As the obesity epidemic continues, more subjects are getting fatter and are therefore at increased risk for metabolic complications, hypertension and cancer-related mortality( Reference Lafortuna, Adorni and Agosti 1 , Reference De Pergol and Silvestris 2 ). The aetiology of obesity is multifactorial, and genetic inheritance and behavioural/environmental causes are considered to be the main factors( Reference Herring, Sailors and Bray 3 ). Dietary fat is considered palatable to humans, and several factors, including its olfactory, visual and textural properties, have been proposed as playing a key role in the attractiveness of fat( Reference Mattes 4 ). Humans and rodents can detect long-chain fatty acids in their diets as gustatory cues( Reference Mattes 4 – Reference Kamphuis, Saris and Westerterp-Plantenga 9 ). Some recent studies have shown that obese subjects exhibit a high preference for dietary lipids as compared to lean subjects( Reference Stewart, Feinle-Bisset and Keast 10 , Reference Stewart and Keast 11 ), which suggests that inappropriate lipid perception might influence obesity risk by impacting feeding behaviour. In fact, obesity is associated with a low sensitivity to the oro-sensorial detection of fat( Reference Stewart, Feinle-Bisset and Keast 10 , Reference Stewart and Keast 11 ).
Lingual cluster of differentiation 36 (CD36), like G protein-coupled receptor 120 (GPR120) and G protein-coupled receptor 40 (GPR40), has been shown to act as a lipid receptor that is involved in a spontaneous preference for fat( Reference Laugerette, Passilly-Degrace and Patris 6 , Reference Cartoni, Yasumatsu and Ohkuri 12 – Reference Abdoul-Azize, Selvakumar and Sadou 15 ). The lingual lipid receptors bind to long-chain fatty acids, which are released by lingual lipases in the buccal cavity( Reference Cartoni, Yasumatsu and Ohkuri 12 – Reference Abdoul-Azize, Selvakumar and Sadou 15 ). We performed the present study on CD36 SNP because CD36 is a high-affinity receptor, whereas GPR120 and GPR40 are low-affinity receptors. In addition, GPR40 could not be detected on human lingual epithelium( Reference Galindo, Voigt and Stein 16 ). Moreover, Sclafani et al. ( Reference Sclafani, Ackroff and Abumrad 17 ) have shown that CD36 is directly involved in early fat detection, whereas GPR120 plays a role in the post-ingestive regulation of fat preference( Reference Sclafani, Zukerman and Ackroff 18 ).
Recent studies have shown that CD36 protein expression is influenced by CD36 gene polymorphism, and it is related to the detection threshold of dietary lipids in obese subjects( Reference Pepino, Love-Gregory and Klein 19 ). Keller et al. ( Reference Keller, Liang and Sakimura 20 ) reported that obese subjects with the CD36 AA genotype (rs1761667) perceived more creaminess in salad as compared to those with the AG or GG genotypes. Pepino et al. ( Reference Pepino, Love-Gregory and Klein 19 ) reported that obese subjects with the AA genotype exhibited higher oral detection thresholds for fat than those with the AG and GG genotypes. These novel findings are changing our view about the pathogenesis of obesity; however, future studies must be conducted to confirm these interesting findings, particularly in developing countries where obesity is quickly rising.
In chronic pathological conditions such as obesity, IL-6 plays a synergic role in inflammation( Reference Dandona, Ghanim and Chaudhuri 21 – Reference Steensberg, Fischer and Keller 23 ), because macrophages within adipose tissue might secrete IL-6( Reference Fried, Bunkin and Greenberg 22 ). An association between the rs1800795 polymorphism of the IL-6 gene and increased adiposity, inflammation and metabolic disturbances has been demonstrated( Reference Poitou, Lacorte and Coupaye 24 , Reference Bouhaha, Baroudi and Ennafaa 25 ). In obesity, adipose tissues also secrete TNF-α abundantly( Reference Qi and Pekala 26 ), and rs1800629 polymorphism of the TNF-α gene( Reference Wilson, Symons and McDowell 27 ) has been reported to be associated with obesity risk( Reference Um and Kim 28 ) as well as a high incidence of type 2 diabetes( Reference Kubaszek, Pihlajamäki and Komarovski 29 ). Because obesity is marked by inflammation, the present study is also designed to explore the relationship between pro-inflammatory markers (IL-6, TNF-α and C-reactive protein) and the oro-sensorial detection of lipids in obese subjects.
