Introduction
Among the raptor species of the world, avian scavengers like vultures have shown some of the most dramatic population declines (Thiollay Reference Thiollay2006, Virani et al. Reference Virani, Kendall, Njoroge and Thomsett2011, Chaudhary et al. Reference Chaudhary, Subedi, Giri, Baral, Bidari, Subedi, Chaudhary, Chaudhary, Paudel and Cuthbert2012, Ogada et al. Reference Ogada, Keesing and Virani2012, Reference Ogada, Shaw, Beyers, Buij, Murn, Thiollay, Beale, Holdo, Pomeroy, Baker, Krüger, Botha, Virani, Monadjem and Sinclair2016). One factor contributing to widespread declines of vulture populations is changes to agricultural practices and sanitary regulations that have fundamentally altered food availability and resulted in lower reproductive output or lower survival probability (Carrete et al. Reference Carrete, Grande, Tella, Sánchez-Zapata, Donázar, Díaz-Delgado and Romo2007, Donázar et al. Reference Donázar, Cortés-Avizanda and Carrete2010, Margalida et al. Reference Margalida, Colomer and Oro2014b). Most prominently, the European Union legislation that mandated the removal of livestock carcasses from the landscape has led to food shortages and demographic changes owing to food now being provided at large, predictable feeding stations (Donazar et al. Reference Donazar, Margalida, Carrete and Sanchez-Zapata2009, Margalida et al. Reference Margalida, Donázar, Carrete and Sánchez-Zapata2010, Margalida and Colomer 2012). Although these feeding stations have supported many vulture populations (González et al. Reference González, Margalida, Sánchez and Oria2006, Oro et al. Reference Oro, Margalida, Carrete, Heredia and Donázar2008, Cortés-Avizanda et al. Reference Cortés-Avizanda, Carrete and Donázar2010), their predictable nature can attract a large number of non-breeding birds that interfere and compete with breeding birds and ultimately reduce productivity (Carrete et al. Reference Carrete, Donázar and Margalida2006, Cortés-Avizanda et al. Reference Cortés-Avizanda, Carrete and Donázar2010, Lieury et al. Reference Lieury, Gallardo, Ponchon, Besnard and Millon2015). Understanding the influence of artificial and predictable food provisioning on the demography of populations is therefore important to inform management strategies (López-López et al. Reference López-López, García-Ripollés and Urios2014, Margalida et al. Reference Margalida, Colomer and Oro2014b).
For many vulture species, accumulating data on important demographic processes becomes logistically challenging once populations have declined and birds nest at low density over vast geographic areas. Obtaining solid baseline information while populations still nest at high density is therefore vital to inform the effects of changing food availability resulting from socio-economic or sanitary policy changes. Such changes are occurring increasingly rapidly in countries that sacrifice natural resources for the purpose of economic growth (Donald et al. Reference Donald, Sanderson, Burfield and van Bommel2006, Reference Donald, Round, Dai We Aung, Grindley, Steinmetz, Shwe and Buchanan2015, Kamp et al. Reference Kamp, Oppel, Ananin, Durnev, Gashev, Hölzel, Mishchenko, Pessa, Smirenski, Strelnikov, Timonen, Wolanska and Chan2015). Turkey is a large country that connects Europe and Asia, and hosts an enormously diverse biodiversity which is under increasing threat of rapid economic expansion (Şekercioğlu et al. Reference Şekercioğlu, Anderson, Akçay, Bilgin, Can, Semiz, Tavşanoğlu, Yokeş, Soyumert, İpekdal, Sağlam, Yücel and Nüzhet Dalfes2011). Besides hosting a vast range of resident wildlife, Turkey is also a gateway for millions of western Palaearctic migratory birds to reach wintering areas in Africa (Cameron et al. Reference Cameron, Cornwallis, Percival and Sinclair1967, Porter and Willis Reference Porter and Willis1968, Sutherland and Brooks Reference Sutherland and Brooks1981, Oppel et al. Reference Oppel, Iankov, Mumun, Gerdzhikov, Iliev, Isfendiyaroglu, Yeniyurt and Tabur2014), and for some migratory species the populations in Turkey may support populations in Europe (Demerdzhiev et al. Reference Demerdzhiev, Stoychev, Dobrev, Spasov and Oppel2015). However, despite harbouring globally significant populations of many species, the demographic consequences of rapidly changing landscape structures on Turkey’s biodiversity are generally poorly understood (Şekercioğlu et al. Reference Şekercioğlu, Anderson, Akçay, Bilgin, Can, Semiz, Tavşanoğlu, Yokeş, Soyumert, İpekdal, Sağlam, Yücel and Nüzhet Dalfes2011).
