Chokeberry, known as Aronia melanocarpa, is found in the eastern parts of North America, as well as Northern and Eastern Europe. Although usually consumed as a fruit, it has also been used in traditional medicine to treat hypertension and atherosclerosis in Russia and Eastern European countries(Reference Kokotkiewicz, Jaremicz and Luczkiewicz1). Chokeberry has attracted scientific interest because of its high content of phenolic phytochemicals. The active compounds found in chokeberry include anthocyanins and flavonoids, some at concentrations over five times greater than those found in cranberries(Reference Wu, Gu and Prior2, Reference Wu, Beecher and Holden3). A comparative in vitro study has shown that chokeberries display higher antioxidant activity with the oxygen radical absorption capacity assay than that obtained with blueberries, cranberries or lingonberries(Reference Zheng and Wang4).
Anthocyanins and anthocyanin-rich extracts exhibit diverse potential health benefits in animal and human studies, including cardioprotective(Reference Bell and Gochenaur5), anti-diabetic(Reference Utzschneider, Carr and Barsness6, Reference Valcheva-Kuzmanova, Kuzmanov and Mihova7) and anti-inflammatory properties(Reference Naruszewicz, Laniewska and Millo8). Although it has been reported that anthocyanins are poorly absorbed and circulate in the blood exclusively as unmetabolised parent glycosides(Reference Cao, Muccitelli and Sanchez-Moreno9), Kay et al. (Reference Kay, Mazza and Holub10) observed that in human subjects, anthocyanins exist in the circulation primarily as metabolites, and cyanidin 3-glycosides are absorbed and transported in human serum and urine primarily as glucuronide and methyl glucuronide derivatives. Moreover, recent studies in rodents have shown that anthocyanins are rapidly absorbed from both the stomach and small intestine(Reference Talavera, Felgines and Texier11), with derivatives found in multiple organs, including adipose tissue(Reference Felgines, Texier and Garcin12).
Adipocyte and adipose tissue dysfunction are primary defects in obesity and may link obesity to several health problems, including increased risk of type 2 diabetes, hypertension, dyslipidaemia and atherosclerosis(Reference Hajer, van Haeften and Visseren13, Reference Blüher14). In cultured adipocytes, anthocyanins enhance adiponectin secretion(Reference Tsuda, Ueno and Aoki15), regulate the expression of multiple adipocyte-specific genes(Reference Tsuda, Ueno and Aoki15, Reference Tsuda, Ueno and Kojo16) and also reverse TNF-α-induced insulin resistance(Reference Guo, Ling and Wang17). In addition, dietary anthocyanins were shown to suppress obesity when administered to mice fed high-fat diets(Reference Tsuda, Horio and Uchida18, Reference Prior, Wu and Gu19).
The aim of the present study was to investigate whether feeding an extract of chokeberry improved metabolic parameters in rats fed a fructose-rich diet (FRD) to induce insulin resistance. Effects on body weight gain and epididymal fat accumulation and the underlying molecular mechanisms of action on the expression of the adipose genes involved in insulin signalling, adipogenic and inflammation pathways were evaluated.
Materials and methods
According to the manufacturer, the chokeberry extract (CBE) used was prepared from frozen Aronia berries from Northern Europe. Berries were stirred at room temperature with 4-fold excess by weight of 60 % ethanol–water for 3–4 h. The mixture was then centrifuged and the supernatant was spray-dried (CellBerry®, the dried CBE, was provided by Integrity Nutraceuticals International (Spring Hill, TN, USA)). This extract contained at least 10 % anthocyanins based on HPLC and MS analyses (lot no. TGB-071020).
