Introduction
Breeding densities and reproductive output of secondary cavity nesting birds can be limited by a shortage of natural nest cavities (Newton Reference Newton1994). One approach to increase the number of nest cavities available is to install artificial nest structures such as nest-boxes. Providing nest-boxes can decrease competition for cavities, allowing for increased breeding activity (Newton Reference Newton1994, Radford and du Plessis Reference Radford and du Plessis2004). In some cases, clutch size and breeding success could be linked to the floor area and the size of the nest-box, respectively (Korpimäki Reference Korpimäki1985). Predators may lack experience with nest-boxes (Mitrus Reference Mitrus2003), or be excluded by the size of the box entrance (Briskie et al. Reference Briskie, Shorey and Massaro2014), allowing pairs occupying nest-boxes to have greater breeding success than those occupying natural cavities. Artificial nests can therefore be used as a conservation tool to improve the reproductive output and population status of threatened bird species with nest-site-limited populations. Artificial nests have assisted population increases in seabirds (Bolton et al. Reference Bolton, Medeiros, Hothersall and Campos2004, Morrison and Gurney Reference Morrison and Gurney2007, Bried et al. Reference Bried, Magalhães, Bolton, Neves, Bell, Pereira, Aguiar, Monteiro and Santos2009), waterfowl (Dennis and Dow Reference Dennis and Dow1984, Pöysä and Pöysä Reference Pöysä and Pöysä2002) and raptors (Ewins Reference Ewins, Bird, Varland and Negro1996, Fargallo et al. Reference Fargallo, Blanco, Potti and Viñuela2001, Catry et al. Reference Catry, Alcazar and Henriques2007), and enhanced reproduction in Gouldian Finches Erythrura gouldiae (Brazill-Boast et al. Reference Brazill-Boast, Pryke and Griffith2013) and Great Hornbills Buceros bicornis (Pasuwan Reference Pasuwan2000). However, the provision of artificial nests may not always be beneficial. The breeding success of Northern Goshawks Accipiter gentilis and Common Buzzards Buteo buteo was reduced when breeding in artificial nests (Björklund et al. Reference Björklund, Valkama, Saurola and Laaksonen2013), although the authors admitted that no clear reason could be found for this result. Nest-boxes created ecological traps for Great Tits Parus major (Mänd et al. Reference Mänd, Tilgar, Lõhmus and Leivits2005, Demeyrier et al. Reference Demeyrier, Lambrechts, Perret and Grégoire2016). Therefore, the conservation benefits of providing artificial nest cavities might be species-specific, and their value in each case needs to be tested.
In addition to nest-site availability, reproductive performance in birds can be affected by various social and environmental factors, which should be considered in any assessment of the role of artificial nests in improving breeding performance. Factors that potentially influence the breeding success in Southern Ground Hornbills Bucorvus leadbeateri (hereafter ground hornbill) include group size, laying date, nest-site characteristics, previous breeding history and nest predation. In cooperatively breeding ground hornbill groups, helpers can increase reproductive output by enhancing nest security, territory defence and provisioning for the female and/or the nestling(s) (Kemp Reference Kemp1988, du Plessis 1991, Blackmore and Heinsohn Reference Blackmore and Heinsohn2007). Larger groups thus can have greater reproductive success (Wilson and Hockey Reference Wilson and Hockey2013).
Generally, food availability is a key factor affecting avian breeding propensity and success (Perrins Reference Perrins1991, Siikamäki Reference Siikamäki1998, Morrison and Bolger Reference Morrison and Bolger2002). In African savannas, invertebrate prey abundance is closely linked with rainfall (Kemp Reference Kemp1976, Sinclair Reference Sinclair1978, Cumming and Bernard Reference Cumming and Bernard1997), with the previous-year’s rainfall influencing vegetation growth (Dudney et al. Reference Dudney, Hallett, Larios, Farrer, Spotswood, Stein and Suding2017), which in turn affects arthropod abundance. Rainfall could therefore be a useful proxy for prey abundance for ground hornbills. Below-average rainfall years may mean leaner times going into the breeding season (Robb et al. Reference Robb, McDonald, Chamberlain, Reynolds, Harrison and Bearhop2008). If conditions are poor, adults may forego breeding, rather investing energy in survival or maintaining body condition, including moult (Langston and Rohwer Reference Langston and Rohwer1996, Dietz et al. Reference Dietz, Rogers and Piersma2013).
The ground hornbill is the largest cooperatively breeding bird in the world. It inhabits tropical and subtropical savanna habitats from Kenya to South Africa, with the South African population estimated to be 1,290–2,380 individuals, and declining (Taylor and Kemp Reference Taylor, Kemp, Taylor, Peacock and Wanless2015). The species is listed as ‘Vulnerable’ globally (BirdLife International 2016), but as ‘Endangered’ in South Africa where habitat loss and secondary poisoning have caused rapid declines (Taylor and Kemp Reference Taylor, Kemp, Taylor, Peacock and Wanless2015). Average group size is 3.5 individuals (range 2–12), and groups occupy large territories year-round of ∼100 km2 (Kemp and Kemp Reference Kemp and Kemp1980). In South Africa, they breed during the austral spring and summer and typically lay two eggs, 3–14 days apart, and if both eggs hatch, only one chick is raised (Kemp and Kemp Reference Kemp and Kemp1980). This species is naturally slow to breed, with one group raising one chick to fledging on average every nine years (Kemp Reference Kemp1988). Adding to this, a lack of nesting sites that are typically large cavities in trees and rocks (Kemp and Begg Reference Kemp and Begg1996) was identified as a factor further limiting population growth (Kemp and Begg Reference Kemp and Begg1996). Wind and African Elephants Loxodonta africana cause both the creation and loss of tree nesting sites (Kemp and Begg Reference Kemp and Begg1996). Given the scarcity of natural nesting cavities (only 13 in the study area), 31 wooden nest-boxes were installed to improve the reproductive output of ground hornbills (Wilson and Hockey Reference Wilson and Hockey2013) and thus to assist conservation efforts aimed at slowing and reversing the population decline in the wild.
