Aggressive behaviour is associated with epilepsy,Footnote a but there is evidence that its prevalence is no higher than in the general population (Reference van Elst, Woermann and Lemieuxvan Elst 2000). Organic brain disease, low socioeconomic status and poor upbringing have all been identified as risk factors for aggression in epilepsy (Reference Hermann and WhitmanHermann 1984). Surgery for intractable epilepsy, such as hippocampectomy or temporal lobectomy, also confers a higher vulnerability to aggression (Reference EngelEngel 1993).
BOX 1 Some definitions
-
Epilepsy: A tendency to unprovoked and recurrent seizures.
-
Seizure/ictus: An abnormal paroxysmal electrical discharge of cerebral neurons.
-
Aura: Simple partial seizures, which may or may not progress into another seizure type.
-
Fit: The physical manifestation of a seizure.
-
Generalised seizures: Epileptic seizures that manifest immediately and spread bilaterally through the cerebral cortex. They may present with or without tonic–clonic convulsions and aura in secondary generalised seizures, or without aura in primary generalised seizures.
-
Partial or focal seizures: Epileptic seizures that start in a specific area of the brain and then spread. A partial seizure may become secondarily generalised if it spreads to both hemispheres.
-
Complex partial seizures: Epileptic seizures that include small areas in the temporal/frontal lobe; they often include automatism (e.g. lip-smacking, picking at clothes, word repeating) and loss of awareness of surroundings. They usually last for 1–2 minutes.
-
Post-ictal phase: A transient period of central nervous system abnormality, which becomes apparent after the ictal phase.
-
Kindling: The tendency of some regions of the brain to react to repeated low-level bioelectrical stimulation by progressively boosting synaptic discharges, thereby lowering seizure thresholds.
-
Sensory–limbic hyperconnection: A neural process in which incoming stimuli are accorded undue emotional significance, resulting in characteristic changes of emotions and behaviour.
A multicentre study in three regions of Italy concluded that aggressive behaviours in people with epilepsy were different from those in the normal population, a feature that we return to later in this article. The presence of compromised intellectual functioning, psychiatric disturbances and disability, as well as the number of medications taken, age, illness duration, education and regional distribution all significantly affected aggressiveness (Reference Piazzini, Bravi and EdefontiPiazzini 2012).
An earlier review (Reference TreimanTreiman 1986) suggested a two to four times greater prevalence of epilepsy in prisoners than in controls, although it maintained that the prevalence was similar to that in the socioeconomic populations from which the prisoners came. The review refutes the belief that violence is more common in epilepsy, finding no greater prevalence in people with epilepsy than in those without or in patients with temporal lobe epilepsy compared with other types of epilepsy.
A retrospective study at a residential epilepsy centre found the prevalence of aggression to be 27.2% over 1 year, compared with age- and gender-matched controls. The overall frequency was estimated at between 121 and 207 incidents per 100 persons per year. Aggressive residents were younger than non-aggressive residents. Gender, age at onset of epilepsy, history of psychosis, degree of mobility, abnormality on magnetic resonance imaging (MRI) scan, intellectual disability and seizure frequency were not related to aggressive conduct. For a very small number of aggressive incidents (0.7%), acute psychosis was noted as the causal factor, and these were more likely to result in significant injury (Reference Bogdanovic, Mead and DuncanBogdanovic 2000). Although this study gives evidence of a higher incidence of aggression in people with epilepsy, it also gives evidence of poor association between epilepsy and person-directed/instrumental aggression or possible consequences of damage.
Temporality and causality
The chronological relationship between seizures and violent behaviours is significant. Violence early in a seizure is very rare and never supported by consecutive series of purposeful movements (Reference TreimanTreiman 1986). A study of violence and ictal psychotic episodes (Reference Kanemoto, Tadokoro and OshimaKanemoto 2010) showed that directed (targeted) violent attacks were most common during post-ictal psychotic episodes (roughly 23%). They were much rarer during inter-ictal psychotic episodes (about 5%) and post-ictal periods of confusion (fewer than 1%). Individuals were more prone to both violence and suicide attempts during post-ictal psychotic episodes. Purposeful, organised violence as a direct manifestation of seizures or ictal automatism was highly unusual. It should be noted that this study, to which we will return later, did not take into account factors such as the effect of anti-epileptic medication on behaviour.
Confusion during and after fits is the usual cause of aggression, as the patient unconsciously resists restraint (Reference TreimanTreiman 1986; Reference Kanemoto, Tadokoro and OshimaKanemoto 2010). Post-ictal psychotic episodes can pose a high risk of injury, especially if violence has been reported in previous episodes.
Aetiology and pathogenesis
Aggression can be fear-induced, territorial or a learned behaviour reinforced by experience. Its purpose can be to achieve gain (instrumental) or prevent loss (fear-induced). Aggressive behaviour can also result from increasing anger in response to a perceived threat or frustration. It can be driven by a disrupted perception of the environment or an internal sense of threat. Cortical neural disturbances can affect perceptual judgement, causing subsequent behavioural changes.
