Introduction
Saline lakes are common landscape features in cold and warm/hot dry regions on every continent (Williams Reference Williams2002). They have a global volume of 104,000 km3, which is almost the same as the world’s fresh water (Florín Reference Florín and Howarth2013). Saline lakes have diverse ecological, sociological, and economic benefits (Wurtsbaugh et al. Reference Wurtsbaugh, Miller, Null, DeRose, Wilcock and Hahnenberger2017). Millions of migratory birds rely on saline lakes to fuel their long migrations with food resources such as brine shrimp Artemia spp. and brine flies Ephydra spp. (Roberts Reference Roberts2013). The water level of a saline lake influences populations of waterbirds in such a way that they are considered important bio-indicators of the lake’s health (Kulshreshtha and Sharma Reference Kulshreshtha and Sharma2008). Furthermore, saline lakes can be important indicators of environmental change because their size, water level, and salinity are sensitive to global circulation phenomena, such as the El Niño Southern Oscillation (ENSO), the North Atlantic Oscillation, or monsoons (Florín Reference Florín and Howarth2013; Williams Reference Williams2002). Due to their productivity and habitat suitability for migratory waterbirds such as flamingos, many saline lakes and wetlands are listed as Ramsar sites (Kshitij Divyansh and Raj Reference Kshitij Divyansh and Raj2019; Ramsar 1970). They are also under threat, and minerals including sodium chloride (salt), magnesium chloride, and sulphate of potassium (potash) are extracted from many saline lakes (Gwynn Reference Gwynn and Gwynn1998).
Despite the large variety of services that saline lakes provide and their global extent in dry regions, they are shrinking worldwide as their hydrological balance is changed by both climatic and anthropogenic factors (Williams Reference Williams1996; Wurtsbaugh et al. Reference Wurtsbaugh, Miller, Null, DeRose, Wilcock and Hahnenberger2017). Using the Köppen–Trewartha (K–T) climate classification and analysing observations from 1900 to 2010, as well as simulations spanning 1900–2100 from 20 global climate models, Feng et al. (Reference Feng, Hu, Huang, Ho, Li and Tang2014) demonstrated a significant redistribution of climate types in the most recent 15-year period (1996–2010) compared with the period 1961–1990. Their observations indicated that precipitation and, notably, temperature have altered the world’s climate since 1990. Based on their observations, the climate change scenarios predict a warmer or drier climate type for 31.4–46.3% of the global land area by the end of the twenty-first century, mostly in the Northern Hemisphere. Temperate, tropical, and dry climates are projected to expand, while polar, subpolar, and subtropical climates are projected to contract (Feng et al. Reference Feng, Hu, Huang, Ho, Li and Tang2014). Increasing temperature and evaporation as a consequence of climate change have caused significant water loss in semi-arid regions (Kirono and Kent Reference Kirono and Kent2011). Meng (Reference Meng2019) suggested that the increased evaporation and low precipitation over the last 50 years are the main reasons for the water decline of the Great Salt Lake in the northern part of the US state of Utah. However, Wurtsbaugh et al. (Reference Wurtsbaugh, Miller, Null, DeRose, Wilcock and Hahnenberger2017) and Wine et al. (Reference Wine, Null, DeRose and Wurtsbaugh2019) rebutted the findings of Meng (Reference Meng2019). Their conclusion is that the Great Salt Lake has been minimally affected by climate change; instead, the primary factor leading to its desiccation has been increased exploitation in the basin, including activities such as irrigation, dam construction, and water abstraction.
By intertwining these climatic elements with human actions like water abstraction for agricultural and domestic needs, the situation of these lakes was further exacerbated. A case in point is the saline lakes in Pakistan (Hussain et al. Reference Hussain, Han, Han, Rodrígue, Ha and Han2019), where these factors have contributed to heightened salinity levels over the past decade. Similarly, the Salton Sea, a shallow, saline lake in the south-west of the USA, is rapidly shrinking due to the reduction of fresh water from its affluent, the Colorado River (Tompson Reference Tompson2016).
Fluctuations in water level as a result of drought lead to frequent and longer periods of high salinity. This causes an enormous reduction in the number of invertebrate fauna because they are only present within a narrow range of salinities (Barnes and Wurtsbaugh Reference Barnes and Wurtsbaugh2015). Lake Albert, in eastern Oregon, hosted hundreds of thousands of waterbirds annually. However, both waterbirds and their invertebrate prey have experienced a reduction of up to 68% over the last 30 years as a result of low lake levels and high salinities (Senner et al. Reference Senner, Moore, Seager, Dougill, Kreuz and Senner2018). In a similar example, almost all of the hypersaline lakes in North America, which are important habitats for grebes, phalaropes, avocets, and stilts, are facing an insecure future because of the diversion of fresh water by humans (Conover and Bell Reference Conover, Bell, Baxter and Butler2020). Surprisingly, the study of Tavernia et al. (Reference Tavernia, Meehan, Neill and Luf2021) has not shown a decline in birds, despite shrinkage of the Great Salt Lake. However, they emphasised the importance of water quantity and quality to continue stable and positive bird population trends.
Together, these factors increase the water stress experienced by waterbirds and can cause a disruption in migration routes and important breeding areas (Haig et al. Reference Haig, Murphy, Matthews, Arismendi and Safeeq2019). The effect on waterbirds of losing a saline lake in arid and semi-arid areas is more dramatic because of a limited number of available migratory stop-overs in these regions (Wilsey et al. Reference Wilsey, Taylor, Michel and Stockdale2017). Integrating surface water information with climate patterns and human water usage factors offers an ecological perspective on evolving flyway conditions and addressing emerging migratory challenges (Donnelly et al. Reference Donnelly, King, Silverman, Collins, Carrera-Gonzalez and Lafón-Terrazas2020). Despite the importance of the saline lake ecosystem for waterbirds, they are not well studied nor protected (Belovsky et al. Reference Belovsky, Stephens, Perschon, Birdsey, Paul and Naftz2011).
Lake Urmia, situated in north-western Iran, exemplifies a drying saline lake that has historically supported substantial migratory waterbird populations. Aerial surveys, such as those by Scott (Reference Scott2001), have consistently documented thousands of waterbirds from diverse species frequenting the lake. Notably, the lake is home to the unique brine shrimp species, a primary food source for visiting migratory waterbirds (Lotfi Reference Lotfi2012). The lake’s surroundings, including mudflats, undulated plains, and eastern areas, provide essential wintering habitats for threatened waterbirds like Great White Pelican Pelecanus onocrotalus, Greater Flamingo Phoenicopterus roseus, White Stork Ciconia ciconia, Marbled Teal Marmaronetta angustirostris, and White-headed Duck Oxyura leucocephala. Therefore, the site aligns with Ramsar Criterion 1 and has the potential to merit Ramsar site designation due to the presence of these declining species. The lake plays a crucial role in bird breeding, hosting a diverse range of waterbird species such as Greater White Pelican, Little Egret Egretta garzetta, Glossy Ibis Plegadis falcinellus, Eurasian Spoonbill Platalea leucorodia, Greater Flamingo, and more. It is also a nesting ground for Greylag Goose Anser anser, Lesser White-fronted Goose Anser erythropus, and Ferruginous Duck Aythya nyroca. Flamingos have exhibited consistent breeding, with their numbers potentially reaching 25,000 breeding pairs, underscoring the significance of Lake Urmia (Ramsar Site Information Service 1997).
