Aflatoxins are a group of mycotoxins, produced by the common fungus Aspergillus flavus, and the closely related species Aspergillus parasiticus, and are categorised as ‘class-I A’ human carcinogens(1). A low level of aflatoxin is linked with the development of occult conditions such as impaired cellular growth, immune malfunction and chronic diseases such as liver cancer. Exposure to aflatoxin-B1 (AFB1), one of the potent mycotoxins and carcinogens, is implicated in the aetiology of liver cancer(Reference Eaton, Ramsdell, Neal, Eaton and Groopman2, Reference Vamio, Heseltine and Wilbourn3). Exposure to AFB1 results mainly from the ingestion of foodstuffs contaminated with aflatoxigenic moulds growing on food ingredients such as maize, peanuts, soyabeans, cereal crops and certain tree nuts(1).
It is therefore desirable to remove aflatoxins occurring naturally in foods. Different strategies to remove aflatoxins and/or combat aflatoxin-mediated toxicity include chemical (e.g. decontamination by ozonisation, ammoniation or other chemical procedures, altering aflatoxin bioavailability at the individual level, using various adsorbents), physical (e.g. more rapid drying, controlled storage, segregation of contaminated lots after aflatoxin analyses, and by sorting out contaminated nuts or grains by electronic sorters) or biological treatments of food and feed ingredients. However, physical and chemical treatments for the removal of mycotoxins are time-consuming and economically impractical, hence they cannot be used on a larger scale(1).
Micro-organisms, especially the bacteria, have the potential to either degrade mycotoxins or reduce their bioavailability; and among them. The use of probiotics has been reported to confer numerous health benefits including removal or detoxification of ingested carcinogens or xenobiotics(Reference Salminen and von Wright4, Reference Kumar, Kumar and Nagpal5). Administration of certain probiotic lactic acid bacteria can increase the activity of antioxidative enzymes such as catalase (CAT), glutathione, glutathione peroxidase (GPx), glutathione reductase (GR), glutathione-S-transferase (GST) and superoxide dismutase (SOD), and modulate circulatory oxidative stress that protect the cells against carcinogen-induced damage(Reference Kumar, Kumar and Nagpal5). Probiotics such as Lactobacillus rhamnosus strain GG, Lactobacillus acidophilus and Bifidobacterium longum have been shown to bind and neutralise AFB1 and aflatoxin M1 in vivo, thus reducing the absorption of ingested toxins in the gut(Reference Haskard, Binnion and Ahokas6).
Incorporation of the probiotic B. longum to the diet of rats has been shown to exert a strong anti-tumour activity on the colonic mucosa by reducing colonic mucosal cell proliferation and colonic mucosal and tumour ornithine decarboxylase activities and the expression level of ras-p21, an oncogene(Reference Singh, Rivenson and Tomita7, Reference Reddy8). Probiotics have also been found to decrease faecal concentrations of enzymes and secondary bile salts, and to reduce the absorption of mutagens that could contribute to colon carcinogenesis(Reference Rafter9).
The lactic acid bacteria inhibit colon cancer through various mechanisms such as enhancing the host's immune response, altering the metabolic activity of the intestinal microflora, binding and degrading carcinogens, producing antimutagenic compounds and altering the physico-chemical conditions in the colon(Reference Kumar, Kumar and Nagpal5, Reference Annuk, Shchepetova and Kullisaar10). Bacillus polyfermenticus have also been shown in vitro to exert antioxidative, antigenotoxic and anticarcinogenic effects(Reference Park, Kim and Kim11), and therefore, consumption of dairy products such as yogurt and fermented milk (FM) containing lactobacilli and bifidobacteria might be correlated with a lower incidence of colon cancer(Reference Shahani and Ayebo12). The oral administration of lactic acid bacteria has also been shown to effectively reduce DNA damage induced by chemical carcinogens in the gastric and colonic mucosa of rats(Reference Tavan, Cayuela and Antoine13), indicating that lactobacilli and B. longum can interfere with the initiation or early promotion of intestinal tumours(Reference Rowland, Rumney and Coutts14, Reference Iyer, Kosters and Sethi15).
