Psychoses affect 2–3% of the population, with schizophrenia occurring in approximately 1% (Perälä Reference Perälä, Suvisaari and Saarni2007). Treatment has been advanced by the introduction of second-generation antipsychotics (SGAs); initially heralded as revolutionary because of their low propensity for extrapyramidal side-effects and seemingly significant improvement of negative and cognitive symptoms, later work tempered this as it became apparent that they had a different, particularly cardiometabolic, harm profile (Leucht Reference Leucht, Corves and Arbter2009) and adherence to them proved just as troubling for patients (Nakonezny Reference Nakonezny and Byerly2006). There has been significant debate regarding the comparable efficacy of first- and second-generation antipsychotics, and much heterogeneity exists within both classes. SGAs arguably provide additional benefit through reduced risk of suicide and all-cause mortality (Sernyak Reference Sernyak, Desai and Stolar2001; Kiviniemi Reference Kiviniemi, Suvisaari and Koivumaa-Honkanen2013).
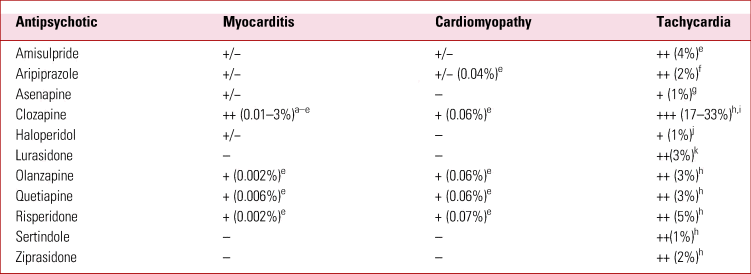
Second-generation antipsychotics have significant cardiac side-effects in common with the first generation of these drugs, as well as additional novel side-effects not seen in the first generation. The most common and/or concerning cardiac side-effects noted with SGAs are sinus tachycardia, QT prolongation, sudden cardiac death, myocarditis and cardiomyopathy. An understanding of the relative frequency (Table 1) and severity of these diagnoses and their subsequent management is important to guide the selection of antipsychotic treatment, balance the risks and benefits of treatment, and recognise and promptly treat complications if they arise. This review aims to provide a practical approach to the diagnosis and management of each of these cardiac side-effects of SGAs. We have, for comparison, included haloperidol, one of the most widely used first-generation antipsychotics.
TABLE 1 Literature reports of cardiac side-effects of common antipsychotics
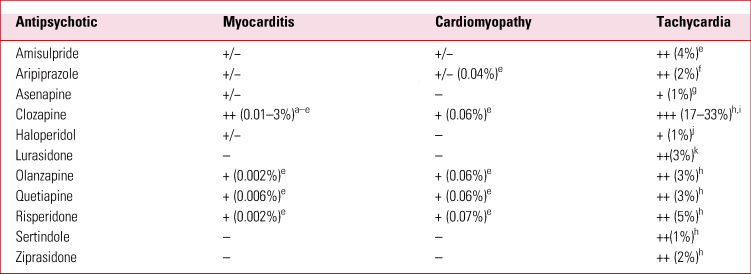
–, not reported in the literature; +/−, isolated case reports; +, <1%; ++, >1%; +++, >10%.
a. La Grenade et al (2001).
b. Reinders et al (Reference Reinders, Parsonage and Lange2004).
c. Ronaldson et al (2015b).
d. Rohde et al (Reference Rohde, Polcwiartek and Kragholm2018).
e. Barnes et al (Reference Barnes, Leeson and Paton2017).
f. Polcwiartek et al (Reference Polcwiartek, Sneider and Graff2015).
g. Saphris FDA label (2019).
h. Polcwiartek et al (Reference Polcwiartek, Kragholm and Schjerning2016).
i. Nilsson et al (Reference Nilsson, Edström and Lindström2017).
j. Haloperidol FDA label (2019).
k. Loebel et al (Reference Loebel, Cucchiaro and Sarma2013).
General considerations
The cardiac side-effects of antipsychotic medication can cause significant anxiety among prescribers – potentially life-threatening conditions can present suddenly with few, if any, warning symptoms and can result in significant morbidity or death. Despite these risks, it is important to note that these are relatively rare complications of a treatment shown to have significant benefits in terms of quality of life and mortality (Sernyak Reference Sernyak, Desai and Stolar2001; Barak Reference Barak, Mirecki and Knobler2004). Furthermore, even in patients treated with antipsychotics, presentation with conditions unrelated to the treatment remains significantly more common than the specific antipsychotic-related side-effects. Therefore, if a patient presents with apparent cardiac symptoms, such as chest pain, palpitations or breathlessness, it is important to exclude medical causes, including pneumonia, asthma, acute myocardial infarction, chronic obstructive pulmonary disease and pulmonary embolism, before arriving at a diagnosis of an antipsychotic-related side-effect.
Before starting antipsychotic treatment in any patient, an assessment of cardiovascular risk factors should be undertaken as this remains the most common cause of cardiac complications and death in patients with schizophrenia. This should include a detailed medical history and examination to screen for pre-existing cardiac disease and a family history of sudden or premature death (at <45 years of age) in first-degree relatives to identify individuals at higher risk. Investigation of these factors before treatment is started is beneficial to reduce cardiovascular risk and to prevent pre-existing cardiac disease from being incorrectly attributed to SGA treatment if discovered after treatment has been started. Guidelines from the National Institute for Health and Care Excellence (NICE; 2018) recommend a baseline electrocardiogram (ECG) as a simple and cost-effective test in patients starting an antipsychotic who have any risk factors for cardiovascular disease detected from the medical history or physical examination or a family history of cardiovascular disease. In addition, individuals being started on antipsychotic medication as in-patients should have an ECG performed on admission. This provides a baseline measure of the QT interval and can identify pre-existing cardiovascular disease. More detailed investigations, such as a transthoracic echocardiogram (TTE), should be reserved for individuals with symptoms or signs suggestive of existing cardiac disease on initial review or with an abnormal baseline ECG.
Tachycardia
Incidence
The development of sinus tachycardia has been reported with most antipsychotic medications, including first- and second-generation therapies. It is most common and most marked with clozapine: between 17 and 33% of patients are affected (Nilsson Reference Nilsson, Edström and Lindström2017). This effect appears to be dose dependent (Lally Reference Lally, Brook and Dixon2014, Reference Lally, Docherty and MacCabe2016) and usually occurs transiently during dose titration, particularly if this is rapid. Clinical trials and dose-finding studies have reported that the majority of SGAs and haloperidol increase the resting heart rate compared with placebo, but the incidence of developing a heart rate >100 beats per minute (bpm) is much lower than that seen with clozapine (Table 1).
Mechanism
The tachycardia caused by SGAs is related to their antagonistic effects on various receptors of the autonomic nervous system, including muscarinic cholinergic (M1) receptors and adrenergic receptors (particularly α1). Antagonism of the former reduces vagal tone and of the latter reduces sympathetic activity and vascular tone, these effects together leading to a reflex tachycardia.
Clinical management
Sinus tachycardia because of SGA treatment is usually benign and self-limiting. However, the development of tachycardia can indicate a systemic illness associated either with the therapy, such as myocarditis or heart failure, or with unrelated causes such as atrial fibrillation, sepsis, hyperthyroidism, pulmonary embolism or anaemia. A persistent marked tachycardia (>120 bpm) or newly developed tachycardia despite no change in dose should prompt further clinical review, with a detailed history and clinical examination. Additionally, thyroid function, full blood count, inflammatory markers, natriuretic peptide levels and electrolytes should be measured to exclude medical causes and an ECG should be performed to ensure that sinus tachycardia is present rather than another arrhythmia.