We investigated the relationship between various factors and breeding attempts (at least one egg laid) as well as breeding success (whether or not a chick fledged) for the ground hornbill in north-eastern South Africa. Is there any evidence to support that groups occupying nest-boxes attempt breeding more often and are more successful in them than groups occupying natural nests? We predicted that groups occupying nests that were positioned higher off the ground would have higher breeding success due to reduced risk of predation (Negro and Hiraldo Reference Negro and Hiraldo1993, Wilson and Cooper Reference Wilson and Cooper1998, Saab et al. Reference Saab, Dudley and Thompson2004). Furthermore, we predicted that rainfall and breeding would have a positive relationship due to the link between rainfall and food availability in the African savannas (Kemp Reference Kemp1976, Sinclair Reference Sinclair1978, Cumming and Bernard Reference Cumming and Bernard1997). By increasing the number of viable nesting sites in an area with only a few natural nests, we expected the population in the study area to grow.
Methods
In this study, a breeding attempt refers to the laying of at least one egg per group per year; while breeding success refers to the fledging of a chick per group per year, but only considering those groups that attempt breeding. The proportion of groups observed per year that attempt to breed, and therein that also succeed, are therefore the outcomes of interest. All average values reported below are accompanied by measured standard deviations (SD).
Study area, climate and nests
This study took place in the Associated Private Nature Reserves (1,800 km2, centred on 24.16°S, 31.18°E) adjacent to the Kruger National Park in north-eastern South Africa during 2000–2015. The study area experiences rainfall during spring and summer (October-March) and is dry during autumn and winter (April-September), with a mean annual precipitation of 500 ± 90 mm, which is patchily distributed. Altitude ranges from 300 to 500 m asl. Daily mean minimum and maximum temperatures range seasonally from 10–20° C and 20–33° C, respectively. The geomorphology is undulating, with rocky outcrops in the north and flat, grassy plains in the south. The vegetation varies from open savanna to closed woodland. Monthly rainfall data were obtained from 14 rain gauges checked daily throughout the study area. Rainfall for each group’s home range was recorded at the nearest rain gauge to the centre of the home range. Rain gauges were situated at a mean distance of 5 ± 3 km (range 1–11 km) from each nest.
Nest-boxes constructed from large Pinus and Eucalyptus spp. logs supplied by local forestry companies were placed throughout the study area in trees large enough to support the heavy structures (Wilson and Hockey Reference Wilson and Hockey2013). Logs were cut in half longitudinally, hollowed using a chisel and hammer to create a cavity approximately 50 cm across by 80 cm high, and the two faces re-joined with metal rods. A rectangular or oval nest entrance was cut in the upper half of the log approximately 30 cm wide by 40 cm high. Fourteen nest-boxes were placed during the winter (non-breeding season) of 2002, 11 during 2003, three during 2005, one during 2009 and two during 2013. Nest-boxes cost ZAR 200 (US$ 15) to construct. At the end of the study period, 20 of the 31 erected nest-boxes were still standing, of which 40%, 35%, 15% and 10% were 14, 13, 11 and 3 years old, respectively. Those that collapsed had lifespans of 9 ± 3 years (range 3–12 years). The location and orientation of the entrance of each nest-site, whether artificial or natural, were recorded with a Garmin 76CSx Map GPS. The diameter at breast height (DBH) of trees containing nests was recorded. In the case of vertical, chimney-like natural tree cavities, nest orientation was scored as ‘top’. Other variables recorded included nest height (ground to the lowest lip of the nest entrance); entrance height and width; floor depth (lowest lip of the cavity entrance to the cavity floor); and floor length and width. The nest floor area and entrance size were calculated as ellipses: area (m2) = π × ½ length × ½ width. Areas for entrances that were rectangular were calculated as length × breadth. Cavity wall thickness was recorded as the average of four measurements of the thickness at the nest entrance measured to the nearest 1 mm using Vernier callipers.
Groups and breeding
Groups were identified by their location (as they are highly territorial), group size and age/sex composition as well as by the presence of known ringed individuals. The number of individuals in each group was recorded annually during 1998–2015, except for 2008 and 2010. Observations of breeding attempts took place during 2000–2015, but data for failed breeding attempts were not available for 2008 and 2009, so these years were excluded from the analyses. The maximum extent of a group’s home range was determined by creating a polygon containing all group sighting data since 1998. Nest checks every 7–10 days commenced at the end of September each year. Laying date was estimated as the middle date between the check when an egg was first discovered and the previous nest check. Each nest was visited again 40–42 days after the estimated lay-date to check for hatching of the first chick, then again after another few days to check for hatching of the second chick. Second chicks are only fed and raised if the first egg does not hatch or if the first chick dies a few days after hatching (Kemp and Kemp Reference Kemp and Kemp1980). Therefore, when the first chick was present and healthy, the redundant second chick was harvested shortly after hatching to be captive reared for a reintroduction programme or to be incorporated into a captive breeding programme. The remaining chick in the nest was checked again 40 days after hatching and before fledging at 80 days. The nest was re-checked 10 days after the predicted fledging date to confirm that the chick had fledged.