One species for which Turkey holds a very important population is the Egyptian Vulture Neophron percnopterus, which has a European population between 3,300 and 5,050 breeding pairs mostly distributed in Spain (1,500 pairs) and Turkey (1,500–3,000 pairs) (Iñigo et al. Reference Iñigo, Barov, Orhun and Gallo-Orsi2008, Şen et al. Reference Şen, Tavares and Bilgin2017). The species is globally ’Endangered’ due to long term declines in Europe, Africa and India (Cuthbert et al. Reference Cuthbert, Green, Ranade, Saravanan, Pain, Prakash and Cunningham2006, Thiollay Reference Thiollay2006, Velevski et al. Reference Velevski, Nikolov, Hallmann, Dobrev, Sidiropoulos, Saravia, Tsiakiris, Arkumarev, Galanaki, Kominos, Stara, Kret, Grubač, Lisičanec, Kastritis, Vavylis, Topi, Hoxha and Oppel2015, Ogada et al. Reference Ogada, Shaw, Beyers, Buij, Murn, Thiollay, Beale, Holdo, Pomeroy, Baker, Krüger, Botha, Virani, Monadjem and Sinclair2016), and these declines are mainly caused by poisoning, direct persecution, decreased food availability, electrocution and collision with power lines and wind turbines (Liberatori and Penteriani Reference Liberatori and Penteriani2001, Mateo-Tomás et al. Reference Mateo-Tomás, Olea and Fombellida2010, Angelov et al. Reference Angelov, Hashim and Oppel2013, Sanz-Aguilar et al. Reference Sanz-Aguilar, Sánchez-Zapata, Carrete, Benítez, Ávila, Arenas and Donázar2015, Velevski et al. Reference Velevski, Nikolov, Hallmann, Dobrev, Sidiropoulos, Saravia, Tsiakiris, Arkumarev, Galanaki, Kominos, Stara, Kret, Grubač, Lisičanec, Kastritis, Vavylis, Topi, Hoxha and Oppel2015). The Egyptian Vulture population in Turkey is not only important due to its size, but also due to its geographic position that connects it to flyways from eastern Europe and central Asia. The declining population on the Balkan peninsula migrates mostly through Turkey (Oppel et al. Reference Oppel, Dobrev, Arkumarev, Saravia, Bounas, Kret, Velevski, Stoychev and Nikolov2015), and immature Egyptian Vultures originating from the Balkans have been prospecting known breeding sites in Turkey (Bougain Reference Bougain2016). Safeguarding and restoring the Balkan population might therefore require a strong and productive population in Turkey, as has been shown for Egyptian Vulture populations elsewhere and for other raptors in the region (Demerdzhiev et al. Reference Demerdzhiev, Stoychev, Dobrev, Spasov and Oppel2015, Lieury et al. Reference Lieury, Gallardo, Ponchon, Besnard and Millon2015, Tauler et al. Reference Tauler, Real, Hernández-Matías, Aymerich, Baucells, Martorell and Santandreu2015). Despite the importance of the Egyptian Vulture population in Turkey, population size and trends are unknown and reliable information is needed to assess the consequences of changing agricultural and land use policies on the species’ status (Kirwan et al. Reference Kirwan, Boyla, Castell, Demirci, Özen, Welch and Marlow2008, Şen et al. Reference Şen, Tavares and Bilgin2017).