There has recently been a rapid upsurge in overweight/obesity and obesity-related diseases in Tunisia, especially in women as compared to men( Reference Popkin, Adair and Ng 30 , Reference El Ati, Traissac and Delpeuch 31 ). This sex gap between women and man differs greatly according to environmental and socio-economic conditions( Reference El Ati, Traissac and Delpeuch 31 ). Keeping in mind the aforementioned literature on lipid oral taste sensitivity and CD36 SNP, we thought it would be worthwhile to investigate whether CD36 SNP in obese Tunisian women is associated with decreased fat taste perception.
Materials and methods
Subjects
Inclusion criteria
Obese women (n 203) were recruited from the group of patients who visited the gynaecology outpatient department (OPD) of Farhat Hached University Hospital, Sousse (Tunisia), in 2012 and 2013 for a general health check-up. Medical records were screened by specialist clinicians. The studied women were between 38 and 43 years old. The women were asked to return to the gynaecology OPD when they were in their first week of menstruation, and they were given an appointment for a particular date so that blood sampling and an analysis of other parameters could be performed.
The exclusion criteria included smoking, diabetes, breastfeeding, pregnancy-related complications, a history of gestational diabetes, the use of oral contraception, chronic illness such as hypertension or any other inflammatory pathology, any autoimmune disease, any lipid-lowering medication, recent weight loss, dieting and the use of any medications known to affect taste. The inclusion criterion constituted a normal glucose tolerance test and electrocardiograms.
Anthropometrics
Body weight and height were measured in the morning while participants were unclothed and not wearing shoes. BMI was calculated as body weight (in kg) divided by height (in m2). Obesity was defined as a BMI of 30 kg/m2 or higher, in accordance with the recommendations of WHO. The characteristics of the women are shown in Table 1.
Table 1 Clinical characteristics of obese Tunisian women (n 203) (Mean values and standard deviations)
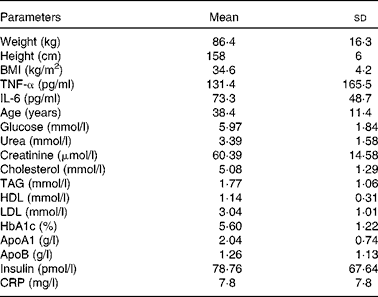
HbA1c, glycosylated Hb; CRP, C-reactive protein.
Ethics
The present study was carried out in accordance with the Declaration of Helsinki (1989) of the World Medical Association and was approved by Farhat Hached Hospital Committee for Research on Human Subjects (Tunisia). Informed written consent was obtained from all of the subjects. The present experimental protocol conforms to the relevant ethical guidelines for human research.
Blood samples
Fasting venous blood samples were collected from each woman to obtain plasma (EDTA tubes) and serum. Serum and plasma were prepared by centrifugation (1000 g at 20 min). Plasma was immediately used for glucose determination. Serum was aliquoted and frozen at − 80°C for further analysis of blood parameters.
Determination of blood parameters
Serum TAG, total cholesterol and free cholesterol concentrations were determined using enzymatic methods, according to the manufacturer's instructions furnished with the kit (Boehringer). HDL-cholesterol was also measured by a kit (Boo Scientific). LDL-cholesterol concentrations were calculated according to Friedewald et al. ( Reference Friedewald, Levy and Fredrickson 32 ). All biochemical parameters were analysed on a Synchron CX7 Clinical System (Beckman). Plasma fasting glucose was determined by the glucose oxidase method with a glucose analyser (Beckman Instruments). Plasma glycosylated Hb (HbA1c) concentrations were determined by isolab column chromatography( Reference Kaplan, Cline and Gartside 33 ). Insulin serum concentrations were determined using an Insulin IRMA kit (Immunotech; Beckman Coulter, Inc.) with a detection limit of 0·5 μIU/ml (3·4725 pmol/l). The inter-assay CV was 3·3 and 4 % for the 13 and 54 IU/ml (90·285 and 375·03 pmol/l) concentrations, respectively.
