LEARNING OBJECTIVES
After reading this article you will be able to:
• recognise the common coexisting sleep disorders in individuals with ASD and ADHD
• develop a working knowledge of sleep disorders that may be encountered in psychiatric practice (with particular focus on individuals with ADHD and ASD)
• demonstrate a broad understanding of how sleep disorders are investigated and treated in children, adolescents and adults with ADHD and ASD.
Fundamentals of normal sleep
Sleep is a fundamental biological function and on average we spend about one-third of our life asleep (Stores Reference Stein, Weiss and Hlavaty2009). Disruptions to a restorative sleep can affect an individual's neurocognition, mood and daytime functioning (Wajszilber Reference Veatch, Sutcliffe and Warren2018). Long-term sleep disturbance can result in broad health consequences, including coronary heart disease, stroke, inflammatory disease and increased likelihood of type 2 diabetes onset (Cappuccio Reference Calhoun, Fernandez-Mendoza and Vgontzas2010, Reference Cappuccio, D'Elia and Strazzullo2011; Irwin Reference Irwin, Olmstead and Carroll2016).
Normal sleep and wakefulness
There are two types of sleep: non-rapid eye movement (NREM) and rapid eye movement (REM) sleep. The stages of sleep are characterised by different patterns of brain activity, eye movements and muscle tone. The basic structure of sleep is referred to as ‘sleep architecture’ and can be represented using a hypnogram (Fig. 1). Typical overnight sleep usually begins with NREM sleep and then follows a cyclical pattern alternating between NREM and REM sleep phases, with brief wakings every 90 min until the person wakes up.
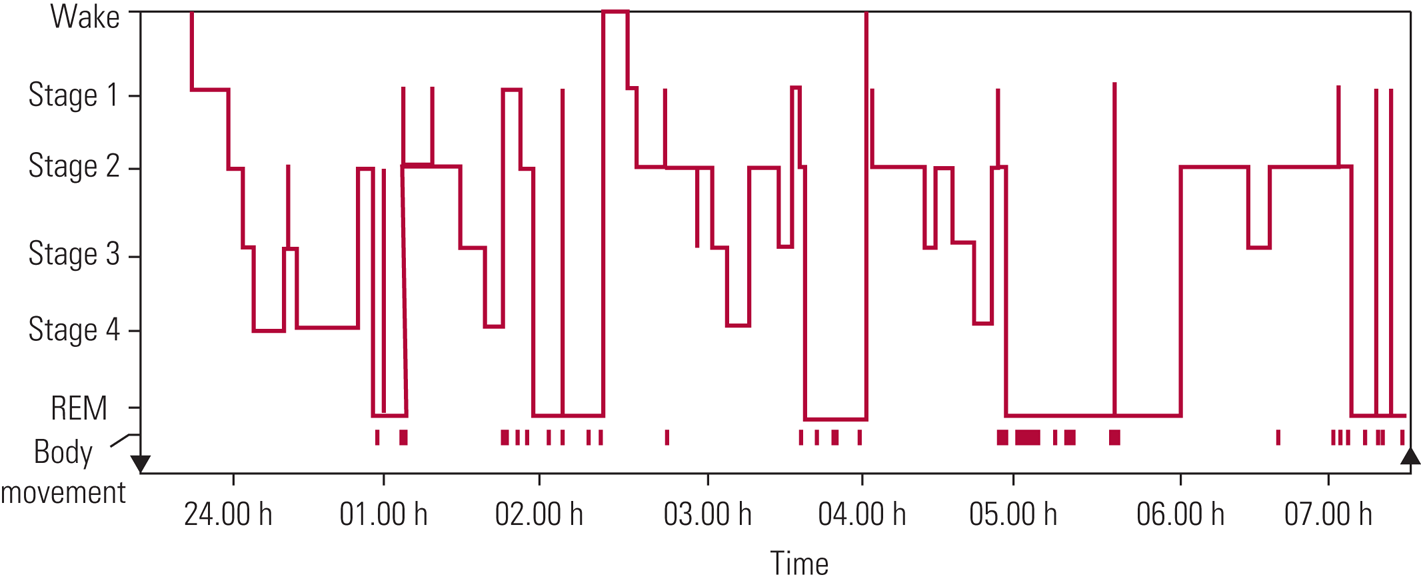
FIG 1 Progression of sleep states across a single night in a young adult. REM, rapid eye movement (Carskadon Reference Carskadon, Dement, Kryger, Roth and Dement2005). © 2006 National Academy of Sciences: reproduced with permission of Elsevier.
The sleep–wake cycle is one of the circadian rhythms and runs roughly every 24 h. These rhythms are generated by the suprachiasmatic nucleus in the hypothalamus, which functions as a biological clock (Dunlap Reference Dunlap, Loros and DeCoursey2004). The sleep–wake system is thought to be regulated by two major processes, one that drives sleep (process S) and one that maintains wakefulness (process C) (Gillette Reference Fuller-Thomson, Lewis and Agbeyaka2005). The need for sleep (process S) accumulates across the day, peaking just before bedtime. This homeostatic drive for sleep is reduced following adequate rest at night and the circadian waking drive (process C) begins to increase, and then the cycle starts again.
As shown in Fig. 2, there are normal variations with age in how sleep is initiated and maintained, the percentage of time spent in each stage of sleep and overall sleep efficiency (Altevogt Reference Altevogt and Colten2006).