Animals
Male Wistar rats (5 weeks old) were housed in a temperature-controlled room according to the Guidelines for Animal Care of the Beltsville Area Animal Care and Use Committee. After a 1-week acclimatisation period, rats were assigned randomly to receive either the CBE at 100 or 200 mg/kg body weight/d (n 6 for each group: CBE100 and CBE200) added to drinking-water or water alone (CON) group. All rats were placed on a FRD for 6 weeks. The diet contained (g/kg diet): casein, 207; dl-methionine, 3·0; fructose, 600; lard, 50; cellulose, 79·8; AIN mineral mix, 50·0; zinc carbonate, 0·04; AIN vitamin mix 10·0; and green food colour 0·15 (89 247-Teklad Animal Diets, Madison, WI, USA). During the experimental period, the consumption of food and fluid and body weight were monitored every other day. At the termination of the feeding experiment, following an overnight fast, blood glucose levels were tested from blood collected from the tail vein. Rats were then anaesthetised and blood was collected from the portal vein in pre-cooled tubes containing EDTA and centrifuged at 5000 rpm for 15 min at 4°C. The epididymal adipose tissues (EAT) were carefully removed and weighed before being snap-frozen in liquid N2 and stored at − 80°C until analysed.
Immunoblotting
For the immunoanalysis of adipose tissue, approximately 100 mg of EAT were homogenised at 4°C for 30 s in lysis buffer containing 20 mm-Tris (pH 7·4), 2 mm-EDTA, 50 mm-NaF, 200 μm-Na3VO4, 250 μm-phenylmethylsulfonyl fluoride, 1 μm-leupeptin, 1 μm-pepstatin and 0·36 μm-aprotinin. Protein concentrations were determined by a commercial assay (Bio-Rad Dc protein assay; Bio-Rad Laboratories, Hercules, CA, USA) using bovine serum albumin as a standard. Rabbit polyclonal antibody against adiponectin was purchased from ProSci (Poway, CA, USA). Rabbit polyclonal antibody against zinc finger protein 36 (ZFP36) was purchased from Genway (San Diego, CA, USA).
Gene expression in epididymal adipose tissues
Total RNA was isolated from EAT using Trizol reagent (Invitrogen, Carlsbad, CA, USA). RNA concentrations and integrity were determined using RNA 6000 Nano Assay Kit and the Bioanalyzer 2100, according to the manufacturer's instructions (Agilent, Santa Clara, CA, USA). The complementary DNA were synthesised from total RNA using SuperScript II RT (Invitrogen). The primers used are described in our previous study(Reference Qin, Polansky and Harry20) and included in Table 1. Real-time quantitative PCR was performed using SYBR Green PCR Master Mix (ABI, Forster, CA, USA). The expression of the housekeeping gene, peptidylprolyl isomerase A, was used to normalise the expression of target genes.
Table 1 Real-time PCR primers

AdipoQ, adiponectin; Lpl, lipoprotein lipase; Fas, fatty acid synthase; Fabp4, fatty acid-binding protein 4.
Biochemistry
Plasma adiponectin was determined with a rat ultrasensitive EIA (Phoenix Pharm, Burlingame, CA, USA). Plasma NEFA were measured using a colorimetric assay (Wako, Richmond, VA, USA). Serum TNF-α and IL-6 were determined with a rat ultrasensitive EIA (Alpco, Salem, NH, USA). Measurement of blood glucose, insulin, TAG and cholesterol were performed as described(Reference Qin, Polansky and Harry20, Reference Qin, Anderson and Adeli21).
Statistical analyses
Data were analysed by one-way ANOVA followed by the least square difference (LSD) test. P values < 0·05 were considered significant.
Results
General observations and plasma biochemistry
Food intake and water consumed did not differ among the three groups. Body weight gain and epididymal adipose weight were reduced at both levels of CBE intake (Table 2, P < 0·05). Fasting blood glucose and plasma insulin, TAG, total cholesterol, LDL-cholesterol and plasma NEFA levels were all reduced in animals consuming both levels of CBE. CBE consumption increased the plasma adiponectin and HDL-cholesterol levels (Table 3). In addition, a significant reduction in the plasma IL-6 and TNF-α occurred in both the CBE groups (Table 3).