Existing data reveal that the Lake Urmia basin has documented 239 bird species, while the national park, encompassing the lake and its satellite wetlands, has recorded 212 species (Lotfi Reference Lotfi2012). The site’s significance was acknowledged in 1994 due to its consistent support of substantial populations of waterbirds (as detailed in Table A1) (Birdlife International 2023). Despite recent droughts, historical records confirm that Lake Urmia and its satellite wetlands typically harbour waterbird populations exceeding 20,000, meeting the Ramsar Convention’s Criterion 6 (Lotfi Reference Lotfi2012). This underscores the global importance of Lake Urmia and several of its satellite wetlands, resulting in its designation as a Ramsar site in 1971 and a biosphere reserve in 1976 (UNEP 2012).
The persistent drought at the lake since the 2000s has led to a significant decline in both migratory (breeding and wintering) and resident bird species that frequent the area (Lotfi Reference Lotfi2012). A decline in water level and increased salinity to more than 300 g/L may have stopped the hatching of brine shrimp, and likely has led to the decline in the number of birds that feed on this crustacean species, especially 40,000–80,000 pairs of breeding Greater Flamingos (Sima et al. Reference Sima, Rosenberg, Wurtsbaugh, Null and Kettenring2021).
The onset of drought in Lake Urmia arises from a combination of natural and human-induced factors. According to Delju et al. (Reference Delju, Ceylan, Piguet and Rebetez2013), the period between 1964 and 1996 witnessed alternating wet and dry cycles impacting Lake Urmia approximately every five years. However, during the 1970s and 1980s, drought events in Lake Urmia displayed heightened frequency but milder intensity. Conversely, from the 1990s onwards, droughts became lengthier and more severe. A discernible rise in temperature and a concomitant decrease in precipitation, notably since the early 1990s, align closely with the established drought cycle in Lake Urmia. The initiation of this pattern in 1997, along with an intensified occurrence between 2000 and 2001, reported by Delju et al. (Reference Delju, Ceylan, Piguet and Rebetez2013), has impeded the lake’s restoration to its typical state since then. During this period, the lake’s water level receded by 6.8 m from January 1992 to August 2010, revealing over 40% of the lakebed. They also observed that the lake experiences seasonal droughts typically spanning June–October annually, a critical timeframe for the agricultural industry, while in times of diminished precipitation, especially during autumn and winter, water scarcity intensifies markedly.
Chaudhari et al. (Reference Chaudhari, Felfelani, Shin and Pokhrel2018) found, respectively, a 98% and 180% increase in agricultural lands and urban areas from 1987 to 2016, with a corresponding shrinkage in lake area by 86%. Their analysis also showed that human water management activities caused a reduction in streamflow of 1.74 km3/year from 1995 to 2010, which accounts for 86% of the total depletion in lake volume during the same period. It is also found that the requirement for irrigation water almost tripled, causing large withdrawals from rivers. These results demonstrate that the ongoing depletion of Lake Urmia is not solely due to prolonged droughts but also due to direct anthropogenic alterations which caused significant changes in land use, streamflow, and water storage within the basin. Moreover, the construction of a causeway in 1979 that divides Lake Urmia into a northern and southern basin restricted water exchange and affected the flow and salinity regimes (Zeinoddini et al. Reference Zeinoddini, Tofighi and Vafaee2009). The combination of these factors could have disrupted the stable conditions of Lake Urmia as a safe habitat for birds.
Despite the significance of Lake Urmia for waterbirds, no comprehensive study has yet examined the long-term impact of the lake’s water crisis on these avian populations. Since waterbirds have shown adverse reactions to the lake’s desiccation, it is anticipated that both bird abundance and species richness have undergone a substantial decline since the year 2000. In this study, we analysed the variations in wintering waterbird species counts from 1970 to 2018, investigating their correlations with changes in environmental parameters, specifically the lake water level, river water discharge, well water levels, well water abstraction, average annual air temperature, and average annual precipitation.
Methods
Study area
Lake Urmia is located in the north-west of Iran between 37°06’15” and 38°15’15”N and 45°00’13” and 45°55’20”E (Figure 1). Lake Urmia is the second largest saltwater lake in the Middle East and the largest inland lake in Iran, with a surface area of 5,000 km2 and a water level at an altitude of 1,276 m.a.s.l (when full). The lake’s average depth is approximately 5.4 m during normal hydrological conditions, with a maximum depth of around 15 m in the northern parts. The basin of the lake comprises 17 permanent rivers, 12 seasonal rivers, and 39 floodways, serving as its primary water sources. The lake is formed in a natural depression within the Urmia basin and shared between the East and West Azerbaijan provinces (Figure 1). The lake basin, encompassing the entire hydrological basin of the lake, occupies 52,000 km2 of mainly mountainous territories in three provinces of East and West Azerbaijan and Kurdistan (Lotfi Reference Lotfi2012). It has a semi-arid climate with a mean annual temperature of 11°C (Delju et al. Reference Delju, Ceylan, Piguet and Rebetez2013), and a mean annual precipitation of about 357 mm (Fazel et al. Reference Fazel, Berndtsson, Uvo, Madani and Kløve2018). The length of the lake varies between 130 km and 140 km, and it reaches a maximum width of 50 km in the southern parts (Lotfi Reference Lotfi2012). Lake Urmia is protected as a national park, Ramsar site, and biosphere reserve. The lake includes 56 islands, most of which are small and uninhabited, and there are 17 satellite fresh or brackish water wetlands around the lake. This ecosystem, comprising Lake Urmia, its surrounding islands, satellite freshwater wetlands, and neighbouring salt lands, forms a unique and essential habitat for breeding and wintering waterbirds (Lotfi Reference Lotfi2012).

Figure 1. Geographical location of Urmia Lake and its basin in north-western Iran.
Environmental parameters
Environmental parameters utilized in our investigation comprised lake water level (LWL) measured in meters above sea level (m.a.s.l), obtained from daily recorded observations at the Golmankhane station (37°36’N, 45°16’E) spanning 1970 to 2018 (Figure 2). Water discharge of the rivers (WDR) was expressed in million cubic meters (mcm), representing the total monthly volume of water flowing from each river to the lake from 1970 until 2015. Well water level (WWL) was measured in meters, representing the distance from the land surface to the water in wells under static conditions, aggregated on a monthly basis. Well water withdrawal (WWW) was quantified in cubic meters per second (m³/second), indicating farmers’ total monthly groundwater extraction from the wells across the lake basin (Faramarzi Reference Faramarzi2012). Additionally, mean yearly air temperature (MYAT) was recorded in degrees Celsius (°C), and mean yearly precipitation (MYPPT) was measured in millimeters (mm).
