Apples are the world’s second most consumed fruit after bananas. They are often an important contributor to the intake of dietary components linked with chronic disease prevention, including flavonoids( Reference Hodgson and Croft 1 , Reference Hooper, Kroon and Rimm 2 ) and soluble fruit fibre( Reference Threapleton, Greenwood and Evans 3 ). Apples also provide vitamin C, K, Mg and other nutrients( Reference Hyson 4 ). Several prospective observational studies have found that apple intake is associated with reduced risk of CVD and specific cancers( Reference Boyer and Liu 5 ). However, data on the relationship of apple intake with all-cause mortality remain limited( Reference Knekt, Kumpulainen and Järvinen 6 ).
Higher fruit intake is associated with lower risk for all-cause and disease-specific mortality in observational cohort studies( Reference Wang, Ouyang and Liu 7 ). Lifestyle change has the potential to substantially reduce the incidence of chronic diseases( Reference Ezzati and Riboli 8 , Reference Renehan and Howell 9 ). Thus, a key component of recommended lifestyle change is increased consumption of fruits( Reference Liu 10 , Reference Lichtenstein, Appel and Brands 11 ).
The primary objective of the present study was to determine whether higher intake of apples was associated with a reduced risk of all-cause and disease-specific mortality in a cohort of Australian women aged 70–85 years and followed-up for up to 15 years. We also explored whether any observed relationships were independent of the known protective nutrients present in apples. In secondary analyses, we explored the relationships of other commonly consumed fruits with mortality due to all-causes, CVD and cancer.
Methods
Study population
The participants involved in this study were recruited in 1998 to a 5-year, double-blind, randomised controlled trial of daily Ca supplementation to prevent osteoporotic fracture – the Calcium Intake Fracture Outcome Study – which has been described previously( Reference Prince, Devine and Dhaliwal 12 ). The women were recruited by mail from the Western Australian general population. A random selection of 24 800 women aged over 70 years on the electoral roll (n 33 336) received a letter inviting them to join the study. Of the women written to, 5586 (22·5 %) responded. Women were excluded if they were receiving bone-active agents including Ca supplements (22·5 %) or if they had a significant current illness (3·5 %). Participants were similar in terms of disease burden and medication use by general population of this age but they were more likely to be from higher socio-economic groups( Reference Prince, Devine and Dhaliwal 12 ). At the completion of the trial, subjects were invited to participate in two 5-year follow-up observational studies. All the participants provided their written informed consent, and ethics approval was granted by the Human Ethics Committee of the University of Western Australia and the Human Research Ethics Committee of the Western Australian Department of Health (approval number 2009/24). The study included 1456 women of a total of 1500 women recruited to the study. There were missing dietary data on fifteen women and implausible energy intakes (<3350 or >17 575 kJ/d) for an additional twenty-nine women, leaving 1456 cases for analysis.
Dietary assessment
At baseline (1998), dietary intake was assessed using a validated semi-quantitative FFQ developed by the Anti-Cancer Council of Victoria( Reference Hodge, Patterson and Brown 13 ). Energy and nutrient intakes were estimated based on frequency of consumption and an overall estimate of usual portion size( Reference Ireland, Jolley and Giles 14 ). Apple intake and intake of other fruits were assessed and estimated in g/d at baseline from separate questions included in the FFQ, which asked ‘Over the last 12 months, how many times did you usually eat apples/pears/oranges and other citrus/bananas/etc?’. Flavonoid intake was estimated from data from the FFQ using the United States Department of Agriculture Flavonoid 2.1 databases, described in detail previously( Reference Ivey, Hodgson and Croft 15 , Reference Ivey, Lewis and Prince 16 ). The intakes of other components present in apples and other fruits that are thought to contribute to their health benefits, including fibre, vitamin C, K and Mg, were also derived from the FFQ( Reference Ireland, Jolley and Giles 14 ).
The FFQ was administered at baseline, 5, 7 and 10 years. Movement between the categories of apple intake was a guide to determine the stability of apple intake over time. At 5, 7 and 10 years, a total of twelve of 1190 (1·0 %), ten of 989 (1·0 %) and ten of 780 (1·3 %) women, respectively, had moved from highest to lowest or lowest to highest apple intake categories, indicating that apple intake for the groups during the follow-up period remained quite stable. Therefore, all the analyses were based on baseline fruit intake alone.