The limbic system is associated with the mediation of basic drives and the expression of emotions (including rage and fear) and behaviours. It is also involved in the processing of information and memory. The frontal lobes organise purposeful behaviour and abstract and executive functions. Changes in the limbic system can increase or decrease the potential for aggressive behaviour. Disturbances in the frontal lobes can result in impaired judgement, personality changes, problems in decision-making, inappropriate conduct and aggressive outbursts. Normal levels of serotonin, dopamine, adrenaline, acetylcholine and gamma-aminobutyric acid (GABA) in the frontal lobes are believed to be essential for behavioural control. Animal studies indicate that raised dopamine and noradrenaline activity can significantly increase the likelihood that the animal will respond to the environment in an impulsively violent manner (Reference MoyerMoyer 1968).
Neurophysiological disturbances
Repeated focal or generalised ictal and inter-ictal epileptiform activity results in an abnormal recurrent synaptic bombardment of distant projection areas. This may induce neuroplastic changes and sensory–limbic hyperconnection, impairing the naturally occurring homoeostasis that maintains the inter-ictal state and thus disturbing normal neuronal and psychological functions (Reference Engel, Shewmon and MorrellEngel 1991, Reference Engel, Wilson, Lopez-Rodriguez, Trimble and Schmitz2002; Reference HamedHamed 2007).
The role of the amygdala and associated limbic structures
The most important brain structures implicated in the mediation of aggressive behaviour are the amygdala and associated limbic structures, the frontal lobes, periaqueductal grey and hypothalamus. The amygdala is involved in normal and abnormal emotional behaviours and serves an important role in anxiety, emotional memory and impulse control (Reference KeeleKeele 2005). It is thought to play a crucial role in the mediation of fear-induced aggression (Reference Eron, Gruder, Reiss, Peterson and EronEron 1977). The hippocampus and surrounding limbic system have also been implicated in seizure-induced behavioural and cognitive disorders and aggression (Reference EngelEngel 1993, Reference Engel, Wilson, Lopez-Rodriguez, Trimble and Schmitz2002; Reference DrevetsDrevets 1998; Reference SteriadeSteriade 2000). Other brain areas involved in generating epileptic discharges and behavioural symptoms include the temporal lobes, orbitofrontal regions, anterior insula, anterior cingulate gyrus and basal ganglia (Reference HelmstaedterHelmstaedter 2001; Reference van Elst, Groffmann and Ebertvan Elst 2009; Reference Broicher, Kuchukhidze and GrunwaldBroicher 2010).
Neuronal hyperexcitability and complex partial seizures
The literature is consistent with the explanation that epilepsy-like neuronal hyperexcitability, particularly in the amygdala, is a biological substrate of disturbed emotional behaviour (Reference KeeleKeele 2005). The hippocampus and the amygdala are common foci from which complex partial seizures (CPS) originate. The emotional behaviour system becomes supersensitised, resulting in a state of neuronal hyperexcitability that produces emotional disturbances and change in behaviour. This state facilitates an adaptive, rapid, subcortical processing of the emotional salience of objects and events in the immediate environment and, in the extreme, the overactivity produces the seizures of CPS (Reference KeeleKeele 2005).
Neuroplastic changes
Mechanisms of synaptic plasticity, including long-term potentiation (Reference Gean, Chang and HuangGean 1993; Reference Huang, Hsu and GeanHuang 1996) and long-term depression (Reference Wang and GeanWang 1999) have been shown to occur at specific synapses in the amygdala following epilepsy. Plastic changes in neurotransmission also occur following epilepsy (Reference Asprodini, Rainnie and Shinnick-GallagherAsprodini 1992; Reference Shinnick-Gallagher, Keele, Neugebauer, Corcoran and MosheShinnick-Gallagher 1998) and following fear-conditioning (Reference McKernan and Shinnick-GallagherMcKernan 1997; Reference Rogan, Staubli and LeDouxRogan 1997).
Neurochemical transmission
Recurrent epileptiform discharges, kindling and related neurobiological abnormalities will result in prolonged aura, hypometabolism of other brain areas, disrupted neuronal plasticity, intractable epilepsy, cognitive deficits and behavioural phenomena (Reference DrevetsDrevets 1998; Reference Engel, Wilson, Lopez-Rodriguez, Trimble and SchmitzEngel 2002; Reference HamedHamed 2007; Reference Mula, Marotta and MonacoMula 2010). Post-ictal psychiatric symptoms are probably a direct manifestation of epileptic discharges in the brain, although they might be a consequence of the inhibitory mechanisms involved in the termination of seizures (Reference Kanner, Stagno and KotagalKanner 1996, Reference Kanner2004; Reference So, Savard and AndermannSo 1990). At subseizure levels, cellular and molecular mechanisms may underlie the abnormal emotional processes that contribute to emotional dysfunction, aggression and lack of impulse control (Reference KeeleKeele 2005). Afferent input to the amygdala activates both excitatory and inhibitory amino acid receptors (Reference Rainnie, Asprodini and Shinnick-GallagherRainnie 1991a,Reference Rainnie, Asprodini and Shinnick-Gallagherb; Reference McKernan and Shinnick-GallagherMcKernan 1997; Reference Mahanty and SahMahanty 1999), and alteration of excitatory and inhibitory transmission may change the functional output of the amygdala. Glutamate and GABA receptors are altered after fully induced (stage 5) seizures (Reference RacineRacine 1972). Neurons in the basolateral amygdala become hyperexcited following kindling (Reference KeeleKeele 2005). Neurobiological processes that depend on the amygdala occur as a result of such changes in the excitability of neurons within the amygdala.