The percentage of open habitat within a 3-km radius around each nest was determined using a vegetation cover map of the study area (van Rooyen Reference van Rooyen2005). Open habitat was maintained by bush-clearing. Open habitat on the vegetation cover map was defined as disturbed areas (old fields, airfields) and open woodland. Four types of open woodland were based on dominant tree species composition: (a) Senegalia nigrescens - Combretum hereroense, (b) C. apiculatum - Sclerocarya. birrea - Strychnos madagascariensis, (c) C. apiculatum - S. birrea and (d) Terminalia sericea - C. zeyheri - Pterocarpus rotundifolius. The amount of riparian habitat (km2) was the area of the group’s home range covered by Phragmites-dominant habitat (van Rooyen Reference van Rooyen2005), identified as being the preferred habitat for ground hornbill groups during the breeding season in the Associated Private Nature Reserves (Wyness Reference Wyness2011).
Statistical modelling of the relationship of environmental and social factors with breeding attempts and breeding success
Generalised Linear Mixed Models (GLMMs, package lme4; Bates et al. Reference Bates, Maechler, Bolker and Walker2015) were fitted to the observational data, using R (R Core Team 2014), to investigate factors related to breeding. To study breeding attempts, data for 19 groups observed during 2000-2015 was considered (n = 235 group-years). To study breeding success amongst those groups that attempted breeding, data for 18 groups observed during 2000–2015 was utilised (n = 117 group-years); this dataset excluded the years 2008 and 2009 as data for unsuccessful nests were not available. The GLMMs allow for estimating systematic relationships between ‘fixed effects’ and breeding, while also allowing less well- understood group-specific or year-specific ‘random effects’ to describe variation in breeding (all described below). The models used a binomial distribution with a logistic link function; i.e. logistic regression models were used. Results in the form of Odds Ratios (ORs) are provided with 95% confidence intervals.
Data exploration followed the protocols described in Zuur and Ieno (Reference Zuur and Ieno2016). Model assumptions were verified using plots of residuals against fitted values as well as residuals against each explanatory variable. Multicollinearity of variables was identified by high variance inflation factors (VIF > 3), and, when this occurred, which one of the collinear variables to retain was decided based on model AIC values.
A ‘full model’ with all identified potential factors was fitted to the data. Additionally, to present a more parsimonious description of relationships, ‘reduced models’ were created by sequentially removing the variable with the largest P-value (in a test for significance), until all P-values were < 0.1. In testing the importance of a variable, P-values were obtained using either Wald approximations (Wald Reference Wald1947) for single parameter variables, or likelihood ratio tests for multiple-parameter variables. When a non-linear or monotonic relationship was suspected, based on initial data exploration or previous study results, the variable was first entered into the full model as a cubic polynomial using B-splines, and AIC comparisons then used to determine whether to simplify to a linear term. Each rainfall variable was included in the models as a continuous variable.
The marginal and conditional R2 values for mixed-effect models (R2m and R2c; Nakagawa and Schielzeth Reference Nakagawa and Schielzeth2013), were computed using the package ‘MuMIn’ in R to estimate the percentage of variation in the model explained by fixed effects (R2m) versus both random and fixed effects (R2c). When random effects were estimated to be zero, a Hosmer-Lemeshow goodness of fit test was performed to assess model fit (Hosmer and Lemeshow Reference Hosmer and Lemeshow1980).
Factors potentially related to breeding attempts
Not all groups had access to a nest-box, and not all groups had access to a natural nest. Therefore, to understand how the availability of nest-boxes or natural nests related to the probability of group breeding attempts, variables were incorporated that described the nesting resources to which each group had access. These variables were the presence of nest-boxes (binary), nest-box age (the age in years of the youngest nest-box in the home range, if any were present) and the presence of natural nests (binary; there was only ever a maximum of 1 natural cavity per home range).
Other variables included in the breeding attempts model were home range size (km2), the amount of riparian habitat (km2), whether a group was successful at raising a chick the previous season (binary), group size (discrete, size of the group during the preceding winter), rainfall over the previous year (mm, total rainfall during July-June), dry season rainfall (mm, total rainfall during April-August) and spring rainfall (mm, total rainfall during September-November). Group identity and year were included as random intercepts to account for group- or year-specific effects on breeding attempt.
Factors potentially related to breeding success
To understand the relationship between breeding success and environmental and social variables, the probability of breeding success was modelled as a function of nest type (binary, 1 for nest-box, 0 for natural cavity), nest height (m), nest entrance orientation (categorical; N, S, W, Top, none were placed facing E), thickness of the nest cavity wall (mm), open habitat around nest (as a percentage), timing of breeding (days after 1 September that the first egg was laid), group size (discrete, group size during winter), the number of breeding groups (discrete, the number of groups that attempted breeding that season), rainfall over the previous year (mm, during July-June) and breeding season rainfall (mm, during September-March).
For the breeding success model, year was also included as a cubic polynomial fixed effect, based on the initial data exploration which suggested a clear trend (non-linear and non-monotonic) in breeding success over the course of the study. Based on findings in a previous study (Wilson and Hockey Reference Wilson and Hockey2013), breeding season rainfall was initially included as a non-linear term, as well as an interaction between nest type and the percentage of open habitat.