In the absence of an identified medical cause for the tachycardia, it usually follows a relatively benign course, improving over 4–6 weeks without the need for treatment (Marinkovic Reference Marinkovic, Timotijevic and Babinski1994). Asymptomatic patients can be safely managed conservatively with regular review of heart rate and enquiry about the development of new symptoms. Patients with symptoms such as palpitation or breathlessness or those with a heart rate persistently above 130 bpm may benefit from treatment with rate-controlling medication. Persistent tachycardia over a prolonged period risks the development of a tachycardiomyopathy – impairment of left ventricular function caused by the tachycardia – although this is rare in sinus tachycardia. This is usually reversible if the heart rate is controlled, and heart rates below 110 bpm are usually tolerated well without long-term consequences.
Treatment
A recent Cochrane review on clozapine-related sinus tachycardia did not find any adequately designed studies to guide the choice of rate control (Lally Reference Lally, Docherty and MacCabe2016). Bisoprolol is the most commonly used effective treatment, although ivabradine (Lally Reference Lally, Brook and Dixon2014) and verapamil (Das Reference Das, Kuppuswamy and Rai2014) have also been used to good effect. If the tachycardia persists despite first-line treatments or patients develop symptoms such as palpitation, breathlessness, chest pain or leg swelling they should be referred to cardiology to consider alternative or combination therapies.
Key points of the clinical presentation and management of tachycardia are summarised in Box 1.
BOX 1 Tachycardia
Symptoms
• Often asymptomatic
• Palpitations
Investigations
• Blood pressure, oxygen saturations, ECG
• Full blood count/thyroid function/electrolytes/C-reactive protein
Who to treat?
• Symptomatic patients
• Patients with heart rate >110 bpm for >3 months
• Patients with heart rate persistently >130 bpm
Management
• Beta-blockers are most common treatment
• Ivabradine and verapamil are alternatives
When to refer to cardiology
• If there is no response to first-line therapy
• If signs or symptoms of heart failure develop
• If the ECG demonstrates a rhythm other than sinus tachycardia
The QT interval
The QT interval is the electrocardiographic representation of the time taken to completely depolarise and repolarise the ventricular myocardium following conduction of an electrical impulse. Prolongation of the QT interval during treatment with psychotropic medication is one of the most common reasons for referral to cardiology from psychiatry departments. QT prolongation is associated with increased risk of mortality due to malignant ventricular arrhythmias in the general population; this association is also present with drug-induced QT prolongation (Yap Reference Yap and Camm2003).
Mechanism
Antipsychotic medications prolong the QT interval because of their effects on inwardly rectifying potassium channels (Kir) on the myocardial cell membrane. The channels, encoded by the human ether-a-go-go-related gene (hERG), permit potassium ions to leave the cardiomyocyte and return the membrane to its resting state after the passage of an action potential. Many antipsychotics block this channel to varying degrees, resulting in a reduction in this repolarising current, which in turn prolongs the action potential and is seen on the surface ECG as prolongation of the QT interval. The affinity of the SGAs at therapeutic doses for this potassium channel correlates with the QT prolonging characteristics of each drug (Kongsamut Reference Kongsamut, Kang and Chen2002).
The resulting increased duration of the action potential increases the risk of developing ventricular arrhythmias, in particular torsades de pointes, which is a form of polymorphic ventricular tachycardia (Fig. 1). This most commonly presents with palpitation and dizziness but can degenerate to pulseless ventricular tachycardia or ventricular fibrillation causing sudden death.

FIG 1 Torsades de pointes, a form of polymorphic ventricular tachycardia.
Measuring the QT interval
The QT interval is measured from the beginning of the QRS complex to the end of the T-wave. Most modern ECG machines automatically calculate the QT interval, which acts as an effective guide; however, they tend to overestimate the QT interval compared with manual interpretation and therefore corroboration by manual measurements is advised to prevent overdiagnosis. In agitated patients where a 12-lead ECG is not possible, it is acceptable to assess the ECG using limb leads for a 6-lead ECG, although a baseline 12-lead ECG should be performed at the earliest opportunity when the patient is sufficiently cooperative.
The QT interval is affected by multiple factors, including differences in autonomic tone, time of day, electrolyte changes, other medications and a recent heavy meal (Magnano Reference Magnano, Holleran and Ramakrishnan2002; Bonnemeier Reference Bonnemeier, Wiegand and Braasch2003; Cirincione Reference Cirincione, Sager and Mager2017). Therefore, if significant prolongation of the QT interval is detected on follow-up ECG recordings, in the first instance recordings should be repeated with an attempt to minimise variability in these factors.
QT correction
The QT interval is inversely correlated with heart rate and therefore to enable comparison it needs to be corrected for any given heart rate. There are multiple correction methods but the most common is Bazett's formula, which corrects for a heart rate of 60 bpm using the formula:
This tends to overcorrect the QT interval at heart rates above 100 bpm, which can result in unnecessarily discontinuing QT-prolonging medications. Therefore, if the heart rate is outside the normal range alternative formulae, such as Framingham, Hodges or Fridericia correction, should be used instead (Patel Reference Patel, Borovskiy and Killian2016; Vandenberk Reference Vandenberk, Vandael and Robyns2016).
Clinical management
The American Heart Association defines a prolonged corrected QT interval (QTc) as above 450 ms in males and 460 ms in females (Rautaharju Reference Rautaharju, Surawicz and Gettes2009).
The recommended thresholds for stopping or reducing treatment with a QT-prolonging agent is an absolute QTc above 500 ms or a relative increase >60 ms (Fanoe Reference Fanoe, Kristensen and Fink-Jensen2014). Continuation of treatment above these thresholds can be considered depending on the clinical benefit of the drug, but this should be in consultation with a cardiologist and with regular cardiac reassessments, including ambulatory ECG monitoring, correction of modifiable risk factors such as electrolytes and discontinuation other QT-prolonging medication (Roden Reference Roden2004).
Patients with a prolonged QTc who present with high-risk symptoms such as dizziness associated with palpitations or unheralded syncopal episodes should be investigated for arrhythmias with ambulatory ECG monitoring to exclude ventricular arrhythmias and referred to cardiology.
Key points of the clinical presentation and management of QT prolongation are summarised in Box 2.
BOX 2 QT prolongation
Symptoms
• Often asymptomatic
• Dizziness or palpitations if there are episodes of torsades de pointes
Investigations
• ECG – calculated QTc
• Use an alternative QT correction formula if the patient is tachycardic
• Check electrolytes: Ca2+, K+, Mg2+
• Ambulatory ECG monitoring if the patient is symptomatic
Management
• Review medication list:
• Have new medications been started recently?
• Is the patient taking other QT-prolonging medication?
• Beta-blockers can be used if torsades de pointes is present
When to stop antipsychotic
• If there is a relative increase in QTc >60 ms
• If the absolute QTc is >500 ms
When to refer to cardiology
• If stopping antipsychotic medication is planned
• If there are symptoms of dizziness, syncope or palpitations associated with prolonged QTc
QT-prolonging drugs and sudden cardiac death
Sudden cardiac death (SCD) is defined as death within an hour of symptom onset in an apparently healthy person where no extra-cardiac causes are identified by post-mortem examination (Priori Reference Priori, Blomström-Lundqvist and Mazzanti2015). The worldwide incidence of SCD in the general population is 1.4/100 000 person-years in females and 6.8/100 000 person-years in males (Priori Reference Priori, Blomström-Lundqvist and Mazzanti2015). It is up to 3 times more common in people with schizophrenia regardless of antipsychotic use (Ifteni Reference Ifteni, Correll and Burtea2014).