Chlorophyllin (CHL), a water-soluble derivative of chlorophyll and a potent antimutagen towards heterocyclic amines, polycyclic aromatic hydrocarbons, aflatoxins and other classes of mutagens, has been regarded as a chemopreventive agent in populations at high risk for exposure to aflatoxin and subsequent development of hepatocellular carcinoma(Reference Dashwood, Negishi and Hayatsu16). CHL was earlier shown to reduce the levels of intracellular reactive oxygen species (ROS) and apoptosis(Reference Sharma, Kumar and Sainis17), and is thought to form molecular complexes with carcinogens, thereby blocking their bioavailability, and hence, could act as an interceptor molecule by forming stable molecular complexes with certain carcinogens including AFB1(Reference Sharma, Kumar and Sainis17, Reference Breinholt, Schimerlik and Dashwood18). Thus, CHL may diminish the bioavailability of dietary carcinogens by impeding their absorption and by enhancing their removal through faeces, leading to reduced DNA damage and tumour formation(Reference Breinholt, Arbogast and Leveland19). In this context, the present study was carried out to explore the biochemical mechanism associated with the hepatoprotective role of probiotic FM alone as well as in combination with CHL on AFB1-induced hepatocellular carcinoma in male Wistar rats.
Materials and methods
Microbial strains and probiotic fermented milk
L. rhamnosus GG was generously gifted by Professor Goldin and Professor Gorbatch (Tufts University, Medford, MA, USA), and has already been reported to possess potential probiotic properties(Reference Goldin, Gualtieri and Moore20). L. casei strain Shirota was procured from the National Collection of Dairy Cultures, Dairy Microbiology Division, National Dairy Research Institute (NDRI), Karnal, India. The strains were propagated in deMann Rogosa Sharpe broth (HiMedia, Bombay, India) at 37°C. For the preparation of FM, an appropriate ratio of 1:2 was inoculated in skimmed milk and incubated at 37°C for 24 h, maintaining the cell number at 108 colony-forming units/g.
Chemicals
Thiobarbituric acid, reduced glutathione (GSH), oxidised glutathione, sodium azide, 1-chloro-2,4-dinitrobenzene, 5,5-dithio-bis-(2-nitrobenzoic acid), AFB1, melatonin, cumene hydroperoxide, NADPH, GR, sodium sulphate, naphthylenediamine dihydrochloride, sulphanilamide, aprotinin, dithiothreitol, HEPES, 3-((3-chloramidopropyl)dimethylammonio)-1-propane sulfonate (CHAPS), CHL and sodium nitrite were all obtained from Sigma (St Louis, MO, USA). Ac-Asp-Glu-Val-Asp-p-nitroanaline and p-nitroaniline were obtained from Calbiochem (Calbiochem-Nova-Biochem Corporation, San Diego, CA, USA).
Animals
Male Wistar rats (4 weeks old) were used in this experiment. The rats were maintained in the Central Small Animal House of the NDRI, Karnal, according to the internationally accepted principles. The rats were allocated into five groups, twenty-five rats in each group. The animals were maintained at a controlled temperature of 22–25°C with about 56–60 % relative humidity and fed with a standard diet (American Institute of Nutrition 76-A) and water ad libitum. A prior approval for study was obtained from the Animal Ethics Committee of NDRI, Karnal, registered under the Committee for the Purpose of Control and Supervision of Experiment on Animals, Government of India.
Dosing
AFB1 was administered intraperitoneally to the rats of selected test groups at the rate of 450 μg/kg body weight per animal twice a week for 6 weeks, maintaining an equal time interval between the two consecutive AFB1 administrations. CHL was given per se orally at the rate of 250 mg/kg body weight per d, and probiotic FM with a final cell concentration of >108 colony-forming units/g of diet was fed to the selected test groups for 25 weeks.
