Introduction
Worldwide, significant effort is being expended on the restoration of islands, particularly by the removal of feral animals (Holmes et al. Reference Holmes, Spatz, Oppel, Tershy, Croll and Keitt2019, Veitch et al. Reference Veitch, Clout and Towns2011). Subsequent colonisation and recolonisation of islands by seabirds are closely linked to the recovery of these degraded island ecosystems. Seabird groups, such as petrels (Procellariiformes), which have strong philopatry (Warham Reference Warham1990) and can increase to numbers in the millions, e.g. Black-winged Petrels Pterodroma nigripennis (Tennyson and Taylor Reference Tennyson and Taylor1990), exert significant influence on the nutrient loads and structural ecology of islands (Holdaway et al. Reference Holdaway, Hawke, Hyatt and Wood2007, Smith et al. Reference Smith, Mulder, Ellis, Mulder, Anderson., Towns and Bellingham2011, Orwin et al. Reference Orwin, Wardle, Towns, St. John, Bellingham, Jones, Fitzgerald, Parrish and Lyver2016). This makes the securing of recovering populations, and encouraging reintroduction of populations to islands from which they were extirpated, an essential restoration goal. Jones and Kress (Reference Jones and Kress2012) and Brooke et al. (Reference Brooke, Bonnaud, Dilley, Flint, Holmes and Jones2017) list examples of active seabird restoration on now predator-free islands, however, recolonisation is more often by chance or social attraction (Buxton et al. Reference Buxton, Jones, Moller and Towns2014) than any deliberate encouragement (Kappes and Jones Reference Kappes and Jones2014). Understanding the dynamics of colonisation, what constitutes good seabird habitat, and how easily initial colonisation can be impacted by moderate predation pressures are fundamental issues for island managers looking to support and encourage the restoration of seabird assemblages. Lewison et al. (Reference Lewison, Oro, Godley, Underhill, Bearhop, Wilson and Ainley2012) recognised the highest priority in conservation management of seabirds was understanding population dynamics which requires basic details of a species’ breeding ecology.
The poorly studied Kermadec Petrel Pterodroma neglecta neglecta breeds across the south Pacific from Lord Howe Island Group, off the Australian east coast, to Juan Fernandez Island Group off Chile (Marchant and Higgins Reference Marchant and Higgins1990), and there is a small population breeding with other surface-nesting petrels on Round Island near Mauritius in the Indian Ocean (Brooke et al. Reference Brooke, Imber and Rowe1999). In the south-west Pacific, small populations occur on islands in the Kermadec Group (Tennyson et al. Reference Tennyson, Scofield and Bell2003) and Balls Pyramid in the Lord Howe Group (Fullagar et al. Reference Fullagar, McKean, Van Tets, Recher and Clarke1974). They were first recorded on Phillip Island in the Norfolk Group in 1986 (Moore Reference Moore1999), a degraded tropical island now recovering after the removal of pigs Sus scrofa (approximately 1856), goats Capra hircus (by 1900), and rabbits Oryctolagus cuniculus (in 1988), all having been present since 1799 (Coyne Reference Coyne2010). By 1900 the island was almost entirely denuded with substantial ongoing soil loss (Coyne Reference Coyne2010), but more recently supports significant vegetation (Figure 1). The species was confirmed breeding there in 1987 (Woods Reference Woods1988).