Psychiatric comorbidities and aggression
Epilepsy is commonly associated with psychiatric disorders, among which depression is the most common, with a prevalence of 20–55% and as high as 80% in some studies (Reference Ettinger, Reed and CramerEttinger 2004; Reference Mensah, Beavis and ThaparMensah 2006). Aggression may be a consequence of a pre-existing psychiatric disorder or a psychiatric disorder that developed subsequent to epilepsy (Reference KannerKanner 2004). Depression and anxiety commonly lead to increased irritability, which can result in verbal or physical aggression (Reference KannerKanner 2004; Reference RiggioRiggio 2010).
The correlation between depression and aggression in epilepsy has been studied using multiple psychometrics in patients with epilepsy and depression compared with patients with idiopathic depression (Reference Beyenburg, Mitchell and SchmidtBeyenburg 2005). Buss–Perry Aggression Questionnaire (BAQ) and Beck Depression Inventory II (BDI-II) scores were closely related in the patients with epilepsy and depression, but were not correlated in the patients with idiopathic depression. This suggests that depression in epilepsy is more likely to lead to aggression than is idiopathic depression. There is some emerging evidence suggesting that depression in epilepsy may modulate drug-induced aggression particularly with levetiracetam (Reference Brodie, Besag and EttingerBrodie 2016).
Aggression in epilepsy can be due to comorbid antisocial personality disorder. High risk of aggressive behaviour in epilepsy has been found to be frequently related to left temporal lobe epilepsy, intractable epilepsy, low IQ (in particular, verbal IQ), early onset of seizures, primary epileptic focus located in the dominant hemisphere and antisocial personality disorder (Reference KannerKanner 2004).
Epilepsy and aggression often present together as a consequence of other neurological or psychiatric conditions, such as traumatic brain injury, vascular disorders, limbic encephalitis, alcohol and drug dependence or intellectual disability. In such situations, epilepsy may not be causally linked to aggression and may be merely an incidental comorbidity (Reference Beyenburg, Mitchell and SchmidtBeyenburg 2005).
Psychotropics and epilepsy
Most antidepressants and antipsychotics are associated with hyponatraemia, which in severe cases can cause seizures (Reference MaramattomMaramattom 2006). However, if hyponatraemia is controlled, some antidepressants (e.g. amitriptyline, dosulepin, clomipramine and bupropion) and antipsychotics (e.g. chlorpromazine) can directly reduce seizure threshold (Reference Pisani, Oteri and CostaPisani 2002).
Worsening epilepsy can complicate existing psychosis and can yield to behavioural changes. Selective serotonin reuptake inhibitors (SSRIs) are generally considered safe in epilepsy (Reference Wedin, Oderda and Klein-SchwartzWedin 1986), as are haloperidol (Reference Marks and LuchinsMarks 1991) and sulpiride (Reference Gazdag, Barna and IványiGazdag 2004).
Anti-epileptic drugs
Table 1 summarises typically reported side-effects of various anti-epileptics. Some anti-epileptics increase the risk of comorbid psychiatric diagnosis, which in turn increases the risk of aggressive behaviour. Case reports give evidence for new-onset psychotic symptoms in patients with epilepsy who were treated with carbamazepine, ethosuximide, gabapentin, lamotrigine (Reference Brandt, Fueratsch and BoehmeBrandt 2007) and levetiracetam (Reference Youroukos, Lazopoulou and MichelakouYouroukos 2003).
TABLE 1 Side-effects of various anti-epileptic drugs
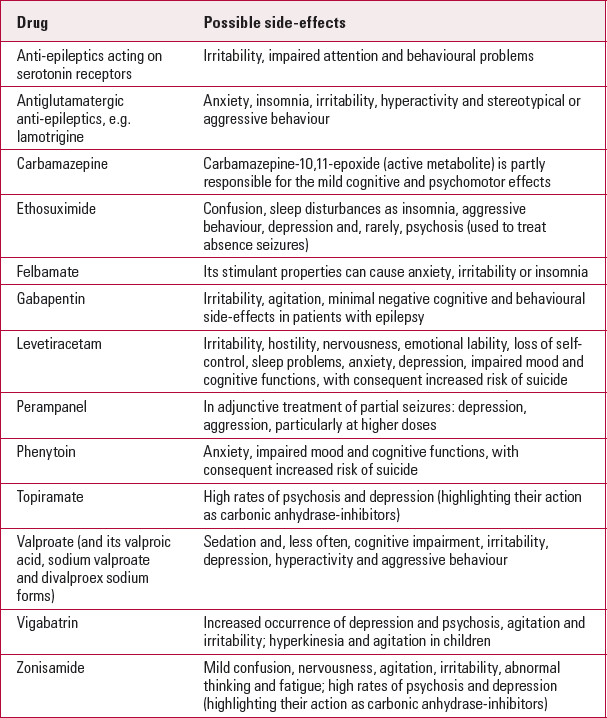
Some anti-epileptics have demonstrated a therapeutic effect on mood disorders in people with epilepsy and in the general population, although levetiracetam, tiagabine, topiramate and vigabatrin are reported to cause new-onset depressive symptoms (Reference Andersohn, Schade and WillichAndersohn 2010).