Serum concentrations of urea, creatinine and C-reactive protein were analysed by routine standard techniques using an automated Synchron CX7 Clinical System (Beckman). Serum concentrations of IL-6 and TNF-α were measured with ELISA kits (Immunotech).
Oleic acid sensitivity analysis
Taste emulsions containing food grade oleic acid (Sigma) were prepared according to Chalé-Rush et al. ( Reference Chalé-Rush, Burgess and Mattes 34 ). EDTA (0·01 %, w/v) was added to prevent fatty acid oxidation. The emulsions were sonicated for 4–5 min in a Labo-Modern sonicator at 4°C in an ice bath. Samples were stored in opaque polypropylene tubes and used for testing within 48 h of preparation. Control samples were prepared in the same way but without added oil.
The women were called on a stipulated date and advised to arrive early in the morning without having eaten breakfast (fasting state). The subjects were weighed, and a blood sample was drawn before the preference test to assess blood parameters. Taste preference tests for dietary lipids were performed by employing oleic acid at different ascending concentrations (0·018, 0·18, 0·37, 0·75, 1·5, 3, 6 and 12 mmol/l) by using a three-alternative forced choice (3-AFC) method( Reference Stewart, Seimon and Otto 35 ). According to the 3-AFC method, the patients were instructed to taste, one by one, three solutions; two of the solutions contained a control substance (acacia gum, 0·01 %), and the third one contained oleic acid in a solution that also included acacia gum (0·01 %). The acacia gum was used to mimic the textural properties of oil in the control solution.
We increased the concentration of oleic acid in the test solution when a single incorrect response was given, and we decreased the quantity of this fatty acid after two correct responses, in accordance with the method described by Pepino et al. ( Reference Pepino, Love-Gregory and Klein 19 ). A reversal in the response was considered when the concentration sequence changed direction. The procedure was terminated when there were four reversals that met the following two criteria. First, there could not be more than two dilution steps between two successive reversals. Second, the series of reversals could not form an ascending pattern. The threshold concentration was calculated as mean of log values for the last four reversals. To avoid visual and olfactory cues, the testing session was conducted under red light and participants used nose clips. The women were not allowed to drink the solutions; rather, they had to spit out each solution after keeping it in their mouths for a few seconds. If they responded that they observed no difference in the taste sensation, we increased the concentration of oleic acid. If they were able to detect a difference, it meant they were capable of detecting the presence of ‘fatty taste’.
Genotyping analyses
Genomic DNA was extracted from 5 ml of whole blood with the use of a commercially available DNA isolation kit (Wizard Genomic DNA purification kit; Promega Corporation) according to the manufacturer's protocol. Genotyping of TNF-α − 308 G/A (rs1800629), IL-6 − 174 G/C (rs1800795) and CD36 A/G (rs1761667) was performed according to methods that have been previously used by our laboratory( Reference Drtilkova, Sery and Theiner 36 , Reference Sery, Hladilova and Novotny 37 ). The PCR primers for the three genotypes were as follows: (TNF-α: 5′-AGG CAA TAG GTT TTG AGG GGC AT-3′ and 5′-CGG GGA AAG AAT CAT TCA ACC AG-3′; CD36: 5′-CAA AAT CAC AAT CTA TTC AAG ACCA-3′ and 5′-TTT TGG GAG AAA TTC TGA AGA G-3′; IL-6: 5′-ACT TTT CCC CCT AGT TGT GTC TTT C-3′ and 5′-AGA ATG AGC CTC AGA CAT CTC CAG T-3′). PCR amplification reactions were performed in a Veriti thermal cycler (Life Technologies). After initial denaturation for 3 min at 95°C, DNA was subjected to further