Introduction
Flamingos are charismatic, gregarious, and extremely specialised birds that have inspired humans for centuries. Indeed, their scientific name, the Phoenicopteridae, or “Phoenix-winged”, reflects their status as the Phoenix, or “firebird”, described by the ancient Greek writer Herodotus (McMillan Reference McMillan1972). Like that mythic bird, their populations seem to be reborn from almost zero to thousands of individuals in just a few days (Balkiz et al. Reference Balkiz, Özesmi, Pradel, Germain, Siki and Amat2007; Krienitz Reference Krienitz2018). In addition, their flight capacity allows them to reach isolated sites where they rapidly gather, and they frequently breed in wetlands near volcanic activity (Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007; Krienitz Reference Krienitz2018; Torres et al. Reference Torres, Marconi, Castro, Moschione, Bruno and Michelutti2019). They are indeed extraordinary birds, being one of the few groups of birds that are filter-feeders (Anderson Reference Anderson2017).
There are three flamingo species, with overlapping distributions in the Southern Cone. The Chilean Flamingo Phoenicopterus chilensis has a broad distribution, occupying diverse high-altitude, lowland, and coastal wetlands, while the Andean Flamingo Phoenicoparrus andinus and the Puna (or James’s) Flamingo Phoenicoparrus jamesi are primarily restricted to wetlands in the high Andes of Argentina, Bolivia, Chile, and Peru (Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007). Owing to their strong association with these wetlands, Puna and Andean flamingos are frequently referred to as “high Andes” flamingos (Caziani and Derlindati Reference Caziani and Derlindati2000), although a portion of their population has been recorded in lowland wetlands in central Argentina (Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007; Cruz et al. Reference Cruz, Barisón, Romano, Arengo, Derlindati and Barberis2013; Derlindati et al. Reference Derlindati, Romano, Cruz, Barisón, Arengo and Barberis2014; Jahn et al. Reference Jahn, Cereghetti, Hallworth, Ketterson, Ryder and Marra2023).
There are large differences in population sizes among the six extant flamingo species in the world. In Africa and Eurasia, there are over 3,000,000 individuals of Lesser Flamingo Phoeniconaias minor, and the overall population of Greater Flamingo Phoenicopterus roseus is estimated at between 550,000 and 680,000 individuals. In the Americas, there are between 260,000 and 330,000 American Flamingo Phoenicopterus ruber, and over 500,000 Chilean Flamingos (BirdLife International 2012; Marconi et al. Reference Marconi, Arengo, Castro, Rocha, Valqui and Aguilar2020; Unterkofler and Blanco Reference Unterkofler and Blanco2016), while the Andean and Puna flamingos have the smallest population sizes, with current population numbers being less than 80,000 and 160,000 individuals, respectively (Marconi et al. Reference Marconi, Arengo, Castro, Rocha, Valqui and Aguilar2020). Based on these estimates, the Andean Flamingo is categorised as “Vulnerable”, while the Puna Flamingo is categorised as “Near Threatened” (IUCN 2023).
The wetland habitats that these two flamingo species use are changing rapidly, which poses potentially serious threats to their survival. Industrial-scale mining in high Andean environments and widespread agro-industrial activities in the lowland Pampas have undergone recent rapid and unprecedented growth (Gajardo and Redón Reference Gajardo and Redón2019; Nanni et al. Reference Nanni, Piquer Rodríguez, Rodríguez, Núñez Regueiro, Periago and Aguiar2020; Tapia et al. Reference Tapia, Murray, Ormachea-Muñoz and Bhattacharya2022; Ugarte-Núñez and Mosaurieta-Echegaray Reference Ugarte-Núñez and Mosaurieta-Echegaray2000). However, the effects of these activities on flamingo populations are still largely unknown (Delfino and Carlos Reference Delfino and Carlos2024; Rocha et al. Reference Rocha, Pacheco, Ayala, Varela and Arengo2021). In addition, climate change models predict that temperatures in the Andean plateau will increase while precipitation will decrease (Zubieta et al. Reference Zubieta, Molina-Carpio, Laqui, Sulca and Ilbay2021), suitable wetland habitat areas will decrease (Delfino Reference Delfino2023), and several studies are beginning to document detectable changes in wetlands in this direction (De los Ríos-Escalante et al. 2024; Gutiérrez et al. Reference Gutiérrez, Moore, Donnelly, Dorador, Navedo and Senner2022). Thus, our objective was to synthesise the information available on the ecology of the Andean and Puna flamingos and highlight the critical conservation issues that need to be tackled immediately at global, regional, and local levels. We describe population trends and distribution of both high Andes flamingo species, and the threats to their associated habitats, including changes in land-use pressures. We describe current conservation strategies (and their limitations) and provide recommendations for further action.
Methods
We obtained Andean and Puna flamingo population numbers and information on wetland sites from a review of the published literature, unpublished reports, field guides, and personal experience. The search considered combinations of the following two terms in different languages: (1) the Andean region (i.e. “high Andean”, “Andes”, “Altiplano”) + (2) the high Andes flamingos by their common names (i.e. “Andean Flamingo”, “Puna Flamingo”, “Flamenco Andino”, “Flamenco Puna”, “James’s Flamingo”, “Parina”, “Parihuana”) or their scientific names (“Phoenicoparrus andinus”, “Phoenicoparrus jamesi”). Since 1997, data on Andean and Puna flamingo distribution and abundance have been collected via a regional collaborative effort by the “Grupo de Conservación Flamencos Altoandinos” (GCFA) that coordinates range-wide censuses of the species in Argentina, Bolivia, Chile, and Peru (Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007; Marconi et al. Reference Marconi, Arengo, Castro, Rocha, Valqui and Aguilar2020). These comprehensive censuses have been carried out every five years since 2005. In these censuses, direct counts are conducted over a 10-day period following a standardised survey protocol during the breeding season (late January–early February) at over 250 wetlands by about 20 teams of 4–5 people (EJD, FA, MM, MCR, HSF, EO, OR, and IMB participated in most censuses). To date, a total of six simultaneous censuses have been carried out covering the global distribution of the Andean and Puna flamingos (Marconi Reference Marconi2010; Marconi et al. Reference Marconi, Arengo, Castro, Rocha, Valqui and Aguilar2020). These censuses provided reliable global population estimates of both high Andes flamingo species, identified flamingo occurrence and abundance at specific wetland sites, and allowed us to evaluate conservation issues and identify priority sites for conservation action (Marconi et al. Reference Marconi, Arengo, Castro, Rocha, Valqui and Aguilar2020).