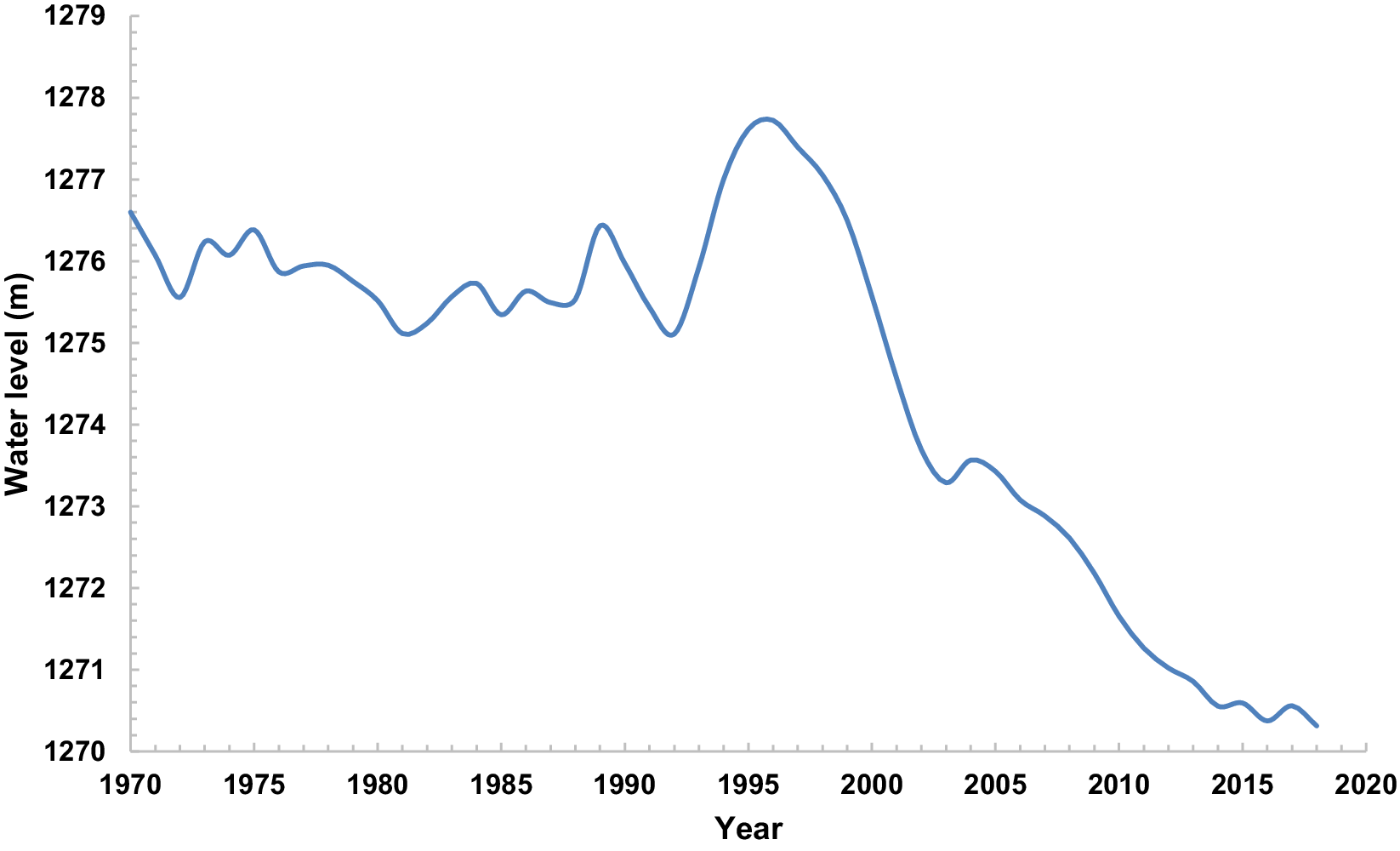
Figure 2. Water level of Lake Urmia (m.a.s.l) in January from 1970 until 2018.
In this study, we used the data from 44 piezometric wells located in the lake basin from 1970 to 2013. The hydrological parameters were obtained from the Urmia Lake Restoration Programme (pers. comm.) and the Iran Water Resources Management Company. Climatic parameters were recorded at the Urmia synoptic station (37°40’00”N – 45°03’00”E) at an altitude of 1,328 m.a.s.l (Delju et al. Reference Delju, Ceylan, Piguet and Rebetez2013). The data from this synoptic station, covering MYAT and MYPPT, were sourced from the Iran Meteorological Organisation (IRIMO) spanning the period from 1970 to 2018.
Bird data
Lake Urmia supports internationally important numbers of wintering water birds. Data are only available for annual winter counts by the Iranian Department of Environment (DoE) in Lake Urmia and bordering Qara-Gheshlaq (Sima et al. Reference Sima, Rosenberg, Wurtsbaugh, Null and Kettenring2021). For this research, we employed bird counting data from January bird counts, starting in 1970 and extending until 2018, with some interruptions during the 1980s. Total counts of the different bird species were recorded by DoE staff by walking or sailing around the lake coasts with 10 × 40 binoculars and 15 × 60 telescopes. Qara-Gheshlaq stands as a multifaceted interconnected wetlands complex spanning approximately 3,000 ha, to the south of the lake, bridging the East and West Azerbaijan provinces. This wetland is characterised by its shallow and freshwater nature. Periodically, when water levels are high, Qara-Gheshlaq is partly submerged by hypersaline water (Lotfi Reference Lotfi2012). To reduce bird recognition and identification biases, the same group of experienced ornithologists carried out the annual census in most of the years. However, the number of survey staff was not fixed and varied depending on vehicle facilities, the number of skilled experts in each region, site area, and bird populations. The counting procedure remained consistent from 1970 until the end of the study in 2018. The bird survey began on 5 January each year and took no longer than two weeks. The birds were counted continuously and without interruption on a sampling day to avoid repeated surveys in nearby sites. Exceptionally, if the team could not finish the survey for a specific location on one sampling day, it continued the next day.
Statistical analysis
Breakpoint analysis
The breakpoint is the location in the time series where the slope changes and is found using single or multiple change point methods (Garmo et al. Reference Garmo, Kaste, Arle, Austnes, de Wi and Fölster2020). Using the bcp package in R (Erdman and Emerson Reference Erdman and Emerson2007), we implemented Bayesian analysis of change point (BCP), a multiple breakpoint method, to identify a change in mean for environmental parameters. The Bayesian approach estimates the posterior means and posterior probability of changes (breakpoints) at any given location along a sequence. Posterior probability of a change shows the proportion of iterations resulting in a change point at each location and posterior means displays the posterior mean of each location (Erdman and Emerson Reference Erdman and Emerson2007). The breakpoint in the BCP method was defined as a point where the posterior probability or the probability of a change exceeded a cut-off point of 60% (Tenan et al. Reference Tenan, Tweedell and Haynes2017). The algorithm in bcp packages is built upon the Barry and Hartigan (Reference Barry and Hartigan1993) product partition model for the normal errors change-point problem, using Markov chain Monte Carlo with a default of 500 iterations. A default value of 0.2 was used as the prior on change-point probabilities occurring at each point in the series. The environmental parameters investigated in the breakpoint analysis included LWL, WDR, WWL, WWW, MYAT, and MYPPT.
Bird assemblages analysis
Initially, we examined the reaction of the waterbird assemblages to the desiccation of Lake Urmia. This involved plotting the count of key waterbird species (based on Ramsar Criterion 6) per observation year in relation to variations in lake water level spanning 1970 to 2018.
Next, we employed canonical correspondence analysis (CCA), a multivariate statistical technique, to explore the connection between environmental factors, namely, LWL, WDR, WWL, WWW, MYAT, and MYPPT, and the abundance of waterbirds. CCA represents an instance of direct gradient analysis, wherein the existing gradient in environmental factors is established beforehand, and species abundances are regarded as responses to this gradient. Furthermore, the primary objective is to identify a set of axes that maximises the correlation between environmental variables and species abundance data (Legendre and Legendre Reference Legendre and Legendre1998). To enhance the reliability of the analysis, we initially applied k-means cluster analysis to eliminate rarely observed avian species from the CCA data set. This pre-processing step is aimed at bolstering the overall robustness of the subsequent analysis.
