Psychiatry has never had a comfortable relationship with movement disorders. Parkinsonism, viewed since the 1960s as an adverse rather than an integral action of antipsychotics, a conditio sine qua non, has largely remained with neurology, whereas akathisia, long the orphan of psychiatric research, was adopted by sleep physicians, rebranded, and disconnected from its psychiatric roots.
Traditionally, psychiatry's interest was tardive dyskinesia (Fig. 1), a group of seemingly iatrogenic and potentially irreversible disorders that raised medico-legal considerations. A body of well-conducted long-term psychiatrically based research, funded independently of industry, emerged throughout the 1980s and early 1990s, solidifying the concept and expanding knowledge of its boundaries and correlates.
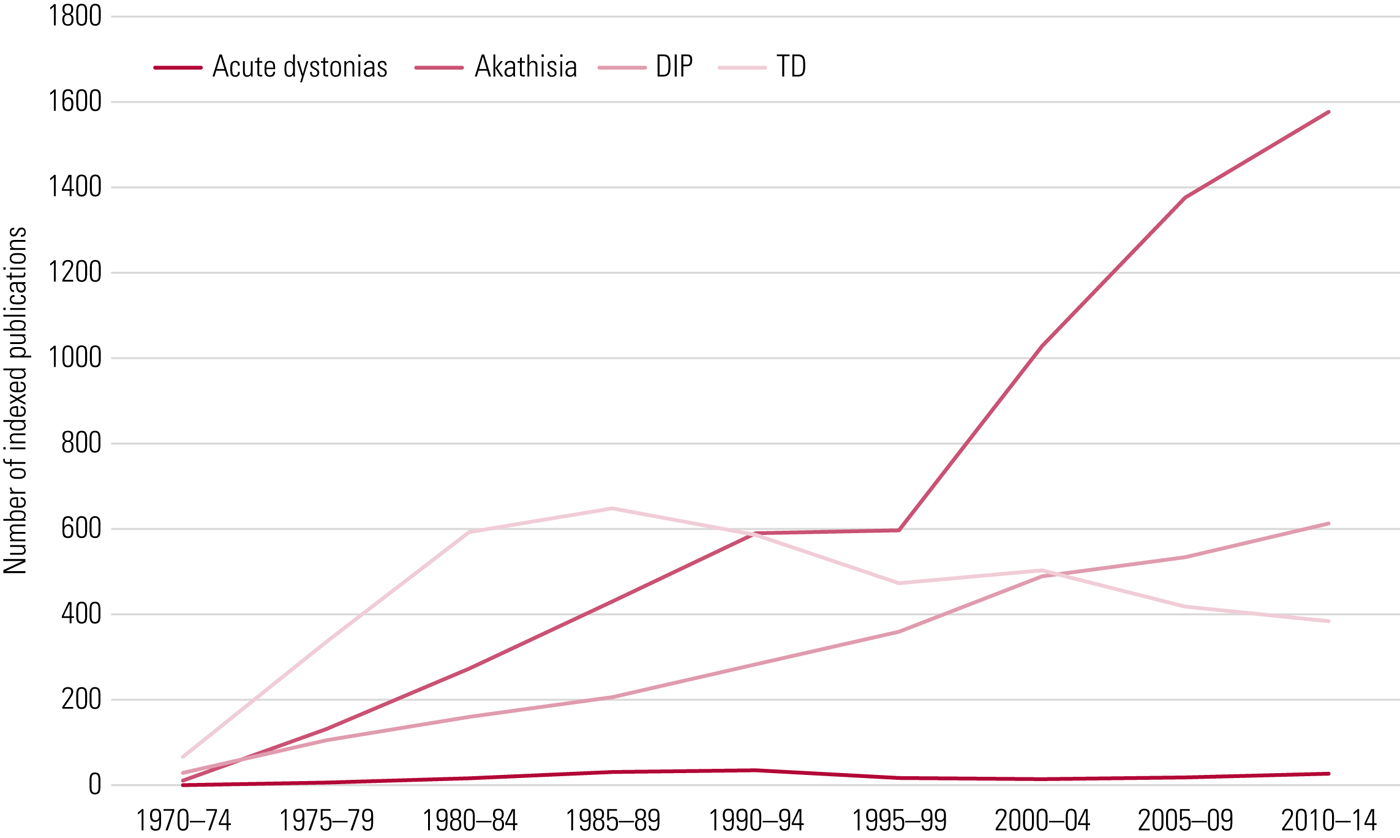
FIG 1 EPS syndromes: literature representation over time. DIP, drug-induced Parkinsonism; TD, tardive dyskinesia. Data from PubMed search.
As this field was finding its scientific credentials it was dealt two blows. First, evidence suggested that relentless progression and irreversibility were not inevitable (Gardos Reference Gardos, Casey and Cole1994) and, with a degree of resolution possible, tardive dyskinesia could be accommodated within a risk–benefit appraisal for antipsychotics that was again acceptable. Second, and more important, new compounds arrived, fanfared as different in their pharmacology, with ‘reduced EPS liability’. This was, on the face of it, a modest claim but to a demoralised profession starved of therapeutic innovation it was sufficient to make a fanciful leap: extrapyramidal side-effects (EPS) were no longer an issue. Tardive dyskinesia faded from the psychiatric literature, although Fig. 1 does not illustrate the extent of this decline. Neurology again took over as cases of drug-induced movement disorder in community patients came to tertiary specialists.
This indifference is dissipating. More detailed – and longer-term – evaluation of tardive dyskinesia with newer antipsychotics reveals a prevalence similar to that with older ones (Carbon Reference Carbon, Hsieh and Kane2017) and the first drugs specifically licensed for its treatment in the USA have come to market, with international promotion likely. It is therefore timely to present again the story of tardive dyskinesia to a generation of psychiatrists who may think this history. Tardive dyskinesia, like all EPS syndromes, is alive and well, living where it always has – comfortably within the pharmacology of all antipsychotic drugs.