amplification as follows for TNF-α and IL-6: denaturation for 30 s at 95°C, annealing for 30 s at 66°C and extension for 30 s at 72°C. After thirty-five cycles, a final extension for 5 min at 72°C was used. Amplified DNA was digested by either endonuclease Ncol (TNF-α) or TaqI (IL-6) and further incubated at either 37°C for 16 h (TNF-α) or 65°C for 5 h (IL-6). For CD36, the conditions were as follows: denaturation for 30 s at 95°C, annealing for 30 s at 56°C and extension 30 s at 72°C. After forty cycles, a final extension for 5 min at 72°C was used. Amplified DNA was digested by Hha1 at 37°C for 30 min. The digestion products were analysed by 2 % (w/v) agarose gel electrophoresis (Elisabeth Pharmacon) containing ethidium bromide, and DNA fragments were visualised under UV light. The following fragments were detected for TNF-α: 264 bp (GG genotype), 264 and 284 bp (AG genotype) and 284 bp (AA genotype). For IL-6, two fragments of 24 and 180 bp (G allele) and an unrestricted fragment of 204 bp (C allele) were obtained. Two fragments of CD36 (138 and 52 bp) in the presence of the G allele were visualised, and an unrestricted fragment (A allele) had a length of 190 bp.
Statistical analysis
CSS Statistica software (StatSoft) was used for statistical analysis. An ANOVA was used for correlation of the different parameters and genotypes. A Kruskal–Wallis test was used for one-way analyses on ranks. The χ2 test was used for the comparison of genotype frequencies. Fisher's exact test was used for the comparison of allelic frequencies. For correlation studies, we used Pearson's correlation coefficient method. Dunn's method was used for all pairwise multiple comparisons between the AA, AG and GG genotypes and the detection thresholds.
Results
Subject characteristics
Table 1 shows the anthropometric measures and concentrations of different blood parameters in the present cohort of obese Tunisian women (n 203). The values of glucose, insulin, urea, creatinine, cholesterol, HDL, LDL, HbA1c, apoA1, apoB, insulin and C-reactive protein were within normal ranges for obese women. Serum TAG, IL-6 and TNF-α concentrations were higher in the women as compared to previously reported control values( Reference Fernandez-Real, Vayreda and Richart 38 , Reference Aukrust, Müller and Lien 39 ).
CD36 genotype and oleic acid detection thresholds
Table 2 shows the genotype frequencies of three polymorphisms in the present cohort of obese women. Fig. 1 shows that the subjects with the GG genotype of the CD36 gene had thresholds for oleic acid detection that were 3·3 times lower than those of subjects with the AA genotype (95 % CI of relative risk 2·5032, 4·4298, OR 9·9615; 95 % CI of OR 6·2101, 15·9793). We did not observe a statistically significant difference in the taste detection thresholds of subjects with the AG or the AA (or GG) genotypes. It is also noteworthy that some subjects, which have been termed non-tasters, could not detect fatty acid even at the highest concentration. There were a total of four non-tasters in the AA, AG and GG genotypes of CD36 gene (Fig. 1).
Table 2 Genotype frequencies in obese Tunisian women
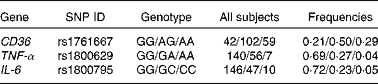
CD36, cluster of differentiation 36.

Fig. 1 Oleic acid detection thresholds in obese Tunisian women. The women (n 203) had either the AA genotype (n 59) or the GG (n 42) or AG (n 102) genotype of the cluster of differentiation 36 (CD36) gene. The figure shows the box plots of the medians, first and third quartiles, standard deviations and extreme values. The difference between the three groups was statistically significant (P< 0·001; Kruskal–Wallis test). * Median value was significantly different from that of the AA genotype (P< 0·05; one-way ANOVA). The difference between the AG and GG genotypes was not statistically significant. NT, non-tasters.