Finally, we conducted a comparative analysis of species richness and waterbird abundance before and after the year 2000 using Student’s t-test. Additionally, we conducted Pearson correlation analysis to ascertain any significant correlation between bird assemblage and lake water level from 1970 to 2018.
Results
Time series
The Bayesian breakpoint analysis identified the commencement of the drought phase for Lake Urmia in the year 2000 (Table 1 and Figure 3). We can observe from Figure 3 that rising air temperature and declining precipitation alongside increased water pumping from wells and a reduction in river flow led to a drop in water level after the year 2000. The year 1987 coincided with maximum water discharges via rivers to the lake (9,551, 55 mcm), and 2001 was synchronous with the dropping of lake water below the normal level (1,276 m.a.s.l). The distinct peak in the change-point analysis indicates the synchronicity of changes in the environmental parameters across the lake. The increase in precipitation and water flow into Lake Urmia in 1994 and 1995 coincided with the water level rising in 1993. Moreover, the beginning of declining trends in lake water level in 1999 was synchronous with an increase in pumping water from wells in 1998 (Table 1 and Figure 3).
Table 1. Change-point analysis using the Bayesian approach

BCP = Bayesian analysis of change point; LWL = lake water level (m.a.s.l); WDR = water discharge of rivers (mcm); WWL = well water level (m); WWW = well water withdrawal (m3/second); MYAT = mean yearly air temperature (°C); MYPPT = mean yearly precipitation (mm).

Figure 3. Bayesian breakpoint analyses for (A) lake water level (m.a.s.l), (B) water discharge of the rivers to the lake (mcm), (C) well water level (m), (D) well water withdrawal (m3/second), (E) mean yearly precipitation (mm), and (F) mean yearly air temperature (°C). The time-series breakpoint analysis was performed using the bcp package in R, with the default (550) number of iterations.
Effect of the lake water crisis on bird assemblages
A total number of 1,380,277 individual waterbirds from 80 species and 14 families was recorded from 1970 until 2018 in Lake Urmia. Out of 80 species, 36 were observed during the whole study period, 37 species only after the year 2000, and seven species only before the year 2000 (Table A2).
In accordance with Ramsar Criterion 6, we identified four prominent waterbird species within Lake Urmia’s ecosystem: the Greater Flamingo, Ruddy Shelduck Tadorna ferruginea, Common Shelduck Tadorna tadorna, and Greylag Goose. These avian species demonstrated a consistent presence throughout the entire duration of the study. While some years experienced intermittent observations, these four species displayed remarkable persistence.
To elucidate the temporal dynamics of these species, we visually represented their population fluctuations spanning the years 1970 to 2018, as depicted in Figure 4. This graphical presentation is combined with an illustration of Lake Urmia’s water level fluctuations, facilitating a comprehensive comparative analysis. Figure 4 reveals a prevailing declining trend in population numbers of the four bird species, mirroring corresponding declines in the lake’s water levels after year 2000. Among these species, the lowest bird numbers after the year 2000 are attributed to the Ruddy Shelduck and Greater Flamingo, with averages of 1,489 and 1,777 individuals, respectively. However, minor increases in the count of these four bird species become noticeable after the year 2000, specifically around 2005, 2012, and 2015, coinciding with slight increments in the lake’s water level. Remarkably, this trend persists even in earlier years (e.g. 1976, 1990, 1996, and 1998), indicating a pre-existing correlation between bird populations and lake water levels (Figure 4).

Figure 4. The population variations of four key waterbird species, Greater Flamingo Phoenicopterus roseus, Ruddy Shelduck Tadorna ferruginea, Common Shelduck Tadorna tadorna, and Greylag Goose Anser anser, meeting Ramsar Criterion 6, alongside Lake Urmia’s water level fluctuations from 1970 to 2018.
Our findings revealed that among the 14 families, Anatidae (ducks, geese, and swans), had a total of 23 species. Laridae (gulls, terns, and skimmers) 13 species, and Scolopacidae (sandpipers, snipes, and phalaropes), comprised 11 species. These families exhibited the greatest species diversity in the lake. On the other hand, Ciconiidae (storks), Pelecanidae (pelicans), Gruidae (cranes), Phalacrocoracidae (cormorants), Phoenicopteridae (flamingos), Recurvirostridae (avocets and stilts), and Threskiornithidae (ibises) showed the lowest species diversity (Figure 5).

Figure 5. The number of observed waterbirds species per family. The blue bar shows the number of species that were observed only before the year 2000, the brown bar shows the number of species that were observed only after the year 2000, and the green bar shows the number of species observed during the whole study period (1970–2018).
In the case of the Laridae and Scolopacidae families, the number of species observed exclusively in the lake after the year 2000 was notably greater compared with species exclusively observed prior to 2000 or those observed throughout the entire study period. Conversely, within the Anatidae family, the majority of species were consistently observed throughout the entire study period. The Recurvirostridae and Pelecanidae families only appeared in the lake after the year 2000 (as shown in Figure 5).
The outcomes of the k-means cluster analysis revealed the presence of 51 rarely observed avian species that were subsequently excluded from the CCA analysis. Consequently, our CCA analysis was conducted on the remaining 30 species – 21 that were occasionally observed and nine that were frequently sighted (see Table A3).
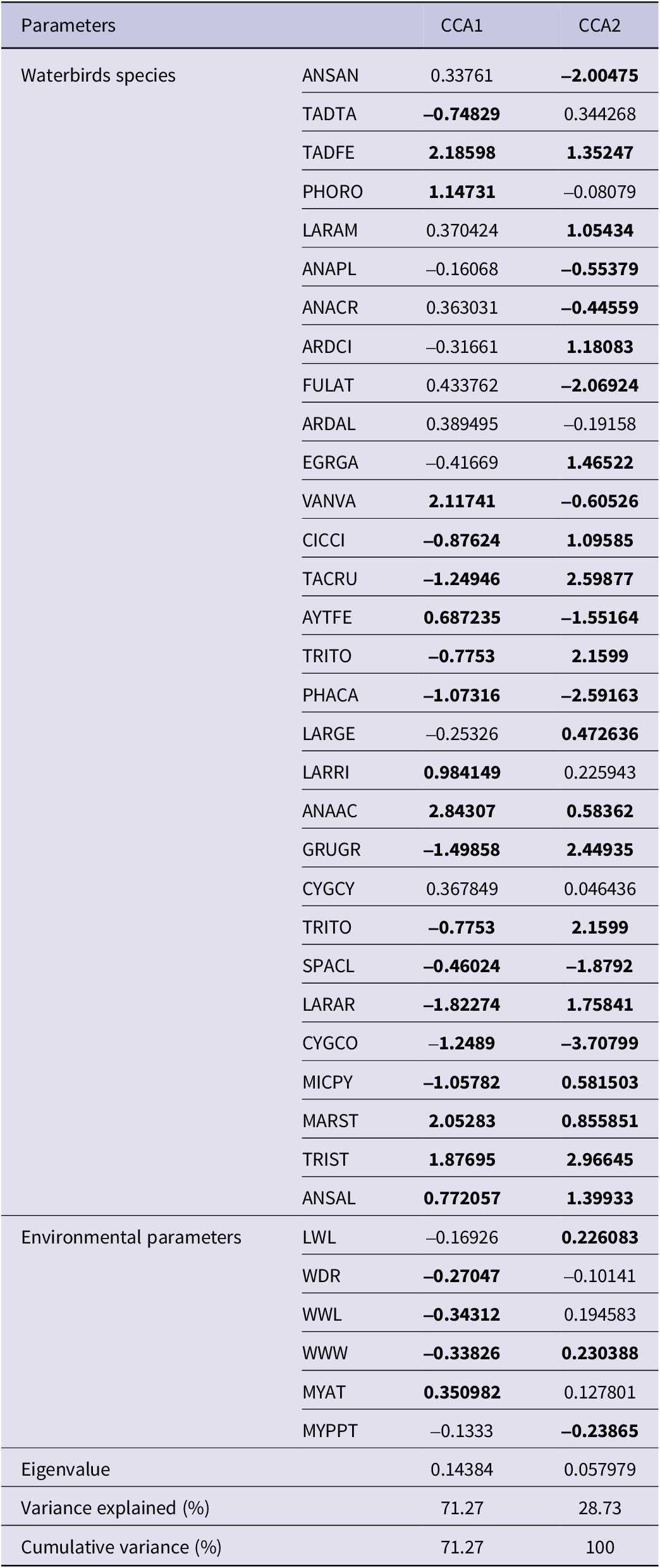
The CCA of bird species and environmental variables unveiled two principal axes accounting for the entire variance (100%). The outcomes of the CCA analysis revealed the substantial roles both axes play in elucidating the connections between bird species and environmental variables. The primary axis, being the most influential, elucidates a significant portion of the variance and underscores robust interrelations among the variables. Although the second axis, characterised by a comparatively lower eigenvalue, maintains meaningful contributions, it does so to a somewhat lesser extent. This apportionment of variance across the axes aligns with the common pattern observed in multivariate analyses like CCA, where the initial axes typically capture the bulk of the information, while subsequent ones capture relatively smaller, yet still valuable, amounts of variation.