Changes in land use, away from traditional livestock farming to a more intensive agriculture, are believed to have detrimental effects on Egyptian Vultures because of reduced food availability (Mateo-Tomás and Olea Reference Mateo-Tomás and Olea2010, Margalida et al. Reference Margalida, Benítez, Sánchez-Zapata, Ávila, Arenas and Donázar2012, Dobrev et al. Reference Dobrev, Boev, Arkumarev, Dobrev, Kret, Saravia, Bounas, Vavylis, Nikolov and Oppel2016), and this degradation of feeding habitats is speculated to pose a key threat for the species in Turkey (Iñigo et al. Reference Iñigo, Barov, Orhun and Gallo-Orsi2008). In addition, sanitary EU legislations (Regulation CE 1774 ⁄ 2002) that prohibit carcass disposal in the countryside, which negatively affected vultures in Spain, were adopted by the Turkish government as part of the EU accession process in March 2010 (Turkish Ministry of Environment and Urbanisation directive on sanitary landfills OG. 26.03.2010/ 27533). These new regulations may affect food availability for vultures because they limit the disposal of livestock carcasses in the landscape and mandate that landfills reduce the amount of accessible food waste. Conversely, Egyptian Vultures have very high dietary plasticity (Margalida and Colomer 2012, Margalida et al. Reference Margalida, Benítez, Sánchez-Zapata, Ávila, Arenas and Donázar2012, Dobrev et al. Reference Dobrev, Boev, Arkumarev, Dobrev, Kret, Saravia, Bounas, Vavylis, Nikolov and Oppel2016), and the removal of a predictable food source such as a rubbish dump may be easily compensated by a breeding population, or indeed have positive effects due to reduced competition and interaction with immature birds frequenting the predictable food source (Cortés-Avizanda et al. Reference Cortés-Avizanda, Carrete and Donázar2010, Lieury et al. Reference Lieury, Gallardo, Ponchon, Besnard and Millon2015). However, there is currently no quantitative information whether the removal of established food sources affects Egyptian Vultures in Turkey.
In this study we examine whether the closure of a predictable food source affected the reproductive parameters of a sizeable Egyptian Vulture breeding population in Central Anatolia, Turkey. We monitored breeding performance of a dense population from 2011 to 2016, and examined whether the closure of a central rubbish dump, which used to contain human food waste and animal carcasses and therefore functioned as a reliable food source for vultures up to 2014, resulted in a change in reproductive parameters in 2015 and 2016. Although this specific rubbish dump did not affect nest site selection of the Egyptian Vulture population (Şen et al. Reference Şen, Tavares and Bilgin2017), recent evidence from Spain suggests that territory occupancy may be influenced by such predictable food sources (Tauler-Ametller et al. Reference Tauler-Ametller, Hernández-Matías, Pretus and Real2017). This study provides the first baseline information on the breeding performance of an Egyptian Vulture population in Turkey and explores the effect of sanitary measures on reproductive parameters that have led to major changes of vulture populations in western Europe (Margalida et al. Reference Margalida, Donázar, Carrete and Sánchez-Zapata2010, Reference Margalida, Colomer and Oro2014b).
Methods
Study area
We studied Egyptian Vultures near the town of Beypazarı in Ankara province in Central Anatolia, Turkey (40°05’N, 31°53’E; Figure 1). The study area (1,293 km2) ranges from 400 m to 1,800 m elevation, and contains high biodiversity which is captured by three Key Biodiversity Areas: Kirmir Valley, Sarıyar Dam and Nallıhan Hills (Eken et al. Reference Eken, Bozdoğan, İsfendiyaroğlu, Kılıç and Lise2006). The southern and western parts of the study area are mainly composed of grasslands with low-intensity livestock grazing along with intensive agriculture, while the northern part is more forested and mountainous. Traditional and industrial agricultural practices provide three principal types of feeding opportunities for vultures (Figure 1): sheep pens, chicken farms, and chicken farm disposal sites. While sheep pens and chicken farms occasionally discard livestock carcasses, the chicken farm disposal sites hold large quantities of chicken remains and excrement which are spread on grasslands to act as fertilizer. The single-most important congregation site for Egyptian Vultures with up to 50 birds attending simultaneously used to be the municipal rubbish dump of Beypazarı. This facility was officially closed in March 2015 because the close proximity of the rubbish dump to existing and developing settlements and recreational areas created a health hazard risk to the public. The rubbish dump closure provided the opportunity to specifically test whether the removal of such a predictable food source affected the reproductive parameters of Egyptian Vultures.