Outcome and covariate assessment
Mortality data were retrieved from the Western Australian Mortality Database by the Western Australian Data Linkage System (WADLS) for each of the study participants from 1 January 1998 until 31 December 2013. The primary cause of mortality was defined using death certificate data and codes from the International Classification of Diseases, Injuries and Causes of Death Clinical Modification (ICD-9-CM)( 17 ) and the International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Australian Modification (ICD-10-AM)( 18 ). The codes included the following: cardiovascular mortality, ICD-9-CM (390-459) and ICD-10-AM (I00-I99); cancer mortality, ICD-9-CM (140-239 excluding 210-229) and ICD-10-AM (C00-D48 excluding D10-D36); and other causes (all other codes). The primary diagnosis text fields from the death certificate were used to ascertain the cause of death where these coded data were not yet available from the WADLS.
Lifestyle questionnaires were completed by participants at baseline. They obtained information on age, physical activity level and smoking status. The participants provided their previous medical history and current medications verified by their general practitioner. These data were coded using the International Classification of Primary Care-Plus method( Reference Britt, Scahill and Miller 19 ). The coding methodology allows aggregation of different terms for similar pathological entities as defined by the ICD-10 coding system. These data were then used to determine the presence of pre-existing diabetes (T89001-90009). Prevalent CVD was determined from hospital discharge records (1980–1998) retrieved from WADLS for each of the study participants. WADLS provides a complete validated record of every participant’s primary diagnosis at hospital discharge using coded data from all hospitals in Western Australia. CVD codes were identified from the International Classification of Diseases, Injuries and Causes of Death Clinical Modification (ICD-9-CM 390-459)( 17 ). Prevalent cancer records were retrieved from the Western Australian Cancer registry by WADLS. For physical activity, the women filled in a questionnaire that allowed estimation of energy used during exercise in kJ/d using a validated method with published energy costs for specific activities( Reference Pollock, Wilmore and Fox 20 , Reference McArdle, Katch and Katch 21 ). The women were asked whether they participated in any sports, recreation or regular physical activities. Women who answered ‘no’ to this question scored 0, and women who answered ‘yes’ were asked to list up to four sports, recreational activities or forms of regular physical activity including walking that were undertaken in the past 3 months. Energy expenditure (in kJ/d) for these activities was calculated using published energy costs. This measure was shown to be associated with bone density( Reference Devine, Dhaliwal and Dick 22 ). Smoking status was coded as non-smoker, ex-smoker (if they had smoked more than one cigarette per day for more than 3 months at any time in their life) or current smoker. Socio-economic status was assessed using relative social advantage related to residential postcodes according to the Australian Bureau of Statistics method( 23 ). This variable was divided into six categories: 1 being the most disadvantaged and 6 being the least disadvantaged. Baseline weight was assessed using digital scales with participants wearing light clothes and no shoes. Baseline height was assessed using a stadiometer, and BMI was calculated as kg/m2. Treatment (placebo/Ca) over the first 5 years was included as a covariate.
Statistical analysis
Analyses were carried out using IBM SPSS Statistics version 21 (2012IBM Corp.). Statistical significance was set at P<0·05 for all tests. Descriptive data are presented as mean values and standard deviations, medians and interquartile ranges or numbers and percentages. Differences in baseline characteristics across categories of apple intake were derived from the ANOVA or Pearson’s χ 2 test. An analytical protocol was developed before the commencement of analysis. The primary analysis explored relationships of apple intake with mortality outcomes, and secondary analyses explored relationships of total and other individual fruit intake with mortality outcomes. Two models of adjustment were used – age-adjusted model and multivariable-adjusted model (age, BMI, treatment code, smoking status (three levels), socio-economic status (six levels), prevalent diabetes, prevalent CVD, prevalent cancer, use of antihypertensive medication, use of cholesterol-lowering medication, use of low-dose aspirin, physical activity, energy intake and alcohol intake( Reference Blekkenhorst, Prince and Hodgson 24 )). We have also considered additional adjustment beyond the multivariable-adjusted model. First, the potential mediating effects of dietary factors found in apples and other fruits that are linked to chronic disease protection were investigated using further adjustment for intakes of flavonoids, total dietary fibre, K, Mg and vitamin C. Second, in separate models, we have adjusted for dietary factors that have been linked with mortality and/or apple intake including SFA intake, total non-apple fruit intake and total vegetable intake. Cox proportional hazard models were used for death outcomes. Initial analyses used apple intake as a continuous variable (presented per sd change in intake). We then used a pre-specified categorical variable with three levels: low, moderate and high. The cut-off values for low intake was <5 g/d (which would equate to an intake of <20 apples/year), for moderate intake 5–100 g/d and for high intake >100 g/d (which would equate to a small apple each day). We tested for evidence of a linear trend for fruit intakes as continuous variables using the median value for each intake category in separate Cox proportional hazards models. No violations of the Cox proportional hazards assumptions were detected using the global test. Secondary analyses used Cox proportional hazard models to assess associations of intake of total fruit and individual fruits (pears, oranges and other citrus fruits and bananas) as continuous variables (presented per sd change in intake) with all-cause mortality, CVD mortality and cancer mortality.