The first CCA axis, characterised by an eigenvalue of 0.14, accounted for 71.27% of the variance across the two data sets. This axis exhibited a robust negative correlation with environmental parameters such as lake water discharge, well water withdrawal, and well water level. Conversely, it displayed a noteworthy positive correlation with the mean annual air temperature (see details in Table 2 and Figure 6). The secondary CCA axis, linked to an eigenvalue of 0.05 and capturing 28.73% of the total variance, exhibited a marked positive correlation with the lake water level and well water withdrawal and conversely, displayed a negative correlation with mean yearly precipitation (Table 2 and Figure 6). Notably, all water-related parameters clustered on the left side of the first axis, while temperature stood alone on the right side (Figure 6).
Table 2. Results of the first two axes of the CCA depicting the relationship between waterbirds and environmental parameters at Lake Urmia, accompanied by their respective factor loadings. The factor loadings exceeding 0.45 for the bird species and loadings surpassing 20 for the environmental parameters within the initial two axes are emphasised by being highlighted in bold. The species name abbreviations are referenced in Table A2
CCA = canonical correspondence analysis; LWL = lake water level (m.a.s.l); WDR = water discharge of the rivers (mcm); WWL = well water level (m); WWW = well water withdrawal (m3/s); MYAT = mean yearly air temperature (°C); MYPPT = mean yearly precipitation (mm).
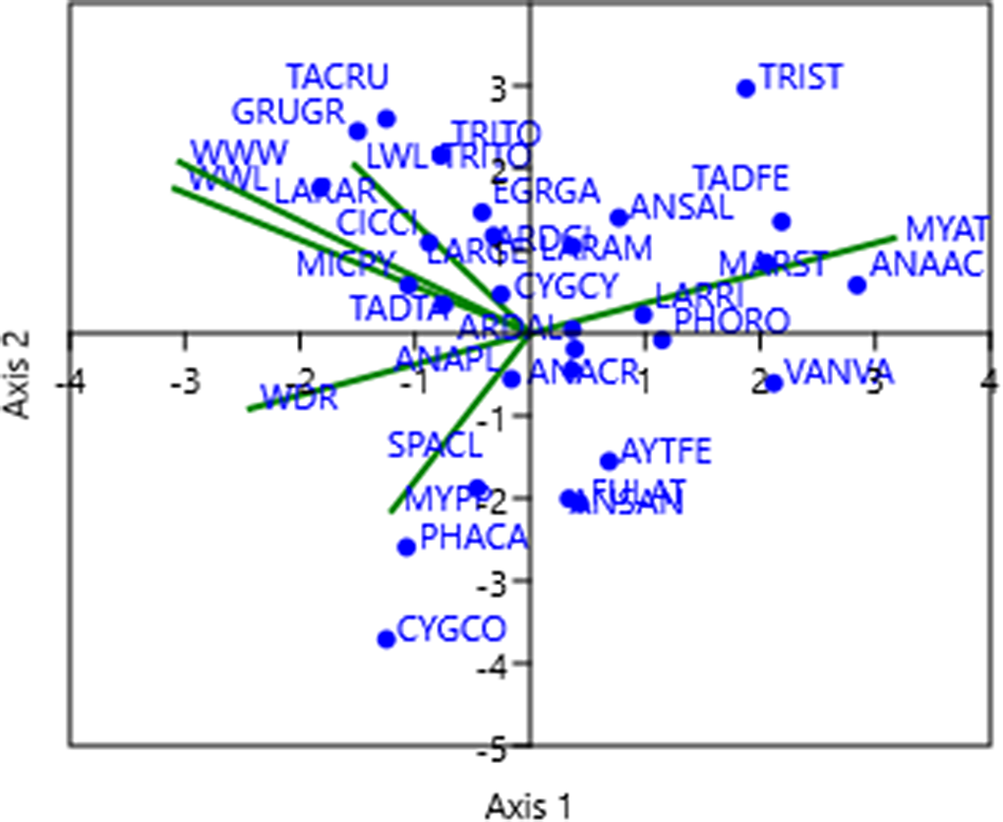
Figure 6. Ordination of waterbirds data and environmental parameters in a canonical correspondence analysis (CCA) space at Lake Urmia. The first (horizontal) and second (vertical) axes of the CCA ordination space show the relationships between waterbirds data and environmental parameters with significant contribution. Blue dots represent waterbirds, and the green lines represent environmental parameters. The abbreviations of species names are given in Table A2. The environmental parameters include lake water level (LWL), water discharge of the rivers (WDR), well water level (WWL), well water withdrawal (WWW), mean yearly air temperature (MYAT), and mean yearly precipitation (MYPPT).
The importance of the species scores lies in their distribution along the first and second CCA axes. Excluding seven avian species, namely, Greater Flamingo, Northern Lapwing Vanellus vanellus, Black-headed Gull Larus ridibundus, Northern Pintail Anas acuta, Ruddy Shelduck, Marsh Sandpiper Tringa stagnatilis, and Gadwall Mareca strepera, all of which exhibited a strong positive correlation with the first CCA axis, representing a measure of temperature; the remaining 23 species showed distinct trends. These trends included pronounced negative correlations with the first CCA axis and/or substantial correlations, both positive and negative, with the second CCA axis. Both the second CCA axis and the negative side of the first CCA axis exhibited correlations with parameters linked to water availability in Lake Urmia. This illumination underscores the crucial role of these parameters in influencing the waterbird dynamics within the area. For a comprehensive overview, refer to Table 2 and Figure 6.