Origins and development of the concept
The German psychiatrist Schonecker (Reference Schonecker1957) often gets credit for the first account but if priority appropriately rests with those who recognise novel implications, Jean Sigwald and colleagues from France better fulfil that requirement (Sigwald Reference Sigwald, Bouttier and Raymondeaud1959). It was, however, the Danes, Uhrbrand and Faurbye (Reference Uhrbrand and Faurbye1960), who brought the condition to life.
The term tardive dyskinesia subsequently appeared in an overview of neurological features associated with antipsychotic use (Faurbye Reference Faurbye, Rasch and Peterson1964). Descriptively, the concept seemed straightforward. Although ‘tardive’ literally means ‘arriving/coming late’, it carries a relative implication. The inference is not of a specific time criterion but of onset later than the other neurological adverse reactions of the class. This clearly left potential for variability, which Faurbye and colleagues addressed by suggesting at least 6 months of antipsychotic exposure as a time criterion (Faurbye Reference Faurbye, Rasch and Peterson1964). The literature tended to greater conservatism – a year or two minimum (Ayd Reference Ayd1967) – while still presenting putative cases with exposure of weeks or even days (Chouinard Reference Chouinard and Jones1979). The generality of ‘later’ could not be squeezed into fixed temporal boundaries.
‘Dyskinesia’, not a word with wide prior usage, generically means ‘any abnormal kinesis’. For this syndromal use, it was restricted to abnormal hyperkinetic movements with ‘co-ordinated, involuntary, stereotyped, rhythmic’ characteristics (Faurbye Reference Faurbye, Rasch and Peterson1964), predominantly targeted on the mouth, although these helpful adjectives relating to form hid a problem that only emerged later. For those neurologists who shared an early interest in this field with psychiatrists, the presentation had mixed characteristics that recalled movements they knew well – chorea and athetosis. These perioral signs were therefore universally referred to as ‘choreoathetoid’ (Marsden Reference Marsden, Mindham, Mackay, Bradley and Hirsch1986) and regarded as authentically ‘involuntary’. However, they were also complex and, as Faurbye et al pointed out, coordinated. These were not in themselves abnormal movements but seemed appropriate oromandibular behaviours that lacked context, such as eating. It was ‘as if the entire template for a set of complex conditioned behaviours is laid vulnerable to release from tonic inhibition’ (Owens Reference Owens2014).
In the mid-1990s, neurologists began to focus on Faurbye et al's third descriptor, referring to the oromandibular component as ‘tardive stereotypies’. This settled one problem – the predominant component was not choreoathetoid – but in the absence of a consensus on what ‘stereotypy’ meant (Ridley Reference Ridley1994; Edwards Reference Edwards, Lang and Bhatia2012), opened another. Much, though not all, of what is overt in tardive dyskinesia may be ‘un-willed’ but it is not ‘involuntary’.
A further debate of historical relevance was fundamental – did tardive dyskinesia actually exist? This argument is too far-reaching to present here (for discussion see Owens Reference Owens2014) but while schizophrenia can itself be associated with motor disorder, the significance to those on antidopaminergics remains unclear. Neither, however, does this distract from the undoubted validity of tardive dyskinesia as a syndrome construct.
In essence, tardive dyskinesia is what clinicians and authors believe it to be (Owens Reference Owens2014). This is not to be over-cynical. The point is that, in considering a differential diagnosis, this disorder – so unpredictable in onset, so phenomenologically varied – is best conceptualised in terms of its inference, and that is clear. Tardive dyskinesia is any hyperkinetic motor disorder (except tremor) in which drugs, especially although not exclusively antidopaminergics, are believed to play a causative role.
Clinical features
Core abnormalities
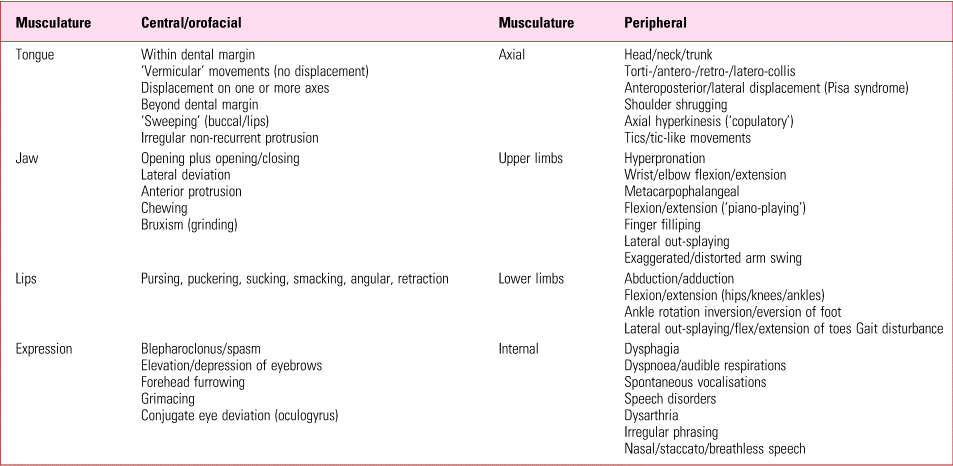
Traditionally, tardive dyskinesia has been a blanket term encompassing the range of hyperkinetic disorders, except tremor (Box 1). These comprise both movements that are clearly involuntary and more complex abnormalities, best considered ‘neurobehavioural’, which span the voluntary/involuntary divide. Tics, for example, like acute akathisia, are preceded by an urge to activate in a way that is ‘suppressible yet irresistible’ (Dure Reference Dure and DeWolfe2006).
BOX 1 Major movement types comprising tardive dyskinesia

Signs
Tardive dyskinesia can affect any voluntary muscle and an elementary but important point is that, as a syndrome, can create kaleidoscopic presentations from diverse constituents (Table 1). The sheer range and combinations of movement types contribute greatly to confusion inherent to categorisation and diagnosis.
Orofacial
Clinically, distribution is an invaluable aid to diagnosis (Fig. 2). In 80% of cases, especially at the milder end, disorder is found in the lower third of the face. Clinical experience suggests this, too, may comprise different components.