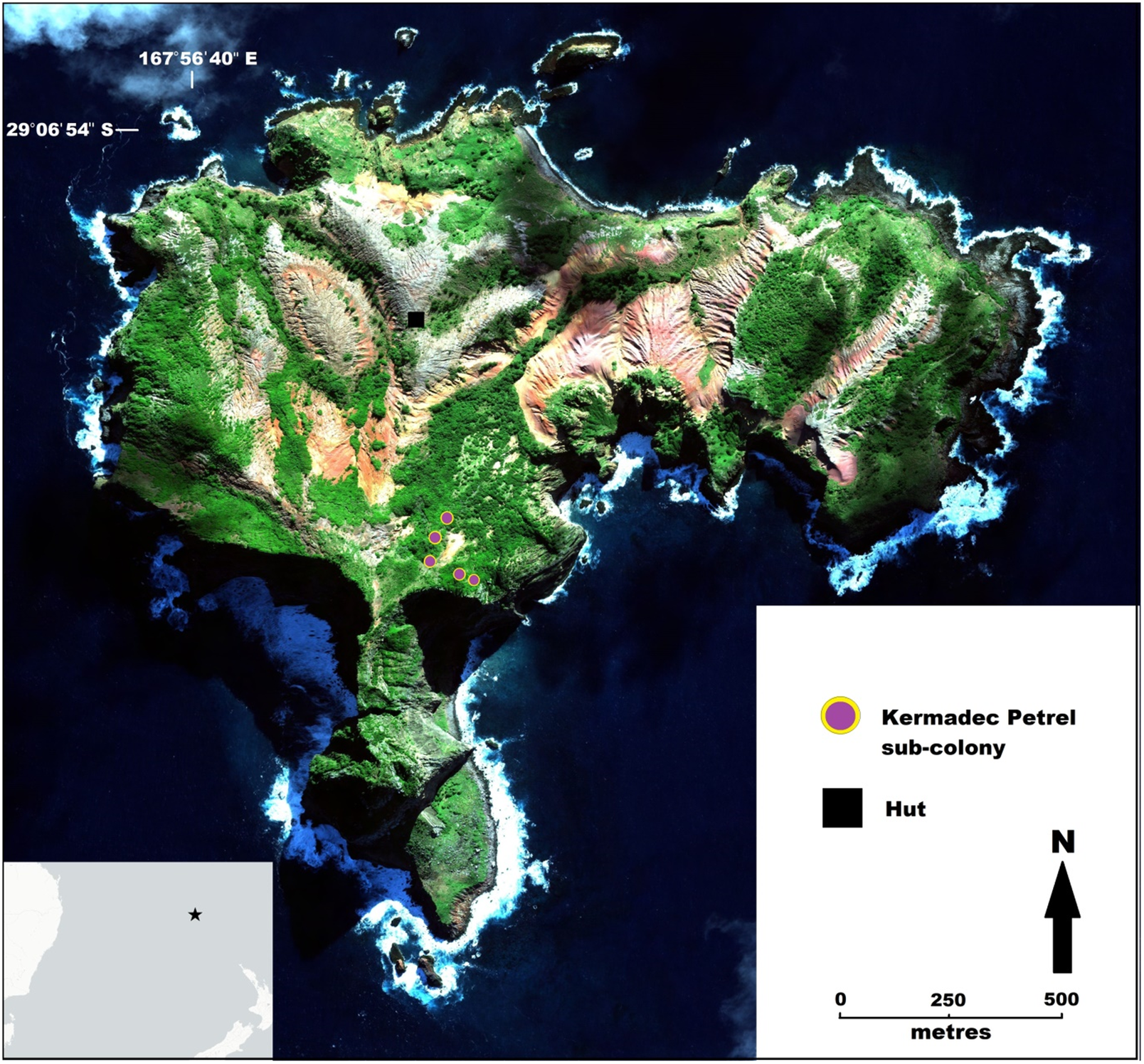
Figure 1. Map of Phillip Island, Norfolk Group, showing the location of Kermadec Petrel sub-colonies by 2021. Inset: Norfolk Island Group (star) relative to Australia and New Zealand.
The south-western Pacific populations of Kermadec Petrel have undergone significant reductions over the last century through the loss of breeding habitat caused by invasive feral mammals (Hindwood Reference Hindwood1940, Merton Reference Merton1970). On Lord Howe Island (31°30´S, 159°05´E), 580 km off the mid-east coast of Australia, they were still breeding in the mountainous areas in 1913 (Bell in Hindwood Reference Hindwood1940). By this time the species had been subjected to feral pig predation for perhaps 100 years (Miller and Mullette Reference Miller and Mullette1985). In 1918 ship rats Rattus rattus were introduced to the island (McCulloch Reference McCulloch1921) and the Kermadec Petrel was thereafter only seen regularly around Ball’s Pyramid, 23 km to the south (Fullagar Reference Fullagar, McKean, Van Tets, Recher and Clarke1974), which has never been surveyed. Following the implementation of the Rodent Eradication Project on Lord Howe Island in 2019 (Harper et al. Reference Harper, Pahor and Birch2020), the possibility of Kermadec Petrels returning to the island at a future date cannot be discounted, as they have been known to overfly the higher parts of the southern mountains (Fullagar et al. Reference Fullagar, McKean, Van Tets, Recher and Clarke1974).
In this study we look at Kermadec Petrel breeding on Phillip Island off Norfolk Island. Internationally the species has decreasing populations and is of Least Concern under IUCN criteria (Birdlife International 2022). The breeding populations within Australian waters are considered Vulnerable (Carlile et al. Reference Carlile, O’Dwyer, Wilson, Clarke, Brown, Baker, Garnett, Garnett and Baker2021). Life history attributes of the species are poorly known (Baker et al. Reference Baker, Gales, Hamilton and Wilkinson2002). Currently there is no understanding of the limitations on population growth. To aid in their conservation we used a range of techniques to bring an understanding of their nesting ecology (i.e. habitat selection, site preferences, and site fidelity). We determined basic breeding ecology (i.e. timing of arrival, their laying, incubation, and fledging periods as well as breeding success), and assessed the impact of avian nest predators on their conservation. A better understanding of the ecology and limitations to breeding will also contribute to efforts to re-establish this species on Lord Howe Island where it has been absent for more than a century following invasion by rodents.