Furthermore, a noteworthy decline in bird abundance was detected after the year 2000 (t = 2.27, n = 40, P = <0.05). In contrast to the reduction in overall bird count, a substantial increase in bird species was observed (t = -4.8, n = 40, P = <0.001). Moreover, a noteworthy and statistically significant positive correlation was observed between bird abundance and lake water level (r = 0.44, n = 41, P <0.01). In contrast, there was a significant, albeit negative, correlation between bird species richness and water level (r = -0.50, n = 41, P <0.001).
Discussion
Breakpoint analyses have shown that the onset of the water crisis in Lake Urmia transpired in the year 2000. Temporal patterns within the time series have revealed a concurrent decline in the lake’s water level, closely paralleling shifts in hydrological and climatic parameters. At the same time, the deleterious effects of the drought have reached the avian assemblages, manifested through a comprehensive reduction in the population counts of all four notable waterbird species, namely the Greater Flamingo, Ruddy Shelduck, Common Shelduck, and Greylag Goose. This decline in population coincides with the diminishing lake water level. Furthermore, our findings underscore a substantial correlation between the majority of waterbird species and the CCA axes, which are intricately linked to water availability within Lake Urmia. These results accentuate the pivotal role played by fluctuations in Lake Urmia’s water levels in shaping the dynamics of its waterbird assemblages.
Analysing the population trends of the Greater Flamingo, Ruddy Shelduck, Common Shelduck, and Greylag Goose in relation to Lake Urmia’s water level fluctuations from 1970 to 2018 unveiled a prominent decline in their numbers post-2000 (Figure 4). As suggested by Savage (Reference Savage1964), the presence of shelducks and flamingos in Lake Urmia is intricately tied to the abundant seasonal food supply, notably brine shrimp and their eggs. Flamingos (Botond et al. Reference Botond2022) and most Anatidae (Nouidjem et al. Reference Nouidjem, Saheb, Bensaci, Bouzegag, Guergueb and Houhamdi2015) require access to shallow and preferably saline water for resting and feeding.
In a study conducted by Sima et al. (Reference Sima, Rosenberg, Wurtsbaugh, Null and Kettenring2021), it was found that the lake’s water level had decreased by 6.8 m, leading to a rise in salinity to nearly 360 g/L. This shift correlated with a notable decrease in the flamingo population at Lake Urmia. Even at a lake level of 1,274 m with salinities around 280 g/L, the flamingo population was less than half that at higher water levels. This can be attributed to the fact that this particular water level might not achieve the salinity levels previously believed to sustain thriving brine shrimp populations. Furthermore, other invertebrates might not attain sufficient densities at a salinity of 263 g/L to provide sustenance for flamingos and other avian species.
Hence, a lake level exceeding 1,275 m.a.s.l. is imperative to reduce salinity, maintain brine shrimps and other invertebrate populations (such as brine flies and molluscs), and safeguard island habitats. Additionally, accurate characterisation of the habitat requirements for flamingos, brine shrimps, invertebrates, and other avian species in Lake Urmia and adjacent wetlands is essential for effective conservation efforts, as emphasised by Sima et al. (Reference Sima, Rosenberg, Wurtsbaugh, Null and Kettenring2021).
While the overarching trend in the abundance of the four prominent waterbird species indicated a decline since 2000, our observations showed distinct peaks in their populations around the years 2005, 2012, and 2015. Notably, these peaks coincided with corresponding spikes in lake water level, suggesting a potential correlation. Importantly, we also identified peaks in their abundance that coincided with the lake’s water level peaks before 2000, particularly during the years 1976, 1990, 1996, and 1998, when the lake’s water level remained within its typical ranges. These observations underscore the intricate relationship between waterbird abundance and oscillations in the lake’s water level, as illustrated in Figure 4.
Saemian et al. (Reference Saemian, Elmi, Vishwakarma, Tourian and Sneeuw2020), reported no notable reduction in the water storage of Lake Urmia between 2010–2012 and 2015–2017. In fact, the trend of decreasing water level of Lake Urmia was less intense in these years, and this trend even showed a slight reverse in 2003–2004 to some extent (0.28 m/year). From 2004 until 2006, the lake water level declined but with a lower slope compared with the years before 2003. We observed almost the same situation between the years 2012 and 2013. The declining trend between the years 2011 and 2013 was less acute compared with the years before 2011 and after 2013. The water level of the lake also had a low increasing trend from 2014 until 2015 and from 2016 until 2017 (Figure 2). This result suggests that waterbirds abundance might be associated with water level fluctuations, as observed by Romano et al. (Reference Romano, Barberis, Pagano and Maidagan2005). Even a small relative improvement in the hydrological conditions of the lake or relatively stable conditions for two consecutive years had a significant impact on increasing the number of wintering waterbirds.
We also found that the variation in species composition in Lake Urmia through the years was associated with water level fluctuations. Waterbirds predominated in those years with lower water levels and the species that fed mainly on plants or vertebrates such as Anatidae predominated in years with higher levels. We observed that the number of waterbirds (i.e. Scolopacidae, Recurvirostridae, and Charadriidae) increased after the year 2000. Among them, the Recurvirostridae family was only observed after the year 2000. The inverse relationship between water levels and waterbird occurrence has also been observed in previous studies (Brennan Reference Brennan2006; Cole et al. Reference Cole, Leslie and Fisher2002; Collazo et al. Reference Collazo, O’Harra and Kelly2002). A decrease in water levels can signify increased habitat availability for waterbirds (Collazo et al. Reference Collazo, O’Harra and Kelly2002; Reske and Yun Reference Reske and Yun2000). Waterbirds tend to occupy shallow water habitats which provide greater access to food resources (Cole et al. Reference Cole, Leslie and Fisher2002).
We also observed that families such as Laridae and Ardeidae (herons) had the highest number of species after the year 2000 (Figure 5). The previous studies found that herons showed a preference for shallow water and shallow shorelines because these provide suitable food resources (Chettibi et al. Reference Chettibi, Bensaci, Mimeche and Djamai2019; Colwell and Taft Reference Colwell and Taft2000; Khaing and Sein Reference Khaing and Sein2019; Nouidjem et al. Reference Nouidjem, Mimeche, Bensaci, Merouani, Arar and Saheb2019). Likewise, the gradual desiccation of saline lagoons along the West African coast (e.g. Ghana) serves as a magnet for migratory birds like sandpipers, terns, and herons. These birds are drawn to these drying lagoons to forage on the benthic fauna found in the shallow peripheries of the lagoons (Van de Kam et al. Reference Van de Kam, Ens, Piersma and Zwarts2004). The results of Jitariu et al. (Reference Jitariu, Dorosencu, Ichim and Ion2022) also showed that the decrease in the water surface had no effect on gulls and terns, and numbers even increased in such conditions.
The outcomes of the CCA analysis revealed a notable concentration of waterbirds on the left side of the CCA plot, indicative of water availability in the lake. This concentration implies that these species are more likely to inhabit years characterised by high water availability in Lake Urmia. However, a few species, including the Greater Flamingo, Ruddy Shelduck, and Marsh Sandpiper, exhibit a positive correlation with the mean annual temperature.
It is crucial to emphasise that the CCA findings provide just a single piece of the puzzle in understanding the relationship between waterbird populations and environmental variables. It is imperative to consider additional lines of evidence, such as examining abundance plotted against lake water levels. For instance, Figure 4 demonstrates that the populations of Greater Flamingo and Ruddy Shelduck experienced a noticeable decline after the year 2000, coinciding with the onset of the Lake Urmia drought. However, due to the very low counts of these two bird species after the year 2000, the CCA may not have effectively extracted a clear correlation between their numbers and the lake’s water level.