Association between cholesterol, LDL and glycosylated Hb and CD36 polymorphism
Fig. 2(a) and (b) shows that cholesterol and LDL concentrations were significantly lower in subjects with the CD36 GG and AG genotypes than in subjects with the AA genotype (P< 0·01). Interestingly, the women with the GG genotype exhibited higher HbA1c plasmatic concentrations than did those with the AA genotype (P< 0·05) (Fig. 2(c)).

Fig. 2 Association between blood parameters and cluster of differentiation 36 (CD36) SNP in obese Tunisian women. Concentrations of cholesterol (a), LDL (b) and glycosylated Hb (HbA1c) (c) in women with the AA, AG or GG genotype of the CD36 gene. Values are means, with standard deviations represented by vertical bars. Mean value was significantly different from that of the AA genotype: * P< 0·05, ** P< 0·01 (one-way ANOVA).
CD36 genotypes and TNF-α and IL-6 concentrations
Serum concentrations of TNF-α was higher in women with the CD36 AA genotype as compared to subjects with the GG genotype (Fig. 3(a)). Interestingly, serum IL-6 concentrations were lower in women with the AA and AG genotypes than they were in women with the GG genotype (Fig. 3(b)).

Fig. 3 Serum TNF-α (a) and IL-6 (b) concentrations in obese Tunisian women with the AA, AG or GG genotype of the cluster of differentiation 36 (CD36) gene. The serum concentrations of cytokines were determined as described in the Materials and Methods section of the present paper. Values are means, with standard deviations represented by vertical bars. *** Mean value was significantly different from that of the AA genotype (P< 0·001; one-way ANOVA). †† Mean value was significantly different from that of the AG genotype (P< 0·01; Fisher's exact test).
Association between TNF-α polymorphism and creatinine serum level and association between IL-6 polymorphism and IL-6 serum level
Fig. 4 shows that the women with the IL-6 GG and TNF-α GG genotypes exhibited higher serum IL-6 and creatinine concentrations, respectively, than did those with the IL-6 CC and TNF-α AA genotypes. Moreover, we did not observe a statistical association between the TNF-α SNP and serum TNF-α concentrations (P>0·05).
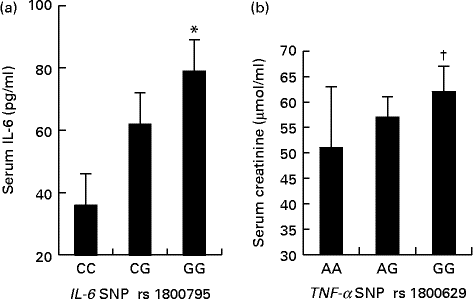
Fig. 4 Serum IL-6 (a) concentrations in obese Tunisian women with the IL-6 polymorphisms CC, CG or GG. Serum creatinine (b) concentrations in obese Tunisian women with the TNF-α polymorphisms AA, AG or GG. Values are means, with standard deviations represented by vertical bars. * Mean value was significantly different from that of the CC genotype (P< 0·05; Fisher's exact test). † Mean value was significantly different from that of the AA genotype (P< 0·05; one-way ANOVA).
Discussion
Evidence suggests that there might be a sixth taste modality that is devoted to the oro-gustatory perception of dietary lipids( Reference Gilbertson and Khan 14 , Reference Running, Mattes and Tucker 40 ). Hence, it seems imperative to explore and better understand the mechanisms that underlie the oro-gustatory detection of dietary fat in order to help prevent and treat obesity( Reference Dramane, Akpona and Simonin 5 , Reference Mattes and Considine 41 ). A number of studies have suggested that lingual CD36, a glycoprotein that is highly expressed in circumvallate papillae, is implicated in the perception of dietary fat taste( Reference Laugerette, Passilly-Degrace and Patris 6 , Reference Cartoni, Yasumatsu and Ohkuri 12 – Reference Abdoul-Azize, Selvakumar and Sadou 15 ). In the present study, we confirm that obese women with the CD36 AA genotype (rs1761667) possess higher thresholds for lipid taste sensitivity than do those with GG genotypes.