Methods
Phillip Island (29°07´S, 167°57´E), is located 6 km south of Norfolk Island in the South Pacific. The 190-ha island rises 280 m above sea level, is highly dissected by eroded gullies (Figure 1), and supports over 70% vegetative cover (Cogger et al. Reference Cogger, Muir and Shea2006). The island is currently home to 14 seabird species (Priddel et al. Reference Priddel, Carlile, Evans, Evans and McCoy2010). Phillip Island was visited for 1–6 days every 2–3 months between January 2017 and June 2021 excepting between April and October 2020 due to an inability to access the island during the global COVID-19 pandemic (Miller-Rushing et al. Reference Miller-Rushing, Athearn, Blackford, Bringham, Cohen and Cole-Will2021). During island visits we searched for Kermadec Petrel nests using two methods: daylight checks around known nesting sites and adjoining areas; more targeted searches in late afternoons, as aerial calling of Kermadec Petrels (Marín et al. Reference Marín, González and Trucco2020) reached peak intensity. Aerial calls typically solicited responses from birds already within the vegetation either in courtship or attending nesting sites. The location of ground calls and landing birds provided indications of potential nesting areas allowing follow-up investigation. Kermadec Petrel nesting appears restricted to a relatively small area on the island as indicated by aerially calling birds. Their vocalisations on low wind days carried more than 500 m (audible from hut) allowing confirmation of no indication of prospecting or nesting other than our study area.
A breeding site was identified by the presence of an incubating adult petrel, or the presence of a petrel chick. Nests were marked with a numbered tag and co-ordinates recorded with a GPS. Where possible, nests were monitored using HC500 HyperFire surveillance cameras (Reconyx®, Holmen, WI, USA), generally set to record images hourly. Where pairs consisted of different morphs, identification of attendance at nests could be made. Nests along public access tracks were not camera monitored for security reasons with up to 12 nests monitored at one time. A nest was considered isolated from those in sub-colonies if its distance from a sub-colony was greater than any single distance between nests in the sub-colony. A sub-colony was considered six or more nests used in the same season where ground calling solicits another nesting bird to call (approximately 10–15 m) and not separated by cleared areas. Nest density within these areas was calculated by drawing a polygon around the outer perimeter of known nests.
All adults encountered were fitted with an Australian Bird and Bat Banding Scheme (ABBBS) stainless steel band on the right tarsus (adults) or left tarsus (fledglings). Adults were handled during hours of darkness to reduce nest desertion and potential interruption of courtship. We deployed Global Light Sensor (GLS) loggers (Migrate Technology Intigeo- C330) to 93 breeding birds. Units (17 × 19 × 8 mm, 3.3 g, 2–3 year battery life) were attached by cable tie to a hook and loop harness which was wrapped around the bird’s tarsus and held in place with a single stitch of rayon thread. Any bird retrapped in a following breeding season had their GLS logger removed (for calibration) and replaced with a new calibrated unit. At the first re-trap, a single blood drop was stored from individuals on to FTATM cards for identification of sex from DNA (Gutiérrez-Corchero et al. Reference Gutiérrez-Corchero, Arruga, Sanz, García, Hernández and Campos2002). Birds were also sexed by cloacal examination detecting post-laying females (O’Dwyer et al. Reference O’Dwyer, Priddel, Carlile, Bartle and Buttermer2006), or from incubation shift duties identified via changes in light levels on GLS loggers (see below). Known partners of sexed birds were considered the opposite sex, even without individual verification.
Adult breeding behaviour from GLS loggers
Light data were downloaded from GLS logger units using Migrate Technology hardware and software in Intigeo-IF kit (© Migrate Technology Limited, Cambridge, UK,2015). We automated sunrise and sunset annotation in raw light-level data using the function preprocess Light in the package TwGeos (Wotherspoon et al. Reference Wotherspoon, Sumner and Lisovski2016). The graphical interpretation of the data using the statistical software environment Flight R version 3.5.1 (R Core Team 2021) was used to assess whether birds were at sea or on-island. Figure 2a, for example, shows clear dawns and dusks and is indicative of a bird at sea. However, obvious periods with diminished light intensity during daylight hours (Figure 2b) is indicative of a bird on-island. Where there is minimal light during the day with only short periods of light recorded (Figure 2c), the bird was assumed to be incubating. From these patterns, breeding behaviour such as laying date and incubation lengths could be determined. Whether a bird was male or female could also be determined based on the order of their incubation shifts.

Figure 2. Outputs of R software showing automated twilight event (i.e. sunrises and sunsets) at Norfolk Island, South Pacific (annotated in GMT) of GLS attached to a Kermadec Petrel: (a) showing dawn to dusk from a bird at sea; (b) a bird on the island without incubating (after first arrival and prior to the honeymoon phase) then departing to sea; (c) transitioning from the end of an adult incubation shift then departing to sea. Dashed line is effective night.
Hatch dates or a complete measure of provisioning behaviour could not be determined based on changes in logger light levels. Parental movement on the egg appeared to vary greatly during the pipping and hatching periods, and birds subsequently fed chicks during both day and night time, meaning only daytime visits were indicated by changes in light levels.
Estimated laying dates were achieved by countbacks from known hatching dates at nests where birds were originally discovered incubating. We calculated breeding success only from nests with an observed egg and subsequent records of advanced chicks (greater than 2/3 grown). A nest was adjudged to have lost a chick where it had been previously recorded as being brooded by an adult or they were observed alive immediately after the post-brood stage.