Some studies reported negative behavioural changes in patients whose epilepsy was treated with anti-epileptics (Reference Ketter, Post and TheodoreKetter 1999; Reference Nadkarni and DevinskyNadkarni 2005). These negative effects result from the direct action of the drugs in altering ion channels and neurotransmitter system functions and modulating electrochemical systems (Reference Kuzniecky, Ho and PanKuzniecky 2002; Reference Nadkarni and DevinskyNadkarni 2005; Reference Koneski and CasellaKoneski 2010). The anti-epileptics were reported to induce behavioural disturbance indirectly in association with successful seizure control. This is clinically known as ‘forced normalisation’.
Many anti-epileptics exert their effect via potentiation of GABA-inhibitory transmission, which can also affect behaviour. Some studies report phenobarbital to have the greatest behavioural toxic potential, with dose-related impairment in attention and reaction time, performance IQ and short-term memory, and induction of a hyperkinetic syndrome in children (Reference Yanai, Fares and GavishYanai 1989). Cross-titration to a non-barbiturate anti-epileptic in patients with depressive, irritable or aggressive symptoms has been successful in alleviating the symptoms with no need for additional psychotropics (Reference HamedHamed 2011).
Levetiracetam exerts a dose-independent stimulating effect that can be positive or negative. Aggression is a prominent feature. Lack of efficacy, intellectual disability, and presumably also pre-intake disposition (organic psychosyndrome, impulsivity) may be helpful in predicting whether additional activation under levetiracetam will be positive or negative (Reference Helmstaedter, Fritz and KockelmannHelmstaedter 2008).
However, more recently, aggression associated with levetiracetam in epilepsy has been shown to correlate to clinical and subclinical depression (Reference Mula and SchmitzMula 2009; Reference Korczyn, Schachter and BrodieKorczyn 2013).
There is evidence of worsening of aggressive behaviours with levetiracetam in children (Reference de la Loge, Hunter and Schiemannde la Lodge 2010), in people with post-stroke seizures (Reference Belcastro, Costa and GallettiBelcastro 2008) and in people with refractory generalised and focal epilepsy (Reference Opp, Tuxhorn and MayOpp 2005; Reference Schiemann-Delgado, Yang and de la LogeSchiemann-Delgado 2012).
Some studies showed variable or even contradictory results. Phenytoin was reported to cause a dose-related decline in concentration, memory, visuomotor function and mental speed, and increased anxiety, aggression, fatigue and depression in people with epilepsy (Reference Cavanna, Ali and RickardsCavanna 2010). In contrast, a prison inmate study involving groups who had committed either impulsive or premeditated aggressive acts suggested that the frequency of impulsive aggressive behaviour was significantly diminished compared with the placebo control condition (Reference Stanford, Houston and MathiasStanford 2001). The study team concluded that the anti-aggressive effect of phenytoin was selective for impulsive aggression, as it was without effect on aggression in the premeditated aggression group. Similar results had been reported in earlier studies, in which phenytoin, carbamazepine and divalproex sodium were efficacious in reducing impulsive aggressive acts in people with personality disorder (Reference Gardner and CowdryGardner 1986; Reference Kavoussi and CoccaroKavoussi 1998). The selective properties of phenytoin on impulsive aggression suggest that biological mechanisms of excitability distinguish impulsive and premeditated aggression (Reference KeeleKeele 2005).
Summary
Available evidence suggests that a wide range of anti-epileptics can be associated with agitation and aggression. Some of these, such a levetiracetam, are more consistently associated with agitation and aggression in a minority of patients, whereas others are associated more rarely or more inconsistently with aggression. Often, the temporal correlation of aggression with the initiation or dose escalation of an anti-epileptic can be a good clue to causation.
Clinical presentation of aggression
Seizure-related behavioural changes, including aggressive behaviour, have been interpreted as the emergence of innate motor patterns (Reference Bronsard and BartolomeiBronsard 2013). Aggression in epilepsy can be classified in relation to its temporality with the fits, as pre-ictal, ictal or post-ictal. It can also manifest as an episodic event independent of observable seizures, making it inter-ictal. It is to be noted that aggression not associated with seizures can occur secondary to anti-epileptics, psychiatric comorbidities or underlying neurological conditions (Reference KannerKanner 2004).
Pre-ictal
A third of patients with epileptic seizures – especially secondary generalised seizures – develop pre-ictal prodromal symptoms hours to days before the seizures. These can include mood changes or depression, irritability, restlessness or motor hyperactivity and poor frustration tolerance, any of which can lead to aggressive or emotional outbursts. These symptoms are often relieved by the occurrence of the seizure, although they might persist for few days afterwards. It has been suggested that these prodromal symptoms represent biophysiological processes involved in initiation of abnormal emotions and seizures or subclinical seizure activity (Reference Blanchet and FrommerBlanchet 1986).
