Introduction
Conservation of migratory species relying on several distant sites during the annual cycle is much more complex than that of non-migratory species (Runge et al. Reference Runge, Martin, Possingham, Willis and Fuller2014). For example, despite growing international commitments, dedicated funding, and conservation efforts (e.g. Western Hemisphere Shorebird Reserve Network; WHSRN), shorebirds in the Americas still experience steep population declines. A recent review found an overall reduction in abundance of 37.4% since 1970, with 68% of species (n = 44) declining (Rosenberg et al. Reference Rosenberg, Dokter, Blancher, Sauer, Smith, Smith, Stanton, Panjabi, Helft, Parr and Marra2019). Although some proximate causes of these declines are known (Baker et al. Reference Baker, González, Piersma, Niles, de Lima Serrano do Nascimiento, Atkinson, Clark, Minton, Peck and Aarts2004) and site-specific adaptive management plans are implemented (McGowan et al. Reference McGowan, Smith, Nichols, Lyons, Sweka, Kalasz, Niles, Wong, Brust, Davis and Spear2015), there is an urgent need for holistic conservation plans which consider the full life cycle (Navedo and Ruiz Reference Navedo and Ruiz2020), including at the subspecies/population level, to better address the drivers of migratory shorebird declines.
The Red Knot Calidris canutus is one of the most extensively studied shorebird species (Thomas et al. Reference Thomas, Székely and Sutherland2003) and is currently ‘Near Threatened’ at the global level (BirdLife International 2018). Within the Americas the situation for two out of three extant C. canutus subspecies is worsening in terms of population size and rate of decrease (U.S. Shorebird Conservation Plan Partnership 2016). C. c. roselaari breeds in Alaska and Wrangel island (Russia) and migrates along the Pacific coast to spend the non-breeding season mainly in Mexico (Carmona et al. Reference Carmona, Arce, Ayala-Perez, Hernández-Alvarez, Buchanan, Salzer, Tomkovich, Johnson, Gill, McCaffery, Lyons, Niles and Newstead2014). Although highly fluctuating in annual numbers (Carmona et al. Reference Carmona, Arce, Ayala-Pérez and Danemann2008) the total population is estimated to be 17,000 birds (Andres et al. Reference Andres, Smith, Morrison, Gratto-Trevor, Brown and Friis2012) and is classified as ‘endangered’ in Mexico (Secretaría de Medio Ambiente y Recursos Naturales 2010) and ‘greatest concern’ in USA (U.S. Shorebird Conservation Plan Partnership 2016). The bulk of roselaari knots winter in Baja California and the Gulf of California (Carmona et al. Reference Carmona, Arce, Ayala-Perez, Hernández-Alvarez, Buchanan, Salzer, Tomkovich, Johnson, Gill, McCaffery, Lyons, Niles and Newstead2014), as well as other areas of the Mexican Pacific coasts where relevant numbers have been reported (Arce et al. Reference Arce, Carmona, Miramontes, Ayala-Pérez, Hernández-Avarez and and Mendoza2015). However, it is unclear whether small groups of knots present elsewhere along the Pacific coast of Central and South America belong to this subspecies. While Carmona et al. (Reference Carmona, Arce, Ayala-Perez, Hernández-Alvarez, Buchanan, Salzer, Tomkovich, Johnson, Gill, McCaffery, Lyons, Niles and Newstead2014) stated that all these groups belong to rufa subspecies, geolocator information retrieved from one bird in a study based in Texas has shown that at least some of these birds are apparently C. c. roselaari - one bird made a non-stop flight of 6 days (8,100 km) from Chiloé (Southern Pacific Coast, Chile) to Texas, then continued onwards to Alaska to breed (Newstead et al. Reference Newstead, Niles, Porter, Dey, Burger and Fitzsimmons2013). By contrast, at least two knots spending the non-breeding season at Chiloé were resighted in the eastern North Atlantic Coasts during northward migration, one in South Carolina (Navedo and Gutiérrez Reference Navedo and Gutiérrez2019) and another in Georgia (Johnson et al. Reference Johnson, Andres, Sitters, Valenzuela, Niles, Dey, Peck and Espinosa2007), and two birds from Delaware Bay were also resighted in Chiloé (Johnson et al. Reference Johnson, Andres, Sitters, Valenzuela, Niles, Dey, Peck and Espinosa2007), all these birds most probably belonging to C. c. rufa.