Results
Habitat selection and nest site use
Kermadec Petrel breeding habitat on Phillip Island was limited to sloping terrain 182–228 m elevation above the shoreline and up to 85 m from the coast (Figure 1). Nests occurred in five loose sub-colonies (mean = 172 m2; range: 46–330 m2) of up to 20 pairs (mean = 0.1 nests/m2; range: 0.02–0.18 nests/m2). Isolated nests were also discovered up to 125 m from the nearest sub-colony. Within the sub-colonies, proximity to the nearest nest averaged 3.0 m (range: 0.4–11.5 m). The nests themselves were approximately 16 cm in width and made up of dried grass and leaves, lining a slight (35 mm) depression in the soil.
Nests were always shaded and mostly under low windswept African Olive Olea africana or more rarely native White Oak Lagunaria patersonia (mean total height of vegetative canopy = 2.2 m; range: 0.5–10 m). In high wind areas, olive formed very thick dense branching, grown close to the ground compared with the more open wind-pruned native oak. Nests were also found on level ground upslope of the vegetative barrier formed by native New Zealand Flax Phormium tenax. More open, under-canopy areas, were only utilised if there were significant physical barriers at ground level. Most of the marked nests (n = 83) were associated with a physical barrier less than a nest-width distant: 38% were formed from very dense impenetrable ground vegetation (olive 60% and flax 40%), often in low thickets, 26% were in vertical or overhanging rock, and 28% were in tree branch or root buttress on the ground; 8% of nests were beneath a low canopy (approximately 1 m) but open at ground level. All nests were established within 3.6 m of cleared areas or cliff edges where adults could become airborne after leaving the nest site. Their preferred habitat was also used by one of only two colonies globally of White-necked Petrel P. cervicalis (Priddel et al. Reference Priddel, Carlile, Evans, Evans and McCoy2010), as well as loose colonies of summer breeding Black-winged Petrel and Wedge-tailed Shearwater Ardenna pacifica, and winter breeding Little Shearwater Puffinus assimilis. However, approximately half of the monitored nests were established in suitable burrowing habitat but were greater than 10 m from other procellariid burrows, suggesting an aversion to burrow-nesting petrels. Those with nests nearby averaged 4.4 m (range: 1.1–8.8 m) distance. Interspecific competition occurs with Red-tailed Tropicbird Phaethon rubricauda. On five occasions where interactions were noted, four resulted in nest failure for the petrel.
Seasonality of nesting
We banded 135 adults and retrapped six birds previously banded before 1995 (two originally banded as adults and four as fledglings). We recorded 58 breeding pairs of which 42 had both partners identified. Active nesting (eggs or nestlings) peaked in February annually. From this we assessed our study covered 4.5 breeding seasons from July to June. Birds laying before June but fledging after July were considered part of the earlier year’s breeding. Egg laying peaked in the austral mid-spring to early summer (72% of nesting pairs).
A total of 129 nesting attempts were documented over the 4.5 years and, excluding any new nesting pairs in the last season of study, all but nine were from birds having nested in at least two previous seasons. The other 120 nesting attempts were documented at 41 sites but only nine of these sites retained the same birds in multiple years (between two and four years). Evidence of a previously used nest lasted several seasons. Re-nesting occurred 0.5–38 m away from the initial nest site. Movements within sub-colonies averaged 3.0 m (SE = 2.7 m; n = 42) and appeared to be regardless of previous breeding outcomes.
Breeding ecology
Over the first year and a half of this study (January 2017 to June 2018), both partners were known for 18 of the 25 breeding events. Two of these known pairs (11%) had both individuals change partners during the following three seasons. Seventeen pairs had at least one partner fitted with a GLS during this period, allowing breeding behaviour to be determined from light data. In the subsequent three seasons of monitoring, only four of these pairs were found breeding in the final year (2020/21) and only a single pair was known to breed in each of the four years. Significant disturbance to breeding from nest predation by the Purple Swamphen Porphyrio melanotus (known locally as “Tarla Bird”) may have impacted the breeding of many pairs (see below).
The sex of breeding birds was determined by DNA analysis of blood for 22 individuals. From 10 of these birds, we cross-referenced with predicted sex based on breeding behaviour gleaned from GLS logger data (see below). The confirmation of the correct sex supports assigning a sex for surface-nesting seabirds using these methods. Additionally, three individual females were confirmed by cloacal examination immediately after laying and three pairs were simultaneously sexed from GLS loggers when both showed complementary incubation activities. Partners of birds not sexed by these methods were considered the alternative sex, by association.
The average adult mass of banded birds was 445 g (range: 340–550 g; n = 153) and included several birds that were weighed more than once in different breeding years. By June 2021, 35 chicks had been banded and a total of eight returned fledglings recaptured. These recaptures were found either unaccompanied or, if unassociated with a nest site, were observed in courtship behaviour with a second bird. Together, with data from ABBBS banding records from the 1990s (one additional bird), the age at first return after fledging was estimated at 2.2 years (range: 1.7–2.8 years; n = 8). From all available data, three known fledglings commenced breeding at an average of 4.5 years (range: 3.8–4.9 years). From ABBBS banding records, the current oldest known bird was seen in 2020 at 31 years since fledging, but from the attached GLS logger it was shown to have not attended a nest for incubation since 2017, aged 27 years.