The risk of SCD or ventricular arrhythmias in people taking SGAs has been estimated in large retrospective cohort studies to be between 1.5 and 2.5 times higher compared with people with schizophrenia not taking antipsychotics (Ray Reference Ray, Chung and Murray2009; Wu Reference Wu, Tsai and Tsai2015). This increase in risk appears to be dose dependent (Ray Reference Ray, Chung and Murray2009), correlates with the QTc-prolonging properties of the drug and is highest when medication has been recently started (Wu Reference Wu, Tsai and Tsai2015).
Haloperidol has also been noted to have an association with SCD, particularly when used in the intravenous preparation (Meyer-Massetti Reference Meyer-Massetti, Cheng and Sharpe2010). However, this needs to be considered in light of its common use in high doses for rapid tranquillisation of acute mania or schizophrenia or for acute delirium in elderly or medically unwell patients who have significantly higher baseline risk of SCD because of electrolyte abnormalities or concomitant QT-prolonging treatments (Meyer-Massetti Reference Meyer-Massetti, Cheng and Sharpe2010). A network meta-analysis revealed that haloperidol at the standard doses used in treatment of schizophrenia appears to have effects on the QTc interval similar to those of the majority of SGAs, with only lurasidone performing significantly better (Leucht Reference Leucht, Cipriani and Spineli2013).
Management of QT-prolonging drugs and risk of SCD
There is limited literature on the practical management of the QT interval when using antipsychotic medication. Abdelmawla & Mitchell (Reference Abdelmawla and Mitchell2006) and Fanoe et al (Reference Fanoe, Kristensen and Fink-Jensen2014) suggest stratified approaches depending on the propensity of the antipsychotic to prolong the QT interval. A classification of common SGAs is outlined in Table 2. All patients starting high-risk SGAs or patients with pre-existing cardiac risk factors starting intermediate-risk SGAs should have their QT monitored using interval ECGs. When starting these treatments, it is important to consider the risk–benefit profiles of these drugs and whether lower-risk alternatives are available. Patients' electrolytes should be optimised and significant polypharmacy addressed before treatment is started. Once established on treatment, patients should have repeat cardiac assessment with a repeat ECG and enquiry about new cardiac symptoms 1–2 weeks after initiation of therapy and following each dose adjustment (Abdelmawla Reference Abdelmawla and Mitchell2006; Fanoe Reference Fanoe, Kristensen and Fink-Jensen2014) (Fig. 2). Additionally, when treating in-patients with known cardiac history, care should be taken when using QT-prolonging agents such as haloperidol for management of acute agitation or delirium. Consideration should be given to using alternative agents if possible or using continuous cardiac monitoring if the use of QT-prolonging agents is unavoidable.
TABLE 2 Classification of second-generation antipsychotics according to risk of cardiac arrhythmia
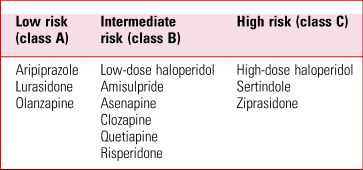
Class A, drugs with no risk of QT prolongation or torsades de pointes; class B, drugs with a propensity of inducing QT prolongation; class C, drugs with pronounced QT prolongation or documented cases of torsades de pointes or other arrhythmia.
Adapted from Fanoe et al (Reference Fanoe, Kristensen and Fink-Jensen2014).
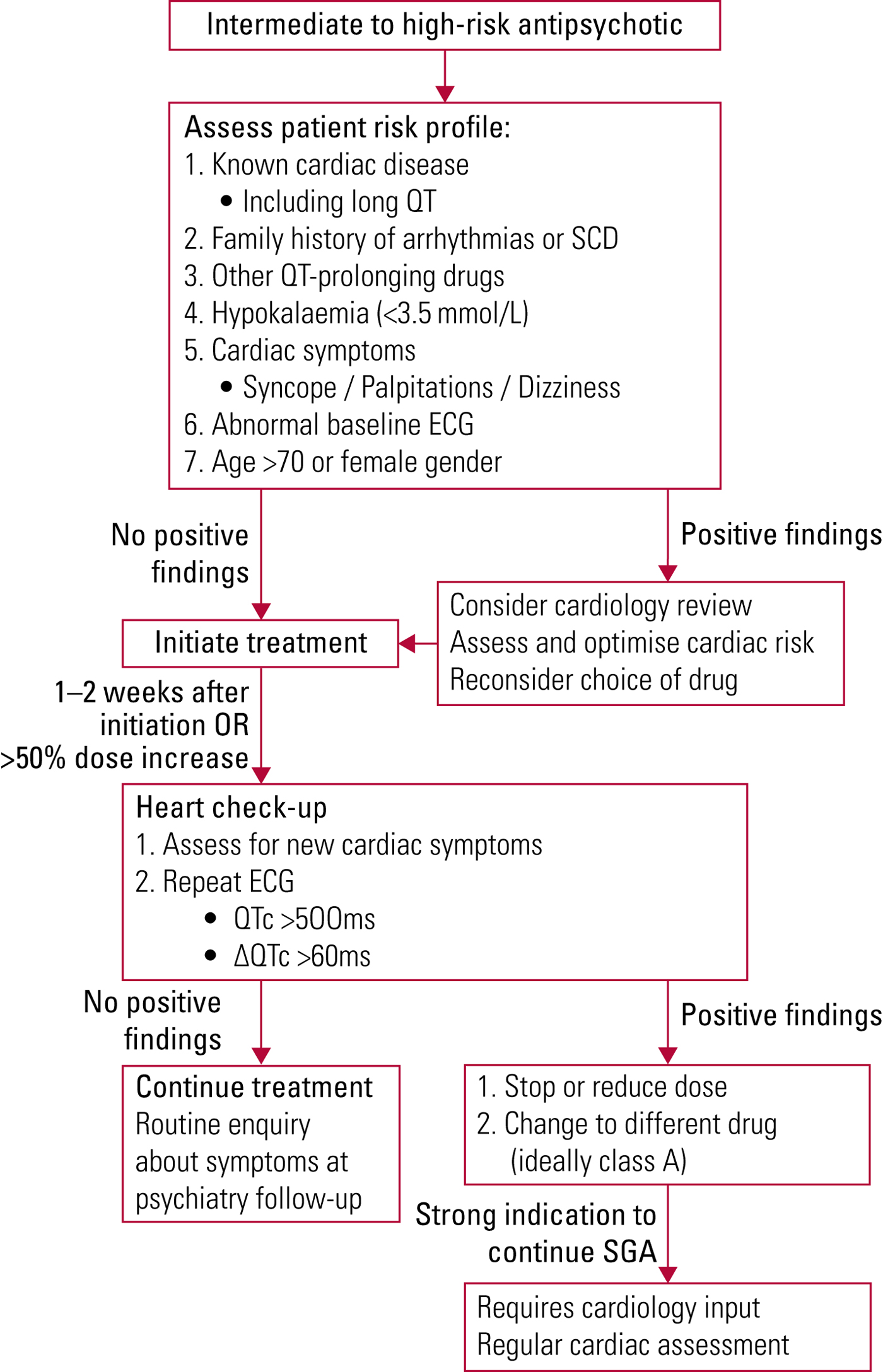
FIG 2 Management of QT-prolonging antipsychotic medication (Abdelmawla Reference Abdelmawla and Mitchell2006; Fanoe Reference Fanoe, Kristensen and Fink-Jensen2014).