Results and Discussion
Population size and geographical range of high Andes flamingos
The Andean Flamingo was first described by Philippi in 1854 from a type specimen from the Salar de Atacama, Chile. The Puna Flamingo was described in 1895 from a type specimen collected in Laguna Parinacota, Chile in 1850 (Allen Reference Allen1956). Until the late 1950s, the scientific community surmised that the Puna Flamingo could be extinct because it had not been observed in many years, however, an expedition to Laguna Colorada, Bolivia, in 1957 reported a few hundred Puna Flamingos (Behn et al. Reference Behn, Johnson and Millie1957; Johnson et al. Reference Johnson, Behn and Millie1958).
Since the early 1960s and until the late 1980s, there were only vague population estimates for the two high Andes flamingo species (Kahl Reference Kahl, Kear and Duplaix-Hall1975; Peña Reference Peña1962), and their distribution was uncertain (Bucher Reference Bucher1992; Fjeldså and Krabbe Reference Fjeldså and Krabbe1990; Hurlbert Reference Hurlbert1978, Reference Hurlbert1981; Parada Reference Parada, Parada, Rottmann and Guerra1990). Estimating the size of flamingo populations is a difficult task because of their flight capacity, their remote wetland habitats, and the extent of their geographical range (Béchet Reference Béchet and Anderson2017). Population estimates were based on extrapolations from counts conducted at a few wetland sites (Cordier Reference Cordier1965; Hurlbert Reference Hurlbert1978, Reference Hurlbert1981; Johnson Reference Johnson and Johnson1967; Kahl Reference Kahl, Kear and Duplaix-Hall1975; Parada Reference Parada, Parada, Rottmann and Guerra1990). Most studies were focused on where these flamingo species were feeding or breeding in high Andes wetlands (Figure 1; see Supplementary material Table S1 for more details). During this period, estimates of total abundances, winter distributions, and movement patterns were mostly hypothetical (Valqui et al. Reference Valqui, Caziani, Rocha and Rodríguez2000).
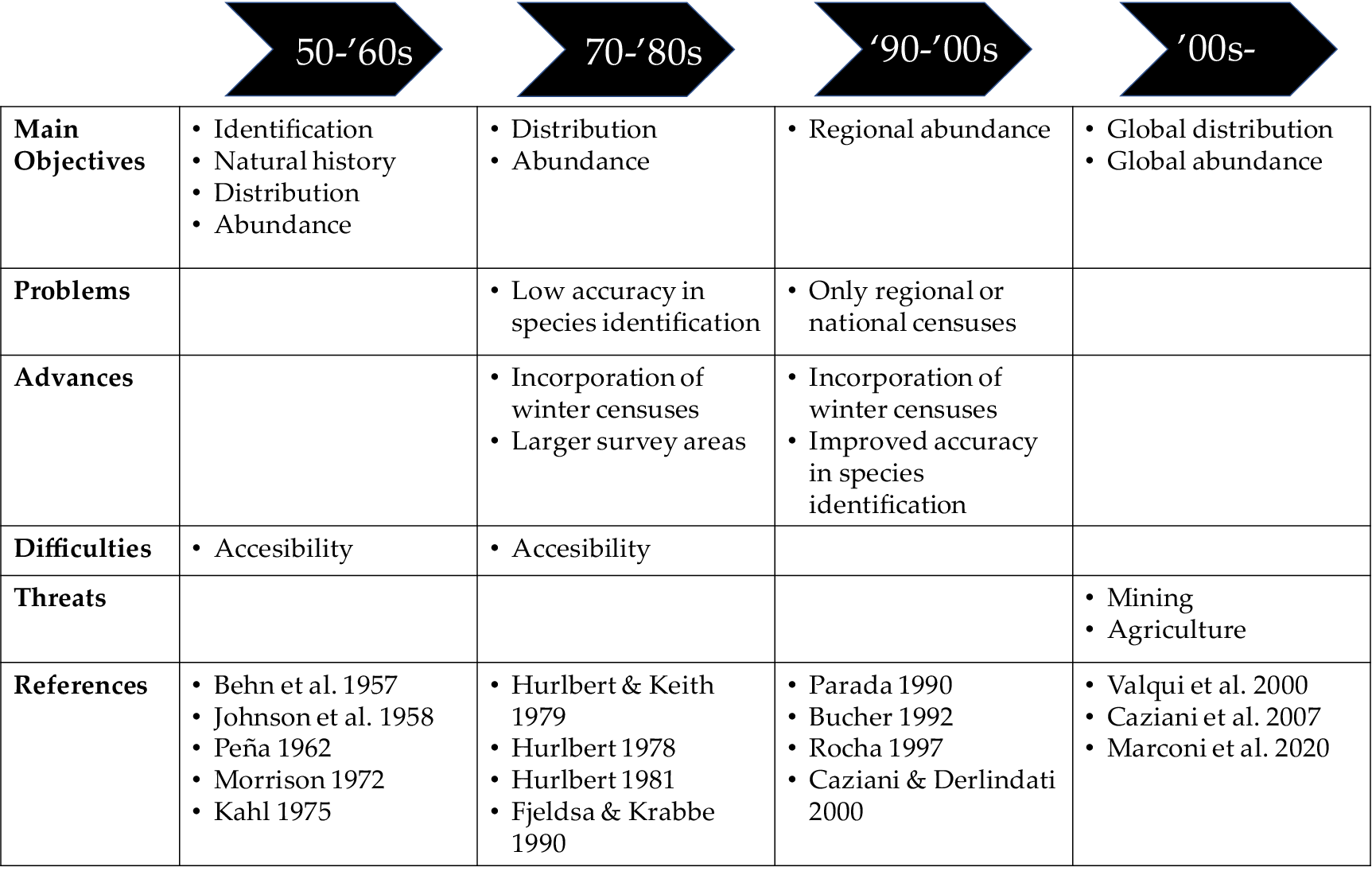
Figure 1. Time arrow of the high Andes flamingo research from the 1950s to the present. Changes in main objectives, problems, advances, difficulties, and threats are shown.