Table 2 Effects of chokeberry extract (CBE) on body weight and epididymal pad weight
(Mean values with their standard errors for six rats)

CON, group fed with water alone; CBE100, group fed CBE at 100 mg/kg body weight daily added to drinking water; CBE200, group fed CBE at 200 mg/kg body weight daily added to drinking water.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Table 3 Effects of chokeberry extract (CBE) on blood and plasma parameters in the fasted state
(Mean values with their standard errors for six rats)
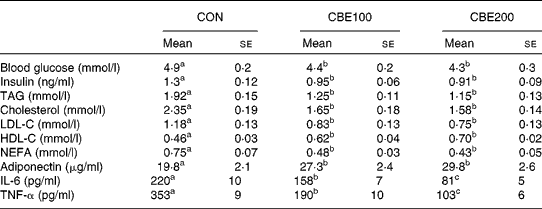
CON, group fed with water alone; CBE100, group fed CBE at 100 mg/kg body weight daily added to drinking water; CBE200, group fed CBE at 200 mg/kg body weight daily added to drinking water; LDL-C, LDL-cholesterol; HDL-C, HDL-cholesterol.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Chokeberry extract altered mRNA expression in the insulin signalling pathway and glucose uptake in epididymal adipose tissues
As shown in Fig. 1(a), CBE consumption (CBE200) with the FRD enhanced mRNA levels of components of the insulin signalling pathway, including increases in mRNA levels of insulin receptor substrate 1 (Irs1) (2·3-fold), Irs2 (1·8-fold) and phosphatidylinositol 3 kinase regulatory subunit 1 (Pi3kr1) (1·5-fold), and inhibited phosphatase and tensin homolog (Pten) mRNA levels (0·61-fold), compared with the FRD-fed control rats.

Fig. 1 Effects of feeding a chokeberry extract (CBE) on the mRNA levels related to insulin signalling, GLUT and glycogen synthesis in epididymal adipose tissue. ![]() , Control group;
, Control group; ![]() , group fed CBE at 200 mg/kg body weight daily (CBE200). (a) Ir, Irs1, Irs2, Pi3kr1, Akt1 and Pten and (b) Glut1, Glut4, Gys1 and Gsk3β. Values are means with their standard errors and are presented as fold of control (n 5–6). * Mean values were significantly different from those of the control group (P < 0·05).
, group fed CBE at 200 mg/kg body weight daily (CBE200). (a) Ir, Irs1, Irs2, Pi3kr1, Akt1 and Pten and (b) Glut1, Glut4, Gys1 and Gsk3β. Values are means with their standard errors and are presented as fold of control (n 5–6). * Mean values were significantly different from those of the control group (P < 0·05).
As shown in Fig. 1(b), CBE consumption (CBE200) induced an increase in Glut1 (1·6-fold), Glut4 (1·5-fold) and glycogen synthase (Gys) (1·5-fold) mRNA expression, and inhibited glycogen synthase kinase 3β (Gsk3β) mRNA levels (0·62-fold), compared with the FRD-fed control rats. Similar trends were observed in the CBE100 group but values were not significant (data not shown).
Consumption of chokeberry extract modulated the expression of genes and proteins involved in epididymal adipose adipogenesis
As shown in Fig. 2(a) and (b), CBE consumption (CBE200) caused a significant increase in adiponectin (AdipoQ) mRNA levels (2·1-fold) and adiponectin protein levels (169 %), compared with the FRD-fed control rats (P < 0·05, both). CBE also induced Pparγ mRNA expression (1·6-fold) and inhibited fatty acid binding protein 4 (Fabp4) (0·7-fold), fatty acid synthase (Fas) (0·63-fold) and Lpl (0·65-fold) mRNA expression (Fig. 2(c)), but did not significantly affect fatty acid translocase (Cd36) mRNA expression. Changes in the CBE100 group were not significant (data not shown).

Fig. 2 Effects of feeding a chokeberry extract (CBE) on the expression of genes and proteins involved in adipogenesis in epididymal adipose tissue. ![]() , Control group;
, Control group; ![]() , group fed CBE at 200 mg/kg body weight daily (CBE200). (a) AdipoQ mRNA, (b) adiponectin protein and (c) Pparγ mRNA, Fabp4, Fas, Lpl and Cd36 mRNA. (b) Representative experiments on the immunoblots of adiponectin were analysed using densitometry. Values are means with their standard errors and are presented as percentage of control (n 5–6). * Mean values were significantly different from those of the control group (P < 0·05).