Despite the increased risk of arrhythmia accompanying antipsychotic treatment, the overall risk of arrhythmic SCD remains low. Identification and management of cardiovascular risk play a much greater role in the reduction of SCD, and effective antipsychotic treatment plays a vital role in encouraging patients' adherence to modification of cardiovascular risk factors.
Myocarditis
The link between myocarditis and antipsychotic medication was first highlighted by Killian et al in 1999, when they reported 15 cases of myocarditis and high associated mortality in patients taking clozapine (Kilian Reference Kilian, Kerr and Lawrence1999). Significant research into the aetiology and incidence of myocarditis related to antipsychotic medication has ensued and significant debate continues regarding the true incidence of myocarditis associated with clozapine use.
Definition
Myocarditis is an inflammatory condition of the myocardium in which necrosis of cardiomyocytes and impairment of myocardial function is not explainable by coronary ischaemia. The gold-standard diagnostic test, proposed by the European Society of Cardiology, is histological identification of cardiomyocyte necrosis combined with inflammatory infiltrate either on endomyocardial biopsy (EMB) or autopsy examination of the heart (Caforio Reference Caforio, Pankuweit and Arbustini2013). However, a tissue diagnosis is not commonly available, and most cases are diagnosed on the basis of a combination of clinical and imaging criteria (Caforio Reference Caforio, Pankuweit and Arbustini2013).
Incidence
The risk of developing myocarditis with non-clozapine antipsychotics is very low. The literature is limited to case reports and large registry-based studies: quetiapine (Roesch-Ely Reference Roesch-Ely, Van Einsiedel and Kathöfer2002; Wassef Reference Wassef, Khan and Munir2015), olanzapine (Vang Reference Vang, Rosenzweig and Bruhn2016), aripiprazole (Vang Reference Vang, Rosenzweig and Bruhn2016) and amisulpride (Sahpaz Reference Sahpaz, Pehlivan and Turkkan2016) have all been reported, although it is difficult to establish a definitive causal relationship between the antipsychotic and myocarditis because of significant polypharmacy and difficulty conclusively excluding other causes of myocarditis unrelated to antipsychotic therapy. A link between haloperidol and myocarditis has only been reported in cases of overdose, and with very weak associations seen in registry studies (Bhatia Reference Bhatia, Gupta and Dhawan2009; Rohde Reference Rohde, Polcwiartek and Kragholm2018).
The incidence of clozapine-associated myocarditis is controversial, with estimates varying from 0.01 to 3% and reportedly up to 9% in some case series (La Grenade Reference La Grenade, Graham and Trontell2001; Reinders Reference Reinders, Parsonage and Lange2004; Ronaldson Reference Ronaldson, Fitzgerald and McNeil2015b). The geographic variation in incidence is particularly remarkable, with a reported incidence in Australia between 1 and 3% (Tirupati Reference Tirupati2006; Haas Reference Haas, Hill and Krum2007; Ronaldson Reference Ronaldson, Fitzgerald and McNeil2015b; Khan Reference Khan, Ashraf and Baker2017) compared with incidences in Europe, Canada and the USA of 0.03%, 0.025% and 0.01% respectively (Degner Reference Degner, Bleich and Grohmann2000; Warner Reference Warner, Alphs and Schaedelin2000; La Grenade Reference La Grenade, Graham and Trontell2001).
Multiple theories have been postulated to explain this difference, including genetic polymorphisms affecting drug metabolism or predisposing to myocarditis, co-administration with other psychotropic medication (Ronaldson Reference Ronaldson, Fitzgerald and Taylor2012a) and regional variation in dose titration (de Leon Reference de Leon, Tang and Baptista2015; Rohde Reference Rohde, Polcwiartek and Kragholm2018), with rapid dose titration being associated with increased risk of myocarditis (Ronaldson Reference Ronaldson, Fitzgerald and Taylor2012b). The most commonly cited explanation is variability in the criteria used to diagnose myocarditis, which makes an accurate estimation of the true incidence of SGA-associated myocarditis extremely challenging. In most cases the diagnosis is based on a set of clinical criteria (Box 3) (Ronaldson Reference Ronaldson, Taylor and Fitzgerald2010) and EMB or CMR are not performed, meaning that these criteria have not been validated against a gold-standard diagnostic test such as histology. We would argue that the specificity of these criteria, particularly a troponin elevation to twice the upper limit of normal, is limited and likely to identify multiple cardiac and non-cardiac pathologies. Therefore, care needs to be taken when making inferences regarding the worldwide incidence of SGA-related myocarditis from studies using these criteria, as they are likely to overestimate the incidence (Ronaldson Reference Ronaldson, Taylor and Fitzgerald2010; Caforio Reference Caforio, Pankuweit and Arbustini2013).
BOX 3 Myocarditis
Symptoms
• Often non-specific
• Chest pain
• Breathlessness
• Flu-like illness
Investigations
• ECG
• Serum troponin
• C-reactive protein (CRP)
• Echocardiogram
• Cardiac MRI
When to refer to cardiology
• Symptoms
AND
• Troponin level is >2 times the upper normal limit
OR
• CRP level is >100 mg/L
OR
• There are abnormalities on echocardiogram
Management
• Supportive care
• ACE inhibitor or beta-blocker for left ventricular impairment
• Corticosteroid in severe cases
Mortality
The reported risk of mortality related to clozapine-associated myocarditis ranges from 10 to 40% (La Grenade Reference La Grenade, Graham and Trontell2001; Haas Reference Haas, Hill and Krum2007; Ronaldson Reference Ronaldson, Fitzgerald and Taylor2011a; Bellissima Reference Bellissima, Tingle and Cicović2018), although these figures are very likely an overestimate. The studies mostly involved either small samples or registry data in which the criteria for diagnosis of myocarditis were reported in retrospect and not strictly defined. This published mortality is thus likely to represent the more severe end of the spectrum of disease rather than patients presenting with non-specific symptoms or minor troponin elevations; the implications of both presentations remain unknown.
Mechanism
The development of myocarditis involves both direct injury to the myocytes by the responsible agent and a dysregulated immune response to this insult by the immune system, causing further myocyte damage and necrosis. Histological samples of antipsychotic-associated myocarditis often show eosinophilic infiltrates (Kilian Reference Kilian, Kerr and Lawrence1999; Bellissima Reference Bellissima, Tingle and Cicović2018), which is unusual in myocarditis in the general population. This suggests that a hypersensitivity reaction to the drug may be the initiating insult in at least a proportion of these cases (Al Ali Reference Al Ali, Straatman and Allard2006; Brambatti Reference Brambatti, Matassini and Adler2017) and is likely to account for the more severe phenotypes, given the high frequency of eosinophilic infiltration in autopsy specimens (Bellissima Reference Bellissima, Tingle and Cicović2018).
Clinical presentation
The clinical presentation of antipsychotic-associated myocarditis is heterogeneous with a wide spectrum of severity, ranging from a transient febrile illness to fulminant myocarditis requiring mechanical cardiac support or leading to SCD. The most common presenting symptoms include chest pain and breathlessness, although non-specific symptoms such as a flu-like illness, fever and fatigue have also been reported (Roesch-Ely Reference Roesch-Ely, Van Einsiedel and Kathöfer2002; Wassef Reference Wassef, Khan and Munir2015; Bellissima Reference Bellissima, Tingle and Cicović2018). In a proportion of cases the patient presents with mild non-specific symptoms that are self-limiting. Many of these cases are likely to go undiagnosed, which also occurs in non-antipsychotic-related presentations of myocarditis to primary care, and the clinical significance of such mild cases is unclear (Patriki Reference Patriki, Gresser and Manka2018).