Analytical procedures
Thiobarbituric acid-reactive substance content
Lipid peroxidation products in liver tissue were estimated as thiobarbituric acid-reactive substances (TBARS) as described by Uchiyama & Mahira(Reference Uchiyama and Mahira21) using malonyl dialdehyde (MDA) as a standard.
Reduced glutathione content
Total GSH was determined in liver at the 15th and 25th week of the study according to the method of Ellman(Reference Ellman22). A known amount of enzyme preparation was allowed to react with H2O2 in the presence of GSH for a specific time interval. The remaining GSH content was measured.
Enzyme assays
The following enzymes were analysed in order to assess the protective effect of probiotic FM and CHL on rat liver. As there is a decrease in the level of these enzymes under stress conditions, therefore, it was expected that probiotic FM and CHL would be able to revert this effect and maintain the normal level of enzymes.
Glutathione peroxidase activity
GPx activity in liver was estimated at 15 and 25 weeks according to the method of Tapple(Reference Tapple23).
Glutathione-S-transferase activity
The assay of GST activity was based on that described by Habig et al. (Reference Habig, Pabst and Jakoby24). It was based on an increase in absorbance at 340 nm owing to the formation of thio-diethyl ether (conjugation of substrate with glutathione).
Superoxide dismutase activity
The activity of SOD was assayed by the method of Marklund & Marklund(Reference Marklund and Marklund25).
Catalase activity
After completion of the experimental trials, rats were euthanised and the liver (10 %) was homogenised in PBS (1 × ). The homogenate was centrifuged at 2800 rpm for 10 min. CAT activity was estimated according to the method of Aebi(Reference Aebi26). CAT activity was calculated using an extinction coefficient of 0·0394 litres/mm per cm and is expressed as μmol H2O2 consumed/min per mg protein.
Caspase activity
Caspase-3 activities were determined using a modified procedure as described earlier(Reference Jaeschke, Fisher and Lawson27, Reference Kim, Hausman and Comton28). Cytosolic extracts were prepared by homogenising the liver in extraction buffer containing 25 mm-HEPES (pH 7·5), 5 mm-MgCl2, 1 mm-EDTA, 0·1 % (w/v) CHAPS and aprotinin (10 μg/ml)(Reference Kunstle, Hentze and Germann29). Subsequently, the homogenates were centrifuged at 13 000 g for 15 min at 4°C. The supernatant was used to determine caspase-3 activity. A colorimetric assay was performed in microplates, where 10 μl cytosolic extracts (1–2 mg/ml protein) were incubated for 3 h at 37°C with 90 μl substrate buffer containing 100 mm-HEPES (pH 7·5), 10 % sucrose, 0·1 % CHAPS, 5 mm-dithiothreitol, 16 μm-caspase-3 substrate (Ac-Asp-Glu-Val-Asp-p-nitroanaline). The amount of chromophore, p-nitroaniline, released by caspase-3 activity was quantified by measuring the optical density at 405 nm. Caspase-3 activity is expressed as μm-p-nitroaniline released/h per mg cellular protein. Protein concentrations of the corresponding samples were estimated, and caspase-3 activity was calculated using serially diluted standards (stock, 10 μm-p-nitroaniline).
Determination of total proteins
Total proteins in liver homogenates were determined by the Lowry method using bovine serum albumin as a standard.
Histopathological examination of liver
Liver tissue was collected after opening the viscera of euthanised animals from all experimental groups. The tissue was fixed in 10 % neutral buffer formalin, and was processed and embedded in paraffin following the standard protocols. The processed tissue was sectioned at 5 μm and stained with haematoxylin and eosin, and examined under light microscopy.
Statistical analysis
Data are presented as means with their standard errors. Differences between the groups were determined using one-way ANOVA with Student's t test. The level of significance was accepted as P < 0·05.
Results
Effect on thiobarbituric acid-reactive substance level
The level of TBARS was increased in the AFB1-administered group II of rats compared with the control group (group I). However, it was observed that the group which received aflatoxin along with probiotic FM (group III), and the group which received aflatoxin along with CHL (group IV) had normal TBARS levels comparable with that of the control group (group I). The group which received probiotic FM in combination with CHL (group V) showed TBARS levels significantly reduced in comparison with the AFB1 group (group II; Table 2).
