Oxidative stress due to abnormal exposure to reactive oxygen species (ROS) plays a key role in the pathogenesis of atherosclerosis and CVD(Reference De Rosa, Cirllo and Paglia1). Endothelial cells lining the vasculature are particularly sensitive to oxidative stress as high concentrations of ROS in the circulating blood directly keep in contact with the endothelium. Overproduction of ROS can damage vascular endothelial cells, and numerous in vivo and in vitro studies have implicated that endothelial cell damage and cell death may play a role in the development of atherogenesis(Reference Clarke, Bennett and Littlewood2). However, the underlying mechanism by which oxidative stress induces endothelial cell damage and cell death has not been fully established. Therefore, further insight into this mechanism is required before a new preventive strategy against oxidative stress-induced endothelial cell injury can be developed for the treatment of atherosclerosis.
Epidemiological evidence suggests that populations consuming soya products rich in isoflavones have a lower incidence of CVD. The benefits of soya isoflavones for the prevention of atherosclerosis and CVD have been examined in many clinical trials and animal models(Reference Walker, Adams and Franke3, Reference Clarkson4). Genistein, the primary isoflavone found in soyabeans, has been highlighted for its antioxidant effects and ability to activate the transcription of various genes. Due to its antioxidative properties, genistein effectively scavenges ROS and inhibits lipid peroxidation(Reference Exner, Hermann and Hofbauer5). In addition, genistein reverses alterations in the protein expression profile induced by cell stress(Reference Wenzel, Fuchs and Daniel6) and protects endothelial cells from oxidative stress-induced damage by maintaining or activating several survival signalling pathways(Reference Xu, Zhong and Ghavideldarestani7). However, there is only limited experimental evidence addressing the impact of genistein on antioxidant gene expression in the vasculature(Reference Borras, Gambini and Gomez-Cabrera8, Reference Siow, Li and Rowlands9). Various other phytochemicals, such as ( − )-epigallocatechin-3-gallate(Reference Wu, Hsu and Hsieh10), quercetin(Reference Chow, Shen and Huan11), resveratrol(Reference Chen, Jang and Li12) and puerarin(Reference Hwang and Jeong13), have been demonstrated to activate antioxidant genes as well as their downstream cytoprotective enzymes. However, the effects of genistein and the underlying mechanisms regarding oxidative stress-induced endothelial cell injury remain unclear.
The most important cellular defence mechanism against ROS involves nuclear factor erythroid 2-related factor 2 (Nrf2), which mediates antioxidant responsive element (ARE) sequences in the promoter regions of phase II and antioxidant genes(Reference Itoh, Tong and Yamamoto14). Among the various antioxidant enzymes regulated by Nrf2/ARE, haem oxygenase-1 (HO-1) has been highlighted for its cytoprotective involvement in the vascular system, including vascular tone regulation, inhibition of smooth muscle proliferation, inhibition of endothelial apoptosis and promotion of angiogenesis(Reference Dulak, Deshane and Jozkowicz15). PPARγ also plays an important role in vascular regulation. Recent studies have indicated that there is a mechanistic link between PPARγ and oxidative stress in various cell types(Reference Almeida, Ambrogini and Han16, Reference Ren, Sun and Sun17), and that the activation of PPARγ exerts beneficial effects in endothelial cells(Reference Sasaki, Jordan and Welbourne18, Reference Verrier, Wang and Wadham19). The present study aims to examine the cytoprotective effects of genistein on oxidative stress-induced loss of cell viability and increased apoptosis in EA.hy926 endothelial cells. Moreover, the present study assesses whether Nrf2, PPARγ, HO-1 and the endogenous antioxidants superoxide dismutase (SOD), catalase (CAT) and glutathione (GSH) are involved in the mechanism of cellular protection.
Experimental methods
Reagents
Genistein, H2O2, Znpp, GW9662, hemin and NADPH were purchased from Sigma. Genistein was prepared as a 0·1 m stock solution in dimethyl sulfoxide; an equal volume of dimethyl sulfoxide was used as a control in each experiment. Polyclonal rabbit anti-human Nrf2 (sc-722) and monoclonal mouse anti-human PPARγ (sc-7273) antibodies were purchased from Santa Cruz Biotechnology. Polyclonal rabbit anti-human HO-1 (SPA-895) antibody was obtained from Stressgen. Rabbit anti-human activated-caspase-3 p17 (BS7004), B-cell lymphoma 2 (Bcl-2, BS1511), β-tubulin (BS1482) and lamin B1 (BS3547) antibodies were purchased from Bioworld Technology. Goat anti-rabbit and goat anti-rabbit IgG peroxidase conjugate were obtained from Pierce Biotechnology (Thermo Scientific). Nrf2 small interfering RNA (siRNA) (sc-37 030), control siRNA (sc-37 007), control siRNA (fluorescein conjugate) (sc-36 869) and siRNA transfection reagent (sc-29 528) were purchased from Santa Cruz Biotechnology. The Cell Counting Kit-8 was purchased from Dojindo Laboratories. The Annexin V-FITC Apoptosis Detection Kit was obtained from BestBio. The In Situ Cell Death Detection Kit was purchased from Roche Diagnostics. The reagent kit used for the measurement of SOD (A001-3), CAT (A007) and GSH (A006) was purchased from Nanjing Jiancheng Bioengineering Institute. The BioEasy SYBR Green I Real Time PCR Kit was obtained from Bioer Technology. The ARE-luciferase reporter gene was kindly provided by Dr Donna D. Zhang (University of Arizona). The PPARγ-luciferase reporter gene was kindly provided by Dr Ronald M. Evans (the Salk Institute for Biological Studies).
