Introduction
In the past, Europe was heavily influenced by agriculture, which led to a high level of biodiversity that was essentially a product of various management forms and measures (Billeter et al. Reference Billeter, Liira, Baile, Bugter, Arens and Augenstein2008). Today, farmland biodiversity is rapidly declining worldwide (Green et al. Reference Green, Cornell, Scharlemann and Balmford2005; Stanton et al. Reference Stanton, Morrissey and Clark2018). Agricultural intensification has resulted in a substantial reduction in management techniques and heterogeneity of farmland, with a concomitant loss of farmland birds (Benton et al. Reference Benton, Vickery and Wilson2003; Donald et al. Reference Donald, Green and Heath2001; Šálek et al. Reference Šálek, Kalinová, Daňková, Grill and Żmihorski2021), and an even more dramatic decline in insects, which are the main food source for insectivorous birds (Benton et al. Reference Benton, Bryant, Cole and Crick2002; Raven and Wagner Reference Raven and Wagner2021). In this context, rural settlements in traditional agricultural land represent important biodiversity habitats especially for birds and insects (Hiron et al. Reference Hiron, Berg, Eggers and Pärt2013; Rosin et al. Reference Rosin, Skórka, Pärt, Żmihorski, Ekner-Grzyb and Kwieciński2016, Reference Rosin, Pärt, Low, Kotowska, Tobolka and Szymański2021; Šálek et al. Reference Šálek, Bažant and Żmihorski2018a, Reference Šálek, Hula, Kipson, Daňková, Niedobová and Gamerob).
Traditional farming villages, including single rural properties around farmsteads, are still an integral part of rural agricultural landscapes, especially in Central and Eastern European countries. Their low-intensity, small-scale, and diverse management can facilitate foraging and breeding opportunities for birds and explains why many species that are declining elsewhere thrive near human settlements (Ambrosini et al. Reference Ambrosini, Bolzern, Canova, Arieni, Møller and Saino2002; Noack et al. Reference Noack, Larsen, Kamp and Levers2021; Rosin et al. Reference Rosin, Skórka, Pärt, Żmihorski, Ekner-Grzyb and Kwieciński2016). However, the conservation potential and suitability of such habitats for bird communities is declining due to renovation and modernisation measures on buildings and their surroundings, reducing the availability of nesting sites and food. These actions may have significant negative impacts on the survival and population development of birds breeding on buildings and their surroundings (Rosin et al. Reference Rosin, Hiron, Żmihorski, Szymański, Tobolka and Pärt2020, Reference Rosin, Pärt, Low, Kotowska, Tobolka and Szymański2021). The modernisation of villages and farmsteads has resulted in significantly lower species richness and abundance of birds (Rosin et al. Reference Rosin, Pärt, Low, Kotowska, Tobolka and Szymański2021; Šálek and Mayer Reference Šálek and Mayer2023). For example, old homesteads harboured more nests of birds such as House Sparrows Passer domesticus, Barn Swallows Hirundo rustica, and House Martins Delichon urbica than new ones (Šálek and Mayer Reference Šálek and Mayer2023). It has also been suggested that village modernisation contributes far more to the decline of building and non-crop nesters than agricultural intensification at different spatial scales (Rosin et al. Reference Rosin, Skórka, Pärt, Żmihorski, Ekner-Grzyb and Kwieciński2016, Reference Rosin, Pärt, Low, Kotowska, Tobolka and Szymański2021). To date, there is little information on whether and how these changes in the design and architecture of human dwellings as a result of modernisation measures affect farmland birds that require habitats other than cropland, such as trees and shrubs (Hiron et al. Reference Hiron, Berg, Eggers, Berggren, Josefsson and Pärt2015). Several of these species are known to prefer nesting within or near active farmsteads, which are characterised by higher habitat heterogeneity and a greater supply of food and safe nesting sites (Hiron et al. Reference Hiron, Berg, Eggers and Pärt2013, Reference Hiron, Berg, Eggers, Berggren, Josefsson and Pärt2015; Šálek et al. Reference Šálek, Bažant and Żmihorski2018a). For example, farmsteads with livestock have been identified as important bird habitats in agricultural ecosystems, as the presence of dung heaps, barns, paddocks, and pastures provides rich foraging opportunities (Ahnström et al. Reference Ahnström, Berg and Söderlund2008; Wirtitsch et al. Reference Wirtitsch, Hoi, Valera and Krištín2001).