Keller et al. ( Reference Keller, Liang and Sakimura 20 ) have provided preliminary evidence that CD36 is involved in human oral fat perception and the human attraction to added fats and oils in food. Pepino et al. ( Reference Pepino, Love-Gregory and Klein 19 ) have demonstrated that CD36 gene polymorphism, which results in a decrease in the gene's expression, is responsible for an increase in the oral detection threshold of dietary lipids in obese subjects. Aside from the present study, no confirming or refuting report is available on this subject, particularly from developing countries where diets are rich in fat. The present data strongly suggested that the oro-sensorial perception of fat taste is altered in some obese subjects. Indeed, we showed that the A allele of CD36 rs1761667 polymorphism in obese women, which was previously associated with decreased expression of the CD36 protein, is associated with a high oro-gustatory threshold detection for oleic acid. Conversely, the subjects with the G allele were more sensitive in their oleic acid lingual detection as compared to the subjects with the A allele. These data corroborate not only the clinical findings of Pepino et al. ( Reference Pepino, Love-Gregory and Klein 19 ) but also experimental data where an association between CD36 gene expression and oral fat detection has been demonstrated( Reference Martin, Passilly-Degrace and Gaillard 42 ). Mice with partial CD36 gene knockout (CD36+/−) had lower CD36 protein expression and a lower oral fat detection threshold than wild type animals (CD36+/+). The CD36 knockout (CD36−/−) failed to exhibit a spontaneous preference for fat.
A low detection threshold for fat in the present study may not have been caused by the low expression of α-gustducin, a marker of taste receptor cells. Indeed, alteration in CD36 expression is not related to changes in α-gustducin expression( Reference Martin, Passilly-Degrace and Gaillard 42 ). Moreover, α-gustducin knockout mice, like wild type animals, exhibited an unaltered preference for dietary fat( Reference Sclafani, Zukerman and Glendinning 43 ). It is possible that other proteins which are likewise involved in fat taste detection, such as GPR120, might also participate in low-fat taste sensitivity( Reference Ozdener, Subramaniam and Sundaresan 44 ). However, the CD36 and GPR120 receptors seem to be differently regulated in lipid taste perception( Reference Sclafani, Zukerman and Ackroff 18 , Reference Ozdener, Subramaniam and Sundaresan 44 ).
In the present study, we also observed that some of the subjects failed to detect oleic acid in the emulsions. These subjects were defined as non-tasters, and they were also reported by Kamphuis et al. ( Reference Kamphuis, Saris and Westerterp-Plantenga 9 ). The mutation responsible for gustatory insensitivity to fatty acid in non-taster subjects deserves further in-depth study.
We performed the present study on Tunisian obese women (who probably eat an above-average amount of fatty food due to cultural customs in Tunisia), because it has been shown that some obese subjects had a low sensitivity to oleic acid( Reference Stewart, Seimon and Otto 35 , Reference Stewart, Feinle-Bisset and Golding 45 ). Oral and gastrointestinal sensitivity to oleic acid are related to each other, and they are decreased in obese subjects( Reference Stewart, Seimon and Otto 35 ). Nonetheless, the present association studies cannot distinguish whether the decreased sensitivity to fat in obese women is a cause or a consequence of obesity. However, Stewart et al. ( Reference Stewart and Keast 11 ) have shown that oral sensitivity towards oleic acid in lean subjects is decreased with a high-fat diet and increased with a low-fat diet. Brennan et al. ( Reference Brennan, Seimon and Luscombe-Marsh 46 ) have reported that acute dietary restriction in obese subjects enhances their gastrointestinal sensitivity to fat, and this is associated with an increased effect of fat on satiation. In addition, a high-fat diet has been shown to decrease the expression of CD36 in mice( Reference Martin, Passilly-Degrace and Gaillard 42 ).