Furthermore, some species might possess adaptations that enable them to cope with fluctuating water levels. Consider the Marsh Sandpiper, a waterbird that tends to inhabit wet mud–shallow water habitats. Shallow waters offer improved access to food resources, which are not as easily accessible in deeper waters (Cole et al. Reference Cole, Leslie and Fisher2002). Hence, this species displays a positive correlation with temperature. Ecological studies often encounter intricate interactions between various environmental variables and species behaviours. Consequently, it is essential to account for multiple factors and potential interpretations when analysing the observed results.
As noted in this study, the conservation value of Lake Urmia is in the provision of habitat both for local birds and to support broader migratory connectivity. Haig et al. (Reference Haig, Mehlman and Oring1998) suggested that the drying of lakes imposes heightened energetic demands on migratory birds by reducing the number of suitable stop-overs (and wintering sites) and increasing the flight distances between them. A reduction in available food resources could result in reduced fitness and continental migration failure (Donnelly et al. Reference Donnelly, King, Silverman, Collins, Carrera-Gonzalez and Lafón-Terrazas2020). Hence, arresting drought progression in Lake Urmia and actively contributing to its restoration becomes crucial for safeguarding the continuity of waterbird migration routes in north-western Iran.
In line with recommendations suggested by Valiallahi et al. (Reference Valiallahi, Soltani and Ahmadi Eghbal2019), any further dam constructions should be halted, and efforts should be directed towards fulfilling the ecological water requirements for Lake Urmia’s restoration. This entails releasing 50% of the water stored behind dams during the wet seasons and 15–25% during dry seasons. Additionally, to facilitate the revitalisation of Lake Urmia, steps such as curbing agricultural demands and effectively managing groundwater wells within the lake basin need to be taken, as outlined by Valiallahi et al. (Reference Valiallahi, Soltani and Ahmadi Eghbal2019). It is important to acknowledge that, given the dependency of many individuals on agriculture for their livelihoods, the implementation of sustainable water management practices becomes pivotal. Striking a balance between human needs and ecological imperatives is essential for ensuring the enduring restoration of Lake Urmia. This involves the implementation of strategies such as altering crop patterns from high water-demand varieties to those requiring less, purifying drainage water for reuse, refraining from cultivating water-intensive crops during crucial periods, and enhancing farmer earnings by opting for high-quality and high-income-return crops. These measures, as outlined by Pereira et al. (Reference Pereira, Oweis and Zairi2002), can effectively curtail water requirements at the farm level.
Moreover, water withdrawal from rivers for agriculture upstream of the lake basin can reduce the lake’s water level. Therefore, implementing policies such as removing subsidies for water resources, imposing tax penalties for illegal water misuse, regular water resource systems control (meaning consistent monitoring and regulation of water usage and distribution), and accurate irrigation schemes upstream could improve water management in the Lake Urmia basin (Pereira et al. Reference Pereira, Oweis and Zairi2002). As suggested by Downard and Endter-Wada (Reference Downard and Endter-Wada2013), policies that focus more on the water needed to sustain wetlands are more effective in protecting them.
Freshwater wetlands adjacent to saline lakes are essential to waterbirds since these balance the physiological demands of saltwater environments (i.e. osmoregulation) (Rocha et al. Reference Rocha, Silva, Villegas, Sanchez-Guzman, Ramos and Masero2016). Therefore, protecting these wetlands around Lake Urmia is important to conserve migratory birds during drought periods because the adaptive capacity of migratory waterbirds to mitigate changing resource distributions has been proved by Rakhimberdiev et al. (Reference Rakhimberdiev, Duijns, Karagicheva, Camphuysen, Castricum and Dekinga2018). Formed through the collaboration of the Department of Environment (DoE), the United Nations Environment Programme (UNEP), and the Conservation of Iranian Wetland Project, the Integrated Management Plan for Lake Urmia Basin outlines a comprehensive 25-year vision for the region. This management plan followed three main objectives: (1) participation of local people in preserving the Lake Urmia basin and wetlands around the lake; (2) sustainable irrigation water management in the lake basin; (3) conservation of biodiversity and the sustainable use of wetland resources (Faramarzi Reference Faramarzi2012).
Unfortunately, recognising the importance of saline lakes as essential habitats for waterbirds has not slowed their degradation (Lemly et al. Reference Lemly, Kingsford and Thompson2000). Since the abundance of waterbirds in the saline lakes is associated with lake level fluctuation (Romano et al. Reference Romano, Barberis, Pagano and Maidagan2005), any management and conservation plan needs a solid baseline to estimate the population trends for each species (Wilsey et al. Reference Wilsey, Taylor, Michel and Stockdale2017) and long-term studies of these ecosystems (Romano et al. Reference Romano, Barberis, Pagano and Maidagan2005). Moreover, conserving saline lake ecosystems for the species that rely on them requires keeping water levels and salinities within the normal range of seasonal inter-annual variation in a way that periods with extremely high or low salinity levels do not occur too frequently or become too prolonged (Senner et al. Reference Senner, Moore, Seager, Dougill, Kreuz and Senner2018). We should take into account that drying of Lake Urmia is not only a threat to bird assemblages but also to humans. The escalation in wind-blown dust and corresponding environmental exposure entails risks such as air and water pollution, as well as exposure to toxic substances such as lead and mercury. This predicament arises from the diminishing saline lake and has the potential to generate a public health crisis and environmental injustices. For instance, as the lake diminishes, the water quality may degrade, potentially leading to increased instances of waterborne diseases among communities reliant on it for drinking water. Moreover, certain marginalized groups, such as low-income communities or indigenous populations, may bear a disproportionate burden from the environmental changes, facing challenges such as loss of livelihoods or unequal access to alternative water sources. Consequently, addressing and safeguarding against this issue necessitate meticulous attention and the formulation of protective measures, as highlighted by Johnston et al. (Reference Johnston, Razafy, Lugo, Olmedo and Farzan2019).
Acknowledgements
This research received financial support from the Iran National Elite Foundation. We are grateful to the Iran Department of Environment for providing bird count data, the Urmia Lake Restoration Programme, the Regional Water Company of Tehran for providing hydrological data, the Iran Meteorological Organisation (IRIMO) for providing meteorological data, and two anonymous reviewers for helpful comments on the first version of the manuscript. We also thank Diana Bowler for her help with the analysis. All authors made substantial contributions to the conception, design, and drafting of the manuscript that led to the writing of the manuscript. The datasets generated and analysed in this study are partially available from the corresponding author on request. The R code used to conduct analyses are available from the corresponding author on request.