Ictal
Ictal behavioural symptoms occur in nearly 25% of auras (especially when long-lasting), and also following a cluster of complex partial seizures and secondary generalised seizures in temporal lobe epilepsy or non-convulsive status epilepticus (Reference Manchanda, Freeland and SchaeferManchanda 2000). Symptoms can include: depression (which ranges from mild sadness to profound hopelessness, despair, anhedonia, guilt and suicidal ideation) (Reference Blanchet and FrommerBlanchet 1986); fear, distress, nervousness, anger, irritability, panic attacks and phobias (Reference Alemayehu, Bergey and BarryAlemayehu 1995; Reference Biraben, Taussig and ThomasBiraben 2001); forced thinking (recurrent intrusive thoughts and ideas, crowding of thoughts), obsession (Reference Bogdanovic, Mead and DuncanBogdanovic 2000; Reference Piazzini, Bravi and EdefontiPiazzini 2012); and aggression (Reference Kanemoto, Kawasaki and MoriKanemoto 1999).
Ictal aggression is unorganised and accidental. Aggression directed towards people almost invariably takes the form of resistive aggression in reaction to physical restraint applied to control the patient during ictal confusion (Reference TreimanTreiman 1999). Ictal behavioural symptoms tend to be stereotyped, paroxysmal, of brief duration and unprovoked by environmental stimuli. They are sometimes associated with other characteristics of temporal discharges, such as bizarre behaviour, mutism, amnesia, depersonalisation and déjà vu, as well as automatism and altered consciousness (Reference KannerKanner 2004).
Post-ictal
Post-ictal behavioural symptoms occur after seizures, particularly with temporal lobe epilepsy, and last hours to days. The symptoms include anxiety, dysphoria, depression, psychosis, suicidal ideas and transient aggression (Reference So, Savard and AndermannSo 1990; Reference Kanner, Stagno and KotagalKanner 1996, Reference Kanner, Trimble and Schmitz2010). Aggression is frequently reported in post-ictal confusional states or post-ictal psychosis.
Post-ictal psychosis can occur between 12 hours and 7 days following the seizure. Symptoms last from a few hours to a few days or, rarely, for up to 4 weeks. Post-ictal psychosis may be associated with irritability and aggression, along with affective and psychotic symptoms (Reference So, Savard and AndermannSo 1990).
Inter-ictal
Inter-ictal behavioural symptoms are often mild and reverse spontaneously (Reference HamedHamed 2011). Complex partial seizures of temporal lobe origin may be accompanied by inter-ictal emotional disturbances (Reference Devinsky, Devinsky and TheodoreDevinsky 1991) such as sadness or dysphoria, anxiety, irritability and aggression (Reference Bear and FedioBear 1977).
The reported prevalence of inter-ictal aggressive behaviour in people with epilepsy ranges from 4.8 to 50%. One study found it to be more common than in the general population, even after controlling for socioeconomic status, family circumstances and ethnic social vulnerabilities (Reference Kanemoto, Tadokoro and OshimaKanemoto 2010). However, the study did not take into account subsyndromic epilepsy, so its findings have poor generalisability.
Personality changes and aggression
A range of personality and behavioural changes have been reported in association with epilepsy. These include increased aggressive behaviour, schizophreniform psychosis, impulse control disorder (including aggression), dissociative amnesia, fugue and psychogenic non-epileptic seizures (Reference HamedHamed 2011). In addition, features of dementia and ‘out-of-body’ experiences have been observed in severe epilepsy, and altered convictions and emotional responses can be a feature of mild epilepsy (Reference JarzebskaJarzebska 2006). Clinical experience suggests that patients with chronic epilepsy may have changes to personality, with traits of viscosity, hyperreligiosity and overvalued ideas. They often focus persistently on philosophical or political issues, but are generally not particularly prone to aggression.
Patients with certain neurological conditions, such as acquired brain injury, may present simultaneously with personality change and epilepsy, but the epilepsy does not generally cause aggression, apart from causing occasional confusion that can lead to agitation.
Investigations and diagnosis
Obtaining a detailed and complete neuropsychiatric history, including history of pre-existing aggressive behaviour or other behavioural disturbances, is crucial. It is also important to obtain confirmation of the diagnosis and type of epilepsy from a neurologist. Reference Kanemoto, Tadokoro and OshimaKanemoto et al (2010) recommend that violent behaviour in epilepsy should be managed in accordance with its pathophysiological causes.
Attempts should be made to identify any comorbid psychopathology, including depression, anxiety, psychosis and personality difficulties. Risk assessment and investigation of factors conferring vulnerability to aggression in epilepsy should be investigated. This should include neuropsychiatric comorbidities, history of head trauma, brain surgery or cerebrovascular event. Early recognition and treatment can help patients and carers to cope better and, in turn, have a better quality of life (Reference Mrabet, Mrabet and ZouariMrabet 2004).
A close examination of the clinical picture and behavioural charts is necessary. This should include a clear description of the patient's most recent fit, the nature of their typical fits and documentation of their thoughts and intentions/premeditation and whether there is an apparent external motive for the behaviour in question. These can provide a good insight into possible causation, temporality and the nature of behavioural changes, leading to early and targeted management.
Neuroimaging can help to detect underlying brain abnormalities and comorbid lesions, which helps determine the patient's vulnerability to developing behavioural changes.