C. c. rufa breeds in the Canadian Arctic and spends the non-breeding season in four distinct areas: south-eastern USA and the Caribbean, Gulf of Mexico (Newstead et al. Reference Newstead, Niles, Porter, Dey, Burger and Fitzsimmons2013), north-eastern Brazil, and along the Argentine Patagonia coast and Tierra del Fuego (Argentina and Chile) (Niles et al. Reference Niles, Sitters, Dey, Atkinson, Baker, Bennett, Carmona, Clark, Clark, Espoz, González, Harrington, Hernández, Kalasz, Lathrop, Matus, Minton, Morrison, Peck, Pitts, Robinson and Serrano2008). Based in isotopic signatures, a small proportion of birds has an unknown non-breeding site (Atkinson et al. Reference Atkinson, Baker, Bevan, Clark, Cole, González, Newton, Niles and Robinson2005). The combined populations of southeast USA, the Caribbean and Brazil has been estimated as a minimum of 20,800 birds (Lyons et al. Reference Lyons, Winn, Keyes and Kalasz2018), the north-west Gulf of Mexico as 2,000 (Andres et al. Reference Andres, Smith, Morrison, Gratto-Trevor, Brown and Friis2012) and Tierra del Fuego as 13,000 birds (in 2012; Baker et al. Reference Baker, Gonzalez, Morrison, Harrington and Billerman2020). The last population estimate of the rufa subspecies was 42,000 (Andres et al. Reference Andres, Smith, Morrison, Gratto-Trevor, Brown and Friis2012). C. c. rufa has been classified as ’endangered’ in Canada (BirdLife International 2018) and ‘threatened’ in the USA (U.S. Fish and Wildlife Service 2015).
An additional regular non-breeding area was recently reported in Chiloé Archipelago (42°S, Chile) (Navedo and Gutiérrez Reference Navedo and Gutiérrez2019), a Site of Hemispheric Importance for the conservation of migratory shorebirds (Humedales Orientales de Chiloé; WHSRN 2011) due to its large numbers of Hudsonian Godwit Limosa haemastica and Whimbrel Numenius phaeopus (Andres et al. Reference Andres, Johnson, Valenzuela, Morrison, Espinosa and Ross2009). Despite the worrying situation of Red Knots in the Americas and the documented presence of knots in Chiloé (Johnson et al. Reference Johnson, Andres, Sitters, Valenzuela, Niles, Dey, Peck and Espinosa2007), this taxon was neither mentioned in the dedicated conservation plan for migratory shorebirds at Chiloé (Delgado et al. Reference Delgado, Sepúlveda and Álvarez2010) nor later considered as a conservation target in this WHSRN site (WHSRN 2011). At least c. 150 Red Knots regularly occupy two studied bays of the main island, one in the north and the other in the central-east coast, showing very high local site fidelity, both intra- and inter- seasonally (Navedo and Gutiérrez Reference Navedo and Gutiérrez2019). Resightings in Chiloé of birds ringed in the Gulf of Mexico (Texas and Louisiana, USA) support strong migratory connectivity between both areas (Navedo and Gutiérrez Reference Navedo and Gutiérrez2019). One additional resighting in Chiloé of a knot ringed in South Carolina and another ringed in Argentina (Navedo and Gutiérrez Reference Navedo and Gutiérrez2019), along with resightings of knots banded in Delaware, and one of two knots banded on Chiloé and resighted in Georgia, respectively (J. Johnson unpubl. data in Andres et al. Reference Andres, Smith, Morrison, Gratto-Trevor, Brown and Friis2012), support a connection with traditional staging sites of migrating rufa in the Atlantic coasts. Therefore, current knowledge reflects the uncertainty regarding the classification and conservation of knots spending the non-breeding season at the Pacific coasts of South America. This uncertainty raises the question of whether those spending the non-breeding season at Chiloé should be considered as a separate conservation unit. It is thus crucial to characterize this endangered migratory population at Chiloé while it is still abundant to identify main threats and establish conservation strategies before it is too late (Wilcove and Wikelski Reference Wilcove and Wikelski2008).
Here we present the first detailed population morphometrics and molecular sexing of captured and ringed Red Knots during the non-breeding season in Chiloé trying to provide insights that can be useful for their conservation. In addition, we compile resightings of these birds outside of Chiloé to improve the understanding of migratory connectivity of one of the most endangered shorebird species within the Americas.