Cell culture and treatment
EA.hy926 cells were obtained from the American Type Culture Collection. Cells were maintained at 37°C in an incubator with a humidified atmosphere of 5 % CO2 and were cultured in Dulbecco's modified Eagle's medium (DMEM), which was supplemented with 10 % heat-inactivated fetal bovine serum, 2 mm-l-glutamine, penicillin (60 μg/ml) and streptomycin (100 μg/ml), until they reached 90–95 % confluence. The culture medium was replaced every 3 d.
Measurement of cell viability
Cell viability was assessed using a Cell Counting Kit-8 assay. WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulphophenyl)-2H-tetrazolium, monosodium salt) is reduced by dehydrogenases in cells to produce a water-soluble formazan dye (orange in colour). The relative amount of formazan dye in cells is directly proportional to the number of living cells. For this assay, cells were seeded at a density of 5000 cells/well in ninety-six-well plates with six replicate wells for each concentration in the same plate. Before detection, the Cell Counting Kit-8 reagent was diluted 10-fold with DMEM, and 100 μl of the Cell Counting Kit-8 solution were then added to each well. After 2·5 h incubation, the optical density (OD) value was read at 450 nm using a multimode microplate reader (Infinite M200; Tecan). The OD450 value is proportional to the degree of cell viability. The results shown are derived from at least three independent experiments.
Flow cytometry assay
Cells grown in six-well plates were harvested, washed and double-stained with annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (Annexin V-FITC Apoptosis Detection Kit, Bestbio), incubated for 15 min at room temperature in the dark, and analysed by flow cytometry.
Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labelling assay
Apoptosis was detected via terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labelling (TUNEL) analysis using the In Situ Cell Death Detection Kit (Roche Diagnostics) according to the manufacturer's instructions. EA.hy926 cells were seeded on sterile glass coverslips in twelve-well plates overnight and pre-incubated with genistein for 10 h before being exposed to H2O2 for 24 h. Cells were then washed twice with PBS, fixed with 4 % methanol-free paraformaldehyde for 10 min, washed twice with PBS and permeabilised with 0·2 % Triton X-100 for 5 min. After two more washes, each glass coverslip was covered with equilibration buffer for 10 min. The buffer was then aspirated, and the glass coverslips were incubated with terminal deoxynucleotidyl transferase buffer at 37°C for 1 h. Chromosomal DNA was stained with 4′,6-diamidino-2-phenylindole, the cells were mounted on glass slides and staining was analysed using confocal laser scanning microscopy.
Assessment of superoxide dismutase, catalase and glutathione levels
Cells were pre-incubated with genistein (0, 20, 100 and 500 nm) for 10 h, while H2O2-treated cells were incubated either with or without 500 nm-genistein for 10 h, followed by additional incubation with H2O2 for 24 h. The medium was then removed, cells were washed twice with cold PBS and cells were removed from the plates while kept on ice. Cell suspensions were sonicated three times for 3–4 s each on ice and then centrifuged at 14 000 rpm (21 913 g) for 25 min at 4°C. Cell supernatants were used for the assessment of antioxidant enzyme activity and GSH levels. The analysis of SOD, CAT and GSH levels was performed according to the manufacturers' instructions. Protein concentration was determined via the Bradford method; bovine serum albumin was used as a reference standard.
Luciferase reporter gene assay
Cells in twenty-four-well plates were transfected using LipofectAMINE™ 2000 reagent (Invitrogen) with either an ARE-luciferase plasmid or PPARγ-luciferase plasmid together with a renilla luciferase expression plasmid (used as an internal reporter to normalise for variations in transfection efficiency) and pGL 4.74 (hRluc/TK) (Promega). The cells were then treated with genistein for 10 h, and the activity of both firefly and renilla luciferases was measured using the dual luciferase reporter assay system from Promega.
Western blot analysis
After the treatment, cells were lysed in lysis buffer (50 mm-Tris, pH 8·0, 150 mm-NaCl, 0·1 % SDS, 1 % Triton X-100, 0·5 % deoxycholate and protease inhibitors), and the protein concentrations of the cell lysates were determined by the Bradford assay. Lysate aliquots (40 μg/well) were separated by 10–12 % SDS-PAGE and then transferred onto polyvinylidene fluoride membranes. Membranes were subsequently incubated with primary antibodies overnight at 4°C, washed with PBS and incubated with horseradish peroxidase-conjugated secondary antibodies (1:5000 dilution). Visualisation was performed using electro chemiluminescence reagents and developed on radiographic film.
Real-time PCR
Total RNA was extracted using Trizol reagent (Bioer), and genomic DNA was digested with DNase. mRNA was reverse-transcribed into complementary DNA using Moloney murine leukaemia virus reverse transcriptase (Bioer). Gene expression was determined by quantitative real-time PCR using a SYBR Green PCR kit. The primer sequences used for expression analysis are as follows: Nrf2, 5′-ATTGCCTGTAAGTCCTGGTCA-3′ (forward) and 5′-ACTGCTCTTTGGACATCATTTCG-3′ (reverse); HO-1, 5′-CGATGGGTCCTTACACTC-3′ (forward) and 5′-GGCTCCTTCCTCCTTT-3′ (reverse); PPARγ, 5′-AGGTTTGGGCGGATGCCACA-3′ (forward) and 5′-AGATCGCCCTCGCCTTTGCT-3′ (reverse); glyceraldehyde-3-phosphate dehydrogenase, 5′-TGCACCACCAACTGCTTAG-3′ (forward) and 5′-GATGCAGGGATGATGTTC-3′ (reverse); 18S rRNA, 5′-GTAACCCGTTGAACCCCATT-3′ (forward) and 5′-CCAT CCAATCGGTAGTAGCG-3′ (reverse); β-actin, 5′-ACCAACTGGGACGATATGGAGAAGA-3′ (forward) and 5′-ACGACCAGAGGCATACAGGGACAA-3′ (reverse). The real-time PCR conditions were as follows: 94°C for 2 min followed by forty-five cycles of 94°C for 10 s and 72°C for 45 s. Data are presented as fold change in gene expression compared with the control group.