Effect on reduced glutathione
GSH contents were found to decrease in group II, which received only AFB1 compared with the control group (group I). Groups III and IV, which received AFB1 along with probiotic FM and CHL, respectively, exhibited an increase in GSH content compared with group II, which received only AFB1. The group which received probiotic FM in combination with CHL (group V) showed a significant increase in GSH level in comparison with the other groups (Table 2).
Effect on the activities of different antioxidant enzymes
Glutathione peroxidase
GPx activity was found to decrease significantly (P < 0·05) in the AFB1-administered group (group II), compared with the control group (group I). However, the activities of this enzyme in group III, which received AFB1 along with probiotic FM, and group IV, which received AFB1 along with CHL, were found to significantly increase compared with the AFB1-treated group (group II). GPx activity was also found to be significantly higher in groups which received probiotic FM in combination with CHL (group V; Table 2).
Glutathione-S-transferase
GST activity was significantly (P < 0·05) lower in the AFB1-administered group (group II), compared with the control group (group I). However, the activities of GST in group III, which received AFB1 along with probiotic FM, and group IV, which received AFB1 along with CHL, were effective. In the group that received probiotic FM in combination with CHL (group V), GST activity was significantly higher than that observed in group II (Table 2).
Superoxide dismutase
SOD activity was decreased in the AFB1-administered group (group II), compared with the control group (group I). However, the activities of SOD in group III, which received AFB1 along with probiotic FM, and group IV, which received AFB1 along with CHL, were increased in comparison with group I. The group which received probiotic FM in combination with CHL (group V) was significantly increased compared with the AFB1-treated group (Table 2).
Catalase
CAT activity was found to be depleted in the AFB1-administered group (group II), compared with the control group (group I). However, the activity of CAT was significantly elevated in group III, which received AFB1 along with probiotic FM, and group IV, which received AFB1 along with CHL, compared with the AFB1 -treated group. The group which received probiotic FM in combination with CHL (group V) was significantly increased compared with the AFB1-treated group (Table 1).
Table 1 Experimental protocol to study the anticarcinogenic effect of probiotic fermented milk (FM) and chlorophyllin (CHL) on aflatoxin-B1 (AFB1)-induced rat liver cancer
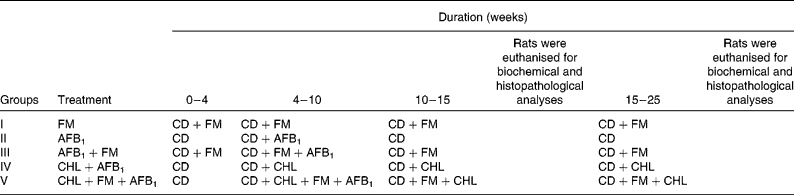
CD, control diet.
Caspase activity
The levels of caspase-3 activities showed high apoptotic rate in the AFB1 group than in the control group.
Histopathological examination
Hepatic lobules with hepatocytes arranged in hepatic strands, hepatic vein, blood vessels and bile ducts were clearly detectable in the control group. No signs of blood congestion in blood vessels were observed in this group. However, a series of changes in AFB1-treated rat liver were discernible during the progression of hepatocellular carcinoma. Histopathological examination of the treatment groups showed chronic liver injury, inflammation, cirrhosis, hyperplasia, dysplasia and liquefactive necrosis (Figs. 1–3). The affected liver showed dilated central vein, dilated sinusoids, blockage of hepatic vein, half moon or shrunk cell nuclei, large vacuoles in the hepatocytes with displacement of the nucleus, irrecoverable metabolic disruption and necrosis of hepatocytes, and pyknotic nuclei (Figs. 4–13), indicating that the cytoplasm had lost definition and the cell margins (plasma membranes) were indistinct. Cirrhosis was marked by the destruction of the normal liver architecture which was replaced by degenerative nodules separated by fibrous tissue. Malignant liver lesions, increased number of inflammatory bodies, dysplasia, hyperplasia, pleomorphic nuclei and multi-nucleated cells were observed as the symptoms of AFB1-mediated hepatocellular carcinoma.