In this study, we investigated the impact of farmstead and settlement modernisation in a traditional rural area on a flagship farmland bird, the Lesser Grey Shrike Lanius minor, which is classified as threatened under Annex I of the European Commission Birds Directive. This species is declining dramatically in Western and Central Europe, and range fragmentation, agricultural intensification, and habitat degradation are considered to be the main reasons for its decline (Bronskov and Keller Reference Bronskov, Keller, Keller, Herrando, Voříšek, Franch, Kipson and Milanesi2020; Kvist et al. Reference Kvist, Giralt, Valera, Hoi, Krištín and Darchiashvili2011; Lefranc and Worfolk Reference Lefranc and Worfolk2022). However, the species is also declining in Slovakia, although it inhabits a typical traditional rural landscape there (Dobrovodská et al. Reference Dobrovodská, Kanka, David, Kollár, Špulerová and Štefunková2019), and the factors explaining this decline at the local level are poorly documented. Our study area was historically excluded from socialist collectivisation due to its mountainous character and therefore was not absorbed into large specialised industrial farms and large field monocultures. As a result, an agricultural area with small private farms and grounds has been preserved. However, with Slovakia’s accession to the EU in 2004 and the subsequent application of the EU’s Common Agricultural Policy, farmers began to renovate or build new houses and farmsteads, stimulated by the Rural Development Programme 2007–2013 (Ministry of Agriculture and Rural Development of the Slovak Republic).
For the purposes of this study, we therefore categorised human dwellings on traditional active farmsteads and modern homesteads according to their occupancy type, management, and renovation, which also reflects differences in surrounding habitats, e.g. vegetation structure and complexity, presence of trees and shrubs, etc. (Hiron et al. Reference Hiron, Berg, Eggers and Pärt2013; Rosin et al. Reference Rosin, Skórka, Pärt, Żmihorski, Ekner-Grzyb and Kwieciński2016). We investigated whether and how the modernisation of human dwellings and settlement over almost three decades affects one of the most important breeding populations of Lesser Grey Shrike in Central Europe (Hoi et al. Reference Hoi, Krištín, Valera and Hoi2012; Krištín et al. Reference Krištín, Hoi, Valera and Hoi2000; Lefranc and Worfolk Reference Lefranc and Worfolk2022). We expected that the breeding area and nesting probability of the species would generally be lower on modernised farmland compared with the situation before the application of the EU Common Agricultural Policy in 2007.
Material and methods
Study species and area
The Lesser Grey Shrike is an insectivorous and long-distance migratory passerine that is declining throughout Europe (Bronskov and Keller Reference Bronskov, Keller, Keller, Herrando, Voříšek, Franch, Kipson and Milanesi2020). In the traditionally agricultural areas of central Slovakia, it nests almost exclusively within farmsteads characterised by a mosaic of farmhouses, backyards, orchards, meadows, and gardens (Hoi et al. Reference Hoi, Krištín, Valera and Hoi2012; Krištín et al. Reference Krištín, Hoi, Valera and Hoi2000; Wirtitsch et al. Reference Wirtitsch, Hoi, Valera and Krištín2001). The species builds its nests on high, dense, mostly fruit trees (mean >8 m) in the periphery of the treetops and near farmhouses (mean distance 21 m) (Krištín Reference Krištín1995, Reference Krištín2010). It is highly philopatric and shows strong nest-site fidelity (Krištín et al. Reference Krištín, Hoi, Valera and Hoi2007). The species is strongly territorial, and usually defends its nest site and mates within a radius of about 50 m around the nest and forages mainly within a radius of up to 100 m around the nests (Krištín Reference Krištín1995; Valera et al. Reference Valera, Hoi and Krištín2003,).
The study area (20 km2) surveyed since 1990 is located on the north-western border of the species’ range in central Slovakia near the town of Hriňová (N 48.553–607°, E 19.437–532°; 450–850 m a.s.l.) on the southern slopes of the Poľana Biosphere Reserve (Figure 1). This traditional agricultural area is characterised by a relatively high biodiversity around scattered settlements, which include dwellings, farms, gardens, barns, stables, other buildings, and the surrounding environment.