Assessment of haem oxygenase-1 activity
After the treatment, cells were washed twice with cold PBS, harvested by gently scraping the cells off the dish (on ice), and centrifuged (1500 g for 15 min at 4°C). Cell pellets were resuspended in cold KH2PO4 buffer (pH 7·4), frozen at − 70°C, thawed three times and sonicated on ice before centrifugation at 14 000 rpm (21 913 g) for 15 min at 4°C. The supernatant (20 μl) was added to a reaction mixture containing 4·5 mm-NADPH, 3 mg protein of rat liver cytosol prepared from the one 5000 g supernatant fraction as a source of biliverdin reductase, 0·1 m-cold KH2PO4 buffer (pH 7·4) and 2 mm-hemin (1·8 ml). The reaction was performed at 37°C for 30 min in the dark and terminated by putting the samples on ice. A reaction without NADPH served as the control. The extracted bilirubin was calculated by determining the difference in absorption between 464 and 530 nm.
Small interfering RNA transfection
Nrf2 siRNA transfection was performed according to the manufacturer's instructions. Cells were seeded in a six-well tissue culture plate (2 × 105 cells/well) in 2 ml antibiotic-free normal growth medium supplemented with fetal bovine serum. Cells were incubated at 37°C in a CO2 incubator overnight. A mixture of 6 μl Nrf2 siRNA and 6 μl transfection reagent was incubated for 45 min at room temperature. The mixture was then added to the cells with 800 μl antibiotic/serum-free medium. The final concentration of Nrf2 siRNA was 60 nm. The culture cells transfected with control siRNA were treated in parallel. Additionally, FITC-labelled siRNA was used as a control.
Statistical analysis
Data are expressed as means and standard deviations. Statistical significance was analysed via ANOVA, and differences among the groups were assessed via Tukey's test using SPSS version 13.0 software (SPSS, Inc.). Student's t test was also used when comparing the means of the two groups. Differences were considered statistically significant at P< 0·05.
Results
Genistein preserves the viability of endothelial cells during oxidative stress
To assess the potential protective effect of genistein at physiological concentrations, cells were pretreated with genistein (1–500 nm) before exposure to 650 μm-H2O2 for an additional 24 h. As shown in Fig. 1, genistein prevented the loss of cell viability in EA.hy926 cells during oxidative stress. Cells treated with genistein (10–500 nm) displayed concentration-dependent protective effects (56·9–67·9 % viable cells) compared with H2O2-treated cells (26·1 % viable cells). These results are consistent with Xu's study(Reference Xu, Zhong and Ghavideldarestani7).

Fig. 1 Genistein attenuates the loss of cell viability during oxidative stress. EA.hy926 cells were pretreated with genistein (1–500 nm) for 10 h and subsequently incubated with H2O2 (650 μm) for an additional 24 h, and cell viability was then assessed by the Cell Counting Kit-8 assay. Values are means (n 6 for each group), with standard deviations represented by vertical bars. Mean values were significantly different compared with the H2O2-treated group: * P< 0·05, *** P< 0·001.
Genistein protects against cell apoptosis induced by oxidative stress
To further demonstrate the protective effects of genistein on endothelial cells during oxidative stress, we evaluated the effects of genistein on apoptosis induced by H2O2 in EA.hy926 cells. Using annexin V-FITC and propidium iodide flow cytometric analysis, we observed that apoptosis induced by H2O2 was significantly inhibited when cells were pretreated with genistein (Fig. 2(a)). In addition, cells exposed to H2O2 demonstrated increased TUNEL staining, whereas the genistein treatment decreased the number of TUNEL-stained positive cells (Fig. 2(b)).

Fig. 2 Genistein suppresses oxidative stress-induced cell apoptosis. (a) EA.hy926 cells were pretreated with genistein (500 nm) for 10 h and then incubated with 650 μm-H2O2 for 24 h. Cells were then harvested and labelled with annexin V-fluorescein isothiocyanate (FITC) and propidium iodide, and apoptosis was subsequently analysed via flow cytometry. Representative flow cytometric histograms of the different groups are shown. Values are means (n 3 for each group) for the level of apoptosis for each treatment group, with standard deviations represented by vertical bars. Mean values were significantly different compared with the negative control (no oxidative stress) group (*** P< 0·001) and the non-genistein group (††† P< 0·001). (b) Cells were seeded on sterile glass coverslips in twelve-well plates overnight and pretreated with genistein for 10 h before stimulation with 650 μm-H2O2 for 24 h. A terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labelling assay was then performed according to the manufacturers' instructions. (c) The cells were exposed to 650 μm-H2O2 for the indicated times and then evaluated for activated caspase-3 p17 expression. *Mean value was significantly different from that at Oh (P< 0.05). (d) Cells were pretreated with various concentrations of genistein for 10 h (the positive control was left untreated) before stimulation with 650 μm-H2O2 for 9 h, and the activated caspase-3 p17 levels were then assessed in cell extracts via Western blot analysis. The expression levels were normalised to β-tubulin expression levels. ***Mean value was significantly different from that of the untreated control (P< 0.001). † Mean value was significantly different from that for OμM-genistein (P< 0.005). (e) Cells were pre-incubated either with or without genistein (500 nm) for 10 h and then exposed to 650 μm-H2O2 for 24 h. Bcl-2 levels in cell extracts were measured by Western blot analysis and were normalised to β-tubulin expression levels. Mean value was significantly different from that of the control: **P< 0.01, *** P< 0.001. † Mean value was significantly different from that of H2O2 alone (P< 0.05). DAPI, 4′,6-diamidino-2-phenylindole; FL1-H, fluoresence 1-height-FITC(log); FL2-H, fluoresence 2-height-propidium iodide (log).