Fig. 1 Effect of aflatoxin-B1 (AFB1) treatment on male Wistar rats. (A, B) Liquefactive hepatic necrosis in an AFB1-treated male Wistar rat.

Fig. 2 Aflatoxin-B1-induced hepatocarcinoma.

Fig. 3 Transverse section of aflatoxin-B1-treated rat liver showing pyknotic nuclei. Cytoplasm has lost definition and the cell margins (plasma membranes) are indistinct (haematoxylin and eosin stain).

Fig. 4 Transverse section of aflatoxin-B1-treated rat liver showing cirrhosis: destruction of the normal liver architecture which is replaced by degenerative nodules of liver separated by fibrous tissue. There may be damage of liver hepatocytes. Cirrhosis also predisposes to the development of hepatocellular carcinoma (haematoxylin and eosin stain).

Fig. 5 Transverse section of aflatoxin-B1-treated rat liver showing cellular carcinoma manifest by malignant lesions: (p) pleomorphic nuclei and (m) multi-nucleated cells (haematoxylin and eosin stain).

Fig. 6 Transverse section of aflatoxin-B1-treated rat liver showing (A) hyperplasia and (B) dysplasia (haematoxylin and eosin stain).

Fig. 7 Transverse section of aflatoxin-B1-treated rat liver showing dilated central vein (haematoxylin and eosin stain).

Fig. 8 Transverse section of aflatoxin-B1-treated rat liver showing (A) dilated sinusoids and (B) blockage of hepatic vein (haematoxylin and eosin stain).

Fig. 9 (A) Transverse section of normal rat liver tissue: (c) central vein, (h) hepatocyte, (n) nucleus and (s) sinusoid (haematoxylin and eosin stain). (B) Transverse section of aflatoxin-B1-treated rat liver: (a) half moon and (b) shrunk nuclei (haematoxylin and eosin stain).

Fig. 10 Transverse section of aflatoxin-B1-treated rat liver showing large vacuoles in the hepatocytes with displacement of the nucleus (haematoxylin and eosin stain).

Fig. 11 Transverse section of aflatoxin-B1-treated rat liver showing irrecoverable metabolic disruption and necrosis of hepatocytes (haematoxylin and eosin stain): (A) distributed all over the tissue and (B) localised near the central vein.

Fig. 12 Transverse section of aflatoxin-B1-treated rat liver showing hepatocellular carcinoma (haematoxylin and eosin stain).

Fig. 13 Transverse section of aflatoxin-B1-treated rat liver cells followed by (A) fermented milk (FM) and (B) FM+chlorophyllin treatment (haematoxylin and eosin stain).
Statistical analysis
Results obtained showed that damage caused by AFB1 was time-dependent. As the period of exposure of mice to AFB1 was increased, enzyme levels were decreased and TBARS levels increased. However, this reduction in the levels of antioxidants and elevation in the levels of TBARS were not much pronounced in the group that received AFB1 and probiotic in combination with CHL (group V). The levels of TBARS were increased significantly (P < 0·05) on aflatoxin administration. The simultaneous administration of the combination of probiotic and CHL (group V) and aflatoxin to rats, however, was able to maintain the levels of TBARS as present in the control group (group I).
The levels of caspase-3 activities showed high apoptotic rate in the AFB1 group than in the control group. The levels of TBARS in liver tissues were significantly increased while the levels of GSH and enzyme activities of GPx and GR in liver tissues were significantly decreased in the AFB1 group, compared with those in the controls. The levels of caspase-3 activities were positively correlated with MDA while negatively correlated with GSH, GPx and GR in rat liver treated with AFB1. The apoptotic rate was significantly reduced when FM and CHL were co-administered with AFB1. In rats receiving FM with AFB1, the levels of TBARS in liver tissues were significantly reduced while GSH levels and GPx, GST, SOD and CAT activities were significantly increased compared with the AFB1-fed group. When FM and CHL were co-administered with AFB1, the reduction of apoptotic rate appeared more effective as detected by the decline of caspase-3 activities.