Individuals with aggressive behaviour show an increase of slow-wave brain activity on electroencephalogram (EEG) (Reference Bronsard and BartolomeiBronsard 2013). Brain-imaging studies have reported that patients with temporal lobe epilepsy and severe inter-ictal aggression (described as intermittent explosive disorder) had a significantly higher incidence of encephalitic brain disease and left-handedness, more bilateral EEG abnormality, and less frequent hippocampal sclerosis than controls. A subgroup of the aggressive patients showed severe amygdala atrophy (Reference van Elst, Woermann and Lemieuxvan Elst 2000). Analysis using voxel-based morphometry showed a reduction of grey matter density over large areas of the left extra-temporal neocortex, maximal in the left frontal neocortex, was observed (Reference Wieser, Hailemariam and RegardWieser 1985).
Aggression associated with epilepsy can be discriminated from a non-ictally related aggression by its directionality. Ictal aggression is not planned or focused, although it can occur if post-ictal delirium is forcibly contained (Reference van Elst, Woermann and Lemieuxvan Elst 2000; Reference Engel, Wilson, Lopez-Rodriguez, Trimble and SchmitzEngel 2002). We suggest the following criteria for diagnosing violent behaviour specific to epilepsy:
-
• the diagnosis of epilepsy should be established by a neurologist with special competence in epilepsy
-
• the case history and video EEG telemetry should confirm the presence of epileptic automatisms and/or aggressive acts
-
• the aggressive act should be characteristic of the patient's habitual seizures
-
• the neurologist should be of the clinical opinion that the aggressive act was part of a seizure.
Box 2 summarises the key elements of the formulation and diagnosis.
BOX 2 Key elements of the formulation and diagnosis of aggression in epilepsy
-
• Detailed history of aggressive behaviour
-
• Detailed history of neuropsychiatric comorbidities
-
• History of the epilepsy and ascertain history
-
• Thorough risk assessment
-
• Review of anti-epileptic drugs
-
• EEG, brain scanning, video EEG telemetry
Treatment
Optimise epilepsy treatment
Adequate seizure control is important when managing any epilepsy-related aggression. If the aggression is believed to be caused by the prescribed anti-epileptic, dose reduction or discontinuing that drug and replacing it with an alternative are indicated.
Treat comorbidities
Untreated comorbid psychiatric disorders can lead to aggressive behaviour. The relationship between psychiatric disorders and epilepsy has therapeutic implications that require a comprehensive biopsychosocial approach. Routine screening in epilepsy clinics can improve the chances of detection of neuropsychiatric comorbidity (Reference Rampling, Mitchell and Von OertzenRampling 2012). Continuous re-evaluation of the management plan and the anticonvulsant treatment are important.
Epilepsy is a risk factor for depression and psychosis (Reference Alper, Devinsky and WestbrookAlper 2001). Seizure-related pre-ictal and ictal depression do not usually require any specific psychopharmacological or psychological treatment (Reference HamedHamed 2011). When treating depression comorbid with epilepsy, preference should be given to anti-epileptics known to improve mood, have antidepressant and attention-enhancing efficacy, and reduce anxiety and suicidal tendencies. Anti-epileptics with antiglutamatergic (e.g. topiramate, gabapentin) and serotonergic (e.g. carbamazepine, lamotrigine) properties may confer antidepressant, mood-stabilising effects (Reference Ketter, Post and TheodoreKetter 1999). Anti-epileptics with serotonergic properties improve mood and reduce anxiety because they exert effects similar to those of antidepressants (i.e. SSRIs). Sedating drugs (e.g. vigabatrin, tiagabine) have anxiolytic, anti-manic and sleep-promoting benefits (Reference HamedHamed 2011).
If the aggression is is related to comorbid depression then use of an SSRI is indicated. If it is linked to emotional lability or impulsivity then mood stabilisers or anti-epileptics with mood-stabilising properties, such as carbamazepine and valproate, can be helpful.
For severe, treatment-resistant or psychotic depression, treatments known to have an effect on seizure frequency might be necessary. These include antipsychotics (Reference McConnell, Duncan, McConnell and SnyderMcConnell 1998), electroconvulsive therapy (ECT) (Reference Baghai and MollerBaghai 2008) and vagus nerve stimulation (Reference Elger, Hoppe and FalkaiElger 2000).
In more severe cases of comorbid personality disorder where aggression is evident, lithium, antipsychotics, carbamazepine or valproic acid should be considered (Reference TrimbleTrimble 2013).
Actively manage aggressive behaviour
Behavioural and psychological management
Education can be effective in controlling aggressive behaviour. This can be achieved by working closely with the patient to identify the problem and devise a plan for appropriate expression of anger. The work should include helping the patient to identify anger, give themselves permission for angry feelings, practice the expression of anger, apply the expression of anger to a real situation, identify alternative ways to express anger, and confront the source of anger while keeping control.
Psychological interventions such as anger management, relaxation training and individual psychotherapy, particularly cognitive–behavioural therapy (CBT), can be effective (Reference HamedHamed 2011).
Pharmacological management
If behavioural and psychological management fail, care must be exercised when considering psychotropic medication to avoid drugs with a known significant effect on the seizure threshold. Adverse effects and drug interactions should be carefully considered (Reference Leppik, Gram and DeatonLeppik 1999).
There are no randomised controlled trials to guide pharmacological treatment of aggression in epilepsy. Generally, the approach followed would be similar to the management of aggression in other neurological settings, including traumatic brain injury. Atypical antipsychotics such as risperidone or quetiapine can be used in low doses. If these are not tolerated or are not effective, other atypicals, such as olanzapine or aripiprazole, again in low doses, can be tried.