In the 1970s, several flamingo censuses were carried out in summer and winter in different wetlands of Peru, Chile, Bolivia, and Argentina (Hurlbert Reference Hurlbert1978, Reference Hurlbert1981) (Figures 1 and 2), showing that the Puna Flamingo was not as rare as believed and that there was marked spatial variation in flamingo abundance. In the 1980s, censuses were carried out in different seasons and years in a set of wetlands in Chile (Parada Reference Parada, Parada, Rottmann and Guerra1990) (Figures 1 and 2). In the 1990s, regional studies were carried out in Bolivia (Rocha Reference Rocha1997) and Argentina (Caziani and Derlindati Reference Caziani and Derlindati2000).

Figure 2. Total numbers of individuals by species derived from censuses conducted in the 1970s through to the second decade of the 2000s (data collated from Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007; Hurlbert Reference Hurlbert1978, Reference Hurlbert1981; Marconi et al. Reference Marconi, Arengo, Castro, Rocha, Valqui and Aguilar2020; Parada Reference Parada, Parada, Rottmann and Guerra1990; Valqui et al. Reference Valqui, Caziani, Rocha and Rodríguez2000). Arrow shows the first International Simultaneous Census (Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007).
The systematic censuses of the GCFA initiated in 1997 showed that high Andes flamingos are present in a diversity of over 200 wetlands across Argentina, Bolivia, Chile, and Peru, including lagoons, salt lakes, salt flats, flooded meadows, marshes, and rivers, from 0 to 4,740 m a.s.l. in the highlands of central Andes (Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007; Marconi et al. Reference Marconi, Sureda, Arengo, Aguilar, Amado and Alza2011, Reference Marconi, Arengo, Castro, Rocha, Valqui and Aguilar2020) (Figure 3). During the breeding season, about 70% of the global population of both flamingo species is concentrated in highland wetlands situated around the tri-national border of Argentina, Bolivia, and Chile (Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007; Marconi et al. Reference Marconi, Sureda, Arengo, Aguilar, Amado and Alza2011, Reference Marconi, Arengo, Castro, Rocha, Valqui and Aguilar2020). Moreover, 50% of the population of both flamingo species is concentrated in just a few wetlands, some of them important nesting sites; thus, any impact on these few wetlands could seriously affect their populations (Caziani and Derlindati Reference Caziani and Derlindati2000). Summer censuses carried out every five years from 2005 until 2020 with similar coverage showed flamingo populations stable and increasing (Marconi et al. Reference Marconi, Sureda, Arengo, Aguilar, Amado and Alza2011, Reference Marconi, Arengo, Castro, Rocha, Valqui and Aguilar2020).
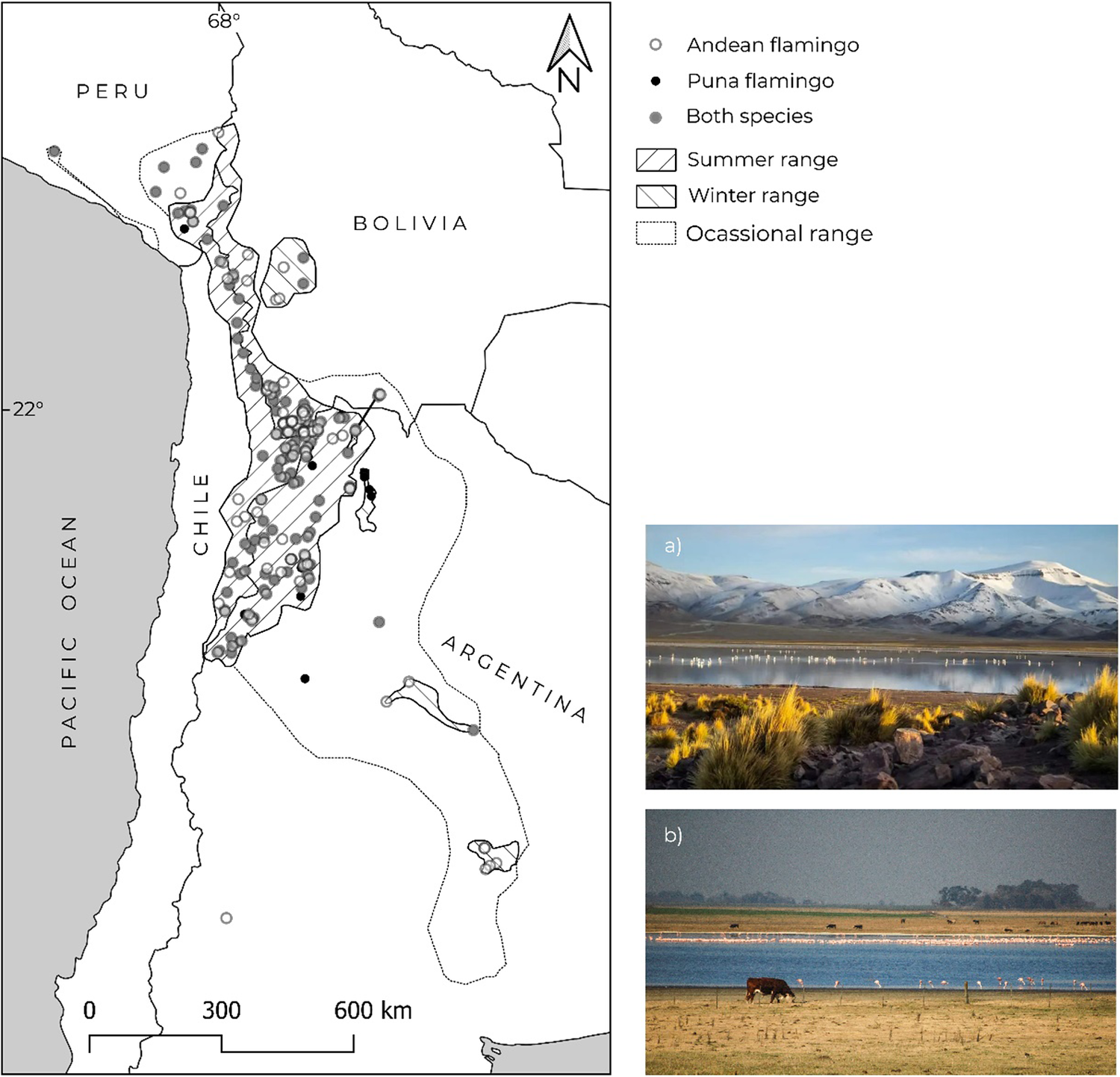
Figure 3. Current known distribution of Andean Flamingo Phoenicoparrus andinus and Puna Flamingo P. jamesi across Argentina, Bolivia, Chile, and Peru: (a) example of a high Andes wetland, Lagunas de Vilama (4,600 m a.s.l.) in north-western Argentina; (b) a lowland wetland, Laguna La Dulce (80 m a.s.l.) in central Argentina. (Photographs: E. J. Derlindati and M. Romano)
The two simultaneous censuses carried out in winter (1998, 2000) showed that almost 40% of the global population of the Andean Flamingo is mainly found in several lowland wetlands in Argentina and a few highland lakes (Poopó and Uru Uru) in Bolivia (Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007). Among the most important lowland wetlands are the Laguna Melincué and the Pampa de las Lagunas systems in southern Santa Fe province, and the Mar Chiquita/Río Dulce wetlands in Córdoba and Santiago del Estero provinces (Bucher Reference Bucher1992; Cabaña et al. Reference Cabaña, Steffolani, Lassaga, Michelutti, Michelutti and Castro2018; Romano et al. Reference Romano, Barberis, Derlindati, Pagano, Marconi and Arengo2009, Reference Romano, Barberis, Pagano, Minotti and Arengo2017). These wintering sites show a large inter-annual variation in the abundance of this flamingo species (Romano et al. Reference Romano, Barberis, Pagano, Minotti and Arengo2017). In contrast, the wintering sites of the Puna Flamingo remain largely unknown (Bucher Reference Bucher1992; Cruz et al. Reference Cruz, Barisón, Romano, Arengo, Derlindati and Barberis2013; Marconi et al. Reference Marconi, Arengo, Castro, Rocha, Valqui and Aguilar2020; Quiroga and Llugdar Reference Quiroga and Llugdar2022).