Caspase-3 plays a pivotal role in the terminal execution phase of apoptosis induced by a wide range of stimuli. Under normal conditions, caspase-3 is undetectable in EA.hy926 cells; however, we observed that H2O2 activated caspase-3 in a time-dependent manner (Fig. 2(c)), while genistein pretreatment significantly inhibited caspase-3 expression induced by H2O2 (Fig. 2(d)). The Bcl-2 family proteins play a critical role in the regulation of cell proliferation and apoptosis under both normal and oxidative stress conditions. Incubation with H2O2 significantly inhibited the expression of Bcl-2 protein. Genistein pretreatment promoted a significant increase in Bcl-2 protein expression levels (Fig. 2(e)) compared with cells treated with H2O2 alone. These data confirm that genistein inhibits H2O2-induced apoptosis in EA.hy926 cells.
Genistein treatment promotes an increase in superoxide dismutase, catalase and glutathione levels
A basic estimation of endogenous antioxidants, including SOD, CAT and GSH, was assessed in genistein-treated EA.hy926 cells in both the presence and absence of oxidative stress. As shown in Fig. 3, genistein pretreatment resulted in a significant increase in SOD (Fig. 3(a)) and CAT (Fig. 3(b)) activity in a dose-dependent manner. H2O2 stimulation led to significant decreases in antioxidant enzyme (SOD and CAT) activity, while pre-incubation with genistein (500 nm) significantly attenuated the decreases in SOD and CAT activity, thereby returning enzyme activity to normal levels. Exposing EA.hy926 cells to genistein caused an increase in GSH levels (Fig. 3(c)). A slight induction in GSH levels was observed after the treatment with 20 and 100 nm-genistein, while treatment with 500 nm-genistein induced significant increases in GSH levels. Although cellular GSH levels decreased during oxidative stress, GSH levels were elevated after the pretreatment with 500 nm-genistein. The present results that display the elevation of antioxidant enzyme activity and GSH levels following genistein treatment alone and the ability of genistein to maintain cellular levels of SOD, CAT and GSH during oxidative stress are consistent with the reports of Choi et al.(Reference Choi, Cho and Park20) and Liu et al.(Reference Liu, Chen and Zhang21).

Fig. 3 Genistein up-regulates superoxide dismutase (SOD), catalase (CAT) and glutathione (GSH) levels. EA.hy926 cells were treated with various concentrations of genistein (0, 20, 100 and 500 nm) for 10 h, while H2O2-treated cells were pretreated with or without 500 nm-genistein, followed by incubation with 650 μm-H2O2 for 24 h. Cell extracts were then prepared for the assessment of the SOD, CAT and GSH levels. The effects of genistein on (a) SOD activity, (b) CAT activity and (c) GSH levels were detected using a commercial kit. One unit of SOD activity is defined as a 50% decrease in the rate of cytochrome C reduction. One unit of CAT will decompose 1.0 μmol H2O2 to O2 and water per min at pH 7.0 at 25°C at a substrate concentration of 50 mμ-H2O2. Values are means (n 3 for each group), with standard deviations represented by vertical bars. Mean values were significantly different compared with both the non-genistein group (* P< 0·05, *** P< 0·001) and the H2O2 control group († P< 0·05).
Genistein activates nuclear factor erythroid 2-related factor 2
Nrf2 signalling plays a major role in the protection of cells against chemical and radiation stress, while it also promotes cell survival. To examine whether genistein activates Nrf2, a plasmid containing an ARE-dependent firefly luciferase reporter gene was utilised as described previously(Reference Du, Villeneuve and Wang22). Genistein treatment induced ARE-dependent luciferase activity in a dose-dependent manner (Fig. 4(a)). A slight induction (1·5-fold) in ARE-dependent luciferase activity was observed at concentrations as low as 20 nm and reached a maximum induction (4·0-fold) at 500 nm. Cells were treated with 500 nm-genistein for the indicated times and proteins were extracted for Western blot analysis. As shown in Fig. 4(c), treatment with 500 nm-genistein led to increased Nrf2 expression for at least 10 h as observed in whole cell extracts. The same phenomenon was observed in the nuclear extract (Fig. 4(d)); the highest level of Nrf2 expression was observed at 6 h. Moreover, treatment with 20–500 nm-genistein for 6 h also induced Nrf2 nuclear translocation (Fig. 4(e)). Of note, treatment with 500 nm-genistein resulted in significant increases in Nrf2 mRNA expression levels (Fig. 4(b)). These data suggest that genistein is a potential inducer of Nrf2 in endothelial cells.

Fig. 4 Genistein activates nuclear factor erythroid 2-related factor 2 (Nrf2). (a) Effect of genistein on Nrf2 promoter activity. EA.hy926 cells were transfected with the antioxidant responsive element-reporter plasmid and treated with genistein (20–500 nm) for 10 h. The cells were then harvested, and luciferase activity was determined. (b) Effects of genistein on Nrf2 mRNA expression levels. Cells were pretreated with genistein (500 nm) for 10 h, and total RNA was extracted. Nrf2 mRNA expression levels were assessed by real-time PCR. (c) Effects of genistein on Nrf2 protein expression levels. Cells were pretreated with genistein (500 nm) for the indicated times, and cell extracts were then prepared for Western blot analysis. (d, e) Effects of genistein on the nuclear translocation of Nrf2 protein. Cells were either pretreated with genistein (500 nm) for the indicated times (d) or treated with the indicated concentrations for 6 h (e), and nuclear extracts were then prepared for Western blot analysis. Nuclear extracts were prepared using the Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime) according to the manufacturer's instructions. Values are means (n 3 for each group), with standard deviations represented by vertical bars. Mean values were significantly different compared with the non-genistein control group: * P< 0·05, ** P< 0·01, *** P< 0·001.