, group fed CBE at 200 mg/kg body weight daily (CBE200). (a) AdipoQ mRNA, (b) adiponectin protein and (c) Pparγ mRNA, Fabp4, Fas, Lpl and Cd36 mRNA. (b) Representative experiments on the immunoblots of adiponectin were analysed using densitometry. Values are means with their standard errors and are presented as percentage of control (n 5–6). * Mean values were significantly different from those of the control group (P < 0·05).
Chokeberry extract inhibited the epididymal adipose inflammation gene expression and induced ZFP36 expression
As shown in Fig. 3(a), CBE consumption (CBE200) caused a significant decrease in Tnfα (0·52-fold), Ilβ (0·38-fold) and Il6 (0·45-fold) mRNA levels, compared with the FRD-fed control rats. In contrast to these inflammatory factors, CBE consumption enhanced Zfp36 mRNA and ZFP36 protein expression (Fig. 3(b) and (c), 1·45-fold and 139 %). Changes in the CBE100 group were not significant (data not shown).

Fig. 3 Effects of feeding a chokeberry extract (CBE) on the expression of genes and proteins involved in inflammation in epididymal adipose tissue. ![]() , Control group;
, Control group; ![]() , group fed CBE at 200 mg/kg body weight daily (CBE200). (a) Tnfα, Ilβ and Il6 mRNA, (b) Zfp36 mRNA and (c) ZFP36 protein. (c) Representative experiments on the immunoblots of ZFP36 were analysed using densitometry. Values are means with their standard errors and are presented as fold of control (n 5–6). * Mean values were significantly different from those of the control group (P < 0·05).
, group fed CBE at 200 mg/kg body weight daily (CBE200). (a) Tnfα, Ilβ and Il6 mRNA, (b) Zfp36 mRNA and (c) ZFP36 protein. (c) Representative experiments on the immunoblots of ZFP36 were analysed using densitometry. Values are means with their standard errors and are presented as fold of control (n 5–6). * Mean values were significantly different from those of the control group (P < 0·05).
Discussion
Consumption of a FRD contributes to insulin resistance, hyperinsulinaemia, dyslipidaemia and hypertension in animal models(Reference Elliott, Keim and Stern22–Reference Qin, Polansky and Anderson24). Furthermore, consumption of a FRD leads to abdominal adipose tissue endocrine dysfunction in normal rats, and increased adipose tissue mass and adipocyte size(Reference Alzamendi, Giovambattista and Raschia25). Growing evidence suggests that adipose tissue plays a crucial role in the regulation of systemic energy homoeostasis, insulin sensitivity and lipid/carbohydrate metabolism(Reference Havel26). In the present study, we used the FRD-fed rat model to investigate the effects of a CBE rich in anthocyanins. The present data indicated that CBE consumption reduced weight gain, epididymal fat accumulation and improved systemic glucose/lipid metabolism. We also demonstrated that CBE consumption increased plasma adiponectin levels and decreased plasma TNF-α and IL-6 levels. In addition, we demonstrated that consumption of CBE regulated gene expression in multiple pathways involved in insulin signalling, adipogenesis and inflammation.