Andean and Puna flamingos breed at high elevations in the Andes in summer (October–February). A portion of the Andean Flamingo population moves to lowland wetlands in the grasslands of Argentina (the Pampas), particularly when the high Andean wetlands freeze completely (Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007). This movement occurs across an altitudinal gradient of over 4,000 m; therefore, these individuals are considered partial altitudinal migrants (Jahn et al. Reference Jahn, Cueto, Fontana, Guaraldo, Levey and Marra2020), a behaviour that sets them apart from other flamingo species (Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007). A portion of the population of the high Andes flamingos spends the entire year at several Andean lakes located at lower elevations or where geothermal activities prevent the water from freezing (Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007; Johnson et al. Reference Johnson, Behn and Millie1958). Most of the lowland wetlands used by Andean Flamingos during the winter season are immersed in a matrix of highly intensive agroecosystems of the Humid Pampas (Nanni et al. Reference Nanni, Piquer Rodríguez, Rodríguez, Núñez Regueiro, Periago and Aguiar2020; Romano et al. Reference Romano, Barberis, Pagano and Maidagan2005, Reference Romano, Barberis, Pagano, Marconi and Arengo2008, Reference Romano, Barberis, Arengo, Caselli, Minotti and Morandeira2011, Reference Romano, Barberis, Pagano, Minotti and Arengo2017). Puna Flamingos are occasionally observed in lowland wetlands and a few sites on the Peruvian coast in winter (Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007; Cruz et al. Reference Cruz, Barisón, Romano, Arengo, Derlindati and Barberis2013; Derlindati et al. Reference Derlindati, Romano, Cruz, Barisón, Arengo and Barberis2014; Dias and Cardozo Reference Dias and Cardozo2014; Hughes Reference Hughes1980). Records of high Andes flamingo species wandering outside their normal range are shown in Appendix S1.
Habitat characteristics, flamingo distribution, and spatial patterning
The main habitats used by Andean and Puna flamingos are unique and specific wetlands that have distinctive characteristics and relatively few variables and conditions are known to affect their abundance and distribution (Boyle et al. Reference Boyle, Caziani and Waltermire2004; Caziani and Derlindati Reference Caziani and Derlindati2000; Derlindati et al. Reference Derlindati, Romano, Cruz, Barisón, Arengo and Barberis2014; Dib et al. Reference Dib, Weiss, Neumann, Ordoñez, Estévez and Farías2009; Hurlbert and Keith Reference Hurlbert and Keith1979; Ortiz et al. Reference Ortiz, Gamboa, Salas and Vera2020; Polla et al. Reference Polla, Di Pasquale, Rasuk, Barberis, Romano and Manzo2018; Valqui et al. Reference Valqui, Caziani, Rocha and Rodríguez2000; Vides-Almonacid Reference Vides-Almonacid1990). These species use habitats with a very narrow range of salinity, productivity, and water levels (Mascitti Reference Mascitti2001; Mascitti and Bonaventura Reference Mascitti and Bonaventura2002; Polla et al. Reference Polla, Di Pasquale, Rasuk, Barberis, Romano and Manzo2018). The inter-annual variation in climatic conditions (e.g. dry and wet years associated with El Niño – La Niña events) modifies the lake water area and water chemistry (Bucher and Curto Reference Bucher and Curto2012; de la Fuente et al. Reference de la Fuente, Meruane and Suárez2021; Guerra et al. Reference Guerra, Martini, Córdoba, Ariztegui and Piovano2019), leading to inter-annual variations in the abundance and distribution of both high Andes flamingos in highland and lowland areas (Bucher Reference Bucher1992; Moschione and Sureda Reference Moschione and Sureda2008; Romano et al. Reference Romano, Barberis, Derlindati, Pagano, Marconi and Arengo2009, Reference Romano, Barberis, Pagano, Minotti and Arengo2017).