Appendix
Table A1. Principal bird species populations regularly present at Lake Urmia
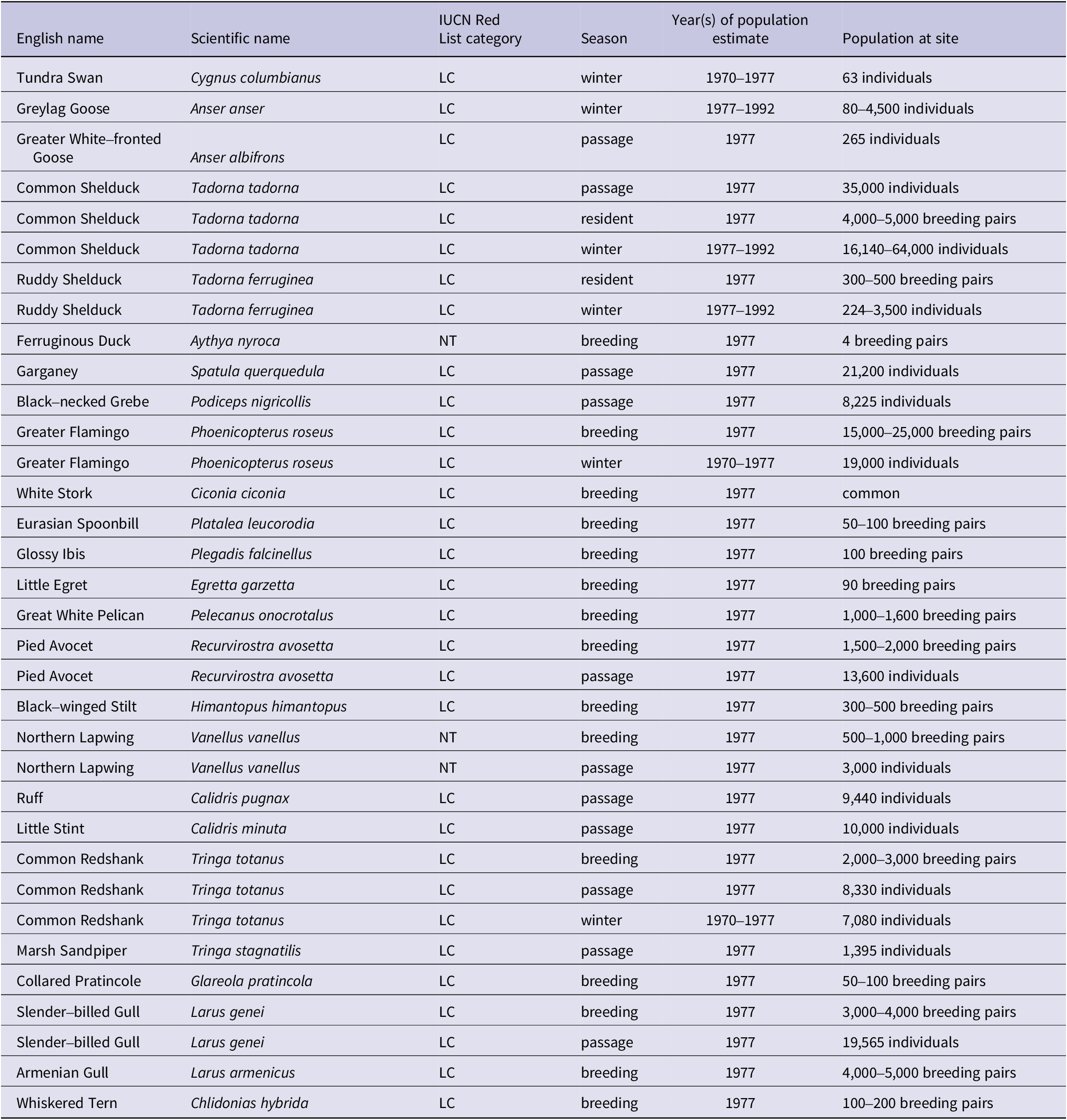
LC = Least Concern; NT = Near Threatened.
Table A2. Waterbirds observed in Lake Urmia from 1970 until 2018. The average number of individuals per observed year has been shown for the periods before and after 2000

The species are sequenced alphabetically within each family, following the alphabetical order of English names. Families are arranged in alphabetical order.
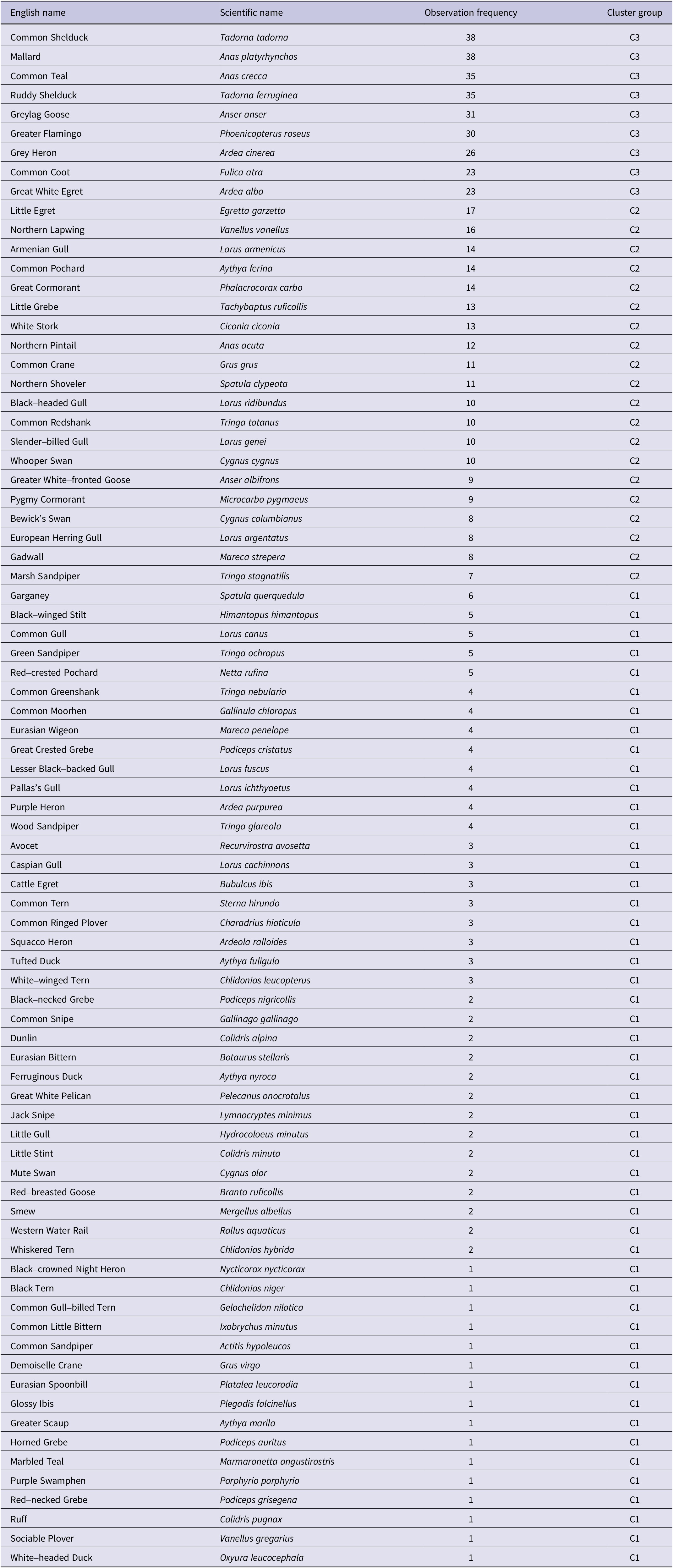
Table A3. Observation frequency of waterbirds from 1970 to 2018 in Lake Urmia. They have been categorised into three cluster groups: the frequently observed birds observed between 23 and 38 times during study period (C3), the occasionally observed birds observed between 7 and 17 times (C2), and the rarely observed birds observed between 1 and 6 times (C1). Species are arranged based on observation frequency, with similar frequencies grouped together and sequenced alphabetically within each group. The overall sequence follows the alphabetical order of English name