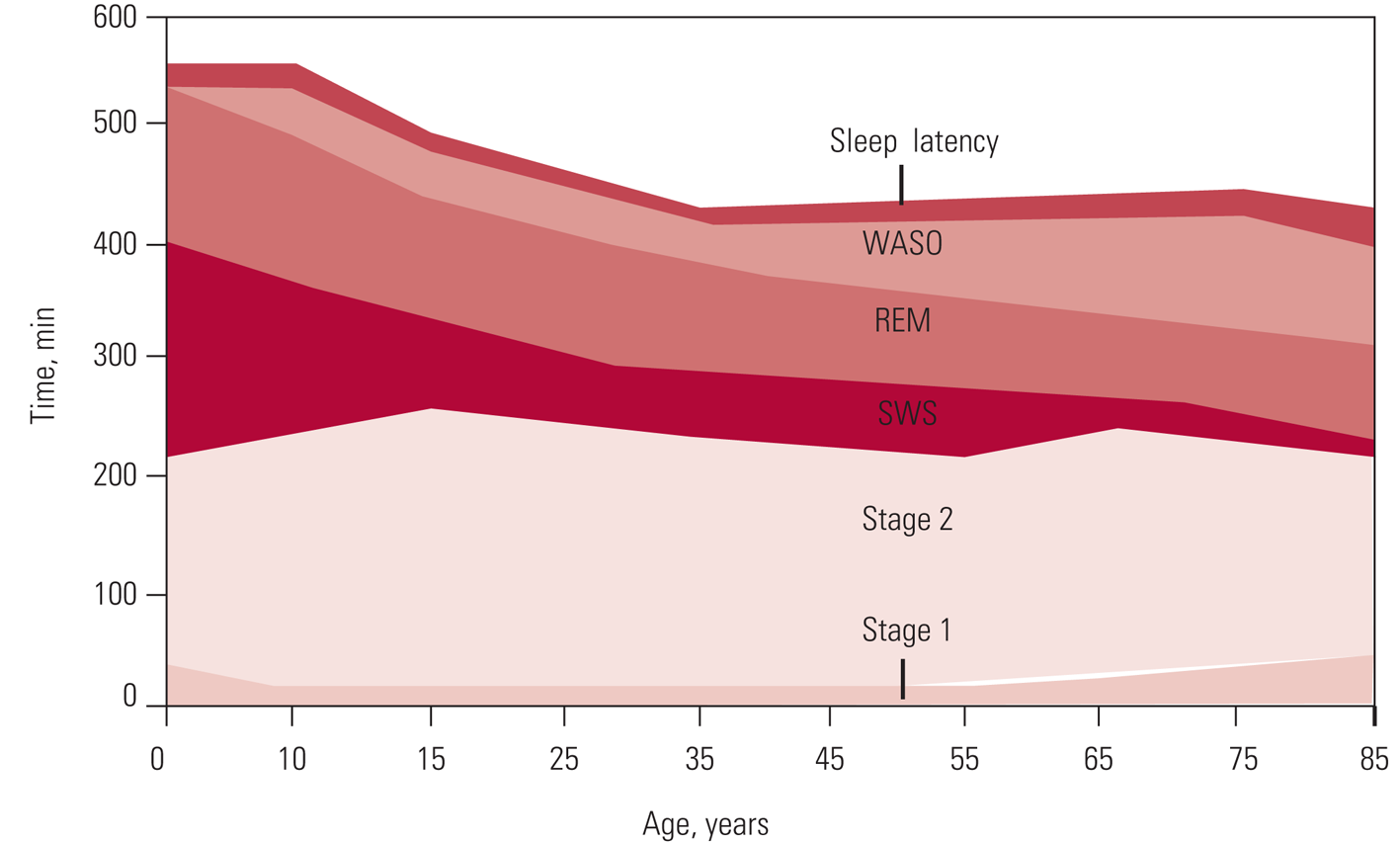
FIG 2 Changes in sleep with age. WASO, wake time after sleep onset; REM, rapid eye movement; SWS, slow-wave sleep (Carskadon Reference Carskadon, Dement, Kryger, Roth and Dement2005). © 2006 National Academy of Sciences: reproduced with permission of Elsevier.
Restorative sleep is important in learning, memory and affect regulation. Sleep deprivation has been shown to have a negative impact on learning, and research suggests that sleep has an important role in regulating the synaptic plasticity and connectivity of the brain. Together with impairments of attention and alertness, sleep deprivation is commonly associated with increased subjective reports of irritability and mood changes (Walker Reference Wajszilber, Santisteban and Gruber2009).
Diagnostic classification of sleep disorders
The International Classification of Sleep Disorders, now in its third edition (ICSD-3), can be used to diagnose insomnia, sleep-related breathing disorders, central disorders of hypersomnolence, circadian rhythm sleep–wake disorders, parasomnias and sleep-related movement disorders such as restless legs syndrome, periodic limb movement disorder and sleep-related leg cramps (American Academy of Sleep Medicine 2014).
The two most commonly reported sleep disorders in individuals with attention-deficit hyperactivity disorder (ADHD) or autism spectrum disorder (ASD) are insomnia and circadian rhythm sleep–wake disorders (ICD-10 and DSM-5 ‘circadian rhythm sleep disorders NOS'). Insomnia is difficulty getting to sleep or staying asleep despite having opportunity and circumstances to sleep, leading to unsatisfactory sleep or daytime consequences (American Academy of Sleep Medicine 2014; Selsick Reference Selsick and O'Regan2018). To be classified as having insomnia the individual must present with these difficulties for at least 3 months and the problems must occur at least three times a week.
Circadian rhythm sleep–wake disorders are defined by ISCD-3 as ‘a chronic or recurrent pattern of sleep–wake rhythm disruption primarily caused by an alteration in the circadian timing system or misalignment between the circadian rhythm and the sleep–wake schedule desired or required causing a sleep–wake disturbance (ie, insomnia or excessive sleepiness, and associated distress or impairment)’ (American Academy of Sleep Medicine 2014). For all circadian rhythm sleep–wake disorders, with the exception of jet lag disorder, a duration criterion of at least 3 months is specified by ISCD-3.
Sleep and its relationship to psychiatric disorders
It is estimated that 40% of people diagnosed with insomnia have a coexisting psychiatric condition (Anderson 2013). A common symptom in the ICD-10 diagnosis of a depressive episode is disturbed sleep, and epidemiological studies have found a 50–90% comorbidity between depression and poor sleep quality (Anderson 2013). Additionally, those who have obstructive sleep apnoea are likely to experience depressive symptoms (BaHammam Reference Axmon, Björne and Nylander2016). ICD-10 guidelines indicate that when psychiatric disorders co-occur with insomnia, both diagnoses should be given (World Health Organization 1992). In the treatment of depression, if there is a comorbid sleep disorder, the greatest risk factor for relapse is the presence of an ongoing untreated sleep disorder (Fang Reference Dunlap, Loros and DeCoursey2018). Other common psychiatric disorders co-occurring with sleep disorders include schizophrenia (30–80%) and bipolar disorder (17–57%) (Anderson 2013).