Nest failure
No formal studies of predation on Southern Ground Hornbill nests had been previously undertaken, but predators were assumed to include species adept at climbing trees such as genets Genetta spp., slender mongoose Galerella sanguine and leopard Panthera pardus. Camera traps identified the predator at one nest, and two predators were inferred based on their presence in the nest when recently broken ground hornbill eggs were found on the ground. Other nests were assumed to have been predated because of the presence of broken egg shells or the remains of chicks found in or near the nest, or by the disappearance of eggs or chicks. We acknowledge that these cues likely overestimate predation events, because some nest contents might be scavenged after nest failure due to other reasons, and therefore nest failure data are presented with this in mind.
Results
Summary statistics for nest characteristics and model variables described below are provided in Tables 1 and 2.
Table 1. Summary statistics for the dimensions of natural tree cavity nests and nest-boxes occupied by Southern Ground Hornbills Bucorvus leadbeateri. SD = standard deviation.

Table 2. Summary statistics for analysed social and environmental factors for Southern Ground Hornbill Bucorvus leadbeateri breeding attempts and breeding success. SD = standard deviation.
Characteristics of natural nests and nest-boxes
Of the 31 nest-boxes installed during 2002–2015, 19 faced north, four faced south and eight faced west. Ten of the 11 known natural nests in the study area resembled chimneys, having no roof to shield the incubating female and/or her brood from the elements. Only one natural nest was not a chimney nest but had a side entrance that faced south. Natural cavities differed from nest-boxes by having larger entrances that were lower to the ground, as well as having deeper, smaller nest floors (Table 1). The average distance separating occupied nest-sites was 5.7 ± 2.4 km (range 3–18 km).
Groups and their home ranges
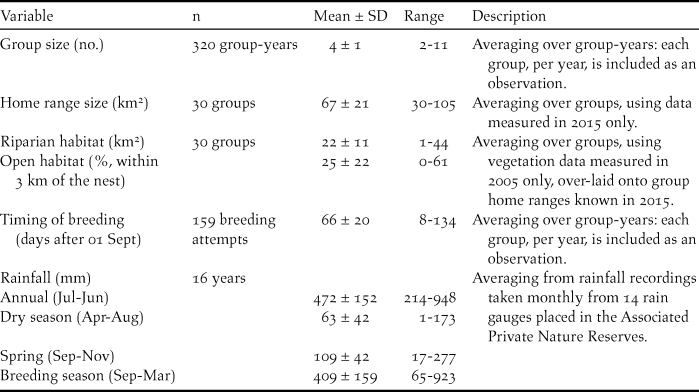
In 2001, the year before nest-boxes were installed, five breeding groups and 10 non-breeding groups were present in the study area (Figure 1). After the first batch of nest-boxes were installed in 2002, the number of breeding groups increased sharply to 20 in the subsequent five seasons. By 2015, 23 breeding groups and seven non-breeding groups were known (Figure 1). Over the entire study period, ground hornbill groups (observed per year) contained an average of 4.0 ± 1.4 individuals each (Table 2).
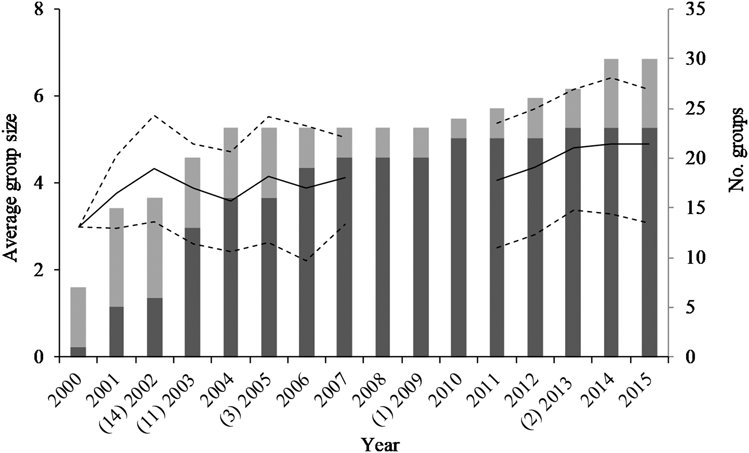
Figure 1. The number of Southern Ground Hornbill Bucorvus leadbeateri groups identified in the Associated Private Nature Reserves over time (breeding groups, dark grey bars; non-breeding groups, light grey; right axis) and the average group size of breeding groups (solid line, with ± 1 standard deviation plotted with dashed lines, left axis). The number of nest-boxes installed per year are provided in parentheses on the x-axis.
In 2015, when home ranges were most accurately recorded, average home range size for breeding groups was 67 ± 21 km2 (Table 2). Home ranges contained an average of 22 ± 11 km2 of riparian habitat typically preferred by ground hornbills during the breeding season (Wyness Reference Wyness2011) and 25 ± 22% open habitat within 3 km of the nest (Table 2).
Summary statistics for breeding
During 2000–2015, the 23 breeding groups laid in 67% of the seasons observed (154 attempts in 235 group-years observed). A total of 36 breeding attempts were made in natural tree cavities and 123 in nest-boxes (2002–2015). On average, 10 ± 5 nests were occupied per season (range: 1–18, natural nests and nest-boxes combined, Figure 2a, b). Occupancy of nest-boxes increased over time and by 2015, 23 of 31 nest-boxes had been occupied at least once (74%, Figure 2a). The 15 groups that occupied mostly nest-boxes attempted nesting twice as often as the eight groups that occupied only natural nests (nest-boxes; one breeding attempt per group every 2.5 ± 2.6 years, natural nests; one breeding attempt per group every 6.0 ± 5.2 years). Of the 154 breeding attempts, 45% came off the back of fledging a chick the previous season and 55% from a failed previous attempt. Eggs were typically laid 66 ± 20 days after the start of spring (mean lay date of 7 November; Table 2).