Results
Baseline characteristics
Characteristics of the participants at baseline across apple intake categories are presented in Table 1. The mean total fruit intake was 244 (sd 129) g/d. Four fruits contributed approximately 75 % of total fruit intake. These included apples (20 %), pears (11 %), oranges and other citrus fruits (23 %) and bananas (21 %). The mean apple intake was 48 (sd 53) g/d. Apple intake at baseline was significantly correlated with apple intake at 5 years (r 0·47; P<0·001), 7 years (r 0·43; P<0·001) and 10 years (r 0·39; P<0·001). Significant differences were observed across the categories of apple intake for smoking status and for energy, alcohol, MUFA, PUFA, total flavonoid, dietary fibre, vitamin C, K and Mg intakes.
Table 1 Baseline characteristics according to apple intake category (Mean values and standard deviations; medians and interquartile ranges (IQR); numbers and percentages)

* P values for difference in baseline characteristics across apple intake categories were derived from Pearson’s χ 2 test or ANOVA.
† Type 2 diabetes.
Apple and other fruit intake and mortality
Over 15 years of follow-up, 607 (41·7 %) women died from any cause. The primary causes of death in this cohort were CVD (n 235; 38·7 % of all deaths) and cancer (n 156; 25·7 % of all deaths). In multivariable-adjusted analyses, each sd increase in apple intake (53 g/d) was associated with a reduced risk of death from all-cause (hazard ratio (HR) 0·89; 95 % CI 0·81, 0·97) and cancer (HR 0·81; 95 % CI 0·67, 0·99) but not CVD (HR 0·91; 95 % CI 0·79, 1·05) (Fig. 1). The relationship of intakes of total and other individual fruits with all-cause, CVD and cancer mortality were also explored (Fig. 1). In multivariable-adjusted models, higher total fruit and banana intakes were associated with lower risk of CVD mortality (P<0·05).
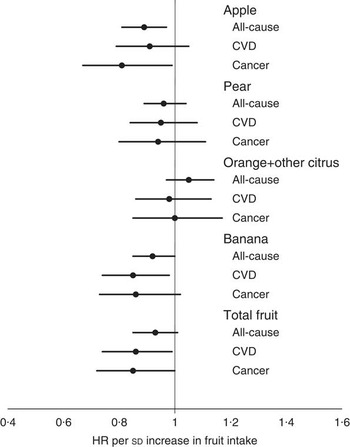
Fig. 1 Multivariable-adjusted hazard ratio (HR) for all-cause mortality (n 601), CVD mortality (n 235) and cancer mortality (n 156) per sd increase in intake of apple (sd 53 g/d), pear (sd 33 g/d), orange and other citrus fruits (sd 59 g/d), banana (sd 41 g/d) and total fruit (sd 129 g/d). The model included age, BMI, treatment code, smoking status (three levels), socio-economic status (six levels), prevalent diabetes, prevalent CVD, prevalent cancer, use of antihypertensive medication, use of cholesterol-lowering medication, use of low-dose aspirin, physical activity, energy intake and alcohol intake.