Sleep disorders are common in children with Tourette syndrome or chronic tic disorder, obsessive compulsions, anxiety, ADHD and ASD. A systematic review reported that sleep difficulties occur in 9.7–80.4% of children with Tourette syndrome/chronic tic disorder, with co-occurring anxiety increasing the risk of sleep disorders (Hibberd Reference Hauck, Ketcheson and Ulrich2020).
Early evidence suggests that mental disorders improve when interventions for sleep disorders are provided. The SENSE study was a randomised controlled trial including 144 adolescents (12–17 years) with high levels of anxiety and sleeping difficulties without a past or current depressive disorder (Blake Reference Baker and Richdale2016). Following a cognitive–behavioural/mindfulness-based intervention for sleep disturbance, improvements were noted on subjective and objective sleep measures and in anxiety symptoms (Blake Reference Baker and Richdale2016). OASIS, a randomised controlled trial of cognitive–behavioural therapy (CBT) for insomnia involving 3755 university students resulted in a reduction in paranoia and hallucinatory experiences (Freeman Reference Faulkner and Sidey-Gibbons2017).
Autism spectrum disorder
Autism spectrum disorder (ASD) is a behaviourally diagnosed neurodevelopmental disorder with qualitative differences in three domains. The domains remain similar across DSM-5 (American Psychiatric Association 2013) and ICD-10 (World Health Organization 1992), and they include reciprocal social interaction, social communication and restricted, repetitive behaviour or interests. Despite the use of differing language across ICD-10 and DSM-5, the core characteristics remain the same. Severity of symptoms, comorbid intellectual impairment and daily functioning often dictate the terms used to describe ASD. Currently, childhood autism, atypical autism and Asperger syndrome exist in ICD-10, but the use of these terms is starting to reduce in clinical practice. Current clinical practice reflects DSM-5 diagnosing of all of these presentations as ‘autism spectrum disorder’. The estimated prevalence of ASD in children is 2.24–3.2%, with an increasing trend in cumulative incidence since 1992 (Zablotsky Reference Zablotsky, Black and Maenner2015; Saito Reference Roberts, Smith and Sherman2020).
Diagnosis of sleep disorders in people with ASD
The prevalence of sleep disorders in ASD is 50–80% in adults and 40–80% in children (Charrier Reference Cappuccio, Cooper and Delia2017; Papadopoulos Reference Papadopoulos, Sciberras and Hiscock2019). There are various hypotheses regarding intrinsic and extrinsic causes for the high prevalence of insomnia in individuals with ASD (Souders Reference Souders, Zavodny and Eriksen2017). These include neurobiological, medical, behavioural and cultural mechanisms, and the cause in any given individual is likely to be multifactorial (Souders Reference Souders, Zavodny and Eriksen2017). The prevalence of sleep disorders in ASD may be misleading owing to the lack of consensus regarding their definition (Richdale Reference Richdale and Schreck2009). The need to assess sleep in those with ASD is highlighted in primary care prescribing. A study of prescribing practices in the UK found that the most commonly prescribed medication (including melatonin) for individuals with ASD is for sleep, and even then rates were low (9.7% of the cohort) (Murray Reference Morgan, Nageye and Masi2014). Insomnia is particularly prevalent in children with ASD, resulting in increasing severity of ASD symptoms (Veatch Reference Taylor and Pruiksma2017). Children's sleep difficulties can have an impact on the family, affecting parents' sleep and mood and resulting in fatigue and strain on relationships. A comparison of sleep patterns between parents of neurotypical children and those with ASD identified reduced sleep quality and quantity in the latter group (Meltzer Reference McDonagh, Holmes and Hsu2008).
It has been suggested that, for some children with ASD, sleep disorders occur as a result of inappropriate sleep-related behaviours and parental mismanagement (Wiggs Reference Walker2004). In adults with ASD, objective evaluation of sleep measures indicates longer sleep onset latency and shorter sleep duration compared with neurotypical controls. Furthermore, some circadian rhythm sleep–wake disorders are significantly more likely in people with ASD than in the general population (Baker Reference BaHammam, Kendzerska and Gupta2017). Finally. it is worth noting that parasomnias are more common in children with ASD (Ming Reference Meltzer2009).
Diagnosis of the various sleep disorders in individuals with ASD can be difficult for a number of reasons. First, given that ASD is a social communication disorder, those who experience sleep disorders, irrespective of intellectual impairment, do not often spontaneously report sleep as a problem (Limoges Reference Lenjavi, Ahuja and Touchette2013). Despite this, actigraphy data and subjective reporting (when explicitly asked) indicate that those with ASD experience lower rates of sleep efficiency and increased duration of sleep onset latency (Morgan Reference Ming, Sun and Nachajon2020). Second, currently there are no validated screening tools specific to the sleep disorders experienced by those with ASD. A caution for the use of screening tools in specialist populations is that they may be misleading because they do not accurately capturing the appropriate sleep disorders relevant to that particular patient group (Faulkner Reference Fang, Tu and Sheng2019). Clinicians may need to take this into consideration when solely relying on screening tools for assessment or onward referral.
Relationship between ASD, sleep and psychiatric disorders
The lifetime prevalence estimates for an anxiety disorder or a depressive disorder in people with ASD are 27–43% and 23–37% respectively, which are higher than the general population (Hollocks Reference Hibberd, Charman and Bhatoa2019). Evidence indicates that, when sleep is improved in ASD, there are reported improvements in anxiety and depression (Loring Reference Loring, Johnston and Shui2018). Rates of obsessive–compulsive disorder in individuals with ASD were also significantly higher than in the general population, at 22–24% (Hollocks Reference Hibberd, Charman and Bhatoa2019).