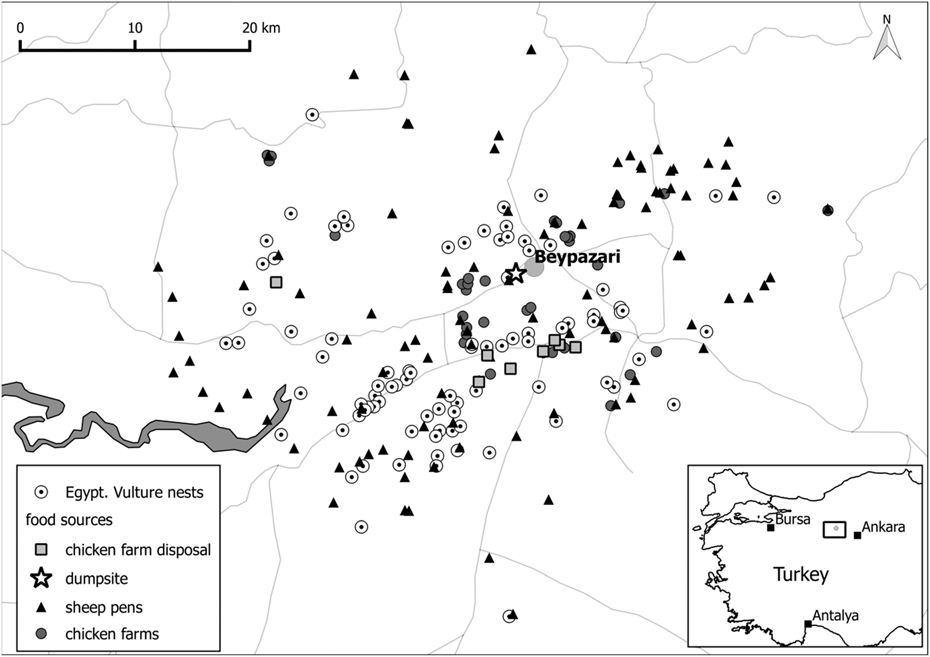
Figure 1. Map of the study area Beypazarı in the Ankara province in Turkey. Egyptian Vulture nest locations are displayed in relation to possible food sources.
Field data collection and calculation of environmental variables
Breeding attempts of Egyptian Vultures were monitored each year from 2011 to 2016, usually from pre-laying until fledging (early April until late August), but a small number of nests were only found after incubation had been initiated. We included only territories where the exact location of the nest could be confirmed. The number of monitored territories increased between 2011 and 2014 due to additive search efforts in every breeding season. Within a breeding season, each monitored territory was visited at least once per month, and we determined the number of fledglings produced by observing dependent young being fed by adults in the vicinity of the nest at the end of the breeding season (August). Observations at nest sites were made with a 20–60× magnification spotting scope at distances varying between 300 and 800 m from nesting cliffs.
We recorded all predictable food source locations (sheep pens, chicken farms and chicken farm disposal sites) using a handheld GPS device during field work and further mapped additional sites using satellite images of the study area. Because exposure to weather may affect breeding performance of raptors (Vlachos et al. Reference Vlachos, Papageorgiou, Bakalaoudis, Chancellor, Meyburg and Ferrero1998, Liberatori and Penteriani Reference Liberatori and Penteriani2001, Sarà and Vittorio Reference Sarà and Vittorio2003, García-Ripollés and López-López 2006), we obtained daily weather variables (mean temperature and precipitation) in our study area for all years from the National Centres for Environmental Prediction downloaded via the R-package RNCEP (Kemp et al. Reference Kemp, Emiel van Loon, Shamoun-Baranes and Bouten2012), and averaged temperature and summed precipitation for each breeding season for analysis.
Because access to food and social interactions have been found to affect Egyptian Vulture demography elsewhere (Vlachos et al. Reference Vlachos, Papageorgiou, Bakalaoudis, Chancellor, Meyburg and Ferrero1998, Liberatori and Penteriani Reference Liberatori and Penteriani2001, Sarà and Vittorio Reference Sarà and Vittorio2003, García-Ripollés and López-López 2006, Carrete et al. Reference Carrete, Grande, Tella, Sánchez-Zapata, Donázar, Díaz-Delgado and Romo2007), we calculated several environmental variables for each monitored vulture nest that could also explain variation in reproductive parameters besides the presence of the rubbish dump. We enumerated the number of sheep pens, chicken farms, and chicken farm disposal sites within a 20-km radius around each nest, reflecting the area that is easily accessible to territorial adult vultures (López-López et al. Reference López-López, García-Ripollés and Urios2014). To characterise intraspecific competition, we calculated the distance to the nearest active Egyptian Vulture nest, and counted the number of active nests within a 2-km radius. To test the effect of the rubbish dump closure we calculated the distance from each nest to the rubbish dump.