In separate analyses, the relationships of apple intake with the risk of all-cause, CVD and cancer mortality were explored using categories of apple intake. Women who consumed more than the equivalent of a small apple each day (>100 g/d) and those with moderate apple consumption (5–100 g/d) were compared with women with low apple consumption (<5 g/d) in the 12 months before baseline (Table 2). In multivariable-adjusted models, moderate apple consumption was associated with a 20 % lower risk of all-cause mortality, whereas those who ate an apple a day had a 35 % lower risk of all-cause mortality compared with women with low apple consumption (Fig. 2). Further adjustment for total flavonoids, total dietary fibre, K, Mg and vitamin C attenuated the association of apple intake with all-cause mortality (P for trend=0·08). Similarly, further adjustment for dietary factors that have been linked with mortality and/or apple intake (SFA intake, total non-apple fruit intake and total vegetable intake) also attenuated the association of apple intake with all-cause mortality (P for trend=0·06).

Fig. 2 Multivariable-adjusted hazard ratio (HR) for all-cause mortality (n 601) for consumption of low (<5 g/d – ![]() ; HR 1·00 (referent)), moderate (5–100 g/d –
; HR 1·00 (referent)), moderate (5–100 g/d – ![]() ; HR 0·80 (95 % CI 0·66, 0·98; P=0·031)) and high (>100 g/d –
; HR 0·80 (95 % CI 0·66, 0·98; P=0·031)) and high (>100 g/d – ![]() ; HR 0·65 (95 % CI 0·48, 0·89; P=0·006)) apple intake. The model included age, BMI (kg/m2), treatment code, smoking status (three levels), socio-economic status (six levels), prevalent diabetes, prevalent CVD, prevalent cancer, use of antihypertensive medication, use of cholesterol-lowering medication, use of low-dose aspirin, physical activity, energy intake and alcohol intake.
; HR 0·65 (95 % CI 0·48, 0·89; P=0·006)) apple intake. The model included age, BMI (kg/m2), treatment code, smoking status (three levels), socio-economic status (six levels), prevalent diabetes, prevalent CVD, prevalent cancer, use of antihypertensive medication, use of cholesterol-lowering medication, use of low-dose aspirin, physical activity, energy intake and alcohol intake.
Table 2 Hazard ratios (HR) for all-cause, CVD and cancer mortality according to apple intake catergoryFootnote * (HR and 95 % confidence intervals; numbers and percentages)

* HR (95 % CI) for all-cause mortality according to the category of apple intake analysed using Cox proportional hazard models.
† Test for trend conducted using median value for each apple intake category.
‡ Significantly different from the lowest category of apple intake: P<0·05.
§ Significantly different from the lowest category of apple intake: P<0·01.
|| The multivariable-adjusted model includes age, BMI, treatment code, smoking status (three levels), socio-economic status (six levels), prevalent diabetes, prevalent CVD, prevalent cancer, use of antihypertensive medication, use of cholesterol-lowering medication, use of low-dose aspirin, physical activity, energy intake and alcohol intake.
Smoking status was markedly different across apple intake categories (Table 1). There was a substantially higher proportion of ex-smokers included in the lowest category of apple intake. The inverse relationship of apple intake with all-cause mortality remained significant after adjustment for smoking status (Fig. 1 and 2). However, we have also explored the relationship of apple intake with all-cause mortality within the non-smoker and ex-smoker groups in separate analyses. In multivariable-adjusted analyses, each sd increase in apple intake (53 g/d) was associated with a reduced risk of death from all-causes among the non-smokers (HR 0·86; 95 % CI 0·75, 0·97), but not among the ex-smokers (HR 0·94; 95 % CI 0·82, 1·07). For total fruit intake, each sd increase in apple intake (129 g/d) was associated with a reduced risk of death from all-causes among the non-smokers (HR 0·88; 95 % CI 0·79, 0·99), but not among the ex-smokers (HR 0·98; 95 % CI 0·86, 1·11). Results were similar for CVD mortality, but the relationships of both apple and total fruit intake with cancer mortality were comparable for non-smokers and ex-smokers (data not presented).
Discussion
Higher fruit intakes are associated with lower risk of all-cause mortality( Reference Wang, Ouyang and Liu 7 ), but the contribution of specific fruits to these benefits is less clear. Apples, which are the world’s second most consumed fruit, often provide a major contribution to total fruit intake. In our cohort, where apple intake provided approximately 20 % of the total fruit intake, we found that higher apple intake was associated with lower all-cause and cancer mortality.