Attention-deficit hyperactivity disorder
Attention deficit hyperactivity disorder (ADHD) is characterised by the core symptoms of inattention, hyperactivity and impulsivity. There are two main sets of diagnostic criteria in current use: ICD-10 and DSM-5. Both ICD-10 and DSM-5 require 6-month duration of symptoms (National Institute for Health and Care Excellence 2018). The diagnostic criteria for ADHD have been updated in DSM-5 to capture more accurately the experience of adults affected by the disorder. DSM-5 states that this revision is based on nearly two decades of research showing that ADHD, although a disorder that begins in childhood, can continue through adulthood for some people (American Psychiatric Association 2013).
There is no separate diagnostic criteria for adults with ADHD in ICD-10. A diagnosis of hyperkinetic disorder may also be made in adult life using the same criteria as in children, although attention and activity must be judged with reference to developmentally appropriate norms (World Health Organization 1992).
Diagnosis of sleep disorders in people with ADHD
Sleep problems are reported in an estimated 25–50% of individuals who have ADHD (Corkum Reference Constantin, Low and Dugas1998). A systematic review of sleep disorders in adolescents with ADHD identified overall association between sleep disorders and poorer outcomes in clinical, cognitive and functional domains (Lunsford-Avery Reference Loring, Johnston and Shui2016). Comorbidities in ADHD such as circadian rhythm sleep–wake disorders, sleep-related breathing disorders, narcolepsy and insomnia are often missed and left untreated. A review by Konofal et al (Reference Kokina and Kern2010) reported that up to 44% of those with ADHD had restless legs syndrome, but the authors advised caution in interpreting this finding owing to the methodological limitations identified in the studies. However, this may be relevant from a clinical evaluation point of view. More studies are needed to understand the prevalence and underlying pathways of symptoms of restless legs syndrome in those with ADHD.
Poor sleep hygiene, disrupted routine and impulse control difficulties may affect the ability of an individual with ADHD to have a winding-down period, leading to bedtime resistance and delayed sleep onset. Delayed sleep–wake phase disorder and late chronotype are frequently comorbid with ADHD in both adults and adolescents (Kooij Reference Konofal, Lecendreux and Cortese2013; Sivertsen Reference Shanahan, Palod and Smith2014). There are proposals suggesting that people with ADHD may have stronger delayed circadian preference and a possible delayed release of endogenous melatonin (Gruber Reference Graham, Banaschewski and Buitelaar2012).
In ADHD, insomnia is reported to be more frequent among children (73.3%) (Sung Reference Stores2008) than among adults (66.8%) (Brevik Reference Boyle, Melville and Morrison2017). These rates are higher than insomnia estimates in children (20–30%) (Calhoun Reference Calhoun, Fernandez-Mendoza and Vgontzas2014) and adults (6–50%) (Ohayon 2002) in the general population. Women with ADHD reported higher prevalence of insomnia than women without ADHD (43.9 v. 12.2%) (Fuller-Thomson Reference Freeman, Sheaves and Goodwin2016). Recent studies indicate that over 30% of individuals with narcolepsy, which is characterised by excessive daytime sleepiness and cataplexy, have comorbid ADHD (Kim Reference Johnson, Turner and Foldes2020). A history of snoring or possible obstructive sleep apnoea during childhood is associated with a twofold difference in the odds of ADHD diagnosis or symptoms (Constantin Reference Constantin, Low and Dugas2014). Other psychopathologies, such as anxiety, depression and tic disorders, are common in those with ADHD, and we recommend that a systematic assessment of coexisting conditions be carried out when evaluating for sleep disorders in those with ADHD.
The relationship between ADHD and ASD
Considerable overlap exists between ASD and other mental disorders. ADHD, anxiety disorders, depression, conduct disorder and behaviours that challenge are among the disorders most commonly associated with ASD (Matson Reference Martin, Papadopoulos and Chellew2013). DSM-5 permits a dual diagnosis of both ADHD and ASD. An estimated 30–80% of children with ASD also meet the criteria for ADHD and, conversely, 20–50% of children with ADHD meet criteria for ASD. Given this overlap, more research is needed to further understand the relationship between the two conditions. It is important that clinicians are aware of the conditions likely to co-occur with ASD, which vary according to the person's age. Clinicians should also be familiar with the assessment methods that are available to assist in diagnosis and keep up to date with new research in this area (Matson Reference Martin, Papadopoulos and Chellew2013).
Assessment and diagnosis
Before assessing for a sleep disorder, the clinician should consider predisposing, precipitating and perpetuating factors that cause sleep disturbance. An example is gastrointestinal disorders, which are commonly associated with sleep problems in individuals with ASD (Klukowski Reference Kim, Lee and Sung2015). Additionally, behaviours that challenge may need considering as a cause of the sleep disorder and can be measured using an appropriate screening tool. The first step in assessing a sleep disorder in children and adults with ASD and ADHD is taking a detailed sleep history. There are a range of factors to consider, outlined in Table 1.
TABLE 1 Sleep assessment

Taking a detailed history may highlight factors that clearly indicate environmental or iatrogenic impact on sleep. The flowchart shown in Fig. 3 can be followed to inform the decision to diagnose or move to a specialist referral.