Buspirone at low doses has been found to be useful for ameliorating aggression, and it does not appear to interact with anti-epileptics. However, in animal models, buspirone has been shown to be proconvulsant (Reference Ninan, Cole, Yonkers, Schatzberg and NemeroffNinan 1998). In addition, a case report cites a patient who sustained a seizure after an overdose of buspirone (Reference Catalano, Catalano and HanleyCatalano 1998).
Beta blockers may be useful for modulating over-stimulation and for treating aggression in people with epilepsy (Reference Yudofsky, Silver, Hales, Yudofsky and HalesYudofsky 1997). Beta blockers reduce hyperarousal, restlessness, tension and anxiety, and cause little cognitive compromise. Nadolol works peripherally, whereas propranolol is more lipophilic and exerts more effects on the central nervous system. Both medications, especially propranolol, may require titration to reach an effective dosage. Propranolol has been noted to be associated with seizure activity in overdose, but in some animal models it appears to raise the seizure threshold (Reference Raju, Gopalakrishna and VenkatadriRaju 1998). In addition, depression is a rare side-effect of propranolol (Reference Yudofsky, Silver, Hales, Yudofsky and HalesYudofsky 1997).
Benzodiazepines and barbiturates should not be used as anxiolytics in people with epilepsy because of the danger of dependence and the potential for withdrawal seizures (Reference HamedHamed 2011).
Deep brain stimulation
In a small series of eight patients given deep brain stimulation (DBS) of the posterior hypothalamus (pHyp) for the treatment of aggressive behaviour associated with epilepsy and below-average IQ, six (75%) benefitted from pHyp stimulation (Reference Franzini, Messina and CordellaFranzini 2010). However, DBS is not at present clinically indicated for the treatment of aggression in epilepsy, owing to the inadequate evidence base.
Dealing with aggressive or violent behaviour
Healthcare staff should respond in a calm, firm but respectful manner, putting space between themselves and the patient. They should avoid making physical or verbal threats and false promises and should try to build rapport with the patient. De-escalation and distraction techniques should be used. Behavioural strategies such as limit-setting, behavioural contracts and a token economy can play a useful role. Time out to a quite area can be initiated by the patient or staff, and it should end when the patient determines that he or she is able to remain calm (Reference TownsendTownsend 2005).
The safety of the patient, staff, other patients and (in psychosis) potential intended victims is most important. In settings in which there is a risk of violence, staff must take care to reduce patients’ access to moveable objects and avoid wearing jewellery and clothing that might add to the risk of injury during an assault: everyday things such as lamps, pens, necklaces, earrings, glasses and neckties can be used to cause injury (Reference BoydBoyd 2011).
In high-risk situations, trained staff can employ restraint techniques, rapid tranquillisation and seclusion if required.
Be mindful of drug interactions
Both anti-epileptic and antidepressant drugs are metabolised by the hepatic microsomal cytochrome P450 oxidases (3A4, 1A2, 2C19, 2C9 and, possibly, 2B6) (Reference Harvey and PreskornHarvey 1996) and also influence the 2D6 cytochrome. The drugs influence these enzymes by either induction or inhibition. The level of anti-epileptic that act as a substrate for the cytochrome may be decreased if an inducing antidepressant drug is taken, with a subsequent decrease in the seizure threshold. An enzyme-inhibiting antidepressant can reduce metabolism of the anti-epileptic, thus increasing its toxicity.
Serotonin syndrome is a rare disorder that can result from drug interactions between anti-epileptics with serotonergic properties and SSRIs. It is caused by excessive serotonergic stimulation. Symptoms include restlessness, myoclonus, hyperthermia and convulsions, and can result in death (Reference Dursun, Mathew and ReveleyDursun 1993).
Careful follow-up of the serum levels of both anti-epileptics and antidepressants may be required while treating comorbid depression and epilepsy. The anti-epileptics most commonly reported for interactions with antidepressants are the cytochrome-inducing drugs and the inhibitory agent valproate (Reference Grimsley, Jann and CarterGrimsley 1991; Reference Lucena, Blanco and CorralesLucena 1998). Significant serum levels of these anti-epileptics have been observed when used in combination with fluoxetine and therefore fluoxetine should not be used in combination with carbamazepine, phenytoin or phenobarbital, or, at the least, serum monitoring is required.
Slow titration of the antidepressant dose is recommended while treating comorbid depression and epilepsy, as fast titration can cause an increase in seizure frequency.
Box 3 summarises key elements in the treatment of aggression and its related causes in epilepsy.
BOX 3 Key elements in the treatment of aggression in epilepsy
-
• Early detection and accurate diagnosis
-
• Behavioural and psychological interventions
-
• Cautious consideration and optimisation of epilepsy treatment
-
• Treatment of comorbid psychiatric conditions
-
• Active management of aggressive behaviour
-
• Cautious consideration of psychotropics and their slow titration
-
• Awareness of drug interactions
-
• Consideration of treatments such as ECT, deep brain stimulation and vagus nerve stimulation in severe cases
Risk: likelihood and impact
Directed attacks on surroundings are unlikely to occur during ictal automatism, but a more organised aggressive response is possible during post-ictal confusion. Sudden serious emotional upset while the individual is clearly conscious is commonly produced by psychic aura, and could result in aggressive behaviour, especially if the amygdala is involved and the episode lasts for an extended period (Reference Hermann and ChhabriaHermann 1980; Reference Wieser, Hailemariam and RegardWieser 1985; Reference KanemotoKanemoto 1997; Reference Takeda, Inoue and TottoriTakeda 2001). Post-ictal violent acts of sudden onset are more likely to occur after a cluster of seizures and are usually related to alcohol misuse.