Diets low in fruits were attributed to more than five million deaths in 2010( Reference Ezzati and Riboli 8 ). A recent meta-analysis including prospective cohort studies found a 6 % risk reduction in all-cause mortality, a 5 % risk reduction in CVD mortality and a non-significant 1 % risk reduction in cancer mortality for each additional serving (80 g/d) of fruit. For all-cause mortality, the relationship was linear up to two servings of fruits, after which there was little additional hazard reduction( Reference Wang, Ouyang and Liu 7 ). Our analysis found a non-significant 7 % lower risk of mortality from any cause for per sd (129 g/d) increase in total fruit intake and a significant 14 and 15 % lower risk of CVD and cancer mortality, respectively. These results are largely consistent with the results of previous studies( Reference Wang, Ouyang and Liu 7 ).
There are limited data on the association of apple intake with all-cause mortality( Reference Knekt, Kumpulainen and Järvinen 6 ). We found that the women who consumed >100 g/d of apple had a 35 % lower risk of mortality compared with those who consumed <5 g/d. CVD and cancer are the two principal causes of death worldwide. Several previous studies have explored the link between apple intake and risk of CVD or cancer outcomes( Reference Boyer and Liu 5 ). A higher apple intake is consistently associated with reduced risk of CHD mortality( Reference Knekt, Kumpulainen and Järvinen 6 , Reference Knekt, Jarvinen and Reunanen 25 – Reference Yochum, Kushi and Meyer 28 ) and incidence of stroke( Reference Knekt, Kumpulainen and Järvinen 6 , Reference Larsson, Virtamo and Wolk 29 , Reference Griep, Verschuren and Kromhout 30 ). Observational studies have also linked higher apple intake with reduced risk of lung cancer( Reference Knekt, Kumpulainen and Järvinen 6 , Reference Knekt, Järvinen and Seppänen 31 – Reference Le Marchand, Murphy and Hankin 33 ) and gastrointestinal cancers( Reference Freedman, Park and Subar 34 , Reference Gallus, Talamini and Giacosa 35 ). Our results indicate that higher apple intakes are associated with reductions in risk of cancer mortality in the elderly women.
If apples do contribute to reduced risk of dying from a chronic disease, available data indicate that both known and unknown food components could be involved. Apples make a contribution to the intake of flavonoids, fibre, vitamin C, K, Mg and several other nutrients( Reference Hyson 4 ). The daily consumption of one small apple (100 g) can provide up to approximately 10 % of the daily intake of total flavonoids and dietary fibre and 2–4 % of the daily intake of vitamin C, K and Mg. In this cohort, adjustment for intake of these dietary components minimally changed the observed association of apple intake with mortality, indicating that the association of apple consumption with mortality is only partially mediated by the increased consumption of these nutrients.
Both flavonoid and fibre intakes have been associated with benefits on chronic diseases and their risk factors. Higher intakes of total and specific flavonoids have been associated with reduced risk of CVD( Reference Hooper, Kroon and Rimm 2 , Reference Knekt, Kumpulainen and Järvinen 6 , Reference Knekt, Jarvinen and Reunanen 25 , Reference Mink, Scrafford and Barraj 27 ), particular cancers( Reference Knekt, Järvinen and Seppänen 31 , Reference Hui, Qi and Qianyong 36 ) and all-cause mortality( Reference Ivey, Hodgson and Croft 15 ). A class of flavonoids consistently related to reduced risk of chronic disease is the flavonols( Reference Ivey, Hodgson and Croft 15 , Reference Hui, Qi and Qianyong 36 , Reference Huxley and Neil 37 ), of which apples often provide a major contribution to overall intake( Reference Knekt, Jarvinen and Reunanen 25 – Reference Mink, Scrafford and Barraj 27 ). The mechanisms by which dietary flavonoids might reduce risk of CVD and cancer are not clearly defined. However, particular flavonoids including the flavonols present in apples enhance nitric oxide status and improve vascular function( Reference Bondonno, Croft and Ward 38 , Reference Bondonno, Yang and Croft 39 ). Higher fibre intakes have been associated with lower risk of CVD( Reference Threapleton, Greenwood and Evans 3 , Reference Streppel, Ocké and Boshuizen 40 ), specific cancers( Reference Aune, Chan and Lau 41 ) and all-cause mortality( Reference Streppel, Ocké and Boshuizen 40 ). There is also evidence that fruit fibre in particular could contribute to these benefits( Reference Threapleton, Greenwood and Evans 3 , Reference Aune, Chan and Lau 41 ). Most of the fibre present in apples is pectin, which can reduce circulating cholesterol concentrations( Reference Theuwissen and Mensink 42 ). Mechanisms of benefit of fruit fibre on specific cancers are less clear. Furthermore, there is evidence that vitamin C( Reference Kobylecki, Afzal and Smith 43 ), K( Reference Yang, Liu and Kuklina 44 ) and Mg( Reference Leone, Courbon and Ducimetiere 45 ) could contribute to the health benefits of fruit. However, given that adjustment for these nutrients together with fibre and flavonoids had little impact on multivariate-adjusted effects suggests that there are other potential mechanisms or interactions involved. An additional possible pathway to health benefits of apples involves interaction between flavonoids, fibre and the gastrointestinal microbiota( Reference Cardona, Andrés-Lacueva and Tulipani 46 , Reference Koutsos, Tuohy and Lovegrove 47 ). There is increasing evidence that the microbiome plays an important role in nutritional determinants of human health( Reference Cho and Blaser 48 ). We speculate that different fruits could have different effects on the microbiome due to the differences in structural characteristics of fibre, flavonoids and/or other components present.