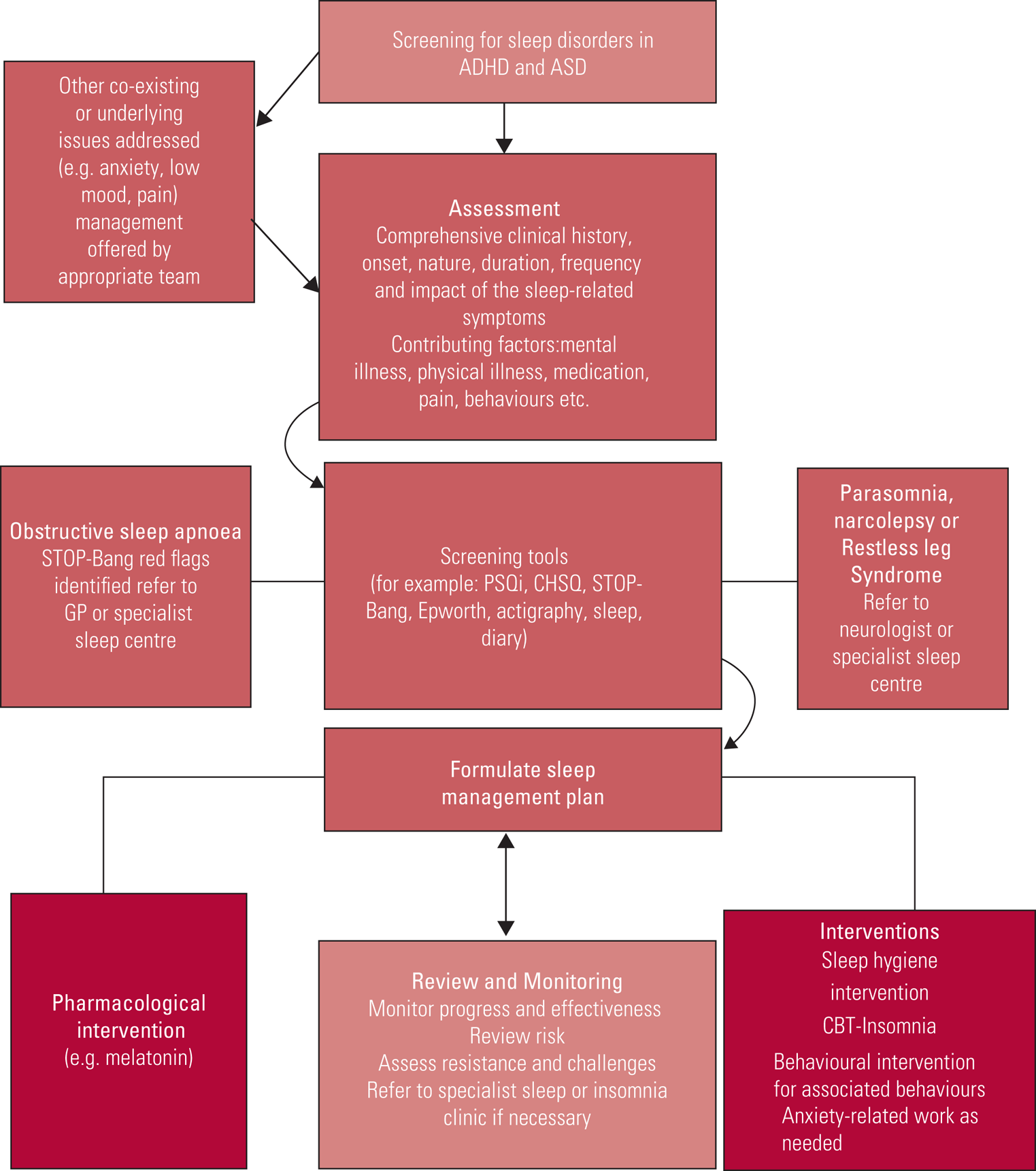
FIG 3 Flowchart for the assessment of sleep disorders. ADHD, attention-deficit hyperactivity disorder; ASD, autism spectrum disorder; GP, general practitioner; PSQi, Pittsburgh Sleep Quality Index; CHSQ, Children's Sleep Habits Questionnaire; CBT, cognitive–behavioural therapy.
Screening tools
Box 1 lists frequently used questionnaires for screening for clinically significant sleep problems. These tools can be found online and some are available to use for clinical and research purposes free of charge.
BOX 1 Commonly used screening questionnaires for clinically significant sleep problems
For children and adolescents
• Bespoke sleep diary (1 week minimum).
• Sleep Disturbance Index (SDI): a four-item, parent-rated questionnaire that covers settling problems, night waking and parental involvement at night.
• Children's Sleep Habits Questionnaire (CSHQ): a 45-item, parent-rated questionnaire that assesses the frequency of behaviours associated with common paediatric sleep disorders.
• Paediatric Daytime Sleepiness Scale (PDSS): self-report questionnaire designed to assess sleepiness in 11- to 15-year-olds.
For adults
• Bespoke sleep diary (1 week minimum).
• Epworth Sleepiness Scale (ESS): a self-administered questionnaire with eight questions. Respondents are asked to rate, on a four-point scale (0–3), their usual chances of dozing off or falling asleep while engaged in eight different activities.
• Pittsburgh Sleep Quality Index (PSQI): a self-report questionnaire that assesses sleep quality over a 1-month period.
• STOP-Bang Questionnaire: an eight-item questionnaire that determines risk for sleep apnoea. It is one of the most widely accepted screening tools for obstructive sleep apnoea.
• Morningness–Eveningness Questionnaire (MEQ): a self-assessment questionnaire to measure whether a person's circadian rhythm (biological clock) produces peak alertness in the morning, in the evening or in between.
Specialist sleep services
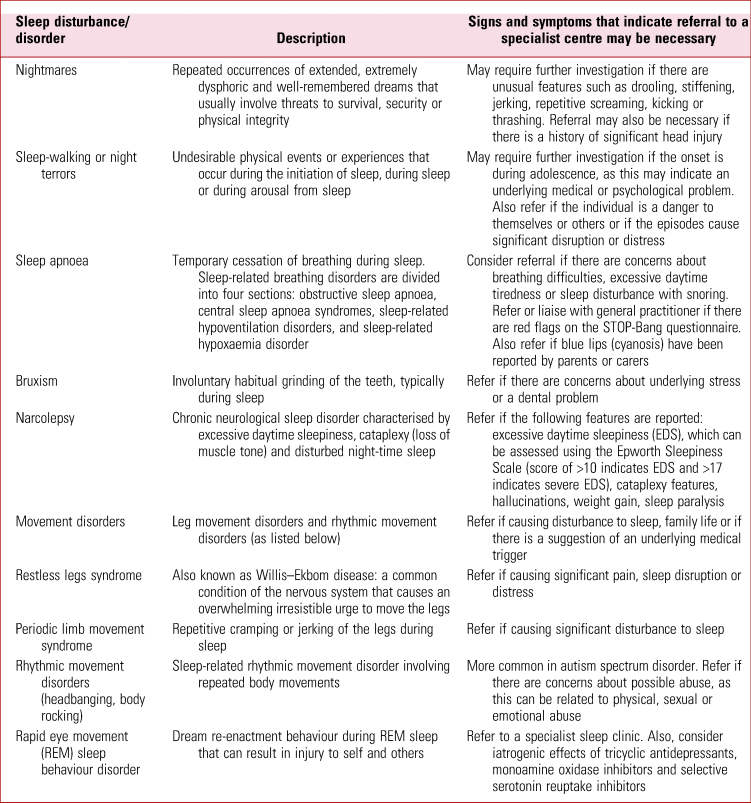
In some cases, it may be necessary to refer individuals with ASD or ADHD for assessment at a specialist centre. Sleep centres in the UK can be located through the National Health Service's ‘Find Sleep Medicine Services’ webpage (www.nhs.uk/service-search/other-services/Sleep-Medicine/LocationSearch/1888). As a rule of thumb, any new onset of parasomnia should trigger further evaluation by a specialist. Table 2 provides an overview of the signs and symptoms that indicate that referral to a specialist centre may be necessary.