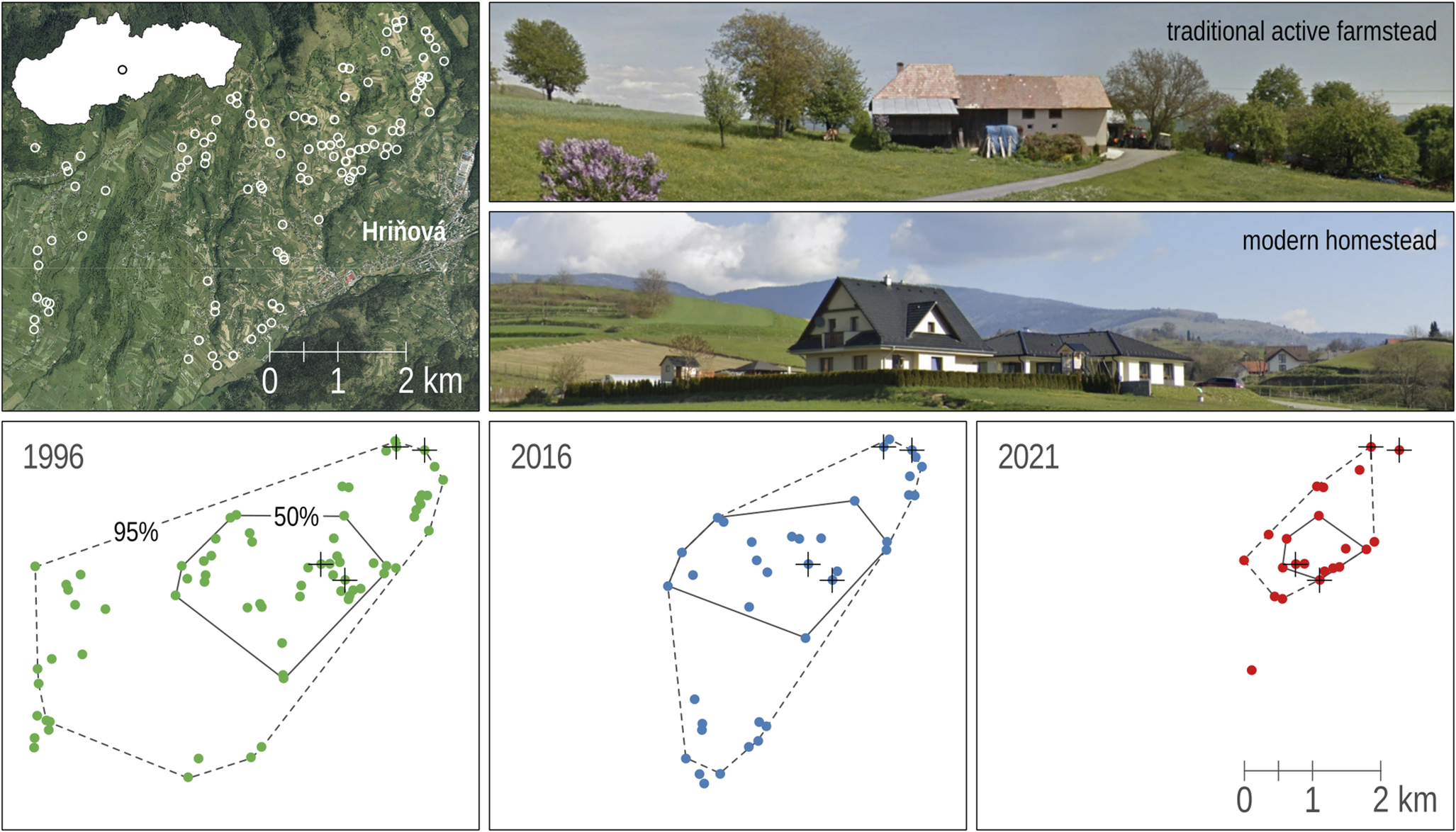
Figure 1. Locations of 106 breeding territories of Lesser Grey Shrike Lanius minor in the study area near the town of Hriňová in central Slovakia and changes in the use of these territories in the three different years of the study. Territories that were occupied during the whole study period are marked with a cross. The change in the size of the breeding range over time is represented by the minimum convex polygons (95% home-range and 50% core-range). The photographs show a typical farmstead and a modern homestead in the study area (map source © Seznam.cz, photo from Google Street View).
Data collection
Data on nest locations and types of human dwellings in breeding territories were collected regularly twice a week from May to June in 1996, 2016, and 2021. Only nests with incubating birds or nests with parents feeding nestlings were recorded. The area within a buffer with a radius of either 50 m or 100 m around the nest tree was considered a breeding territory (GPS coordinates of central positions are available at Figshare repository https://doi.org/10.6084/m9.figshare.24421024.v4). These areas were defined according to the expected distance for nest defence or foraging, respectively (Krištín Reference Krištín1995; Valera et al. Reference Valera, Hoi and Krištín2003). For each territory, field surveys and aerial photographs were used to record the number of human dwellings according to their type, i.e. traditional active farmstead (hereafter farmstead) or modern homestead (hereafter homestead). A farmstead was characterised by the presence of active farmhouses, stables or barn houses, livestock, tall fruit trees or orchards, gardens, and grassland with infrequent mowing (Figure 1). For detailed environmental characteristics of farmsteads and their surroundings as habitat for breeding territories of Lesser Grey Shrike in the Poľana Biosphere Reserve, see Wirtitsch et al. (Reference Wirtitsch, Hoi, Valera and Krištín2001). In contrast, the homestead was only used for living, sometimes only at weekends, and there were no agricultural activities in the area. On the other hand, frequent lawn mowing and planting of low, non-native ornamental trees (unsuitable for breeding of Lesser Grey Shrike) were typical activities. In this new type of dwelling, stables, the keeping of domestic animals, and the cultivation of tall fruit trees and vegetables were completely absent (Figure 1). In some cases, breeding pairs used different nest trees within the same territory during the three years of the study. However, each nest was in close proximity to the previous one, so that the change of nest site was negligible and was not influenced by the number of houses in the defined areas (radius of 50 m or 100 m). Since the character of the surrounding habitats is strongly dependent on the type of dwelling (Wirtitsch et al. Reference Wirtitsch, Hoi, Valera and Krištín2001), we used this as a proxy for the complex of factors (e.g. availability of suitable nesting trees, intensity of land use, proportion of semi-natural habitats, food availability or predators) that limit the suitability of the area for the species (Rosin et al. Reference Rosin, Skórka, Pärt, Żmihorski, Ekner-Grzyb and Kwieciński2016).