Myocarditis usually develops early during the initiation of SGAs (Bellissima Reference Bellissima, Tingle and Cicović2018): with clozapine treatment most cases present within the first 4 weeks of therapy and with the other SGAs presentation normally occurs within the first 6 months of therapy. Fulminant myocarditis can cause severe cardiac impairment or death within days and therefore it is important that patients reporting chest pain or rapidly progressive symptoms of breathlessness or peripheral oedema are referred urgently for assessment in an accident and emergency department to exclude myocarditis.
Investigations
Electrocardiogram
A 12-lead ECG should be performed on patients presenting with symptoms suggestive of myocarditis. The most important role for the ECG is to exclude alternative serious diagnoses, such as acute myocardial infarction or arrhythmias, which might explain the presenting symptoms. Global saddle-shaped elevation in the ST segment provides the most specific finding for myocarditis (Fig. 3), although ECG findings in myocarditis are usually non-specific.
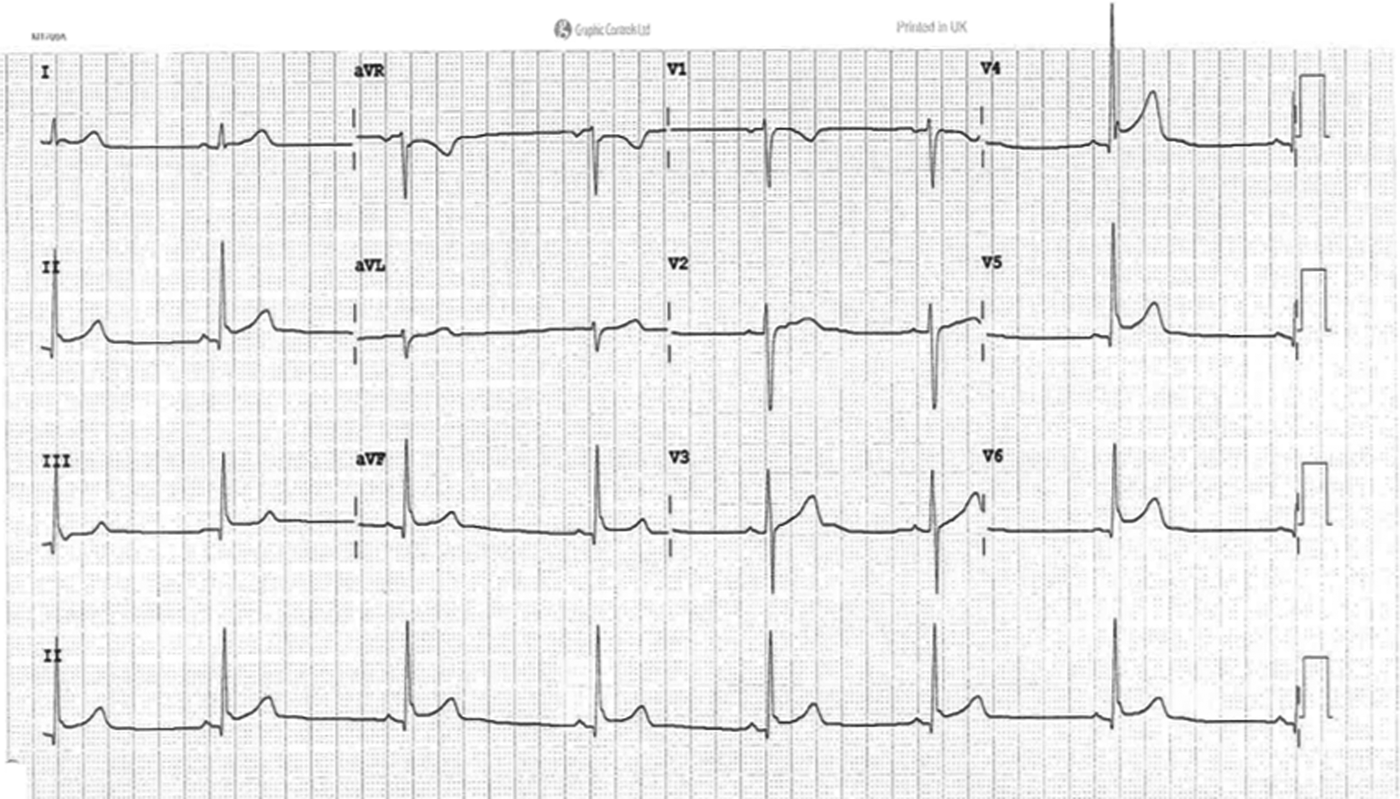
FIG 3 Electrocardiogram (ECG) demonstrating global saddle-shaped ST elevation which is the most specific ECG finding in myocarditis.
Blood tests
Elevation of cardiac enzymes such as troponin suggests active myocardial necrosis and, in patients with a consistent clinical history, measurement of serum levels of cardiac troponin is a highly sensitive test for myocarditis (Lauer Reference Lauer, Niederau and Kühl1997), although lacking in specificity. C-reactive protein (CRP) is elevated in approximately half of the cases of myocarditis and eosinophil levels can be useful if significantly elevated, although these tend to peak after the onset of myocardial damage (Ronaldson Reference Ronaldson, Fitzgerald and McNeil2015a).
Cardiac imaging
The presence of new ventricular dysfunction or regional wall motion abnormalities on echocardiogram, although not specific for myocarditis, is supportive of new myocardial damage. Echocardiography also determines the degree of ventricular dysfunction, which correlates with clinical outcomes and risk of complications (Quigley Reference Quigley, Richardson and Meany1987).
The gap between clinical diagnosis and histological diagnosis of myocarditis is increasingly being bridged by cardiac magnetic resonance imaging (CMR), using its ability to characterise myocardial tissue and identify myocardial scar and oedema. In cardiology practice, CMR is now often undertaken in lieu of pursuing a histological diagnosis.
Monitoring
Non-clozapine antipsychotics
Routine monitoring for myocarditis in patients taking non-clozapine antipsychotics is not recommended as the incidence is too infrequent to make this clinically useful or cost-effective.
Clozapine
In the case of clozapine, Ronaldson et al (Reference Ronaldson, Fitzgerald and Taylor2011b) have suggested an approach that involves baseline echocardiography, alternate-day measurements of vital signs and weekly measurement of C-reactive protein (CRP) and troponin levels for the first 4 weeks after clozapine initiation.
Screening of asymptomatic patients for a troponin rise has numerous challenges. Most importantly the natural progression of clozapine-associated myocarditis is not well understood; it is not clear whether small troponin elevations early in treatment will develop into significant ventricular dysfunction or have a significant risk of mortality if the treatment is continued. Second, the test or combination of tests proposed for myocarditis are not sufficiently specific to exclude other potential diagnoses and, given the low incidence of cases that are fatal or result in significant morbidity, it is questionable whether this is cost-effective or instead creates unnecessary barriers to the prescription of clozapine, with the potential to do significant damage to the mental health of this group of patients.
We therefore do not recommend routinely measuring troponin and CRP levels in asymptomatic patients taking clozapine. However, symptoms such as chest pain, breathlessness, palpitations, persistent fever or malaise should prompt measurement of troponin and CRP levels and a 12-lead ECG recording.