Discussion
Mycotoxins are ubiquitous and produced by several fungi. Humidity, high temperatures and other environmental conditions such as insect infestation can encourage aflatoxin growth on crops. Consequently, aflatoxins can invade the food supply at any time during production, processing, transport and storage. Because aflatoxins, particularly AFB1, are potent carcinogens in some animals, there is interest in the effects of long-term exposure to low levels of these important mycotoxins on humans.
The present study aims to explore the hepatoprotective effect of probiotic FM in combination with CHL on experimental liver damage caused by AFB1 in Wistar rats.
Fermented foods have been considered beneficial to human and animal health. In the present study, feeding of FM alone, as well as in combination with CHL, was found to significantly (P < 0·05) increase in the activities of antioxidative enzymes compared with the group of animals which received AFB1 alone (Table 2).
Table 2 Effect of probiotic fermented milk (FM) and chlorophyllin (CHL) on non-enzymatic and enzymatic antioxidants and the level of thiobarbituric acid-reactive substances (TBARS) and caspases in the liver of rats administered aflatoxin-B1 (AFB1)
(Mean values with their standard errors, n 8)

GST, glutathione-S-transferase; SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase; CDNB, 1-chloro-2,4-dinitrobenzene; GSH, reduced glutathione; pNA, p-nitroaniline.
a,b,c,d,e,f,g,h Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Mean values in rows at 25-week intervals were significantly different from the corresponding values at 15-week intervals (P < 0·05).
Non-lethal and inheritable mutations in cells mediated by the interaction of ingested carcinogens with DNA are the initial events in the initiation of cancer. The necessary step for this process is the activation of a carcinogen to an electrophilic DNA-damaging moiety. Substantial experimental evidence exists which implicates both oxygen and organic free-radical intermediates in the multiple stages of chemical carcinogenesis(Reference Trush and Kensler30). ROS generated in the body are believed to mediate the activation of such carcinogens through various mechanisms including hydroperoxide-dependent oxidation that can be mediated by peroxyl radicals(Reference Trush and Kensler30). This occurs with AFB1, aromatic amines and polycyclic aromatic hydrocarbon dihydrodiols(Reference Trush and Kensler30). ROS, or their by-products of lipid peroxidation such as MDA, have been found to induce mutations in bacterial and mammalian cells and cancer in rats(Reference Chaudhary, Nokubo and Marnett31). MDA can also directly react with deoxynucleosides in vitro and the monomeric adduct of MDA with deoxyguanosine is the major adduct formed(Reference Chaudhary, Nokubo and Marnett31).
The presence of carcinogen–DNA adducts and oxidative DNA adducts generated by chemical carcinogens suggests an interactive role of ROS in initiation. In present study, there was a significant (P < 0·05) increase in GSH levels (Table 2) with a concomitant decrease in TBARS levels (Table 2) in the groups of animals, particularly (AFB1+FM) group III and (CHL+FM+AFB1) group V, compared with the experimental group administered AFB1. Thus, it was found that the level of lipid peroxidation was decreased and that of antioxidants increased after feeding of FM singly or in combination with CHL. These results are similar to those of Yadav et al. (Reference Yadav, Jain and Sinha32) who observed that oral ingestion of probiotic ‘dahi’ (yogurt) reduced the oxidative stress marker TBARS in rat intestinal tissues (P < 0·05). Moorthy et al. (Reference Moorthy, Murali and Devaraj33) and Kamat et al. (Reference Kamat, Boloor and Devasagayam34) also showed that CHL could act as an antioxidant and can inhibit lipid peroxidation.