Methods
Knots were captured in the north of Chiloé main Island (41°49 S, 73°63 W) during the austral summer of 2017/18, 2018/19 and 2019/20, in two different periods (November and March). Birds were captured at high tide using cannon nets and maintained in cages until being processed, following approved bioethics protocol and animal capture permissions. Birds were ringed with a metallic ring and an engraved-red PVC flag. Each bird was weighed and morphological measurements (bill length, head-bill length, wing cord, ‘maximum tarsus’ length; (Redfern and Clark Reference Redfern and Clark2001)) were taken, always by the same researcher. Age class was determined based on the plumage at capture: 1) yearlings: one-year-old birds hatched the same or previous year (i.e. in their first or second calendar year); 2) adults: birds at least two-years-old (i.e. third calendar year or older) (Pyle Reference Pyle2008, Baker et al. Reference Baker, Gonzalez, Morrison, Harrington and Billerman2020, Martínez-Curci et al. Reference Martínez-Curci, Isacch, D’Amico, Rojas and Castresana2020). A blood sample was taken from the jugular vein (~ 0.7 ml, < 1% of body weight) and kept in an Eppendorf tube with 96% ethanol at 4°C. Birds were then molecularly sexed using primers P0, P2, and P8 (Griffiths et al. Reference Griffiths, Double, Orr and Dawson1998, Han et al. Reference Han, Kim, Kim, Park and Na2009).
Morphometrics were compared by sex, and adult weight was compared by period (i.e. ‘maintenance’ (November) and ‘pre-migratory fuelling’ (March), since weight varies significantly during the non-breeding season) and sex through analyses of variance (ANOVA). All analyses were done in R (R Core Team 2020).
We also estimated expected daily rate of weight gain during fuelling by first discarding from the analysis six individuals with no external signs of alternate breeding plumage in March, as well as yearlings, because they probably aborted migration (e.g. Martínez-Curci et al. Reference Martínez-Curci, Isacch, D’Amico, Rojas and Castresana2020). With departure dates from Chiloé concentrated in the second week of April (J. G. Navedo pers. obs.), we assumed knots at Chiloé keep fuelling at a similar rate of daily weight gain to reach a departure mass over 210–230 g, similar to C. c. canutus in South Africa that have to fly 6,000 km up to their first staging site in Guinea-Bissau (Summers et al. Reference Summers, Underhill, Waltner and Swann2010). For knots to achieve this departure mass in c.30 days from capture in early March, we estimated daily mass gains using as reference the average body mass of the five lightest and five heaviest birds in those captures (following Hua et al. Reference Hua, Piersma and Ma2013).
We then compiled all resightings of these PVC-flagged birds obtained from scientists and birdwatchers (see Acknowledgements) from May 2017 up to May 2020 to increase understanding of migratory connectivity in this population. Photos of all but one resighted individuals from Chiloé preclude misidentifications (see Tucker et al. Reference Tucker, McGowan, Robinson, Clark, Lyons, DeRose-Wilson, du Feu, Austin, Atkinson and Clark2019).
Results
A total of 42 knots (18 females and 22 males, 2 not sexed; 39 adults, 3 yearlings) were captured. Males were smaller than females in all measurements, and we found significant differences in bill length and head-bill, but not in tarsus length or wing chord (Table 1). Body weight in the maintenance period were on average (± SD) 115.0 ± 3.72 (range 110.5–120.5, n = 7) for females and 109.6 ± 4.5 (range 103.4–116.5, n = 6) for males, while it reached 139.3 ± 7.0 (range 126.6–166.6, n = 11) in females and 125.5 ± 9.3 (range 103.6–62.0, n = 15) in males captured during the pre-migratory fuelling period. We found differences in body weight both in females and males (ANOVA, F1,16 = 23.75, adjusted R2 = 0.57, P < 0.05), and between periods (ANOVA, F1,19 = 7.776, adjusted R2 = 0.25, P < 0.05), with females being slightly heavier than males in the pre-migratory fuelling period (ANOVA, F1,23 = 6.90, adjusted R2 = 0.20, P = 0.01), but not in the maintenance period (ANOVA, F1,11 = 3.99, adjusted R2 = 0.19, P = 0.07).
Table 1. Morphometrics (in mm) of Red Knots captured in Chiloé Archipelago. We present the mean and standard deviation (SD), range of values, and sample size (n). Results of the analysis of variance (ANOVA) are presented with F and P values.