Data analysis
Food availability, weather, and competition may affect three biological processes that comprise overall annual productivity: whether territorial pairs initiated a breeding attempt (breeding propensity), whether pairs that did initiate a breeding attempt raised any fledglings (breeding success), and whether successfully breeding pairs managed to raise two rather than just one fledgling (fledging rate). Because our monitoring was mostly initiated around nesting pairs, we here only investigate the effects of environmental variables on breeding success and fledging rate, and provide summaries of annual productivity, which we define as the number of fledglings raised by a territorial pair (including non-breeding pairs).
To test whether the rubbish dump closure in 2015 could effectively explain variation in breeding success and fledging rate, we constructed two competing models for each reproductive parameter to examine the main hypothesis of management interest, and compared these two models using a likelihood-ratio test (Lewis et al. Reference Lewis, Butler and Gilbert2011). Because many different factors can affect Egyptian Vulture reproductive parameters (Oppel et al. Reference Oppel, Dobrev, Arkumarev, Saravia, Bounas, Manolopoulos, Kret, Velevski, Popgeorgiev and Nikolov2017), our basic model considered that breeding success or fledging rate could vary with the number of nearby active nests and the distance to the nearest nest (intraspecific competition); the number of sheep pens, chicken farms and chicken farm disposal sites within a 20-km radius (food availability); and total rainfall and average temperature of a breeding season (weather effects). Our competing model included all the effects of the basic model, plus the effect of the presence (2011–2014) or absence (2015–2016) of the rubbish dump. Because the effect of the rubbish dump may have depended on the distance between the nest and the rubbish dump, we specified this effect as the distance from each nest to the rubbish dump for years when the rubbish dump was available (2011–2014) and zero for years when the dump was not available (2015–2016). An alternative parameterisation using the availability of the rubbish dump as a binary variable yielded virtually identical results that are not presented here.
We used generalised linear mixed models with a binomial error distribution to evaluate these hypotheses on two different reproductive parameters (breeding success, fledging rate) while accounting for variation between territories and years by including the territory and the year as random intercepts in each model (Gillies et al. Reference Gillies, Hebblewhite, Nielsen, Krawchuk, Aldridge, Frair, Saher, Stevens and Jerde2006, Bolker et al. Reference Bolker, Brooks, Clark, Geange, Poulsen, Stevens and White2009). We standardized all continuous variables and fitted all models using the Laplace approximation in R 3.2.5 (R Core Team 2016) with the package ’lme4’ version 1.1-7 (Bates et al. Reference Bates, Maechler, Bolker and Walker2015).
To account for the varying exposure time of nests in our analysis of breeding success, we used the Mayfield logistic regression (Hazler Reference Hazler2004), which incorporates the exposure time of each nest in an adapted link function (available at: http://rpubs.com/bbolker/logregexp) to address the problem that nests that fail earlier may be less likely to be accounted for during monitoring. We removed the data from 2012 for the analysis of breeding success, because in 2012 nest monitoring was carried out only in July and August.
We evaluated the explanatory power of models by predicting the response variable based on the full model and data set, and then calculated the area under the receiver-operated characteristic curve (AUC), a common performance metric for binary data that indicates whether the model has a poor (0.5) or good (1.0) discriminative ability (Fielding and Bell Reference Fielding and Bell1997, Jiménez-Valverde Reference Jiménez-Valverde2012).
Results
Breeding population size and overall productivity
The number of Egyptian Vulture territories monitored in the Beypazarı population increased from 37 in 2011 to 81 in 2014, but because the survey effort was not exhaustive, the actual population size may be higher. The density of Egyptian Vultures in the study area was therefore at least 6.26 pairs per 100 km2. Breeding propensity ranged between 0.83 and 0.97 with an average of 0.87 before and 0.91 after the dump closure; breeding success ranged from 0.61 to 0.76 with an average of 0.64 before and 0.71 after the closure; and annual productivity ranged from 0.71 to 0.92 fledglings/territorial pair, with an average of 0.78 before the dump closed and 0.82 after the closure (Table 1).
Table 1. Mean (± 95% confidence interval) reproductive parameters of the Egyptian Vulture population around Beypazarı, Turkey, during a period with an open rubbish dump (2011–2014) and after the rubbish dump had been closed (2015–2016). Note that the number of nests monitored is a consequence of survey effort and not related to changes in population size. See text for definition on how reproductive parameters were calculated.