In our opinion, the screening criteria could prove useful in selected populations who are at higher risk of developing cardiac complications from clozapine therapy. Currently identified risk factors include older age (>75 years), rapid titration of dose, pre-existing structural heart disease and a previous cardiac side-effect with antipsychotic therapy (Ronaldson Reference Ronaldson, Fitzgerald and Taylor2012b; Sanchez Reference Sanchez, Foster and Plymen2016). The benefit and yield of regular routine investigation in this group of patients is likely to be significantly higher. For these patients we would advocate the approach suggested by Sanchez et al (Reference Sanchez, Foster and Plymen2016), which involves performing a TTE at baseline and again 6 weeks after the final dose increase (Fig. 4). In the interim, they recommend daily monitoring of observations and the measurement of CRP and troponin levels and an ECG at baseline and 24 hours after each dose increase. Echocardiography and ECG should be undertaken if there is a persistent fever or abnormalities in the troponin or CRP levels.
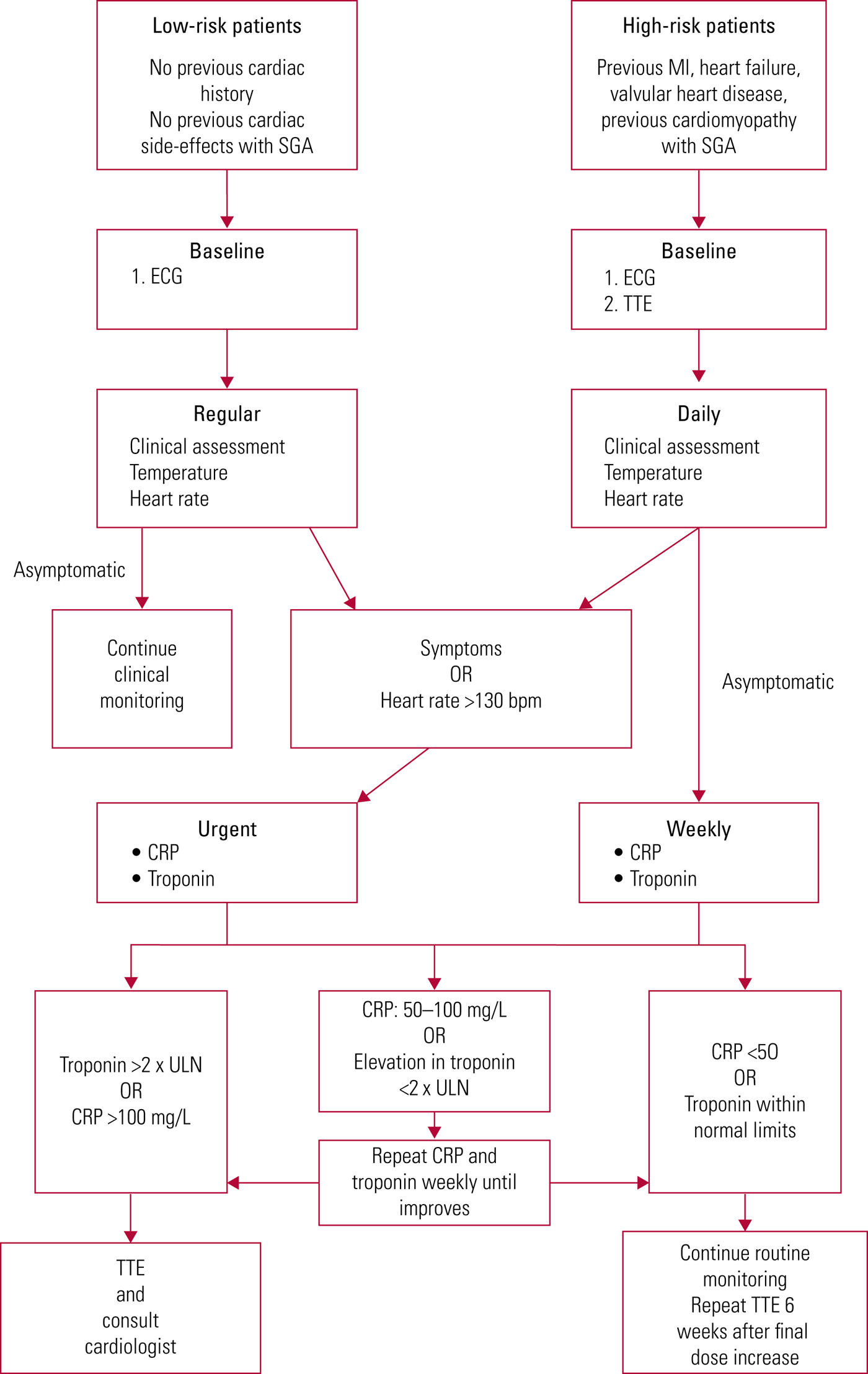
FIG 4 Routine monitoring for cardiac complications in patients on clozapine therapy (based on Sanchez Reference Sanchez, Foster and Plymen2016).
Treatment and discontinuation of antipsychotics
Myocarditis, once identified, requires cardiology input; in-patient management should be considered for those with significant symptoms or very high troponin levels (Berk Reference Berk, Fitzsimons and Lambert2007). Presentation with ventricular arrhythmias or heart failure carries a less favourable prognosis and these individuals should be admitted to a cardiology ward for management (Grün Reference Grün, Schumm and Greulich2012).
Deciding when to discontinue an SGA suspected of causing myocarditis is challenging. Presentation with high-risk symptoms such as syncope, heart failure or ventricular arrhythmias should prompt discontinuation of treatment at least temporarily and early cardiology assessment to identify the aetiology. Defining a threshold for troponin level is more problematic; most commonly a threshold of double the upper limit of normal has been suggested as a trigger for intensifying monitoring, with elevation greater than 4 times the upper limit of normal signifying the need to discontinue treatment (Ronaldson Reference Ronaldson, Fitzgerald and Taylor2011b). However, other factors will play a role in this decision, including the severity of symptoms, the presence of left ventricular dysfunction and the extent of scarring on CMR, if available.
If an equally efficacious alternative antipsychotic is available this should be used in preference. However, there is a lack of alternatives for clozapine in treatment-resistant schizophrenia and up to 80% of individuals in this situation who have clozapine discontinued will experience a deterioration in their mental health (Conley Reference Conley1998). Weighing the risk of myocarditis against the risk of deterioration in mental health can be exceptionally challenging clinically, and the benefit of including a cardiologist in the multidisciplinary team for cases such as this cannot be overstated.
If SGAs are to be continued or reintroduced, closer monitoring is warranted during the period of dose titration. Recommendations on the frequency of this monitoring vary (Ronaldson Reference Ronaldson, Fitzgerald and Taylor2011b, Reference Ronaldson, Fitzgerald and Taylor2012a; Sanchez Reference Sanchez, Foster and Plymen2016). Some recommend daily temperature measurement and observations, with troponin and CRP measurement following any dose increase; more conservative recommendations suggest daily troponin and CRP measurement, regardless of dose change. It is important to have access to echocardiography to investigate persistent or worsening abnormalities on blood tests, as severe or progressive deterioration in left ventricular function will usually dictate that SGA therapy should be discontinued.
Key points of the clinical presentation and management of myocarditis are summarised in Box 4.