With birds weighing between 123.2 g and 153.2 g respectively in early March, the expected daily mass gain during the fuelling period before departing by the second week of April ranged between 2.9 and 3.6 g per day for the lightest birds, and between 1.9 and 2.6 g for the heaviest ones.
We ringed a total of 37 birds, from which 24 have been resighted, all but one in Caulín bay or in a nearby (10 km distant) bay. Three of the 13 birds which have never been seen again in Chiloé were resighted in other countries (Perú, Guatemala, and USA). In addition, we have received 12 resightings of individual Red Knots across the Americas (Figure 1). During northward migration two individuals were separately resighted in Paracas, Perú, on different occasions in early and late May, the latter bird resighted again there in mid-July. There were resightings of different knots in the coasts of the Gulf of Mexico from late April until the end of May. In late May there were also resightings inland in North America, both in lakes of Minnesota, USA, and Manitoba, Canada. During southward migration there were fewer resightings. One knot was observed in Florida, USA, in late April and then again in August, where it was resighted multiple times throughout at least 15 days. Other two knots were resighted in early September in Escuintla, Guatemala, and in Esmeraldas, Ecuador.

Figure 1. Resightings of Red Knots banded in Chiloé Archipelago, Chile. Light dots represent resightings during northward migration, dark dots represent resightings during southward migration, and the star represents non-breeding area in Chiloé. Each number corresponds to: 1) Chiloé, Chile; 2) Yucatán, México; 3) Texas, USA; 4) Louisiana, USA; 5) Florida, USA; 6) Paracas, Perú; 7) Minnesota, USA; 8) Manitoba, Canada; 9) Escuintla, Guatemala; 10) Esmeraldas, Ecuador.
Discussion
Our results provide first detailed morphometrics of the Red Knot population spending the non-breeding season in Chiloé Archipelago. In addition, multiple resightings during both northward and southward migration periods helped to increase our understanding of migratory connectivity of an endangered species within the Americas.
Comparatively, Chiloé knots had bill length values within the ranges reported for rufa and roselaari knots captured in other non-breeding areas (Table 2). Bill length and head-bill length varied between sexes, which has been described for both subspecies (Baker et al. Reference Baker, Gonzalez, Morrison, Harrington and Billerman2020). Males and females showed similar weights during the maintenance period and significantly increased during the pre-migratory fuelling period, as expected in long-distance migrant shorebirds preparing for a non-stop endurance flight, with larger females being slightly heavier than males. Our estimated daily mass gains during fuelling are high, but similar to those of Red Knots fuelling in high-latitude non-breeding sites (reviewed in Piersma et al. Reference Piersma, Rogers, González, Zwarts, Niles, de Lima Serrano Do Nascimiento, Minton, Baker, Greenberg and Marra2004), and probably can be reached at least by competitive individuals attaining high intake rates given the comparatively high prey biomass available in Chiloé intertidal areas (Micael and Navedo Reference Micael and Navedo2018).
Table 2. Comparison of the bill length (mm) of red knots captured in different non-breeding areas.