* nest monitoring in 2012 started in July, and may have therefore overestimated breeding success and underestimated breeding propensity because early nest failures went unrecorded.
During the Egyptian Vulture breeding season from May to August in 2011–2016, average daily temperatures were 24°C (range: 11–34°C) and precipitation ranged from 203 to 590 mm per season. The full model explaining variation in breeding success of 244 monitored breeding attempts had a reasonable discriminative capacity (AUC = 0.707), but there was no evidence that breeding success was related to the distance from the rubbish dump in years when the dump was operational (Likelihood ratio test, χ2 = 0.634, P = 0.426). The only parameter that was estimated to be significantly different from zero indicated that nest failure rate increased with increasing distance to the nearest occupied Egyptian Vulture nest (Table 2).
Table 2. Parameter estimates of the fixed effects of two generalised linear mixed models evaluating the effect of the closure of the rubbish dump (in bold) while accounting for other factors affecting reproductive parameters of Egyptian Vultures around Beypazarı, Turkey, 2011–2016. See text for description of models; note that breeding success is modelled as nest failure rate. SE = standard error of the estimate.

The mean fledging rate of 199 successful breeding attempts was 1.22 ± 0.41 (Table 1). The full model explaining variation in fledging rate had good discriminative capacity (AUC = 0.813), but there was no evidence that fledging rate was related to the distance from the rubbish dump in years when the dump was operational (χ2 = 0.074, P = 0.786). Parameter estimates of all other environmental factors had too low precision to be statistically different from zero (Table 2).
Discussion
Our analysis of Egyptian Vulture reproductive parameters shows annual variability in breeding success but no statistical support for a distinct effect of the closure of a main food source, the communal rubbish dump. Instead, we found that Egyptian Vultures in this dense breeding population appear to have higher breeding success when nesting in close proximity to other pairs, which may be a consequence of spatially aggregated ideal nesting opportunities (Şen et al. Reference Şen, Tavares and Bilgin2017).
Our estimate of mean annual productivity (0.79) is slightly lower than the mean productivity of European populations (Iñigo et al. Reference Iñigo, Barov, Orhun and Gallo-Orsi2008). Across Europe, productivity ranges from 0.6 to 1.04 fledglings per territorial pair among both declining (Grubač et al. Reference Grubač, Velevski and Avukatov2014, Oppel et al. Reference Oppel, Dobrev, Arkumarev, Saravia, Bounas, Manolopoulos, Kret, Velevski, Popgeorgiev and Nikolov2017) and increasing populations (Mateo-Tomás et al. Reference Mateo-Tomás, Olea and Fombellida2010, Tauler et al. Reference Tauler, Real, Hernández-Matías, Aymerich, Baucells, Martorell and Santandreu2015). The productivity for the Beypazarı population was very similar to the productivity of an increasing population in southern France that was aided by vulture restaurants (Lieury et al. Reference Lieury, Gallardo, Ponchon, Besnard and Millon2015). However, the French population benefited from immigration and productivity suffered from compensatory density feedback possibly due to increased interference of floaters (Lieury et al. Reference Lieury, Gallardo, Ponchon, Besnard and Millon2015). We found that breeding success appeared to increase when nests were closer to a neighbouring nest (Table 2), which is contrary to the expectation that interference competition with conspecifics might adversely affect productivity. However, this result might explain why the distance to the nearest nest in this population is much lower than in other European populations and that conspecific attraction appeared to peak at distances ∼1,500 m from the nearest nest (Şen et al. Reference Şen, Tavares and Bilgin2017). Whether this aggregated nesting is due to the spatial proximity of certain topographic or habitat attributes that Egyptian Vultures prefer for nesting, or whether nesting in close proximity actually confers a fitness advantage by some other process will require further study.