Statistical analyses
To estimate the breeding area we calculated the 95% home-range and the 50% core-range from the nest locations using the minimum convex polygon (MCP) method, implemented in the package adehabitatHR 0.4.20 (Calenge Reference Calenge2006) in the R 4.1.2 environment for statistical computing (R Core Team 2021). The output of MCP provided a more realistic picture (no overestimation) of the breeding range size for the study (species and area) when compared preliminarily with an alternative such as kernel density estimation. We used logistic regression to analyse the effect of human habitation on nesting. The binomial response variable represented the presence or absence of nesting (yes or no) in a potential territory during three separate years of monitoring (1996, 2016, and 2021). Nesting probability was assessed for two sizes of nesting territory (50 m and 100 m buffer radius). For each territory area, the same set of five models with different predictors was constructed to explain nesting probability. In order to account for the global population decline of the species, we took the year as a factor (ordinal variable). The year as a single fixed factor constituted the first model. The second model had only two fixed factors, i.e. the number of farmsteads and homesteads in a territory. The third model also included a fixed effect of year as an ordinal variable. The fourth model did not include year, but included an interaction between farmsteads and homesteads. In addition to this interaction term, the fifth model included year and territory ID as random effects. The first four generalised linear models (GLMs) were fitted using the “glm” function of the core R package stats, while the last generalised linear mixed-effects model (GLMM) was fitted using the “glmer” function of the R package lme4 1.1-31 (Bates et al. Reference Bates, Maechler, Bolker and Walker2015). Using the R package sjPlot 2.8.11 (Lüdecke Reference Lüdecke2022), we calculated the odds ratios of the predictors and the goodness-of-fit (R 2) of all models and plotted the predicted values for the best ranked model according to Akaike Information Criterion (AIC) values. For this model, we also performed a simple slopes analysis with the Johnson–Neyman interval properly managing type I and II error rates to test significance of the conditional slope of the focal predictor (number of modern homesteads) when the number of farmsteads was held at a certain value, using the R package interactions 1.1.0 (Long Reference Long2019).
Results
A total of 106 territories of the Lesser Grey Shrike were recorded in the study area, of which 78% were occupied in one study year, 18% in two, and only 3% in all three study years. The number of occupied territories (presence of nesting) has declined dramatically over the 25 years of monitoring, from 73 in 1996 to 38 in 2016 and 22 in 2021. Only in 1996 were more than half of the territories (54) occupied. Despite this negative trend, some territories were only classified as positive in later years (Table 1). Interestingly, as the population size has declined, the breeding range has also decreased. This was true for both the 95% home-range (17.5 km2 in 1996, 9.6 km2 in 2016, 2.2 km2 in 2021) and the 50% core-range (4.7 km2 in 1996, 4.1 km2 in 2016, 0.7 km2 in 2021) (Figure 1). The number of dwellings has changed during the study period depending on the type of habitation. While there were no modern homesteads in Lesser Grey Shrike territories in 1996, the number of homesteads had increased to that of traditional active farmsteads by 2021. However, in occupied territories (50-m buffer around the nest), the average number of farmsteads was still higher (range 1.50–2.05) than that of homesteads (0.00–0.41) (Figure 2). This pattern was also true for the more distant surroundings (100-m buffer), but was less pronounced, 1.82–2.84 (farmsteads) and 0.04–1.55 (homesteads) (Supplementary material Figure S1).