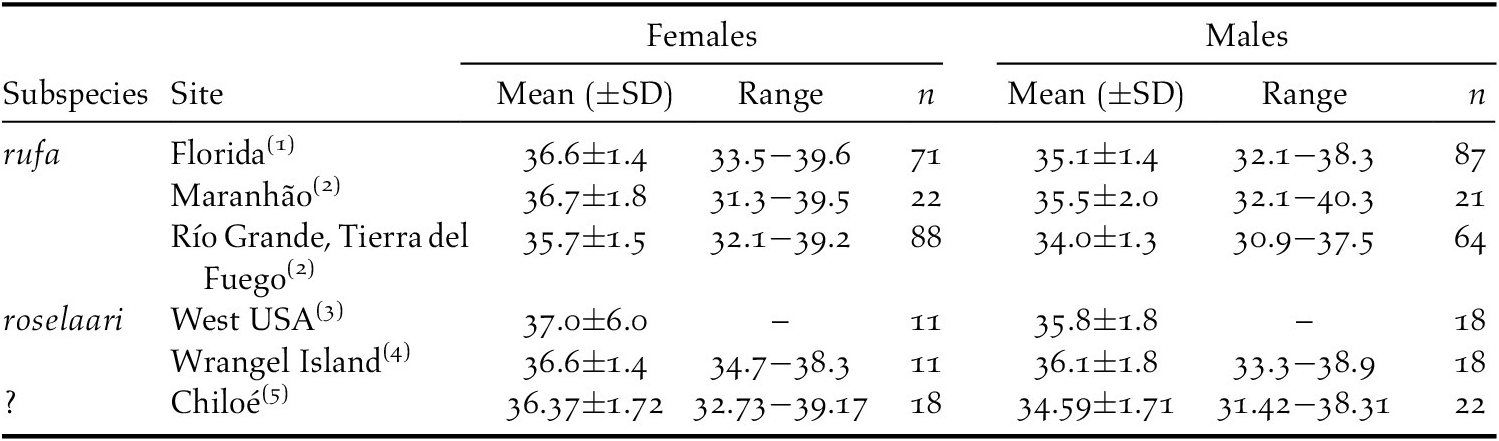
Source: (1) Niles et al. Reference Niles, Dey, Douglass, Clark, Clark, Gates, Harrington, Peck and Sitters2006, (2) Baker et al. Reference Baker, Gonzalez, Morrison, Harrington and Billerman2020, (3) Niles et al. Reference Niles, Sitters, Dey, Atkinson, Baker, Bennett, Carmona, Clark, Clark, Espoz, González, Harrington, Hernández, Kalasz, Lathrop, Matus, Minton, Morrison, Peck, Pitts, Robinson and Serrano2008 , (4) Tomkovich Reference Tomkovich1992, (5) this study.
Knots banded in Chiloé were resighted early during the northward migration period mainly in the Gulf of Mexico, at coastal wetland staging areas in Texas and Louisiana (USA) (Navedo and Gutiérrez Reference Navedo and Gutiérrez2019). Individuals with adequate body condition seem to make a non-stop flight of several days from Chiloé to Texas (e.g. Newstead et al. Reference Newstead, Niles, Porter, Dey, Burger and Fitzsimmons2013), probably by flying under favourable wind conditions as do other knots (e.g. Tomkovich et al. Reference Tomkovich, Porter, Loktionov and Niles2013), since this distance surpasses the theoretical limits of non-stop power flight for the species (Hedenström Reference Hedenström2010). However, our earliest resighting in Yucatán Península suggests that at least some birds stop over before finally crossing the Gulf. Resightings in Paracas, 3,000 km from Chiloé, one of them a bird seen in late May and again in mid-July, might alternatively suggest individuals that entered in an oversummering stage (e.g. Navedo and Ruiz Reference Navedo and Ruiz2020, Martínez-Curci et al. Reference Martínez-Curci, Isacch, D’Amico, Rojas and Castresana2020). The northernmost resightings, one in Minnesota (USA) and the other in Manitoba (Canada), indicate that some birds migrate further north through the Mid-Continental Flyway, as do other Red Knots that spent the non-breeding season in north-west Gulf of Mexico (Newstead et al. Reference Newstead, Niles, Porter, Dey, Burger and Fitzsimmons2013). However, this does not give clear support about the breeding destination of the birds since this flyway is also used by other shorebirds that breed in different areas along the Arctic (e.g. Senner Reference Senner2012, Farmer et al. Reference Farmer, Holames, Pitelka and Billerman2020, Parmelee Reference Parmelee, Poole, Stettenheim and Gill2020), including both the breeding grounds of rufa in central Canadian Arctic (Lathrop et al. Reference Lathrop, Niles, Smith, Peck, Dey, Sacatelli and Bognar2018) and roselaari in Alaska (Tomkovich and Dondua Reference Tomkovich and Dondua2008). By contrast, the scarce resightings of these knots during northward migration in eastern Atlantic coasts (Georgia, Delaware Bay) (see also Navedo and Gutiérrez Reference Navedo and Gutiérrez2019), when resighting efforts are intensive (e.g. Tucker et al. Reference Tucker, McGowan, Robinson, Clark, Lyons, DeRose-Wilson, du Feu, Austin, Atkinson and Clark2019), suggest that C. c. rufa knots that spent the non-breeding season in Chiloé may follow a different route to the Arctic.