Kanemoto et al 's work, which we mentioned earlier, sheds light on violence in epilepsy. The team compared individuals with post-ictal confusion, post-ictal psychosis or inter-ictal psychosis matched for age and age at onset of epilepsy (Reference Kanemoto, Tadokoro and OshimaKanemoto 2010). All patients had complex partial seizures and temporal lobe foci on EEG. Incidents of violent behaviour were defined as well-documented irrational, directed attacks against other people that resulted in a severe injury (e.g. bone fracture) or a life-threatening situation (e.g. strangulation). Aggressive behaviour such as cursing and menacing gestures without physical attacks were not included. Episodes of resistive aggression were counted separately. Directed violent attacks occurred during 22.8% of the post-ictal psychotic episodes, 4.8% of the inter-ictal episodes, and 0.7% of the episodes of post-ictal confusion. Thus, compared with the two other situations, proneness to violence stood out in the post-ictal psychotic episodes. Resistive violence was observed only during episodes of post-ictal confusion (3.0%).
Targeted, highly organised violent acts have not been reported in patients with frequent, hyperkinetic seizures of frontal lobe origin. However, as already mentioned, violent acts can occur during post-ictal confusion, as an expression of unconscious, vigorous resistance to protective restraint. If physical contact with the patient can be avoided, unorganised behaviour during post-ictal confusion usually ceases in less than 30 minutes and the individual becomes settled (Reference Kanemoto, Tadokoro and OshimaKanemoto 2010).
Unpredictable post-ictal psychotic episodes targeted at anyone who happens to be nearby can occur without apparent provocation. Such episodes are biologically based and closely linked to seizures; thus, successful seizure control also controls violent acts. In contrast, in inter-ictal psychotic episodes, aggression based on frank psychotic experiences is typically not directed at anyone who is incidentally in the area, but rather at an imagined culprit suggested by an hallucinatory voice or delusional idea (Reference Kanemoto, Tadokoro and OshimaKanemoto 2010).
Intermittent explosive disorder (also known as episodic dyscontrol syndrome) has been described as a condition with associated cranial neurophysiological abnormality, although the validity of the concept has been disputed. It presents as sudden episodes of spontaneously released violence, often in the setting of minimal provocation, which tend to be short-lived. Episodes can be provoked by even small amounts of alcohol, and patients may feel remorse after the event. Generally, the condition is associated with non-specific abnormalities that are also seen in epilepsy, with evidence of minimal neurological damage, soft neurological signs and abnormal EEG studies, although there is no evidence that these episodes have the same pathophysiology as epileptic seizures (Reference MonroeMonroe 1970).
Conclusions
Aggressive behaviour in people with epilepsy is not uncommon and is often emphasised in medico-legal settings. Epilepsy itself is only rarely linked directly to aggression, and this is unlikely to be directed or planned. Causes may include epilepsy-related factors (specific subtypes such as temporal lobe epilepsy), anti-epileptics, neuropsychiatric comorbidities or other brain abnormalities. A comprehensive history, exploration of seizure symptomatology and investigations, including EEG and brain imaging, can help. Occasionally, in more difficult cases video EEG telemetry may be needed. Management depends on the underlying aetiology of the aggression. Since some anti-epileptics can themselves cause behavioural disturbance, their pros and cons should be carefully considered before prescribing. There is a role for routine screening for neuropsychiatric comorbidities and regular review of anti-epileptics. The evidence for pharmacological treatment of aggression in epilepsy continues to be very limited and anecdotal, and expert neuropsychiatric opinion should be sought in complex cases. Treatment should consist of management of possible comorbidities and existing symptomatology using pharmacological, psychological and behavioural methods.
MCQs
Select the single best option for each question stem
-
1 The anti-epleptic commonly reported to have the greatest dose-related behavioural adverse effects in children is:
-
a pregabalin
-
b phenobarbital
-
c lamotrigine
-
d sodium valproate
-
e valproic acid.
-
-
2 The reported prevalence of aggression in patients with epilepsy is nearly:
-
a 27%
-
b 37%
-
c 17%
-
d 47%
-
e 7%.
-
-
3 Alteration of the following receptors may change the functional output of the amygdala after seizures:
-
a GABA receptors
-
b glutamate receptors
-
c serotonin receptors
-
d a and b
-
e b and c.
-
-
4 The antiglutamatergic anti-epileptics include:
-
a vigabatrin
-
b phenobarbital
-
c a and b
-
d lamotrigine
-
e none of the above.
-
-
5 In patients with epilepsy and aggression, hyperarousal, restlessness and tension can be modulated by:
-
a escitalopram
-
b procyclidine
-
c oral morphine
-
d bisoprolol
-
e propranolol.
-
MCQ answers

| 1 | b | 2 | a | 3 | d | 4 | d | 5 | e |




eLetters
No eLetters have been published for this article.