A total of 86 tracking devices were attached to 52 adult breeding birds between January 2017 and June 2021. Fourteen of these birds have not been retrapped since initial attachment. The remaining 38 birds were intercepted at least once during this study, resulting in 26 recordings of variation in light levels during periods of breeding from which nesting behaviour could be ascertained (Table 1). The period spent on the island to commence breeding varied between two and six days. The following honeymoon period was estimated at a mean of 40 days (range: 34–47 days; n = 29), with no significant difference between males (n = 18) and females (n = 11) (t = 0.59, P = 0.56). For females alone, this egg-formation period was 38.4 days. The length of first incubation shift was not significantly different between sexes, at 14.3 days for males (n = 14) and 13.3 days for females (n = 9) (t = 0.45, P = 0.66). Both sexes had shorter subsequent shifts (Table 1). From observed egg laying to observed hatching (n = 4), the incubation period was 50 days (range: 48–51 days). Hatching to fledging of chicks (n = 15) averaged 96 days (range: 85–113 days).
Table 1. Island attendance and pre-chick provisioning activities of Kermadec Petrels from 4.5 years of surveys on Phillip Island, Norfolk Group.

A schematic of the calendar weeks of adult attendance on the island (Figure 3) was developed using all the average measures of days for breeding attributes (i.e. pre-honeymoon visit, laying, incubation shifts, hatching date, and nest visitation during provisioning period) combined with the full range of laying dates of known pairs from all seasons. Nest failure was approximated using breeding success of the 2020/21 season, following nest predator control. Nest attendance during the provisioning period was based on two nests where camera surveillance on motion sensor mode captured breeding adult attendance, individually identified by variations of feather morphs. The schematic assumes all breeding pairs attempt to breed in one calendar year. Egg laying peaks in the last week of November, and the estimate of on-ground activity of breeding birds’ peaks in the following February, which was confirmed by our observations of active nests.

Figure 3. Seasonality of Kermadec Petrel breeding on Phillip Island, Norfolk Group, from all nesting pairs aggregated into one 12-month period. Radar circle represents weeks and months within a single year with internal radar indicating the week in which eggs were laid. External radar represents the number of weekly bird movements as determined by approximate dates of: pre-breeding arrival; egg laying; incubation shifts; pairs brooding; feeding and nest visits over provisioning period; parents and fledglings departure. Data are based on 50% breeding success but include pre-breeding bird attendance. Developed with ggplot2 (Wickham et al. Reference Wickham, Averick, Bryan, Chang, McGowan and Francois2019) with appropriate colours (Garnier et al. Reference Garnier, Ross, Rudis, Camargo, Sciaini and Scherer2021).
Impact of avian nest predators
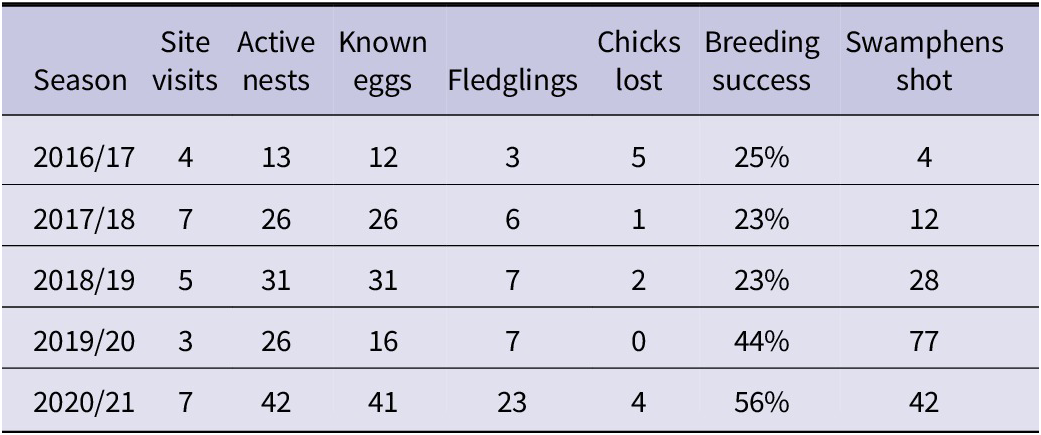
The number of breeding pairs and breeding success increased in the latter seasons of this study (Table 2). A major impact on earlier breeding success was probably nest predation by the Purple Swamphen. Although surveillance cameras were set to capture hourly images on active nests, many captured the presence of swamphen in front of incubating adults – an indication of the high frequency of nest visitation. Occasionally, an unattended petrel chick was seen in an image that included a swamphen but in the following image the chick had disappeared. On most other camera-monitored nests, hatchlings disappeared shortly after brooding was completed, presumably taken by swamphen. From all the nests we marked (n = 83) most were associated with a physical barrier (less than a nest-width away). Successful nests were more often associated with dense ground vegetation that likely impeded swamphen access (see above). Management of swamphen populations on Phillip Island was implemented by the Parks Australia staff and trained volunteers at the end of the 2018/19 breeding season and is ongoing. After the population of swamphens was reduced by more than 40 birds in a 12-month period, the petrels breeding success began to improve (Table 2). Due to the proximity of the source population on Norfolk Island, regular movements of swamphens between the two islands occur, leading to culling of swamphens on the main island as an additional conservation measure.