Cellular glutathione and related enzymes such as GPx, GST and GR are among the principal protective mechanisms against endogenous and exogenous toxic substances and free-radical-mediated damage in hepatic and other tissues(Reference Hayes and McLellan35, Reference Murphy, Scholich and Sies36). The significant reduction (P < 0·05) in the activities of antioxidant enzymes (GPx, GST, SOD and CAT) and the non-enzymatic antioxidant system (GSH) in the AFB1-treated group compared with the control and treatment groups (Table 2) could be responsible for the increased levels of TBARS observed during aflatoxin-induced hepatic damage. Meki et al. (Reference Meki, Abdel-Ghaffar and El-Gibaly37) and Shen et al. (Reference Shen, Shi and Lea38) provided an in vivo evidence that AFB1 could cause lipid peroxidation in rat liver.
The liver occupies a vital role in the main functions of the organism. It is particularly susceptible to chemically induced injury due to its extensive metabolic capacity and cellular heterogeneity. The increase in TBARS levels in the liver of AFB1-treated rats observed in the present study could be due to such toxic nature of AFB1. The significant (P < 0·05) increase in TBARS levels (Table 2) in AFB1-treated rats is similar to the earlier reports(Reference Abdelali, Cassand and Soussotte39, Reference El-Gibaly, Meki and Abdel-Ghaffar40). Moreover, in the present study, the levels of TBARS increased significantly while the levels of antioxidants (GSH, GPx and GST) were significantly lower in the liver tissues of AFB1-treated animals than in the other groups (Table 2). The increase in TBARS levels in the liver of AFB1-treated animals could be due to toxicity caused by AFB1. However, there was a significant (P < 0·05) decrease in TBARS level in (AFB1+FM) group III and (CHL+FM+AFB1) group V compared with the AFB1-administered groups. CHL had a better ameliorative effect, and both probiotic FM and CHL were able to overcome toxicity induced by AFB1. In addition, the levels of caspase-3 activities (Table 2) were positively correlated with TBARS while negatively correlated with GSH and GPx in rat liver treated with AFB1. However, besides oxidative damage directly inflicted on DNA by free radicals, there could be other indirect mechanisms by which radicals can cause genotoxicity. This is another interesting area of future research.
The decrease in SOD as observed in AFB1-treated rats (Table 2) in the present study could be due to the increase in oxidative stress and generation of free radicals. The two ‘remedy’ groups, i.e. (AFB1+FM) group III and (CHL+FM+AFB1) group V, which were fed probiotic FM from the start of the experiment and CHL along with AFB1, and probiotic FM as a curative measure exerted control on the effect of AFB1 and the activities of the antioxidant enzymes continued to increase during the experiment. As expected, the combination of FM and CHL displayed a synergistic effect against carcinogencity caused by AFB1 such that it surpassed even the FM-alone treatment. Kamat et al. (Reference Kamat, Boloor and Devasagayam34) also observed the inhibition of lipid peroxidation by CHL.
CHL is a food-grade derivative of the green plant pigment chlorophyll, and is regarded as a potent in vivo inhibitor of hepatic AFB1–DNA adduction and hepatocarcinogenesis(Reference Breinholt, Schimerlik and Dashwood18). It has also been shown to reduce carcinogen bioavailability, biomarker damage and tumorigenicity in trout and rats. These findings were partially extended to humans, where CHL reduced the excretion of AFB1–DNA repair products in human subjects who were unavoidably exposed to dietary AFB1(Reference Jubert, Mata and Bench41). Initial results suggest that chlorophyll or CHL co-consumption may reduce the bioavailability of ingested aflatoxin in human subjects, as they do in animal models(Reference Jubert, Mata and Bench41). Another study(Reference Zhang, Guan and Wang42) has reported that CHL could induce antioxidant enzymes and confer protection against oxidative damage. More specifically, CHL could induce haem oxygenase-1 and NADPH-quinone-1 expression in human umbilical vein endothelial cells in a time- and dose-dependent manner and protect them against oxidative damage caused by hydrogen peroxide. The induction of haem oxygenase-1 and NADPH-quinone-1 by CHL was accompanied by the accumulation of the transcription factor nuclear factor erythroid 2-related factor 2 in the nucleus and the activation of the phosphatidylinositol 3-kinase/Akt signalling pathway. Additionally, the specific inhibitor of phosphatidylinositol 3-kinase/Akt could obviously decrease not only the induced expression of haem oxygenase-1 and NADPH-quinone-1 but also the antioxidant effect of CHL(Reference Zhang, Guan and Wang42).