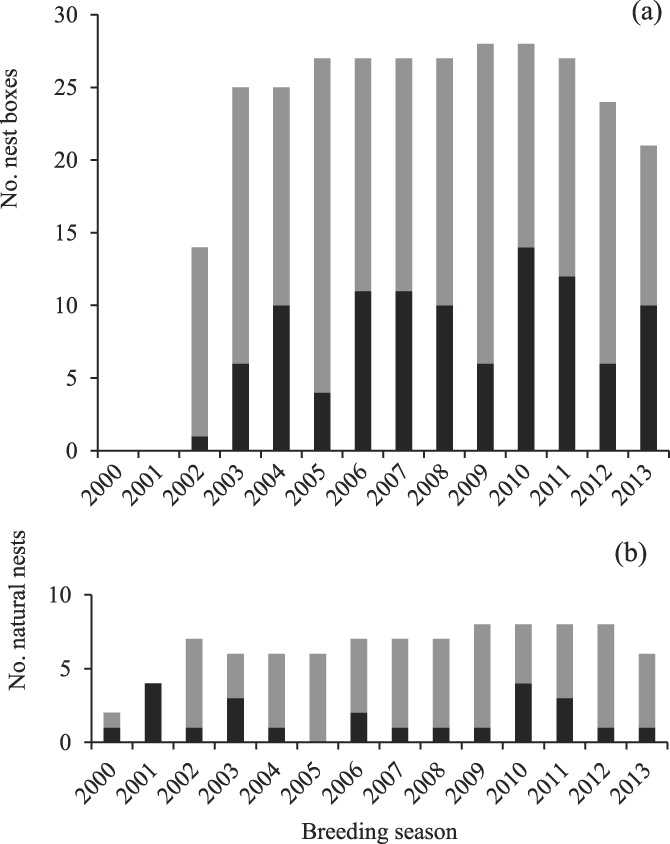
Figure 2. Number of breeding attempts each year in nest-boxes (a) and natural cavities (b) available to Southern Ground Hornbills Bucorvus leadbeateri. Grey fill indicates the number of nests available, black fill indicates the number of available nests that were occupied.
Over the study period, 66% of breeding attempts in natural nests and nest-boxes combined fledged a chick (107 chicks fledged from 117 breeding attempts). A total of 66% of breeding attempts by groups using natural nests fledged a chick, and 68% of attempts by groups using nest-boxes fledged a chick. On average, 7 ± 4 chicks fledged from nests in the study area annually (range 1–13 chicks fledged; Figure 3). Of the 107 chicks fledged over the entire study period, 79% were from nest-boxes (Figure 4). On average, each of the 15 groups occupying mostly nest-boxes fledged a chick once every two years (84 chicks fledged by 15 groups over 14 seasons = 0.40 chicks per group per year). Each of the groups occupying mostly natural nests took twice as long, fledging a chick once every five years (23 chicks fledged by eight groups over 16 seasons = 0.18 chicks per group per year).

Figure 3. The number of Southern Ground Hornbill Bucorvus leadbeateri chicks fledging per year since nest-boxes were installed during 2002–2003.

Figure 4. Cumulative total of Southern Ground Hornbill Bucorvus leadbeateri chicks fledged from natural nests and nest-boxes over 16 breeding seasons.
Model interpretations – breeding attempts
The GLMM investigating factors related to breeding attempts (Table 3) identified a positive relationship between breeding attempts and each of home range size and the previous year’s rainfall. Specifically, the odds of a group attempting to breed increased by 33% for every 10 km2 increase in home range size (OR: 1.33, 95% CI: 1.03, 1.71; P = 0.03), and by 38% for every 100 mm increase in previous year’s rainfall (OR: 1.38; 95% CI: 1.01, 1.88, P = 0.04).
Table 3. Generalised Linear Mixed-effect model results: Relationship of environmental and social factors with breeding attempts in Southern Ground Hornbill Bucorvus leadbeateri groups (19 breeding groups over 235 group years during 2000-2015). R-squared values: full model R2m = 0.13 and R2c = 0.42; reduced model R2m = 0.09 and R2c = 0.44.

1 In this model, this term was included only when interacting with the presence of a nest-box, to ensure it only entered the model when at least one nest-box was present.
Breeding decisions did not appear to be related to nesting resources (including access to an artificial nest), the amount of preferred habitat in the home range, whether a group fledged a chick in the previous season, group size or rainfall (all P-values > 0.25 in full model). All three rainfall variables were initially included as cubic polynomials but were simplified to linear terms.
The inclusion of random effects for year and group contributed largely to explaining the variation in breeding attempts (R-squared values: full model R2m = 0.13 and R2c = 0.42; reduced model R2m = 0.09 and R2c = 0.44). This suggests that there are still substantial year-specific and group-specific attributes, related to breeding attempts, that are not adequately described by the fixed effects included in the model.