Table 2. Monitoring results from 4.5 years of breeding surveys for Kermadec Petrel on Phillip Island, Norfolk Group, and the tally of annual reduction of principal nest predator.
Discussion
Habitat selection and nest sites
On Phillip Island the population of Kermadec Petrel has specific requirements relating to elevation and proximity to areas cleared of shrubs for launching sites. There is suitable habitat elsewhere on the island that is currently not used, and perhaps conspecific social attraction is currently restricting where they are found. On Henderson Island (3,700 ha) in the Pitcairn Group, Brooke (Reference Brooke1995) found that the species was <1,000 m from cliff edges but always associated with open rocky patches. Brooke (Reference Brooke1995) observed that the species differed from Henderson Petrel Pterodroma atrata and Murphy’s Petrel P. ultima there, by not climbing trees to affect take-offs (contra to Iredale Reference Iredale1910). On Raoul Island (2,900 ha) in the Kermadec Group, Iredale (Reference Iredale1910) observed them nesting in thick forest, whereas on the adjacent Meyer islets (area = 11.5 ha), they were found in thick low scrub, on slopes similar to their habitat on Phillip Island. Maintaining the open areas for the petrels’ egress to nesting habitat is considered a significant future management consideration for their persistence on Phillip Island.
Nest attributes recorded in our study give a clear indication of the requirements for the petrel to breed successfully in the presence of a predatory ground-foraging bird. Branch tangles from the introduced olive provided a barrier to foraging swamphen in low shrub habitat. Targeted regeneration efforts on Phillip Island have been ongoing for several decades (Parks Australia 2000). As suggested by Priddel et al. (Reference Priddel, Carlile, Evans, Evans and McCoy2010) and supported by our empirical data, the removal of the introduced olive will need to be carefully considered so that Kermadec Petrels are not disadvantaged. The absence of other burrowing seabirds in Kermadec Petrel sub-colonies on Phillip Island is perhaps an additional requirement in nest selection for this species. The importance of this requirement needs to be determined from further studies at other colonies. We know that this petrel shares limited habitat on the Meyer islets with approximately 10,000 pairs of burrowing Wedge-tailed Shearwaters (Merton Reference Merton1970), indicating their tolerance to intraspecific competition for breeding space may be greater than we found.
Nesting density
The approximate nesting density of 0.1 nests/m2 found here is similar to the average density on San Ambrosio Island off northern Chile, where Marín et al. (Reference Marín, González and Trucco2020) found 0.17 nest/m2 (n = 10 plots) in an arid landscape denuded by goats. This is much higher than densities of 0.02 nests/m2 found by Imber (Reference Imber2005) on the heavily vegetated Meyer islets which are home to over 6,000 pairs. However, densities seen by early observers like Iredale (Reference Iredale1910) and others on Raoul Island in the early twentieth century of 0.4 nests/m2 (Venables in Merton Reference Merton1970), gives an indication of conspecific proximity that this species will tolerate. This density seems plausible with Iredale’s (Reference Iredale1914) estimate of 250,000 pairs on Raoul Island, before Norway rats Rattus norvegicus arrived in 1921 (Merton Reference Merton1970). Images depicting high densities of incubating birds from the islands off northern Chile shows high conspecific proximity (Marín et al. Reference Marín, González and Trucco2020). While uncommon in our study, there is potential for nest site competition with tropicbirds, which have similar nest attributes on Phillip Island (Priddel et al. Reference Priddel, Carlile, Evans, Evans and McCoy2010). In general, the Kermadec Petrel population on Phillip Island has ample space to expand given sufficient recruitment.
Seasonality of nesting
Kermadec Petrels on Phillip Island lay predominantly in the austral spring and summer (Figure 3). However, low numbers of austral autumn and winter breeding pairs may be due to historic nest predation by the swamphen during the cooler months. Summer breeding Black-winged Petrels depart in May and the winter breeding Little Shearwaters lay in July. Swamphens target both species and in their absence nest predation on the surface-nesting Kermadec Petrel may increase. From our initial season of monitoring all autumn-winter nests (n = 6) were predated prior to swamphen control. Further monitoring, with fewer swamphens, may see an increase in Kermadec Petrel breeding in the cooler seasons. Kermadec Petrel breeding has been noted both as summer or winter breeding on Raoul Island (Iredale Reference Iredale1910) and Henderson Island (Brooke Reference Brooke1995), as well as year-around on Meyer islets (Veitch and Harper Reference Veitch and Harper1998). The phenotypically similar surface-breeding Trindade Petrel Pterodroma arminjoniana breeds all year round, with laying peaks in September–October and again in February–March (Fonseca-Neto Reference Fonseca-Neto2004, Luigi et al. Reference Luigi, Bugoni, Fonseca-Neto and Teixeira2009).