Table 1. The presence (yes) and absence (no) of nesting in 106 breeding territories during three separate years of monitoring
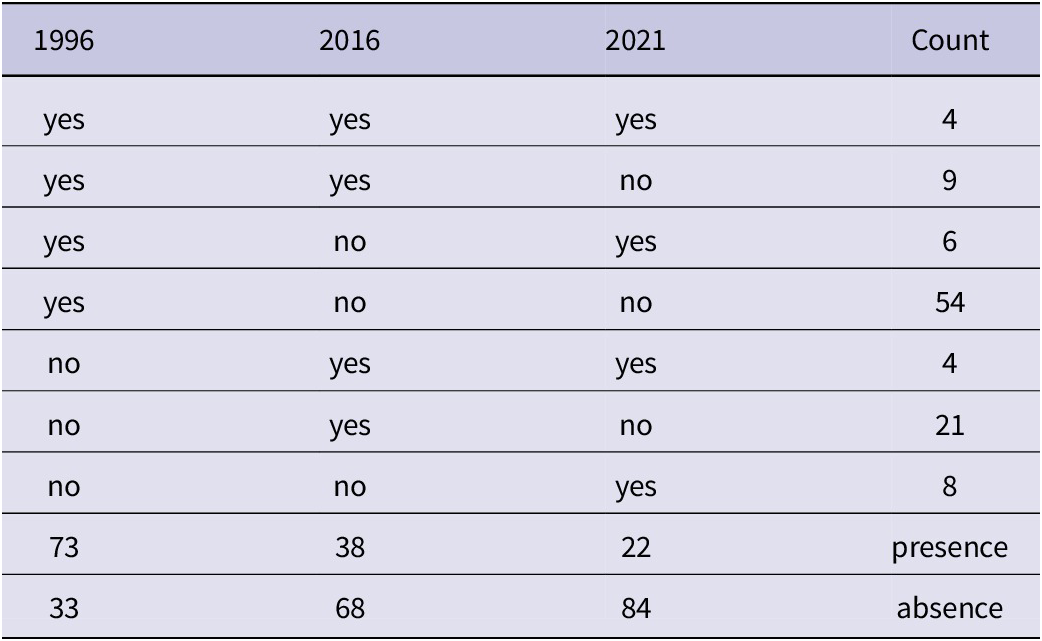
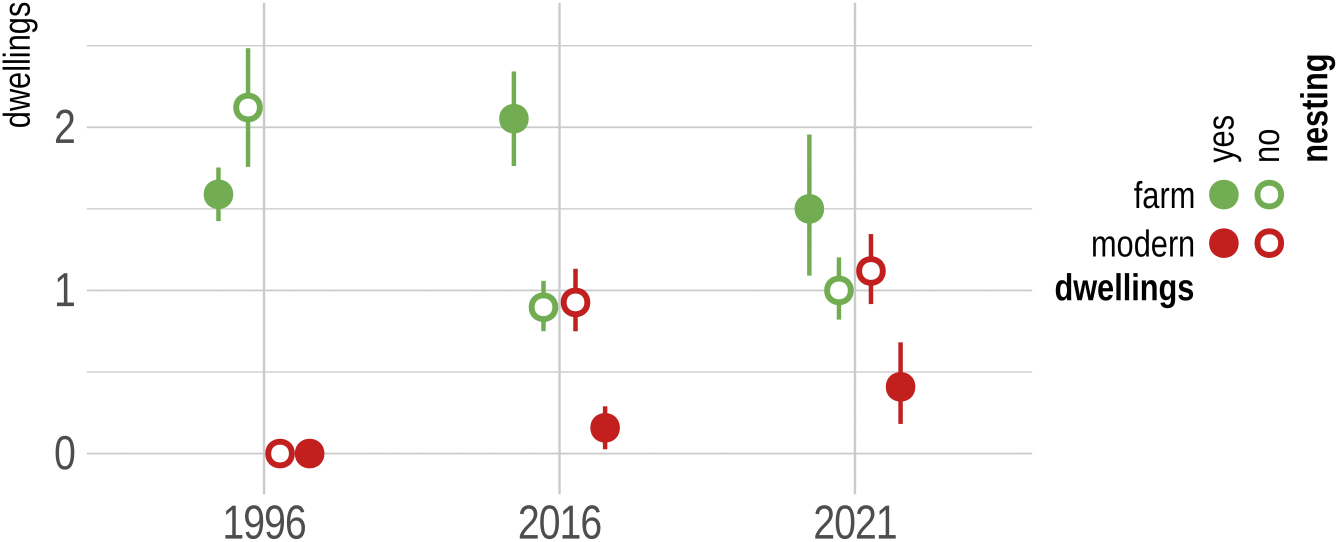
Figure 2. The average number of traditional active farmsteads (farm) and modern homesteads (modern) with 95% confidence intervals in breeding territories of Lesser Grey Shrike Lanius minor, measured in a buffer of 50-m radius around the nest. Nesting refers to whether the territory was occupied or not in a given year.
The goodness-of-fit of all three GLM models (#1–4) without repeated measures to the data from the 50-m buffer indicated relatively weak explanatory power (Tjur’s R 2 = 0.17–0.27). The lowest power was found in model #1 which contained only the year as a fixed factor. In contrast, the overall explanatory power of the GLMM model (#5) was twice as good (conditional R 2 = 0.51) as that of the GLM models, with a substantial part accounted for by the fixed effects alone (marginal R 2 = 0.49). Although the ΔAIC of the second-ranked model (#4) was only 0.5, the inclusion of random effects in model #5 significantly improved the model’s performance. Since the random effects of the territory ID were zero and the model was therefore overfitted (singularity problem), we dropped this term and retained only the random effects of the year in the final model (Table 2). When there was no modern homestead, the probability of nesting in a territory was 57%, regardless of the number of farmsteads. However, building a single modern house in a territory without a farmstead reduced the probability of nesting to about 6%, and this effect was also strong when there were still one or two farmsteads present (17% and 40%, respectively). Another modern homestead in the territory reduced the nesting probability to almost zero, even if there was still a farmstead present (Figure 3). When exploring the interaction term in model #5, the slope of modern homesteads was significant (P <0.05) if there were fewer than two traditional farmsteads present (Table S1).