Genistein activates PPARγ
PPARγ plays an important role in vascular regulation. PPARγ activation inhibits endothelial inflammation by suppressing inflammatory gene expression, thereby hindering endothelial damage. To assess whether genistein activates PPARγ, a plasmid containing the PPAR response element X3-luciferase reporter gene was transfected in EA.hy926 cells, and gene activation was examined using a dual luciferase reporter gene assay. As shown in Fig. 5(a), activation of the PPARγ promoter was enhanced (1·7-fold in the 500 nm-treated group). Additionally, PPARγ protein expression was analysed using Western blotting in EA.hy926 cells after the treatment with genistein (500 nm) for the indicated times. The genistein treatment induced PPARγ expression in a time-dependent manner (Fig. 5(c)). According to the present results, the ratio of PPARγ:tubulin in non-genistein-treated cells was 0·7, cells treated with genistein for 1 h displayed a slight induction (ratio 1·3), and the expression induction ratio reached 2·0 after a 4–10 h treatment with genistein. As expected, genistein led to significant increases in PPARγ accumulation within the nucleus of EA.hy926 cells (Fig. 5(d)). Moreover, the genistein treatment also significantly increased PPARγ mRNA expression levels (3·3-fold) compared with the control cells (Fig. 5(b)). Above all, these data demonstrate that genistein is able to activate PPARγ in endothelial cells. However, the effect that genistein displayed on PPARγ activation was less pronounced than its effect on Nrf2 activation, which suggests that genistein is able to induce the gene expression of various antioxidant genes, albeit at varying degrees.

Fig. 5 Genistein activates PPARγ activity. (a) Effect of genistein on PPARγ promoter activity. EA.hy926 cells were transfected with a PPARγ-reporter plasmid and treated with genistein (20–500 nm) for 16 h. Cells were subsequently harvested, and then luciferase activity was determined. F/R, firefly/renilla (b) Effect of genistein on PPARγ mRNA expression levels. Cells were pretreated with genistein (500 nm) for 10 h, and total RNA was extracted. The PPARγ mRNA expression levels were assessed by real-time PCR. (c) The effects of genistein on PPARγ protein expression levels. Cells were pretreated with genistein (500 nm) for the indicated times, and cell extracts were prepared for the analysis of PPARγ expression via Western blot. (d) Effects of genistein on the nuclear translocalisation of PPARγ protein. Cells were pretreated with genistein (500 nm) for 4 h, and nuclear extracts were prepared for Western blot analysis. Values are means (n 3 for each group), with standard deviations represented by vertical bars. Mean values were significantly different compared with the non-genistein control group: * P< 0·05, *** P< 0·001.
Genistein up-regulates haem oxygenase-1 expression and activity
As HO-1 is an important component of cellular defence against oxidative stress and is regulated by the Nrf2/ARE-mediated defensive pathway or PPARγ, we also assessed whether genistein could induce HO-1 gene expression. Cells were again exposed to 500 nm-genistein for the indicated times, and time-dependent (Fig. 6(a)) and dose-dependent (Fig. 6(b)) increases in HO-1 protein expression levels were observed. The genistein treatment for 5–10 h led to increases in HO-1 mRNA expression levels compared with the control cells (Fig. 6(c)). In addition, HO-1 activity was also tested, and as shown in Fig. 6(d), a dose-dependent induction of HO-1 activity was observed after the treatment with genistein for 10 h.
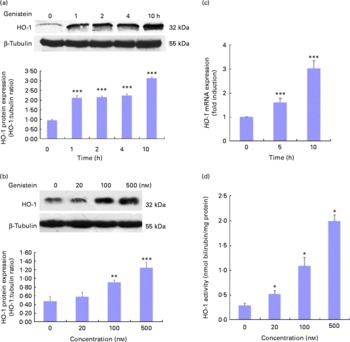
Fig. 6 Genistein up-regulates haem oxygenase-1 (HO-1) expression. (a, b) Effect of genistein on HO-1 protein expression levels. EA.hy926 cells were either pretreated with genistein (500 nm) for the indicated times (a) or treated with various concentrations of genistein for 10 h (b). Cell extracts were then prepared, and HO-1 expression levels were analysed using Western blotting. (c) Effect of genistein on HO-1 mRNA expression levels. Cells were pretreated with genistein (500 nm) for the indicated times, and total RNA was extracted. The HO-1 mRNA expression levels were assessed by real-time PCR. (d) HO-1 activity was measured 10 h after exposure to various concentrations of genistein. Values are means (n 3), with standard deviations represented by vertical bars. Mean values were significantly different compared with the non-genistein control group: * P< 0·05, ** P< 0·01, *** P< 0·001.
Nuclear factor erythroid 2-related factor 2, PPARγ and haem oxygenase-1 mediate the protective effect of genistein
To further investigate whether Nrf2 plays a role in genistein-induced protection, EA.hy926 cells were transfected with Nrf2 siRNA, while FITC-labelled control siRNA-transfected cells served as a control (Fig. 7(a)). Nrf2 protein expression levels were significantly reduced by Nrf2 siRNA transfection, and the up-regulation of Nrf2 by genistein (500 nm) was lost after siRNA transfection (Fig. 7(b)). Moreover, the protective effect against H2O2-induced cell death was reduced after the transfection with Nrf2 siRNA (Fig. 7(c)), thereby suggesting that the activation of Nrf2 plays a role in the genistein-mediated protection against oxidative stress-induced cell damage.