In the late 1990s, lakes in north-western Argentina and northern Chile showed marked variations in depth, transparency, and pH, and these variations in salinity and depth were strongly related to the presence of waterbird communities, especially flamingos (Boyle et al. Reference Boyle, Caziani and Waltermire2004). In the high Andes, all three flamingo species forage in saline wetlands (Hurlbert and Keith Reference Hurlbert and Keith1979), and there are wetlands where all three species occur. However, Andean and Puna flamingos tend to forage in hypohaline lakes characterised by 3–20 g/L of salt, dissolved organic matter of low molecular weight, high silicon concentration, and high dissolved oxygen (e.g. lakes Purulla, San Francisco, and Grande in Catamarca province, Argentina), whereas Chilean Flamingos tend to forage in mesohaline lakes characterised by 20–50 g/L of salt, high total organic carbon concentration (e.g. lakes Archibarca and Las Peñas in Catamarca province, Argentina) (Frau et al. Reference Frau, Battauz, Mayora and Marconi2015). With a range of occurrences according to pluriannual cycles or seasons, there are differences in the distributional overlap among the lakes, with the highest overlap observed between Chilean and Andean flamingos, and the lowest between Chilean and Puna flamingos (Hurlbert and Keith Reference Hurlbert and Keith1979). Within a lake, flamingos typically form single-species flocks, although mixed flocks also form, with the highest overlap occurring between Andean and Puna flamingos (Hurlbert and Keith Reference Hurlbert and Keith1979). In lowlands, all three flamingo species are found foraging in some wetlands, forming single species flocks, but both Andean and Puna flamingos are most often associated with Chilean Flamingos in mixed flocks (Castro and Torres Reference Castro and Torres2014).
Foraging behaviour and diet
High Andes flamingos mainly forage in shallow waters using different foraging modes, but mainly forage with their beak, head or neck submerged, walking along the coast filtering water and mud (Hurlbert Reference Hurlbert1982; Hurlbert and Keith Reference Hurlbert and Keith1979). Puna Flamingos walk slowly (10–15 steps/minute), spending more time feeding at each spot, while Andean Flamingos walk faster (20–30 steps/minute) (Hurlbert Reference Hurlbert1982).
Andean and Puna flamingos have a deep-keeled bill and mainly feed on diatoms (Frau et al. Reference Frau, Moran, Arengo, Marconi, Battauz and Mora2021; Hurlbert Reference Hurlbert1982; Hurlbert and Chang Reference Hurlbert and Chang1983; Mascitti Reference Mascitti1998; Mascitti and Kravetz Reference Mascitti and Kravetz2002; Ortiz et al. Reference Ortiz, Gamboa, Salas and Vera2020; Tobar et al. Reference Tobar, Rau, Iriarte, Villalobos, Lagos and Cursach2012), but they likely ingest considerable amounts of mud with microbes when feeding (Mascitti Reference Mascitti1998; Polla et al. Reference Polla, Di Pasquale, Rasuk, Barberis, Romano and Manzo2018). In the high Andes, Andean Flamingos primarily filter the larger (>80 μm long) diatoms while Puna Flamingos primarily filter smaller ones (<60 μm long; Hurlbert Reference Hurlbert1982). In lowland wetlands, Andean Flamingos showed a high positive selection for diatoms, and a strong negative selection for microinvertebrates (Polla et al. Reference Polla, Di Pasquale, Rasuk, Barberis, Romano and Manzo2018).
Nesting sites and breeding biology
Although the breeding ranges of the Andean and Puna flamingos extend over large areas in the Andes of Chile, Bolivia, and Argentina, the currently known nesting sites are limited to very few isolated, shallow hypersaline lakes (Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007; Childress Reference Childress2005; Derlindati et al. Reference Derlindati, Moschione and Cruz2010; Marconi and Clark Reference Marconi and Clark2011; Marconi et al. Reference Marconi, Arengo, Castro, Rocha, Valqui and Aguilar2020; Rocha et al. Reference Rocha, Pacheco, Ayala, Varela and Arengo2021; Torres et al. Reference Torres, Marconi, Castro, Moschione, Bruno and Michelutti2019; Valqui et al. Reference Valqui, Caziani, Rocha and Rodríguez2000). Until 2006, the most important breeding sites for the Andean Flamingo were five wetlands in northern Chile (Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007; Childress Reference Childress2005; Parada Reference Parada, Parada, Rottmann and Guerra1990), while for the Puna Flamingo, it was Laguna Colorada, a lake in south-western Bolivia (Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007; Rocha et al. Reference Rocha, Aguilar, Vargas and Quiroga2009; Valqui et al. Reference Valqui, Caziani, Rocha and Rodríguez2000). Such a limited number of nesting colonies, plus the frequent colony failures due to natural (e.g. predation by foxes; Lagos et al. Reference Lagos, Villalobos, Vianna, Espinosa-Miranda, Rau and Iriarte2023) or human-induced (e.g. egg harvesting by local people; Rocha and Quiroga Reference Rocha and Quiroga1997; Torres et al. Reference Torres, Marconi, Castro, Moschione, Bruno and Michelutti2019) causes, are likely to play a central role in the present population dynamics of high Andes flamingos, resulting in unique conservation challenges.
Intermittent breeding and shifting of nesting colony locations are common events for flamingos worldwide (Baldassarre and Arengo Reference Baldassarre and Arengo2000; Balkiz et al. Reference Balkiz, Özesmi, Pradel, Germain, Siki and Amat2007; Boucheker et al. Reference Boucheker, Samraoui, Prodon, Amat, Rendón-Martos and Baccetti2011). Similar patterns have been reported for Andean and Puna flamingos at a regional scale (Parada Reference Parada, Parada, Rottmann and Guerra1990; Torres et al. Reference Torres, Marconi, Castro, Moschione, Bruno and Michelutti2019). For example, since 2005, the Andean Flamingo has been reported breeding in south-western Bolivia in several wetlands, with Laguna Colorada being one of the most important breeding sites (Rocha et al. Reference Rocha, Aguilar, Vargas and Quiroga2009, Reference Rocha, Pacheco, Ayala, Varela and Arengo2021). In contrast, both the number of nesting pairs and the number of fledglings of Andean Flamingos have declined in Chile (Marconi et al. Reference Marconi, Arengo, Castro, Rocha, Valqui and Aguilar2020). In Argentina, Andean Flamingo nesting activity was first recorded in 1986 in Mar Chiquita, where 100 active nests were mixed within a Chilean Flamingo nesting colony (Cobos et al. Reference Cobos, Miatello and Baldo1999). This finding caught the attention of flamingo specialists since this lake is located at 64–71 m a.s.l. in the Chaco ecoregion (Cobos et al. Reference Cobos, Miatello and Baldo1999), whereas Andean Flamingos were previously known to only nest from 2,300 to 4,300 m a.s.l. (Parada Reference Parada, Parada, Rottmann and Guerra1990; Rocha et al. Reference Rocha, Pacheco, Ayala, Varela and Arengo2021; Torres et al. Reference Torres, Marconi, Castro, Moschione, Bruno and Michelutti2019). Subsequently, the Andean Flamingo was recorded nesting in Laguna La Brava (La Rioja Province, 4,200 m a.s.l.) in 1998 (Bucher et al. Reference Bucher, Chani and Echevarría2000), and in Los Aparejos and Purulla lakes (Catamarca Province, 4,200 m and 3,500 m a.s.l.) in 2001 and 2010, respectively (Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007; Marconi and Clark Reference Marconi and Clark2011), and Laguna Llancanelo (Mendoza Province, 1,300 m a.s.l.) in 2010 (Sosa and Martín Reference Sosa and Martín2010). Subsequently, six new breeding sites for this species were reported in Argentina (Torres et al. Reference Torres, Marconi, Castro, Moschione, Bruno and Michelutti2019). These nesting colonies never exceeded 200 individuals, unlike the main colonies in Chile with thousands of individuals, which have declined in number and size in concert with increasing mining activities over the last four decades (Torres et al. Reference Torres, Marconi, Castro, Moschione, Bruno and Michelutti2019).