Table 2. Odds ratios with 95% confidence intervals and P values of different logistic regression models with number of traditional active farmsteads (farm) and modern homesteads (modern) in a 50-m buffer around the nest tree as fixed factors (the first three models contained no interaction). Year was used as a fixed effect of an ordinal variable (models #1 and #3) or as a random effect factor (model #5)
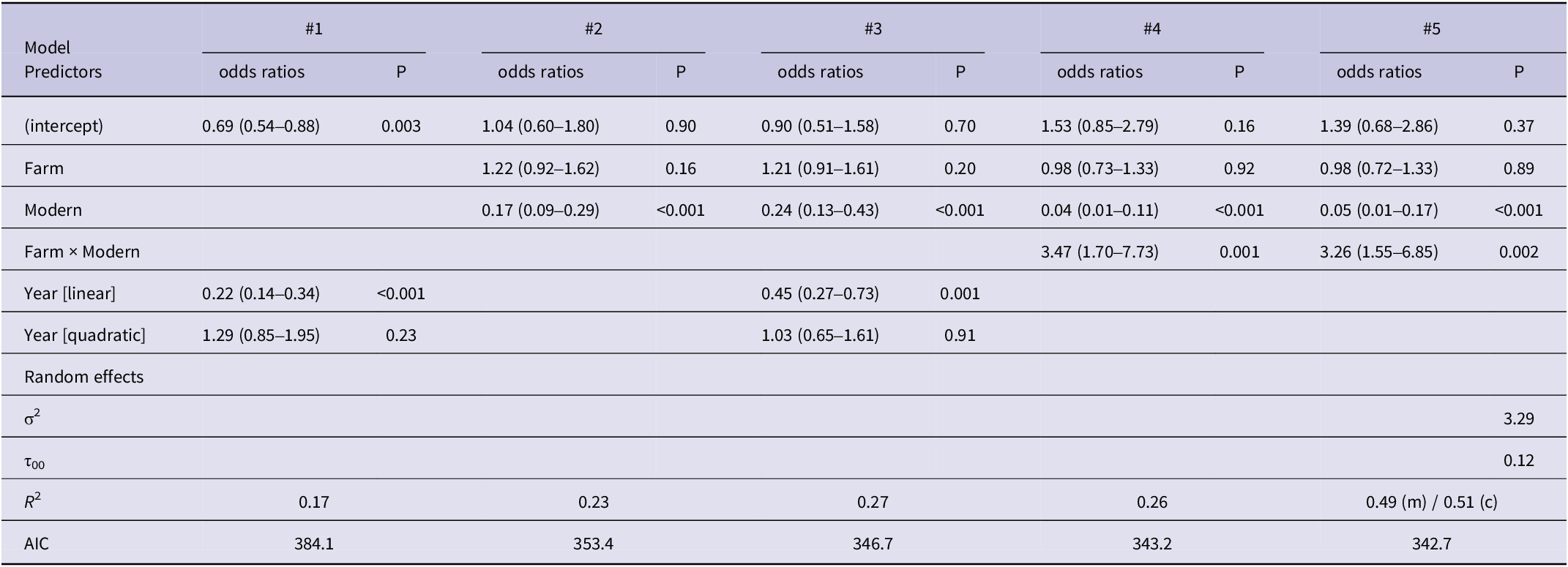
σ2, mean variance; τ00, between-subject variance; m, marginal; c, conditional.
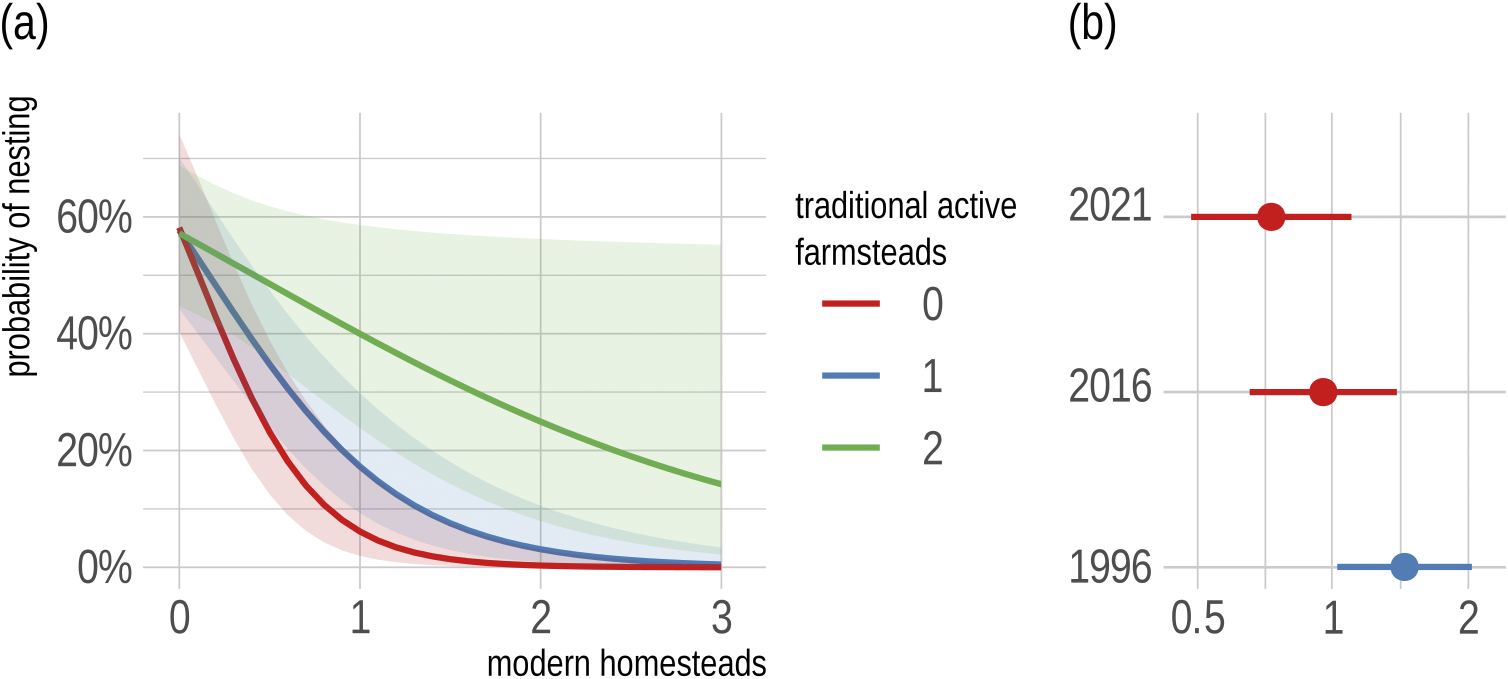
Figure 3. (a) Prediction of nesting probability of Lesser Grey Shrike Lanius minor with 95% confidence intervals as a function of the number of traditional active farmsteads and modern homesteads (fixed factors) in the potential territory using the generalised linear mixed-effects model. (b) Random effect of the different years in this model.
Using the data from the 100-m buffer, the results were similar, but the GLMM model was overfitted. In addition, an additive effect of farmstead (P <0.05) was found in models without an interaction term, and the significance of the fixed effect of year in GLM model #3 had decreased (Table S2).
Discussion
The Lesser Grey Shrike is relatively faithful to its traditional breeding territories, with more than 50% of nests built in the same or a neighbouring tree (Hoi et al. Reference Hoi, Krištín, Valera and Hoi2012; Krištín et al. Reference Krištín, Hoi, Valera and Hoi2007). In the study area, the species breeds almost exclusively near farmhouses (Krištín Reference Krištín1995, Krištín et al. Reference Krištín, Hoi, Valera and Hoi2000). We used this strong nest-site fidelity or even “conservativeness” to investigate the impact of the modernisation of human settlements on the negative local population trend over the last 25 years. As the character of human habitation has changed dramatically, homestead density has increased at the expense of reduced availability of suitable breeding and foraging sites, we found little re-use of the same territories after 20 and 25 years of monitoring (18% and 3%, respectively). This is a significantly