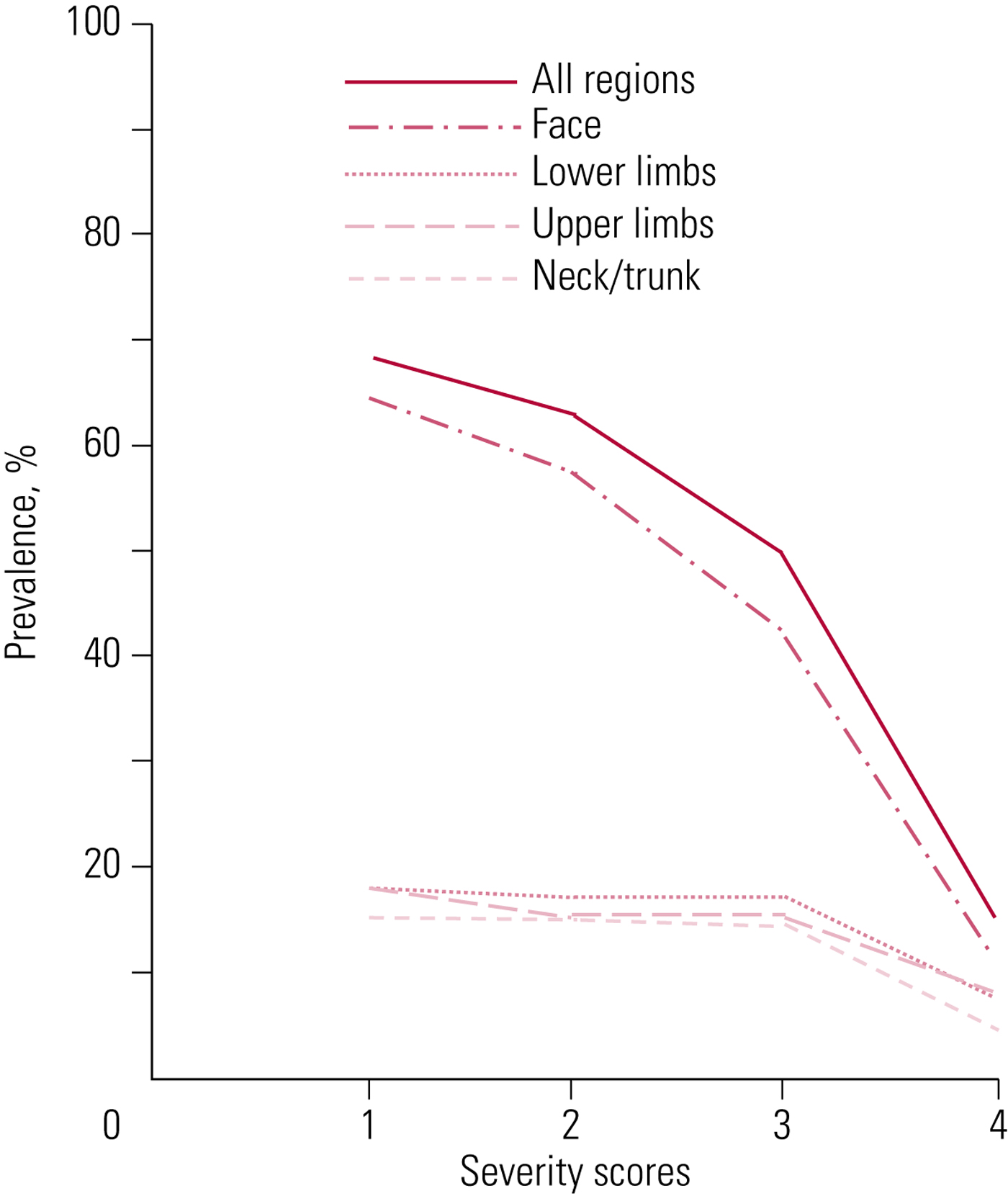
FIG 2 Prevalences of movement disorders in different body regions at different severity scores on the Abnormal Involuntary Movement Scale (AIMS) (Owens Reference Owens, Johnstone and Frith1982).
Disorder has always been thought of as starting with the tongue, although this has never been confirmed. The author has certainly seen patients in whom other movements were gross but tongue abnormality minimal or absent. These early signs are usually described as vermicular (worm-like) and in the tongue. In the early stages individual parts of the tongue's surface can be seen to move, with progression, turning it into a ‘bag of worms’. The structure itself, however, is not strikingly displaced, remaining within the floor of the mouth. Displacement becomes evident with progression, when activity causes curling along its transverse axis, arching and/or rotation on its longitudinal axis or lateral movement, in more severe instances sweeping inner surfaces, especially of the cheeks (‘bon-bon’ sign) and sometimes the lips, usually the lower lip. Protrusion beyond parted lips can be central or to one side and may be slow and rhythmic, or darting (‘fly-catcher’ sign). Mouth-opening, forced jaw closure, bruxism, lateral jaw deviation and a range of lip movements, including puckering, pouting, smacking and bridling (retraction of the angles of the mouth), with or without accompanying sounds, complete a characteristic picture.
Some of this activity seems choreoathetoid and authentically involuntary. However, in the more extravagant activity, such as buccal sweeping and chewing, we see the basis of complex behaviours, where intra-oral activity is coordinated with lips and jaws. The combination of complex, oral-focused disorder has traditionally been referred to as the bucco–linguo–masticatory (BLM) triad, although the buccal component is a passive participant compared with the often active involvement of lips, and the triad might better be described as labio–linguo–masticatory (Owens Reference Owens2014).