We observed an association between the CD36 AA genotype and high serum levels of cholesterol and LDL in obese women. Because the CD36 A allele was previously associated with reduced expression of the CD36 gene, it is possible that high blood lipid concentrations are the result of their curtailed uptake by adipocytes that also express CD36; in this case, it acts as a fatty acid transporter( Reference Nakamura, Yudell and Loor 47 , Reference Zhou, Samovski and Okunade 48 ). Moreover, CD36 gene polymorphisms have been significantly associated with high TAG concentrations among ethnic Chinese in Taiwan( Reference Chien, Hsu and Liu 49 ).
Interestingly, obese women with the CD36 G allele had higher plasma HbA1c concentrations than women with the A allele. These observations corroborate the findings of Rać et al. ( Reference Rać, Krupa and Garanty-Bogacka 50 ), who have shown that the GG genotype was significantly associated with higher HbA1c concentrations as compared to the AA genotype of CD36 in obese children.
We observed that obese women had high IL-6 and TNF-α serum concentrations. Homozygous women with the CD36 AA genotype had higher TNF-α serum concentrations than did those with the GG or AG genotypes. Conversely, IL-6 serum concentrations were higher in women with the GG genotype than they were in women with the AA or AG genotypes. The importance of the association of high serum levels of TNF-α with the CD36 AA genotype and the association of high serum levels of IL-6 with the CD36 GG genotype is not well understood. These cytokines play a key role in the regulation of insulin sensitivity in subjects who are suffering from obesity and metabolic syndrome( Reference Bougnères 51 ). The SNP of these cytokines have been suggested to predispose for obesity( Reference Curti, Jacob and Borges 52 ). The homozygous subjects with the GG genotype exhibited high serum IL-6 concentrations. These observations are in close agreement with the results of Pereira et al. ( Reference Pereira, Garcia and Narciso 53 ), who studied the association between the IL-6 gene and plasma IL-6 concentrations in community-dwelling and institutionalised older women. Those authors reported that women with the IL-6 GG genotype had high IL-6 serum concentrations. High serum IL-6 concentration in homozygous subjects with the GG genotype might take part in increased fat oxidation in response to fat load in obesity, as has been suggested elsewhere( Reference Hoene and Weigert 54 ). With regards to TNF-α, we noticed a positive relationship between circulating creatinine concentrations and the TNF-α GG genotype, which indicates that the present subjects might be at risk for renal complications. Chang et al. ( Reference Chang, Lu and Yang 55 ) have shown that the G allele of the TNF-α gene was associated with high serum creatinine concentrations that increased the risk for contrast-induced nephropathy. We also observed a significant association between IL-6 and TNF-α gene polymorphisms in obese women, which indicates that inflammatory status, as indicated by pro-inflammatory cytokines, is a key element of obesity in these women. Curtis & Singh( Reference Curtis and Singh 56 ) have likewise shown that the SNP of these two cytokines predispose for obesity.
Finally, we can state that a major value of the present study is that it validates the importance of a common CD36 SNP rs1761667 in obese women. The present results must be confirmed by additional studies in other developing countries. It is also possible that in the present study, there might be an influence of female sex hormones on fat taste perception and other parameters. At this stage, it is difficult to determine whether oral fat perception sensitivity affects fat intake or body weight. Future studies are needed to answer these important questions. The stimulation of taste receptors, such as CD36, by synthetic fatty acid analogues within the oral cavity may provide a new target for obesity treatment.
Acknowledgements
The present study was partly supported by funds from the Ministry of Higher Education and Research, France (to N. A. K.), and by the Specific Research Project of Masaryk University Brno (to O. S.). The present research received no grants from the commercial or not-for-profit sectors. The funders had no role in the design, analysis or writing of the present article.
The authors' contributions are as follows: N. A. K. designed the research (project conception, development of the overall research plan and study oversight); I. M. conducted the research (hands-on conduct of the experiment and data collection); N. A. K. and O. S. wrote the manuscript; O. S. supervised the SNP research and statistical analysis; J. P. completed the technical part of the SNP analysis and participated in writing the manuscript; A. A., M. F., A. B. and M. Z. provided the facilities in the sample collections; N. A. K. and Z. T. supervised the study. All authors have read and approved the final content of the manuscript.
The authors declare no conflicts of interest.









