021). The climate has warmed significantly since the 1980s (Silva et al. Reference Silva, Cabral Júnior, Rodrigues and Silva2023). Deforestation and removal of large trees leaves the remaining fragments of forest less able to buffer adverse climatic conditions (Betts et al. Reference Betts, Phalan, Frey, Rousseau and Yang2018). Many tropical rainforest invertebrates, – the prey of the Alagoas Antwren – are sensitive to extremes of dry or wet conditions (Newell et al. Reference Newell, Ausprey and Robinson2023). Aerial pesticide applications over sugarcane may have resulted in drift of pesticides into Murici and other forest fragments in past decades, affecting insects and insectivores (Silveira et al. Reference Silveira, Olmos and Long2003; personal communications from local population).
It is perhaps already too late to save the Alagoas Antwren, but there are examples of species that were recovered from similarly low numbers of individuals, including the Mauritius Kestrel Falco punctatus, Chatham Island Black Robin Petroica traversi, and Echo Parakeet Psittacula eques (Jones and Merton Reference Jones, Merton, Ewen, Armstrong, Parker and Seddon2012). Success in such cases depended on interventions such as control and exclusion of predators from nest sites, captive breeding, supplementary feeding, and translocation. Further projects are currently working to save other “Critically Endangered” birds using similar methods (Ghestemme et al. Reference Ghestemme, Matohi, Blanvillain, Portier, Barh and O’Brien2019; Paxton et al. Reference Paxton, Crampton, Vetter, Laut, Berry and Morey2022). Inspired by these examples, and aided by a multi-stakeholder collaborative conservation planning process (Phalan et al. Reference Phalan, Aguiar, Andrade, Barbosa, Bichinski and Bosso2021), we are working to implement a conservation response, with three main elements.
The first element is to find and protect nests of the species, as reproductive success is apparently very low. Even with substantial effort, finding nests has proved a challenge, with only five active nests located to date, four of which failed. We are testing the effectiveness of barriers (plastic tubes and funnels) to exclude predators, as well as cutting connections (vines and branches) to impede access by arboreal predators. We are testing these methods with Blue-backed Manakin Chiroxiphia pareola and Plain Antvireo, which construct similar nests in similar locations to those of the Alagoas Antwren. The successful nest was one at which we installed protective barriers, but another nest with barriers installed was predated. We have improved the barrier design, aware that success will need to be repeated consistently for a chance of population recovery. In the current breeding season (2023/24) we have also begun predator removal, using nest boxes and traps to capture and translocate snakes and small marsupials away from the area around Alagoas Antwren nests. Successful recruitment of fledglings from two or three nests would make a real difference to the population trajectory of the Alagoas Antwren, and give more time to improve other strategies such as captive breeding (see below).
The second element is to strengthen conservation of the protected area at Murici within which the last known individuals persist, including lobbying and communication efforts to ensure that the importance of conserving this area is widely recognised. This protected area exists largely because of efforts by conservationists to raise the profile of the area in the late 1990s (with the support of the British BirdFair and BirdLife International’s Brazil Programme, later SAVE Brasil, as well as other organisations (Develey et al. Reference Develey, Goerck, Campanili and Girard2020), and forest condition is likely improving now that logging and hunting have been brought to an end through the actions of local personnel from the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio). What is needed now is for landowners to be compensated so that deforested areas within the protected area, currently managed as pasture, can be restored as rainforest. In the long term, forest restoration is essential to expand and connect suitable habitat for the Alagoas Antwren and other forest-dependent species.
The third element is to develop methods for breeding antwrens in captivity, working with Plain Antvireo and White-flanked Antwren as model species. This is a challenge as there are no established captive populations of any antwren species (Phalan et al. Reference Phalan, Aguiar, Andrade, Barbosa, Bichinski and Bosso2021; but see Lima et al. Reference Lima, Pacheco and Cohn-Haft2007; Touchton et al. Reference Touchton, Seddon and Tobias2014). Up to the time of writing in late 2023, we had captured a total of 35 individuals of these species, of which 12 died, 6 were released because they were not adapting well to captive conditions, 15 were released for other reasons or escaped, and 2 were maintained alive with the aim of breeding them. Given the high failure rate, taking the last Alagoas Antwrens into captivity cannot be recommended at this time. We continue to refine methods and protocols for maintaining and breeding such species, including from the egg stage, and the results of this work will be described elsewhere.
The data presented here highlight the perilous situation of the Alagoas Antwren. Two species with which it co-existed in the recent past, Alagoas Foliage-gleaner and Cryptic Treehunter, are gone forever. Our findings indicate that the Alagoas Antwren is now also very close to extinction. Its chances of survival do not appear high, given its rapid decline, tiny population, and the limited success so far with conservation interventions. Captive breeding may offer some hope, but until greater success is achieved with captive management and reproduction of model species, this is not a viable option. In the short term, the most promising interventions are those to protect nests from predation and improve breeding success. Despite all of these concerns and challenges, while there are still birds, there is still a chance to save the Alagoas Antwren from extinction.
Acknowledgements
We thank all of the fieldworkers and bird observers who have contributed to knowledge of this species, the Bolsas FUNBIO programme, Márcio Efe and his LABECAN research group at the Federal University of Alagoas, and participants at the conservation planning workshops for this species in 2016 and 2019 – the latter facilitated by the IUCN SSC Conservation Planning Specialist Group. Work was carried out under licence from the Chico Mendes Institute for Biodiversity Conservation (SISBIO 78775). We thank Nigel Collar and an anonymous referee for their comments which greatly improved the paper. We are grateful for financial support from the National Geographic Society, American Bird Conservancy, BirdLife International, Aage V. Jensen Charity Foundation, Fondation Segré through IUCN Save Our Species, Neotropical Bird Club, Mohamed bin Zayed Species Conservation Fund, Brazilian National Council for Scientific and Technological Development (CNPq), “Conservando o Futuro” fund of FUNBIO and Instituto Humanize, SAVE Brasil, Instituto Claravis, and Parque das Aves.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0959270924000078.