Only one study (Glazer Reference Glazer, Morgenstern and Neidzwiecki1988) supports the author's impression that two different phenomenologies comprise the oral component of tardive dyskinesia: one, authentically involuntary and of choreoathetoid type, predominantly localised to the tongue but with the potential to involve jaw and lips; the other, a coordinated pattern of ‘release’ conditioned behaviours related to normal oromandibular/lingual activity in the preparation of food for swallowing. However, such a distinction could explain why patients frequently seem unaware of often disfiguring disorder. This observation, much commented on (Alexopoulos Reference Alexopoulos1979; McPherson Reference McPherson and Collis1992; Sandyk Reference Sandyk, Kay and Awerbuch1993), is in contradistinction to other EPS syndromes (Owens Reference Owens2014) but may be understandable if relevant to automatic behaviours as opposed to genuinely involuntary movements. In the author's experience, patients with dominant independent tongue activity are very poorly tolerant of what is both annoying and, because of local irritation, sometimes uncomfortable, whereas those with complex oromandibular activity which, because it is coordinated does not isolate the tongue or subject it to injury (although it may stimulate hypertrophy), are more likely to be indifferent.
Breaking down motor disorders phenomenologically has merit in demonstrating the rich disorder tardive dyskinesia presents but does introduce an element of artificiality. Forced tongue protrusion clearly requires the lips, and in those with teeth the jaws, to part. So, when are these separate components or part of a package? Many such examples arise when recording with multi-item, as opposed to global impression, rating scales (Owens Reference Owens2014).
Tics may rarely affect the upper face but more commonly movements here are choreiform or dystonic and genuinely involuntary. Involvement of eyelids, periocular and temporal musculature gives rise to blepharoclonus, partial or complete blepharospasm, frowning, furrowing, grimacing, etc. Marked upper face involvement produces especially disfiguring disorder.
Peripheral
In 20% of cases, distribution is predominantly trunk and/or limbs, although peripheral disorder may develop in combination with orofacial abnormality. With the exception of tics (which are relatively uncommon), peripheral disorder is characteristically involuntary and dystonic in type, with sustained (static) or kinetic attitudinal changes in the head/neck and limbs and/or postural distortions of the trunk. Torticollis is often best spotted from behind, in the compensatory kyphoscoliosis evident on walking, where the shoulder on the side of the turn is raised and promoted forward, or by the presence of antagonistic gestures (touching/pressing/massaging the opposite side of the neck to relieve the extent or discomfort of the displacement). Involvement of the upper limb girdle produces irregular shoulder shrugging or rotations, and of pelvic musculature, so-called copulatory movements. Arms may pronate, fingers show lateral out-splaying or flexion/extension piano-playing movements. Legs may likewise rotate, with intermittent, sustained rotational movements of ankles and out-splaying of toes. The lateral truncal leans, usually associated with slight rotation, of Pisa (or Ekbom) syndrome are rare as the sole abnormality but can seriously impede mobility. Peripheral disorder centred on lower limbs can be hard to distinguish from tardive akathisia.
Internal voluntary muscles of the oropharynx, larynx and diaphragm can also be involved in tardive changes, resulting in some of the most medically concerning complications, such as dysphagia (with or without inhalation), stridor and speech distortions (laryngeal adductor or abductor spasms), along with less sinister hiccups, grunts and groans from intercostal or diaphragmatic involvement.
Words cannot adequately describe the varied and intricate presentations of tardive dyskinesia, and inability to categorise all aspects of its component parts should not unnerve the generalist. The important point is to be sensitive enough to this variability to recognise when iatrogenic disorder is taking root without misattributing it.
Subtypes
Tardive dyskinesia may come on during drug exposure (treatment-emergent) or following discontinuation or dose reduction (withdrawal-emergent). Sometimes the latter type persists but withdrawal-emergent disorder is, overall, more likely to resolve and is the most common presentation in children (Owens Reference Owens2014). Resolution usually occurs over a couple of weeks but may take months.
In some instances, dystonia is the predominant or exclusive abnormality and in such cases a diagnosis of tardive dystonia is appropriate. This distinction is important as tardive dystonia is much more functionally incapacitating than generic tardive dyskinesia, especially if that term is restricted to perioral disorder, and may have different treatment and other characteristics. Clinically, a classification based on how prominent dystonia is to the overall presentation can be useful in treatment planning (Adityanjee Reference Adityanjee, Aderibigbe and Chowdary1999) (Box 2).
BOX 2 Subdivision of tardive dystonia
Type 1 Pure dystonia: no other movement disorder type
Type 2 Coexisting dyskinetic movements in same or different body part(s), but dystonia predominates
Type 3 Coexisting dyskinetic movements in same or different body part(s) more prominent than dystonia
Type 4 Part of a mixed picture: coexisting Parkinsonism, akathisia, etc. with no one predominating
Rarely, tics form the greater part of the presentation and, again, separate consideration is justified. Only a handful of cases of tardive Tourette syndrome have appeared in the literature but they are worth knowing about as they can be among the most dramatic tardive dyskinesia presentations of all.
Analysis of rating scale data consistently shows at least two components to tardive dyskinesia – perioral, most strongly associated with age, and limb/truncal, more directly attributable to drug factors (e.g. Kidger Reference Kidger, Barnes and Trauer1980; Glazer Reference Glazer, Morgenstern and Neidzwiecki1988; Gureje Reference Gureje1989). In line with the view presented above, Glazer et al (Reference Glazer, Morgenstern and Neidzwiecki1988) found that orofacial disorder itself comprised two components, although ‘jaw–tongue’ and ‘face–lips’ are not entirely consonant with my experience. Nonetheless, data supporting distributional differences are sufficiently robust that assessment of interventions should take them into account.
Recent trends in classification
The concept of tardive dyskinesia has undergone expansion and contraction over the years, initially including what would become akathisia (Faurbye Reference Faurbye, Rasch and Peterson1964), which was subsequently stripped out, although the most common movement disorder, tremor, was always specifically excluded (Marsden Reference Marsden, Tarsy, Baldessarini, Benson and Blummer1975). Recently, neurologists have proposed subclassification of all tardive presentations under the single rubric of ‘tardive syndrome’ (Frei Reference Frei, Truong and Fahn2018), where the core concept is not later onset but persistence (Box 3). This is justified on the basis of varied phenomenologies.