Breeding ecology
Nest failure due to predation may have reduced the probability of pairs consistently breeding (Warham Reference Warham1990). Therefore, breeding frequency of individual pairs of Kermadec Petrel was difficult to determine. Pair bonds were strong with almost 90% of pairs (n = 16) remaining faithful to one partner despite the high likelihood of breeding failure (Table 2). Although breeding Kermadec Petrels are on Phillip Island year-around, it appears that most birds only vary their nesting schedule by one to two weeks. Imber (Reference Imber2005) posited that if the two temporarily separated populations on Meyer islets formed a single biological unit, the birds may be able to switch breeding seasons depending on food availability. Our data suggest this does not occur on Phillip Island. Knowing that birds have a consistent breeding schedule allows for targeted conservation actions. For example, predator control may be most appropriate on Phillip Island when alternative prey (small burrowing procellarids) are least available, which would increase the reproductive output of autumn-winter breeding Kermadec Petrels. The peak egg-laying period (austral spring) for the petrel appears to be little impacted by swamphen predation as a more abundant, alternative prey, is available.
By 2021 we recorded 56 breeding pairs (over two seasons) with an annual maximum of 42 in the final year (Table 2). The population grew from one breeding pair in 1989 to five breeding pairs (including one formed from two returned fledglings) in 1995 (O. Evans, unpub.). The slow rate of growth over the last 26 years is not unexpected in the absence of immigration, for example Bermuda Petrel Pterodroma cahow (Madeiros et al. Reference Madeiros, Carlile and Priddel2012). However, that the population has grown since first colonising, indicates that despite the impacts of nest predators, there is sufficient fledgling survival to allow recruitment. Alternatively, growth in the colony may be due to immigration from elsewhere. Further monitoring of colony expansion and knowledge of fledgling return rates will determine which factors are allowing the species to persist here.
Incubation shifts within breeding pairs were of similar duration (Table 1) and fit within those reported by Priddel et al. (Reference Priddel, Carlile, Evans, Evans and McCoy2010). An incubation shift of 18–19 days was reported for this species on Henderson Island (Brooke Reference Brooke1995), which is at the upper end of the ranges we recorded. Although these longer shifts require sufficient energy stores, they allow the partner to forage for longer and potentially go further afield (Kim et al. Reference Kim, Priddel and Carlile2018). Breeding success for the Phillip Island birds only reached above 50% in the final season of the study (Table 2). This level of success is as expected for petrels in general (Warham Reference Warham1990), and is similar (54%; n = 28) to that found in a small population on islets in the Juan Fernández Archipelago, off Chile (Hodum and Wainstein Reference Hodum and Wainstein2003). Maintaining this success on Phillip Island will be key to the species persisting here.
In the seasons following the first record of nesting, 78% of pairs nested at least once in a different location, on average 3 m from the previous nesting attempt. Our data indicate that birds are not as tied to their nest site as burrow-nesting petrels (Warham Reference Warham1990), and may indicate a change in nesting location related to their increased vulnerability, a developed behaviour to prevent predators learning their annual locations. We were unable to investigate whether successful breeders are more likely to retain nest sites in following seasons due to the small sample size and high level of nest predation. The transitory nature of breeding site choice has implications for installation of artificial habitat for conservation purposes as several units would be necessary for each breeding pair to accommodate their movements.
The age of first return for Kermadec Petrels on Phillip Island is less than three years, which is younger than the 4–6 years estimated by Imber (1985) and younger than other gadfly petrels. For example, Bermuda Petrel has first return age of approximately four years (males 4.0 years; n = 41: females 4.4 years; n = 55; range: 1.9–8 years: J. Madeiros pers. comm.), and in Gould’s Petrel P. leucoptera, the average age at return is five years (combined sexes range: 1–19 years; n = 455: N. Carlile unpub. data). If return rates and early breeding age continue, we could see considerable colony growth and resilience. More knowledge of the life-history of Kermadec Petrels could be gained by deployment of GLS tracking devices on pre-breeding birds. This would fulfil a gap in tracking knowledge for this species and provide insights into potential threats the species might be exposed to during the initial years of oceanic travel.