lower re-use of the same breeding territory than in the previous but shorter period 1989–2000 (30–57%) (Hoi et al. Reference Hoi, Krištín, Valera and Hoi2012; Krištín et al. Reference Krištín, Hoi, Valera and Hoi2007). We suspect that the main reason for this is the modernisation of settlements, which is associated with the reduction of suitable breeding and foraging habitats, manifested in the loss of vegetation structures (i.e. trees, orchards, and unmown meadows) and the heterogeneity of habitats near modern homesteads (Hiron et al. Reference Hiron, Berg, Eggers and Pärt2013; Rosin et al. Reference Rosin, Skórka, Pärt, Żmihorski, Ekner-Grzyb and Kwieciński2016). The likelihood of nesting in a traditional territory declined significantly with the construction of modern homesteads, accompanied by the loss of traditional agriculture, which was replaced by modern gardening (lawns). Our results thus confirm that changing lifestyles, including the modern design of buildings, but even more so, the significant changes in land use of the environment have a negative impact on the threatened Lesser Grey Shrike. There is also increasing evidence from other studies that agricultural land surrounding modernised villages has 50–60% fewer birds than around comparable older villages (Rosin et al. Reference Rosin, Pärt, Low, Kotowska, Tobolka and Szymański2021). The abundance of birds on farmland has reduced almost threefold in modernised farmsteads compared with older farms (Šálek and Mayer Reference Šálek and Mayer2023). The renovation or replacement of buildings with modern construction methods and styles eliminates nesting opportunities for a number of species that depend on structures in old buildings for their long-term survival (e.g. House Sparrow, Eurasian Tree Sparrow Passer montanus, Barn Swallow Hirundo rustica) (Ambrozini et al. 2002; Rosin et al. Reference Rosin, Hiron, Żmihorski, Szymański, Tobolka and Pärt2020; Šálek and Mayer Reference Šálek and Mayer2023). However, the impact of rural modernisation on species that require trees or shrubs for nesting, such as the Lesser Grey Shrike, has been insufficiently documented.