BOX 3 Tardive syndrome
Encompassing
• Tardive dyskinesia (oro–buccal–lingual, plus choreiform movements in any other body parts)
• Tardive stereotypy
• Tardive akathisia
• Tardive dystonia
• Tardive myoclonus
• Tardive tremor
• Tardive tics (Tourette-like)
• Tardive pain (chronic oral/genital pain/dysaesthesia)
• Withdrawal-emergent dyskinesia
In this system, tardive dyskinesia refers to ‘classical oro-buccal-lingual dyskinesia’ but also comprises ‘choreic movements in other body parts’, although tardive stereotypy ‘fails to fulfil the definition of stereotypy’ (Aquino Reference Aquino and Lang2014), so is somewhat left in the air! Tardive akathisia, having started life as a discrete entity, becomes subsumed within the generality of tardive dyskinesia. A tardive gait, described as ‘dancing’ or ‘duck-like’, is proposed as ‘a new entity’ (Kuo Reference Kuo and Jankovic2008), while so-called tardive tremor is also incorporated. Tardive Parkinsonism is, furthermore, considered legitimate by some.
Simplicity might justify such changes but phenomenology does not necessarily correlate sufficiently with pathophysiology to support the validity of cross-sectional, clinically led micro-classification. It seems peculiar to lump together all oromandibular with peripheral disorder when, as noted, multivariate analysis of rating scale data consistently suggests a syndrome of at least two components, and the question of whether tardive akathisia has pathophysiologically more in common with akathisia or tardive dyskinesia was much debated (Munetz Reference Munetz and Cornes1982; Barnes Reference Barnes and Braude1985; Dufresne Reference Dufresne and Wagner1988) but never answered. As the psychiatric literature shows, gait disorders can be striking in tardive dyskinesia but the merit of considering them a discrete new entity is questionable, while tardive tremor is exceedingly rare (Ure Reference Ure, Dhanju and Lang2016; Frei Reference Frei, Truong and Fahn2018) and of doubtful relevance to the long-established tardive dyskinesia concept. Finally, the notion of tardive parkinsonism (Melamed Reference Melamed, Achiron and Shapira1991) seems contemplatable only when the prolonged binding characteristics of high-potency dopamine D2 receptor blockers and the revealing effect of drugs on idiopathic disease are overlooked (Owens Reference Owens2014).
One innovation within this system is the highlighting of ‘tardive pain’ (Frei Reference Frei, Truong and Fahn2018). While conventional pain is a frequent accompaniment of tardive, especially craniocervical, dystonia, sometimes discomfort in the mouth seems disproportionate. Furthermore, the author has encountered a small but memorable group of patients, usually female, with bizarre complaints – more dysaesthesia than pain and usually genitally focused – that at the time the author never associated with tardive dyskinesia. This may not arise often but occasionally may be diagnostically illuminating.
For psychiatrists who deal daily – and, most importantly, prospectively – with the consequences of long-term antipsychotic use, the traditional concept of tardive dyskinesia, defined in terms of movement types and distribution, is best retained until alternatives can demonstrate utility and validity.
Differential diagnoses and diagnostic criteria
There is always a differential diagnosis in someone presenting with new-onset movement disorder and this can be extensive (Table 2). A similar pattern of predominantly oromandibular disorder can develop in older individuals, formerly referred to as senile chorea but more accurately called simply spontaneous dyskinesias. Likewise, 16% of edentulous individuals can be similarly afflicted, where disorder may result from loss of proprioceptive input from dental apices (Koller Reference Koller1983). These states have been poorly studied, although in psychiatric cohorts, data suggest a much lower prevalence (2.6%) in the unimpaired elderly than similarly appearing movements in those on antidopaminergic medication (Woerner Reference Woerner, Kane and Lieberman1991). In younger patients with untreated schizophrenia the relationship between spontaneous dyskinesias and a range of apparently involuntary movements that seem indistinguishable from tardive dyskinesia (Owens Reference Owens, Johnstone and Frith1982; McCreadie Reference McCreadie, Thara and Kamath1996) is also unclear.
TABLE 2 Tardive dyskinesia: some differential diagnoses
Having reassured oneself of the validity of the diagnosis, it is useful, even in routine practice, to apply standardised diagnostic criteria, the most widely utilised being those of Schooler & Kane (Reference Schooler and Kane1982) (Box 4). This is less about diagnosis, which should be established clinically, but allows for monitoring progression, persistence etc. and can contribute to prognostication.
BOX 4 Tardive dyskinesia: the Schooler and Kane criteria
A. Prerequisites
1 Minimum 3 months cumulative antipsychotic exposure (continuous or discontinuous)
2 Abnormal movements at least ‘moderate’ in one body part or ‘mild’ in two or more (standardised rating)
3 No other potential cause
B. Diagnoses
(a) Probable: Fulfils 1–3 on a single examination
(b) Masked probable: Fulfils 1–3 at first examination but fails on 2 above on second examination within 2 weeks of drug increment or reintroduction
(c) Transient: Fulfils 1–3 at first examination but fails on 2 above on second examination within 3 months with no increase or reintroduction of drug (reduction possible)
(d) Withdrawal: Does not fulfil 2 while on medication but fulfils 1–3 within 2 weeks of stopping drugs of average half-life or 5 weeks with long half-life preparations (e.g. depots)
(e) Persistent: Fulfils 1–3 at first examination and over at least a subsequent 3-month period
(f) Masked persistent: As (e) but fails to fulfil 2 with 3 weeks of dose increment or reintroduction
Epidemiology
After a shaky start, the tardive dyskinesia literature covering the period of older antipsychotics produced a body of quality research, funded independently of industry, relating to prevalence, incidence and risk factors. No comparable research base exists for the newer, so-called atypical drugs but as no evidence has emerged to support the hope that (with the sole exception of clozapine) these drugs differ sufficiently in their pharmacology to affect the epidemiology of tardive dyskinesia, it is legitimate to present what we knew then as what we ought to know now.
It is important to realise that, owing to the intimate relationship all EPS syndromes bear to practice principles (how drugs are used), there is no such thing as the prevalence, incidence, etc. – only what is relevant to how antipsychotics are/were used there and then.