BOX 4 Ronaldson et al.'s proposed diagnostic criteria for clozapine-associated myocarditis
Onset of new symptoms within 45 days of initiating clozapine
PLUS
Histological evidence of myocarditis at post-mortem or on endomyocardial biopsy
OR
Signs of cardiac dysfunction, such as:
(a) persistent tachycardia
(b) third heart sound
(c) basal crepitations
(d) peripheral oedema
PLUS
ONE of the following five findings on investigation:
(a) elevated troponin level or muscle/brain creatine kinase (CK-MB) level >2 times the upper limit of normal
(b) ECG changes consistent with myocarditis (>1 mm ST depression or T-wave inversion on two contiguous leads)
(c) chest radiograph suggestive of heart failure
(d) evidence of ventricular dysfunction or regional wall motion abnormalities
(e) MRI tissue characterisation suggestive of myocarditis
(Ronaldson 2010)
Rechallenging with clozapine
After an episode of SGA-associated myocarditis, avoidance of that particular antipsychotic is normally recommended and an alternative antipsychotic administered (Bellissima Reference Bellissima, Tingle and Cicović2018). However, in the case of clozapine there may be occasions where rechallenging after recovery is required because of deterioration in the patient's mental health, and this has been successfully undertaken in a limited number of reported cases. In such situations increased monitoring during the rechallenge is vital (Ronaldson Reference Ronaldson, Fitzgerald and Taylor2012c; Alawami Reference Alawami, Wasywich and Cicovic2014) and should ideally be undertaken on an in-patient basis, with regular clinical assessment, monitoring of troponin, CRP and vital signs. The left ventricular function should have normalised before rechallenge and any recurrence of symptoms should prompt reassessment of cardiac function. Continuation of clozapine despite a small elevation in troponin levels with no change in left ventricular function is permissible with cardiology supervision; however, deterioration in left ventricular function should prompt discontinuation of treatment (Hassan Reference Hassan, Brennan and Carroll2011; Ronaldson Reference Ronaldson, Fitzgerald and Taylor2011b).
Rechallenge with clozapine appears to be a viable option for selected patients, albeit one that should be carried out under close joint supervision and reserved for patients where the mental health benefits significantly outweigh the risks of myocarditis recurrence (Bellissima Reference Bellissima, Tingle and Cicović2018).
Dilated cardiomyopathy
Dilated cardiomyopathy (DCM) is characterised by dilation of the ventricles, with impaired myocardial performance in the absence of explanatory ischaemic, valvular, congenital or hypertension-related heart disease. There are multiple aetiologies that result in DCM, including familial cardiomyopathies, toxins such as alcohol or cytotoxic chemotherapy, an inflammatory cardiomyopathy such as myocarditis, or peripartum cardiomyopathy. The annual incidence in the general population is approximately 0.002% (Rakar Reference Rakar, Sinagra and Di Lenarda1997).
Incidence
SGAs are associated with an increased incidence of DCM (Alawami Reference Alawami, Wasywich and Cicovic2014), with a reported annual incidence between 0.06 and 0.2% (Kilian Reference Kilian, Kerr and Lawrence1999; La Grenade Reference La Grenade, Graham and Trontell2001; Rohde Reference Rohde, Polcwiartek and Kragholm2018). The most well-reported association is with clozapine; however, this may represent a reporting bias following the initial reports of SGA-associated cardiomyopathy (Kilian Reference Kilian, Kerr and Lawrence1999). In retrospective registry studies of out-patient antipsychotic prescriptions, the annual incidence of DCM was similar for clozapine, olanzapine, quetiapine and risperidone, at between 0.06 and 0.09% (Montastruc Reference Montastruc, Favreliere and Sommet2010; Rohde Reference Rohde, Polcwiartek and Kragholm2018). Aripiprazole had a lower annual incidence of DCM, at approximately 0.05% (Montastruc Reference Montastruc, Favreliere and Sommet2010) and haloperidol was not been reported to be associated with cardiomyopathy either in case reports or registry studies. However, these studies rely on coded data from primary care and hospital records, which can be unreliable and may underestimate the incidence of DCM as milder cases often go unreported.
Mechanism
The precise mechanism behind the development of DCM is unclear, although several possibilities have been suggested. The cardiac dysfunction may represent a mild form of indolent myocarditis causing progressive low-level myocardial damage or it may represent the sequelae of a previously unrecognised episode of myocarditis (Merrill Reference Merrill, Dec and Goff2005; Knoph Reference Knoph, Morgan and Palmer2018). Alternatively, SGAs may have a direct toxic effect on the myocytes throughout the body, including the cardiomyocytes, resulting in cell necrosis and apoptosis that weakens the contractile function of the heart (Scelsa Reference Scelsa, Simpson and McQuistion1996; Merrill Reference Merrill, Dec and Goff2005).
Clinical presentation
The most common presenting symptom of heart failure is breathlessness or decreasing exercise tolerance, with other common symptoms including palpitations and orthopnoea (Alawami Reference Alawami, Wasywich and Cicovic2014). A detailed clinical history is the most useful tool to identify the aetiology of heart failure, including DCM, and helps guide further diagnostic tests. It includes enquiring about exertional chest pain or cardiovascular risk factors suggestive of coronary artery disease, history of excessive alcohol consumption, exposure to other known cardiotoxic medication, such as chemotherapy, and a family history of cardiomyopathy. Patients who are acutely unwell with signs of pulmonary oedema on examination require hospital admission for emergency management (Ponikowski Reference Ponikowski, Voors and Anker2016).
Investigation
If new-onset heart failure is suspected in any patient taking antipsychotic medication, first-line tests should include ECG and serum troponin levels to exclude acute myocardial infarction or myocarditis; these tests most commonly require attendance at an accident and emergency department. In subacute presentations measurement of serum B-type natriuretic peptide (BNP) or N-terminal pro-BNP (NT-proBNP) is extremely helpful (Ponikowski Reference Ponikowski, Voors and Anker2016). These proteins are released by the heart in response to ventricular stretch, which occurs in decompensated heart failure, and are an effective ‘rule-out’ test for heart failure – if normal heart failure is unlikely and alternative causes for the presenting symptoms should be sought (Cowie Reference Cowie, Struthers and Wood1997). If the BNP level is elevated in the context of symptoms or signs suggesting heart failure an echocardiogram should be arranged to investigate further (Ponikowski Reference Ponikowski, Voors and Anker2016).
Echocardiography
Echocardiography is the mainstay for diagnosis and monitoring of individuals with cardiomyopathy and can provide information about the aetiology and severity of left ventricular dysfunction. Those with symptoms or signs of heart failure with objective evidence of cardiac dysfunction on echo should be referred to cardiology for further assessment and treatment.
Monitoring
Given that many people can be clinically well despite significant left ventricular impairment, prophylactic screening of asymptomatic individuals on antipsychotic treatment using echocardiography has been suggested in a number of publications (Berk Reference Berk, Fitzsimons and Lambert2007; Knoph Reference Knoph, Morgan and Palmer2018). However, the prognostic benefit of diagnosing and treating asymptomatic left ventricular dysfunction is unproven and thresholds for stopping antipsychotic treatment in this situation are not known. Consequently, this approach may result in patients with mild asymptomatic systolic dysfunction having effective antipsychotic therapy discontinued unnecessarily with little evidence to support these decisions. Therefore, we would not recommend routine echocardiographic screening, although clinical screening for symptoms and signs of heart failure is important and should be carried out at least four times a year (Alawami Reference Alawami, Wasywich and Cicovic2014). We recommend that patients presenting with signs or symptoms of heart failure should have natriuretic peptides measured in the first instance, as per the NICE guidelines for heart failure (National Institute for Health and Care Excellence 2018). Reserving the use of echocardiography for patients with abnormal natriuretic peptide results will be much more cost-effective than using echocardiography as the first-line investigation for suspected cardiomyopathy (Murch, Reference Murch, Tran and Liew2013).