There are a number of strengths to this study. The long follow-up and resulting high mortality over this time helped increase the power of the study. In addition, mortality outcomes were ascertained using the Western Australian Mortality Database via the WADLS, and therefore all the participants remaining in the state had complete follow-up independent of self-report. Finally, multiple measures of apple consumption were recorded and remained relatively consistent in this cohort over time, suggesting that the observed associations are likely to be a true representation of the relationship between long-term habitual apple intake and mortality.
There are, however, a number of limitations to this study. First, the study included only women, and was an observational study, and thus causality cannot be firmly established. There remains the possibility of residual confounding and selection bias, although the women were mostly representative of the general population. In addition, although the validated FFQ used to assess fruit intake is likely to provide a reasonable estimate of fruit intakes, there will be error in the measurement. Any measurement error would, however, be likely to be non-differential and would therefore only lead to more conservative effect estimates. Furthermore, the questionnaire was not followed-up each year, but multiple measures of apple consumption indicated relatively consistent apple intake in this cohort over time. Finally, although the women recruited to this study are likely to be representative of elderly women at high risk of mortality, additional studies are needed in younger women and in men.
In conclusion, we have shown that a higher apple intake was dose-dependently associated with lower risk of all-cause and cancer mortality in elderly women. Our results support the concept that an apple a day protects against death in elderly women, particularly via reductions in the risk of cancer.
Acknowledgements
The authors thank the staff at the Data Linkage Branch, Hospital Morbidity Data Collection and Registry of Births, Deaths and Marriages for their work on providing the data for this study.
The study was supported by research grants from Healthway Health Promotion Foundation of Western Australia, Sir Charles Gairdner Hospital Research Advisory Committee and by the project grants 254627, 303169 and 572604 from the National Health and Medical Research Council of Australia. The salary of J. M. H. is supported by a National Health and Medical Research Council of Australia Senior Research Fellowship. The salary of N. C. W. was supported by a MRF/UWA Post-doctoral Fellowship. None of these funding agencies had any input into any aspect of the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication. J. M. H., C. P. B. and K. D. C. have received funding support from the Department of Agriculture and Food, Western Australia and Fruit West to support research into the development and evaluation of new apple varieties.
The authors’ responsibilities were as follows – J. M. H., R. L. P., R. J. W., C. P. B., N. B., N. C. W., K. D. C. and J. R. L. were responsible for the project conception; J. M. H., R. L. P. and J. R. L. developed the research plan; R. L. P. and J. R. L. collected data; J. M. H., R. J. W. and J. R. L. analysed the data; J. M. H., R. L. P., R. J. W., C. P. B., K. L. I., N. B., E. B. R., N. C. W., K. D. C. and J. R. L. interpreted the data, J. M. H. prepared the manuscript; J. M. H., R. L. P., R. J. W., C. P. B., K. L. I., N. B., E. B. R., N. C. W., K. D. C. and J. R. L. critically reviewed the manuscript. All the authors read and approved the final version of the manuscript. J. M. H. had the primary responsibility for the final content.
None of the other authors has any conflicts of interest.