Model interpretations – breeding success
The GLMM investigating factors related to breeding success (Table 4) identified positive relationships between breeding success and each of nest height, thickness of the nest cavity walls, and earlier laying. For every 1 m increase in the height of the nest entrance above ground, the odds of raising a chick to fledging increased by 39% (OR: 1.39; 95% CI: 1.02,1.96; P = 0.04). For every 10 mm increase in the thickness of the nest cavity wall, the odds of raising a chick to fledging increased by 17% (OR: 1.17; 95% CI: 1.03,1.35; P = 0.02). A delay in laying by one week reduced the odds of fledging a chick by 14% (OR: 0.86; 95% CI: 0.73,1.00; P = 0.06).
Table 4. Generalised Linear Model results: Relationship of environmental and social factors with breeding success in Southern Ground Hornbill Bucorvus leadbeateri groups (18 breeding groups over 117 group years during 2000-2015). Hosmer-Lemeshow goodness-of-fit test (10 groups) for the full model: P = 0.80; reduced model: P = 0.69.

1 The non-linear relationship between year and breeding success was accommodated by a cubic polynomial, entered into the model using B-spline basis functions. Although odds ratios for individual basis functions appear large, the overall shape of the fitted cubic trend was reasonable and consistent with the data (see results).
Nest type and orientation were found to be strongly related with 80% of nest-boxes opening N and 18% S, and 86% of natural cavities having top entrances, resulting in orientation being removed from the analysis. Similarly, number of breeding groups and year were strongly correlated, with the number of breeding groups increasing steadily over time, and therefore number of breeding groups was removed from the analysis.
An additional effect identified was a trend in breeding success over time, both non-linear and non-monotonic (P = 0.05). The shape of the fitted cubic relationship was investigated, in both the full and reduced models, and found to consistently resemble the data on breeding success over time (Figure 5). An initial upward trend in breeding success (2000–2007) was followed by a distinct downward trend (2010–2015), with the transition occurring in 2008–2009, although there were no data available for these two years against which to compare the fitted shift in trend. This trend may relate to the number of breeding groups, where breeding success markedly declined when > 20 groups occupied the study area (Figure 5; see discussion). Annual breeding success was 80% during 2004–2007, and 20% lower at 60% during 2010–2015.
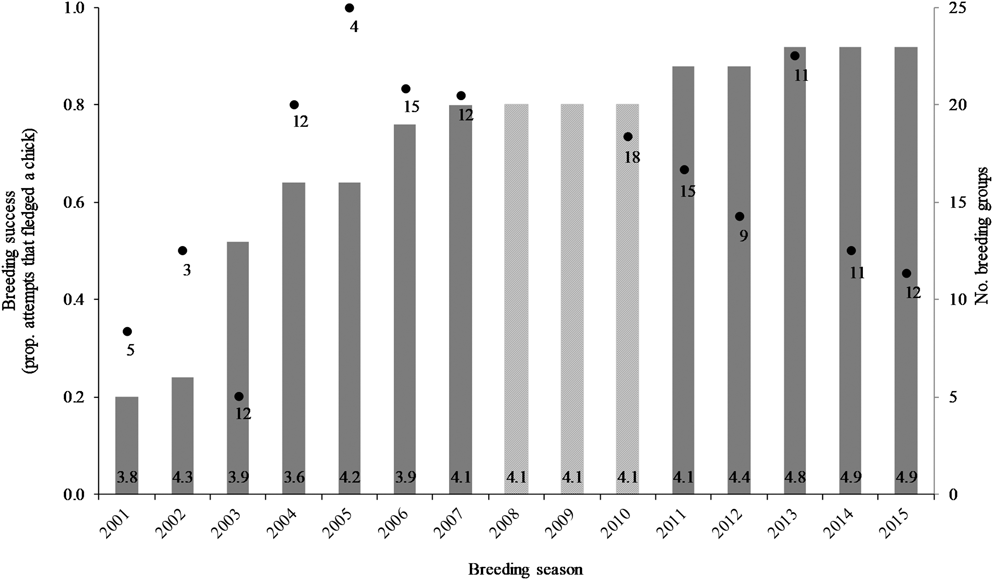
Figure 5. Variation in seasonal breeding success for Southern Ground Hornbill Bucorvus leadbeateri groups (black dots with the number of breeding attempts indicated; left axis) relative to the number of breeding groups (grey bars, average group size at the base; right axis). Data for group sizes were not available during 2008–2010.
Breeding success did not appear to be related to rainfall before or during the breeding season, group size, nest type, or the percentage of open habitat around the nest (all P-values > 0.17 in full model, Table 4). Although initially included based on a previous study (Wilson and Hockey Reference Wilson and Hockey2013), we found no evidence to retain breeding season rainfall as a non-linear term, or the interaction between open habitat and nest type. While group identity was initially included as a random intercept, the effect was found to be zero.
Harvesting second-hatched chicks
Harvesting of redundant second chicks occurred during 2002–2003 and again during 2010–2015, and at 33% of the active nests during these periods (n = 92 nests). In all cases where harvesting occurred, the first chick hatched successfully and looked healthy at the time of harvesting. In harvested nests (n = 30 nests), 83% were successful at fledging the first-hatched chick. In nests where no harvesting occurred and where, like in harvested nests, the first-laid egg was confirmed to have hatched and to be alive and healthy a few days after hatching (n = 27 nests), 81% were successful at fledging the first-hatched chick.
Nest failure
A total of 54% of failed attempts were at the egg stage, and this did not differ for nests initiated late or early in the season. Of the nests that failed at the chick stage, chicks in nests initiated later in the season (after the mean laying date) were older at the time of nest failure (mean nestling age = 35 ± 29 days, range 1–82) than chicks in nests initiated earlier in the season (16 ± 7 days, range 5–26).