The results of the present study showed that GSH was significantly (P < 0·05) reduced in the AFB1-induced group (Table 2) compared with the other control FM and treatment groups (CHL+FM+AFB1) group V, (CHL+AFB1) group IV and (FM+AFB1) group III. It could be inferred that FM and CHL provide a substrate for the synthesis of GSH that protects the cells from oxidative damage by the ROS moiety. The results are in concurrence with those of Kot & Bezkorovainy(Reference Kot and Bezkorovainy43) who demonstrated that Bifidobacterium thermophilum was able to abate the increased activity of free radicals, not only by scavenging them, but also by removing Fe from their environment. Earlier reports have also indicated that GSH and its cooperating enzymes are important in neoplastic diseases and play a crucial role in the defence against ROS(Reference Oberley and Oberley44–Reference Abou Ghalia and Fouad46).
The enzymatic antioxidant defence systems are the natural protectors against lipid peroxidation. SOD, CAT and GPx are important scavengers of superoxide ion and H2O2. These enzymes prevent the generation of hydroxyl radical and protect the cellular constituents against oxidative damage(Reference Scott, Lubin and Zuo47). The significant reduction (P < 0·05) in the activities of antioxidant enzymes (GPx, GST, SOD and CAT) and the non-enzymatic antioxidant system (GSH) in the AFB1-treated group compared with the control group could be responsible for the increased levels of TBARS observed during aflatoxin-induced hepatic damage.
Gratz et al. (Reference Gratz, Taubel and Juvonen48) also demonstrated the modulation of AFB1 uptake in rats by the administration of the probiotic L. rhamnosus GG. Faecal AFB1 excretion in GG-treated rats was increased via bacterial AFB1 binding. Furthermore, AFB1-associated growth faltering and liver injury were alleviated with GG treatment(Reference Gratz, Taubel and Juvonen48). Administration of FM in combination with CHL in the aflatoxin-administered group showed an increase in the activities of antioxidant enzymes compared with the group of rats that received aflatoxin alone. There was also a significant (P < 0·05) increase in GSH levels with a concomitant decrease in TBARS levels in these groups of animals compared with the other groups. In addition, the levels of caspase-3 activities were positively correlated with TBARS while negatively correlated with GSH and GPx in rat liver treated with AFB1. Although, due to the lack of available literature on probiotic FM together with CHL v. AFB1-induced hepatocarcinogenesis, the present results may not be evaluated at large, yet the overall ameliorative effect could not be ruled out. However, further studies in which AFB1 is administered, mixed into feed ingredients and at naturally occurring levels are needed before we fully realise the potential of probiotics or FM and CHL to alleviate or reduce the hepatocarcinogenic effects of AFB1.
Conclusion
The present study indicates that an increase in apoptotic rate in the liver of rats treated with AFB1 is associated with biochemical disturbances in the oxidant/antioxidant balance system, which may be interlinked with the pathogenic network of AFB1 toxicity. However, the overall information obtained from the present study indicates that probiotic FM that is administered individually or jointly with CHL to experimental rats possesses a potent protective effect against AFB1-induced hepatocarcinogenesis.
Acknowledgements
The authors wish to thank the NDRI, Karnal, and the Indian Council of Agricultural Research, New Delhi, India, for financial support. The probiotic culture L. rhamnosus GG provided generously by Professor Goldin and Professor Gorbatch, Tufts University, Medford, MA, USA, is also cordially acknowledged. P. K. A., V. V. and B. S. contributed to the supervision and guidance of the study. R. N. and P. V. B. helped during experimentation and animal handling. A. K. contributed to the writing of the manuscript. The authors declare that there is no conflict of interest.

