The Puna Flamingo breeds in a few salt flats above 4,000 m a.s.l. (Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007; Derlindati et al. Reference Derlindati, Moschione and Cruz2010). Up to 1998, the main known breeding sites were in Bolivia (Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007), but in 2000 a breeding colony was found in the Laguna Brava Provincial Reserve, La Rioja Province (Argentina), which has been more recently abandoned due to red fox (Pseudalopex culpeus) predation (H. Sosa, personal communication). Subsequently, new colonies were found in Surire and San Pedro de Atacama (northern Chile) in addition to those already known in southern Bolivia (Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007). Between 2007 and 2009, new breeding sites were reported from Laguna Vilama (Jujuy Province) and Laguna Santa María (Salta Province), Argentina (Derlindati et al. Reference Derlindati, Moschione and Cruz2010). Despite this, Laguna Colorada in Bolivia remains a key and central site for the Puna Flamingo, with thousands of pairs breeding there annually (Rocha et al. Reference Rocha, Pacheco, Ayala, Varela and Arengo2021). We have observed Puna Flamingos breeding in Laguna Grande (Catamarca Province), Argentina, regularly since 2010, although there is anecdotal evidence that they had bred there in the early 2000s.
The reasons for the apparent tendency towards more frequent and widespread breeding events in recent years are unclear. Records of nesting colonies at several new sites suggest high behavioural plasticity, although breeding success at lower altitudes remains unknown. Some individuals may prevent others from settling in high-quality habitats so that the group of individuals arriving last at an established breeding colony is forced to use lower-quality habitats in which their fitness is lower. This frequently occurs in Greater Flamingo colonies during years of extreme environmental conditions and resource constraints when older birds displace younger age classes toward suboptimal habitats (Boucheker et al. Reference Boucheker, Samraoui, Prodon, Amat, Rendón-Martos and Baccetti2011; Rendón et al. Reference Rendón, Garrido, Ramírez, Rendón-Martos and Amat2001). The recorded variability in food availability and nesting suitability for flamingo species has major monitoring and management implications, because the maintenance of a stable flamingo population depends on the availability and connectivity of multiple foraging and nesting sites, although any given site may not be continually active (Cortés-Avizanda et al. Reference Cortés-Avizanda, Almaraz, Carrete, Sánchez-Zapata, Delgado and Hiraldo2011; Latta et al. Reference Latta, Rimmer and McFarland2003). For instance, Greater Flamingos are opportunistic breeders able to change their breeding sites spatially and temporally in response to variations in conditions and resources (Rendón et al. Reference Rendón, Garrido, Ramírez, Rendón-Martos and Amat2001), a relationship that remains unknown for high Andes flamingos.
Inter-annual variation in the number of flamingos nesting at a given site is likely in part a response to the quantity and quality of habitat that flamingos find in these wetlands (Gálvez Aguilera and Chávez-Ramírez Reference Gálvez Aguilera and Chávez-Ramírez2010; Li et al. Reference Li, Wang and Ge2013; Stirnemann et al. Reference Stirnemann, O’Halloran, Ridgway and Donnelly2012). The effects of human activities on these activity patterns are poorly understood but could negatively affect flamingos by decreasing the time they spend foraging or courting, potentially negatively affecting their fitness.
Global and regional pressures, threats, and drivers
Global climatic conditions have changed significantly over the past 50 years and models suggest future increases in temperature and changes in precipitation regimes throughout the world, which will directly affect the number, extent, seasonality, and salinity of wetlands (Guevara et al. Reference Guevara, Santander, Espinosa and Graham2021). In this scenario, many species will have to adapt or change their distributions in response to climate change (La Sorte and Jetz Reference La Sorte and Jetz2012). Even though high Andes flamingos can move between wetlands with adequate resources and conditions, the trade-offs involved when undertaking such movements, or how climate change will transform wetland characteristics and their availability remain unknown. Recent research from Chile suggests that high Andes flamingos are negatively impacted by climate change (Gutiérrez et al. Reference Gutiérrez, Moore, Donnelly, Dorador, Navedo and Senner2022), although the mechanisms involved are not identified. A recent study based on habitat suitability models predicts that climate change will lead to a decrease in the distribution of both Andean and Puna flamingos. In the short term (2021–2040), both high Andes flamingo species will lose area (area change: -2.02% to -10.78% and -8.16% to -21.6%, respectively), and these values will be higher in the medium to long term (Delfino Reference Delfino2023).
During the non-breeding season, changes in meteorological conditions may result in mortality and dispersal into highly unsuitable habitats. In spring 2017 (September–November), Andean Flamingos were recorded stopping in unsuitable areas at intermediate sites along their migratory routes (E. J. Derlindati, personal observation). Unfortunately, these individuals were very weak or incapable of flying to complete their seasonal movements. These results are likely to be the effects of climate conditions on their wintering habitat, exacerbated by human-caused encroachment into their wetlands. In November 2023, during a regional avian influenza outbreak, 237 dead individuals of Puna Flamingo were recorded in north-west Argentina (220 individuals in Catamarca Province and 17 individuals in La Rioja Province). Based on limited sampling, the mortality of at least 23 of the Catamarca flamingos was attributed to HPAI H5 virus infection (EFSA et al. Reference Adlhoch, Fusaro and Gonzales2023). This report of avian influenza on Puna Flamingo raises concern about the populations of both high Andes flamingos.