Predation accounted for 20% of nesting attempts failing in nest-boxes and 18% in natural nests. Groups rarely made a second breeding attempt in the same season if the first attempt failed; only 3% of breeding attempts were second attempts (four in 159 breeding attempts). Of the four groups that attempted to breed a second time, the eggs in the first attempt were laid early in the season: their first attempt was initiated on average 16 ± 8 days earlier than the mean lay-date for each season (range 10–27 days), and all four first attempts failed during incubation. Three of the four groups that attempted nesting twice during the same season had access to an alternative nest, and in all three cases they switched nests for the second attempt. Females re-laid ∼17 ± 5 days after the last attempt failed (range 11–23 days). Groups did not make a second attempt in a season when their attempt failed at the chick stage, and no second attempts were made later than 19 November, which is 80 days after the start of spring.
Discussion
Southern Ground Hornbill groups occupying nest-boxes fledged a chick once every two to three years (0.40 chicks fledged annually per group per year), compared to groups using natural nests that fledged a chick once every five years (0.18 chicks fledged annually per group per year). This reaffirmed one of Wilson and Hockey’s (Reference Wilson and Hockey2013) findings at the same study area up until 2008, that groups using nest-boxes fledged twice as many chicks over time (0.33 chicks fledged annually per group per year) as those occupying natural nests (0.15 chicks fledged annually per group per year). However, when modelled with environmental and social factors, breeding success in this study was not related to the type of nest used. Wilson and Hockey (Reference Wilson and Hockey2013) found that, for groups studied during 2001–2008, the proportion of open habitat adjacent to the nest-site moderated the relationship between breeding success and the type of nest used. We found no relationship between open habitat and breeding success at either natural and artificial nesting sites. This may be due to the addition of another seven years data or it may be because of changes in habitat structure since 2005. There has been a net increase in woody vegetation in South Africa’s savannas since 1990, linked to increasing atmospheric concentrations of CO2 (Skowno et al. Reference Skowno, Thompson, Hiestermann, Ripley, West and Bond2016). However, there are no recent vegetation data for the study site, and thus the reason for this disparity between our results and Wilson and Hockey’s (Reference Wilson and Hockey2013) remains unsolved.
Other studies on reproductive output have found the use of artificial nests to have both negative (Catry et al. Reference Catry, Franco and Sutherland2011, Björklund et al. Reference Björklund, Valkama, Saurola and Laaksonen2013, Demeyrier et al. Reference Demeyrier, Lambrechts, Perret and Grégoire2016) and positive effects (Brazill-Boast et al. Reference Brazill-Boast, Pryke and Griffith2013, Sutherland et al. Reference Sutherland, Dann and Jessop2014, Bourgeois et al. Reference Bourgeois, Dromzée and Vidal2015). Ground hornbill groups used nest-boxes more frequently than natural nests once nest-boxes were provided, and fledged chicks more often than groups using natural nests. Similarly, Eurasian Kestrels Falco tinnunculus used nest-boxes more frequently than natural nest-sites and fledged more chicks in artificial nests than in natural nests, with nest predation being higher in natural nests than in artificial nests (Fargallo et al. Reference Fargallo, Blanco, Potti and Viñuela2001). For ground hornbills, predation levels in nest-boxes and natural nests were similar.
There were some findings that highlighted the importance of long-term studies on conservation interventions, such as the change in breeding success over time. Initially, ground hornbill breeding success increased following the provision of nest-boxes, but later declined. It is not clear which factors influenced this trend for ground hornbills. Reduced productivity in birds can be affected by, among other things, above-optimal breeding density (Mänd et al. Reference Mänd, Tilgar, Lõhmus and Leivits2005, Flockhart et al. Reference Flockhart, Mitchell, Krikun and Bayne2016), foraging constraints (Sorato et al. Reference Sorato, Griffith and Russell2016), or conflict, where social conflict occurs with nest disturbance caused by aggressive interactions between dominant individuals and helpers (López-Sepulcre et al. Reference López-Sepulcre, Norris and Kokko2009). Future studies could investigate whether ground-hornbills exhibit density-dependent reproductive output, since a decline in productivity was observed during a period when breeding group density and average group size was higher than the average for the species in the region. Furthermore, our findings highlighted the importance of density regulation for ground hornbills, supported by the positive relationship between home range size and the odds of a group attempting to breed.
Rainfall is out of the control of land managers, but did have some positive impact on productivity. There was a positive relationship between rainfall over the previous year and the odds of a group attempting to breed. However, it is likely that rainfall was not the cue, but rather the lagged effect it had on vegetation growth (Dudney et al. Reference Dudney, Hallett, Larios, Farrer, Spotswood, Stein and Suding2017), and ultimately arthropod abundance (Kemp Reference Kemp1976). This might also explain the positive relationship between territory size and the odds of a group attempting to breed, since larger territories are more likely to buffer varying food availability by having a larger area for groups to utilise in their search for food. Interestingly, rainfall did not appear to be related to breeding success. Thus, once breeding, it appears that ground hornbills may be more resilient to variable rainfall conditions than was suggested by Wilson and Hockey (Reference Wilson and Hockey2013), who found a negative relationship between breeding success and rainfall of < 300 mm or > 500 mm during the breeding season in the same study area during 2000–2008. By including an additional eight years of data, we unexpectedly found that rainfall was not related to breeding success. The varied diet of ground hornbills, which includes invertebrates, reptiles, amphibians and small mammals, likely assists them to switch between prey items under varying rainfall conditions during the breeding season, ultimately facilitating breeding success during seasons of high rainfall or low rainfall. This is reassuring given the predicted changing environmental conditions in north-eastern South Africa due to climate change (Cunningham et al. Reference Cunningham, Madden, Barnard and Amar2016).