Prevalence
By the late 1980s/early 1990s there was concurrence that the point prevalence of tardive dyskinesia was 20% in chronically treated samples in both the USA (Woerner Reference Woerner, Kane and Lieberman1991) and Europe (Muscettola Reference Muscettola, Pampallona and Barbato1993). Individuals were defined by Schooler and Kane criteria, implying more than one examination, which likely lowers overall prevalence as it removes patients with mild or equivocal signs. Nonetheless, this corresponds well to the less impressive literature relating to newer compounds, recent review of which found a headline mean global prevalence of 25.3% (Carbon Reference Carbon, Hsieh and Kane2017). Standardised criteria were mostly not applied in this work and some contamination with unrelated and spontaneous movements would be inevitable. It seems reasonable to conclude that the prevalence of tardive dyskinesia remains at 20%.
The modern literature is of insufficient quality to allow comment on severity (Carbon Reference Carbon, Hsieh and Kane2017) but these worrying figures are tempered by the fact that most abnormality in point/period prevalence tardive dyskinesia studies is of mild severity, usually requiring specific examination to elicit. In one study, only 43% of those affected were rated as moderate or moderately severe at follow-up and none was rated severe (Kane Reference Kane, Woerner and Lieberman1988). Severe tardive dyskinesia is the exception.
Incidence
Two prospective US studies found cumulative annual incidences of 5% for persistent disorder: the Hillside study (Kane Reference Kane, Woerner and Weinhold1982) and the Yale study (Morgenstern Reference Morgenstern and Glazer1993). The Hillside (New York) study comprised diagnostically heterogeneous patients, mainly treated with high-potency compounds but the same group subsequently followed up patients with first-episode schizophrenia and confirmed a cumulative incidence of 4.8% at 1 year and 15.6% at 4 years (Chakos Reference Chakos, Alvir and Woerner1996). The exceptionally long follow-up of the original Hillside sample allowed confirmation of a fact for many years viewed sceptically. Early cross-sectional assessments of predominantly institutional patients reported prevalences of over 50% (Owens Reference Owens, Johnstone and Frith1982). Hillside data found a cumulative figure of 52% over 15 years, again mostly of mild disorder (Fig. 2). However, by the late 1980s amber flags were flying.
Concern was emphasised by projections from the Yale study (Glazer Reference Glazer, Morgenstern and Doucette1993). One of the many frustrations of tardive dyskinesia is its apparently random occurrence in patients otherwise asymptomatic for years. The Yale study, focusing on schizophrenia, reported a 5-year incidence of 5.3% (Morgenstern Reference Morgenstern and Glazer1993) but because of variable durations of antipsychotic exposure, permitted calculation of the long-term risk of tardive dyskinesia emerging in those who remained free of disorder for increasingly longer periods of time. These projections (Table 3) showed that the risk never really disappears. Even after two decades of uneventful antipsychotic treatment, the risk with continued exposure is still around 10% over the subsequent 5 years.
TABLE 3 Estimated risk (%) of persistent tardive dyskinesia in the context of prior exposure
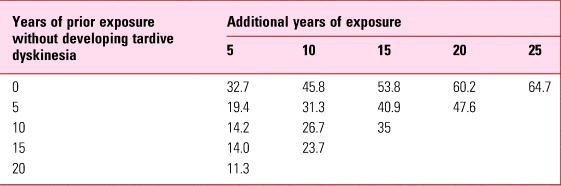
Source: data from Glazer et al (Reference Glazer, Morgenstern and Doucette1993).
A further important finding from the Hillside group is that risk over time is not linear. Incidence accelerates in the second 6 months of exposure, with only gradual increment thereafter until a further ‘jump’ early in the fifth year (Chakos Reference Chakos, Alvir and Woerner1996). That most of this is mild/moderate disorder leaves the possibility that relationships with risk are log-dose ones, where risk plateaus above moderate doses (Tarsy Reference Tarsy and Baldessarini2006), which is both theoretically and clinically plausible.
Risk factors
A great deal of effort went into establishing risk factors for tardive dyskinesia. The older literature was largely retrospective and blighted by the flaws of such approaches. Nonetheless, there is some consistency between what it suggested and factors identified from later, largely prospective data. Box 5 presents a composite of those factors shared across both designs (for details, see Owens Reference Owens2014).
BOX 5 Tardive dyskinesia: predisposing factors
The following predisposing factors are evident in both retrospective and prospective literatures:
• Age
• Antipsychotic potency
• Antipsychotic-free intervals (number)
• Previous (early) extrapyramidal symptoms
• Affective disorders
• Organicity: leucotomy, structural brain change, negative (deficit) symptoms
• Genetics
Age
The most consistent factor predisposing to the development of tardive dyskinesia is age – the older the patient the greater the risk. It is likely that age operates independently of duration of illness and exposure, as annual incidence is much greater (up to six-fold) in those first exposed to antipsychotics in middle to late life (Jeste Reference Jeste, Caligiuri and Paulson1995, Reference Jeste2000). Furthermore, in elderly patients the cumulative incidence of orofacial disorder is twice that of peripheral disorder (Paulson Reference Paulson, Caligiuri and Palmer1996), supporting the idea that antipsychotics may in part be bringing forward focal, age-related changes.
Antipsychotic variables
Neat correlations with antipsychotic drug variables (daily dose, duration of exposure, cumulative exposure, potency, polypharmacy), which seem obvious, proved hard to pin down, although this is hardly surprising. Not only is it unlikely that associations are linear, the pharmacokinetics of antipsychotics are so variable across individuals as to introduce a major obstacle to establishing risk relationships. However, in general, prospective data do suggest that the more antipsychotic a patient receives, in terms of daily exposure – especially as reflected in potency – more than duration, the greater the likelihood of tardive dyskinesia. In the Yale study, the risk was 2.5 times greater in those receiving 500 mg/day chlorpromazine equivalent (CPZeq) antipsychotic than those on 100 mg CPZeq or less (Morgenstern Reference Morgenstern and Glazer1993), and the Hillside study, although establishing only a trend significance for dose overall, reported that each 100 mg CPZeq dose increase was associated with a 5% increase in at least ‘presumptive’ disorder (Chakos Reference Chakos, Alvir and Woerner1996). This emphasises that, for tardive dyskinesia risk, what we consider standard doses are sufficient to sow the seeds of dopaminergic disruption.