Treatment and discontinuation of antipsychotics
The treatment of left ventricular systolic dysfunction, irrespective of aetiology, involves the use of prognostic heart failure medications. Angiotensin-converting enzyme (ACE) inhibitors and beta-blockers both significantly reduce mortality and improve symptoms and should be started in all symptomatic patients. For those who remain symptomatic despite these treatments, mineralocorticoid antagonists such as spironolactone are added, which further reduces mortality and morbidity. Diuretics are used as required to manage fluid overload, thus improving symptoms (Ponikowski Reference Ponikowski, Voors and Anker2016; National Institute for Health and Care Excellence 2018). There is currently no evidence for prophylactic treatment with heart failure therapies.
Deciding when to discontinue antipsychotic treatment in patients with SGA-associated cardiomyopathy is challenging. Given the limited experience in this area, published recommendations tend to be relatively conservative, suggesting discontinuation of therapy as soon as the left ventricular function is seen to deteriorate. However, although it may be necessary to stop therapy in patients who have significant symptoms or severe impairment of their left ventricular function, in milder cases this decision becomes less clear-cut and must be balanced against the deleterious mental health effects of stopping the antipsychotic. Protocols for monitoring cardiotoxic chemotherapies such as doxorubicin and trastuzumab in oncology patients have suggested using an absolute ejection fraction cut-off of 45% to withhold treatment, as below this level recovery of left ventricular function was less reliable on stopping treatment (Suter Reference Suter, Procter and van Veldhuisen2007). However, in asymptomatic patients in particular it is important to involve the cardiology team in the decision-making process (Merrill Reference Merrill, Dec and Goff2005).
Key points of the clinical presentation and management of DCM are summarised in Box 5.
BOX 5 Dilated cardiomyopathy
Symptoms
• Fatigue
• Breathlessness:
• orthopnoea
• paroxysmal nocturnal dyspnoea
• Ankle swelling
• Palpitations
Investigations
• ECG
• Serum troponin level to exclude myocarditis
• Serum BNP or NT-proBNP levels
• Chest X-ray
• Echocardiogram
When to request an echocardiogram
• Symptoms
PLUS
• BNP >100 pmol/L
OR
• NT-proBNP > 400 pmol/L
When to refer to cardiology
• Absolute left ventricular ejection fraction is <50%
• Relative reduction in left ventricular ejection fraction is >10%
Management
• ACE inhibitor
• Beta-blocker
• Mineralocorticoid receptor antagonist
Pre-existing cardiac problems and rechallenging
It is important not to universally exclude all patients with pre-existing cardiac conditions or ventricular impairment from treatment with SGAs. In the first-instance, an antipsychotic such as aripiprazole should be considered, as it has a very infrequent association with DCM and is likely to be the safest treatment if there is a significant concern. Patients with pre-existing cardiac disease who require treatment with SGAs should have an up-to-date assessment of their left ventricular function before starting treatment (Sanchez Reference Sanchez, Foster and Plymen2016).
There is very limited experience of rechallenging patients who have discontinued SGAs because of the development of cardiomyopathy – it has mainly been described in case reports with mixed results (Floreani Reference Floreani and Bastiampillai2008; Nederlof Reference Nederlof, Benschop and de Vries Feyens2017). If the patient's mental health is compromised by withholding an effective antipsychotic, rechallenging remains an option, but careful monitoring is required as the rate of recurrence of cardiac symptoms appears to be high. Before considering rechallenging, the patient should be asymptomatic, the ejection fraction should have recovered to at least 45% and they should be maintained on prognostic heart failure therapies including both an ACE inhibitor and beta-blocker at the highest tolerated doses, even if the left ventricular function has normalised (Zamorano Reference Zamorano, Lancellotti and Rodriguez Muñoz2016).
In both groups, the antipsychotic should be up-titrated slowly, with careful regular assessment of clinical symptoms, heart rate and temperature. Troponin and/or BNP should be measured at weekly intervals along with weekly ECGs. Repeat echocardiography should be performed following completion of dose escalation or earlier if there are any concerning symptoms or abnormalities in the blood tests. The key to managing treatment is the early involvement of cardiology teams to allow individualised decisions to be taken based on the relative risks of harm to the physical and mental health of the patient (Sanchez Reference Sanchez, Foster and Plymen2016).
Conclusions
Second-generation antipsychotics are an effective treatment for schizophrenia and awareness of the cardiovascular side-effect profile of these medications is important for clinicians prescribing them. Knowledge of the relative risks of these side-effects compared with the benefits to the patient's mental health derived from antipsychotic treatments is important so as not to terminate effective therapy or withhold it unnecessarily. It must be remembered that most patients take these potentially life-saving treatments with no cardiovascular consequences.
Routine screening of asymptomatic individuals remains controversial and in many cases is difficult to justify from a cost–benefit analysis. The Maudsley Prescribing Guidelines recommend yearly ECGs for patients taking SGAs and these are a relatively low-cost intervention to monitor the QT interval. However, routine screening for myocarditis or cardiomyopathy in all patients taking SGAs is not cost-effective and may discourage the use of these treatments in patients for whom it may be a highly effective therapy, particularly in resource-limited healthcare systems.
An important consideration for patients showing antipsychotic-related cardiac symptoms is whether the offending medication should be stopped indefinitely or whether its side-effects might be managed with either temporary discontinuation or careful monitoring. These are challenging decisions that need to be made on an individual patient basis, balancing the risks of further cardiac dysfunction against the risk of significant deterioration in mental health when an effective antipsychotic discontinued. Parallels can be drawn between the use of potentially cardiotoxic SGAs and the use of cardiotoxic chemotherapy in cancer, for which a new cardio-oncology subspecialty has developed. In both areas, important and highly effective drugs carry a risk of significant cardiovascular morbidity and mortality, and the role of the cardiology team in the oncology multidisciplinary team is to facilitate the safe continuation of treatment where possible. We would suggest similar integration of cardiologists into the psychiatric multidisciplinary team to provide regular and early input to enable continuation of antipsychotic treatment wherever possible and to help quantify risk or assuage concerns as necessary.
MCQs
Select the single best option for each question stem
1 The most common cardiac cause of death in patients with schizophrenia is:
a myocarditis
b torsades de pointes
c cardiovascular disease
d cardiomyopathy
e atrial fibrillation.
2 Antipsychotic-induced persistent sinus tachycardia cannot be treated with:
a beta-blockers
b ivabradine
c diltiazem
d verapamil
e doxazocin.
3 The European Society of Cardiology gold-standard definition of myocarditis requires:
a a rise in troponin levels to >4 times the upper limit of normal
b regional wall motion abnormalities on echocardiogram
c late gadolinium enhancement on cardiac MRI
d C-reactive protein levels >100 mg/L
e histological evidence of inflammatory infiltrate and cardiomyocyte necrosis on endomyocardial biopsy.
4 Symptoms or prognosis in heart failure due to dilated cardiomyopathy are not improved by:
a aspirin
b diuretics
c beta-blockers
d ACE-inhibitors
e mineralocorticoid antagonists.
5 In a patient taking antipsychotic medication, urgent cardiology assessment should be prompted by:
a a QTc interval >480 ms
b a heart rate of 105 bpm
c a left ventricular ejection fraction <60%
d unexplained syncope
e an eosinophil count >2.5 × 106 cells/L.
1 c 2 e 3 e 4 a 5 d








eLetters
No eLetters have been published for this article.