Fig. 7 Inhibition of nuclear factor erythroid 2-related factor 2 (Nrf2), PPARγ and haem oxygenase-1 attenuates the cytoprotective effect of genistein. EA.hy926 cells were seeded on sterile glass coverslips in twenty-four-well plates overnight, and small interfering RNA (siRNA) transfection was then performed according to the manufacturers' instructions. (a) A control siRNA (fluorescein-conjugated) transfection was used as a control. (b) Cells were transfected with either Nrf2 siRNA or control siRNA, treated with genistein (500 nm) for 10 h, and harvested. Nrf2 protein levels were assessed by Western blot analysis. (c) After transfection with Nrf2 siRNA (![]() ) or control siRNA (
) or control siRNA (![]() ), cells were pretreated with genistein (500 nm) for 10 h and then incubated with H2O2 (650 μm) for 24 h. Cell viability was determined by the Cell Counting Kit-8 (CCK-8) assay. Values are means (n 6), with standard deviations represented by vertical bars. *** Mean values were significantly different compared with the control siRNA transfection (genistein plus H2O2) group (P< 0·001). (d) Cells were treated with genistein (500 nm) for 10 h and then incubated with H2O2 (650 μm) with the addition of either Znpp (10 μm) for 24 h or GW9662 (10 μm) for 30 min. Next, cells were treated with genistein (500 nm) for 10 h and then incubated with H2O2 (650 μm) for 24 h. Cell viability was assessed using the CCK-8 assay. Values are means (n 6 for each group), with standard deviations represented by vertical bars. Mean values were significantly different compared with both the H2O2-only control group (** P< 0·01, *** P< 0·001) and the genistein plus H2O2 control group (†† P< 0·01, ††† P< 0·001).
), cells were pretreated with genistein (500 nm) for 10 h and then incubated with H2O2 (650 μm) for 24 h. Cell viability was determined by the Cell Counting Kit-8 (CCK-8) assay. Values are means (n 6), with standard deviations represented by vertical bars. *** Mean values were significantly different compared with the control siRNA transfection (genistein plus H2O2) group (P< 0·001). (d) Cells were treated with genistein (500 nm) for 10 h and then incubated with H2O2 (650 μm) with the addition of either Znpp (10 μm) for 24 h or GW9662 (10 μm) for 30 min. Next, cells were treated with genistein (500 nm) for 10 h and then incubated with H2O2 (650 μm) for 24 h. Cell viability was assessed using the CCK-8 assay. Values are means (n 6 for each group), with standard deviations represented by vertical bars. Mean values were significantly different compared with both the H2O2-only control group (** P< 0·01, *** P< 0·001) and the genistein plus H2O2 control group (†† P< 0·01, ††† P< 0·001).
To investigate the involvement of PPARγ and HO-1 in the protective mechanism of genistein, we applied a PPARγ-specific antagonist (GW9662) and an HO-1-specific antagonist (Znpp) to determine whether they would block the protective effects of genistein. Both GW9662 (10 μm) and Znpp (10 μm) partially blocked the protective effects of genistein (Fig. 7(d)), indicating that the activation of PPARγ and HO-1 is at least partly involved in genistein-mediated protection. The involvement of HO-1 further suggests that Nrf2 and PPARγ, two antioxidant genes, play a fundamental role in genistein-induced protection.
Discussion
In the present study, we have shown that treatment with low (physiological) concentrations of the soya isoflavone genistein significantly protects vascular endothelial cells against oxidative stress-induced loss of cell viability and apoptosis. This protective effect was mediated by both the gene regulation of Nrf2 and PPARγ, and their downstream gene HO-1, as well as cellular antioxidants, including SOD, CAT and GSH. These findings shed light on the mechanism by which genistein protects vascular endothelial cells against oxidative stress.
Endothelial cell damage and subsequent apoptosis are key events in the pathogenesis of various vascular diseases, such as oxidative stress-induced atherosclerosis(Reference Norata, Tonti and Roma23). Genistein (μM, pharmacological concentrations) has previously been demonstrated to hinder oxidative stress-induced endothelial damage, as displayed by the inhibition of cell proliferation and secretion(Reference Busik, Mohr and Grant24) as well as a decrease in cell apoptosis(Reference Xu, Zhong and Ghavideldarestani7, Reference Si and Liu25–Reference Huang, Liu and Chang27) and permeability(Reference Ho, Lin and Chen28). Here, the present results show that genistein displayed cytoprotective effects at physiological concentrations (nm) and significantly inhibited oxidative stress-induced cell apoptosis. Furthermore, the 100–500 nm concentration of genistein found to exert the potent cytoprotective effects is within the concentration range found in the plasma of Asian populations with a diet rich in soya protein (300–500 nm)(Reference Borras, Gambini and Gomez-Cabrera8, Reference Adlercreutz, Markkanen and Watanabe29), which suggests that a soya-rich diet may be beneficial for atherosclerosis patients.
However, controversy exists regarding the effect of genistein-mediated activity (antioxidant or pro-oxidant) on apoptosis. Genistein has been shown to induce apoptosis in many cancer cell types, including breast cancer cells, prostate cancer cells, non-small cell lung cancer cells, head and neck squamous carcinoma cells, and pancreatic cancer cells(Reference Ramos30). This genistein-induced pro-apoptotic effect may be due to its regulation of apoptosis-associated genes, such as Bcl-2, Bax, Bcl-xL, PARP (poly-ADP-ribose polymerase) and caspase-3 (Reference Ramos30). There is limited direct evidence that genistein's pro-apoptotic effect is attributed to its pro-oxidant activity. Ullah et al. (Reference Ullah, Ahmad and Zubair31) have found that genistein induced breast cancer cell death through the mobilisation of endogenous Cu ions and generation of ROS. However, genistein exerts an antioxidant effect via the inhibition of ROS generation, thereby resulting in the inhibition of NF-κB activation, which plays a role in angiogenesis and metastasis. Genistein also acts as a chemopreventive agent against CVD, osteoporosis and Alzheimer's disease. It has been reported that genistein reduces the levels of apoptosis by enhancing the expression of anti-apoptotic proteins in endothelial cells, human retinal pigment epithelial cells and neuronal PC12 cells. In addition to the direct scavenging of ROS and the inhibition of the oxidation of LDL, the ability of genistein to activate cell signalling pathway molecules, including endothelial No synthase, nuclear factor erythroid 2-related factor 1 (Nrf1) and Nrf2, makes it an optimal natural antioxidant with potential clinical use. The observation that genistein exerts a pro-apoptotic effect on cancer cell while normal cells remain unaffected may be due to differences in cell type, pathophysiology and behavioural gene expression in cancer and CVD. Unlike normal cells, cancer cell types are characterised by deregulated cell growth and cell proliferation, invasive and metastatic potential, and constitutively active NF-κB signalling, resulting in high levels of oxidative stress. It has previously been reported that genistein concentrations that induce apoptosis range from approximately 30 to 200 μm, based on studies in various cancer cell lines. Interestingly, these high genistein concentrations are non-toxic to normal cells. According to the present observations, genistein (50 μm) has little influence on endothelial cell proliferation and apoptosis. Moreover, curcumin has been shown to induce apoptosis and enhance the activity of oxaliplatin on colon cancer cells, while immortalised normal colon cells remain unaffected(Reference Howells, Mitra and Manson32). Additionally, (–) epigallocatechin gallate causes apoptosis in epidermoid carcinoma cells, but not in normal keratinocytes(Reference Ahmad, Gupta and Mukhtar33). Therefore, we could not simply analyse genistein as an antioxidant or a pro-oxidant while studying its anti-apoptotic or pro-apoptotic effect under different pathophysiology. This is especially true for mechanistic studies, which require careful consideration and realistic approaches with respect to dose, choice of cell types and growth conditions.