There was a positive relationship between breeding success and each of nest height and the thickness of the cavity wall. For birds in general, higher nests are typically favoured as they may be less vulnerable to predation presumably due to reduced accessibility (Negro and Hiraldo Reference Negro and Hiraldo1993) and birds tend to be more successful in them (Negro and Hiraldo Reference Negro and Hiraldo1993, Wilson and Cooper Reference Wilson and Cooper1998, Saab et al. Reference Saab, Dudley and Thompson2004). For birds like ground hornbills breeding in the savannas during summer, nests with thicker walls likely buffer against high temperatures experienced. The critical maximum temperature for avian embryos and chicks is 41° C, and prolonged exposure to temperatures at or above this is fatal (Webb Reference Webb1987). The negative effect of exceptionally hot temperatures on ground hornbill egg failure was demonstrated in the study area during the 2015/2016 season when frequent maximum temperatures of 45° C were reported (C. Rowles pers. comm. 2016). During that season, 24% of eggs laid (n = 17 eggs) failed to hatch at a site where, on average only 6% of eggs laid fail to hatch (n = 217 eggs; data from 2000–2015). In a similar scenario, 22% of Lesser Kestrel Falco naumanni nests experienced hatching failures associated with atypically high temperatures (Catry et al. Reference Catry, Franco and Sutherland2011). Given that thicker cavity walls improved breeding success for ground hornbills, this feature should be considered when designing nest-boxes that will buffer the negative effects of exceptionally hot conditions during the breeding season. Therefore, in addition to the careful spacing of nest-boxes to create a suitable density of breeding groups, nest height and nest wall thickness are additional factors to consider when designing and installing nest-boxes for this species.
There was a negative relationship between late breeding and breeding success, with the likelihood of failure increasing as the breeding season progressed. This differs from studies of other species in the southern hemisphere which either show no relationship between the timing of breeding and breeding success (Heinsohn Reference Heinsohn1992), or a similar trend to the north-temperate zone with increased breeding success as the breeding season progresses (Reyer Reference Reyer1984, Garnett et al. Reference Garnett, Pedler, Crowley, Garnett, Pedler and Crowley2001). For ground hornbills, this supports the view that food availability for chicks likely diminishes towards the end of the breeding season (Kemp Reference Kemp1976).
Recommendations
Nest-boxes are a useful conservation tool to reverse the population decline of the Southern Ground Hornbill. Although nest-boxes can be placed as close as 3 km apart, we recommend that nest-boxes be spaced roughly 10 km apart to facilitate a territory size of ∼100 km2 (Kemp et al. Reference Kemp, Joubert and Kemp1989) and thus avoid risking the possibility of density-dependent impacts on breeding performance. Once territories have been established at a suitable density, a second nest-box can be provided to allow for switching nest-boxes after a predation event to increase the likelihood of a second breeding attempt in each season. Switching nest-sites is common in other birds following nest failure, and one explanation is that this reduces predation risk (Sonerud Reference Sonerud1985, Lima Reference Lima2009). We also recommend placing nest-boxes at least 6 m off the ground and using thick cavity walls (at least 6 cm) to maximising reproductive output.
Harvesting the redundant second-hatched chick appeared to have no negative impact on breeding outcomes. In nests where the first chick hatched and was healthy a few days after hatching, the likelihood of that chick fledging was comparable between nests where harvesting occurred and nests where no harvesting occurred. Therefore, harvesting of the second chick for conservation purposes can continue to support the wild-release and captive breeding programmes vital to slowing the decline of the species in the wild.
Although the provision of nest-boxes is a substitute for the loss of large trees in the landscape, it does not address the cause of the loss of those large trees. Humans and African Elephants are the major contributors to high tree-fall rates in African savannas (Mograbi et al. Reference Mograbi, Asner, Witkowski, Erasmus, Wessels, Mathieu and Vaughn2017). The loss of, or damage to, large trees places higher pressure on woody vegetation in the lower height classes, thereby compromising ecosystem resilience (Mograbi et al. Reference Mograbi, Asner, Witkowski, Erasmus, Wessels, Mathieu and Vaughn2017). If nest-boxes are considered as a tool to bridge the gap until new suitable tree cavities are formed, then a further important conservation action for Southern Ground Hornbills is promoting the protection and growth of large, mature trees for roosting (Zoghby et al. Reference Zoghby, Little, Ryan and Hockey2016) and more importantly, for nesting.
Acknowledgements
We thank the landowners and wardens of the Associated Private Nature Reserves for access to the properties. We thank the many field assistants who contributed to data collection during the project’s long history. We also thank Robert Thomson and two additional reviewers who greatly improved the quality of this manuscript. The research was funded by the Department of Trade and Industry Technology and Human Resources for Industry Programme, Dow Southern Africa (Pty) Ltd, the Hans Hoheisen Charitable Trust and Senelala Estates. Permission to harvest second-hatched chicks was given by the Mpumalanga Tourism and Parks Association, permit numbers: MPB. 5290, 5760, 6005, 6352.