The natural heterogeneity and seasonality of their habitats cause variations in habitat availability and water quality (e.g. salinity), and thus in the density, diversity, and availability of potential food items for them (Caziani and Derlindati Reference Caziani and Derlindati2000). These variations directly affect flamingo activity patterns, especially the balance between time spent foraging and breeding (Derlindati et al. Reference Derlindati, Romano, Cruz, Barisón, Arengo and Barberis2014), which may ultimately impact their abundance, fitness, and reproductive success. Although we have robust population estimations for both high Andes flamingo species, and information on their presence and abundance at sites throughout their range since 2000, details on mechanisms and/or drivers of their population dynamics remain unknown.
Flamingo habitats in the high Andes are increasingly threatened by human activities, notably mining for lithium which has experienced exponential growth since 2016 throughout the so-called “Lithium Triangle” that encompasses high Andes wetlands in Argentina, Bolivia, and Chile (Gutiérrez et al. Reference Gutiérrez, Moore, Donnelly, Dorador, Navedo and Senner2022; Marconi et al. Reference Marconi, Arengo and Clark2022). Mining lithium impacts wetlands directly in terms of habitat loss, conversion, and degradation (Garcés and Álvarez Reference Garcés, Álvarez and Casares2020; Liu et al. Reference Liu, Agusdinata and Myint2019; Sticco Reference Sticco, Guerra, Kwaterka and Valdés2021), and sustained extraction of groundwater and surface water will likely affect hydrological balance, chemical composition, and ultimately the rich biodiversity dependent on these wetlands (Gajardo and Redón Reference Gajardo and Redón2019; Marconi et al. Reference Marconi, Arengo, Castro, Rocha, Valqui and Aguilar2020, Reference Marconi, Arengo and Clark2022; Torres et al. Reference Torres, Marconi, Castro, Moschione, Bruno and Michelutti2019), especially in arid ecosystems where the water balance is negative, and uncertainties in the fundamental hydrology remain (Moran et al. Reference Moran, Boutt, Munk and Fisher2024). Lithium mining is occurring in the areas with the highest concentration of high Andes flamingos, including protected areas and breeding sites (Gajardo and Redón Reference Gajardo and Redón2019; Liu et al. Reference Liu, Agusdinata and Myint2019; Marconi et al. Reference Marconi, Arengo, Castro, Rocha, Valqui and Aguilar2020, Reference Marconi, Arengo and Clark2022), Mining permits have been granted without a robust understanding of hydrological cycles and the effects of extraction and changes in chemical composition on biological communities, and without consideration of cumulative and synergistic effects of multiple mining projects on water sources (Gutiérrez et al. Reference Gutiérrez, Moore, Donnelly, Dorador, Navedo and Senner2022; Marconi et al. Reference Marconi, Arengo and Clark2022; Petavratzi et al. Reference Petavratzi, Sánchez-López, Hughes, Stacey, Ford and Butcher2022).
It is important to note that when the first studies on these birds were conducted (during the 1980s and 1990s), there was little human disturbance (Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007). The current situation is very different, especially in the last two decades during which mining pressure has increased and tourism has grown with limited regulation (Marconi et al. Reference Marconi, Arengo and Clark2022) (Figure 1). On the other hand, several of the lowland wetlands used by high Andes flamingos have been affected by channelling and drainage for agriculture, resulting in a drastic reduction in landscape heterogeneity, and therefore, in biodiversity (Brandolin et al. Reference Brandolin, Ávalos and de Angelo2013; Romano et al. Reference Romano, Barberis, Guerra, Piovano and Minotti2014, Reference Romano, Barberis, Pagano, Minotti and Arengo2017) (Figure 1). A hydrological management plan that includes wetland restoration and conservation should be developed and implemented (Canevari et al. Reference Canevari, Blanco, Bucher, Castro and Davidson1999) and must consider the long-term cycles that dictate the local hydrological dynamics (Romano et al. Reference Romano, Barberis, Guerra, Piovano and Minotti2014). Strong El Niño Southern Oscillation conditions regularly affect the central plains of Argentina, and therefore large areas of lowland wetlands experience cycles of flooding and drought. Variation in rainfall directly affects flamingos, as changes in water levels modify salinity levels and the availability of nesting and fledgling habitats (Romano et al. Reference Romano, Barberis, Pagano, Minotti and Arengo2017). Human actions can modify the hydrology of wetlands and therefore affect flamingo abundance. For example, in the 1990s and 2000s, the abundance of Andean Flamingos in Laguna Melincué (in the central plains of Argentina) increased during the dry cycles of the El Niño Southern Oscillation due to a reduction in lake water area and an increase in water salinity. Water pumping in the 2010s also reduced the lake water area, but as salt water was pumped out and the lake was then replenished by rainfall, water salinity dropped markedly leading to a strong reduction in the abundance of Andean Flamingos (Romano et al. Reference Romano, Barberis, Pagano, Minotti and Arengo2017).
Global and regional conservation strategies
The main successes in the conservation of the high Andes flamingos in South America are synthesised in two strategies proposed by regional experts in high Andes flamingos (Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007; Marconi et al. Reference Marconi, Arengo, Castro, Rocha, Valqui and Aguilar2020; Rodríguez Reference Rodríguez2006). The first strategy is based on the evaluation and estimation of the size of populations and their geographical distributions. Despite this robust information, more detailed research is needed to identify specific actions that are necessary to effectively conserve these species, update conservation criteria, and update the conservation status of specific populations. As part of this, the status of population connectivity still needs to be described (Cohen et al. Reference Cohen, Hostetler, Hallworth, Rushing, Sillett and Marra2018; Knight et al. Reference Knight, Harrison, Scarpignato, Van Wilgenburg, Bayne and Ng2021).
The other strategy is through the Network of Wetlands for Flamingo Conservation, a network of priority sites of importance because of flamingo numbers, presence of breeding sites, biodiversity value, conservation status, and functional connectivity with other sites (Marconi and Sureda Reference Marconi and Sureda2008; Marconi et al. Reference Marconi, Sureda, Rocha Olivio, Rodríguez Ramírez, Derlindati and Romano2007). Since their identification, these sites have been focal areas for the implementation of management and action plans, including the establishment of new Ramsar Sites and subnational reserves (Marconi et al. Reference Marconi, Arengo and Clark2022), but additional steps that result in a commitment by different stakeholders, particularly governments, to undertake conservation actions throughout the network are necessary.