Bcl-2 is an anti-apoptotic protein that plays an important role in sustaining cell viability and function(Reference Metrailler-Ruchonnet, Pagano and Carnesecchi34). Both Bcl-2 and Bax, another Bcl-2 family member, have been shown to be regulated by oxidative stress(Reference Xu, Zhong and Watson35). However, Bcl-2 regulation by phyto-oestrogens during oxidative stress conditions has only been reported in a few studies(Reference Xu, Zhong and Ghavideldarestani7, Reference Xu, Zhong and Watson35). Here, we verified that Bcl-2 protein expression was up-regulated by genistein in EA.hy926 cells, thereby suggesting that this effect may play a role in the mechanism by which genistein protects against oxidative stress-induced endothelial cell injury.
Genistein is the most prominent soya isoflavone and has been shown to inhibit the growth of cancer cells via the regulation of genes associated with the homeostatic control of cell-cycle progression and apoptosis. At high concentrations (10–50 μm), genistein has also been shown to act as an inhibitor of protein-tyrosine kinases(Reference Sarkar and Li36). Although we used a relatively low genistein concentration (100–500 nm) in the present study, we observed a significant cytoprotective effect against oxidative stress in endothelial cells, which correlates with the results described by Xu et al. (Reference Xu, Zhong and Ghavideldarestani7). Interestingly, plasma genistein levels of patients receiving genistein or dietary soya supplementation were previously found to be 18 (sd 20) nm(Reference Bhakta, Higgins and Sevak37), while plasma genistein concentrations ranged from 50 to 800 ng/ml (equal to 185 nm–3 μm) in Japanese subjects with traditional soya-rich diets(Reference Adlercreutz, Markkanen and Watanabe29, Reference Morton, Arisaka and Miyake38). Therefore, the beneficial effects of genistein occur at dietary concentrations. In addition, previous studies have indicated that a 10–50 μm-genistein concentration is required for the protein-tyrosine kinases-inhibitory effects of genistein, thereby suggesting that the protective effects observed in the present study may be a result of the antioxidative properties of genistein rather than its inhibitory effects on protein-tyrosine kinases. Moreover, as it is difficult to take in such high levels (μM) simply through dietary soya supplementation and may also be difficult to achieve via drug intervention, physiologically relevant concentrations (nm) of genistein that can be obtained with either a reasonable diet or pharmacologically relevant supplementation are more realistic for the clinical setting. Therefore, a change in diet or the use of supplements may help to improve vascular dysfunction in patients by decreasing oxidative stress.
Nrf2 has been implicated in the regulation of genes involved in response to oxidative stress(Reference Zhang39). Nrf2-null mice exhibit an increased susceptibility to oxidants, colitis, lupus-like immune nephritis, emphysema, carcinogenesis and vascular damage(Reference Kobayashi and Yamamoto40). Based on the involvement of Nrf2 in chemoprevention, a high-throughput screening for small-molecule Nrf2 activators represents an innovative strategy to enhance resistance to environmental insults. HO-1 plays a key role in the maintenance of antioxidant homeostasis during cellular stress(Reference Alam and Cook41). Human atherosclerotic lesions have been shown to exhibit enhanced gene expression of HO-1, while the overexpression of HO-1 in vascular tissues has been shown to protect against atherogenesis and restenosis in rodent models of hypercholesterolaemia and vascular injury(Reference Juan, Lee and Tseng42). Until now, evidence displaying that genistein may regulate the expression of genes encoding for phase II and antioxidant enzymes was limited and controversial. In vivo studies have shown that Nrf2 protein levels were not altered by dietary genistein in the liver of feeding rats(Reference Wiegand, Wagner and Boesch-Saadatmandi43). A pharmacological dose of genistein (50 μm) was previously found to activate Nrf1, but not Nrf2, in EA.hy926 cells(Reference Hernandez-Montes, Pollard and Vauzour44). Genistein treatment has been shown to lead to increased quinine reductase activity, although daidzein and equol displayed a greater impact on Nrf2 activity at physiological concentrations (1 and 5 μm) compared with genistein(Reference Froyen and Steinberg45). Borras et al. (Reference Borras, Gambini and Carmen Gomez-Cabrera46) have reported that genistein (0·5 μm) up-regulated antioxidant and longevity-related genes as well as MnSOD levels in MCF-7 cells. However, we herein provide the first demonstration that a low, physiologically relevant concentration of genistein (20–500 nm) can activate Nrf2 and a downstream gene, HO-1, in endothelial cells. Moreover, we demonstrated that the effect of genistein on H2O2-induced loss of cell viability was blocked by Nrf2 siRNA transfection and the HO-1 antagonist Znpp, which suggests that low concentrations of genistein may protect cells via the activation of the Nrf2/HO-1 pathway. Furthermore, the present findings indicate that physiologically relevant concentrations of genistein, commonly found in the plasma of Eastern populations who regularly consume soya-based foods, may improve cellular redox imbalance via the activation of an antioxidant gene. It remains to be determined whether these plasma levels can also be achieved in Western populations, as these populations generally do not consume soya products, and thus there may be variations in the disposition of genistein in different populations.