Over the last 25 years, an 11% decline in the breeding range of the Lesser Grey Shrike has been recorded in Europe, which is the greatest decline of all European shrike species. However, the factors explaining this trend are missing or based on assumptions derived from studies on other farmland birds (Bronskov and Keller Reference Bronskov, Keller, Keller, Herrando, Voříšek, Franch, Kipson and Milanesi2020). In this context, we also documented a negative demographic trend in our study area over the same period from 1996 to 2021, noting a population decline of almost 70% (expressed in the number of breeding territories) and an even greater reduction in breeding range (-87% and -85%, expressed in 95% home-range and 50% core-range, respectively). The year had the least explanatory power in the models compared and its effect as a random factor in the best-ranked GLMM model was surprisingly negligible (only 2%). This suggests that the global population decline documented in recent decades is not a confounding influence in our analysis. Conversely, our results suggest that population decline may be a consequence of habitat changes or land use in the breeding range rather than a loss during migration or wintering. The modernisation of the countryside and the abandonment of traditional agriculture were most pronounced at the lower altitudes on the western and southern edges of the study area, near the town of Hriňová, which is the most populated settlement there.
Currently, the negative impact on the population of Lesser Grey Shrike due to modernisation measures is exacerbated by the current Rural Development Programme 2014–2022 and by the Agri-environment Schemes (AES) of the EU’s Common Agricultural Policy. Therefore, such a decline of local populations and reduction in distribution areas can also be expected in other Central and Eastern European countries where traditional settlements are an integral part of the rural landscape. One consequence could be an increasing isolation of populations and fragmentation of the species’ range (Bronskov and Keller Reference Bronskov, Keller, Keller, Herrando, Voříšek, Franch, Kipson and Milanesi2020). Agri-environment schemes were designed for intensively managed landscapes in Western Europe, but seem to be ineffective or may even have negative impacts on biodiversity in extensively managed areas in the new EU Member States (Batáry et al. Reference Batáry, Dicks, Kleijn and Sutherland2015; Sutcliffe et al. Reference Sutcliffe, Batáry, Kormann, Báldi, Dicks and Herzon2015; Tryjanowski et al. Reference Tryjanowski, Hartel, Báldi, Szymański, Tobółka and Herzon2011). The general lesson from the European experience is that AES can be effective for wildlife conservation on agricultural land, but needs to be carefully adapted to local differences and specificities (Batáry et al. Reference Batáry, Dicks, Kleijn and Sutherland2015). Therefore, an update of AES for Central and Eastern Europe and the promotion of suitable breeding and foraging habitats for declining farmland birds there is urgently needed. If the negative demographic trend for Lesser Grey Shrike continues, local extinction of the species at the northern limit of its distribution can be expected in a short time, as known from Western Europe (Bronskov and Keller Reference Bronskov, Keller, Keller, Herrando, Voříšek, Franch, Kipson and Milanesi2020; Lefranc and Worfolk Reference Lefranc and Worfolk2022).
It can be concluded that traditional human dwellings in rural areas provide important breeding habitats for declining farmland birds. Detailed knowledge of the impact of their modernisation on biodiversity is crucial for the effective conservation of species. The significant change in settlement patterns from predominantly rural to modern areas is characterised by loss of habitat heterogeneity. In line with the Lesser Grey Shrike’s breeding habitat requirements, this is mainly associated with the absence of animal husbandry and horticulture, the loss of tall fruit trees and, on the other hand, the creation of “sterile” lawns and the planting of low ornamental trees not suitable for nesting. Our study has shown that farmsteads offer an opportunity to promote hands-on nature conservation awareness to farmers, who are important stakeholders in agro-ecosystems. Based on our findings, we propose the following conservation measures for such historic landscape areas: (1) the density of modern homesteads should be limited in rural areas inhabited by endangered species; (2) the heterogeneity and mosaic of rural habitats (orchards, arable soil, gardens, and grassland) should be maintained in areas inhabited by shrikes (Wirtitsch et al. Reference Wirtitsch, Hoi, Valera and Krištín2001); (3) the planting of tall fruit trees and local agriculture should be supported and encouraged by the authorities; (4) the use of agrochemicals (e.g. pesticides, artificial fertilisers) for lawns should be restricted as these reduce the availability of potential prey of shrikes in their breeding territories; (5) the application of conservation measures on farms and the local environment that increase the availability of nesting and feeding sites for birds should be promoted through public education.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S095927092400011X.
Acknowledgements
We thank the landowners who allowed us access to their land during the fieldwork and Peter Tuček for his help with the fieldwork. This work was supported in part by the Slovak Scientific Grant Agency VEGA (2/0065/20 and 2/0018/24) and the bilateral scientific programme between KLIVV and IFE SAS. We are grateful to three anonymous reviewers for valuable comments and suggestions that helped to improve a previous version of the manuscript. The data presented in this study and R code are available at the Figshare repository https://doi.org/10.6084/m9.figshare.24421024.v4.