TABLE 2 Referral to specialist sleep services
Diagnosis of a sleep disorder
Typically, specialist services will identify and diagnose a sleep disorder by taking a detailed history (especially about the sleep problem), performing a physical and behavioural examination, and monitoring the 24 h sleep–wake pattern using a sleep diary and possibly objective sleep studies such as actigraphy or polysomnography (Stores Reference Stores2015). Information about family circumstances and history will also be sought. For children, parenting practices and the child's development history will be assessed. Figure 4 outlines patterns observed in common circadian rhythm sleep–wake disorders.
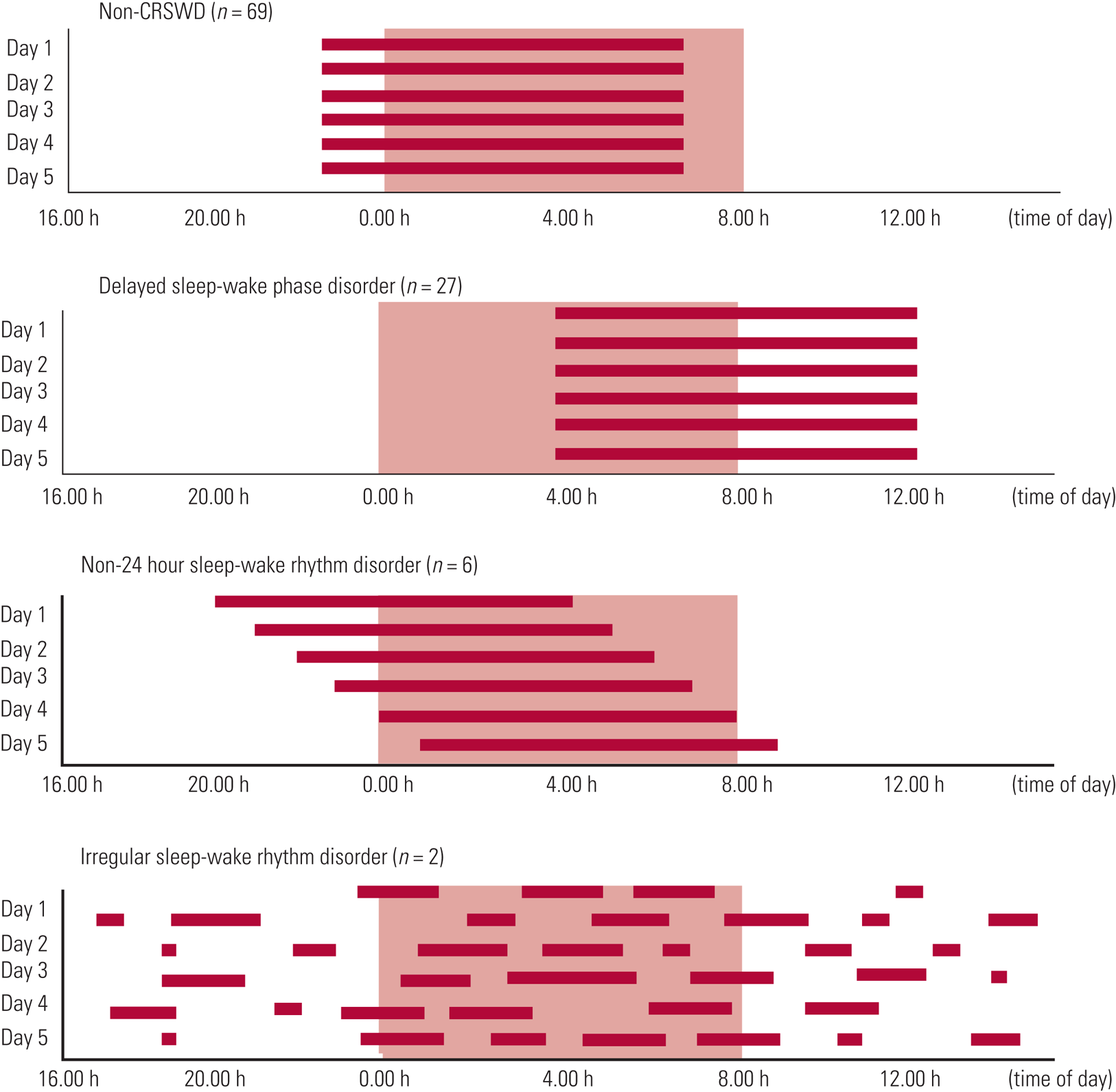
FIG 4 Circadian rhythm sleep–wake disorders (CRSWD) (Takaesu Reference Sung, Hiscock and Sciberras2016). © 2016 Takaesu et al: reproduced under the terms of the Creative Commons Attribution License (CC BY 4.0).
Treatment options
One of the problems noted in the literature is the use of an array of terminologies to describe sleep symptoms, such as sleep disturbance, sleep difficulties and sleep disorders. Making a clear distinction between them will be useful in clinical practice. Aspects of sleep should be carefully assessed before starting treatment or medication. This requires the clinician to use structured assessments and evaluation tools, which will help them to understand the baseline state and choose a suitable treatment option.