Knowledge gaps and recommendations for further actions
There is an urgent need for further research and action that can serve to create effective and adaptive conservation planning for these unique bird species. At the population level, these include continuous monitoring of flamingo occurrence and abundance throughout their range, combined with a collection of detailed movement data to estimate season- and age-specific survival probabilities (Rushing et al. Reference Rushing, Hostetler, Sillett, Marra, Rotenberg and Ryder2017). Most of the census effort has been focused on the breeding season distribution of flamingos. However, results from winter regional censuses (1998 and 2000) and localised counts (Romano et al. Reference Romano, Barberis, Pagano and Maidagan2005, Reference Romano, Barberis, Pagano and Romig2006, Reference Romano, Barberis, Pagano, Marconi and Arengo2008, Reference Romano, Barberis, Derlindati, Pagano, Marconi and Arengo2009) show that winter monitoring yields essential information on distribution and habitat use for both flamingo species, but especially Andean Flamingos (Marconi et al. Reference Marconi, Sureda, Arengo, Aguilar, Amado and Alza2011). Given the rapid changes in the environment since the last comprehensive range-wide winter census in 2000, periodic winter censuses at key sites would contribute valuable information on the response of flamingo populations. Understanding the spatial and temporal drivers of mortality is a basic requirement for effective conservation planning because mortality may be driven by mechanisms operating within a given season (e.g. nest predation) or between seasons (e.g. winter habitat quality affecting reproductive success in summer; Hostetler et al. Reference Hostetler, Sillett and Marra2015; Rushing et al. Reference Rushing, Hostetler, Sillett, Marra, Rotenberg and Ryder2017). Quantifying these demographic links among seasons requires a full-annual cycle approach that relies on tracking data with high spatial and temporal resolution (Marra et al. Reference Marra, Cohen, Loss, Rutter and Tonra2015), allowing the quantification of seasonal population connectivity (Knight et al. Reference Knight, Harrison, Scarpignato, Van Wilgenburg, Bayne and Ng2021). Remote sensing tools and specialised software programs could be used to understand these major habitat requirements, including responses to climate and human land use (De los Ríos-Escalante et al. Reference De los Rios-Escalante, Esse, Correa-Araneda, Rodríguez, Fernández, Prado, Singh, Jamal and Ahmad2024).
Population genetics research would also help to determine the extent to which different populations are genetically distinct, whether gene flow is sufficient to avoid inbreeding, and whether the genetic variation is sufficient to overcome current and future rapid environmental change (Bay et al. Reference Bay, Harrigan, Underwood, Gibbs, Smith and Ruegg2018). Results from a recent study show that individual Andean Flamingos move between lakes located hundreds of kilometres apart, sometimes visiting all three countries in the central Andes (Argentina, Bolivia, and Chile) in a matter of a few months (Jahn et al. Reference Jahn, Cereghetti, Hallworth, Ketterson, Ryder and Marra2023). Thus, international research collaborations in these topics will be vital to developing meaningful results.
At the individual level, it is necessary to measure behavioural and physiological responses to change, as well as activity patterns at different sites throughout the year to anticipate future challenges to their survival and proactively implement conservation plans. This could also include research on various aspects of their ecology (e.g. diet) and physiology (e.g. immune function), exposure to toxins (Rocha et al. Reference Rocha, Pacheco, Ayala, Varela and Arengo2021), as well as detailed studies of the relationship between their body condition, climate, wetland hydrology, and food availability.
Combining such individual- and population-level data will ultimately allow an assessment of the drivers of flamingo population dynamics. Linking demographic, climate, and movement data within an integrated population model can be used to inform a regional index of population size and recruitment rates in driving overall population dynamics (Saracco et al. Reference Saracco, Cormier, Humple, Stock, Taylor and Siegel2022).
After almost two decades of research, important gaps remain. More research is needed to understand their ecology and the effects of rapidly changing environmental conditions and resource availabilities on their fitness and population dynamics. It will be important to quantify the environmental characteristics that determine the choice of nesting colony location, the drivers of breeding success, the behavioural responses to regional climatic patterns, the functional role these birds play within their habitats, and the ages of the individuals in the new breeding colonies. Thus, further research is needed on the foraging ecology and reproductive physiology of high Andes flamingos, as well as detailed studies on wetland hydrology and food availability.
Both high Andes flamingo species generally have limited distributions and specific habitat requirements, making them extremely vulnerable to climate change and other regional-scale environmental stressors (Caziani et al. Reference Caziani, Rocha Olivio, Rodríguez Ramírez, Romano, Derlindati and Tálamo2007; Gutiérrez et al. Reference Gutiérrez, Moore, Donnelly, Dorador, Navedo and Senner2022). One of the main threats is water overexploitation from these systems (e.g. Gutiérrez et al. Reference Gutiérrez, Moore, Donnelly, Dorador, Navedo and Senner2022); therefore, a commitment from mining companies and better oversight of mining activities by public agencies are necessary to conserve these wetlands. Specifically, it is key to adjust wetland use and management plans to address flamingo conservation, accompanied by up-to-date and adequate legislation that protects environmental services and biodiversity. Only through a combination of interdisciplinary research, monitoring, and effective conservation planning that extends across political boundaries, with input from various stakeholders, including local communities, will the future of high Andes flamingo populations be safeguarded.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0959270924000273.
Acknowledgements
We thank the following people, who were indispensable in the fieldwork: Hugo Asencio, Andrés Elias, Sebastián Martín, Marcelo Cuevas Amorelli, Marcelo Gallego, Lucila Castro, Laura Álvarez Borla, Patricia Marconi, Ricardo Clark, Mario Mosqueira, Ana Laura Sureda, Rodrigo Valdez, Lucas Aros, Francisco Estive, Jerónimo Sosa, Pedro Barrera, and Cirilo Urriche. We thank the staff from Secretaría de Ambiente Gobierno de La Rioja, Salta, and Jujuy and park rangers from Laguna Brava (la Rioja), Los Andes (Salta), and Las Chinchillas (Jujuy) Reserves. Funding was provided by Consejo de Investigación de la Universidad Nacional de Salta (Grants # 2.229/0 and 1151) and Universidad Nacional de Rosario (Grant # 80020190300097UR). Author contributions: EJD and IMB conceived the idea and design. EJD, FA, HSF, and IMB wrote the paper, and AEJ, EJD, FA, HSF, IMB, MM, MMC, MCR, EO, and OR edited the paper.