A resighting in Florida during southward migration period matched with a staging site used by rufa knots en route through the Atlantic coasts to Brazil and Tierra del Fuego (Baker et al. Reference Baker, Gonzalez, Morrison, Harrington and Billerman2020). In this light, the resightings of two individuals obtained from Guatemala and Ecuador (Pacific Flyway) in this period are noticeable given the fact of a bird breeding in Alaska and using wetlands within Pacific coasts en route to Chiloé (D. Newstead pers. comm.), thus not belonging to rufa subspecies. Molecular analyses, isotopic signatures and individual GPS tracking would decipher this surprisingly complex subspecific puzzle (Buehler and Baker Reference Buehler and Baker2005, Carmona et al. Reference Carmona, Arce, Ayala-Perez, Hernández-Alvarez, Buchanan, Salzer, Tomkovich, Johnson, Gill, McCaffery, Lyons, Niles and Newstead2014) concerning knots that spend the non-breeding season in the Austral Pacific coasts of Americas.
Conservation implications
Resightings of knots out of Chiloé suggest that several individuals fly non-stop the impressive distance of 8,000 km to the main staging area in the Gulf of Mexico, supporting previous results (Navedo and Gutiérrez Reference Navedo and Gutiérrez2019, Newstead et al. Reference Newstead, Niles, Porter, Dey, Burger and Fitzsimmons2013), only comparable to the extreme 10,100 km non-stop flight by the rogersi subspecies from New Zealand to China (Tomkovich et al. Reference Tomkovich, Porter, Loktionov and Niles2013). Besides the strong connection with some areas used by rufa subspecies in the Atlantic, our results show morphometric differences within the ranges of both rufa and roselaari. Although our small sample size presents some limitations to reach robust conclusions, individuals with retrieved geolocators (Newstead et al. Reference Newstead, Niles, Porter, Dey, Burger and Fitzsimmons2013) and specific resightings shows that the Chiloé non-breeding population is composed of different subspecies.
In this light, in Chile C. c. rufa is classified as ‘endangered’ (Inventario Nacional de Especies de Chile 2008) and C. c. roselaari is not reported. Given the threats faced by rufa subspecies (Niles et al. Reference Niles, Sitters, Dey, Atkinson, Baker, Bennett, Carmona, Clark, Clark, Espoz, González, Harrington, Hernández, Kalasz, Lathrop, Matus, Minton, Morrison, Peck, Pitts, Robinson and Serrano2008), and the crucial importance of maintaining the most diverse genetic pool in highly endangered migratory species (Wilcove and Wikelski Reference Wilcove and Wikelski2008), the population in Chiloé Archipelago should be treated as a separate conservation unit within interhemispheric conservation programmes for endangered shorebirds within the Americas. It is urgent to assess main threats (e.g. Navedo et al. Reference Navedo, Verdugo, Rodríguez-Jorquera, Abad-Gómez, Suazo, Castañeda, Araya, Ruiz and Gutiérrez2019), overall population abundance, and trends to design adequate conservation plans in bays used by this qualitatively important but poorly understood population of Red Knots.
Acknowledgements
We thank C. Verdugo, E. Basso, V. Araya, G. Biscarra, C. Navarrete, G. Torres-Fuentes, N. Martínez-Curci, J.M. Abad-Gómez, J.S. Gutiérrez, J.A. Masero and everyone in the Bird Ecology Lab who assisted in fieldwork. We also thank Jorge Valenzuela and CECPAN members for their long-last support at Chiloé. César Mansilla helped us to carry out fieldwork. Tyler McFadden helped edit the English language. We thank E. Pacheco (Yucatán, Mexico); D. Newstead (Texas, USA); E. Ortiz, R. Huayanca and Y. Tenorio (Paracas, Perú); E. Gallien and R. Russell (Minnesota, USA); D. LeBlanc, C. Wright and B. Keeler (Louisiana, USA); R. Ahlman (Esmeraldas, Ecuador); M.T. Smith, J. Hall, A. DiNuovo and J. Hall (Florida, USA); V. Sagastume (Escuintla, Guatemala), I. Ward and Donna Danyluk (Manitoba, CA) for their resightings of Chiloé Red Knots along the Americas. Comments by V. D´Amico and P. Atkinson greatly improved the manuscript. Captures and banding were made under bioethics approval #260/2016 from UACh and permissions #5932/2016 from SAG, Gobierno de Chile. CGF was funded by CONICYT grant #21180828 during writing. All fieldwork and analyses were funded by FONDECYT #1161224 (JGN).