Non-pharmacological interventions
In the management of insomnia and circadian rhythm sleep–wake disorders in individuals with ADHD or ASD, lifestyle factors, including exercise and diet, should be considered, followed by consideration of strategies to ensure adherence to treatment plans (National Institute for Health and Care Excellence 2018). The use of personalised reminders, incentives and clearly documented instructions may help (Hauck Reference Hauck, Ketcheson and Ulrich2016). Social Stories™ (Kokina Reference Klukowski, Wasilewska and Lebensztejn2010) and visual schedules (Malow Reference Lunsford-Avery, Krystal and Kollins2014) have shown good short-term effectiveness in reducing behaviours that challenge related to bedtime routines and facilitating interventions such as the bedtime pass programme for children.
Interventions for children with ASD primarily focus on education of parents and carers (Johnson Reference Johnson, Turner and Foldes2013). Training programmes include providing information about normal sleep, recommendations for establishing good stimulus control in the bedroom environment, sleep scheduling and managing maladaptive behaviour at night-time (Johnson Reference Johnson, Turner and Foldes2013). Providing two educational sessions covering sleep hygiene and relaxation strategies to adolescents with ASD and their carers resulted in improvements in sleep latency and efficiency (Loring Reference Loring, Johnston and Shui2018). In all interventions outlined above, homework tasks are set and reviewed at subsequent sessions (Cortesi Reference Corkum, Tannock and Moldofsky2012; Loring Reference Loring, Johnston and Shui2018). Online parent training has also shown promising short-term sleep improvements in children with ASD (Roberts Reference Roberts, Smith and Sherman2019).
Sleep hygiene is a term used widely in sleep disorders, but it will look different for each patient. It focuses on the principles of stimulus control and nurturing bodily changes to encourage sleep during natural cycles. Stimulus control refers to ensuring that the place of sleep is associated with sleeping and other places are associated with wakefulness. An example of an intervention to promote stimulus control among adolescents or adults is leaving the bedroom after 15 min if not sleeping and then returning to bed only when tired. Any non-sleep-related behaviours that occur in bed (e.g. laptop use, video games or watching TV) should be relocated to other living areas where possible.
In adults with ASD and ADHD, as circadian rhythm sleep–wake disorders are more common, the use of light therapy has shown effectiveness, involving 10 000 lux light to promote wakefulness and 400 lux dim red light prior to sleep onset. In advanced sleep–wake phase disorder, 10 000 lux can be considered between 18.00 and 21.00 h; for delayed sleep–wake phase disorder 10 000 lux can be considered between 10.00 and 13.00 h; and for irregular sleep–wake disorder 10 000 lux at 09.00 h for 30 minutes might be used (Burgess Reference Brevik, Lundervold and Halmøy2016). There is growing evidence for the use of CBT for insomnia (CBT-I) face to face or online (National Institute for Health and Care Excellence 2017). A consideration that might affect the likelihood of behaviour change in individuals with ASD or ADHD is the extent to which restricted and repetitive behaviours affect daily living and sleep routines.
Finally, sleep disorders are highly associated with daytime distress and maladaptive behaviours in individuals with ASD (Abel Reference Abel, Schwichtenberg and Brodhead2018). In adults as well as children with a diagnosis of ASD, a functional analysis of behaviours that challenge followed by a function-based intervention plan is recommended (National Institute for Health and Care Excellence Reference Murray, Hsia and Glaser2012). If a functional analysis is not feasible, other functional assessment procedures can be used aiming to understand the relationship between the maladaptive behaviour and its maintaining consequences. In adults, who are very likely to be able to describe the contingencies, this may be sufficient to understand maintaining consequences.
A recent systematic review of non-pharmacological interventions for non-respiratory sleep disorders in children with neurodisabilities (including ASD and ADHD) identified that, despite clinical guidance recommending parent-directed interventions as the first approach to managing sleep disorders, there is a lack of high-quality evidence assessing the effectiveness of non-pharmacological interventions for these populations (Scantlebury Reference Saito, Hirota and Sakamoto2018). Given the limitations of the existing evidence, the authors highlighted the importance of prioritising research in this area.
Pharmacological interventions
From a clinical perspective when prescribing, thorough consideration must be given to predisposing, precipitating and perpetuating factors and differential diagnosis. Some psychiatric drugs have insomnia as a side-effect or even unmask a subclinical REM sleep behaviour disorder. If there are underlying coexisting mental illnesses such as anxiety or depression, the choice of medication with sedative effects may be worth considering, but not as a stand-alone treatment for sleep disorders.
Stimulants are the first-line medication for treatment of ADHD. The effects of stimulant medication on sleep in those with ADHD vary, which may be a reflection of the underlying complexity of the link between ADHD and sleep disorders (Graham Reference Graham, Banaschewski and Buitelaar2011). Stimulants are associated with disturbed sleep, such as increased sleep onset latency, difficulties falling asleep and decreased total sleep time in those with ADHD (Stein Reference Souders, Zavodny and Eriksen2012). However, some studies have also shown that ADHD symptom control can promote sleep (Jerome Reference Jenabi, Ataei and Bashirian2001). Adjusting the dose or changing to another formulation have all been proposed (Konofal Reference Kokina and Kern2010). In contrast to stimulants, the most common side-effect associated with atomoxetine and guanfacine is somnolence. Dosing in the evening rather than in the morning has been found to reduce daytime somnolence with atomoxetine (Block Reference Blake, Waloszek and Schwartz2009).
Medications can be effective in treating circadian rhythm sleep–wake disorders and insomnia. Melatonin has been shown to reduce the symptoms related to insomnia, to improve sleep latency and improve sleep efficiency and total sleep duration (Jenabi Reference Irwin, Olmstead and Carroll2019). This observation was replicated in a systematic review of pharmacological treatments for sleep disorders in children, which concluded that melatonin was useful in improving sleep latency, sleep duration and wake time after sleep onset at least in the short term, particularly with children with comorbid ASD and other neurodevelopmental disorders (McDonagh Reference Matson and Williams2019). However, there was no improvement noted in the number of awakenings per night with melatonin. More research is required to establish how and when to stop melatonin use in individuals with neurodevelopmental disorders. Utilising melatonin should be considered with caution as it is not currently licensed in the UK for use in adults between 18 and 55 years of age (National Institute for Health and Care Excellence 2015).