FRD-induced adipose tissue dysfunction in studies of high-polyphenol extracts of other plants caused alterations in the production of many adipocyte-derived factors such as NEFA and adiponectin(Reference Qin, Polansky and Anderson24). These dysregulated factors induced local and systemic insulin resistance, which is a major contributor to the pathogenesis of type 2 diabetes and plays a key role in associated metabolic abnormalities, such as obesity, dyslipidaemia and hypertension(Reference Guilherme, Virbasius and Puri27). Evidence from human and animal studies demonstrated that loss of body weight is associated with an increase of insulin sensitivity(Reference Utzschneider, Carr and Barsness6, Reference Bogardus, Ravussin and Robbins28, Reference Kong, Oh and Ahn29). In the present study, we found that consumption of a CBE, high in polyphenols, reduced weight gain and epididymal fat accumulation, and improved systemic insulin sensitivity-related factors, such as fasting glucose, plasma insulin and lipids. At the molecular level, although the FRD impaired insulin signalling pathways in multiple tissues, such as liver(Reference Taghibiglou, Rashid-Kolvear and van Iderstine30), skeletal muscle(Reference Qin, Nagasaki and Ren23) and adipose tissue(Reference Qin, Polansky and Anderson24), the consumption of CBE improved the impaired gene expression related to insulin signalling and glucose uptake in EAT, and up-regulated the decreased Irs1, Irs2 and Pi3k mRNA expression and the expression of other genes related to carbohydrate metabolism, such as Glut1, Glut4 and Gys1. CBE also inhibited the expression of Pten and Gsk3β mRNA, which are increased in insulin-resistant adipose tissue(Reference Komazawa, Matsuda and Kondoh31, Reference Eldar-Finkelman, Schreyer and Shinohara32) (Fig. 1).
In contrast to many other factors derived from adipose tissue, circulating adiponectin and adipose adiponectin expression are decreased in insulin resistance(Reference Ukkola and Santaniemi33, Reference Hu, Liang and Spiegelman34). In most clinical reports, primate studies and genetic models, serum adiponectin levels have been reported to be negatively correlated with body weight, visceral fat mass and resting insulin levels(Reference Berg, Combs and Scherer35–Reference Hotta, Funahashi and Bodkin37). Transgenic mice overexpressing AdipoQ have increased insulin sensitivity and improved glucose tolerance and TAG clearance(Reference Combs, Pajvani and Berg38). We have reported previously that FRD feeding significantly decreased plasma adiponectin and AdipoQ mRNA expression(Reference Qin, Polansky and Anderson24). In the present study, we observed that feeding CBE significantly increased plasma adiponectin levels and the mRNA and protein expression of adipose adiponectin of FRD-fed rats (Fig. 2). These results support the previous finding in human subjects that combination therapy of statins with an extract of chokeberry increased plasma adiponectin levels in patients after myocardial infarction(Reference Naruszewicz, Laniewska and Millo8). Adiponectin possesses insulin sensitising and anti-atherogenic properties(Reference Berg, Combs and Scherer35).
It is well known that increased adipose tissue mass and adipocyte dysfunction associated with obesity are linked to the abnormal regulation of adipogenesis(Reference Rodriguez-Acebes, Palacios and Botella-Carretero39). PPARγ, a master regulator of adipogenesis(Reference Tontonoz, Hu and Graves40), plays a critical role in glucose metabolism and energy homoeostasis(Reference Tontonoz and Spiegelman41, Reference Rosen and MacDougald42). Fructose feeding induced a lower Pparγ mRNA expression in white adipose tissue, and a PPAR ligand reversed the down-regulated expression of PPARγ and systemic insulin resistance(Reference Shih, Lin and Lin43). In the present study, we observed that CBE consumption increased Pparγ mRNA expression in EAT. PPARγ regulates multiple genes in the adipose tissue regulating adipogenesis, including those encoding the adipocyte fatty acid-binding protein 4 (FABP4), fatty acid synthase (FAS) and lipoprotein lipase (LPL). FABP4 is postulated to be an early marker of the metabolic syndrome and the future onset of type 2 diabetes(Reference Tso, Xu and Sham44, Reference Xu, Tso and Cheung45). Fabp4-deficient mice are protected from insulin resistance, hyperglycaemia, dyslipidaemia and atherosclerosis(Reference Boord, Maeda and Makowski46, Reference Makowski, Boord and Maeda47). We have reported that FRD feeding induced the overexpression of Fabp4 mRNA(Reference Qin, Polansky and Anderson24). FAS is a key enzyme in de novo lipogenesis. Studies have reported that Fas mRNA and protein levels are elevated in obese Zucker rats(Reference Guichard, Dugail and Le48), and polyphenols from cinnamon(Reference Qin, Polansky and Anderson24) and dietary green tea inhibit Fas mRNA expression in diet-induced insulin-resistant animals(Reference Lee, Kim and Kim49). LPL, the rate-limiting enzyme in TAG-rich lipoprotein catabolism, provides TAG-derived fatty acids to adipose tissue for storage. Studies have shown that polyphenols from cinnamon and green tea also inhibit Lpl mRNA expression and other genes of lipogenesis(Reference Qin, Polansky and Anderson24, Reference Lee, Kim and Kim49). In the present study, the data show that feeding polyphenols from chokeberry suppressed Fabp4, Fas and Lpl mRNA levels in EAT. CD36, referred to as fatty acid translocase, is a transmembrane protein present in many tissues that is believed to play a role in facilitating fatty acid transport(Reference Ibrahimi and Abumrad50). In ob/ob mice, and FRD-fed rats, CD36 mRNA(Reference Memon, Fuller and Moser51) and protein(Reference Qin, Polansky and Anderson24) levels in the adipose tissue were increased. CBE consumption did not affect CD36 mRNA expression in the adipose tissue.