Impact of avian nest predators on Phillip and Lord Howe Islands
The Purple Swamphen is self-introduced to the Norfolk Island Group (Smithers and Disney Reference Smithers and Disney1969, Schodde et al. Reference Schodde, Fullagar and Hermes1983) and has long been treated as a pest species on Phillip Island (Priddel et al. Reference Priddel, Carlile, Evans, Evans and McCoy2010). Although they were not present in the late 1970s (Schodde et al. Reference Schodde, Fullagar and Hermes1983), their numbers have now been considered high for more than a decade (Priddel et al. Reference Priddel, Carlile, Evans, Evans and McCoy2010). No measure of swamphen predation on surface-nesting seabirds has previously been determined on the island although this had been recognised as an ongoing issue since the 1990s (Director of National Parks 2010). However, since culling of swamphens from 2019 there has been a clear rise in breeding success of Kermadec Petrels accompanied by increased numbers of nesting pairs. It is likely that the impact of chick loss and possible disturbance to nesting of Kermadec Petrels has been ongoing for many years and has resulted in low fledging success, despite available adult birds within the population. As Priddel and Carlile (Reference Priddel and Carlile2007) found with Gould’s Petrel, the removal of an avian predator can provide an immediate increase in breeding pairs because previously displaced individuals are then able to establish lasting pair bonds. It is not known if predation pressure on Kermadec Petrels by swamphens was equal throughout the year. Significant influxes of other ground-nesting seabirds (i.e. Black-winged Petrel, Little Shearwater, and Sooty Terns Onychoprion fuscatus) vary over the seasons and provide alternative prey. Current efforts to reduce the swamphen population are planned to continue (Director of National Parks 2022). This will also advantage other ground- nesting seabirds (Priddel et al. Reference Priddel, Carlile, Evans, Evans and McCoy2010) and allow increased breeding participation of autumn-winter nesting petrels, which are currently few in number (Figure 3). As the vegetative recovery of Phillip Island continues, it is likely that in future the Purple Swamphen will find the reduction in open habitat less conducive for foraging and breeding. As long as open areas are maintained near breeding sub-colonies of Kermadec Petrels such regeneration will not impact their breeding.
Kermadec Petrel formerly bred on Lord Howe Island prior to the introduction of rodents (Hindwood Reference Hindwood1940). Following recent efforts to eradicate rodents (Harper et al. Reference Harper, Pahor and Birch2020), reintroduction of the Kermadec Petrel is a conservation objective (Carlile et al. Reference Carlile, O’Dwyer, Wilson, Clarke, Brown, Baker, Garnett, Garnett and Baker2021) and could be achieved through the use of seabird attraction techniques (e.g. Kappes and Jones Reference Kappes and Jones2014). However, consideration must be given to the endemic Lord Howe Woodhen Hypotaenidia sylvestris, which is known to feed on petrels (Bester et al. Reference Bester, Priddel, Klomp, Carlile and O’Neill2007, O’Dwyer et al. Reference O’Dwyer, Carlile, O’Neill and Halpin2022). Our elucidation of nest attributes that contribute to predator avoidance will assist in establishing suitable locations for this conservation initiative but includes identifying areas that are infrequently inhabited by woodhens. Sites may be enhanced with artificial habitat, designed to encourage breeding. Artificial habitat should also be designed to reduce potential encounters from ground-foraging woodhens as well as aerial identification of nests by Lord Howe Pied Currawong Strepera graculina crissalis, also a known predator of seabird nestlings (Carlile and Priddel Reference Carlile and Priddel2015). If the Phillip Island population can maintain or increase fledgling production, it is likely to be the main source of recolonisers to Lord Howe Island due to its proximity (900 km north-east) and being within the bird’s foraging range (Carlile and O’Dwyer in litt.). There is an existing small breeding population with limited habitat on Balls Pyramid, south of Lord Howe Island, but it is unlikely to provide sufficient numbers of fledglings for colony establishment.
This species once bred in the South Pacific region in their 100,000s (Imber Reference Imber2005). The careful management of the population on Phillip Island and the re-establishment of the former colony on Lord Howe Island could aid in their regional recovery. In the process, it will assist the restoration of Phillip Island by significantly increasing the transfer of marine-derived nutrient (e.g. Wright and Metson Reference Wright and Metson1959), and pave the way for the return of the full complement of seabird fauna that inhabited Lord Howe Island prior to human arrival.
Acknowledgements
The Phillip Island surveys would not have been possible without the assistance and support of the staff from the Parks Australia Norfolk Island office. Access to the estate of the late Owen Evans OAM, through Mark Hallam by Arthur Evans was invaluable. Norfolk Island Clay Target Club assisted with Tarla Bird control. Luke Halpin, Emily Mowat, and Dean Portelli assisted with GLS attachment and recovery and nest monitoring. Thanks to ABBBS for access to banding records. DNA sexing was facilitated by Tammy Steeves through the School of Biological Sciences, University of Canterbury, New Zealand (export permit 2017064856). Grant funding for this research was through NSW Environmental Trust 2015/SSD/0002, NSW Government’s Conservation and Restoration Science (2017–2021), and Save Our Species programme (2018–2019). Funding for swamphen control was made to Parks Australia by the Australian Government Department of Infrastructure. The paper benefited from review of an earlier draft by Geoffrey Smith and Lisa O’Neill, and the final manuscript by Vincent Bretagnolle and an anonymous referee. This study was conducted under DPE Scientific licence SL100668 and AEC 021028/02 and Parks Australia permits.