Drug-free intervals
One unexpected finding from the older literature was an association with drug-free intervals. This was at a time when ‘drug holidays’ were widely advocated to diminish exposure and hence risk but the best discriminator between ‘reversible’ and ‘persistent’ tardive dyskinesia was the greater number of periods, 2 months or longer, the latter group experienced off antipsychotic (Jeste Reference Jeste, Potkin and Sinha1979). This was replicated with off intervals as short as 1 month and supported prospectively (Branchey Reference Branchey and Branchey1984; Chakos Reference Chakos, Alvir and Woerner1996). The risk of tardive dyskinesia may increase as much as threefold with upwards of three interruptions to treatment (van Harten Reference van Harten, Hoek and Matroos1998). This is important for clinicians – and patients – to appreciate. It is better to decide early in relapsing–remitting illnesses what ‘long-term’ medication recommendations actually mean.
Pre-existing neurological problems
Logically, those who develop long-term neurological problems would most likely be those who experienced neurological issues earlier in treatment, although this again took time to emerge (DeVeaugh-Geiss Reference DeVeaugh-Geiss and DeVeaugh-Geiss1982). In general, the literature supports an association, especially with prior akathisia in older patients. Woerner et al (Reference Woerner, Alvir and Saltz1998) found a cumulative tardive dyskinesia incidence of 26% in those without akathisia that inflated to 71% if akathisia had been present earlier. Nonetheless, while a similar increase in tardive dyskinesia risk (twofold) was found in the Schizophrenia Outpatient Health Outcomes (SOHO) study over only 12 months (Reference Tenback, van Harten and SchloofTenback 2006), its authors found ‘acute-end’ EPS to be a weak predictor of later tardive dyskinesia. One obvious problem is the composite nature of acute-end EPS, which may comprise disorders with sufficient pathophysiological variability to invalidate lumping them together. A single report, relating to newer antipsychotics, supports the intuitive belief that acute dystonias predispose to tardive dystonia (Ryu Reference Ryu, Yoo and Kim2015).
Mood disorders
There is a long-reported association between increased tardive dyskinesia risk and mood disorders, which extends to newer drugs (Gardos Reference Gardos and Casey1984). This creates an obvious paradox: if schizophrenia is associated with an inherent risk of movement disorders, why is the risk of tardive dyskinesia greater in those with mood disorders? Although replicated, this may be a confounded observation (Owens Reference Owens2014), the most obvious reason being that those with mood disorders are more likely to experience intermittent antipsychotic exposure.
Damaged neural substrate
A further association with face validity is that vulnerability may result from a damaged brain substrate. A wide range of parameters reflecting organic brain change, both primary and secondary to psychiatric disorder, have been evaluated and, although positive relationships have emerged, the association remains weak and general (Owens Reference Owens2014).
Genetic predisposition
Since not everyone at risk (i.e. exposed) develops tardive dyskinesia, liability has long been put down to individual susceptibility. While any one, or combination, of the above risks might constitute predisposition, by far the most likely factor(s) underlying individual susceptibility are genetic. These fall into two broad categories – mutations that directly predispose regardless of drug exposure (e.g. receptor/signalling proteins; enzymes) and those that predispose by altering exposure itself (e.g. pharmacokinetics) (Tables 4 and 5).
TABLE 4 Tardive dyskinesia: negative or unreplicated gene mutations suggested as predisposing
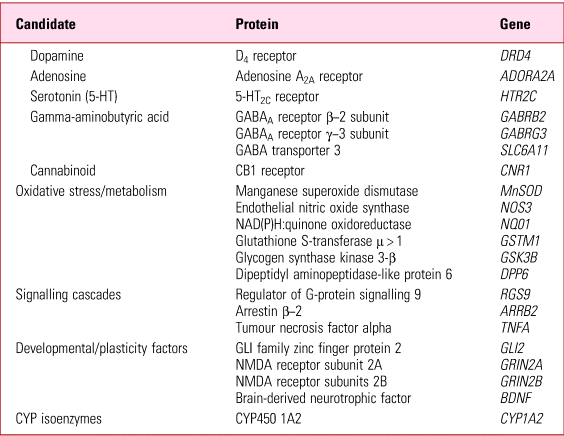
Source: Owens (Reference Owens2014).
TABLE 5 Tardive dyskinesia: replicated gene mutations suggested as predisposinga
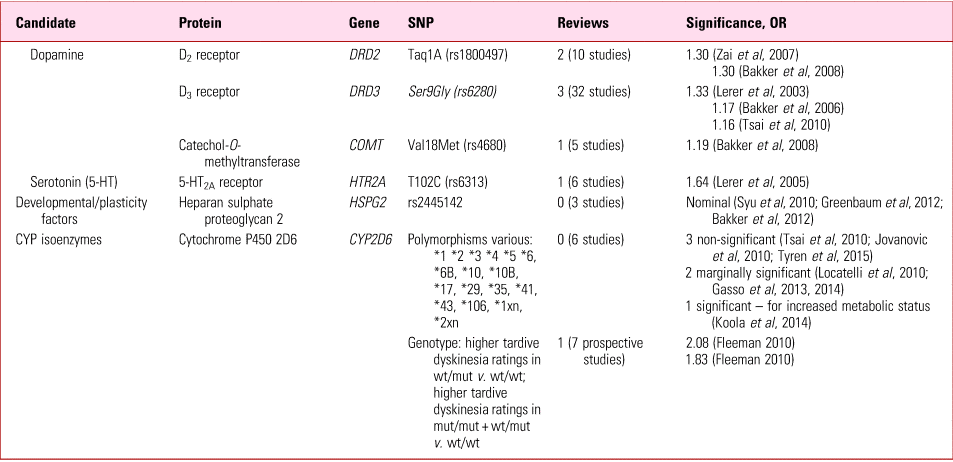
a The references cited in this table can be found in Owens (Reference Owens2014).