Both human genetic analyses and studies in transgenic mice have demonstrated the importance of PPARγ in vascular disorders(Reference Duan, Usher and Mortensen47). Angiotensin II has been implicated in both hypertension and atherosclerosis in mice, and studies on endothelial cell dysfunction display decreases in PPARγ mRNA and protein expression(Reference Tham, Martin-McNulty and Wang48). However, the impact of oxidative stress on PPARγ activity is not clear. Recent studies have shown that oxidative stress attenuates PPARγ expression and activity in endothelial cells through the suppression of PPARγ transcription at least partly as a result of the activation of inhibitory redox-regulated transcription factors(Reference Blanquicett, Kang and Ritzenthaler49). PPARγ activation in endothelial cells inhibits inflammation by suppressing the expression of inflammation-associated genes, thereby reducing endothelial damage(Reference Sasaki, Jordan and Welbourne18, Reference Verrier, Wang and Wadham19). Genistein has been shown to act as an agonist of PPARγ in an in vitro reporter gene assay experiment(Reference Dang, Audinot and Papapoulos50), while the oestrogen-induced production of a PPAR ligand has also been reported(Reference Ma, Sprecher and Kolattukudy51). In our studies, we observed that genistein (80 μm), a natural PPARγ ligand, could induce HT29 cell apoptosis via the activation of PPARγ (R Rong unpublished results). In the present study, we demonstrated that low concentrations of genistein also induced PPARγ promoter activity and led to increases in PPARγ protein expression and transcription levels in EA.hy926 cells, while the effects of genistein during cellular oxidative stress were attenuated by a PPARγ-specific antagonist, GW9662. These results are consistent with studies that displayed that genistein (0·5 μm) activates PPARγ in cultured astrocytes(Reference Valles, Dolz-Gaiton and Gambini52). In addition, it should be noted that PPARγ ligands are also able to up-regulate HO-1(Reference Ferguson, Thatcher and Olsen53). The up-regulation of HO-1 by genistein further suggests the involvement of the PPARγ signalling pathway in genistein-induced endothelial cell protection. Several clinical trials have demonstrated the apparent cardiovascular benefits of thiazolidinediones (especially rosiglitazone), a class of drugs used to treat type 2 diabetes mellitus that functions by binding to PPAR(Reference Duan, Usher and Mortensen47). Genistein may also exert cardiovascular benefits through PPAR proteins. Therefore, the present results provide a basis for new approaches in the treatment of oxidative stress-induced atherosclerosis.
Low concentrations of genistein have been shown to activate the oestrogen receptor (ER). At high concentrations, genistein inhibits the growth of both ERα- and ERβ-positive MCF-7 cells as well as ERα-negative and ERβ-positive (MDA-MB-231) breast cancer cells via an ER-independent mechanism; however, the stimulation of MCF-7 cell growth by treatment with low concentrations of genistein functions via the ER(Reference Moiseeva and Manson54). Similar reports of ERα and ERβ immunoreactivity were first identified in human umbilical vein endothelial cells (HUVEC) by Joy et al. (Reference Joy, Siow and Rowlands55). Genistein has also been reported to up-regulate ERβ expression in HUVEC(Reference Xu, Zhong and Ghavideldarestani7). Moreover, studies have suggested that oestrogenic activity is involved in genistein-mediated antioxidant effects (quinine reductase and MnSOD)(Reference Froyen and Steinberg45, Reference Borras, Gambini and Carmen Gomez-Cabrera46) and that endothelial cells also represent an important oestrogen target. However, the present data display conflicting results regarding the interactions between oestrogen and the antioxidant genes mediated by soya isoflavones. Nutritionally relevant plasma concentrations of equol stimulated NO synthesis in an ER-independent manner(Reference Joy, Siow and Rowlands55). This mounting evidence suggests that the mechanism of genistein activity is more complex than simple receptor interactions or antioxidant activation. Therefore, further analysis should be focused on the possible involvement of ER-mediated antioxidant genes (Nrf2 and PPARγ) in endothelial cells.
In conclusion, genistein can protect against oxidative stress-induced endothelial cell injury by altering the expression of antioxidant genes and elevating antioxidant enzyme and GSH levels. The present results provide the basis for further evaluation of the effects of soya isoflavones with regard to their potential in the treatment of CVD. Moreover, the present findings suggest that the lower incidence of CVD in Asian populations compared with Western populations may be at least partially due to the higher plasma concentration of genistein in Asian individuals.
Acknowledgements
This study was supported by the Major State Basic Development Program of China (no. 2010CB529403) and the National Natural Science Foundation of China (no. 81102129), China. The authors thank Wei Sun, Yang Xia, Bin Yu, Maohua Hu, Wang Li and Hongxin Hao for their extensive technical assistance and acknowledge Changjun Zhao and Minliang Chen for their assistance in the experimental work. T. Z., M.-T. M., L. Y., H.-X. X., Q.-Y. Z. and H. C. were involved in the study design. T. Z., F. W. and Y. Q. conducted the experiments and the statistical analyses. T. Z., F. W. and M.-T. M. wrote the first draft of the manuscript. All authors contributed to the final version of the manuscript. M.-T. M. had primary responsibility for the final content. The authors declare that there are no conflicts of interest.