Substantial evidence indicates that a state of low-grade chronic inflammation typically is associated with obesity, and the increased production of pro-inflammatory cytokines by adipose tissue plays a crucial role in the development of insulin resistance(Reference Hotamisligil52, Reference Schenk, Saberi and Olefsky53). FRD feeding also induces the overexpression of plasma TNF-α and IL-6, which both contribute to the development of CVD by promoting insulin resistance, dyslipidaemia and endothelial dysfunction(Reference Wilson54). TNF-α is known to be a potent negative regulator of adipogenesis and PPARγ function(Reference Zhang, Berger and Hu55). The present results suggest that consumption of CBE not only inhibited the plasma levels of TNF-α and IL-6 but also induced a decrease of mRNA expression of Tnfα and Ilβ and Il6. This is in agreement with a human study in which chronic chokeberry consumption reduced the severity of plasma inflammation, increased anti-inflammation factor and increased plasma adiponectin levels(Reference Naruszewicz, Laniewska and Millo8). Previous studies suggested that Zfp36, an anti-inflammatory protein, increased Tnfα mRNA degradation by binding to its 3′ untranslated region(Reference Carballo, Lai and Blackshear56), and that omental adipose Zfp36 mRNA levels were correlated with insulin, insulin resistance index and adiponectinaemia in women(Reference Bouchard, Vohl and Deshaies57). In the present study, we found that CBE consumption increased adipose Zfp36 protein and mRNA expression, which is consistent with CBE feeding-induced decreases in TNF-α expression and increases in adiponectin expression in plasma and adipose tissue.
In summary, the present study provides evidence that an anthocyanin-rich extract of chokeberry reduced body weight gain and abdominal fat, improved the risk factors related to the metabolic syndrome in plasma and modulated multiple signalling pathways related to adipose dysfunction in an animal model. The present findings suggest that chokeberry or its extract might be beneficial in preventing or decreasing obesity and the metabolic syndrome. Further studies are needed in human volunteers at increased risks for diet-related chronic disease to ascertain the beneficial effects from consumption of polyphenols such as those found in chokeberry.
Acknowledgements
The present study was supported in part by a USDA Cooperative Research and Development Agreement (CRADA no.58-3K95-7-1184) with Integrity Nutraceuticals International. The authors gratefully appreciate Marilyn M. Polansky and Dr Harry Dawson for their assistance. They would also like to thank Dr Joseph F. Urban, Jr for critically reviewing the manuscript. R. A. A. was involved with planning and evaluation of the study and writing of the manuscript. B. Q. was involved in all phases of the study. B. Q. is a visiting scientist, working at the USDA/ARS Beltsville Human Nutrition Research Center, and employed by Integrity Nutraceuticals International; R. A. A. has no conflicts of interest to declare. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. The USDA is an equal opportunity provider and employer.