Hypnotics are effective in treatment of insomnia, however they are for short-term use and only work for as long as they are used (Riemann Reference Richdale and Schreck2009). Short-acting hypnotics may be helpful for sleep-onset insomnia. In the UK, hypnotics for the management of insomnia are only licensed for short-term use. Clinicians should consider the risk of side-effects, including dependency, cognitive impairment, falls and withdrawal symptoms. NICE guidance suggests only to use hypnotics if insomnia is severe and to use the lowest possible dose for the shortest period of time (National Institute for Health and Care Excellence 2015). Insomnia can be a chronic condition, so behavioural interventions and CBT-I are suggested as treatment choices (Riemann Reference Richdale and Schreck2009).
Neurodevelopmental disorders and sleep disorders in intellectual disabilities
For individuals with intellectual disabilities, there is a fourfold higher likelihood of having ADHD than for the general population (Axmon Reference Anderson and Bradley2018). There is an even greater likelihood of having a comorbid diagnosis of ASD. The prevalence of sleep disorders among people with intellectual disabilities is high and increased further in populations with specific chromosomal disorders (Boyle Reference Block, Kelsey and Coury2010).
Sleep disorders in this population can present as behaviours that challenge and are associated with poor quality of life for the patient and caregivers (Lenjavi Reference Lenjavi, Ahuja and Touchette2010). Current guidance for adults with intellectual disabilities highlights the completion of a functional analysis prior to the use of melatonin (National Institute for Health and Care Excellence 2015). However, there is no evidence for the use of functional analysis in identifying or assessing sleep-related behaviours that challenge and, as mentioned above, melatonin only has a licence for those under 18 and over 55 (National Institute for Health and Care Excellence 2015). Before assessment for a sleep disorder, a comprehensive assessment of physical health, including review of current medications and epilepsy management, may be needed.
A systematic review of interventions for sleep difficulties, including sleep hygiene, behavioural interventions and use of melatonin, found that existing literature is of low quality (Shanahan Reference Selsick and O'Regan2019). However, there is some evidence that educating parents about interventions such as stimulus control and other sleep-related behaviours improves sleep in children and should be considered for parents and carers of adults with ASD and comorbid intellectual disabilities.
Clinical implications and future directions
Children and adults presenting with neurodevelopmental disorders should be offered assessment for primary sleep disorders which may need separate treatment strategies. The problems of circadian rhythm sleep–wake disorders and insomnia can persist into adulthood if not treated. The focus questions outlined in Table 1 may assist in taking the clinical history. Baseline records of sleep onset latency, night-to-night variability and total sleep duration before treatment begins will be helpful to evaluate progress and formulation of a management plan. Most intervention plans should include psychoeducation and behavioural interventions such as sleep hygiene. Sleep studies may be required for those with new or complex sleep disorders.
Clinicians may face additional challenges on continued prescriptions of melatonin. Shared-care treatment protocols and transition meetings between children's and adult services or primary care physicians may be required.
A diagnosis of chronic insomnia disorder should only be considered when the insomnia is especially prominent or unexpectedly prolonged and is the focus of clinical assessment and treatment. In such cases, care is best offered in consultation with sleep clinics.
Behavioural interventions should be considered for long-term sleep disorders. One such is CBT for insomnia (CBT-I), an evidence-based effective treatment for insomnia in those with comorbid psychiatric illness (Taylor Reference Takaesu, Inoue and Murakoshi2014). There are several web-based apps available, such as Sleepio, Headspace and Calm, which incorporate aspects of CBT, relaxation, meditation and mindfulness. There is insufficient evidence supporting the use of apps such as Sleepio, Headspace and Calm in this population and there are risks of high drop-out rates (Freeman Reference Faulkner and Sidey-Gibbons2017; National Institute for Health and Care Excellence 2017). More research into the effectiveness of online CBT-I or sleep apps should be considered, given the high likelihood of cost savings and benefits of a non-face-to-face approach for people with ASD and also in situations that restrict a face-to-face consultation, such as pandemic. Longitudinal research is required to inform the adaptation of pharmacological and non-pharmacological sleep interventions to better meet the needs of children and adults with ADHD or ASD and their families (Martin Reference Malow, Adkins and Reynolds2019).
Clinical evaluations and management plans based on comprehensive assessment and multidisciplinary team evaluation, timely consultations with other appropriate specialists in primary care, neurology and respiratory medicine, and referral to specialist sleep centres remain the mainstay in identifying and managing sleep disorders in people with ASD or ADHD.
Acknowledgements
We thank Dr Swapnil Palod (Mackay Base Hospital, Queensland, Australia) for his contribution to information about prescribing and initial coordination of the project.
Author contributions
All three authors contributed to writing sections of this article.
Declaration of interest
None.
ICMJE forms are in the supplementary material, available online at https://doi.org/10.1192/bja.2020.65.
MCQs
Select the single best option for each question:
1 The prevalence of sleep disorders in adults with autism spectrum disorder is:
a 20–50%
b 30–60%
c 40–70%
d 50–80%
e 60–90%
2 As regards the statement that delaying treatment in adolescents with circadian rhythm sleep–wake disorders is acceptable as the sleep disorder will resolve in adulthood:
a this is true only for those with ADHD
b this is true only for those with ASD
c this is true only for those without ADHD and ASD
d this is true
e this is false.
3 In advanced sleep–wake phase disorder, use of a light box with 10 000 lux can be considered:
a never
b at 03.00 h
c at 09.00 h
d at 15.00 h
e at 20.00 h.
4 As regards the prevalence of insomnia in children and adults with ADHD:
a insomnia is not a reported sleep disorder in children with ADHD
b the prevalence in adults is lower than the prevalence in children
c the prevalence in adults is the same as the prevalence in children
d the prevalence in adults is higher than the prevalence in children
e insomnia is not a reported sleep disorder in adults with ADHD.
5 The STOP-Bang Questionnaire is a widely used screening tool for identifying:
a narcolepsy
b restless legs syndrome
c obstructive sleep apnoea
d parasomnias
e circadian rhythm sleep–wake disorders.
MCQ answers
1 d 2 e 3 e 4 b 5 c









eLetters
No eLetters have been published for this article.