In Beypazarı, Egyptian Vultures are breeding at a density of more than six pairs per 100 km2, which is considerably higher than early records from Spain when Ceballos and Donázar (Reference Ceballos and Donázar1989) found approximately 1.4 pairs/100 km2, and significantly denser than the current figures from Northern Spain where only 0.14 territories/100 km2 exist (Mateo-Tomás and Olea Reference Mateo-Tomás and Olea2009). The rubbish dump may have benefitted floaters and immature birds more than territorial breeding pairs (Oro et al. Reference Oro, Margalida, Carrete, Heredia and Donázar2008, Weiser and Powell Reference Weiser and Powell2011, Lieury et al. Reference Lieury, Gallardo, Ponchon, Besnard and Millon2015), and the closure of the dump may therefore alleviate interference competition by reducing the number of floaters in the study area, which could explain why we did not observe a decrease in breeding success after the closure of the rubbish dump. Alternatively, high plasticity in diet choice, which has been found in Egyptian Vultures (Dobrev et al. Reference Dobrev, Boev, Arkumarev, Dobrev, Kret, Saravia, Bounas, Vavylis, Nikolov and Oppel2016), may allow adult birds to rapidly shift to other food sources and therefore compensate for the loss of a food source such as the communal rubbish dump.
Our estimates of fledging rate are similar to the range of European populations of Egyptian Vultures (Mateo-Tomás et al. Reference Mateo-Tomás, Olea and Fombellida2010), and we also found no effect of the closure of the rubbish dump on fledging rate. The main threat to Egyptian Vultures in Turkey is seen as food shortages arising from declining traditional animal husbandry (Iñigo et al. Reference Iñigo, Barov, Orhun and Gallo-Orsi2008), but in our study area there are still a large number of small agricultural units that can provide food for vultures (Figure 1). These small agricultural units likely provide a temporally heterogeneous supply of potential food sources, and therefore replicate the less predictable opportunistic foraging opportunities to which vultures are adapted (Cortés-Avizanda et al. Reference Cortés-Avizanda, Carrete and Donázar2010). As a result of these other foraging opportunities, the rubbish dump may not have played a critical role in food acquisition for territorial adult vultures, explaining why we detected no effect of the rubbish dump closure on fledging rate. Maintaining the heterogeneous landscape mosaic of small animal husbandry operations that can provide small amounts of food at unpredictable times may therefore be more beneficial to the Egyptian Vulture population than large and permanent central feeding stations such as rubbish dumps or vulture restaurants due to reduced effects of interference competition (Cortés-Avizanda et al. Reference Cortés-Avizanda, Jovani, Carrete and Donázar2012). However, some of the chicken farm disposal sites that are promoted as grassland fertilization appear to be industrial waste disposal sites in the open landscape. If the chickens reared in those industrial chicken farms that dispose waste material on grassland are treated with pharmaceuticals that can be harmful to vultures, then survival probabilities of adult vultures may be negatively affected despite short-term improvements in fledging rate (Green et al. Reference Green, Taggart, Das, Pain, Sashi Kumar, Cunningham and Cuthbert2006, Ogada et al. Reference Ogada, Keesing and Virani2012). We recommend that animal products made available for vultures in the open landscape are tested for substances that are known to be harmful to vultures, and that problematic pharmaceuticals are banned for veterinary use in Turkey (Margalida et al. Reference Margalida, Bogliani, Bowden, Donazar, Genero, Gilbert, Karesh, Kock, Lubroth, Manteca, Naidoo, Neimanis, Sanchez-Zapata, Taggart, Vaarten, Yon, Kuiken and Green2014a).
In summary, we conclude that the closure of the rubbish dump in Beypazarı has not led to a substantial increase or decrease in reproductive parameters, but we recommend continued monitoring as the effects of a lower floater population may potentially improve breeding success over time. In addition, because feeding stations are known to benefit the survival of immature birds (Oro et al. Reference Oro, Margalida, Carrete, Heredia and Donázar2008, Margalida et al. Reference Margalida, Colomer and Oro2014b, Lieury et al. Reference Lieury, Gallardo, Ponchon, Besnard and Millon2015), a better understanding of other demographic parameters such as adult and juvenile survival and recruitment is required to assess whether the population is sustainable with the current levels of productivity.
Acknowledgements
We acknowledge the support of colleagues helping to monitor territories, especially Adem Akyol, Mustafa Akyol, Fatih Bük, Şenol Uzunoğlu, İzzet Koçak, Osman Türkdoğan, Bahattin Özcan, Emrah Varol, Onur Güngör, and Mehmet Ertuğrul. This work was financially supported by the LIFE+ project “The Return of the Neophron” (LIFE10 NAT/BG/000152) funded by the European Union and co-funded by the AG Leventis Foundation. We appreciate constructive comments by Antoni Margalida, Metodija Velevski, and an anonymous reviewer on an earlier draft of the paper.