SNP, single-nucleotide polymorphism; mut, mutated allele; wt, wild-type allele.
Source: Owens (Reference Owens2014).
It is beyond my present remit to review the relevant genetic literature and interested readers are referred to specialist publications (MacNeil Reference MacNeil and Muller2016). It is fair to say, however, that the situation has not advanced substantially since I concluded, on the basis of data up to 2012, that ‘work to date does not point to a clear, single abnormality that might provide a key to unlocking “the” genetic predisposition to tardive dyskinesia at the receptor, enzyme or kinetic levels’ (Owens Reference Owens2014).
Much of this work explores theoretically obvious candidates, such as dopamine receptor genes and those of systems known to modify dopaminergic function (serotonin, adenosine, GABA, cannabinoid, etc.), although more recently genome-wide association studies (GWAS) have emerged. Results from either method thus far point at best to marginal effects, at worst to statistically artefactual, incidental findings.
The most statistically robust (though still weak) effects relate to cytochrome P450 (CYP450) genotypes (Table 5). Because of their metabolic requirements (hydroxylation/demethylation/dealkylation) many psychotropic compounds are particularly dependent on the highly polymorphic CYP2D6. Although susceptible to inhibition, unusually, 2D6 is resistant to induction, so the impact of polymorphisms on overall metabolic capability can be strikingly magnified. Focusing on just 7 prospective studies (from a mixed group of 20), Fleeman et al (Reference Fleeman, McLeod and Bagust2010) found a significant reduction in tardive dyskinesia ratings in extensive metabolisers, compared with intermediate and poor metabolisers – i.e. those with two functioning wild-type (wt) alleles were relatively spared compared with those with one or two non-functioning or poorly functioning mutant (mut) alleles respectively. Genotyping 2D6 is easy, relatively inexpensive and commercially available, although currently evidence is insufficient to justify routine testing prior to commencing long-term antipsychotics.
This is a fledgling literature containing major teething problems, the most obvious of which relate to imprecise clinical characterisation of movement types, generalisation (assuming a single causative mechanism for every presentation) and, most damning of all, serious under-powering.
Course and outcome
Following emergence, tardive dyskinesia tends to plateau in severity, usually over weeks. Although progression by extension may occur, once stable, progression by severity is uncommon.
There has never been unanimity on the potential for reversibility (Gardos Reference Gardos and Cole1980). One problem in addressing this is whether, in an iatrogenic disorder secondary to necessary long-term treatment, reversibility refers to what happens when the drug is stopped or when it is maintained.
Evidence suggests that, on cessation of an antipsychotic, reversibility can occur but figures vary – from 33% after 2 years (Kane Reference Kane, Woerner and Borenstein1986) to only 12% over an average of 6.7 years (Kang Reference Kang, Burke and Fahn1986). Such variability relates to types of disorder (e.g. orofacial versus peripheral, especially dystonia) and durations of drug exposure and movement disorder. Recently, Zutshi et al (Reference Zutshi, Cloud and Factor2014) reported very low rates of spontaneous reversibility: 2.8% over an average of 4.3 years. The paediatric literature strongly suggests that reversibility on antipsychotic cessation is the rule (Campbell Reference Campbell, Adams and Perry1988), whereas in working-age adults, it is much less predictable and in the elderly it is probably not likely (Gardos Reference Gardos and Cole1983). Age, therefore, is the crucial factor.
Nonetheless, in patients who remain on medication long-term (up to 10 years), the incidence of new cases tends to be offset by cases in whom disorder ameliorates (Gardos Reference Gardos, Casey and Cole1994). Whether this represents genuine resolution or suppression is unclear but age-related pharmacokinetic and other changes favour the latter.
Tardive dyskinesia can be socially stigmatising and debilitating. Importantly, its physical outcomes can be highly negative (Box 6), with the suggestion of a 1.5- to 2.5-fold increase in mortality risk (Chong Reference Chong, Tay and Subramaniam2009; Dean Reference Dean and Thuras2009).
BOX 6 Serious medical adverse outcomes of tardive dyskinesia
Trauma
• Falls
• Fractures
• Myoglobinuria
• Renal failure
Overactivity
• Weight loss
• Cachexia
• Lingual hypertrophy
Dysphagia
• Choking
• Inhalation
Respiration
• Stridor
• Speech impairment
Possible death
This is not a condition whose neglect, in research or clinical terms, is justified.
MCQs
Select the single best option for each question stem
1 With regard to the clinical features of tardive dyskinesia:
a 80% of patients have choreoathetoid-type perioral movements
b internal voluntary muscles are selectively spared
c peripheral tremor at a frequency of <6 Hz is characteristic
d the earliest signs are lingual
e withdrawal-emergent disorder is irreversible.
2 Tardive dyskinesia:
a characteristically progresses by severity focally rather than by extension
b comprises homogeneous phenomenology
c is not seen in children exposed to causative agents
d is associated with increased mortality risk
e is deeply troubling to sufferers.
3 The incidence of tardive dyskinesia:
a is gender-specific
b is greatest in those with organic brain disorders
c is higher in the first decade of antipsychotic exposure
d is inversely proportionate to age
e is non-linearly related to duration of antipsychotic exposure.
4 An increased risk of developing tardive dyskinesia is associated with:
a antipsychotic potency
b cumulative antipsychotic exposure
c drug-free periods longer than 2 years
d high antipsychotic blood levels
e use of two or more antipsychotics concurrently.
5 Genetic predisposition to tardive dyskinesia is suggested in replicated associations with polymorphisms in:
a BDNF (brain-derived neurotropic factor gene)
b CNR1 (CB1 cannabinoid receptor gene)
c CYP 1A2 (cytochrome P450 1A2 gene)
d DRD4 (dopamine D4 receptor gene)
e HTR2A (5-HT2A receptor gene).
MCQ answers
1 d 2 d 3 e 4 a 5 e










eLetters
No eLetters have been published for this article.